Sample Neurons And Dendrites Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
1. Introduction And Scope
At the beginning of the twenty-first century, neural tissue remains arguably the most powerful substrate for information processing in existence. Unfortunately, the principles underlying brain function are still known only in rough outline. To unlock the many secrets of the brain, and to help explain the panoply of neural functions ranging from simple motor reflexes to conscious thought and action, neuroscientists have amassed an extensive body of experimental data bearing on the properties of nerve cells (neurons) and their interconnections (synapses), which together form the building blocks of neural tissue. At the same time, the functional significance of the neurobiological data has been explored in computer modeling studies, which have often been used to test hypotheses inaccessible to direct experimental approaches. This research paper summarizes what is known about the intracellular physiology of individual nerve cells, and interprets these data in relation to the information processing functions of single neurons. For comprehensive treatments of many of these same issues, see Stuart et al. (1999) and Koch (1999).
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Given the morphological and functional diversity of neurons across the invertebrate and vertebrate phyla, this research paper is focused on the principal neuron types of the vertebrate central nervous system (CNS), though much of what is discussed applies to neurons in general. This focus is justified, first, by the fact that neurons of the vertebrate CNS have been among the most frequent objects of correlated physiological, behavioral, and modeling studies, and second, by the fact that these cells are those which most directly underlie the cognitive and behavioral phenomena.
2. Basic Construction Of A Neuron
The typical neuron of the vertebrate CNS is composed of (a) a cell body, or soma, which contains the nucleus and other molecular machinery present in any living cell, (b) the dendritic tree, which emanates from the cell body and acts as the main input-receiving surface of the neuron, (c) the axon which carries output signals generated by the neuron over long distances to other neurons, and (d) a large number of synapses, the specialized sites where axons make contacts with the dendrites and cell bodies of other neurons for subsequent processing (Fig. 1).
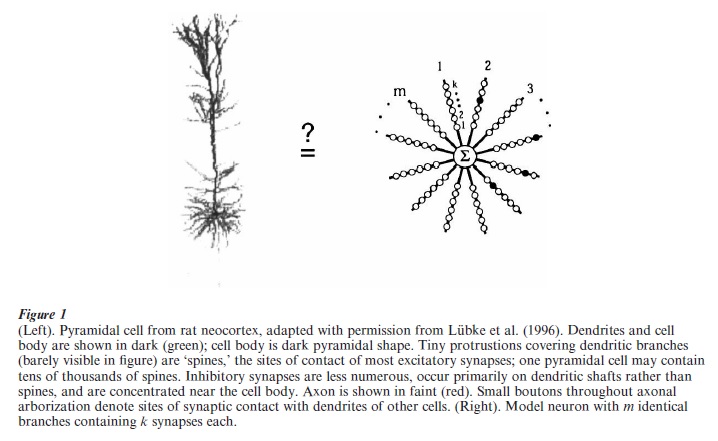
The axon, a thin fiber emanating from the cell body, is the cell’s final common output used to convey messages to other neurons. The axon carries trains of ‘action potentials’ (APs), all-or-none voltage spikes 60–100 mV in height and 1–2 ms in duration which propagate undecremented along the length of the axon at speeds up to 100 m/s. The single axon of a neuron, which may travel over distances ranging from millimeters to meters depending on the neuron, and which may branch profusely at one or more sites along its run, gives rise to many thousands of synaptic terminals impinging on the dendrites and cells bodies of hundreds to thousands of other neurons. Neurons can fire APs at rates up to 1,000 impulses per second, though the maximum firing rate for most neurons in the CNS lies in the range of 100–200 Hz. APs are triggered when the membrane potential at the site of spike initiation reaches a firing threshold. In a typical neuron, this threshold is around – 50 mV, that is, about 20 mV more positive than the cell’s resting potential of – 70 mV. While APs are thought to be triggered in the axon near the cell body, the electric currents which flow there represent the combined influences of many widely scattered synaptic inputs acting on the soma/dendritic membrane.
