View sample tuberculosis research paper. Browse research paper examples for more inspiration. If you need a health research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Mycobacteria are Gram-positive, nonmotile rods whose species show great microbiological diversity. Many have natural habitats in water and soil. However, some have become intracellular pathogens of higher vertebrates causing disease in animals and humans. Mycobacterium tuberculosis belongs to this group and has been in our midst since antiquity, but our ability to fight the organism is less than 150 years old.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Charting The Disease Through History
A study that determined the frequency of genetic polymorphisms in clinical strains led to the hypothesis that M. tuberculosis originated around 15 000 years ago and that M. bovis was the likely pathogen in humans until 1000 BC (Sreevatsan et al., 1997). After this, widespread M. tuberculosis infection emerged. Early evidence of infection in mankind dates from examinations of the spines of Egyptian mummies and of tomb paintings from 3500 BC, which confirm tuberculosis as a common disease in Egypt at this time (Zink et al., 2003). Around 2000 BC, tuberculosis was established in Europe, but the earliest evidence of the disease in Britain comes from graves dating from approximately 200 BC (Taylor et al., 2005).
There are references in the book of Deuteronomy, thought to have been written in the seventh century BC, to tuberculosis: ‘‘The lord shall smite thee with a consumption, and with a fever, and with an inflammation, and with an extreme burning’’. In the Dark Ages (400–1000 AD), references to tuberculosis are infrequent. As the Roman Empire declined, King Clovis of 5th century France founded the Merovingian dynasty of kings and claimed to heal tuberculosis by the touch of his hand. The King’s evil became the term used for tuberculosis of the lymph glands as English and French monarchs laid hands on scrofulous patients in regular healing services. Queen Elizabeth I, for one, was faithful to this custom. However, the powers of the monarchy were not enough to protect themselves. King Edward VI succeeded to the throne in 1547 as the last in the male line of the house of Tudor. The king’s reign was cut brutally short when he died from tuberculosis at Greenwich in 1553, aged 15 (Holmes and McMorrough, 2001).
Although it has been suggested that colonial Europe brought tuberculosis to the Americas, there is evidence from Peruvian and Chilean mummified tissue a millennium old, that humans inhabiting the Americas had been exposed to mycobacterial disease before the advent of the European ships. Unlike bubonic plague, which in the Middle Ages swept through Europe in short, swift frenzies, tuberculosis burned with a steady, consuming flame. The disease was known as the white plague because of the anemia of chronic disease that rendered sufferers pale. Urbanization across Europe in the sixteenth and seventeenth centuries provided the perfect breeding ground for tuberculosis, as poor populations with bad sanitation gathered in close contact. Tuberculosis was responsible for 20% of deaths in London in the 1600s, as recorded in the Bills of Mortality. Characters in Shakespeare’s Much Ado About Nothing (1600) and Macbeth (1606) were afflicted with consumption. The British heavily colonized Australia in the eighteenth and nineteenth centuries, leading to bitter conflict with the native Aboriginal populations. More devastating than the conflict, however, was the impact of European diseases to which Aboriginal people had no immunity, including tuberculosis. Aboriginal numbers decreased from an estimated 1 000 000 in 1788 to approximately 93 000 in 1901.
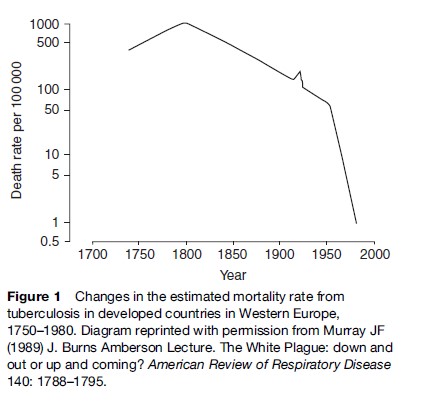
The epidemic of tuberculosis in the nineteenth and early twentieth centuries in Europe and the Americas is illustrated in Figure 1 and underscored by the many people who succumbed to the disease, both real and in the arts. The heroine, Mimi, of Puccini’s La Bohe`me suffers from tuberculosis (a theme carried over in the modern film adaptation ‘Moulin Rouge’). Violetta, heroine of Verdi’s La Traviata also dies of the disease. Dickens described tuberculosis in Nicholas Nickleby as a ‘‘disease in which death and life are so strangely blended, that death takes the glow and hue of life.’’ Tuberculosis patients were frequent characters in nineteenth-century Russian literature, and even inspired a character type; the consumptive nihilist, examples of which include Bazarov from Turgenev’s Fathers and Sons, Katerina Ivanovna from Dostoevsky’s Crime and Punishment, Kirillov from Dostoevsky’s Demons, and Ippolit and Marie from Dostoevsky’s The Idiot.
