View sample typhoid fever research paper. Browse research paper examples for more inspiration. If you need a health research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
History
The name typhoid fever is derived from the Greek word meaning smoke, obscurity, stupor, and refers to the apathy, confusion, stupor, and neuropsychiatric symptoms that are seen in severe infection. Typhoid fever was probably described for the first time by Willis in 1659, but before the nineteenth century, typhoid fever and typhus were confused. The preeminent involvement of the intestinal tract was noted by Louis and subsequently by Jenner. In 1869, the more accurate term enteric fever was proposed by Wilson as an alternative to typhoid fever given the anatomic site of infection. In 1873, Budd demonstrated the food and waterborne transmissibility of typhoid fever. The typhoid bacillus was described for the first time by Eberth in 1880 (the name Eberth bacillus is sometimes used) and isolated from the spleens of infected patients by Gaffkey in 1884. In 1896, Pfeiffer and Kalle obtained the first heat-killed organism vaccine, and Widal described the agglutination reaction of typhoid bacilli caused by serum of convalescent patients. Based on this phenomenon, from the 1020s to 1940s, Kauffman and White studied the antibody interactions with bacterial surface antigens and established the antigenic classification of Salmonella genus still used today. The modern age for typhoid fever treatment began in 1948 when Woodward successfully treated some Malaysian typhoid patients with chloramphenicol, just synthesized by Burkholder.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Epidemiology
Typhoid fever continues to be a serious public health problem throughout the world. It has been estimated that typhoid fever caused about 22 million cases and more than 200 000 deaths globally in 2000. Until the twentieth century, the disease had a worldwide distribution. Afterward, the number of typhoid cases in developed countries greatly decreased, as a result of changes in sanitation and hygiene. Today, cases of typhoid fever occur throughout the world. Countries with a high endemicity, classified as high-risk regions (incidence >100 cases per 100 000 population per year), include south-central Asia, southeast Asia, and possibly southern Africa (more detailed incidence studies are needed). Medium risk areas (10–100 cases per 100 000) are the rest of Asia, Africa, Latin America, and Oceania, except for Australia and New Zealand. In the other parts of the world, the incidence of typhoid fever is low (<10 per 100 000), and most cases in these countries occur in travelers who visit regions in which typhoid fever is highly endemic, particularly the Indian subcontinent. Outbreaks of typhoid fever occur when a relatively high number of cases are observed due to the contact with a common source of infection. Epidemics are less common than sporadic cases. In the United States they account for 7% of total cases.
Paratyphoid fever, a less severe enteric fever caused by Salmonella paratyphi A, S. paratyphi B, and S. paratyphi C, is estimated to have caused roughly 6 million cases in 2000. It has been reported that S. paratyphi A may cause up to half of all cases of enteric fever in some endemic countries. Some studies report that the incidence of disease caused by S. paratyphi may be higher among travelers, probably due to a vaccine effect, which gives protection only for Salmonella typhi.
In endemic areas, the incidence of typhoid fever is highest in children from 5 to 19 years of age, but in some settings typhoid can also be a significant cause of morbidity from 1 to 5 years of age. In children younger than 1 year, the disease is often more severe and is associated with a higher rate of complications. An increased risk of severe illness is also related to some preexisting diseases (immunosuppression, biliary and urinary tract abnormalities, hemoglobinopathies, malaria, schistosomiasis, bartonellosis, histoplasmosis). Travelers from industrialized countries who visit endemic areas are at particular risk of developing the disease, probably because of their lack of the background immunity that the local population has acquired as a result of multiple subclinical infections.
S. typhi and S. paratyphi colonize only humans. People can transmit the disease as long as the bacteria remain in their body, from prior to the onset of symptoms to the first week of convalescence. Approximately 10% of untreated patients will discharge bacteria for up to 3 months and 1–5% of typhoid patients become chronic carriers.
The disease is most often acquired by ingestion of food or water contaminated by the feces of patients and carriers. Transmission related to contamination with infected urine can occasionally occur. Direct person-to-person transmission is also possible. The pathogen can survive for days in water and for months in contaminated eggs and frozen oysters. Polluted water is the most common source of typhoid transmission. Contaminated raw fruits and vegetables, shellfish, milk and milk products such as ice creams have been shown as a source of infection. Waterborne transmission of S. typhi usually involves the ingestion of fewer microorganisms and therefore has a longer incubation period and lower attack rate than foodborne transmission, which is associated with larger inocula.
