View sample measles research paper. Browse research paper examples for more inspiration. If you need a health research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Disease Description
Measles is an acute viral illness caused by a virus in the family Paramyxoviridae, genus Morbillivirus. The highly contagious measles virus is transmitted following airborne or droplet exposure. The average incubation period from exposure to onset of the symptoms is 8 to 12 days.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Clinical Features
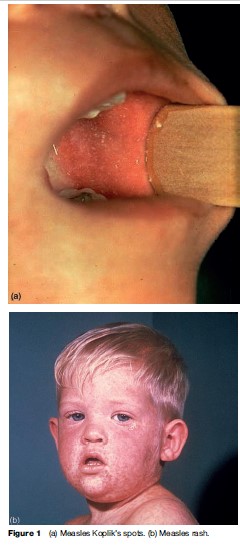
Measles is characterized by a prodrome of fever and malaise, cough, coryza, and conjunctivitis that lasts 2 to 4 days. Koplik’s spots, an enanthem considered pathognomonic for measles, usually appears on the buccal mucosa 1 to 2 days before rash onset (Figure 1(a)). The rash is an erythematous maculopapular eruption which usually appears 14 days after exposure and spreads from the head over the trunk to the extremities during 3 to 4 days (Figure 1(b)). The rash is usually most confluent on the face and upper body and fades during the next 3 to 4 days in order of appearance.
Complications
Measles can be severe and is most frequently complicated by diarrhea (8%), middle ear infection (7%–9%), and pneumonia (1%–6%). The most serious complications include blindness and encephalitis. Encephalitis frequently results in permanent brain damage, and can occur in 1 per 1000–2000 cases of measles. The most severe sequela of measles virus infection is subacute sclerosing panencephalitis, a rare degenerative central nervous system disease that can occur in 1 per 100 000 cases and usually develops 7 to 10 years after infection. In developing countries, mortality rates due to measles are usually 1 to 5 per 1000 cases but may reach 10% to 30%.
Pneumonia is the most common complication from measles associated with death. The risk of severe complications and death is higher among children less than 5 and adults greater than 20 years of age. In the United States, measles has resulted in encephalitis in 1 in 1000 reported cases during 1987–2000. One third of cases (29%) had some complication, with 6% of cases complicated by pneumonia and 19% of cases being hospitalized. During that period, death was reported in 0.3% of the cases (Perry and Halsey, 2004).
Global Public Health Burden
Measles is now rare in many industrialized countries; however, it remains a common illness in many countries of the world, mostly in developing countries. The World Health Organization (WHO, 1999) estimates that more than 20 million people are affected each year by measles. In 2005, it was estimated that there were 345 000 measles deaths globally: this translates to more than 945 deaths every day or 39 deaths every hour from measles. The overwhelming majority (>95%) of measles deaths occur in countries with per capita gross national income of less than US$1000. The primary reason for the continuing high childhood measles morbidity and mortality is the failure to deliver at least one dose of measles vaccine to all infants. In countries where measles has been largely eliminated, cases imported from other countries remain an important source of infection.
Diagnosis
Clinical Diagnosis
Measles should be suspected in patients with an acute erythematous a prodrome of cough, coryza, and conjunctivitis. Several clinical features support the diagnosis of measles: a characteristic prodrome of intensifying symptoms over 2 to 4 days, the presence of Koplik’s spots, a rash that pro- gresses from the head to trunk and extremities, and the appearance of fever shortly after rash onset. In the United States, a clinical case definition used for public health surveillance includes the presence of a generalized maculopapular rash and fever preceded by maculopapular rash lasting 3 or more days; a temperature 101 F (38.3ºC) or higher; and cough, coryza, or conjunctivitis. Other countries use a less specific clinical definition that does not require a 3-day duration of rash. Laboratory diagnosis is often used to confirm the diagnosis, especially for sporadically occurring cases.
Laboratory Diagnosis
Measles Immunoglobulin M (Igm) Antibody
In a susceptible person exposed to measles virus, an IgM serologic response is usually detected around the time of rash onset. In the first 72 hours after rash onset, however, up to 30% of tests for IgM may give false-negative results; therefore, tests that are negative on serum specimens taken in the first 72 hours after rash onset should be repeated. IgM is detectable for at least 28 days after rash onset and frequently longer.