The real death toll was much more profound. Consumption was probably the most common killer of American colonial adults and accounted for more than 25% of deaths in New York City between 1810 and 1815 (Holmberg, 1990). James McGill, the founder of McGill University, reported in his journal the culmination of months of hopeless vigilance ‘‘At 2:00 am on July 4, 1812, my adopted daughter died of decay.’’ John Keats died from tuberculosis at the age of 25 in 1821, as did Frederic Chopin at 39 years in 1849. Tuberculosis killed Emily Bronte in 1848 (Wuthering Heights), Anne Bronte in 1849 (Agnes Grey) and Charlotte Bronte in 1855 (Jane Eyre). Doc Holliday, the American gunfighter and gambler, worked as a dentist until 1872 when tuberculosis forced him to move to the West for its dry climate, where he eventually succumbed to the disease in 1887. Robert Louis Stevenson died at 44 years in 1894 from tuberculosis despite a partial recovery while in Saranac in 1887–88. Franz Kafka, the Austrian Jewish novelist, contracted tuberculosis in 1917 and died in a sanatorium in Kierling, Austria in 1924. Jimmie Rodgers was American country music’s first important recording star but died from tuberculosis in 1933 shortly after writing one of his last songs, ‘TB Blues.’ Mohammed Ali Jinnah, the founding father of Pakistan and its first governor-general, died of tuberculosis in Karachi in 1948. George Orwell died of tuberculosis in January 1950, 1 year after the publication of Nineteen Eighty-Four. Of course, these were the famous; the death toll on the huddled masses was much more far-reaching.
During World War II, increases in tuberculosis were evident in all great cities. In Poland, the rate of tuberculosis tripled and the death rate in Warsaw from tuberculosis in 1944 was 500 per 100 000 people, a sharp increase compared with the background mortality in Western Europe at the time (Figure 1). By April 1945, British Army forces had advanced across Lower Saxony toward Bergen-Belsen. That day, the Germans opened negotiations for the surrender of the concentration camp, stating that the camp had 9000 sick inmates. When the British took the camp on 15 April, the first sights that they encountered were distressing. The appalling conditions of the camp had been a perfect breeding ground for tuberculosis.
Describing The Disease
An early written description of pulmonary tuberculosis was from the last great king of ancient Assyria, Assurbanipal (668–627 BC): ‘‘The patient coughs frequently; his sputum is thick and sometimes contains blood. His breathing is like a flute.’’ Two centuries later, Hippocrates (460–370 BC), in his writings, described phthisis (a Greek word meaning chronic wasting or consumption) as the most prevalent disease of the times, and believed that it killed almost everyone affected. During the reign of the Roman Empire, Claudius Galen (129–216 AD) served as personal physician to Marcus Aurelius and recognized that tuberculosis was contagious.
Advances in the understanding of the disease were few and far between for several centuries thereafter. However, from the origins of the Renaissance came a revolution in the knowledge of disease. In 1546, Girolamo Tracastoro wrote the book De Morbis Contagiosis. Tracastoro wrote that the seeds of contagion remain in such bodies as articles of clothing and bedsheets used by the infected. In the seventeenth century, the anatomical revolution produced knowledge about the body from the study of the dead. In his Opera Medica of 1679, the Dutch physician Dr. Sylvius was the first to identify tubercles in the lungs of consumptive patients. An edict issued by the Italian Republic of Lucca in 1699 states that, ‘‘henceforth, human health should no longer be endangered by objects remaining after the death of a consumptive.’’ In 1720, the English physician Benjamin Marten speculated, in his publication, A New Theory of Consumption, that tuberculosis could be caused by ‘‘wonderfully minute living creatures’’ (Doetsch, 1978). In 1761, Leopold Auenbrugger of Austria, the father of percussion, published a book about the relation of pathological changes and clinical signs of tuberculosis. In 1782, the physicist Graumann presented a treatise where he proved tuberculosis and syphilis were not identical.
