Sample Neural Representations Of Movement Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The motor cortex consists of a number of functionally and connectionally interrelated areas that participate in the planning and production of voluntary movements. Functions like conditional association, coordinate transformation, and linking of movements occur in multiple parietal, cingulate, and frontal areas.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
There is a general organization that abstract aspects of planning and spatial organization occur in parietal cortex, while sequencing and execution of movement occurs in frontal areas. However, these functions are extensively intermingled across the set of motor areas, so that intended movements are products of continual interactions across a broad region of cerebral neocortex.
1. What Is The ‘Motor Cortex’?
The cerebral neocortex is frequently considered to be the primary site where voluntary movements are planned, initiated and, to some extent, learned. These ideas have been supported by studies using a variety of methods, including single and multiple neuron recording, lesion effects, and human functional brain imaging (Sanes and Donoghue 1997). This body of work has significantly advanced our understanding of the means by which plans for voluntary movement are assembled and used for muscle control. This research paper will define the term motor cortex and the cortical areas that comprise it, then describe how various motor areas participate in voluntary movement. Our present knowledge leads to the conclusion that a vast cortical territory cooperates in fashioning voluntary movements from external cues or mental intentions.
The motor cortex comprises a collection of more or less distinct areas or ‘fields,’ which are united by function and connectivity. A field is typically, but not exclusively, included as motor if neurons in that area modulate their discharge in close conjunction with the contraction of voluntary muscles, it receives inputs from other motor structures in the central nervous system (CNS) and it contributes output through the corticospinal tract. More detailed differences in the functional properties, connections, and cytoarchitecture distinguish one motor area from its neighbors. Ongoing anatomical and functional studies of frontal and parietal cortex add to a progressively increasing list of motor separate cortical fields; probably more than a dozen can be identified. The blending of sensory and motor properties across the neocortex means that many cortical fields could be classified within several different domains, pointing out the vagaries of forcing divisions in a region of the CNS that is inherently integrative. Among motor fields, oculomotor and somatomotor control areas are often separated. This review deals with those areas of cortex that control the somatic musculature, in particular those involved in arm reaching movements, which have been most thoroughly studied.
1.1 Subdivisions Of Motor Cortex
Figure 1 summarizes the regions where neurons related to arm reaching are likely to be present in the human brain. Localization of motor areas in humans is inexact because their identification is based upon data obtained directly from single neuron recordings in non-human primates. Similar data is difficult to obtain in the normal human brain. Localization is made by correlating areas of blood flow changes from imaging techniques in humans with a presumed homolog in other species. Traditionally, the primary motor cortex (MI, Brodmann’s area 4), which is located on the precentral gyrus of many primates (including humans), is considered a primary site for movement generation. It is ranked ‘primary’ largely because movements of the opposite side of the body can be elicited by the lowest levels of electrical stimulation anywhere in the cortex. Among cortical areas, MI also has the bulk of the direct projections to the spinal cord, including some direct projections to alpha motor neurons ( ~ 5 percent). In addition, MI neurons are active with voluntary movement, and lesions of MI lead to paralysis of the contralateral musculature. Lying in front of MI are a collection of frontal lobe non-primary areas, where stimulation thresholds are higher, neurons may have more complex properties, and lesions result in movement defects other than frank paralysis, such as incoordinations called apraxia. While there is some diversity in the naming of these other motor areas, a dorsal and ventral premotor (PM), a supplementary motor (SMA), and presupplementary motor area (pSMA) are commonly recognized as distinct areas; each of these may be subdivided further (Geyer et al. 2000). More recently it has become apparent that the cortex on the medial wall of the cerebral hemisphere along the cingulate sulcus is related to movement planning and preparation (CMA; Strick et al. 1998), which also appears to contain multiple subdivisions.
