Sample Pre-Motor Cortex Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The frontal lobe of primates is formed by two large regions, a rostral region involved in cognitive functions (prefrontal lobe) and a caudal one devoted to motor functions. Histologically, the caudal region is characterized by the absence of granular cells. It is referred to also as the agranular frontal cortex (AFC). The AFC consists of two main sectors: area 4 (the primary motor cortex) and area 6. Recent data showed that area 6 is neither anatomically nor functionally unitary, but is formed by a mosaic of independent ‘premotor’ areas (see He et al. 1993, Rizzolatti et al. 1998). The aim of this research paper is to review the general organization of the premotor areas in monkeys and to discuss in detail the operations of two of them. A comparison between the monkey and human premotor cortices will be made in the last section.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. The General Organization Of The Premotor Area: Classes And Connectivity
The existence of a cortex with motor functions lying rostral to the primary motor cortex (the ‘premotor cortex’) has been postulated since the beginning of the twentieth century. However, following the stimulation studies of Woolsey and Penfield, the prevalent view was that AFC functionally consists of two large motor areas: the ‘primary motor cortex’ located on the lateral brain convexity (MI) and the ‘supplementary motor area’ (SMA) located on its mesial surface (see Porter and Lemon 1993). Anatomical and functional evidence collected from 1980–2000 showed that this view is incorrect. The AFC is formed by seven independent areas (F1–F7). F1 corresponds to the Brodmann area 4 (the ‘primary motor cortex’); the other areas are located within the Brodmann area 6 (the ‘premotor areas’). This new subdivision of the AFC is shown in Fig. 1.
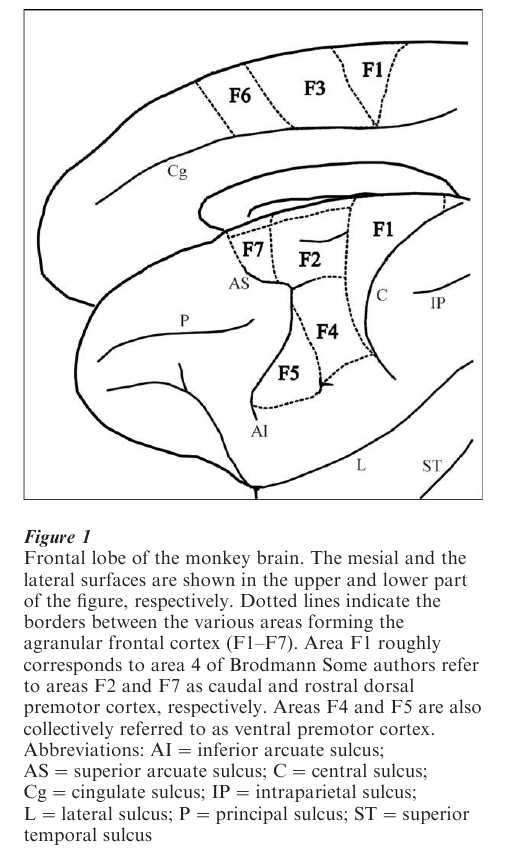
The premotor areas can be subdivided into two main classes: areas that receive their predominant cortical input from the parietal lobe (parieto-dependent premotor areas) and areas that receive their predominant cortical input from the prefrontal and the cingulate cortex (fronto-dependent premotor areas). Areas F2, F3, F4, and F5 belong to the first class, areas F6 and F7 to the second (Rizzolatti et al. 1998).
The organization of corticospinal projections is in accord with this subdivision (He et al. 1993). The parieto-dependent areas have direct access to the spinal cord, whereas the fronto-dependent areas send their output to the brain stem. Furthermore, the parieto-dependent areas have direct connections with F1, whereas the fronto-dependent areas lack this link.
