Sample Neural Representations Of Direction Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
1. The Head Direction Signal
To navigate successfully animals require knowledge about their location and directional heading before embarking on a route. While location information may be provided by place cells in the hippocampus which discharge with respect to the animal’s location, another group of limbic system neurons discharge in relation to the animal’s directional heading in the azimuthal plane, independent of the animal’s behavior and location (Taube et al. 1990a, Muller et al. 1996, Taube 1998). For example, a head direction (HD) cell might discharge whenever the animal points its head northeast, no matter where it is in the environment. The direction at which the cell fires maximally is referred to as the preferred firing direction. The position of the animal’s trunk does not affect the cell’s firing, nor does pitch or roll of the animal’s head up to 90 degrees. HD cell firing is motion-independent— cells will discharge whether the rat is moving or remains still, as long as the animal is pointing its head in the proper direction. Very little, if any, adaptation of firing rate is noted when the head remains fixed at the preferred firing direction.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
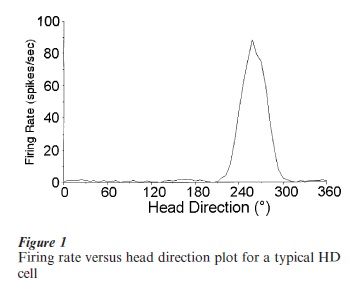
HD cells are tuned to different directional headings, and within a particular brain area the population of these cells encodes all 360 degree directions. Each HD cell can be described by its tuning curve, constructed by plotting firing rate as a function of directional heading. Figure 1 shows a typical tuning curve for an HD cell that fired maximally at 258 degrees. For each HD cell several properties can be characterized from its tuning curve: (a) the cell’s preferred firing direction; (b) the maximal (peak) firing rate of the cell at the preferred direction; and (c) the angular range over which cell firing is elevated from background firing rate. Some HD cells have low peak firing rates (5–10 spikes sec−1) while other HD cells have high peak firing rates (100 spikes sec−1). Intermediate peak firing rates are also observed. The functional significance of different cells having different peak firing rates is unknown. The firing rate of each HD cell decreases linearly as one moves away from the preferred firing direction. The range of directional headings where firing is elevated above background firing levels is about 90 degrees for most cells. The firing characteristics of an HD cell usually remain stable indefinitely, as long as the environmental parameters remain unchanged.
2. Where Are HD Cells Located In The Brain?
HD cells were originally identified in the dorsal presubiculum of rats. They have subsequently been identified in several limbic system areas in the rat, including the anterior dorsal thalamic nucleus, lateral dorsal thalamic nucleus, lateral mammillary nuclei, and posterior cingulate cortex (Taube 1998). Note that many of these areas are considered part of the classical Papez circuit. HD cells have also been identified in one nonlimbic structure—the striatum. In addition, HD cells have been reported in the presubiculum of nonhuman primates. With few exceptions, HD cells in each of the different brain areas have similar tuning curves, and behave similarly following manipulations of the animal’s environment. However, HD cells in the anterior dorsal thalamus and lateral mammillary nuclei appear to discharge optimally to the animal’s future directional heading, while HD cells in the dorsal presubiculum discharge maximally to the animal’s current directional heading (Blair and Sharp 1995). Similarly, in contrast to HD cells in the dorsal presubiculum, HD cell firing in the anterior dorsal thalamus and lateral mammillary nuclei is secondarily correlated to the rat’s angular head velocity, where firing rates within the cell’s preferred direction are higher for faster head turns than slower head turns (Taube 1995). It is also noteworthy that there are distinct populations of cells in the lateral mammillary nuclei that, independent of directional heading, encode for either pitch of the animal’s head or angular head velocity (Stackman and Taube 1998).
3. HD Cell Responses In 3-D
How HD cells respond in three dimensions is understood less well. HD cells will continue to discharge in the vertical plane, if the vertical locomotion begins with the rat’s orientation corresponding to the preferred firing direction (Stackman et al. 2000). But if the vertical plane locomotion begins with the animal’s head orientation outside the cell’s preferred direction, then the cell will not discharge on the vertical wall until the animal turns its head to the proper orientation. Thus, HD cells are capable of maintaining their discharge in planes outside Earth horizontal and their firing is dependent on the directional orientation of the rat as it moves out of the horizontal plane. One model consistent with these findings is that HD cells define the horizontal reference frame as the animal’s plane of locomotion, but this leaves open the question of which cue source(s) determine the horizontal reference frame: vestibular, somatosensory, visual, motor cues, or some combination of these sources. It is not known how HD cells respond when an animal locomotes upside down on a ceiling. In terms of height, if an animal locomotes back and forth between two horizontal surfaces that are separated by about one meter in height, the preferred firing direction is the same on both surfaces, but the peak firing rate increases a little on the higher surface.