The dendritic tree is the main input-receiving do-main of the neuron, generally accounting for more than 95 percent of the cell’s total surface area, and receiving many thousands of synaptic inputs from other cells. With a few notable exceptions, the dendrites of CNS neurons are purely input structures (Shepherd 1998). A synaptic input to a dendrite can be categorized roughly as either ‘excitatory’ or ‘inhibitory,’ meaning that when the synapse is activated, the membrane on the ‘postsynaptic,’ that is, dendritic, side of the synaptic junction is driven transiently to a more positive (excitatory) or more negative (inhibitory) potential. Most of the contacts on a dendritic tree are excitatory (80–90 percent); inhibitory contacts can occur anywhere, but tend to be concentrated on or around the cell body. A synaptically evoked change in membrane potential can have a time course ranging from one to hundreds of milliseconds depending on the type of synapse. In addition, synaptic inputs can trigger ‘active’ dendritic responses, including complex spike-like events involving both fast and slow components (e.g., Kamondi et al. 1998, Golding et al. 1999). Thus, the rules which govern how excitatory and inhibitory synaptic inputs are combined and transformed within the dendritic tree to produce outbound action potentials can be quite complex. This process commonly is referred to as synaptic or dendritic ‘integration,’ and is the focus of the remainder of this research paper.
3. The Membrane Potential
To understand how synaptic and other electrical events combine to generate APs, it is important to understand the basic electrical properties of ‘neurites’ (axons and dendrites).
The resting membrane potential of a neuron, as for any living cell, is established by molecular pumps which actively maintain concentration gradients of sodium (Na+) and potassium (K+) ions across the cell membrane. Relative to the extracellular fluid, the inside of the cell contains a high concentration of K+ ions and a low concentration of Na+ ions. The constant pressure on these electrically charged ions to flow across the membrane down their respective concentration gradients (Na+ in, K+ out), is analogous to the pressure electrons feel which propels them from the negative to the positive terminal of a battery given a suitable path for current. The electrical pressure on each of these ions can thus be represented by a battery, whose voltage conveys the magnitude and direction of the ion’s concentration gradient; the fact that the Na+ and K+ batteries point in opposite directions will turn out to be critical for electrical signaling.
Since the membrane lipid bilayer is itself impervious to ions, currents can only flow through ‘ion channels,’ specially designed pores in the membrane formed by large, membrane-spanning protein complexes. Ion channels are generally quite selective as to which ions may flow through them, giving rise to the nomenclature ‘sodium channels,’ ‘potassium channels,’ and so on. Furthermore, certain ion channels are tonically open, while others can open and close under the control of various signals. For example, some channels open and close in response to changes in the membrane potential, such as occurs in the production of action potentials, others in response to chemical signals, such as occurs in the production of synaptic potentials.
The resting membrane potential of a neuron is a weighted average of ionic battery values, where the weight given to each ion’s battery is determined by the membrane’s total electrical conductance gI to ion I through all open channels
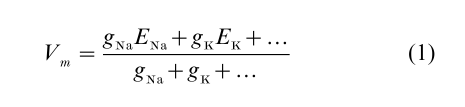
The usual resting potential around Vm = – 70 mV is dominated by a K+ conductance, whose associated battery value is EK = – 90 mV. An additional small resting Na+ conductance, whose associated battery value ENa = + 60 mV or higher, pulls the resting potential some 20 mV in the positive direction.
4. Electrical Signaling Results From Conductance Changes
Electrical signaling in a neuron is caused by transient changes in the conductances of various ion channels, which cause transient fluctuations in the membrane potential about its resting value. At every moment in time, the membrane potential moves towards the equilibrium value given by Eqn. (1)—not instantaneously—but at a rate limited by the need to charge or discharge the membrane capacitance. The classical signal generated by a neuron is the action potential. When a synaptic or other injected current drives the membrane potential to a threshold of approximately – 50 mV, a population of voltage-dependent Na+ channels, normally closed at rest, opens abruptly to a large conductance value resulting in a large influx of current. As predicted by Eqn. (1), this leads to a rapid voltage upswing as the membrane potential spikes toward the Na+ battery potential of 60 mV. Within a fraction of a millisecond, however, the Na+ channels begin to close, or ‘inactivate,’ of their own accord. At the same time, voltage-dependent K+ channels sense the voltage upswing on the leading edge of the AP, and open to produce a large outward current. The combined effects of Na+ channels closing and K+ channels opening results in an abrupt downswing in the membrane potential to terminate the AP.
