View sample lyme disease research paper. Browse research paper examples for more inspiration. If you need a health research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Introduction
Lyme disease (LD), a multisystem zoonotic illness caused by the spirochete Borrelia burgdorferi, is now recognized as the most common vectorborne illness in the United States and Europe, and the number of cases per year continues to increase in much of the northern hemisphere. Clinical manifestations are protean, often involving the skin, joints, central nervous system (CNS), and heart.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Borrelia burgdorferi sensu lato (‘in the broad sense’) can be further divided into three closely related genospecies based on DNA relatedness: (1) Borrelia burgdorferi sensu stricto (‘in the strict sense’), herein referred to as B. burgdorferi, the causative agent of Lyme disease in the United States, Asia, and Europe, (2) Borrelia afzelii, and (3) Borrelia garinii, two additional agents of LD in Asia and Europe, but not yet isolated in the United States. At least 37 additional Borrelia species have been identified, many of which are agents of relapsing fever; two species, B. valaisiana and B. lusitaniae, have been detected in skin biopsies in patients with skin findings but their role in the pathogenesis remains unclear. Infection with different B. burgdorferi sensu lato genospecies results in varying degrees of pathogenicity, probably due to different organotropism, and therefore distinct disease patterns.
Lyme disease was first recognized as a clinical syndrome in Lyme, Connecticut in 1975 and described in 1977 by Steere et al. as a cohort of 27 children and 8 adults in a tight geographic cluster with oligoarticular arthritis seen in a seasonal distribution, 25% of which developed the typical erythema migrans (EM) rash (Steere et al., 1977). Similar dermatologic symptoms were previously described by Afzelius from the bite of the sheep tick, Ixodes ricinus, in Europe, as a rash associated with multifocal neurologic disease termed ‘tickborne meningopolyneuritis’; this was found to be penicillin-responsive and given the eponym ‘Bannwarth syndrome.’ Dr. Rudolph Scrimenti reported the first description of an erythema chronicum migrans (ECM) in the United States in 1970, in a north-central Wisconsin physician bitten by a tick while grouse hunting; he was successfully treated with penicillin G benzathine (Scrimenti, 1970).
The causative spirochete was initially identified in ticks captured on Long Island, New York and grown from patient samples from New York, Connecticut, and other endemic areas. It was named Borrelia burgdoferi after Willy Burgdorfer at The Rocky Mountain Laboratories in Hamilton, Montana, who was an expert in tickborne diseases and was the first to culture the Lyme disease agent (Burgdorfer et al., 1982).
Clinical Presentation Of Lyme Disease
Stages Of Lyme Disease
Lyme disease has been described as having three phases: early localized, disseminated, and late persistent infection; patients may seek treatment at any point. These phases fall along a continuum and may overlap. The host immune system may occasionally eradicate the infection, or the Borrelia may incite a localized reaction at the skin. This reaction, presenting as a red migrating rash in humans may go unnoticed, especially if on a hidden area, such as the base of the neck, scalp, or back. Borrelia travel from the site of inoculation and if not contained, may hematogenously disseminate to a variety of organ systems, including the CNS, musculoskeletal system, and/or the heart, with broad clinical implications.
Early Lyme Disease: Stage I
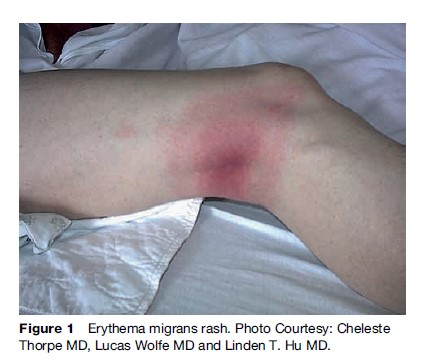
The first stage of Lyme disease, or early localized disease, manifests as an expanding rash and is the most easily recognized symptom when present. The classic erythema migrans (see Figure 1), whose name was shortened from erythema chronicum migrans due to a lack of chronicity, was first described in Europe by Lipshutz in 1913 and Afzelius in 1921 and described in the United States in Wisconsin and Connecticut in 1970 and 1975, respectively, as recounted by Campbell et al. in 1998. EM usually appears within a few days to a few weeks after prolonged feeding (>48 hours) by an infected tick. The rash may be asymptomatic or associated with vague, systemic symptoms including myalgias, arthralgias, headache, stiff neck, and fever in 80% of cases (Steere, 2001). Within 3–4 weeks, the EM usually resolves. Classically, the EM rash is sufficient for diagnosis, but for extracutaneous manifestations of Lyme disease, diagnostic testing in qualified laboratories may be necessary.
Early Disseminated Disease: Stage II
The second stage, early disseminated disease, may occur days to weeks after transmission of Borrelia, even in the absence of EM lesions. Hematogenous dissemination is a common and important feature of early Lyme disease in the United States. Symptoms may involve the skin, joints, muscles, peripheral or central nervous system; joint involvement occurs in 60% of untreated people. The central or peripheral nervous system may become involved at this stage, and present as meningitis, cranial nerve VII palsy (Bell’s palsy), or sharp or tingling pains in the extremities (neuropathy).
Late Lyme Disease: Stage III
The third stage, late Lyme disease, is a diagnosis of exclusion, and generally affects the central nervous system and joints. In Europe, it may involve the skin as well, particularly with B. afzelii infection. This stage is characterized by inflammatory arthritis of large joints, most commonly the knee. Cognitive dysfunction of late LD can appear as nonspecific depression, anxiety, fatigue, and sleep disturbances. In the United States, arthritis is the most common late sequela, but in Europe, radiculopathy, peripheral neuropathy, and late skin findings are associated with Borrelia garinii infection.