4. Both Landmark Cues And Internally-Derived Self-Motion Cues Affect HD Cell Firing
While HD cells bear a similarity to a navigational compass, they are not dependent on the Earth’s magnetic field. They respond to several different types of sensory stimuli, some of which are external to the animal and others of which are self-generated. Visual landmarks that are stable over time play the predominant role in controlling the HD cell’s preferred firing direction. For example, if an animal is first disoriented and then returned to a familiar environment where all the visual landmarks have been rotated, the HD cell’s preferred firing direction will also be shifted by a similar amount (Taube 1995). However, HD cells continue to respond normally when the animal moves about in darkness or when the prominent visual cues have been removed, indicating that self-motion cues are capable of maintaining cell firing as well as the cell’s preferred firing direction (Goodridge et al. 1998). HD cells’ discharge is not environmentor context-specific, and they will discharge at some direction in any environment. If an animal locomotes from a familiar to a novel environment, the preferred firing direction will be similar in both environments (Taube and Burton 1995). These findings indicate that HD cells receive information concerning the animal’s movements, either through the vestibular or proprioceptive systems, or through motor corollary discharge. This movement information is integrated over time so that the animal can keep track of its directional heading given an initial reference point. When an animal is passively transported from a familiar to a novel environment, the preferred firing direction of a cell will often shift substantially, indicating the important role that motor systems play in maintaining a stable preferred firing direction (Taube 1998). Under this condition, the animal is deprived of normal motor, proprioceptive, and kinesthetic cues which accompany self-locomotion, but vestibular cues are left intact. Thus, while the vestibular system may play a crucial role in the generation of the HD cell signal (see below), these findings indicate that vestibular cues alone are not sufficient to allow maintenance of a stable preferred direction and suggest a critical role for motor information.
Most studies have shown that when spatial information derived from landmarks conflicts with information derived from internally generated self-movement, the landmark information usually predominates and overrides the spatial signals developed through self-generated movements (Goodridge and Taube 1995). Thus, when a salient, familiar cue is reintroduced into an animal’s environment into a position that conflicts with the cell’s current preferred firing direction, HD cells will shift their preferred direction to reflect their originally established orientation with the cue.
When two or more HD cells are monitored simultaneously in the same animal, the effects of an environmental manipulation on the preferred direction for one cell are similar to the responses observed in the other cell(s) (Taube et al. 1990b). This finding indicates that afferent input driving one HD cell will similarly influence other HD cells, and indicates that HD cells within a particular brain area behave as a network and that their preferred directions always remain a fixed angle apart (in-register) from one other.
5. Generation Of The Directional Signal
Given that HD cells have been identified in several brain areas, many of which are interconnected with one another, the question arises as to which areas are critical for generating the directional signal. By recording from one brain area containing HD cells, and lesioning various afferent areas, a number of studies have concluded that (a) the anterior dorsal thalamus, but not the hippocampus or lateral dorsal thalamus, is critical for the generation of directional activity in the dorsal presubiculum; (b) lesions of the lateral mammillary nuclei disrupt the HD cell signal in the anterior dorsal thalamus; and (c) lesions of the vestibular labyrinth abolish all direction-specific firing in areas containing HD cells (Stackman and Taube 1997, Taube 1998). It is also noteworthy that lesions of the anterior dorsal thalamus or the dorsal presubiculum do not abolish location-specific firing in hippocampal place cells, indicating that spatial representations concerning location can be generated independently of HD cell information.
The vestibular lesion data suggest that the neural code for directional bearing is critically dependent upon vestibular information, and that the loss of HD cell information may account for the orientation and navigational deficits observed following vestibular dysfunction in humans. Researchers have suggested that vestibular information is conveyed to the brain areas containing HD cells via a subcortical pathway starting in the medial vestibular nuclei and then projecting to the dorsal tegmental nucleus directly (or indirectly via the nucleus prepositus), and then to the lateral mammillary nuclei, which in turn project to the anterior dorsal thalamus. In this scheme, the anterior dorsal thalamus is viewed as a convergence site where subcortical information representing self-movement is combined with spatial information concerning landmarks that originate from projections in the posterior cingulate cortex. Attempts to model HD cell firing have used attractor-type neural networks (Skaggs et al. 1995). These models have been able to replicate most, but not all, the empirical data.
In animals with lesions of the hippocampus each HD cell’s preferred firing direction remained stable across days when the animal was placed into a novel environment (Golob and Taube 1997). This result suggests that extrahippocampal structures are capable of creating and maintaining a novel representation of the animal’s spatial context. This representation shares features in common with mnemonic processes involving episodic memory, which are thought to require an intact hippocampus.
