View sample vaccination research paper. Browse research paper examples for more inspiration. If you need a health research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Arguably, in the entire history of humanity the concept and practice of vaccination against infectious diseases has resulted in greater benefits to the health of mankind than any other cultural, social, or scientific advance. In testament to their historical importance, vaccines were ranked first among the ten greatest public health achievements of the twentieth century (Centers for Disease Control and Prevention, 1999). Through their use, scourges of nature have been eradicated, controlled, or rendered irrelevant, and generations of children have survived into adulthood, unscathed by diseases that earlier in history would have been lethal. Vaccines harnessed the human immune system to its fullest extent long before the fundamental tenets of immunology were described; the concepts that form the basis of vaccine science have since been extended to a plethora of infectious and noninfectious diseases.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
The history of vaccination parallels the history of human scientific endeavor and illustrates a number of important precepts common to all scientific inquiry: major advances generally stem from incremental progress that itself derives from the accumulation of ordered, experimental observations that have been synthesized from a variety of fields; landmark discoveries generally do not occur in a vacuum but are instead based on expanding pre-existing scientific thought; advances in technology inherently drive advances in science, and both are frequently products of specific unmet needs. Figure 1 provides a timeline of vaccine development within the historical context of world events over the last 200 years.
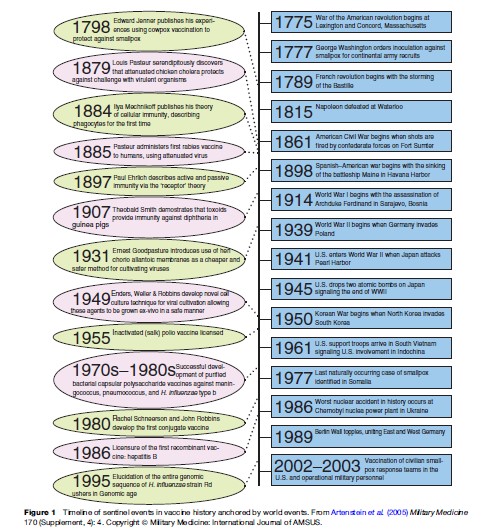
The Foundations Of Vaccinology
The history of vaccination, from a scientific standpoint, is traditionally dated from the publication, in 1798, of Edward Jenner’s landmark experiments with cowpox in which he inoculated a neighbor’s boy with purulent material from a milkmaid’s hand lesion in Berkeley, England (Stern and Markel, 2005). The boy, 8-year-old James Phipps, was subsequently shown to be protected against a smallpox challenge. However, the concept that humans could be protected against disease by intentionally exposing them to the supposed cause of the malady probably arose many centuries before Jenner, although this remains poorly documented. Legend has it that Mithridates VI, King of Pontus from 120 to 63 BC, protected himself against assassination by ingesting sub-lethal doses of poison in order to build his tolerance to such agents (Parish, 1965). Buddhists in India in the seventh century supposedly ingested snake venom to protect themselves from its fatal effects (Plotkin and Plotkin, 2004). These early forays into practical vaccination were based on empiricism; although Jenner and his immediate predecessors also appear to have based their theories on empiric observations from nature, their observations were probably supported by a more substantial experiential tradition.
In many ways smallpox represented a natural choice for the earliest explorations into systematic vaccination because of its historical position as the greatest disease scourge of mankind. The impact of smallpox on human history has been well documented and has been the subject of numerous textbooks, works of literature, objects of art, and theses regarding the rise and fall of civilizations (Barquet and Domingo, 1997; Fenner et al., 1988). Because it was commonly observed, as early as ancient times, that survivors of smallpox were protected against further episodes of the disease, attempts to harness this information to prevent infection were practiced long before Jenner. Documentation in Chinese medical texts of the eighteenth century reference practices in place in China in the late seventeenth century for inoculating the noses of healthy individuals with powdered scabs or lesional contents from infected individuals (Parish, 1965). Other forms of inoculation, later referred to as variolation (after the word ‘variola,’ derived from the Latin word meaning ‘mark on the skin’ (Parish, 1965)) were probably used in Africa, India, and the Ottoman Empire before being introduced into Europe in the early eighteenth century. It was common practice for women recruited from the Caucasus for the Turkish sultan’s harem to be inoculated in childhood in inconspicuous areas of their bodies (Barquet and Domingo, 1997).
Although the technique of variolation may have been introduced into other parts of Europe through various travelers returning from Istanbul, the credit for its introduction into England is generally given to Lady Mary Wortley Montague in 1721 (Barquet and Domingo, 1997). She had witnessed and been favorably impressed by the practice during her husband’s tenure as ambassador to the region. Material from the pustules of afflicted persons was introduced into the arm of a healthy person through multiple scratches or punctures, later known as scarification. Lady Montague, herself scarred by smallpox and therefore always pictured wearing a headpiece, had her young son inoculated while in the Ottoman court and upon the family’s return to England, had her daughter inoculated in public, under the scrutiny of physicians of the royal court. The interest of the royal family was thus piqued and, following several other successful public inoculation experiments, including one on prisoners in exchange for their freedom and one involving two royal children, the practice of variolation gained widespread acceptance in England by the 1740s and from that point on began to disseminate throughout Europe (Barquet and Domingo, 1997).
