View sample yellow fever research paper. Browse research paper examples for more inspiration. If you need a health research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
This sample Yellow Fever Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Introduction
Yellow fever (YF) is a viral hemorrhagic fever with high mortality transmitted by mosquitoes. The term ‘yellow fever’ refers to the yellow color, or jaundice, seen in people with the hepatitis (liver disease) of YF. YF virus is a member of the Flavivirus genus and is related to other mosquitoborne viruses including dengue, West Nile, and Japanese encephalitis viruses. Yellow fever disease occurs now only in Africa and Central and South America, though historically large outbreaks occurred in Europe and North America. Mosquitoes capable of transmitting YF exist in regions where disease does not presently occur and regions, such as Asia, where yellow fever has never occurred. Vector control strategies once successful in eliminating YF from many areas have faltered, leading to re-emergence of disease (Robertson et al., 1996). Consequently, immunization is now the most important method of prevention of YF, supplemented by prevention of mosquito bites.
Effective vaccines against YF have been available for almost 70 years and are responsible for significant disease reduction worldwide. Currently available vaccines protect against all YF virus strains and are attenuated live-virus vaccines derived from a virus originally isolated in 1927.
This virus strain was attenuated by passage in mouse embryo tissue culture, then chicken embryo tissue culture, resulting in the 17D strain from which all current vaccines are derived. Yellow fever is the only disease for which immunization is regulated by international law. Recently, newly recognized serious but rare adverse events to YF vaccines have been described, and have prompted investigation into the mechanisms of these adverse reactions and into clarifying the most appropriate indications for YF vaccine.
Virology
Yellow fever virus is a member of the genus Flavivirus, small (40–60 nm) single-stranded RNA viruses. Yellow fever virus is antigenic ally and evolutionarily distinct from other flaviviruses. A single serotype exists, so vaccine protects against all strains of the virus. Seven genotypes have been identified, and entire genomes have been sequenced for two: the Asibi strain, from which the 17D vaccine is derived, and the French viscerotropic virus, from which the French neurologic vaccines were derived (Monath, 2004).
Epidemiology
Approximately 200 000 cases of YF occur annually, resulting in about 30 000 deaths; 90% of cases occur in Africa. Large epidemics, with over 100 000 cases, have been recorded repeatedly in Sub-Saharan Africa, and multiple outbreaks have occurred in the Americas. The virus has never appeared in Asia or the Indian subcontinent (Barnett, 2007). Historically, epidemics of yellow fever occurred in the Americas beginning probably in the seventeenth century, introduced by ships carrying infected vector mosquitoes. Large outbreaks occurred in Philadelphia in 1793, the lower Mississippi Valley in 1878, and New Orleans in 1905. Identification of the mosquito as the vector of transmission of yellow fever, as a result of the work of Carlos Findlay in Cuba and Walter Reed and colleagues in Panama, led to the aggressive vector-control measures that resulted in elimination of the disease from the United States and reduction in the areas in which outbreaks of yellow fever occurred. Aedes aegyptii is the major YF mosquito vector species. Vaccine development occurred rapidly following isolation of the yellow fever virus in 1927 and, combined with mosquito control, contributed to significant reduction of disease in South America and Africa in the first half of the twentieth century (Monath, 2004).
A significant resurgence of YF has occurred since the 1980s, in both Sub-Saharan Africa and South America (Robertson et al., 1996). A series of epidemics and smaller outbreaks throughout West Africa were primarily responsible for the increased incidence of YF in Africa, but the first epidemic reported in Kenya in more than two decades signaled that a change in the distribution of disease was also occurring. Transmission in Africa is maintained by a high density of vector mosquito populations in proximity to human populations that are, for the most part, unvaccinated. Although some countries have incorporated YF vaccine into childhood immunization programs, vaccine coverage is not optimal.
