Sample Hippocampus And Related Structures Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The hippocampus is part of the cortex of the forebrain in mammals and is closely linked to a restricted number of related cortical areas which are here collectively referred to as the parahippocampal region. This collection of cortical regions, which in humans is located in the most medial portion of the temporal lobe, is involved critically in learning and memory processes, in particular in long-term explicit memory. The hippocampus also has strong functional links with subcortical structures believed to play a role in the selection of appropriate behavior to favor either survival of the individual or of the species. In this research paper, the wiring of the hippocampus and the parahippocampal region underlying these two proposed functions will be described, and experimental data that support the proposed functions will be summarized.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. What Is The Hippocampus And What Are Related Structures?
The hippocampus is a macroscopically defined cortical structure which is present in the brains of all mammals. Grosso modo, its appearance looks alike in all species in that it is an enrolled continuation of the most medial rim of the cortical surface of the hemisphere. The cortices directly adjacent to the hippocampus, which are referred to collectively as the para (next-to) hippocampal region, are considered as the related structures. The particular arrangement of these different cortical structures can be appreciated easily in sections taken perpendicular to the long axis of the hippocampus (see Fig. 1).
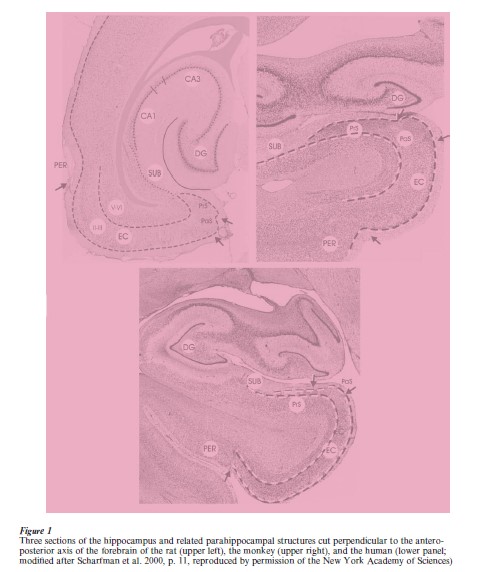
The hippocampus and the parahippocampal region of different species are positioned differently in the brain, such that in species with a clearly developed temporal lobe, the hippocampus is positioned more ventrally and anteriorly (humans and monkeys) compared to the situation in, for example, the rat, where the hippocampus looks more like a c-shaped structure positioned in the caudal third of the hemisphere, such that its dorsal one-third has a marked spatial relation with the corpus callosum. However, these differences in position do not alter the major characteristics and the topological relations between the hippocampus and the parahippocampal structures (see Sects 2 and 3). Nor does it influence the fact that the most anterior ventral portion of the hippocampus has a close spatial relationship with the amygdala.
2. General Features Of The Hippocampus Parahippocampal Region And Species Differences
The hippocampus consists of three major subdivisions, which can be recognized easily in all species (see Fig. 1). The first field, the dentate gyrus, is a c-shaped, three-layered cortical structure which is characterized by a densely packed cell layer, mainly consisting of granular cells, i.e., cells with a round soma, and an apical dendritic tuft and no, or only sparse, basal dendrites. Enclosed in this c-shaped cortical blade is a polymorphic cell group generally referred to as the hilus of the dentate gyrus. The second subfield is the Ammon’s Horn or Cornu Ammonis, generally abbreviated as CA-field. The CA-field is generally subdivided into CA1, CA2, and CA3. The third subdivision is the subiculum. Note that, according to some authors, a prosubiculum can be differentiated from the subiculum proper. Also, readers may find texts in which reference is made to the subicular complex, consisting of (pro)subiculum, presubiculum, and parasubiculum. Based on connectional arguments, it is preferred to consider the presubiculum and parasubiculum as being functionally different from the subiculum (cf. Amaral and Witter 1995; and for a very detailed comparison between species the reader is referred to Stephan 1975).