Measles Immunoglobulin G (Igg) Antibody
The IgG response to measles infection starts more slowly, beginning about 7 days after rash onset, but typically persists for a lifetime. Diagnosis of measles through measurement of IgG antibody titers requires two serum specimens, the first taken at the time of diagnosis (acute) and the second collected 14 to 30 days after the first (convalescent). Laboratory confirmation of measles requires paired testing of acute and convalescent specimens and the demonstration of a fourfold rise in IgG antibody titer against measles.
Measles Virus Detection
Measles can also be confirmed by isolation of measles virus in culture or detection of measles virus by reverse transcription polymerase chain reaction (RT-PCR) in clinical specimens such as throat swabs, nasopharyngeal aspirates, or urine. Measles virus is more likely to be detected when the specimens are collected within 3 days of rash onset. Practically, clinical specimens should be obtained within 7 days of rash onset to increase the likelihood of detecting virus if present. If measles virus is cultured or detected by RT-PCR, the viral genotype can be determined and used to identify the genotypes associated with imported cases of measles.
Treatment
There is no specific antiviral therapy for measles. The basic treatment consists of providing necessary supportive therapy such as hydration and antipyretics and treating complications such as pneumonia. Vitamin A supplementation has been shown to decrease mortality and morbidity from measles in communityand hospital-based studies (D’Souza and D’Souza, 2002). WHO recommends treatment with vitamin A to all children diagnosed with measles in communities where vitamin A deficiency is a problem or the measles case-fatality rate is 1% or greater. Because low serum concentrations of vitamin A have been found in children with severe measles in the United States, the American Academy of Pediatrics recommends vitamin A supplementation for hospitalized measles patients 6 months to 2 years of age, and for measles patients 6 months or older with any of the following conditions: immunodeficiency, clinical evidence of vitamin A deficiency, impaired intestinal absorption, moderate to severe malnutrition, or recent immigration from areas where high measles mortality rates have been observed.
Prevention
Vaccination
Measles vaccine contains live, attenuated measles virus. It is available as a single-antigen preparation and in combination formulations, such as measles-rubella (MR), measles-mumps-rubella (MMR), and measles-mumpsrubella-varicella (MMRV). Measles vaccine, as a singleantigen or combined, is given subcutaneously in a dose of 0.5 mL. A single dose of measles-containing vaccine administered in the second year of life induces immunity in about 95% of vaccinees (King et al., 1991), and approximately 95% of persons who fail to respond to the first dose respond to a second dose (Watson et al., 1996).
Indications
According to WHO recommendations, the first dose of measles vaccine should be given at 9 months old in most developing countries because of the high morbidity and mortality of measles in the first year of life. A second opportunity for measles immunization is also recommended. In the United States, the first dose of MMR is routinely administered at 12 to 15 months of age and the second dose at 4 to 6 years of age (CDC, 2006), the minimum interval between doses being 28 days. Combined vaccines are recommended whenever one or more of the individual components are indicated to provide protection against mumps, rubella, and/or varicella.
Adverse Reactions To Vaccination
Fever greater than 39.4 C (>103 F) can occur in 5% to 15% of susceptible vaccinees, usually beginning 7 to 12 days after measles vaccination. Transient rashes, usually appearing 7 to 10 days following vaccination, occur in 5% of the individuals vaccinated with measles-containing vaccines. Mild allergic reactions such as urticaria or wheal and flare at the injection site, generalized rash, and pruritis can occur after measles vaccination. Severe anaphylactic reactions are estimated to occur less than once per million doses distributed. Clinically apparent thrombocytopenia has been reported at a rate of approximately 1 case per 30 000 vaccinated children. The risk for febrile seizures is approximately 1 case per 3000 doses of measles vaccine administered. Encephalopathy has also been attributed to measles containing vaccination with an estimated frequency of 1 case per 2 million doses distributed.
Precautions And Contraindications To Vaccination
- Severe illness: Vaccination of persons with moderate or severe febrile illness should generally be deferred until they have recovered from the acute phase of their illness.