Paratyphoid transmission usually needs a higher infective dose than typhoid and thus is more frequently associated with the ingestion of contaminated food from street vendors.
A major problem is the emergence of antimicrobial resistance involving both S. typhi and S. paratyphi strains, particularly in endemic areas.
Etiology
Salmonella is a genus of the family Enterobacteriaceae. According to the recommendations from the World Health Organization (WHO) Collaborating Centre for Reference and Research on Salmonella, the genus Salmonella contains two species named S. enterica and S. bongori. S. enterica is divided into six subspecies (I–VI); the majority of Salmonella serotypes, including serotypes S. typhi and S. paratyphi A, B, and C, belong to S. enterica subsp. I (S. enterica subsp. enterica).
S. typhi is a Gram-negative, non-spore-forming, facultative anaerobic bacillus 2–3 0.4–0.6 mm in size, motile by peritrichous flagella. Unlike other Salmonellae, it does not produce gas on sugar fermentation. The bacterium is characterized by its flagellar antigen, H, its lipopolysaccharide O antigens 9 and 12, and its polysaccharide capsular Vi (for virulence) antigen, found at the surface of freshly isolated strains. The cell wall O antigens are components of lipopolysaccharide (LPS), which elicits a variety of inflammatory responses. Vi antigen is associated with increased infectiousness and virulence, but Vi-negative strains can also cause the disease.
After treatment with different substances, antibodies to the flagellar H antigen and to the somatic O antigen can be used to agglutinate the organism. Serotyping of all surface antigens can be used for formal identification.
The complete DNA sequence of a multidrug-resistant isolate of S. typhi has been determined and shows that up to 80% of the chromosome is similar to that in Escherichia coli. Species or serotype-specific genes are scattered along this conserved core. All types of S. enterica have two large clusters of genes known as Salmonella pathogenicity island 1 (SPI-1) and SPI-2, that facilitate invasion of and survival inside host cells. The S. typhi genome also contains SPI-7, which has genes that code for Vi polysaccharide production. Two plasmids are present in some strains: pHCM1, which encodes several drugresistance determinants, and pHCM2, which shows homology with the virulence plasmid of Yersinia pestis. Sensitivity to specific bacteriophages can be used to distinguish S. typhi among other serotypes.
S. paratyphi A, B, C cause a very similar but often less severe disease than S. typhi. S. paratyphi A and B share the somatic O12 antigen with the serovar Typhi; some S. paratyphi C strains can possess the Vi antigen. The analysis of the S. paratyphi A genome indicates that it is similar to the S. typhi genome but suggests that it has a more recent evolutionary origin.
Pathogenesis
After ingestion of contaminated water or food, S. typhi must overcome the host’s defenses to result in infection. First, bacteria must transverse the acid barrier of the stomach. Decreased gastric acidity seems to be the most important factor in lowering the infectious dose, because salmonellae survive poorly at normal gastric pH (i.e., <1.5), while they tolerate a pH 4.0 or higher well. Then salmonellae must cross the mucus layer overlying the epithelium of the small intestine and evade secretory product of the intestine, pancreas, and gallbladder as well as secretory IgA.
S. typhi invades the gut mucosa in the terminal ileum, interacting with both enterocytes and microfold cells (M cells) that overlie the ileal Peyer patches. A key, early step in the infectious process is the induction of the intestinal epithelial cells to increase the levels of the membrane receptor through which S. typhi interacts with them. Salmonellae are then internalized within membrane-bound vacuoles and move from the apical to the basolateral surface by transcytosis. Then bacteria enter mononuclear and dendritic cells in Peyer patches or are taken up by macrophages, by inducing generalized macropinocytosis, and are carried to the mesenteric lymph nodes. After internalization within macrophages, organisms multiply in the cytosol and move toward adjacent cells. Intracellular salmonellae have the ability to induce macrophage cell death by activating the pro-apoptotic protease caspase 1 while also initiating a pro-inflammatory cytokine response. The ability to induce phagocytosis by macrophages and epithelial cells protects salmonellae from phagocytosis by neutrophils, which can rapidly kill these bacteria. Vi antigen also inhibits phagocytosis of S. typhi by neutrophils while not interfering with internalization by more permissive macrophages and epithelial cells.