In South America, disease occurs less frequently than in Africa in part because of higher vaccine coverage occurring primarily as part of mass immunization campaigns in response to outbreaks. The largest outbreak in South America since the 1950s occurred in Peru in 1995, and cases were reported in Bolivia, Brazil, Colombia, Ecuador, and Peru from 1985 to 1994. Resurgence of disease in Brazil in the late 1990s and early 2000s prompted mass vaccination campaigns. Transmission of yellow fever in South America involves monkeys and daytime biting mosquitoes that live in the forest canopy, usually in relative isolation from humans. Forest clearing and expanding agricultural activities are occurring with increased frequency in areas of yellow fever transmission, attracting workers who migrate from nonendemic areas. Thus, factors related to resurgence of disease in South America include relatively low vaccine coverage in areas in which outbreaks occur, migration of susceptible individuals into forested regions where disease is transmitted, and increasing urbanization of YF. Dengue fever is transmitted by Aedes aegyptii as well, and there are concerns that YF may become epidemic again in regions where dengue has become epidemic.
Accurate data about burden of YF are difficult to obtain because of under-reporting of disease especially from isolated areas, limitations of passive surveillance, lack of diagnostic capability in many YF endemic areas, and occurrence of asymptomatic infection. Such challenges bolster support for immunization programs as the mainstay of prevention in endemic areas.
Yellow fever has occurred in unvaccinated travelers. From 1970 to 2002, nine cases were reported in unimmunized travelers from the United States and Europe; disease was acquired in Brazil (three cases), Senegal (two cases), Venezuela, Ivory Coast, the Gambia, and West Africa. The mortality rate was 89%. Another case occurred in 1987 in an immunized traveler from Spain who visited four countries in West Africa (Monath and Cetron, 2002; Wilson et al., 2004). Estimation of risk of YF associated with travel is made difficult by fluctuation of disease by year and season, vaccine coverage of the local population, making it more challenging to estimate risk to the unimmunized, and incomplete surveillance data (Barnett, 2007). Areas of current risk for disease are shown in Figures 1 and 2.
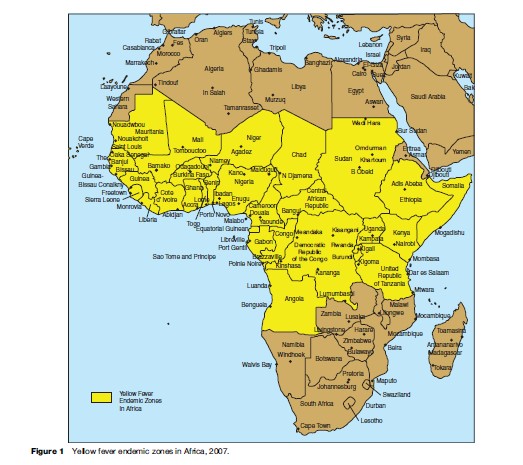
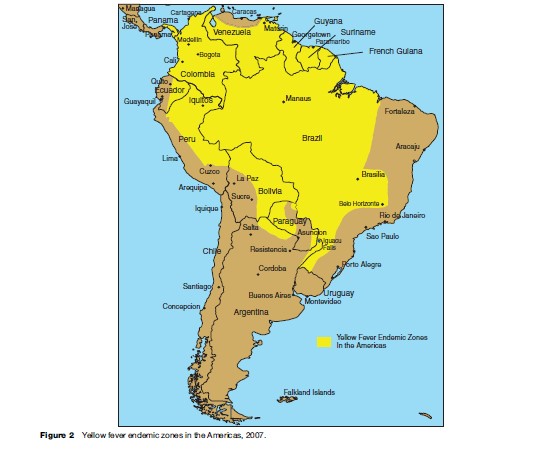
Clinical Description Of Yellow Fever
Yellow fever disease ranges from subclinical infection to life-threatening systemic disease with fever, jaundice, hemorrhage, and renal failure. Individuals of all ages are affected, but disease is most severe, and mortality the highest, in the elderly. Differences in virus strains as well as incompletely understood host immune factors are likely responsible for the range of clinical symptoms. Genetic factors may also be important in susceptibility to YF virus disease.
Three phases of YF are described. The first, during which virus is present in blood (viremia), is characterized by fever, malaise, generalized myalgia, nausea, vomiting, irritability, dizziness, and a generally toxic appearance. Laboratory abnormalities include leukopenia, present at the onset of illness, and elevation of serum liver transaminase levels on days 2–3 of illness, before the onset of jaundice. Viremia peaks 2–3 days after infection, with fatal cases having a longer duration of viremia than survivors. The second phase, the period of remission, is characterized by improvement in symptoms with a reduction of fever; this may last up to 48 h, but is not noted in all cases. Some infected individuals recover at this phase without developing jaundice; these cases are referred to as abortive infections. Because of the nonspecific nature of the illness when it resolves at this point, diagnosis cannot be made clinically; it is therefore not known what proportion of YF cases are subclinical or abortive.