- Allergy: Persons with severe allergy to gelatin or neomycin (components of the vaccine) or who have had a severe allergic reaction to a prior dose of measles vaccine should not be vaccinated except with extreme caution.
- Pregnancy: There is no evidence that measles vaccine causes any damage to the fetus. However, it should not be administered to women known to be pregnant because of theoretical risks to the fetus with administration of a live attenuated vaccine. Pregnancy should be avoided for 1 month after receipt of measles vaccine.
- Immunosuppression: Severely immunosuppressed individuals should not be vaccinated with measles vaccine because of potentiated replication of viruses in persons who have deficiency disorders.
- Steroids: Persons receiving high daily doses of corticosteroids (>2 mg/kg per day or >20 mg/day of prednisone) for 14 days should not receive measles vaccine because of concern about vaccine safety. Measles vaccine should be avoided for at least 1 month after cessation of high-dose therapy.
- Other immunosuppressive therapy: In general, measles vaccine should be withheld for at least 3 months after cessation of immunosuppressive therapy.
- HIV: Measles vaccine is not recommended for HIV-infected persons with evidence of severe immunosuppression (i.e., CD4þ T-lymphocyte count <15%).
- Immune globulins or other antibody-containing blood products: Measles vaccine should be administered at least 14 days before the administration of antibodycontaining blood products, such as immune globulin, because passively acquired antibodies may interfere with the response to the vaccine. Measles vaccination should be delayed until 3 to 11 months after administration of blood products, depending on the type of blood product received (Kroger et al., 2006).
- Thrombocytopenia: Avoiding a subsequent dose of measles vaccine may be prudent if an episode of thrombocytopenia occurred within approximately 6 weeks after a previous dose of vaccine.
- Tuberculosis: Measles vaccine can suppress the response to skin testing in a person infected with Mycobacterium tuberculosis. Tuberculosis skin testing can be done on the day of vaccination. Otherwise, it should be delayed for 4 to 6 weeks after measles vaccination.
Care Of Exposed Persons
Use Of Vaccine
Measles vaccine, if administered within 72 hours of initial measles exposure, may provide some protection. If the exposure does not result in infection, the vaccine should induce protection against subsequent measles infection.
Use Of Immune Globulin
Immune globulin can be used to prevent or modify measles in a susceptible person if given within 6 days of exposure. Immune globulin is indicated for household contacts of patients with measles, particularly contacts younger than 1 year, pregnant women, and immunocompromised persons for whom the risk of complications is higher (American Academy of Pediatrics, 2006).
Public Health Impact Of Vaccination Programs
Despite the availability of an effective vaccine for over 40 years, measles remains the leading cause of vaccinepreventable deaths in children. Nevertheless, remarkable reduction of measles morbidity and mortality is being achieved regionally and globally.
In the United States, measles vaccine was introduced in 1963. Before its introduction, roughly half a million cases of measles were reported each year. In 1989 a two-dose schedule was recommended and in 1998, the Advisory Committee on Immunization Practices and the American Academy of Pediatrics jointly recommended that states ensure second-dose coverage of children in all grades by 2001. The two-dose strategy has led to a dramatic decline in measles cases. Current surveillance data indicate that indigenous measles transmission has stopped, and measles was declared eliminated in the United States in 2000 (Katz and Hinman, 2004). Fewer than 150 cases were reported each year during 1997–2005 and measles incidence has decreased to a record low of 37 reported cases in 2004 (Figure 2).
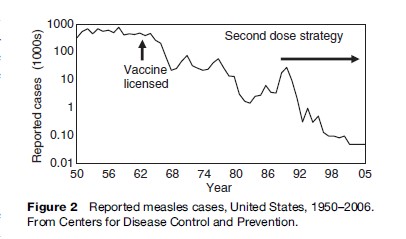
In recent years, outbreaks of measles in the United States have been small, with less than 35 cases reported. Recent outbreaks do not have a predominant setting but mostly involve people who are exposed to imported measles cases and who are unvaccinated or have received only one dose of measles vaccine. Moreover, recent outbreaks have often been associated with a lack of adherence to existing recommendations for measles prevention among high-risk groups such as travelers, healthcare workers, and communities that reject vaccination.