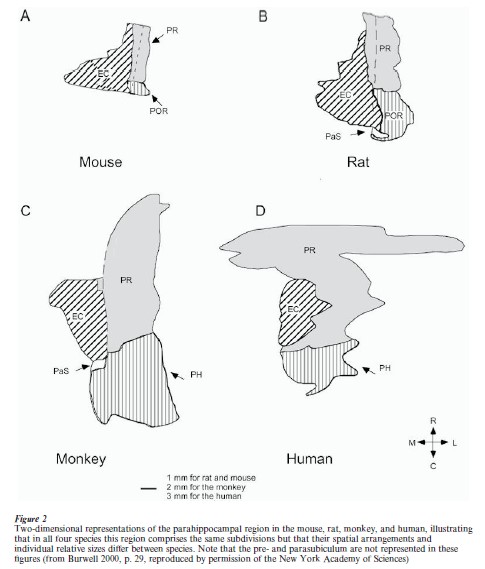
The parahippocampal region, in contrast to the hippocampus, comprises cortical regions with more than three layers—generally, five or six layers are distinguished. In all species, the parahippocampal region comprises five different regions, the preand parasubiculum, the perirhinal and entorhinal cortices, and a fifth area which in primates is commonly referred to as the parahippocampal cortex. In nonprimates, the most likely homolog for the latter area is the so-called postrhinal cortex (cf. Burwell et al. 1995, Witter et al. 1989). The respective names of some of the areas that make up the parahippocampal region refer to the rhinal sulcus, which can be distinguished in all mammals. However, its particular features differ considerably between species. Whereas in rats it is a distinguishable fissure that runs along the rostrocaudal extent of the hemisphere, in humans it is only present in about 50 percent of the cases, as a shallow and short indentation in the most medio-anterior temporal lobe. It is of interest that the spatial relationships among the different fields of the parahippocampal region appear similar in, for example, mouse, rat, monkey, and human (see Fig. 2).
A remark is appropriate concerning the question of whether nonmammalian species do have a hippocampus and related structures. In reptiles, cortical areas have been described which resemble, both in terms of celltype as well as connectivity, the mammalian dentate gyrus and Ammon’s Horn. Part of the olfactory-related cortex of reptiles may be considered homologous to the mammalian parahippocampal region (see Ten Donkelaar 1997 for further reading). Interestingly, there is some evidence that lesions of these structures in reptiles interfere with learning. With respect to birds, there is convincing evidence that a particular region in the forebrain, homologues to the reptilian cortex, is functionally important for spatial memory. This region is generally referred to as the hippocampus, but structurally it looks quite different from the mammalian hippocampus. Connection-wise, though, it is quite similar (see Clayton and Krebs 1995 for further details).
3. Wiring Of The Hippocampus And Parahippocampal Region
Most detailed information about the connectivity of the system comes from anatomical tracing studies in the rat, although for some pathways, relevant detailed information is available for the monkey, and for the cat as well. The overall picture which emerges from all these studies is that this connectivity is rather conservative, such that a general non-species specific description may suffice. For further details, readers are referred to Witter et al. (1989, 2000a, 2000b), Amaral and Witter (1995), Burwell (2000), and Suzuki and Amaral (1994a, 1994b). The hippocampus has two major pathways through which it is connected to the rest of the brain. The first pathway makes use of the so-called cortico–parahippocampal–hippocampal circuit, and the second is formed by the fornix (see Fig. 3).