6. Relationship Of HD Cell Activity To Behavior
One important issue concerning HD cells is what role they play in an animal’s behavior. Are they actively involved in guiding navigational behavior? Evidence suggesting a link between HD cells and behavior has been produced using a radial arm maze in a spatial reference memory task (Dudchenko and Taube 1997). Animals were trained to run down a particular maze arm relative to a visible landmark cue to obtain a water reward. Following rotation of the cue, the animal’s maze arm selection and the HD cell’s preferred direction shifted in tandem relative to the salient visual cue. Furthermore, when the HD cell’s preferred direction did not shift relative to the cue, neither did the animal’s behavioral response. When an HD cell was recorded over several days as the animal learned the task, there was little change in the firing properties of the cell. These findings indicate that the coding of directional heading information is independent of the neural systems involved in task acquisition, and that over time, an animal learns to use information provided by the directional system to guide its behavioral responses.
Recent experiments that have tested this hypothesis more rigorously have surprisingly found that HD cell activity and behavioral choice are not linked together on two different types of spatial tasks (Golob et al. 2001). One task was a spatial reference memory task where animals were required to generalize appropriately between a square and a rectangular enclosure to receive a reward. The other task involved a spatial working memory task. In both cases HD cell activity in the anterior dorsal thalamus did not correlate very well with the animal’s behavioral responses. While it appears that information derived from HD cells is not used in these two types of spatial tasks, it is likely that future studies will find that HD cell information will be more utilized in spatial tasks that require path integration or navigation over larger distances. Clearly, additional experiments that explore this issue are warranted.
7. Summary
In summary, HD cells represent a high-level spatial signal that encodes the animal’s directional heading. HD cells have been identified in several brain areas— mostly within the limbic system, but the functional role served by each brain area remains to be elucidated. HD cells receive multimodal sensory and motor information. It appears that the signal arises from the lateral mammillary nuclei, or structures afferent to it, and that both vestibular and motor information play important roles in generating the signal. Visual landmarks can exert control over the preferred firing direction of an HD cell. The nature of the HD cell signal makes it an ideal candidate for studying the neural mechanisms underlying navigation.
Bibliography:
- Blair H T, Sharp P E 1995 Anticipatory head direction signals in anterior thalamus: Evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. Journal of Neuroscience 15: 6260–70
- Dudchenko P A, Taube J S 1997 Correlation between head direction cell activity and spatial behavior on a radial arm maze. Behavioral Neuroscience 111: 3–19
- Golob E J, Taube J S 1997 Head direction cells and episodic spatial information in rats without a hippocampus. Proceedings of the National Academy of Sciences of the United States of America 94: 7645–50
- Golob E J, Stackman R W, Wong A C, Taube J S 2001 On the behavioral significance of head direction cells: Neural and behavioral dynamics during spatial memory tasks. Behavioral Neuroscience 115: 285–304
- Goodridge J P, Taube J S 1995 Preferential use of the landmark navigational system by head direction cells. Behavioral Neuroscience 109: 49–61
- Goodridge J P, Dudchenko P A, Worboys K A, Golob E J, Taube J S 1998 Cue control and head direction cells. Behavioral Neuroscience 112: 749–61
- Muller R U, Ranck J B Jr, Taube J S 1996 Head direction cells: Properties and functional significance. Current Opinion in Neurology 6: 196–206
- Skaggs W E, Knierim J J, Kudrimoti H S, McNaughton B L 1995 A model of the neural basis of the rat’s sense of direction. In: Tesauro G, Touretzky D S, Leen T K (eds.) Advances in Neural Information Processing Systems. MIT Press, Cambridge, MA, Vol. 7, pp. 173–80
- Stackman R W, Taube J S 1997 Firing properties of head direction cells in rat anterior thalamic neurons: Dependence upon vestibular input. Journal of Neuroscience 17: 4349–58
- Stackman R W, Taube J S 1998 Firing properties of rat lateral mammillary nuclei single units: Head direction, head pitch, and angular head velocity. Journal of Neuroscience 18: 9020–37
- Stackman R W, Tullman M L, Taube J S 2000 Maintenance of rat head direction cell firing during locomotion in the vertical plane. Journal of Neurophysiology 83: 393–405
- Taube J S 1995 Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. Journal of Neuroscience 15: 70–86
- Taube J S 1998 Head direction cells and the neurophysiological basis for a sense of direction. Progress in Neurobiology 55: 225–56
- Taube J S, Burton H L 1995 Head direction cell activity monitored in a novel environment and during a cue conflict situation. Journal of Neurophysiology 74: 1953–71
- Taube J S, Muller R U, Ranck J B Jr 1990a Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. Journal of Neuroscience 10: 420–35
- Taube J S, Muller R V, Ranck J B Jr 1990b Head direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. Journal of Neuroscience 10: 436–47