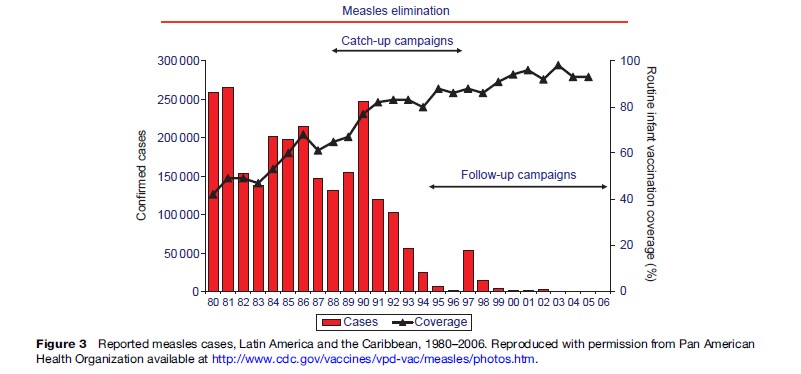
In the Americas, under the leadership of the Pan American Health Organization (PAHO), Ministries of Health of the member countries implemented an aggressive measles elimination program. In 1994, ministers of health of countries of North and South America established the goal of eliminating measles from the Western Hemisphere by the end of 2000. To accomplish this goal, PAHO developed a strategy with three essential vaccination components: (1) catch-up – a one-time mass vaccination covering all children ages 1 to 14 years regardless of prior disease or vaccination status; (2) keep-up – achievement of 90% or greater immunization coverage in each successive birth cohort; and (3) follow-up – subsequent mass campaigns conducted every 3 to 5 years covering all children ages 1 to 5 years irrespective of prior disease or vaccination history. In addition to the vaccination strategy, case-based surveillance with laboratory confirmation of suspected measles cases has been established in all countries of the Americas. Implementation of the PAHO strategy in the Western Hemisphere has resulted in a greater than 99% decline in reported measles cases from a high of almost 250 000 cases in 1990 to 85 cases in 2005, the lowest annual total ever (Figure 3).
In May 2003, the 56th World Health Assembly unanimously adopted a resolution to reduce measles deaths by 50% by the end of 2005 compared with 1999 levels. This goal was established a year earlier by the United Nations General Assembly Special Session on Children, ‘‘World Fit for Children.’’ In May 2005, the 58th World Health Assembly adopted the WHO/UNICEF Global Immunization Vision and Strategy (GIVS). GIVS calls on countries to reduce global measles deaths by 90% by 2010 compared with 2000 estimates. The strategy recommended by the WHO for sustainable measles mortality reduction includes four components:
Strong routine immunization. In countries with mortality reduction goals, the first dose of measles vaccine should be given to children at the age of 9 months or shortly thereafter through routine immunization services. This is the foundation of the sustainable measles mortality reduction strategy. At least 90% of children should be reached by routine immunization services every year, in every district.
‘Second opportunity’ for measles immunization. Recommendation of a second opportunity for measles immunization to assure measles immunity in children who failed to receive a previous dose of measles vaccine, as well as in those who were vaccinated but failed to develop immunity following vaccination (approximately 10% to 15% of those children vaccinated at 9 months of age).
The second opportunity prevents the accumulation of susceptible children because many older children may have missed measles vaccination and may not have been infected, so they are not immune. The second opportunity for measles immunization can be implemented either through routine immunization services (if high coverage can be achieved and maintained over time) or through periodic supplementary immunization activities (SIAs). SIAs target large populations of children (entire nations or large regions) and aim to achieve immunization coverage of over 90%.
Adequate surveillance. Prompt recognition and investigation of measles outbreaks provides important information about program impact and assures the implementation of appropriate outbreak response activities. Standard measles surveillance guidelines have been developed and should be implemented (WHO, 1999).
Improvement of clinical management of measles cases. This includes vitamin A supplementation and adequate treatment of measles complications, if needed, with antibiotics.