The third phase, the period of intoxication, occurs in about 15% of cases, and is characterized by return of fever, nausea, vomiting, jaundice, and bleeding diathesis. Severity of symptoms is related to the degree of liver dysfunction that occurs. Antibodies appear in the blood as virus disappears. Multi-organ system involvement is typical and may include renal dysfunction, bleeding diathesis, myocardial injury, and central nervous system dysfunction. Serum liver transaminase and bilirubin levels are proportional to the severity of disease; they peak early in the second week of illness and then decline rapidly in patients who recover. In contrast to other forms of viral hepatitis, AST levels typically exceed ALT levels. Casefatality rates vary widely, but were in the range of 20% in West African patients with jaundice in several studies (Monath, 2004).
Diagnosis
Clinical diagnosis of yellow fever is possible when the pathognomonic features of biphasic/triphasic acute illness and typical clinical features occur in unvaccinated individuals with a compatible exposure history. Unfortunately, these features are present only in a minority of patients. Laboratory diagnosis of YF is made by detection of either virus or virus antigen or genome (by enzymelinked immunosorbent assay (ELISA), polymerase chain reaction (PCR), or inoculation virus into suckling mice, mosquitoes, or cell cultures), or by serology (IgM capture ELISA), though cross-reactions with other flaviviruses complicate serologic methods of diagnosis. Postmortem examination of the liver reveals pathognomonic features of YF, including mid-zonal necrosis, and definitive diagnosis can be made by immunohistochemical staining of tissues (liver, heart, kidneys) for yellow fever antigen. It is important to note that liver biopsy should never be used for diagnosis during YF illness because of the risk for fatal hemorrhage at the biopsy site (Monath, 2004).
Treatment
Treatment of YF is generally supportive, as no specific therapy for YF exists. Information about potential treatment modalities is available from studies of treatment of YF in humans, from animal models, and from retrospective epidemiologic studies of YF virus adverse events (Barnett, 2007). Rhesus monkeys were protected from YF virus challenge when given YF antiserum; administration of immune serum after onset of clinical disease had no beneficial effect (Monath, 2004). Clinical experience with use of immune globulin in cases of vaccine-induced viscerotropic disease (a rare and severe side effect, described below) has not been promising, but use of the product very early in the clinical illness, when there remains potential for affecting the level of viremia, has not been studied. Use of interferons for prevention and treatment of YF is limited by the need to use these products before infection or during the incubation period in order to have therapeutic benefit (Monath, 2004). Ribavirin is active in vitro against YF, but high doses are required to achieve a beneficial effect. Ribavirin was ineffective in monkey and mouse models, but did show reduced mortality in a hamster model. A retrospective study of corticosteroid therapy in 11 cases of vaccine induced viscerotropic disease identified a higher rate of survival in patients receiving stress-dose steroids (75%; 3 of 4) compared with those who received no steroids, or high or low-dose steroids (29%; 2 of 7).
Prevention Of Yellow Fever
Vector Control Strategies
Methods to control YF have focused on elimination of possible breeding areas. Vector control strategies, initially highly successful, have foundered due to lack of coordinated political will, the shifting balance of human populations and development of rural areas, and global warming, all of which have contributed to reducing barriers to the spread of the mosquito vectors. It is unlikely that vector control strategies alone will result in elimination of yellow fever; such strategies must be combined with effective vaccination programs.
Yellow Fever Vaccines
Vaccine Development
YF virus was isolated in 1927 in Lagos, Nigeria (Asibi strain) and Dakar, Senegal (French strain). Research into development of YF vaccine began soon thereafter in England, the United States, West Africa, and Brazil. Efforts to produce inactivated vaccines early in the twentieth century were unsuccessful, and subsequent work on vaccine development focused on live virus products. Use of French neurologic vaccine began in the 1930s and proved effective, especially in curtailing epidemic disease in West Africa, but this vaccine was discontinued in 1982 because of an unacceptably high incidence of adverse events, especially encephalitis.