Significant research into the causes and cure of tuberculosis began in earnest in the nineteenth century. In 1810, Carmichael, a London physician, declared that the tuberculosis of cattle is transmissible to man through the use of tuberculoid meat or milk. The French physician Gaspard Bayle described the damage caused by tuberculosis in 900 autopsies. Rene´-The´ophile-Hyacinthe Lae¨nnec, most famous for designing the stethoscope, described the evolution of the disease from the initial tubercle through its final stages. Lae¨nnec died of pulmonary tuberculosis in 1826 at the age of 45. Known until then as scrofula or consumption, the name tuberculosis seems first to have been used in 1839 by Johann Schoenlein. The Lancet in 1847 blamed London’s milk supply for the rise in scrofula. In 1865, the French military doctor Jean-Antoine Villemin demonstrated that consumption could be passed from humans to cattle. On the basis of this evidence, he postulated a specific microorganism as the cause.
In 1880, Robert Koch was appointed director of the bacteriological laboratories at the Imperial Health Office in Berlin. In 1882, Koch discovered a staining technique that enabled him to see Mycobacterium tuberculosis under a microscope and showed that bacilli could be isolated in pure cultures and produce tuberculosis in animals that were inoculated. He published his work under the title ‘The Etiology of Tuberculosis,’ in the Berliner Klinische Wochenschrift in which he proved the infectious nature of the disease which ultimately earned him the Nobel Prize in physiology or medicine in 1905 (Kaufmann, 2005). Although meticulously documented, his research met with some opposition; because of its chronic nature, tuberculosis until then was widely believed to be inherited rather than inhaled. However, the description of humanity’s deadliest enemy had now truly begun and a century later, in June 1998, the genome sequence of M. tuberculosis was published (Cole et al., 1998).
Beginning The Fight Against The Disease
Early Measures
While the real fight against the disease began in the nineteenth century, the foresight of Pliny the Elder (28–79 AD), the Roman Senator, cannot be understated. He sent one of his freedmen, who suffered from tuberculosis, on a cruise to Egypt and when the dry air of the desert did not cure the patient, he sent him to a chalet in the Alps. However, his dietary prescription was less conventional: ‘‘For phthisis one had the liver of a wolf in wine, the lard of a thin sow fed on herbs, the flesh of an ass with a bouillon made from it.’’
Eighteen centuries later, Hermann Brehmer, a Silesian botany student suffering from tuberculosis, traveled to the Himalayan Mountains to pursue his botanical studies. He returned home cured and began to study medicine. In 1854, he presented his doctoral dissertation, Tuberculosis is a Curable Disease. In the same year, he built the first sanitorium in Gorbersdorf. In 1883, Dr. Edward Livingston Trudeau, dying from pulmonary tuberculosis, traveled to Saranac Lake in the Adirondack Mountains of New York to spend his final days. He attributed his subsequent recovery to the fresh air of the mountains and in 1885, built the first American sanatorium. By 1930, the United States had 600 sanatoriums with a total of 84 000 beds. For the wealthy, the sanatorium was a combination luxury hotel and hospital, as immortalized in Thomas Mann’s novel The Magic Mountain. The sanatoria provided the first effective step to control tuberculosis by separating those infected from those who were not and providing a healthy diet and climate for those who had the disease.
The Italian Forlanini in 1890 observed that lung collapse tended to have a favorable impact on the outcome of the disease. This is because M. tuberculosis is an obligate aerobe and the absence of oxygen in the collapsed lungs led to its demise. With the introduction of artificial pneumothorax (Figure 2) and other surgical methods to reduce lung volume, the depressing era of helplessness in the face of tuberculosis was over, and active therapy had begun.

In 1886, de Cerenville introduced rib resection to collapse the underlying lung. In 1900, Dr. Edward Archibald, the father of thoracic surgery in North America, had tuberculosis and was treated in the Trudeau Sanatorium at Saranac Lake. In 1912, Archibald was the first surgeon to perform thoracoplasty in North America at Montreal. Thoracoplasty emerged from the array of collapse techniques to become the predominant form of collapse therapy. Furthermore, the discovery of radiation by Von Ro¨ ntgen in 1895 meant the progress and severity of a patient’s disease could now be accurately followed.