Major progress has been made in reducing global measles mortality by implementation of these strategies. Worldwide, more than 360 million children received measles vaccine through SIAs during 2000–2005. During the same period, improvements in routine measles vaccination coverage and implementation of measles SIAs have resulted in a 60% decrease in the estimated number of global measles deaths. This reduction was highest in Africa where measles morbidity and mortality decreased by nearly 75%. Therefore, the goal to reduce global measles deaths between 1999 and 2005 by 50% has been not only achieved but exceeded.
Based on the success in the Americas using PAHO’s strategies, measles elimination targets have been established in the European and Eastern Mediterranean regions for the year 2010, and the Western Pacific region for 2012. The African and South-East Asian regions have set goals for sustainable reductions in measles mortality.
Bibliography:
- American Academy of Pediatrics (2006) Measles. In: Pickering LK (eds.) Red Book: 2006 Report of the Committee on Infectious Diseases. 27th edn. Elk Grove Village, IL: American Academy of Pediatrics.
- Centers for Disease Control and Prevention (CDC) (2006) Recommended childhood and adolescent immunization schedule—United States, 2006. MMWR Morbidity and Mortality Weekly Report 54.
- D’Souza RM and D’Souza R (2002) Vitamin A for the treatment of children with measles—a systematic review. Journal of Tropical Pediatrics 48: 323–327.
- Katz SL and Hinman AR (2004) Summary and conclusions: Measles elimination meeting, 16–17 March 2000. Journal of Infectious Diseases 189(supplement): S43–S47.
- King GE, Markowitz LE, Patriarca PA, and Dales LG (1991) Clinical efficacy of measles vaccine during the 1990 measles epidemic. Pediatric Infectious Disease Journal 10: 883–888.
- Kroger AT, Atkinson WL, Marcuse EK, and Pickering LK (2006) General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and Mortality Weekly Report 55: RR-15.
- Perry RT and Halsey NA (2004) The clinical significance of measles: A review. Journal of Infectious Diseases 189(supplement): S4–S16.
- Watson JC, Pearson JA, Markowitz LE, et al. (1996) An evaluation of measles revaccination among school-entry-aged children. Pediatrics 97: 613–618.
- World Health Organization (WHO) (1999) WHO Guidelines for Epidemic Preparedness and Response to Measles Outbreaks. Geneva, Switzerland: WHO.
- Bellini WJ, Rota JS, Lowe LE, et al. (2005) Subacute sclerosing panencephalitis: More cases of this fatal disease are prevented by measles immunization than was previously recognized. Journal of Infectious Diseases 192: 1686–1693.
- Centers for Disease Control and Prevention (2006) Measles. Epidemiology and Prevention of Vaccine Preventable Diseases: The Pink Book, 9th edn. Washington, DC: Public Health Foundation.
- Helfand RF, Heath JL, Anderson LJ, et al. (1997) Diagnosis of measles with an IgM capture EIA: The optimal timing of specimen collection after rash onset. Journal of Infectious Diseases 175: 195–199.
- Hinman AR, Orenstein WA, and Papania MJ (2004) Evolution of measles elimination strategies in the United States. Journal of Infectious Diseases 189(supplement): S17–S22.
- Institute of Medicine (1994) Measles and mumps vaccines. In: Stratton KR, Howe CJ, and Johnston RB (eds.) Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Washington, DC: National Academy Press.
- Papania MJ, Seward JF, Redd SB, et al. (2004) Epidemiology of measles in the United States, 1997–2001. Journal of Infectious Diseases 189(supplement): S61–S68.
- Parker AA, Staggs W, Dayan GH, et al. (2006) Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. New England Journal of Medicine 355: 447–455.
- Peltola H and Heinonen OP (1986) Frequency of true adverse reactions to measles-mumps-rubella vaccine: A double-blind placebo-controlled trial in twins. The Lancet 8487: 939–942.
- Strebel PM, Papania MJ, and Halsey NA (2003) Measles vaccine. In: Plotkin SA and Orenstein WA (eds.) Vaccines. 4th edn. Philadelphia, PA: WB Saunders.
- Watson JC, Hadler SC, Dykewicz CA, et al. (1998) Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and Mortality Weekly Report 47: 1–57.