Within the parieto-dependent group of premotor areas, each area is the target of a different set of parietal areas. Typically, each premotor area receives strong afferents from one parietal area. Conversely, each parietal area projects predominantly (but not exclusively) to a single premotor area. Thus, the parietal and premotor areas form a series of anatomical circuits largely independent one from another. The functional correlate of this anatomical organization is that each parieto-premotor circuit is dedicated to a specific sensorimotor transformation (see Rizzolatti et al. 1998). The result of this operation is the generation of a ‘potential motor action,’ that is of a representation of an action that may or may not be executed.
The fronto-dependent premotor areas (F6 and F7) have only weak connections with the parietal lobe. F6 is mostly connected with the prefrontal area 46 and the rostral cingulate cortex (area 24c). F7 is richly connected with the dorsal sector of the prefrontal cortex. The functions of prefrontal and cingulate areas are much less known than those of the parietal cortex. There is consensus, however, that these areas are not involved in the analysis of sensory stimuli, but rather play a role in cognitive functions such as working memory, temporal planning of actions, and motivation. It is clear, therefore, that the functions of the fronto-dependent premotor areas ought to be different from those of the parieto-dependent ones. The hypothesis presented here is that the fronto-dependent premotor areas relay information on the individual’s motivations, long-term plans, and memory of past action from the prefronto/cingulate cortex to the parieto-dependent premotor areas. On the basis of this information, the potential motor actions, formed in the posterior premotor areas, are either executed or not.
2. The Parieto-Dependent Premotor Areas (Fig. 1: F2, F3, F4, And F5)
As a model of information processing of the parietodependent areas, the functional properties of one of them (area F5) will be reviewed in some detail (see Rizzolatti et al. 1999). The properties of the other parieto-dependent areas will only be dealt with in short.
2.1 Area F5
Area F5 occupies the rostroventral part of the AFC (Fig.1). Its electrical stimulation evokes hand and mouth movements. F5 neurons typically discharge in association with goal-directed motor actions, such as object grasping, holding, tearing, and manipulating. The neuron firing correlates with specific actions (e.g., grasping) or action segments (e.g., hand opening, hand closure during grasping) rather than with the individual movements forming the action. Most of the F5 neurons code specific types of hand prehensions such as precision grip or whole-hand prehension.
A considerable part of F5 neurons respond to visual stimuli. Visually responsive F5 neurons fall into two classes: ‘canonical neurons’ and ‘mirror neurons.’ Canonical neurons discharge to the presentation of 3-D objects. Typically, their response is also present when there is no subsequent action on the presented object. Most canonical neurons are selective for one or a small set of visual objects similar in size and shape. Typically, the visual selectivity is congruent with the motor selectivity. A neuron that is selective for precision grip responds also to the presentation of small, but not of large objects. Mirror neurons do not discharge in response to object presentation but to action upon objects (see Sect. 2.2).
The parietal area that predominantly sends its input to area F5 is the anterior intraparietal area (AIP). Neurons of this area have been subdivided into three main classes: ‘motor dominant,’ ‘visual dominant,’ and ‘visual and motor’ neurons. ‘Motor dominant’ neurons are functionally similar to F5 motor neurons. Like F5 neurons, their discharge is associated with actions rather than individual movements. Often they are selective for object size, shape, and/or orientation. ‘Visual dominant’ neurons are active only when the task is performed in light, but not in dark. They respond also to object presentation in the absence of movements. Many of them are selective for size, shape, and/or orientation of 3-D objects. ‘Visual and motor’ neurons discharge in both light and dark, but less in the last condition. Like F5 canonical neurons, ‘visual and motor’ neurons show congruence between their visual and motor properties (Sakata et al. 1995).
From this summary of the AIP neuron characteristics, it is clear that the AIP and F5 have many functional properties in common. There are, however, also some important differences between the two areas. First, in F5 there are no ‘visual dominant’ neurons. Second, purely motor neurons are much more frequent in F5 than in the AIP. Third, most AIP neurons discharge during the whole action leading to the grasping of objects, being active often also during object holding. By contrast, most F5 neurons are active only during a specific phase of the grasping holding action, not during the whole action.