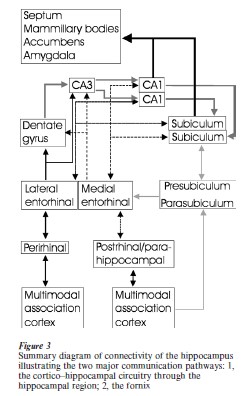
3.1 Cortico–Parahippocampal–Hippocampal Circuit
A striking feature of the cortico–parahippocampal– hippocampal circuit is that the hippocampus does not have direct connections with the majority of the functionally different cortical regions. In contrast, this communication is mediated by way of the parahippocampal region. Of this region, the perirhinal and postrhinal/parahippocampal cortices maintain the highest number of cortico–cortical connections, including connections within all major sensory realms, such as visual, auditory, somatosensory, and gustatory ones. Also, massive connections exist with the other two major higher-order association domains, the parietal and prefrontal cortices, but connections with the motor cortex are less prevalent. The perirhinal and postrhinal parahippocampal cortices, in turn, provide a major input to the superficial layers II and III of the entorhinal cortex, which are further reached by inputs from olfactory structures, the amygdala, and from the remaining two components of the parahippocampal region, i.e., the preand parasubiculum. Neurons in entorhinal layers II and III, which are the recipients of this wide variety of inputs, give rise to the major cortical input to the hippocampus, the so-called perforant pathway. This name is derived from the traditional descriptions by Ramon y Cajal (1911), who noted that fibers from the entorhinal cortex perforate the underlying white matter and the cortical lamina of the subiculum to gain access to the dentate gyrus molecular layer. In order to understand the potential functioning of the system, it is critical to point out that the perforant pathway projection harbors two different systems. Layer II cells distribute their axons to the dentate gyrus and CA3, whereas layer III cells send their axons to CA1 and the subiculum. In addition to this intriguing difference in target specificity, the terminal distributions of these two components of the perforant pathway are entirely different. Layer II cells give rise to a widespread projection to all dentate granular cells. In contrast, axons of layer III cells target only restricted groups of the available neurons in CA1 and the subiculum (see Sect. 5.1 for functional relevance; see also Fig. 3). This particular feature is of interest, since field CA1 and the subiculum constitute the major output structures of the hippocampus. Hippocampal output from these two hippocampal fields is distributed by way of projections back to the entorhinal cortex, from where ongoing output projections originate to a host of cortical and subcortical structures, including projections to perirhinal and parahippocampal postrhinal cortices, prefrontal areas, amygdala, ventral striatum, and midline thalamus.
A further refinement has to be added to the overall organization of the connections between the parahippocampal region and the hippocampus. It has been established that the input–output system consists of at least two parallel pathways. One of the pathways carries inputs from the perirhinal cortex into and away from the hippocampus, whereas the second mediates transfer of information from and to the parahippocampal postrhinal cortex. In view of the anatomical organization outlined above, it has thus been suggested that in the hippocampus these two pathways converge at the level of the dentate gyrus and CA3, whereas they are kept more or less separate at the level of CA1 and the subiculum (cf. Witter et al. 2000b).
3.2 The Fornix
The fornix is a major fiber bundle which connects the hippocampus to the hypothalamus, in particular the mammillary bodies. On its way, the fornix also issues fibers to the septal complex, the ventral striatum, and the amygdala. As indicated above, the fornix is not a pure output pathway, since projections from the septal complex (see Sect. 5.2) to the hippocampus and in part to the entorhinal cortex travel by way of the fornix. In addition, the commissural connections between the left and right hippocampi also partially travel by way of the fornix. The hippocampal connection to the mammillary bodies is part of the traditionally described limbic or Papez circuit, which includes the mammillo-thalamic tract connecting the mammillary bodies with the anterior complex of the thalamus, which in turn project to large portions of the limbic cortex, including anteriorand posterior-cingulate cortex, and preand parasubiculum. All these structures, in turn, provide input to the hippocampus, either directly, or indirectly by way of the entorhinal cortex.