By 1890, Koch had published a series of four papers describing a remedy for tuberculosis – a glycerol extract of pure culture of tubercle bacilli – as well as the reaction of animals infected with M. tuberculosis and the differential responses to tuberculin in healthy and diseased persons. Koch noted that the extract ‘‘will enable us to diagnose questionable cases of early consumption even when we fail to detect bacilli’’ (Kaufmann, 2005). Arthur Conan Doyle correctly challenged the therapeutic value of Koch’s lymph, arguing that it might remove traces of the enemy, but it left deadly germs ‘‘deep in the invaded country.’’ Its real value, Conan Doyle asserted, was as ‘‘an admirable aid to diagnosis’’ to determine if a patient was ‘‘in any way tubercular’’. In Paris, French bacteriologists Albert Calmette and Camille Guerin serially passaged M. bovis 230 times from 1908 to 1919 at the Pasteur Institute (Hagwood, 1999). The resulting strain, bacille Calmette-Gue´rin, known commonly as BCG, was found to be avirulent. It was first administered to humans in 1921 and is still applied today with varying efficacy.
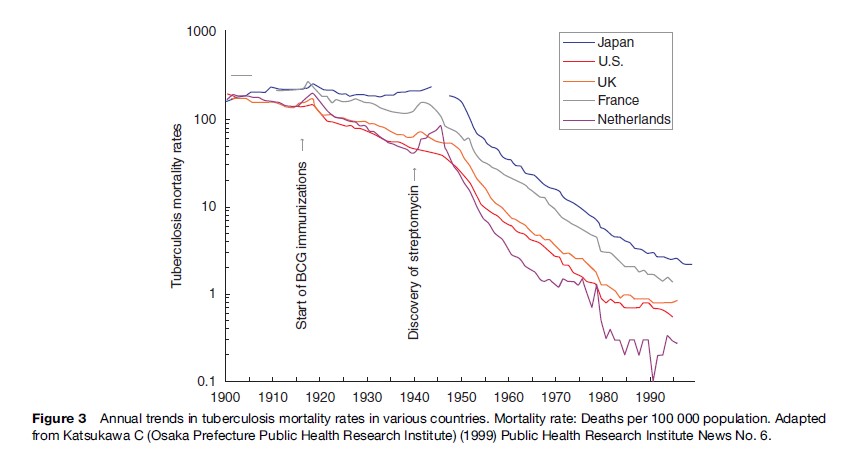
Wade Hampton Frost’s epidemiologic studies in the 1930s showed that, even without chemotherapy, the incidence of tuberculosis in the United States was declining steadily (Frost, 1995). In Western countries, deaths from tuberculosis fell from 200 per 100 000 in 1900 to 50 per 100 000 by 1950 (Figure 3).
The Antibiotic Era
In 1885, the Italian bacteriologist Cantani had tuberculosis patients inhale cultures of nonpathogenic bacteria, which led to a fall in the quantity of tubercle bacilli in patients’ sputum. In 1939, Selman A. Waksman had discovered the inhibitory effect of certain fungi, especially actinomycetes, on bacterial growth. In 1940, his team at the University of California were able to isolate an effective antituberculous antibiotic, actinomycin; however, this proved to be too toxic for clinical use (Waksman, 1969). In the years that followed, research with actinomycetes, aspergillus, and other microorganisms demonstrated the antituberculous activities of their products. In January 1944, Waksman announced the discovery of a drug called streptomycin isolated from a fungus, Streptomyces lavendula and Patricia, a 21-year-old woman was the first patient to be successfully treated with the drug (Schatz, 1944).
In 1946, Lehmann announced the discovery of paraamino-salicylic (PAS). By 1947, strains of M. tuberculosis had developed resistance to streptomycin. In 1948, the combination of streptomycin and PAS was found to be very effective against M. tuberculosis. In 1952 at Seaview Hospital, New York, a new drug called isoniazid was used to treat tuberculosis patients by Robitzek and Selikoff. Other antituberculous drugs followed with the discovery of pyrazinamide in 1954, cycloserine in 1955, ethambutol in 1962, and rifampicin in 1963. The combination of the early bactericidal activity of isoniazid and the sterilizing activity of rifampicin shortened treatment to less than a year, resulting in short-course chemotherapy. Several studies from Madras and elsewhere demonstrated that treatment was effective when given at home rather than in the sanitorium and that antituberculous drugs were effective when given intermittently rather than daily. The clinical effectiveness of antituberculous drugs led to the closure of the sanatoria in the 1950s and 1960s.