On the basis of these findings and inactivation data, it has been proposed that areas AIP and F5 form the key elements of a circuit that transforms visual information on intrinsic (physical) properties of objects into grasping movements (Jeannerod et al. 1995). The following model has been proposed to explain how this transformation occurs.
AIP neurons code visual information on the object’s intrinsic features. This information is then sent to specific sets of F5 neurons that transform the received information into potential hand movements appropriate to the size, shape, and orientation of the presented object. In turn, F5 neurons transmit this information to F1 (and the spinal cord) for movement execution. The movement occurs, however, only if there is a concomitant ‘go’ signal coming from the fronto-dependent premotor areas.
Objects can be grasped in different ways according to the use one wants to make of them. Because different AIP neurons are selective for different object features, F5 receives a multiple descriptions of each seen object from the AIP. The decision on how to grasp that object is determined by the motivations of the acting agent. This decision comes from the fronto cingulate cortex and reaches the AIP via F5. Thus, a corollary discharge from F5 activates the AIP. In this view, the AIP ‘visual and motor’ neurons are memory neurons that maintain the representation of the selected object feature active during the entire execution of movement. Finally, the ‘motor dominant’ neurons would represent an intermediate stage in the transmission of F5 corollary discharge to the AIP ‘visual-and-motor’ neurons.
2.2 Mirror Neurons
F5 mirror neurons are neurons that discharge when the monkey makes a specific hand action and when it observes another individual making a similar action. The simple presentation of 3-D objects does not activate them. Similarly, the observation of actions that are not directed toward an object, or the observation of actions made using tools, fail to activate them (for a review, see Rizzolatti et al. 1999).
The visual and motor properties of mirror neurons are usually congruent. A neuron that discharges when the monkey grasps an object discharges also when the monkey observes another individual making the same action. In some neurons the way in which the object is precisely grasped does not matter (broad congruence). Other neurons fire only if the observed action is identical to that coded by that neuron (e.g., executed precision grip and observed precision grip; strict congruence).
There is evidence that mirror neurons are mostly located in the caudal part of F5. This sector is anatomically connected with parietal area PF, where, unlike in area AIP, there are neurons with mirror properties. It appears, therefore, that mirror neurons are part of a parieto-premotor circuit separate from that of canonical F5 neurons.
The discharge evoked by the observation of actions is most likely, as in the case of canonical neurons, not a pictorial description of the stimuli, but the motor representation of the action coded by the neuron. However, while canonical neurons use this representation to guide successive motor actions, this is not true for mirror neurons. This, obviously, raises the question of what this motor representation is for. Monkeys, unlike humans, are unable to imitate hand actions. Thus, the imitation hypothesis can be ruled out (at least for hand F5 neurons). The most accepted interpretation of mirror neuron activity is that it is used for action understanding. The assumptions underlying this hypothesis are: (a) individuals understand actions made by other individuals because they are able to react to them internally; and (b) the individuals know the outcome of their actions.
In conclusion, mirror neurons data indicate that the parieto-premotor circuits intervene in higher order cognitive functions and, very interestingly, that the same mechanisms that are at the basis of sensorimotor transformation underlie cognitive functions.
2.3 Areas F2, F3, And F4
Area F2 forms the caudal part of superior area 6 (Fig. 1). F2 receives a rich somatosensory input from area 5. Its ventro-rostral sector also receives visual information from the parietal areas MIP (medial intraparietal area) and V6A (see Rizzolatti et al. 1998). The dorsal part of F2 is related to hind limb movements, whereas the ventral part is related to forelimb movements. Single neuron studies of the arm field showed that some F2 neurons discharge during the preparation of reaching movements, others during movement execution. F2 neurons code the direction of reaching movement rather than a specific position in space (Weinrich et al. 1984). It is likely that F2 uses information on limb position in order to organize (and or control) appropriate limb trajectories.