Despite an observed mortality rate of 2 to 3% related to variolation, the procedure still offered better odds than the 15 to 30% mortality from naturally acquired smallpox. The practice reached the New World as well; in 1721 Reverend Cotton Mather successfully advocated for its use to abort a smallpox epidemic in Boston. Variolation gained the support of American thought leaders such as Benjamin Franklin and became widespread in the colonies. In 1777, with his army decimated by smallpox and inoculated British troops boasting a significant tactical advantage, General George Washington, with the support of one of America’s preeminent physicians and statesmen, Dr. Benjamin Rush of Philadelphia, adopted the bold and unprecedented plan to inoculate all susceptible members of the Continental Army, a strategy that is believed to have tipped the balance in favor of the Americans (Artenstein et al., 2005).
Alternatives to the practice of variolation with its attendant risks arose from observations commonly recognized among rural agricultural societies of the seventeenth and eighteenth centuries. It was believed, although not necessarily widely known, that milkmaids who developed cowpox, generally a benign disease in humans manifested by pustular lesions on the hands or forearms resulting from contact with infected cow udders, were protected against smallpox. In addition, it was reported that these women failed to demonstrate cutaneous responses to variolation (Barquet and Domingo, 1997). In 1774, Benjamin Jesty, a farmer and cattle breeder in Yetminster, England, noted first-hand that his two milkmaids failed to acquire smallpox after caring for family members who had the disease (Hammarsten et al., 1979). Armed with this knowledge, a healthy dose of intestinal fortitude, and the threat of epidemic smallpox in the region, Jesty inoculated his wife and two sons with infected material from a neighbor’s cow. All survived the experiment and resisted natural smallpox, and the two boys were shown to be immune to subsequent variolation challenges 15 years later (Hammarsten et al., 1979). Jesty, though recognized as the earliest inoculator of cowpox by the Jennerian Society in 1805, was probably neither the first nor the last to perform such pragmatic, local experiments.
Jenner, although aware of Jesty’s work, became the first to systematically study and document the effect of vaccination by using cowpox and to formally propose and provide data to support his hypothesis that cowpox infection protected against subsequent smallpox infection (Riedel, 2005). Jenner, known as a country doctor but trained in the scientific method by the renowned surgeon John Hunter, performed his now famous experiments with cowpox in 1796 and subsequently published his findings, at his own expense after having his manuscript rejected by the Royal Society, in 1798 (Barquet and Domingo, 1997). In his work, entitled An Inquiry into the Causes and Effects of the Variolae Vaccinae, a disease discovered in some of the western counties of England, particularly Gloucestershire and Known by the Name of Cow Pox, Jenner coined the term ‘vaccination’ (from the Latin word vacca, for cow) to describe the procedure (Riedel, 2005). The monograph describes in detail his vaccination of 10 individuals and an additional 17 who resisted variolation after acquiring natural cowpox infection (Baxby, 1999); it also details seven individuals who were vaccinated with material derived from a previous vaccinee’s lesion, thus obviating the continued need for the animal intermediary in potential larger-scale vaccinations. Jenner’s work was met with considerable skepticism, but subsequent investigations and experiences by himself and others largely confirmed his findings and led to the dissemination of vaccination against smallpox in the Western world by the early part of the nineteenth century (Barquet and Domingo, 1997). As is the case with subsequent major advances in vaccinology, the landmark work of Jenner drew incrementally upon that of his predecessors to advance science to a new level and create the nascent field of vaccinology.
Although scientific inquiry regarding vaccination began in earnest with the dissemination of Jenner’s findings, it would be 87 years before the next major advance would take place. During that interval, however, incremental steps were occurring that would lay the groundwork for future major discoveries. The concepts of attenuation, or weakening of a pathogen strain, and strain passage, the transmission from one animal to another in order to attenuate, as they related to smallpox vaccination were discussed in scientific circles throughout the world during this period (Plotkin and Plotkin, 2004). In addition, as the practice became more widespread the risks of transmitting other diseases through person-to-person vaccination were becoming more apparent. It was in this milieu that Louis Pasteur, a French chemist, drew on the concepts promulgated by Jenner as he embarked on experiments with attenuated cultures of organisms in the latter part of the nineteenth century.