4. The Hippocampus Is Not A Homogeneous Structure
When one looks at the overall anatomical organization of the parahippocampal–hippocampal system, it appears that the hippocampus is rather homogeneous along its long axis. However, differences in numbers of neurons between the dorsal posterior and ventral anterior hippocampus have been reported, indicative for differences in the overall neuronal network. Moreover, in the rat and in the monkey, it has been established that the dorsal posterior portion of the hippocampus receives its major cortical input from the more sensory-related or exteroceptive lateral and caudal portions of the entorhinal cortex, whereas the ventral anterior hippocampus receives inputs from more medial parts of the entorhinal cortex, which most likely convey information that reflects the interoceptive status of the individual. In addition, direct reciprocal connections with the amygdala are limited to the ventral anterior and intermediate levels of the hippocampus and are absent for the dorsal posterior portion of the hippocampus (cf. Witter 1986). Behavioral data in support of this notion are the findings that in rats the dorsal hippocampus is critical for the initial learning and long-term memory of spatial information, whereas the ventral hippocampus is not essential (cf. Moser and Moser 1998). Recently, brain imaging studies have revealed that in humans functional differences are also present between anterior and posterior halves of the hippocampus; however, the precise nature of these differences is still an issue for debate (Lepage et al. 1998, Schacter and Wagner 1999).
5. Functions Of The Hippocampus And Parahippocampal Region
5.1 Functions Of The Cortico–Hippocampal System
Ample clinical and experimental evidence supports the view that the hippocampus and parahippocampal structures are essential for memory processes, although the unique contributions of each of the different subfields are as yet incompletely understood (Scoville and Milner 1957, Squire 1992, Eichenbaum et al. 1994). The results of the behavioral studies, in particular, have provided insights into how each of the components of the hippocampal system contributes to memory processes. Instead of contributing in a unitary way to memory, it appears evident that individual structures make selective contributions. Such studies indicated that the hippocampal formation itself does not contribute critically to object recognition memory. Instead, it is the laterally positioned component of the parahippocampal region, the perirhinal cortex, that appears to be involved critically in this task (Murray et al. 2000). In contrast, the more posterior portions of the parahippocampal region, the parahippocampal cortex in primates or the postrhinal cortex in nonprimates, may be involved critically in spatial or topographical memory (Bohbot et al. 2000, Vann et al. 2000, cf. Witter et al. 2000b). Concerning the role of the entorhinal cortex, the results of lesion studies appear inconclusive; still, it is most likely that the entorhinal cortex operates cooperatively with the hippocampal formation, such that both structures process information in different, yet complementary ways. With respect to the hippocampal formation, it has been proposed that it plays a critical role in mediating memory for relevant relationships among conjunctions or associations unique to a particular episode (Eichenbaum et al. 1994).
5.2 Functions Of Hippocampus–Fornix Connectivity
As indicated above, the fornix provides the hippocampus with another pathway, beside the cortical route, to communicate with a host of subcortical brain structures, including the mammillary bodies. Behavioral data indicate a clear functional dichotomy between the cortical versus the fornix route. However, the role of the fornix pathway in learning and memory is as yet poorly understood. Lesions of the main target of the fornix, i.e., the mammillary complex in animals, do not result in marked memory deficits, although in Korsakoff’s patients, which do show a marked anterograde as well as retrograde memory deficit, the mammillary bodies show a marked decrease in volume (cf. Aggleton and Brown 1999). In addition, there is convincing human evidence from patients with thalamic infarctions in favor of a role in memory processes of the connection from the mammillary complex to the anterior complex of the thalamus (van der Werf et al. 2000).
The fornix also mediates connectivity from the hippocampal–parahippocampal structures to the ventral striatum and the amygdala. In the ventral striatum, interactions may take place between hippocampal, amygdaloid, and prefrontal outputs, leading to the selection of appropriate behavioral strategies, for example, in learning paradigms (consult Groenewegen et al. 1999 for further reading). The functional relevance of the reciprocal connections between the amygdala and hippocampus, and for that matter also those between the amygdala and the parahippocampal region, is still poorly understood (Pitkanen et al. 2000, Stefanacci et al. 1996). It is assumed that amygdala inputs may provide the hippocampal system with a marker for relevance to a particular memory episode.