Active electrical responses of the neuronal membrane have historically been most closely associated with the long-range signal transmission function of the axon. It is now apparent, however, that the dendrites of many neuron types contain voltage-dependent channels capable of generating spikes which are independent of the cell body (Amitai et al. 1993, Golding and Spruston 1998, Kamondi et al. 1998). Unlike the stereotyped all-or-none form of the classical AP, however, synaptically-evoked dendritic spikes are of variable magnitude and can consist of complex superpositions of fast and slow events. As discussed below, this highly nonlinear electrical behavior of dendritic origin is likely to profoundly influence the integrative properties of nerve cells.
5. Synaptic Inputs
Synaptic potentials, the principal inputs signals to which a neuron responds, likewise are evoked by changes in the conductance of ion channels in the postsynaptic membrane. In this case, the conductance changes are triggered by the binding of neurotransmitter molecules to receptors exposed on the extracellular surface of the membrane. The normal sequence of events in synaptic transmission is as follows. An AP invades the presynaptic axon terminal. The voltage upswing in the presynaptic terminal opens voltage-dependent calcium (Ca++) channels leading to a rapid influx of Ca++ ions. A small number of vesicles (usually zero or one) containing neurotransmitters fuse with the presynaptic membrane at specialized release sites and disgorge their contents into the synaptic cleft. The neurotransmitter (e.g., glutamate or GABA) diffuses the several nanometers across the synaptic cleft in a fraction of a millisecond, and binds with receptors on the postsynaptic membrane. Upon binding, the receptor-channel complex undergoes a conformational change, causing the channel to open. Alternatively, in the case of ‘second messenger’ transmission, the protein to which the neurotransmitter binds is not the channel itself, but instead triggers a cascade of biochemical reactions inside the postsynaptic cell which lead indirectly to the opening or closing of ion channels. In either case, the resulting conductance change(s) cause the membrane potential in the postsynaptic compartment to fluctuate toward the characteristic battery value for that synapse, often called the synaptic ‘reversal potential.’ The reversal potential at an AMPA or NMDA-type excitatory synapse is typically 0 mV, 70 mV more positive than the resting potential. This value results from an approximately equal conductance to Na+ and K+ ions. A synapse with a reversal potential around 0 mV is termed ‘excitatory’ since under normal operating conditions with a negatively polarized membrane potential, activation of the synapse drives the membrane transiently to more positive potentials (i.e., towards 0 mV). This ‘depolarization’ increases the likelihood (or rate) of outbound AP production in the postsynaptic cell. Conversely, the reversal potential is – 65 mV for GABAA-type inhibitory synapses which conduct chloride ions, and – 90 mV for GABAB-type inhibitory synapses which conduct K+ ions. In either case, activation of the synapse opposes synaptic excitation by transiently pulling the membrane potential to more negative values, thus reducing the likelihood (or rate) of AP generation.
6. Synaptic Integration
To this point we have seen how transmitter-dependent and voltage-dependent gating of ion channels can produce the fluctuations in membrane potential that underlie both synaptic responses and action potentials. But what are the rules governing synaptic integration?
The simplest ‘linear’ model of dendritic integration holds that the neuron sums its excitatory and inhibitory influences across the dendritic arbor, followed by a global thresholding nonlinearity at the cell body (McCullough and Pitts 1943, Rosenblatt 1962, Rumelhart and McClelland 1986). Despite its simplicity (Fig. 1), this formulation has influenced thinking about neuronal function in both the experimental and computational communities for many years. Extensions to this idea that explicitly include the ‘passive cable’ properties of the neuron—accounting for attenuation of signals with time and distance (Rall 1964, Koch et al. 1982, Agmon-Snir and Segev 1993)—have also been highly influential, and have led to a variety of productive interchanges between theory and experiment (Rall et al. 1992).
From the perspective of currently available neurophysiological data, however, much of it derived from direct intradendritic recordings, synaptic integration is likely to be more complex than can be captured by linear or passive models of dendritic function (Segev and Rall 1998, Mel 1999). The question is complicated for three reasons, principally relating to uncertainties as to how or to what extent synaptic inputs interact with each other through shared voltage or chemical signals within the dendrites.
First, the spatio-temporal patterns of synaptic activation driving neurons in the CNS in vivo are not well known, so that current best guesses as to the electrical and chemical environments in which synapses normally operate remain largely rooted in conjecture.