Pasteur had spent much of the 1860s and 1870s developing the germ theory in studies of fermentation, thus laying the framework for modern microbiology. In the summer of 1879, after inadvertently leaving a laboratory culture of chicken cholera (now known to be the bacterium Pasteurella multocida) exposed to air over a long holiday, Pasteur noted that the attenuated strain, weakened after prolonged exposure to air, protected chickens against challenge with live, virulent organisms (Plotkin and Plotkin, 2004). He correctly deduced that this concept was similar to Jenner’s and those of Jenner’s predecessors, allowing for the fact that Pasteur had immunized animals with an attenuated version of the challenge organism as opposed to a related species as in Jenner’s work. This, again, demonstrated Pasteur’s own axiom, stated years earlier in relation to his basic chemistry experiments, that in the realm of observation ‘‘chance only favors the prepared mind.’’
In 1881 Pasteur applied his findings to the larger goal of preventing the transmission of a disease of great medical and economic importance at the time – anthrax. Drawing on the landmark work of the German physician Robert Koch, who five years earlier had demonstrated the transmissible nature of Bacillus anthracis, Pasteur performed a series of public experiments on livestock using attenuated bacteria and a control group of animals that did not receive inoculations (Plotkin and Plotkin, 2004). Within days after both groups of animals were challenged with virulent anthrax spores it was clear that only the vaccinated animals were protected against disease and death. Thus, using his own discoveries regarding attenuation as well as the technical advances of Koch and other early microbiologists, Pasteur not only demonstrated the efficacy of a live, attenuated bacterial vaccine, but he also laid a foundation for future vaccine science through the creation of a standardized, self-perpetuating, laboratory-derived product. Pasteur followed up his animal work involving a live, attenuated anthrax vaccine by developing an attenuated product for rabies, a fatal disease of suspected bacterial etiology that was only much later found to be a viral infection. The fact that these 1885 experiments involved human subjects, not farm animals, and that there were clearly vaccine-related deaths caused public outcry and foreshadowed one of the persistent controversies engendered by vaccines – the balance between safety versus efficacy. Nonetheless, proof of principle was achieved, rabies vaccine soon became widely accepted, and Pasteur was credited with extending the concept of vaccination beyond cowpox and smallpox to the general practice of using a form of microbe to prevent an infectious disease.
Pasteur, Jenner before him, and of course, all of their predecessors derived their hypotheses regarding vaccine-induced prevention of transmissible diseases from reasoned observations. Around the time that Pasteur was performing his pioneering human experiments with rabies, investigations were unfolding that would explain their empiric findings and launch the fledgling field of immunology. The work of two European scientists in particular, Ilya Mechnikoff and Paul Ehrlich, established the basis for the modern schools of cellular and humoral immunity, respectively; their work led to significant and rapid advances in vaccinology (Parish, 1965). Mechnikoff, a Russian zoologist, embryologist, and student of Pasteur, published the first descriptions of phagocytic cells and other forms of cell-based defense in 1884; Ehrlich, a German academic physician, pioneered the concepts of active and passive immunization and through his famous ‘side-chain’ theory, promulgated in 1897, sought to explain the interaction between toxins and antitoxins, positing an argument that would later expand to form the basis for the pervasive immunologic concept of surface ligands and receptors (Winau et al., 2004). Although both shared the 1908 Nobel Prize in Medicine for their research on immunity, the work of other scientists during the late nineteenth and early twentieth centuries incrementally advanced the extant knowledge of immunology that would drive vaccine science for the next century.
Shortly after Pasteur’s successful, yet controversial experiments with live, attenuated rabies vaccines in humans, Daniel Salmon and Theobald Smith, in the United States, reported the successful immunization of pigeons against the hog cholera agent, later found to be a bacterium, using a heat-killed vaccine. The concept of killed vaccines appears to have developed concurrently with that of live vaccines; scientists in Pasteur’s institute, competing with Salmon and Smith, published their results a year later in 1887. Over the next two decades or so, and paralleling advances in microbiology, killed vaccines were developed that afforded protection against typhoid, plague, and cholera, in most instances, through the combined and competitive efforts of multiple scientific groups working independently (Plotkin and Plotkin, 2004). These forays into expanding the range of vaccines and the obstacles encountered therein had the effect of informing the field of medicinal chemistry and the conduct of clinical trials, and presaged future vaccine controversies such as patent and discovery claims, informed consent, ethics, and vaccine contamination by adventitious agents.
By the beginning of the twentieth century, the fundamental concepts of vaccinology were firmly rooted. Following the independent discoveries of Emile Roux, a Pasteur associate, and Alexandre Yersin that diphtheria bacteria elaborate a soluble protein toxin during infection that caused significant tissue destruction, and the independent findings by Emil von Behring and Shibasaburo Kitasato, prote´ge´s of Koch, that antitoxin in the serum of surviving animals neutralized diphtheria toxin in vitro, much of the active vaccine work during the early part of the century was devoted to the development of antitoxins and toxoids as vaccines against diphtheria and tetanus. Theobald Smith, an early pioneer in the concept of killed vaccines, also had an important role in the development of toxoids through animal studies. Further modifications to these products through medicinal chemistry, including the discovery of the adjuvant effect of aluminum compounds on toxoid immunogenicity in 1926, ultimately led to toxoid vaccines for human use.