All current YF vaccines derive from the 17D strain. In the initial phase of YF vaccine production in the United States and Brazil between 1937 and 1941, two main lineages of the 17D line, 17D-204 and 17DD, were used for vaccine production (Monath, 2004). Recognition that continued serial passage could result in substrains with unacceptably high rates of adverse events led to the adoption of the ‘seedlot’ system of vaccine production. Primary and secondary seed lots were prepared and characterized, and all vaccine lots were prepared from a single passage from the secondary seed. In 1957 WHO published ‘‘Requirements for Yellow Fever Vaccine,’’ which standardized the seed lot and manufacturing procedures. These procedures, and the concept of using a stable source of seed stock, are now also used for other vaccines such as measles. New seed lots are tested for neurovirulence and viscerotropism before being used for vaccine production. The vaccine contains no antibiotics or preservatives such as thimerosol, but some preparations do contain gelatin, and latex is present in the stopper of the vaccine vial (Monath, 2004).
Vaccine Response
Vaccination against YF produces high levels of protection, with seroconversion rates of greater than 95% in children and adults and duration of immunity of at least 10 years (Poland et al., 1981). Ninety percent of vaccine recipients develop neutralizing antibody within 10 days after immunization, and 99% within 30 days. Although immunity is likely to be lifelong after a single dose, international health regulations recommend revaccination at 10-year intervals for those remaining at risk. Neutralizing antibody titers at the time of booster immunization may affect response to the booster dose: patients with lower prevaccine titers developed a more robust antibody response and had a more rapid decline in antibody titer in a recent study of U.S. Army laboratory workers. Serologic response to YF vaccine is not diminished by simultaneous administration of tetanus, diphtheria, pertussis, measles, polio, Bacillus Calmette-Gue´rin, hepatitis A, hepatitis B, Vi antigen capsular polysaccharide typhoid, oral Ty21a typhoid vaccines, or oral cholera vaccine (Monath and Cetron, 2002). Data on response to YF vaccine when administered with Japanese encephalitis ( JE) vaccine are lacking, though prior infection with JE does not interfere with protection. Prior dengue infection may decrease response to YF vaccine (Monath and Cetron, 2002). Immune globulin did not decrease the antibody response to YF vaccine when given 0–7 days before immunization.
Chloroquine does not affect adversely the antibody response to YF vaccine. Mild viremia with the YF vaccine strain occurs 3 to 7 days after immunization in individuals receiving their first dose of vaccine, lasting for 1 to 3 days. Elevations of interferon-alpha, tumor necrosis factoralpha, and markers of T-cell activation occur at this time and are likely mediators of common, mild side effects of YF vaccine. A recent study from Brazil investigated phenotypic responses of major and minor peripheral blood lymphocyte subpopulations and identified features associated with both activation events and modulatory pathways following initial immunization with the 17DD YF vaccine. The balance of these events may be important in the development of an adequate immune response and the prevention of adverse events to vaccine. Resolution of viremia following first-time immunization occurs as neutralizing antibody develops. Viremia does not occur with subsequent doses of vaccine, and side effects are milder. No data are available about levels or duration of viremia in children or immunosuppressed individuals (Monath, 2004).
Common Vaccine Side Effects
Side effects are generally mild, and include headaches, myalgia, and low-grade fever occurring 5 to 10 days after immunization in less than 25% of those participating in clinical trials of YF vaccine.
Severe Vaccine Adverse Events
Cases of severe multi-organ failure following YF vaccine were reported beginning in 1996, raising awareness in the medical community of adverse events associated with YF vaccine. Identifying the spectrum of adverse events and risk factors for severe reactions is the subject of intense investigation (Barnett, 2007).
The three kinds of severe adverse events to YF vaccine are immediate hypersensitivity reactions, neurologic disease, and viscerotropic disease.
Hypersensitivity Reactions
YF vaccine is prepared in embryonated eggs, and individuals with egg allergy should not receive vaccine. Individuals able to eat eggs or egg products can receive vaccine. Systemic allergic reactions, such as anaphylaxis and urticaria, have been reported in 1 in 58 000 to 131 000 individuals following administration of YF vaccine. Sensitivity to other vaccine components, especially gelatin, but also potentially latex found in the stopper of the vaccine vial, may play a role in these events.