Survival within macrophages is essential to typhoid fever pathogenesis and the spread of the organisms beyond the bowel to the systemic circulation. This primary bacteremia within 24 h of ingestion results in the organisms reaching the liver, spleen, bone marrow, and other parts of the reticuloendothelial system, where they survive and replicate in cells of monocytic lineage. The increase in generalized macropinocytosis may also be important in the development of neutropenia, anemia, and thrombocytopenia because of stimulation of hemophagocytosis. During the asymptomatic incubation phase most organisms are localized intracellularly. Symptoms of typhoid fever occur when a critical number of organisms have replicated, inducing the secretion of cytokines by macrophages. Cytokine release may also activate the pathophysiologic mechanism of the neuropsychiatric manifestations of typhoid fever. The characteristic enlargement of the liver and spleen is probably related to S. typhi survival or replication within reticuloendothelial cells, the pathologic recruitment of mononuclear cells, and the development of a cell-mediated immune response. Recruitment of additional mononuclear cells and lymphocytes can also result in marked enlargement and necrosis of the Peyer patches after several weeks of infection. This process is probably the cause of the abdominal pain that is characteristic of typhoid fever.
Clinical Manifestations
The clinical presentation of typhoid fever is highly variable, ranging from fever with little other morbidity to a severe systemic illness with marked toxemia and associated complications involving many systems (Table 1).
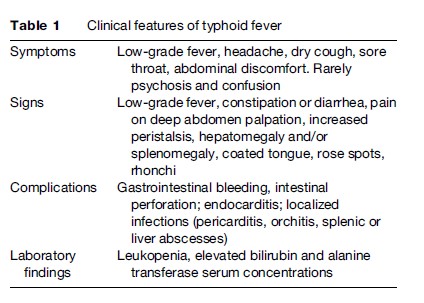
The incubation period ranges from 5 to 21 days depending on the inoculum ingested and the person’s health and immune status. After ingestion of the organism, enterocolitis may develop and usually resolves before the onset of fever. The clinical picture of acute noncomplicated typhoid fever is characterized by prolonged low-grade fever, dull frontal headache, a dry cough, sore throat, and nonspecific symptoms such as malaise, dizziness, myalgia, anorexia, and nausea, which are frequently present before the onset of fever. Alterations of bowel habits varying from constipation in adults to diarrhea in children and tender abdomen are typical symptoms, even if abdominal pain is initially present in only 20–40% of cases.
Neuropsychiatric manifestations, including psychosis and confusion, occur in 5–10% of patients. This so-called typhoid state has been described as muttering delirium and coma vigil. Seizures and coma are very infrequent and may represent febrile seizures of childhood.
The fever might rise progressively in a stepwise manner, with 5–7 days of daily increments in maximal temperature of 0.5–1 C, to become persistent and high grade (39–41 C) by the 2nd week of illness. Continuous high-grade fever can continue for up to 4 weeks if left untreated, followed by a return to normal temperature. Weakness and lethargy can continue for 2 months thereafter. Although fever is a classic sign of typhoid, it does not always develop, and its pattern is not always useful because typhoid may also have an abrupt febrile onset instead of the typical subacute trend.
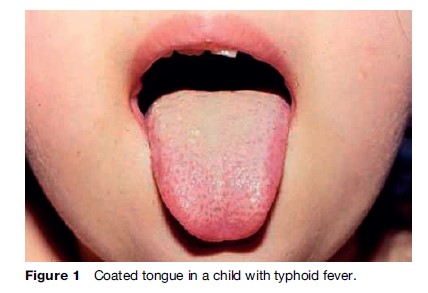
On physical examination, coated tongue is a frequent finding (Figure 1). Small erythematous maculopapular lesions 2–4 mm in diameter (rose spots) are seen on the abdomen and chest in 25–30% of cases late in the first week of fever. Organisms can be cultured from punch biopsies of these lesions. Rhonchi and scattered rales might be heard on chest auscultation, with normal chest radiographs. Relative bradycardia at the peak of high fever, previously considered an indicator of typhoid fever, is neither a sensitive nor a specific sign and occurs in less than 50% of patients. Examination of the abdomen usually reveals pain on deep palpation, and peristalsis is frequently increased. Hepatomegaly and splenomegaly are often present. In approximately 3% of adults, cholecystitis develops.