Other than the important work with bacterial antitoxins and the development, by disciples of Pasteur, Calmette and Guerin, of a live, attenuated, partially protective vaccine for human tuberculosis (BCG), the first 25 years of the twentieth century produced significantly fewer major advances in vaccinology than had the last 25 years of the nineteenth century. The discovery of viruses led to the next great period in vaccinology. In the latter part of the nineteenth century the German scientist Adolf Mayer noted that the agent of tobacco mosaic disease in plants did not fulfill Koch’s postulates that had been established earlier for bacterial organisms: although clearly transmissible, it was unable to be cultivated via existing microbiological methods. Dimitri Ivanofsky of Russia extended these observations in 1892 with the finding that the agent was small enough to pass through porcelain filters that would typically block bacterial passage, yet still retained its infectivity. With the seminal, albeit incremental finding by Martinus Beijernick of the Netherlands in 1898 that these agents were capable of reproducing themselves in living cells (Manrubia and Lazaro, 2006), a new class of infectious agents, the ‘filterable agents,’ was recognized, although it would not be until after the first decades of the twentieth century that the modern connotation of ‘virus’ would become widespread in scientific circles.
In large part the nascent field of virology was limited by technical considerations related to the inability to cultivate viruses in laboratory environments. In 1931 Ernest Goodpasture, a professor of pathology at Vanderbilt University and later the director of the U.S. Armed Forces Institute of Pathology, developed the technique of growing viruses in the chorioallantoic membranes of fertile hen’s eggs (Goodpasture et al., 1931), a procedure that provided an inexpensive and safer alternative to whole animal inoculation and represented a major advance in virology that would lead to substantial dividends for vaccine science.
The Golden Age Of Vaccines: 1949–1980s
The advances of the so-called ‘golden age’ of vaccinology, while descended from branches of the Jenner-Pasteur lineage, were a direct outgrowth of Goodpasture’s work, although additional methodological refinements would be necessary first. Safe, attenuated yellow fever, influenza A, and killed typhus vaccines for human use were developed in the 1930s via the technique described by Goodpasture; nonetheless, the field began searching for further improvements to the laboratory methods for viral culture, a search that eventually culminated in the seminal work of John Enders, Thomas Weller, and Frederick Robbins at Boston Children’s Hospital in 1949. These investigators, building upon and refining the flask culture and continuous rolling bottle techniques devised by other contemporaries in the field, demonstrated the successful cultivation of Lansing type II poliovirus in human fibroblasts (Enders et al., 1949), thus permitting the practical, safe, and efficient ex vivo growth of viral pathogens and representing a major leap forward in vaccine research.
The novel method for growing viruses outside of living hosts in tissue culture led to an immediate period of rapid and sustained growth in vaccinology that continued for decades in which scientific advances were used to fill pressing public health needs. The vaccines that were developed in this period quickly and substantially reduced the burden and mortality associated with infectious diseases, especially among children, and these products continue to be used in global immunization programs. During this period, a variety of technical improvements were employed to produce effective products by several methods: live attenuated, inactivated, and subunit; over the course of the ensuing four decades, all contributed to the scientific database and the development of important vaccines that are still used extensively today.
The significance of the discovery of Enders and his colleagues was immediately apparent to scientific observers, as is evident by the fact that they were awarded the Nobel Prize in Medicine a mere five years after their discovery. Perhaps more important, though, was that their breakthrough provided the critical technical piece necessary to take the first giant steps toward conquering the most medically significant contagious diseases of the time. The first domino to fall was polio. Backed by the financial support of the National Foundation for Infantile Paralysis, which was also responsible for supporting the basic research that defined the correlate of protection in polio, Jonas Salk developed a formalininactivated polio vaccine (IPV) in 1954 and published his findings of a safe and highly effective whole virus vaccine (Salk et al., 1954). Clinical effectiveness was demonstrated in the largest clinical trial in history up to that time, directed by Salk’s mentor and perhaps the most respected epidemiologist and clinical trialist of the era, Thomas Francis of the University of Michigan. The field trial included over 1.8 million children from 44 states; the trivalent vaccine proved to be 60–70% effective against type 1 poliovirus and over 90% effective against types 2 and 3 (Markel, 2005), the three serotypes responsible for clinical illness, and was licensed and in widespread use within the year. Salk’s IPV was the first effective product against polio but was not without controversy, requiring technical modifications due to numerous incidents of incomplete viral inactivation that resulted in cases of paralysis in vaccinees and intermittent problems with variable and suboptimal potency. It was supplanted in much of the world by the live attenuated, oral polio vaccine (OPV), developed by Albert Sabin and introduced in 1961 (Sabin et al., 1954). The story of the polio vaccine was dominated by these two strong-willed and accomplished scientists and their career-long, professional competition to develop an effective vaccine by using disparate approaches. The history of polio vaccine also reinforced the importance of carefully designed and executed clinical trials in vaccinology and introduced the concept of large-scale collaborations between government, industry, and academics in the development of vaccines of public health import, a concept that has been revisited most recently in the field of biodefense. The widespread use of these vaccines led to the eradication of polio in the Western hemisphere and is the ongoing strategy being applied to the worldwide eradication of polio.