Area F3 (or SMA proper) is located on the mesial cortical surface. Its main input originates from somatosensory parietal areas (mostly mesial area 5). F3 contains a complete representation of body movements, with a prevalence of axial and proximal, arm and leg, movements. Electrical stimulation of F3 frequently evokes complex movements, involving both proximal and distal joints. Single joint movements are rarely evoked (Luppino et al. 1991). It has been proposed that F3 uses the knowledge of actual limb position to prepare the posture necessary for the incoming movements, thus playing a fundamental role in predictive postural adjustments. SMA also appears to contribute to the initial stages of the learning of motor sequences improving their performance (Hikosaka et al. 1999) and, according to others, in the execution of certain motor sequences (Tanji 1994).
Area F4 forms the caudal part of inferior area 6. F4 receives its main input from the ventral intraparietal area (VIP), a parietal area that contains visual and bimodal, visual and tactile, neurons. Electrical stimulation of F4 evokes mostly head and proximal arm movements. One of the most interesting characteristics of F4 is the presence of neurons with tactile and visual receptive fields. The tactile receptive fields are typically large and located on the face, arms, and chest. The visual receptive fields are limited in depth and are located in the space immediately around the animal. They are registered with the tactile fields. The visual receptive fields remain anchored to the tactile fields regardless of the eye and the body part position. These properties show that the F4 visual receptive fields are coded in egocentric coordinates. It has been suggested that F4 plays a crucial role in reaching movements, transforming spatial locations coded in egocentric coordinates into movement directed toward those locations (Graziano et al. 1994, Fogassi et al. 1996).
3. The Fronto-Dependent Premotor Areas (F6, F7)
3.1 Area F7
Very little is known on the functional properties of area F7, except for its dorsal sector where eye movements are represented (supplementary eye field, SEF). The SEF is connected with the frontal eye field and other centers controlling eye movements. In contrast to the SEF, the F7 ventral part is linked to the premotor areas controlling body movements. In both the SEF and ventral F7 many neurons respond to visual stimuli. The precise role of F7 in motor control is unknown.
Interestingly, joint ablation of F7 and (rostral) F2 determines a profound deficit in learning arbitrary stimulus-movements associations. This finding stresses once more that the premotor areas are involved in cognitive, as well as motor functions (Passingham 1993).
3.2 Area F6
Area F6 (or pre-SMA) corresponds to the rostral part of the SMA of Woolsey and Penfield. Motor responses can be evoked from it only with rather high current intensities. Typically, they consist of slow, complex arm movements (Luppino et al. 1991). Visual responses are common in F6, while somatosensory responses are rare. F6 neurons, recorded during active arm movements, show frequently a long leading activity preceding movements (Tanji 1994).
Studies carried out in a naturalistic condition where monkeys interacted with objects presented close to and far from them, showed that F6 neurons exert a global control on arm actions, rather than firing in association to specific, distal (like F5), or proximal (like F4) actions. Some discharge at visual presentation of graspable objects independent of their location, but increase their firing as the stimulus moves toward the monkey. Others, on the contrary, are inhibited at the presentation of the graspable objects, but start to discharge as soon as an object is brought close to the monkey. Summing up: there are several facilitation/inhibition combinations that characterize different F6 neurons. What appears to be constant is the modulation of the neuron discharge according to whether an object can or cannot be grasped (see Rizzolatti et al. 1998).
Recent data showed that F6 plays an important role in the early stages of motor learning, precisely when the cognitive aspect of the procedure to be learned is acquired. This conclusion is based on the behavior of single neurons and on inactivation experiments showing that the number of errors for learning new sequences increases after muscimol injections of this area. In contrast, the performance of learned sequences is not affected by pre-SMA inactivation (Hikosaka et al. 1999).