Hematological abnormalities associated with typhoid fever include leukopenia and anemia. In children and in the first 10 days of illness, leukocytosis can be present. Thrombocytopenia that usually resolves spontaneously develops in some patients. Liver involvement is common with elevated concentrations of serum bilirubin and alanine transferase. Rarely proteinuria and immune complex glomerulonephritis without irreversible loss of renal function are noted.
Many of the complications of untreated typhoid fever occur in the third or fourth week of infection. The commonest complications are gastrointestinal bleeding and intestinal perforation. Gastrointestinal bleeding occurs in 10–20% of cases due to erosion of the Peyer patch into an intestinal vessel and can express with either occult blood in stool or melena. Intestinal perforation develops in 1–3% of hospitalized cases and is characterized by recurrent fever, abdominal pain, and intestinal hemorrhage. In such cases, the patient’s blood should be recultured and antimicrobial therapy broadened to cover aerobic and anaerobic enteric organisms.
Other infectious complications include endocarditis and localized infections such as pericarditis, orchitis, and splenic or liver abscesses.
Vertical transmission of typhoid fever during late pregnancy is a rare but often life-threatening event. Neonatal typhoid usually begins within 3 days of birth with fever, vomiting, diarrhea, and abdominal distention. There might be significant hepatomegaly and jaundice. Seizures can occur. Asymptomatic excretion can also be the only consequence of infection.
The diagnosis of typhoid fever should be strongly considered in travelers returning from tropical and subtropical areas with fever. Because of early access to medical care, the stepwise fever curve is usually not seen in this population. The differential diagnosis of gradual onset of fever and abdominal pain with hepatosplenomegaly also includes malaria, amebic liver abscess, visceral leishmaniasis, and viral syndromes such as dengue fever.
In the preantibiotic era, approximately 15% of patients with typhoid fever died. With the introduction of early and appropriate antibiotic therapy, the average case fatality rates are less than 1%. However, mortality rates of 10–30% have been reported in certain Asian and African countries and have been associated with multidrug-resistant strains and delays in starting antimicrobial therapy. In children aged 1–5 years, typhoid fever can be milder and can mimic a viral syndrome. The rate of severe complications is lower than at later ages.
Chronic biliary or urinary carriage may occur in 2–5% of cases, even after treatment. The long-term carrier state is defined as the persistence of S. typhi in stool or urine for periods longer than 1 year. The frequency is higher in women and in persons with biliary abnormalities or concurrent bladder infection with Schistosoma. Long-term carriage of S. typhi is a public health risk, especially for infected individuals who work in the food industry. Moreover, it has been associated with an increased incidence of carcinoma of the gallbladder and other intestinal malignancies. Some persons excreting S. typhi have no history of typhoid fever.
Diagnosis
Definitive diagnosis of typhoid fever requires the isolation of S. typhi.
Blood culture is the foundation of the diagnosis. Using standard broth cultures, a positive result is yielded in 30–90% of cases, probably because small quantities of S. typhi are present in patients’ blood. Sensitivity decreases with increasing duration of fever. Scarce volume of cultured blood, unsuitable ambient temperature, and antimicrobials compromise the result. Culture of the blood mononuclear cell-platelet fraction obtained by centrifugation reduces the time to isolation of S. typhi, but does not increase the sensitivity.
The sensitivity of bone marrow culture is 80–95%. Bone marrow cultures are particularly useful for lengthy illnesses and prior antibiotic treatment (up to 5 days), since the sensitivity is not reduced.
Stool isolation alone is insufficient for diagnosis of typhoid fever. However, it is helpful for carrier detection. Children have a higher incidence of positive stool cultures than adults (60% vs. 27%).