Using variations and improvements on the viral cultivation methodologies of Goodpasture and Enders, the late 1950s through the 1960s represented a period of great accomplishments in vaccine science. Live virus vaccines were developed for a variety of important childhood diseases: measles, by Katz, Enders, Hilleman, Schwarz, and their colleagues using an attenuated Edmonston strain; mumps, also by Hilleman and colleagues, using an attenuated version of a viral isolate from one of his daughters, the Jeryl Lynn strain; and rubella, a significant cause of congenital disorders. Following its isolation in culture in 1961 by Paul Parkman, Malcolm Artenstein, and Edward Buescher at the Walter Reed Army Institute of Research (WRAIR) and independently by Thomas Weller and Franklin Neva at Harvard Medical School, the rubella virus was propagated and attenuated in duck embryo cells, which led directly to several effective vaccine strains (Plotkin and Plotkin, 2004). By the early 1970s a vaccine combining the measles, mumps, and rubella products (MMR) was licensed, in widespread pediatric use, and shown to confer long-lasting immunity (Weibel et al., 1973).
Prior to his important contributions to childhood viral vaccines and while still serving at WRAIR, Maurice Hilleman serendipitously discovered adenoviruses during an investigation of an outbreak of influenza at Fort Leonard Wood in 1953 (Artenstein et al., 2005). These agents were subsequently found to be the major etiologic agent of acute respiratory disease in military recruits and a significant cause of atypical pneumonia in civilian settings. Within 3 years of the initial description of the infectious agent, a formalin-inactivated, whole-virus vaccine against the two most clinically relevant serotypes was produced, studied, and found to be more than 90% effective in large field trials by the military. The adenovirus vaccine suffered a major setback in 1963 when seed lots were found to be contaminated by SV40, an oncogenic simian virus that also caused significant problems with Salk’s polio vaccine during the same period. However, adenovirus vaccine resurfaced in the early 1970s as a live, attenuated, bivalent oral product that halted any further adenoviral outbreaks in military camps.
Influenza vaccine efforts that had begun in earnest following the work of Goodpasture were active throughout the latter part of the twentieth century and remain so. The primary vaccine for human influenza is a killed one and, as a consequence of the biology of this RNA virus, requires annual antigenic manipulation followed by cultivation in embryonic hen’s eggs. A live attenuated vaccine for influenza derived from cold-adapted mutant strains is licensed and gaining acceptance as an alternative, practical product for certain individuals. Because of the persistent dilemma of antigenic drift and shift with influenza that requires frequent vaccine antigen reformulation, novel approaches to vaccines for this virus are likely to be brought to bear to solve this problem in the future.
The final live virus vaccine that targeted a major childhood disease, varicella, was developed by Michiaki Takahashi in 1974 at the Biken Institute in Japan (Takahashi, 2001). He attenuated the Oka strain of the varicella zoster virus derived from the vesicular fluid of a 3-year-old boy with classic chickenpox. After extensive clinical studies demonstrated its safety and efficacy, the vaccine was licensed for routine use in children in Japan and Europe, but only after further, complicated study was licensure obtained in the United States in 1995. Recently, a new live, attenuated varicella-zoster virus vaccine greater than 15-fold more potent than the earlier formulation has shown efficacy in reducing the incidence of zoster and post-herpetic neuralgia in older adults (Oxman et al., 2005) and represents a novel vaccine against a chronic infection.
By the late 1960s, following the rapid successes of vaccine development involving childhood viral diseases, the scientific community began to explore the use of bacterial subunits, such as proteins and polysaccharides, to induce protective immune responses. Although the subunit hypothesis, in essence, had been tested with the toxoid-based vaccines for diphtheria and tetanus that were developed in the late 1920s, attention was now turned toward encapsulated bacteria, which were notorious and prevalent human pathogens and attractive targets because of their antigenic capsules. Theoretically, subunit vaccines would confer protective immunity without the inherent risks and toxicities associated with live attenuated and whole virus vaccines.
The concept of polysaccharides as immunogens derived from experiments performed by Michael Heidelberger at Columbia and Oswald Avery and Colin MacLeod at the Rockefeller Institute in the late 1930s and early 1940s using Streptococcus pneumoniae (Artenstein et al., 2005). Avery and MacLeod were two-thirds of the team that subsequently identified DNA as the carrier of genetic information in their further, seminal work on pneumococci. Heidelberger tested a multivalent pneumococcal polysaccharide immunogen at the Army Air Base in Sioux Falls, South Dakota in 1945 and found that it reduced the incidence of pneumonia among vaccinated troops (Artenstein et al., 2005). His colleague at Columbia, Elvin Kabat, expanded further on the potential role of the bacterial capsule by demonstrating anticapsular antibodies in the convalescent sera of animals recovering from infection with Neisseria meningitidis, also an encapsulated organism.