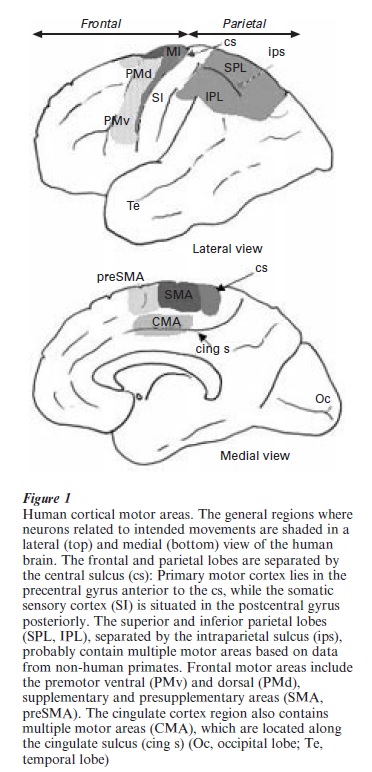
The parietal lobe can be subdivided into a number of areas associated with somatic sensation in its anterior part (SI or primary somatic sensory cortex), and for all senses more posteriorly. The posterior parietal cortex is divided into superior and inferior lobes which, on the basis of non-human studies, is likely to contain multiple somatic motor areas. The parietal lobe is heavily engaged in spatially guided actions. In the anterior parietal cortex, the postcentral gyrus contains a collection of four distinct somatic sensory areas—Brodman’s areas 3b,1, 2, and 3a. The former two areas seem mainly concerned with proces- sing of touch, while areas 2 and 3a process deep or kinesthetic information from the joints, muscle spindles (muscle length and velocity), and golgi tendon organs (muscle tension receptors). Neurons related to the production of arm movement are also in this region, particularly in area 2. More posterior areas of parietal cortex (both IPL and SPL) contain areas related to eye and arm movement, often blended with various types of visuo-spatial or other sensory modality related neurons. In monkeys, group areas related to reaching and grasping movement are located in the intraparietal sulcus; whether such areas are in the same location in humans is not resolved. Most posteriorly in the parieto-occipital area, the PO region of monkeys is an additional arm related area; also termed V6 for its visual responsiveness (Burnod et al. 1999).
1.2 Connections Of Motor Areas
Motor areas are densely interconnected with each other and with other cortical areas; the thalamus and the basal ganglia, brainstem and, for most areas, the spinal cord. All motor cortical areas are interconnected within at most two or three synaptic relays, but this is also true of most of the CNS, making connectivity difficult to be used as a sole differentiator of dedicated motor areas. Direct connections exist from parietal to frontal lobes with a general pattern that regions close to the central sulcus (dividing SI and MI, see Fig. 1) in the parietal cortex project to regions close to the central sulcus in the frontal cortex, creating a rough pattern of mirror symmetry of connections (see review of Geyer et al. 2000). It is essential to emphasize the vastly parallel nature of possible interactions among motor areas, rather than any simplistic view of a stepwise serial process from one to the next area. Beyond the clear picture of vast interconnectivity of areas, studies of the timing of neurons during movement planning and action demonstrate that neurons in most or all of the motor areas interact extensively (Wise et al. 1997).
2. Cortical Areas Engaged In Movement Imaging Methods
A broad perspective of the activation patterns of motor areas can be gained from brain imaging studies. Both positron emission (PET) and functional magnetic resonance imaging (fMRI) studies in humans show that primary motor cortex is engaged when voluntary actions are produced. By contrast, premotor and supplementary motor areas can be activated simply by planning motor actions, although they are also engaged during movement production (Sanes 2000). Recently, human cingulate cortex has been tied to movement based on activation in PET and fMRI studies. Cingulate motor areas (CMA, Fig. 1) can be activated by grasp and reaching actions; some functions may be segregated within rostral and caudal cingulate areas (Picard and Strick 1996). Other results suggest that cingulate cortex may play a role in performance monitoring and in selection of actions (Carter et al. 2000). Similarly, the parietal lobe is heavily engaged in voluntary arm movements when viewed by brain imaging methods. Numerous areas of the parietal lobe activate with arm movement (Connolly et al. 2000), suggestive of multiple subdivisions, as reported in monkeys (see later).
3. Activity Of Single Neurons In Cortical Motor Areas
Single neuron recordings in nonhuman primates give the most direct evidence for the role of cortical areas in movement. In addition, they verify conclusions based upon the indirect PET and fMRI measures. Neuronal activity has been measured during movement planning by instructing movement first, then, after a delay, requesting its enactment (an instructed delay task) during movement sequences and after various aspects of kinematics and dynamics have been varied. Neuron activity has been correlated with direction, amplitude, various types of loads, sequence structure, and sensory-motor contingencies and attention. Rather than describe the patterns of neural activity in each area under each of these conditions, only general patterns of engagement of each area will be described. One of the most pronounced features of motor cortex neurons is the relationship of firing modulation to direction of a reaching movement.