S. typhi can also be isolated from gastric or intestinal secretions, urine, or rose spots biopsies.
Serologic tests include the classic Widal test and new rapid tests. The Widal test identifies the agglutinating antibodies against the O (somatic) and H (flagellar) S. typhi antigens, which appear 7–10 days after disease onset. False-positive results stem from sharing of O and H antigens and cross-reacting epitopes with other Enterobacteriaceae. Results from a single acute sample should be interpreted against the appropriate local cut-off value, or there should be a fourfold rise in the antibody titer of a second sample collected 2 weeks later.
New simple and rapid serologic tests have been developed: Tubex, Typhidot, and Typhidot M, as well as the S. typhi IgM dipstick test. Tubex can detect IgM O9 antibodies from patients within a few minutes. The O9 antigen is extremely specific because it has been found in serogroup D salmonellae but not in other microorganisms. A positive result given by Tubex invariably suggests a serotype D Salmonella infection. Infections caused by other serotypes, including S. paratyphi A, give negative results. In a preliminary study, the test performed better than the Widal test in both sensitivity and specificity.
Typhidot can detect specific IgM and IgG antibodies against a 50-kDa antigen of S. typhi and takes 3 h to perform. The detection of IgM reveals acute typhoid in the early phase of infection, while the detection of both IgG and IgM suggests acute typhoid in the middle phase of infection. False-positive results attributable to previous infection may occur, since IgG can persist for more than 2 years after typhoid infection. False-negative results may occur in cases of reinfection, if the significant boosting of IgG masks the detection of IgM.
For solving these problems Typhidot-M was developed.
This test detects specific IgM antibodies only, by inactivating total IgG in the serum sample. The detection of specific IgM suggests acute typhoid infection. High specificity (75%), sensitivity (95%), and negative and positive predictive values suggest that this test could be used as the gold standard in laboratory diagnosis of typhoid fever.
The typhoid IgM dipstick assay detects specific IgM antibodies to S. typhi LPS antigen. It is a rapid and simple alternative for the diagnosis of typhoid fever. Sensitivity seems to be lower than the culture method. Specific antibodies usually only appear 1 week after the onset of symptoms and signs.
Urinary Vi antigen can be detected by ELISA within the first febrile week, but the specificity is low (false-positive results in patients with brucellosis).
DNA probes and PCR-based tests are used for research purposes.
Therapy
Treatment of typhoid fever reduces mortality and severe complications drastically. It is also important to obtain the rapid resolution of clinical disease and to eradicate the organism promptly to prevent relapses and fecal carriage.
Chloramphenicol has been the treatment of choice for typhoid fever since its introduction in 1948. After oral administration of 500 mg four times daily, the duration of fever is reduced from 14–28 days to 3–5 days and mortality from 20 to 1%. Problems with chloramphenicol therapy are the emergence of plasmid-mediated resistance, a high rate of chronic carriage, bone marrow toxicity, and the withdrawal of oral formulation in some countries.
Amoxicillin and trimethoprim-sulfamethoxazole were also used for the treatment of typhoid fever, but the efficacy of these drugs has also diminished due to the emergence of multidrug-resistant strains of S. typhi (plasmid-mediated resistance).
Fluoroquinolones (ciprofloxacin, ofloxacin, pefloxacin) are greatly effective for treatment of typhoid fever because they are highly active against salmonellae in vitro, effectively penetrate macrophages, and achieve high concentrations in the bowel and gallbladder. Ciprofloxacin 500 mg orally twice daily for 10 days is the drug of choice for the treatment of multidrug-resistant typhoid fever. The clinical cure rate is approximately 98%, fever clearance time is roughly 4 days and relapse and fecal carriage rates are less than 2%. A relative fluoroquinolone resistance due to chromosomal mutation has emerged in some countries. Such S. typhi strains are nalidixic acid-resistant (NAR) and have a higher minimal inhibitory ciprofloxacin concentration (0.125–1 mg/dl). Short courses of ofloxacin 10–15 mg/kg divided twice daily given for 2, 3, or 5 days appears to be simple, safe, and effective in the treatment of uncomplicated multidrug-resistant typhoid fever, with a fever clearance time of 4 days and relapse and fecal carriage rates less than 3%, when the isolate strain is nalidixic acid-susceptible. Patients infected with NAR strains should be treated either with parenterally administered third-generation cephalosporins or higher doses of fluoroquinolones. Therapeutic options include ceftriaxone (1–2 g daily for 10–14 days), ciprofloxacin (10 mg/ kg twice daily for 10 days), or ofloxacin (10–15 mg/kg divided twice daily for 7–10 days).