Drawing on such previous work and facing the serious clinical problem of epidemic and increasingly drugresistant meningococcal meningitis in military recruit camps, Malcolm Artenstein and colleagues at WRAIR, in a series of classic investigations, elucidated the correlate of protective immunity to meningococci, purified a polysaccharide vaccine candidate, and subsequently performed two, large clinical trials among recruits that demonstrated definitively the safety and efficacy of their meningococcal group C polysaccharide subunit vaccine (Artenstein, 1969; Artenstein et al., 1970). The U.S. military immediately began using the newly developed vaccine; a tetravalent vaccine, protective against four of the five major serogroups of meningococci, was subsequently licensed in 1981.
Other polysaccharide vaccines followed the meningococcal success. Robert Austrian and colleagues, again armed with knowledge from Heidelberger and Kabat’s previous work on the pneumococcal capsule and the revelation that pneumococcal disease was a significant cause of mortality despite the availability of antibiotics, developed a multivalent vaccine that incorporated 23 of the most clinically relevant serotypes. Several groups working in the U.S. and Finland developed a capsule-based vaccine against Haemophilus influenzae type b in the 1970s that was licensed in the mid-1980s; the routine childhood use of advanced generations of this vaccine essentially eradicated this bacterial disease as a significant cause of pediatric morbidity and mortality.
Polysaccharide-based vaccines, although safe and effective for older children and adults, act on mature B cells independently of T-cell help, and thus as a group are poorly immunogenic in children younger than 2 years of age and induce poor memory responses. The need for improved vaccine properties drove many of the most recent advances in vaccinology that have resulted in licensed products in clinical use: conjugate vaccines that improve upon the immunogenicity and durability of polysaccharides by linking them to antigenic protein carriers, such as diphtheria or tetanus toxoid. Through these incremental refinements to extant vaccine technology, driven by unmet needs, conjugate vaccines for H. influenzae, pneumococcus, and groups A and C meningococci have proven safe and effective.
Perhaps the most recent major advance of the golden age of vaccines came in 1986 with the introduction of the first recombinant vaccine (Hilleman, 2000). The hepatitis B vaccine (HBV), a yeast-derived recombinant product, was developed to supplant the plasma-derived vaccine with its attendant safety concerns involving the transmission of incompletely inactivated blood-borne pathogens. The recombinant vaccine was derived by cloning the gene for hepatitis B surface antigen (HBsAg), the subunit viral protein that represents the correlate of protective immunity, into yeast cells and was shown to be as effective as the plasma-derived product but lacked the toxicity concerns of the latter. HBV represented the first recombinant expressed vaccine to be licensed for use – a landmark achievement in vaccine science. The molecular technology allowing for the in vivo synthesis of the protective microbial subunit, in antigenic form, leads the way for other, genetically advanced vaccine strategies. Also, HBV holds another distinction that presages the future of vaccinology: it is the first vaccine shown to prevent cancer, hepatoma caused by chronic hepatitis B infection.
The Future Of Vaccinology
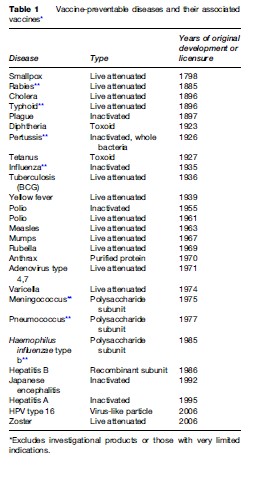
In vaccinology, as in all realms of science, major advances are driven by emergent technologies coupled with unmet needs. As previously noted, the advent of modern microbiology and immunology in the late nineteenth century and the quantum leap that occurred after the Nobel Prize winning work on viral culture methodology of Enders et al. in the mid twentieth century had the immediate effects of triggering a flurry of vigorous activity in vaccine science that led directly to the successful development and licensure of a host of vaccines against important infectious diseases of the time (Table 1). Further scientific developments, such as the recognition and exploitation of subunit protection and the harnessing of recombinant technology, also led to major progress in vaccines. As molecular technology has become more advanced, especially with the wealth of data accessible from genomics, the search for vaccines against problematic pathogens, emerging infectious diseases, and other, novel diseases has expanded once again and may be expected to yield significant dividends.