Second, synaptic inputs may trigger secondary events in the dendrites mediated by voltage-dependent NMDA, Na+, K+, and Ca++ channels, which could influence significantly how excitatory and inhibitory synaptic inputs are combined to produce overall neuronal responses. Synaptically-evoked dendritic spikes have been observed in vitro (Golding and Spruston 1998, Schiller et al. 2000) and in vivo (Kamondi et al. 1998), though it is not yet known whether such events occur commonly in awake, behaving animals, and if so whether they are localized to one or many dendritic branches, and so on. In addition, the spatial distributions of these channels on the cell surface, their kinetic properties, their dependence on voltage and other modulatory factors, and their efficacies under normal in vivo operating conditions, are not sufficiently well known to make firm predictions regarding the contributions of these channels to the integrative processes of the cell.
Third, cable theory (Jack et al. 1975, Koch 1999) informs us that the dendritic morphology of a typical CNS neuron—typically consisting of many thinbranch subtrees radiating outward from the cell body and/or main dendritic trunks—is ideally suited to isolate voltage signals within specific subregions of the dendritic arbor. Thus, the electrical coupling between a thin branch and a main trunk (or soma) is expected to produce a significant attenuation of distally generated voltage signals when measured at the trunk, with the most profound suppression of signal transmission reserved for rapid voltage transients associated with fast synaptic potentials or dendritic spikes. Thus, if thin branches, which receive the vast bulk of the excitatory input in most neurons, routinely generate fast spike-like events in response to synaptic excitation, the cable structure of these cells appears designed to ensure that distally-generated spikes are seen only in strongly attenuated form in other parts of the cell. Clearly, this type of functional compartmentalization could complicate the issue of synaptic integration, since the overall response of the cell would depend on whether synaptic inputs were delivered to the same or different dendrites, and whether dendritic spikes were generated or not.
7. Computational Implications
In spite of many uncertainties, currently available experimental data, combined with insights gained from theoretical and modeling studies, seem to support the hypothesis that dendritic trees are compartmentalized functionally, and could consist of a moderately large number of, for example, up to 100, independent integrative ‘subunits.’ The idea that dendritic trees are compartmentalized functionally has been discussed in a variety of forms over the years (Koch et al. 1982, Shepherd et al. 1985, Rall and Segev 1987, Mel 1992a, 1992b, 1993, Mel et al. 1998, Archie and Mel 2000, Poirazi and Mel 2001). In the continued absence of empirical evidence sufficient to confirm or deny this ‘‘nonlinear subunit’’ hypothesis, several recent modeling studies have provided support for one simple version of the idea. According to this model, (a) each dendritic subunit, corresponding to a single branch or small thin-branch subtree, acts as a separately thresholded neuron-like summing unit, and (b) the many subunit responses are summed in the main trunks and the cell body to produce the cell’s final output (Fig. 1). This formulation ignores differences in the locations of synapses within a subunit, as justified by theoretical considerations and biophysical modeling studies indicating that voltage signals are communicated far more effectively within a dendritic branch than between branches—especially for branches separated by a main trunk or the cell body. In mathematical terms, the neuron’s overall output y is given by a sum of m independently-thresholded subunit responses. Each subunit response is given by a weighted sum of k inputs followed by a fixed subunit nonlinearity, g, which is typically modeled as a sigmoid or power function
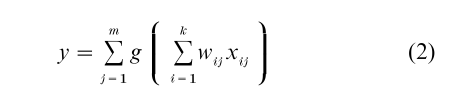
This simple formula, which is similar to the expression describing a conventional two-layer neural network (Bishop 1995), was found to predict the time-averaged firing rates produced by a biophysically detailed compartmental model of a pyramidal cell, a model which included passive membrane properties, a spatially-extended dendritic morphology, synapses with realistic conductance models, and voltage-dependent ion channels in the dendrites (Archie and Mel 2000).
From a functional perspective, it is interesting to note that the sum-of-thresholded-subunits model captures the underlying structure of a variety of classical and extraclassical receptive field properties found in cortical neurons, suggesting that dendritic subunits could contribute in important ways to the many quotidian signal-processing functions of cortical tissue (Mel 1999). In a different vein, a recent modeling study has shown that subunit-containing neurons as described in Eqn. (2) could increase the trainable storage capacity of neural tissue by orders of magnitude (Poirazi and Mel 2001).