Deaths from tuberculosis in the United States dropped from 188 per 100 000 in 1904 to approximately 1 per 100 000 in 1980. M. tuberculosis was no longer a bacteriological exception. It could be assailed and beaten into retreat.
Tuberculosis Resurgent
From the 1950s to 1985, the Western world saw a rapid decline in the incidence of tuberculosis. For example, 84 304 contracted the disease in 1953 in the United States, falling to 22 201 in 1985. In 1960, Dr. John Crofton at the University of Edinburgh proposed that a combination of streptomycin, PAS, and isoniazid made tuberculosis completely curable and he declared all-out war to conquer the disease. In 1970, William Stewart, the U.S. Surgeon General, is reported to have told the U.S. Congress that the United States was ‘‘ready to close the book on infectious disease.’’ In 1984, the Public Health Service reported that the number of tuberculosis cases reported annually in 1953 had declined by 74% (Huebner and Castro, 1995). In 1987, the Advisory Council for the Elimination of Tuberculosis put forth a plan to eliminate tuberculosis (1 case per 1 million population) by the year 2010 (Anonymous, 1993). These advances contributed to the sense that mankind had prevailed over M. tuberculosis and funding for the fight against tuberculosis was dramatically reduced in Western countries. Humanity had won the war … or had it?
M. tuberculosis was not ready to leave without a fight. Patients recovering rapidly after starting treatment and then defaulting from further treatment as well as inadequate therapy of tuberculosis in counties with poor access to antituberculous drugs led to the development of multidrug resistance. A survey (1961–1968) in the United States that examined drug susceptibility results found 3.5% drug monoresistance and the United States reported the first outbreak of drug-resistant tuberculosis in 1970. In 1985, for the first time in that century, the decline in tuberculosis stagnated and then began a slow, steady increase. Tuberculosis had outsmarted its smartest opponent.
From 1990 to 1992, the Centers for Disease Control in Atlanta investigated outbreaks of multidrug-resistant tuberculosis in eight hospitals in Florida, New York, and New Jersey (Young and Wormser, 1994). Tuberculosis was found to be transmitted not only from patient to patient, but also from patient to health-care worker. The WHO reported that the death rate of patients with multidrug-resistant tuberculosis in the United States was approximately 70%. The typical interval from diagnosis to death was 4–16 weeks and 80% of cases were HIV-positive. HIV and tuberculosis had forged a secret alliance.
The International Union against Tuberculosis and Lung Disease (IUATLD) tested a countrywide system for tuberculosis control by treating tuberculous patients in several poor developing countries. It was proven effective even in difficult settings. As part of this system, it was noted that the emergence of multidrug resistance could be avoided by supervision of drug taking. The World Health Organization (WHO) adopted the strategy and named it DOTS (Directly Observed Therapy, shortcourse) as a framework for global prevention and control of tuberculosis that can be modified according to local needs in its implementation. DOTS was crucial to the restoration of tuberculosis control during the multidrugresistant outbreaks of tuberculosis in North America.
The possibility of an untreatable form of tuberculosis attracted extensive media attention and the funding that had been pulled from tuberculosis management was reinstated in many Western countries. From 1993 to 2003, the incidence of tuberculosis in the United States decreased 44% and is now occurring at a historic low level (14 093 cases in 2005) (Taylor et al., 2005). However, the WHO estimated that in 1995, there were more global deaths from tuberculosis than in any previous year in history. Once again, tuberculosis had adapted to its environment and found new potential for survival in developing countries, where poor health infrastructure and poverty have contributed to a sustained increase in the incidence of tuberculosis.
Currently, the WHO estimates that 2 billion people (one-third of the world’s population) are infected with tuberculosis. There are 2 million deaths annually from tuberculosis and 98% of these are in the developing world. If left unchecked, within 20 years tuberculosis will kill a further 35 million people. In 2003, 8.8 million new cases of tuberculosis arose, with 80% occurring in 22 countries. There are approximately 425 000 new multidrug-resistant cases every year, with the highest rates in the former USSR and China. Tuberculosis has reestablished its position as the leading infectious killer in the world, simply by changing its predominant hunting ground.