Based on their biology and pathogenesis, many pathogens have proven to be especially problematic regarding the development of effective vaccines. Some of these agents, such as HIV-1, tuberculosis, herpes simplex, and hepatitis C, are associated with a significant global burden of disease but reside intracellularly and cause chronic infections; others, such as influenza, owing to its frequently mutating antigenic targets, and group B meningococcus, by inducing immunologic tolerance through molecular mimicry between the bacterial capsule and a human self-antigen, have proven elusive to classic vaccine strategies and require novel approaches to vaccine design. Some of these innovative strategies target key virulence factors in the pathogenesis of the organism; others may employ vectored antigens in order to access sequestered sites in cells. Most of the approaches under active investigation have been informed by microbial genomics and new insights into host immunity, with the goals of targeting antigens derived by the former and optimizing specific responses in the latter arena. To this end strategies include the use of recombinant proteins, replication-defective viral particles, ‘naked’ DNA, and peptides (Plotkin, 2003). One approach, reverse vaccinology, employs a genomics and informatics-based methodology to identify potential antigenic components for use in a subunit vaccine (Abu-Bobie et al., 2003). Such strategies may permit a nimble and flexible vaccine posture that is required to respond rapidly to new and emerging threats, such as agents of bioterrorism or pandemic influenza.
Using novel molecular approaches has led to efficacious vaccines against selected chronic infectious diseases. In a double-blind, controlled study, a human papillomavirus type 16 (HPV-16) virus-like particle vaccine expressed as a recombinant in yeast demonstrated significant benefit in reducing the incidence of both persistent HPV-16 infection and cervical intraepithelial neoplasia in young women (Koutsky et al., 2002). Thus, as with the recombinant hepatitis B vaccine, the HPV vaccine has the potential to prevent a form of cancer resulting from a chronic infection. Other recently developed vaccines with efficacy against chronic infections include a herpes zoster vaccine, discussed previously, and a recombinant Lyme disease vaccine, with the gene for the spirochete’s outer surface protein expressed in E. coli (Plotkin and Plotkin, 2004). However, the latter product was withdrawn from the market shortly after its licensure due to persistent controversy over unproven toxicities and poor public acceptance (Nigrovic and Thompson, 2007).
Molecular technologies hold the promise of extending vaccinology to realms beyond those of infectious diseases. Although the concept of vaccines against various forms of cancer is not entirely new and has been clinically tested in selected viral-associated neoplasms (see previous discussion), progress has intensified over the past decade with the introduction of advanced molecular and genetic technologies. Numerous potential tumor antigens, multiple potential delivery methods, including dendritic cells and viral vectors, and several different disease targets have been investigated (Stevenson, 2005). DNA-based vaccines have received much of the attention, largely in phase I or early phase II clinical trials in patients with advanced malignancies, such as melanoma, prostate cancer, and colon cancer, with limited evaluable immunologic data to date (Stan et al., 2006). It is anticipated that further progress will be based on future insights into cancer-associated immune surveillance and protection.
Controversies related to vaccines have been an integral part of their history since at least the early eighteenth century, pre-Jennerian experiences with variolation. Landmark events in vaccinology, such as Pasteur’s public experiments with anthrax vaccine in livestock and rabies vaccine in humans, and the intense, scientific competition that played out in the media surrounding the polio vaccine effort, have been met with equal parts of praise and criticism by the world community. There continue to be substantial public misperceptions regarding vaccines, such as questions concerning possible associations between vaccine constituents and autism, autoimmune disease, or allergies, despite the lack of significant scientific evidence for a causal relationship in most cases (Hivid et al., 2003; Offit and Hackett, 2003). Similarly, there exists an additional and perhaps growing number of controversial issues related to vaccines: vaccine shortages and production problems, related to production costs, return on investment by industry, and liability concerns (National Vaccine Advisory Committee, 2003); risk versus benefit questions, which paradoxically have become more polarized as vaccines have become more effective, thus rendering the ‘benefit’ side of the equation more difficult for society to discern while accentuating cases in which the ‘risk’ side is highlighted (Maldonado, 2002); and compulsory childhood vaccination, which has always been a contentious issue among some portions of society but has once again revealed itself in the current controversy over compulsory HPV vaccination of teenage girls in some states in the United States.
The next ‘golden age’ of vaccinology may very well be poised to begin and is likely to derive from the rapidly accruing wealth of information acquired from molecular genetics and a deeper and more detailed understanding of the human immune system. The science, coupled with inevitable technical advances, will spur new insights into vaccine protection and will likely contribute to the solutions to new and difficult disease problems, as occurred during previous, fertile periods in the history of vaccines.
Bibliography:
- Abu-Bobie J, Capeddhi B, Serruto D, Rappuoli R, and Pizza M (2003) Two years into reverse vaccinology. Vaccine 21: 605–610.
- Artenstein MS (1969) Human Immunity to the Meningococcus. (Reprinted from Journal of Experimental Medicine (1969) 129(6): 1307–1385) Baltimore, MD: Waverly Press.
- Artenstein MS, Gold R, and Zimmerly JG (1970) Prevention of meningococcal disease by group C polysaccharide vaccine. New England Journal of Medicine 282: 417–420.
- Artenstein AW, Opal JM, Opal SM, et al. (2005) History of U.S. military contributions to the study of vaccines against infectious diseases. Military Medicine 170(4:3): 3–11.