The connections to and from the septal complex of both the hippocampus and parahippocampal structures use the fornix as well as a number of other white matter pathways. Although much about these interactions has yet to be resolved, it is clear that the normal functioning of the hippocampal–parahippocampal-cortical interactions depends strongly on a functional septal complex. It is in particular the fact that the septal complex supplies the hippocampus and parahippocampal region with most of its cholinergic input, as well as the role of the septal complex in the synchronization of electrical activity which relates to its functional relevance (see Sect. 5.3).
5.3 Oscillatory Activity
The hippocampus shares with the entorhinal cortex the presence of strong rhythmical activity, in particular theta rhythm, which is coexpressed with trains of gamma waves during exploring behavior in the awake animal as well as during a particular sleep phase called Rapid Eye Movement (REM) sleep. Both structures also share the occurrence of so-called sharp waves, a high-frequency discharge which occurs predominantly during the alternating sleep phase, the so-called slow wave sleep phase. Although the electrophysiological mechanisms underlying the occurrence of such oscillatory phenomena have been clarified to some point, the functional relevance, for example, in relation to learning, is still debated. There appears to be consensus though that the normal functioning of the cortico-hippocampal learning system depends strongly on the normally occurring alternations between the theta and the sharp wave state (cf. Buzsaki 1996).
5.4 Clinical Relevance
The hippocampal–parahippocampal structures play an established role in learning and memory processes, which is supported by reports concerning pathological changes seen in diseases that are associated with learning and memory disorders such as Alzheimer’s disease. However, other neurological psychiatric disorders, such as epilepsy, schizophrenia, Huntington’s, Parkinson’s and Pick’s disease are associated with pathological alterations in these regions as well. The most striking observation, however, is the specificity of the pathology in each of these diseases. In all except epilepsy and schizophrenia, the first pathological changes are seen in the anterolateral part of the entorhinal cortex, also called the transentorhinal cortex. However, in all these diseases, the layer showing the most dramatic alterations seems to be disease specific (Braak et al. 2000). In the case of epilepsy, the most striking initial pathology is observed in layer III of the medial entorhinal cortex (Schwarcz et al. 2000), whereas in the case of schizophrenia, the pathology appears to start in layer II throughout the entorhinal cortex (Arnold 2000). In all these disorders, the hippocampus becomes involved as well, leading to an overall dramatic reduction in neuronal numbers and/or connections.
Bibliography:
- Aggleton J P, Brown M W 1999 Episodic memory, amnesia and the hippocampal–anterior thalamic axis. Behavavioral and Brain Sciences 1999: 425–89
- Amaral D G, Witter M P 1995 Hippocampal formation. In: Paxinos G (ed.) The Rat Nervous System. Academic Press, San Diego, CA
- Arnold S 2000 Cellular and molecular neuropathology of the parahippocampal region in schizophrenia. Annals of the New York Academy of Science 911: 275–92
- Bohbot V D, Allen J B J, Nadel L 2000 Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Annals of the New York Academy of Science 911: 355–68
- Braak H, Del Tredici K, Bohl J, Bratzke H, Braak E 2000 Pathological changes in the parahippocampal region in select non-Alzheimer dementias. Annals of the New York Academy of Science 911: 221–39
- Burwell R D 2000 The parahippocampal region: corticocortical connectivity. Annals of the New York Academy of Science 911: 25–42