Concerning parenterally administered third-generation cephalosporins, short-course therapy for 5–7 days gives an excellent response rate in uncomplicated typhoid fever, but the relapse rate seems to be high. Currently, quinolones are not recommended in children younger than 10 years or pregnant women because of evidence of cartilage damage in young animals; therefore the preferred treatment of multidrug-resistant typhoid fever in children is a parenteral third-generation cephalosporin. However, quinolones have been used to treat multidrug-resistant typhoid fever in children and pregnant patients without adverse effects.
Azithromycin seems to be comparable to fluoroquinolones and ceftriaxone. It could also be an acceptable therapeutic option for quinolone-resistant typhoid fever in children.
Supportive treatment includes maintenance of hydration, appropriate nutrition, and antipyretics. The use of glucocorticosteroids for 48 h, i.e., dexamethasone, 3 mg/kg intravenously, followed by eight doses of 1 mg/kg every 6 h, should be considered for the treatment of severe typhoid with altered mental status or shock, because this treatment was associated with a significant reduction in mortality.
Treatment of chronic carriers is a public health topic because it is useful to reduce the transmission potential. Long courses and high doses of quinolones (ciprofloxacin 750 mg orally twice daily or norfloxacin 400 mg twice daily for 28 days) can obtain negativization of stool and bile cultures in 80% of carriers without gallstones. In people with gallstones, cholecystectomy along with antibiotic therapy might be required.
Prevention And Control
Safe water, safe food, personal hygiene, and appropriate sanitation are the key preventive strategies against typhoid fever. Vaccination is an additional tool and not a substitute for avoiding high-risk food and beverages because the protective efficacy is not 100%; furthermore, immunity can be overcome by high inoculum dose. Vaccination is recommended in travelers from developed countries to typhoid-endemic countries, particularly those traveling to small cities, villages, and destinations off the usual tourist itineraries for 2 weeks or more. It is also useful in preventing and controlling epidemics, as well as for children in endemic settings aged 2–19 years.
The parenteral heat-phenol-inactivate whole-cell vaccine is no longer used in developed countries, but it is still licensed in many countries in spite of its reactogenicity. It showed to be effective in preventing typhoid fever, but local and systemic adverse reactions occur frequently (fever, severe headache, significant local pain at the site of injection).
Two other vaccines are currently licensed, the attenuate Ty21a live oral vaccine and the purified Vi polysaccharide parenteral vaccine. Both vaccines are safe and relatively well tolerated (Table 2).
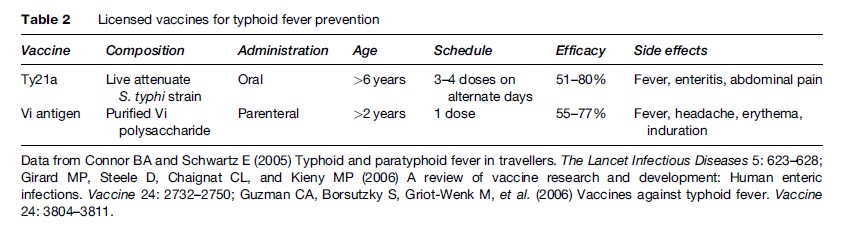
The Ty21a live oral vaccine is an attenuated S. typhi strain that lacks the Vi antigen and is thus avirulent but contains immunogenic cell wall polysaccharides. The vaccine stimulates vigorous secretory IgA, serum IgG, and cell-mediated immune response. Primary vaccination consists of one enteric-coated capsule or lyophilized sachet on alternate days for three to four doses. The vaccine needs to be refrigerated. The overall protective efficacy varies widely in different studies (51–80%). The multidose administration schedule and the requirement for refrigeration may be a problem in some settings. The safety and tolerability are excellent: The most common adverse events reported were mild and transient gastroenteritis, abdominal pain, and pyrexia. No serious adverse reactions have been observed. The live oral vaccine is not recommended for children younger than 6 years, pregnant women, and the immunosuppressed. The concurrent use of antibiotics or antimalarials (mefloquine, proguanil) may interfere with the antibody response, so antimicrobials should be avoided for 7 days before or after vaccination. Concomitant administration of other vaccination is allowed.