While it is clear that many questions remain to be answered regarding the information processing functions of single neurons, one may be confident that with the steady advance of experimental methods available to study neuronal function at the required level of detail, coupled with the enormous flexibility of hypothesis testing provided by the computer modeling enterprise, the many fascinating secrets of neural information processing will gradually yield in the coming years.
Bibliography:
- Agmon-Snir H, Segev I 1993 Signal delay and input synchronization in passive dendritic structures. Journal of Neurophysiology 70: 2066–85
- Amitai Y, Friedman A, Connors B, Gutnick M 1993 Regenerative electrical activity in apical dendrites of pyramidal cells in neocortex. Cerebral Cortex 3: 26–38
- Archie K A, Mel B W 2000 An intradendritic model for computation of binocular disparity. Nature Neuroscience 3(1): 54–63
- Bishop C 1995 Neural Networks for Pattern Recognition. Oxford University Press, Oxford, UK
- Golding N L, Jung H-Y, Mickus T, Spruston N 1999 Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel sybtypes in CA1 pyramidal neurons. Journal of Neuroscience 19: 8789–98
- Golding N L, Spruston N 1998 Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron 21: 1189–200
- Jack J, Noble D, Tsien R 1975 Electric Current Flow in Excitable Cells. Clarendon Press, Oxford, UK
- Kamondi A, Acsady L, Buzsaki G 1998 Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. Journal of Neuroscience 18: 3919–28
- Koch C 1999 Biophysics of Computation. Oxford University Press, New York
- Koch C, Poggio T, Torre V 1982 Retinal ganglion cells: A functional interpretation of dendritic morphology. Philosophical Transactions of the Royal Society of London B 298: 227–64
- Lubke J, Markram H, Frotscher M, Sakmann B 1996 Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: Comparison with synaptic innervation of adjacent neurons of the same class. Journal of Neuroscience 16: 3209–18
- McCullough W, Pitts W 1943 A logical calculus of the ideas immanent in nervous activity. Bulletin of Mathematical Biophysics 5: 115–33
- Mel B W 1992a The clusteron: Toward a simple abstraction for a complex neuron. In: Moody J, Hanson S, Lippmann R (eds.) Advances in Neural Information Processing Systems, Vol. 4. Morgan Kaufmann, San Mateo, CA, pp. 35–42
- Mel B W 1992b NMDA-based pattern discrimination in a modeled cortical neuron. Neural Computation 4: 502–16
- Mel B W 1993 Synaptic integration in an excitable dendritic tree. Journal of Neurophysiology 70(3): 1086–101
- Mel B W 1999 Why have dendrites? A computational perspective. In: Stuart G, Spruston N, Hausser M (eds.) Dendrites. Oxford University Press, Oxford, UK, pp. 271–89
- Mel B W, Ruderman D L, Archie K A 1998 Translationinvariant orientation tuning in visual ‘complex’ cells could derive from intradendritic computations. Journal of Neuroscience 17: 4325–34
- Poirazi P, Mel B W 2000 Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29: 779–96
- Rall W 1964 Theoretical significance of dendritic trees for neuronal input-output relations. In: Reiss R (ed.) Neural Theory and Modeling. Standard University Press, Stanford, CA, pp. 73–97
- Rall W, Burke R, Holmes W, Jack J, Redman S, Segev I 1992 Matching dendritic neuron models to experimental data. Physiological Reviews 72(4): S159–86
- Rall W, Segev I 1987 Functional possibilities for synapses on dendrites and on dendritic spines. In: Edelman G, Gall W, Cowan W (eds.) Synaptic Function. Wiley, New York, pp. 605–36
- Rosenblatt F 1962 Principles of Neurodynamics. Spartan, Washington
- Rumelhart D, McClelland J 1986 Parallel Distributed Processing. MIT Press, Cambridge, MA
- Schiller J, Major G, Koester H J, Schiller Y 2000 NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature 404: 285–89
- Segev I, Rall W 1998 Excitable dendrites and spines—earlier theoretical insights elucidate recent direct observations. Trends in Neuroscience 21: 453–60
- Shepherd G 1998 The Synaptic Organization of the Brain. Oxford University Press, Oxford, UK
- Shepherd G M, Brayton R K, Miller J P, Segev I, Rinzel J, Rall W 1985 Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proceedings of the National Academy of Science USA 82: 2192–5
- Stuart G, Spruston N, Hausser M 1999 Dendrites. Oxford University Press, Oxford, UK