Neurons in frontal and parietal areas are directionally tuned so that they fire most to one direction and less so to other directions, and typically are least modulated in the direction opposite to their preferred direction. Neurons in motor areas are also modulated by force, and kinematic features (amplitude, position, and velocity changes) (Kalaska et al. 1997, Fu et al. 1993, Ashe and Georgopoulos 1994). Each of these features is correlated with neuronal activity, but causal links to the actual computation of these aspects of movements are less certain. The current experimental data cannot fully dissociate whether or where conversion from an abstract notion of movement in space to muscle activity patterns occurs. However, the relatively weaker influence of movement force on neural activity in the parietal areas suggests that motor plans are more abstract here, compared to the frontal lobe.
Correlation of force and neural activity is common in MI, but many neurons are influenced by kinematics as well, so that there is no clear cut neuronal segregation of these features. The activity of neurons during motor preparatory delays has provided further evidence for a role in planning actions. Neurons with delay activity are found across motor cortex, but they are more prevalent in the nonprimary regions. However, other work also suggests that even primary motor cortex may be involved in cognitive aspects of a motor plan (Carpenter et al. 1999), so it is difficult to accept a simple notion that plans are formulated in nonprimary cortex and then passed to MI for command generation. Rather, the set of areas continually cooperate throughout the planning and production of movement. This does not negate the finding that certain functions predominate in specific subdivisions of the motor cortex.
4. Parietal–Frontal Engagement In Movement
It is clear from electrophysiological, lesion, and imaging data that the computations concerning the spatial coordinates of movement are processed in the parietal lobe, while movement execution is more weighted towards frontal areas; planning occurs across the parietal–frontal network (Kalaska et al. 1997). Knowledge of arm position in space is available from tactile and prophoceptive information in SI, while the location of visual cues is delivered from occipital visual areas. There is strong evidence that the posterior parietal cortex combines signals related to arm position and planned arm movements, as well as for gaze direction and location of sensory cues. (Batista and Andersen 2001, Burnod et al. 1999). In parietal cortex multiple frames of reference are present, although how these are interconverted is not clear (Boussaoud and Bremmer 1999). Further, planning for visually guided actions begins very early in ‘visual’ pathways, such as PO, only two synapses from primary visual cortex.
Nonprimary motor areas within the frontal lobe appear to be engaged in planning processes, based on the observation that activity in these regions occurs during instructed delay periods. Frontal areas are important for linking stimuli to movements or to link various movements together in space or time. These more complex actions seem less coded in primary motor cortex, where neuronal activity is more tightly coupled to the actual performance of intended arm movements. However, neurons related to planning and production of motion can be found intermingled throughout motor cortex; it appears as if only the relative proportions change across motor areas. There is considerable evidence that supplementary motor cortex (SMA) is important in formulating sequences of movements. Neurons in SMA can be selective for particular movement sequences or transitions and these same neurons may not be active when the same movements are made in other sequences (Shima and Tanji 2000). The pre-SMA, lying just in front of SMA, appears to be critical for learning new sequences, but the pre-SMA’s role seems to fade as sequences are learned (Hikosaka et al. 1996). The dorsal premotor cortex PMD is also engaged in forming conditional associations between sensory cues and movement. While the entire PM cortex seems to be engaged in planning in various coordinate systems, the ventricle premotor cortex (PMv) seems to be a region for sensorimotor processing for stimuli near the body (Graziano and Gross 1998).
The primary motor cortex is often considered as the final common pathway and home of the upper motor neuron for movement commands to be issued. The widespread origin of the corticospinal path from most of the motor cortex coupled with the broad activation of other motor areas during movement is sufficient to reject these ideas in favor of a more distributed role for motor cortex in movement. Nevertheless, the preponderance of neurons closely coupled to the details of muscular action and the ability to activate muscles easily with electrical stimulation from MI suggest a special role for MI in voluntary movement. As stated earlier, MI neurons modulate their activity with nearly every aspect of movement including force, velocity, acceleration, and distance. It remains a considerable challenge to identify whether MI represents movements in terms of muscle activity or more abstract variables (Todorov 2000). On the one hand, up to 5 percent of MI’s output directly contacts alpha motor neurons so that it provides a very powerful direct route to the muscles. On the other hand, some MI neurons are active with very abstract aspects of motor plans, such as the serial order of stimuli, suggesting that it participates in the cognitive events leading to action.