Bibliography:
- Anonymous (1993) Tuberculosis control laws – United States, 1993. Recommendations of the Advisory Council for the Elimination of Tuberculosis (ACET). MMWR Recommendations Report 42(RR-15): 1–28.
- Cole ST, Brosch R, Parkhill J, et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393(6685): 537–544.
- Doetsch RN (1978) Benjamin Marten and his ‘‘New Theory of Consumptions’’ Microbiology Review 42(3): 521–528.
- Frost WH (1995) The age selection of mortality from tuberculosis in successive decades. 1939. American Journal of Epidemiology 141(1): 4–9.
- Hawgood BJ (1999) Doctor Albert Calmette 1863–1933: Founder of antivenomous serotherapy and of antituberculous BCG vaccination. Toxicon 37(9): 1241–1258.
- Holmberg SD (1990) The rise of tuberculosis in America before 1820. American Review of Respiratory Diseases 142(5): 1228–1232.
- Holmes G, Holmes F, and McMorrough J (2001) The death of young King Edward VI. New England Journal of Medicine 345(1): 60–62.
- Huebner RE and Castro KG (1995) The changing face of tuberculosis. Annual Review of Medicine 46: 47–55.
- Kaufmann SH (2005) Robert Koch, the Nobel Prize, and the ongoing threat of tuberculosis. New England Journal of Medicine 353(23): 2423–2426.
- Murray JF (1989) J. Burns Amberson Lecture. The White Plaque: down and out or up and coming? American Review of Respiratory Disease 140: 1788–1795.
- Schatz A, Bugie E, and Waksman SA (1944) Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. 1944. Clinical Orthopaedics and Related Research 437: 3–6.
- Sreevatsan S, Pan X, Stockbauer KE, et al. (1997) Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proceedings of the National Academy of Sciences USA 94(18): 9869–9874.
- Taylor GM, Young DB, and Mays SA (2005) Genotypic analysis of the earliest known prehistoric case of tuberculosis in Britain. Journal of Clinical Microbiology 43(5): 2236–2240.
- Taylor Z, Nolan CM, and Blumberg HM (2005) Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recommended Report 54(RR-12): 1–81.
- Waksman SA (1969) Successes and failures in the search for antibiotics. Advanced Applied Microbiology 11: 1–16.
- Young LS and Wormser GP (1994) The resurgence of tuberculosis. Scandinavian Journal of Infectious Disease Supplement 193: 9–19.
- Zink AR, Grabner W, Reischl U, Wolf H, and Nerlich AG (2003) Molecular study on human tuberculosis in three geographically distinct and time delineated populations from ancient Egypt. Epidemiology and Infection 130(2): 239–249.
- Barnes D (1995) The Making of a Social Disease. Tuberculosis in Nineteenth-Century France. Berkeley, CA: University of California Press.
- Crofton J, Horne N, and Miller F (1999) Clinical Tuberculosis. 2nd ed. London: Macmillan.
- Davies PDO (2003) Clinical Tuberculosis. 3rd edn. London: Arnold.
- Dormandy T (2000) The White Death: A History of Tuberculosis. New York: New York University Press.
- Enarson DA (1991) Principles of IUATLD collaborative TB programmes. Bulletin of the International Union of Tuberculous and Lung Diseases 66: 195–200.
- Frieden T (ed.) (2004) Toman’s Tuberculosis. Case detections, Treatment and Monitoring – Questions and Answers, 2nd edn. Geneva, Switzerland: World Health Organization.
- Hecker JFC (2003) The Black Death and the Dancing Mania. https://biotech.law.lsu.edu/Books/hecker/Death_c.htm
- Mitchison DA (2005) The diagnosis and therapy of tuberculosis during the past 100 years. American Journal of Respiratory and Critical Care Medicine 171: 699–706.
- Murray JF (2001) A thousand years of pulmonary medicine: good news and bad. The Millennial Lecture. European Respiratory Journal 17: 558–565.
- Murray JF (2004) A century of tuberculosis. American Journal of Respiratory and Critical Care Medicine 169: 1181–1186.
- http://www.stoptb.org/ – Stop TB Partnership.
- https://www.who.int/ – World Health Organization.