- Burwell R D, Witter M P, Amaral D G 1995 Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus 5: 390–408
- Buzsaki G 1996 The hippocampal–neocortical dialogue. Cerebral Cortex 6: 81–92
- Clayton N S, Krebs J R 1995 Food-storing memory and the hippocampus. Current Opinion in Neurobiology 5: 149–54
- Eichenbaum H, Otto T, Cohen N J 1994 Two functional components of the hippocampal memory system. Behavioral and Brain Sciences 17: 449–72
- Groenewegen H J, Mulder A B, Beijer A V J, Wright C I, Lopes da Silva F H, Pennartz C M A 1999 Hippocampal and amygdaloid interactions in the nucleus accumbens. Psychobiology 27: 149–64
- Lepage M, Habib R, Tulving E 1998 Hippocampal PET activations and memory encoding and retrieval: The Hiper model. Hippocampus 8: 313–22
- Moser M B, Moser E I 1998 Functional differentiation in the hippocampus. Hippocampus 8: 608–19
- Murray E A, Bussey T J, Hampton R R, Saksida L M 2000 The parahippocampal region and object recognition. Annals of the New York Academy of Science 911: 166–74
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A 2000 Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex and postrhinal cortex in the rat. Annals of the New York Academy of Science 911: 369–91
- Ramon y Cajal S 1911 Histologie du Systeme Ner eux de l’Homme et des Vertebres. Maloine, Paris
- Schacter D L, Wagner A D 1999 Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9: 7–24
- Scharfman H E, Witter M P, Schwarcz R 2000 Preface. Ann. New York Acad. Sci 911: ix–xiii
- Schwarcz R, Eid T, Du F 2000 Neurons in layer III of the entorhinal cortex. A role in epileptogenesis and epilepsy? Annals of the New York Academy of Science 911: 328–42
- Scoville W B, Milner B 1957 Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry 20: 11–21
- Squire L R 1992 Memory and hippocampus: A synthesis of findings with rats, monkeys and humans. Psychology Review 99: 195–231
- Stefanacci L, Suzuki W A, Amaral D G 1996 Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology 375: 552–82
- Stephan H 1975 Allocortex. In: Borgman W (ed.) Handbuch der Mikoskopischen Anatomie des Menschen IV-9. Springer-Verlag, Berlin, pp. 1–998
- Suzuki W A, Amaral D G 1994a The perirhinal and parahippocampal cortices of the Macaque monkey: Cortical aff Journal of Comparative Neurology 350: 497–533
- Suzuki W A, Amaral D G 1994b Topographic organisation of the reciprocal connections between the monkey entorhinal and the perirhinal and parahippocampal cortices. Journal of Neuroscience 14: 1856–77
- Ten Donkelaar H J 1997 Reptiles. In: Nieuwenhuys R, Ten Donkelaar H J, Nicholson C. (eds.) Central Nervous System of Vertebrates. Springer-Verlag, Berlin, Vol 2, pp. 1315–524
- van der Werf Y D, Witter M P, Uylings H B M, Jolles J 2000 Neuropsychology of infarctions in the thalamus: A metaanalysis. Neuropsychologia 38: 613–27
- Vann S D, Brown M W, Erichsen J T, Aggleton J P 2000 Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. Journal of Neuroscience 20: 2711–18
- Witter M P 1986 A survey of the anatomy of the hippocampal formation, with emphasis on the septotemporal organization of its intrinsic and extrinsic connections. In: Schwarcz R, BenAri Y (eds.) Advances in Experimental Medicine and Biology, Vol. 203: Excitatory Amino Acids and Epilepsy. Plenum Press, New York, pp. 67–82
- Witter M P, Groenewegen H J, Lopes da Silva F H, Lohman A H M 1989 Functional organisation of the extrinsic and intrinsic circuitry of the parahippocampal region. Progress in Neurobiology 33: 161–254
- Witter M P, Naber P A, van Haeften T, Machielsen W C M, Rombouts S A R B, Barkhof F, Scheltens Ph, Lopes da Silva F H 2000a Cortico-hippocampal communication by way of parallel parahippocampal–subicular pathways. Hippocampus 10: 398–410
- Witter M P, Wouterlood F G, Naber P A, van Haeften T 2000b Anatomical organization of the parahippocampal–hippocampal network. Annals of the New York Academy of Science 911: 1–24