5. Conclusions
The combination of cortical motor areas combine signals related to proprioceptive and tactile signals of arm position, vision, gaze angle, attention and motor plan, which conveys a patterned outflow of information that guides somatic musculature for action.
The parietal lobe deals with the spatial aspects of movement: keeping track of the configuration of the arm in space, its relationship to the direction of gaze and to visual cues. In addition, it participates in formulating upcoming actions, possibly in a form more abstract than found in the frontal lobe. The frontal lobe motor areas contribute to learning and assembling sequences of motor actions and are also involved in non-spatial motor planning where arbitrary cues (or perhaps thoughts) are linked to particular motor actions. However, parietal–frontal motor areas also combine many types of signals, such as gaze or attention, which may have overt, or sometimes more subtle or conditional influences upon the activity of motor cortex neurons. Finally, this complex of areas is nearly simultaneously engaged in many forms of motor action so that they act in concert when intention leads to action. The essence of understanding how an intention becomes an action depends on further understanding of the functional interactions among the collection of motor areas.
Bibliography:
- Ashe J, Georgopoulos A P 1994 Movement parameters and neural activity on motor cortex and area 5. Cerebral Cortex 4: 590–600
- Batista A P, Andersen R A 2001 The parietal reach region codes the next planned movement in a sequential reach task. Journal of Neurophysiology 85: 539–44
- Boussaoud D, Bremmer F 1999 Gaze effects in the cerebral cortex: reference frames for space coding and action. Experimental Brain Research 128: 170–80
- Burnod Y, Baraduc P, Battaglia-Mayer A, Guigon E, Koechlin E, Ferraina S, Lacquaniti F, Caminiti R 1999 Parieto-frontal coding of reaching: an integrated framework. Experimental Brain Research 129: 325–46
- Carpenter A F, Georgopoulos A P, Pellizzer G 1999 Motor cortical encoding of serial order in a context-recall task. Science 283: 1752–7
- Carter C S, Macdonald A M, Botvinick M, Ross L L, Stenger V A, Noll D, Cohen J D 2000 Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Science USA 97: 1944–8
- Connolly J D, Goodale M A, Desouza J F, Menon R S, Vilis T A 2000 Comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. Journal of Neurophysiology 84: 1645–55
- Fu Q G, Suarez J I, Ebner T J 1993 Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. Journal of Neurophysiology 70: 2097–116
- Geyer S, Matelli M, Luppino G, Zilles K 2000 Functional neuroanatomy of the primate isocortical motor system. Anatomy and Embryology 202: 443–74
- Graziano M S, Gross C G 1998 Spatial maps for the control of movement. Current Opinion in Neurobiology 8: 195–201
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B 1996 Activation of human presupplementary motor area in learning sequential procedures: a functional MRI study. Journal of Neurophysiology 76: 617–21
- Kalaska J F, Scott S H, Cisek P, Sergio L E 1997 Cortical control of reaching movements. Current Opinion in Neurobiology 7: 849–59
- Picard N, Strick P L 1996 Motor areas of the medial wall: a review of their location and functional activation. Cerebral Cortex 6: 342–53
- Sanes J N 2000 The relation between human brain activity and hand movements. Neuroimage 11: 370–4
- Sanes J N, Donoghue J P 1997 Static and dynamic organization of motor cortex. Advances in Neurology 73: 277–96
- Shima K, Tanji J 2000 Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. Journal of Neurophysiology 84: 2148–60
- Strick P L, Dum R P, Picard N 1998 Motor areas on the medial wall of the hemisphere. Novartis Foundation Symposium 218: 64–75
- Todorov E 2000 Direct cortical control of muscle activation in voluntary arm movements: a model. Nature Neuroscience 3: 391–8
- Wise S P, Boussaoud D, Johnson P B 1997 Caminiti Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annual Review of Neuroscience 20: 25–42R