Yellow Fever Vaccine-Associated Neurologic Disease
Yellow fever vaccine-associated neurologic disease (YEL- AND) (formerly termed postvaccinal encephalitis) was historically the most common severe adverse event, especially in infants. Between 1945, when the seed-lot system for vaccine development was introduced, and 2002, encephalitis was reported in 25 patients worldwide among more than 200 million doses of vaccine distributed. One patient died; the others recovered without sequelae. Sixteen cases occurred in those under 9 months of age before age restrictions were placed on the use of YF vaccine (Centers for Disease Control and Prevention, 2002a; Monath, 2004). An additional five cases have been reported in the literature: two cases of encephalitis, one of Guillain-Barre´ syndrome, and one of bulbar palsy, in Europe from 1991 to 2001 (1.3 cases of YEL-AND per million doses distributed), and a fatal case of encephalitis in a man from Thailand with unrecognized HIV infection. Incidence of YEL-AND in very young infants is estimated to be 0.5 to 4 per 1000 (Monath, 2004). In the United States, the reported rate for YEL-AND following yellow fever immunization is approximately 0.5 per 100 000 doses distributed (Eidex et al., 2007). The Vaccine Information Statement (VIS), prepared by the U.S. Centers for Disease Control and Prevention (CDC) for distribution to vaccine recipients, states the incidence as 1:150 000–250 000 doses.
Onset of YEL-AND in recent cases has ranged from 4 to 23 days after vaccination, and the syndrome is associated with fever, headache, and either focal or generalized neurologic dysfunction. Laboratory findings include CSF pleocytosis (100–500 WBCs per mL) and elevated protein, indicating inflammation of the central nervous system. Liver function tests are usually normal. Laboratory methods used to make a diagnosis of YELAND have included finding virus, viral genome, or YFspecific IgM in cerebrospinal fluid (Centers for Disease Control and Prevention, 2002b). The case-fatality rate is less than 5%, and most affected individuals recover without sequelae. A recent review of 15 cases of neurologic disease associated with YF immunization in the United States from 1990 to 2005 identified cases of acute disseminated encephalomyelitis and Guillain-Barre´ syndrome in addition to encephalitis (McMahon et al., 2007). Twelve cases of aseptic meningitis were reported in association with the 2001 YF mass immunization campaign in Juiz de Fora, Brazil.
Yellow Fever Vaccine-Associated Viscerotropic Disease
Yellow fever vaccine-associated viscerotropic disease (YEL-AVD), a syndrome of fever, jaundice, and multiple organ system failure following YF vaccine, was reported in ten patients worldwide, ranging in age from 5 to 79 years, from 1996 to 2001 (Centers for Disease Control and Prevention, 2001). In 2002 two additional suspected cases of viscerotropic disease and four of neurologic disease were reported in U.S. recipients of YFV, and two fatal cases, both in young women, were reported in 2005 and 2007 (Centers for Disease Control and Prevention, 2002b). As of August 2006, more than 30 cases were described worldwide (Eidex et al., 2007). Though identified first in 1996, characterization of a YF virus strain as vaccine-derived – from what was thought initially to be a fatal case of YF in Brazil in 1975 – suggests that this syndrome has been present for at least several decades.
Yellow fever vaccine-associated viscerotropic disease (YEL-AVD), initially called febrile multiple organ system failure, ranges in severity from moderate disease with focal organ dysfunction to severe multisystem failure and death, and may include neurologic disease. The syndrome resembles severe wild-type yellow fever and laboratory evidence indicates overwhelming infection with vaccine strain YF virus. It has occurred only in first time nonimmune vaccine recipients. Symptoms begin 2 to 5 days after immunization, and include fever, elevated hepatocellular enzymes, respiratory failure, blood dyscrasias, and in some cases renal failure. Initial crude estimates placed incidence of YEL-AVD in the range of 3 to 5 cases per million doses distributed (Eidex et al., 2007).