The Vi antigen parenteral vaccine contains only the purified capsular polysaccharide antigen. A single subcutaneous or intramuscular dose induces rapid anti-Vi serum antibody production, but neither mucosal immunity nor immunological memory. The vaccine is licensed for use in adults and children older than 2 years. The protective efficacy ranges between 55 and 77%. Rare side effects include fever, headache, and local erythema or induration. It is safe to be coadministered with other vaccines as well as antimalarials, with no diminution in antibody response.
The lack of guaranteed efficacy of the licensed vaccines in children younger than 2 years has prompted the development of a conjugate Vi vaccine using several protein carriers to enhance immunogenicity. The median protection rate after two parenteral doses seems to be approximately 91.5%.
New vaccines are being developed based on outer membrane proteins and new live oral vaccines.
Currently, there is no licensed vaccine for paratyphoid fever. S. paratyphi A and B lack the Vi antigen, rendering Vi polysaccharide-based vaccines ineffective. Some cross-protection may be elicited by S. typhi live attenuated vaccines, because S. paratyphi A and B share the somatic O12-antigen with serovar Typhi.
Bibliography:
- Bhan MK, Bahl R, and Bhatnagar S (2005) Typhoid and paratyphoid fever. The Lancet 366: 749–762.
- Brenner FW, Villar RG, Angulo FJ, Tauxe R, and Swaminathan B (2000) Salmonella nomenclature. Journal of Clinical Microbiology 38: 2465–2467.
- Connor BA and Schwartz E (2005) Typhoid and paratyphoid fever in travellers. The Lancet Infectious Diseases 5: 623–628.
- Crump JA, Luby SP, and Mintz ED (2004) The global burden of typhoid fever. Bulletin of the World Health Organization 82: 346–353.
- Fadeel MA, Crump JA, Mahoney FJ, et al. (2004) Rapid diagnosis of typhoid fever by enzyme-linked immunosorbent assay detection of Salmonella serotype Typhi antigens in urine. The American Journal of Tropical Medicine and Hygiene 70: 323–328.
- Girard MP, Steele D, Chaignat CL, and Kieny MP (2006) A review of vaccine research and development: Human enteric infections. Vaccine 24: 2732–2750.
- Guzman CA, Borsutzky S, Griot-Wenk M, et al. (2006) Vaccines against typhoid fever. Vaccine 24: 3804–3811.
- Ismail TF (2006) Rapid diagnosis of typhoid fever. The Indian Journal of Medical Research 123: 489–492.
- Lin FY, Ho VA, Khiem HB, et al. (2001) The efficacy of a Salmonella typhi Vi conjugate vaccine in twoto five-year-old children. The New England Journal of Medicine 344: 1263–1269.
- Mandell GL, Bennett JE, and Dolin R (eds.) (2000) Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone.
- Meltzer E, Yossepowitch O, Sadik C, Dan M, and Schwartz E (2006) Epidemiology and clinical aspects of enteric fever in Israel.
- The American Journal of Tropical Medicine and Hygiene 74: 540–545.
- Siddiqui FJ, Rabbani F, Hasan R, Nizami SQ, and Bhutta ZA (2006) Typhoid fever in children: Some epidemiological considerations from Karachi, Pakistan. International Journal of Infectious Diseases 10: 215–222.
- Walia M, Gaind R, Mehta R, et al. (2005) Current perspectives of enteric fever: A hospital-based study from India. Annals of Tropical Paediatrics 25: 161–174.
- World Health Organization (2003) Background document: The diagnosis, treatment and prevention of typhoid fever. www.who. int/vaccines-documents/ (accessed January 2008).
- https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/typhoid-and-paratyphoid-fever – Centers for Disease Control and Prevention. Traveler’s Health: Yellow Book, Typhoid and Paratyphoid Fever.
- https://www.cdc.gov/typhoid-fever/index.html – Centers for Disease Control and Prevention, Typhoid Fever.
- https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/typhoid – World Health Organization.