The data reviewed above suggest that F6 plays a double role in motor control. A first role consists in controlling the posterior premotor areas. Normally, even when the posterior areas are activated, movement does not start. Only when external contingencies and motivational factors allow it, F6 renders the movement onset possible. A second possible role of F6 is more cognitive. It consists in the control of visuomotor associations underlying the declarative phase of motor learning.
4. The Premotor Cortex In Humans
Data from brain imaging studies indicate that the organization pattern of the AFC in humans is similar to that of non-human primates. As in monkeys, there is a multiplicity of premotor areas in humans. Some of them become active during the execution of simple movements (Fink et al. 1997); others require more complex tasks.
The distinction between premotor areas that elaborate sensory information for action (parieto-dependent areas) and areas that control action execution (fronto-dependent areas) appears to hold true also for humans. For example, recent data (Binkofski et al. 1999) showed that in humans there is a specific circuit for hand-object interactions. As in the monkeys, this circuit includes a parietal area—the probable homologue of monkey area AIP—and a ventral premotor area, area 44, the probable homologue of monkey F5. Conversely, data on the pre-SMA (F6) indicate that this premotor area is involved in humans, as it is in the monkey, in complex actions, rather than in the execution of simple movements (Picard and Strick 1996, Hikosaka et al. 1999).
Bibliography:
- Binkofski F, Buccino G, Posse S, Seitz R J, Rizzolatti G, Freund H J 1999 A fronto-parietal circuit for object manipulation in man: Evidence from an fMRI-study. European Journal of Neuroscience 11: 3276–86
- Fink G R, Frackowiak R S J, Pietrzyk U, Passingham R E 1997 Multiple nonprimary motor areas in the human cortex. Journal of Neurophysiology 77: 2164–74
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G 1996 Coding of peripersonal space in inferior premotor cortex (area F4). Journal of Neurophysiology 76: 141–57
- Graziano M S A, Yap G S, Gross C G 1994 Coding of visual space by premotor neurons. Science 266: 1054–7
- He S Q, Dum R P, Strick P L 1993 Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the lateral surface of the hemisphere. Journal of Neuroscience 13: 952–80
- Hikosaka O, Sakai K, Nakahara H, Lu X, Miyachi S, Nakamura K, Rand M K 1999 Neural mechanisms for learning of sequential procedures. In: Gazzaniga M S (ed.) The New Cognitive Neurosciences. MIT Press, Cambridge, MA, pp. 553–72
- Jeannerod M, Arbib M A, Rizzolatti G, Sakata H 1995 Grasping objects: the cortical mechanisms of visuomotor transformation. Trends in Neurosciences 18: 314–20
- Luppino G, Matelli M, Camarda R, Gallese V, Rizzolatti G 1991 Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study. Journal of Comparative Neurology 311: 463–82
- Passingham R E 1993 The Frontal Lobe and Voluntary Action. Oxford University Press, Oxford, UK
- Picard N, Strick P L 1996 Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex 6: 342–53
- Porter R, Lemon R 1993 Corticospinal Function and Voluntary Movement. Clarendon Press, Oxford, UK
- Rizzolatti G, Fogassi L, Gallese V 1999 Cortical mechanisms subserving object grasping and action recognition: A new view on the cortical motor functions. In: Gazzaniga M S (ed.) The New Cognitive Neurosciences. MIT Press, Cambridge, MA, pp. 539–52
- Rizzolatti G, Luppino G, Matelli M 1998 The organization of the cortical motor system: New concepts. Electroencephalography-and-clinical-neurophysiology 106: 283–96
- Sakata H, Taira M, Murata A, Mine S 1995 Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cerebral Cortex 5: 429–38
- Tanji J 1994 The supplementary motor area in the cerebral cortex. Neuroscience Research 19: 251–68
- Weinrich M, Wise S P, Mauritz K M 1984 A neurophysiological study of the premotor cortex in the rhesus monkey. Brain 107: 385–414