A study of reports submitted to the Vaccine Adverse Event Reporting System in the United States identified advanced age as a risk factor for adverse events associated with YF immunization, though cases have occurred in younger individuals (Martin et al., 2001). The U.S. CDC VIS states incidence as 1:200 000–300 000 doses for all first-time vaccine recipients; for those aged 60 years or older, 1:40 000–50 000 doses. An update on advanced age as a risk factor for serious adverse events to YF vaccine was published in 2005, affirming the increased risk and documenting a reporting rate ratio of 5.9 (95% CI 1.6–22.2) in first-time vaccine recipients aged 60 years and older (Khromava et al., 2005). Disease of the thymus gland has been identified as another risk factor for developing severe reactions after YF vaccine. Fifteen percent of 26 individuals with YEL-AVD described in a 2004 paper had a history of thymus disease, including thymoma and myasthenia gravis (Barwick, 2004). The CDC’s Health Information for International Travel describes thymus disease as a contraindication to YF vaccine (Eidex et al., 2007).
YEL-AVD is characterized by a widespread inflammatory response with exuberant viral replication. Antibody levels are significantly higher than expected. Examination of tissues from fatal cases of YEL-AVD reveals widespread dissemination of YF vaccine strain virus and active viral replication in multiple organs; sequencing studies do not identify mutations in vaccine virus to explain these adverse events (Monath, 2004). The occurrence of two cases in one family in Brazil supports the hypothesis that host genetic factors may be associated with a predisposition to YF vaccine adverse events (Monath, 2004).
Treatment Of YEL-AND And YEL-AVD
There are no standardized treatment protocols for treatment of adverse events to YF vaccine. Supportive care remains the mainstay of treatment, and patients should be managed in settings where intensive care is available.
Indications, Precautions, And Contraindications For Yellow Fever Immunization
Table 1 lists contraindications and precautions for use of YF vaccine. Travelers to countries or regions where there is increased risk of YF should receive a single dose of vaccine at least 10 days before departure unless there are specific contraindications (Centers for Disease Control and Prevention, 2002b; Wilson et al., 2004; Marfin et al., 2005; Eidex et al., 2007). YF vaccine must be given at official YF vaccine centers and documented in an International Certificate of Vaccination, valid from 10 days through 10 years after the date of immunization. Vaccine is contraindicated absolutely in children under 6 months of age, and should be given to infants 6 to 9 months of age only if risk of disease is significant and other methods of prevention cannot be employed. The single most important step in judicious use of YF vaccine is to immunize only individuals traveling to YF-endemic areas: in one report two out of five individuals with multi-organ system failure were traveling to areas where YF had never been described (Centers for Disease Control and Prevention, 2001). Individuals should also use personal protective measures to prevent mosquito bites, such as mosquito repellants and protective clothing.
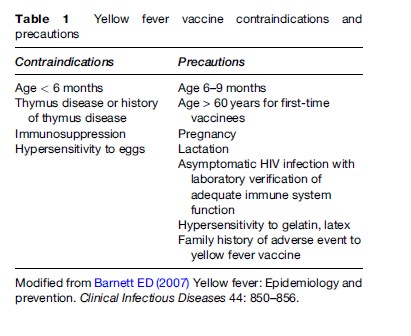
History of thymus disease is a contraindication to YF vaccine (Eidex et al., 2007). Immunocompromised individuals also should not be immunized. Asymptomatic HIV-infected individuals with CD4 counts greater than 200/mm3 who face increased risk of YF infection and cannot avoid potential exposure should be offered the choice of immunization (Eidex et al., 2007). Such individuals, however, may have impaired response to vaccine and if they remain at risk for YF, testing for neutralizing antibody may be advisable. Individuals immunized for the first time after age 60 are at increased risk for adverse events. Those who have a family member who sustained a severe adverse event to YF vaccine may also be at increased risk. Use of YF vaccine in such individuals requires careful review of risk during the travel itinerary and elucidation of information about the likelihood of severe adverse events.
Safety of YF immunization during pregnancy has not been established, and little is known about the potential of vaccine-associated virus strains to infect the fetus (Tsai, 2006). Fetal infection was documented in 1 out of 41 infants exposed to maternal vaccination, and increased risk of spontaneous abortion was found in a Brazilian study of 39 pregnant women immunized with YF vaccine compared with 74 control patients. Immunization during pregnancy may result in antibody concentrations inferior to those obtained following immunization of nonpregnant women. A mass vaccination campaign in Brazil in early 2000 resulted in inadvertent immunization with 17DD vaccine of 480 pregnant women who were followed until the infants were 1 year of age. Maternal seroconversion was high, and no infants were found to be infected at birth (no IgM antibodies were detected and no placental or umbilical cord blood was found to contain YF vaccine strain virus by PCR). Therefore, YF immunization should be avoided during pregnancy except when there is a clear and unavoidable increased risk of infection. Inadvertent administration of vaccine during pregnancy is not an indication for termination of pregnancy.