- Barquet N and Domingo P (1997) Smallpox: The triumph over the most terrible of the ministers of death. Annals of Internal Medicine 127(8): 635–642.
- Baxby D (1999) Edward Jenner’s inquiry: A bicentenary analysis. Vaccine 17: 301–307.
- Centers for Disease Control and Prevention (1999) Achievements in public health, 1900–1999 impact of vaccines universally recommended for children – United States, 1990–1998. Morbidity and Mortality Weekly Reports 48(12): 243–248.
- Enders JF, Weller TH, and Robbins FC (1949) Cultivation of the Lansing strain of Poliomyelitis virus in cultures of various human embryonic tissues. Science 109: 85–87.
- Fenner F, Herderson DA, Arita I, Zdeneˆ k J, and Ladnyi ID (1988) Smallpox and Its Eradication. Geneva, Switzerland: World Health Organization.
- Goodpasture EW, Woodruff AM, and Buddingh GJ (1931) The cultivation of vaccine in the chorio-allantoic membrane of chick embryos. Science 74: 371–372.
- Hammarsten JF, Tattersall W, and Hammarsten JE (1979) Who discovered smallpox vaccination? Edward Jenner or Benjamin Jesty? Transactions of the American Clinical and Climatological Association 90: 44–55.
- Hilleman MR (2000) Vaccines in historic evolution and perspective: A narrative of vaccine discoveries. Vaccine 18: 1436–1447.
- Hviid A, Stellfeld M, Wohlfahrt J, and Melbye M (2003) Association between thimerosal-containing vaccine and autism. Journal of the American Medical Association 290(13): 1763–1766.
- Koutsky LA, Ault KA, Wheeler CM, et al. (2002) A controlled trial of a human papillomavirus type 16 vaccine. New England Journal of Medicine 347(21): 1645–1651.
- Maldonado YA (2002) Current controversies in vaccination. Journal of the American Medical Association 288(24): 3155–3158.
- Manrubia SC and Lazaro E (2006) Viral evolution. Physics of Life 3: 65–92.
- Markel H (2005) April 12, 1955: Tommy Francis and the Salk vaccine. New England Journal of Medicine 352(14): 1408–1410.
- National Vaccine Advisory Committee (2003) Strengthening the supply of routinely recommended vaccines in the United States. Journal of the American Medical Association 290(23): 3122–3128.
- Nigrovic LE and Thompson KM (2007) The Lyme vaccine: a cautionary tale. Epidemiology and Infection 135: 1–8.
- Offit PA and Hackett CJ (2003) Addressing parents’ concerns: Do vaccines cause allergic or autoimmune diseases? Pediatrics 111: 653–659.
- Oxman MN, Levine MJ, Jonson GR, et al. (2005) A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. New England Journal of Medicine 352: 2271–2284.
- Parish HJ (1965) A History of Immunization. London: Livingstone Ltd. Plotkin SA (2003) Vaccines, vaccination, and vaccinology. Journal of Infectious Diseases 187: 1349–1359.
- Plotkin SL and Plotkin SA (2004) A short history of vaccination. In: Plotkin SA and Orenstein WA (eds.) Vaccines, 4th edn., pp. 1–15. Philadelphia, PA: Elsevier.
- Riedel S (2005) Edward Jenner and the history of smallpox and vaccination. Baylor University Medical Center Proceedings 18: 21–25.
- Sabin AB, Hennessen WA, and Winsser J (1954) Studies on variants of Poliomyelitis virus. Journal of Experimental Medicine 99: 551–576.
- Salk JE, Krech U, Youngner JS, et al. (1954) Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. American Journal of Public Health 44: 563–570.
- Stan R, Wolchok JD, and Cohen AD (2006) DNA vaccines against cancer. Hematology/Oncology Clinics of North America 20: 613–636.
- Stern A and Markel H (2005) The history of vaccines and immunization: Familiar patterns, new challenges. Health Affairs 24(3): 611–621.
- Stevenson FK (2005) Update on cancer vaccines. Current Opinion in Oncology 17: 573–577.
- Takahashi M (2001) 25 years’ experience with the Biken Oka strain Varicella vaccine. Paediatric Drugs 3(4): 285–292.
- Weibel RE, Buynak EB, Stokes J, and Hilleman MR (1973) Persistence of immunity following monovalent and combined live measles, mumps, and rubella virus vaccines. Pediatrics 51(3): 467–475.
- Winau F, Westphal O, and Winau R (2004) Paul Ehrlich-in search of the magic bullet. Microbes and Infection 6: 786–789.
- De Kruif P (1996) Microbe Hunters. San Diego, CA: Harcourt Brace.
- Oshinsky DM (2005) Polio: An American Story. New York: Oxford University Press.
- Srivastava IK and Liu MA (2003) Gene vaccines. Annals of Internal Medicine 138(7): 550–559.