There are no reports of transmission of YF vaccine virus from nursing mothers to their infants, and it is not known whether YF vaccine virus is excreted into breast milk. Lactating women who travel to YF-endemic areas and whose risk of YF infection exceeds the theoretical risk of transmission of vaccine virus to their infants may be immunized (Eidex et al., 2007).
Conclusion
Yellow fever continues to occur in parts of Africa and South America. Immunization of susceptible populations with YF vaccine is likely to be the most effective method of reducing the prevalence of the disease. Recent descriptions of serious adverse events to YF vaccine have lent new urgency to defining criteria for judicious use of YF vaccine. Future research is focused on defining the spectrum of adverse events to YF vaccine and host factors that would increase risk to these events, and on identifying potential treatment modalities for YF and for YF vaccine-associated viscerotropic and neurologic disease.
Bibliography:
- Barnett ED (2007) Yellow fever: Epidemiology and prevention. Clinical Infectious Diseases 44: 850–856.
- Barwick RE (2004) History of thymoma and yellow fever vaccination. The Lancet 364: 936.
- Centers for Disease Control and Prevention (2001) Fever, jaundice, and multiple organ system failure associated with 17D-derived yellow fever vaccination, 1996–2001. MMWR Morbidity and Mortality Weekly Report 50: 643–645.
- Centers for Disease Control and Prevention (2002a) Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and Mortality Weekly Report 51(No. RR–17): 1–10.
- Centers for Disease Control and Prevention (2002b) Adverse events associated with 17D-derived yellow fever vaccination – United States, 2001–2002. MMWR Morbidity and Mortality Weekly Report 51: 989–993.
- Eidex RB, Hayes EB, and Russell M (2007) Yellow fever. In: Centers for Disease Control and Prevention. Health Information for International Travel 2008, pp. 362–379. Atlanta, GA: Department of Health and Human Services, Public Health Service.
- Khromava AY, Eidex RB, Weld LH, et al. (2005) Yellow fever vaccine: An updated assessment of advanced age as a risk factor for serious adverse events. Vaccine 23: 3256–3263.
- Marfin AA, Eidex RS, Kozarsky PE, and Cetron MS (2005) Yellow fever and Japanese encephalitis vaccines: Indications and complications. Infectious Disease Clinics of North America 19: 151–168.
- Martin M, Weld LH, Tsai TF, et al. (2001) Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerging Infectious Diseases 7: 945–951.
- McMahon AW, Eidex RB, Marfin AA, et al. (2007) Neurologic Disease Associated with 17D-204 Yellow Fever Vaccination: A Report of 15 Cases. Vaccine 25: 1727–1734.
- Monath TP (2004) Yellow fever vaccine. In: Plotkin SA and Orenstein WA (eds.) Vaccines, 4th ed., pp. 1095–1176. Philadelphia, PA: WB Saunders.
- Monath TP and Cetron MS (2002) Prevention of yellow fever in persons traveling to the tropics. Clinical Infectious Diseases 34: 1369–1378.
- Poland JD, Calisher CH, Monath TP, et al. (1981) Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bulletin of the World Health Organization 59: 895–900.
- Robertson SE, Hull BP, Tomori O, et al. (1996) Yellow fever: A decade of reemergence. Journal of the American Medical Association 276: 1157–1162.
- Tsai T (2006) Congenital arboviral infections: Something new, something old. Pediatrics 117: 936–939.
- Wilson ME, Chen LH, and Barnett ED (2004) Yellow fever immunizations: Indications and risks. Current Infectious Disease Reports 6: 34–42.
- https://www.cdc.gov/yellowfever/prevention/index.html – Centers for Disease Control and Prevention (CDC): Prevention of Yellow Fever.
- https://www.cdc.gov/vaccines/hcp/vis/vis-statements/yf.html – Centers for Disease Control and Prevention (CDC): Yellow Fever Vaccine: What You Need to Know.