Manifestations Of Lyme Disease
Skin
Three cutaneous manifestations of LD involve the skin: the prototypical EM, which is translated as ‘red migrating rash,’ Borrelia lymphocytoma (BL), and acrodermatitis chronica atrophicans, more commonly seen in elderly patients.
After an incubation period of 7–14 days (range of 3–32 days), an expanding skin lesion may be seen at the site of the tick bite. The EM rash is typically present in 70–90% of the patients, but may be undetected or forgotten. The EM lesion is often found near the axilla, inguinal region, behind the knee, or at belt lines, as the tick has a predilection to attach at warm, moist areas. It may demonstrate central clearing, with a ‘bull’s-eye’ appearance; however, this is seen in only a minority of cases. The rash may progress at a rate of 1–2 cm per day. Not every annular rash is an erythema migrans. A local allergic reaction may occur at the site of tick attachment and can appear as an erythematous macular expanding lesion; differentiation is important. Hypersensitivity reactions may occur while the tick is still attached, but generally disappear by 24–48 hours and are less than 5 cm. Approximately 10% of patients with EM have multiple skin lesions, which represent dissemination of the organism. If EM goes unrecognized and untreated, patients may develop early or late disseminated disease.
Borrelia lymphotcytoma is a bluish-purple rash seen in early Lyme disease, which resembles dermal lymphoma. Histopathology demonstrates infiltration with polyclonal lymphocytes. BL is rare, with a prevalence of 0.6–1.3%.
During late infection, an erythematous, atrophic plaque (acrodermatitis chronica atrophicans) may develop, most commonly seen in Europe with B. afzelii infection. It presents as a localized edema and bluish-red discoloration on extensor surfaces of the hands, feet, elbows, and knees.
Musculoskeletal
In the United States, arthritis is the most common manifestation, usually with monoor oligoarticular infection affecting the knee, ankle, and elbow. Approximately 50% of untreated patients develop migratory polyarthitis, and 10% develop monoarthritis.
Sacroiliac or spinal involvement is not seen with Lyme disease and therefore favors an alternative diagnosis.
Neurological
The neurologic manifestations of Lyme disease were first described in France by Garin-Bujadoux in the 1920s, and later by Bannwarth in 1941, associating neurologic symptoms with the preceding EM rash. The first systematic study of chronic neuropathy in LD was reported by Hopf in 1966 in 201 German patients with acrodermatitis chronicum atrophicans and a concomitant 10-year asymmetric sensory neuropathy in 40% of the affected patients.
Early disseminated disease may produce neurological symptoms such as meningitis (inflammation of the meninges resulting in headaches, photophobia, or stiff neck), neuropathy (inflammation of peripheral nerves), or radiculoneuritis, resulting in pain or numbness or tingling sensation or weakness. Lyme meningitis is typically lymphocytic. Neuropathy may affect both cranial and peripheral nerves. In the United States, cranial neuropathy is the most common symptom of early LD, and typically affects cranial nerve (CN) VII, the facial nerve, presenting as a unilateral Bell’s palsy. Occasionally, Lyme disease may present as a bilateral Bell’s CN VII palsy, which helps differentiate it from idiopathic Bell’s palsy.
The most prominent clinical symptom is pain caused by inflammation of a spinal nerve, or radiculoneuritis, seen more often in Europe than in the United States, and more frequent and severe in adults than in children. Chronic Lyme neuropathy, as compared with acute neuropathy, is generally less severe with mild sensory and rare motor or autonomic involvement, and is associated with a reversible, mild axonal sensorimotor polyradiculoneuropathy or polyradiculopathy.
Cardiac
Lyme carditis occurs in less than 5% of patients, and presents with symptoms such as palpitations, lightheadedness, dizziness, or syncope. This correlates with the electrophysiologic manifests of atrioventricular conduction delay or block, most commonly Mobitz type 1. For patients with advanced heart block, a temporary pacer may be required. Conduction system involvement usually reverses rapidly with antibiotic treatment.
Post-Lyme Syndrome
There is no accurate definition of ‘post-Lyme disease syndrome.’ Controversy exists as to evidence of a chronic, persistent infection in the skin and other sites after antibiotic treatment. The results of a National Institutes of Allergy and Infectious Diseases-supported, randomized, placebo-controlled, double-blind study demonstrated a 90-day course of oral or intravenous antibiotics was no better than placebo in improving the persistent musculoskeletal, neurologic, or cognitive problems reported after a full course of treatment for a documented case of Lyme disease (Klempner et al., 2001). However, survival of the spirochete has been demonstrated in mice after antibiotic therapy, and B. burgdorferi-infected mice demonstrate reactivation of the spirochete after administration of an anti-TNF-a agent. For a recent evaluation of what has been termed chronic Lyme disease, see Feder et al. (2007).
Lyme Disease In Pregnancy
Case reports have suggested poor fetal outcome in women with Lyme disease during pregnancy; however, several large studies have failed to demonstrate definitive adverse neonatal outcomes with maternal Lyme disease. The evidence-based recommendations reviewing published literature do not suggest altering treatment, except for the avoidance of doxycycline in pregnant or breast-feeding women. An alternative agent such as amoxicillin should be used.
Coinfection
Coinfection with Babesia and/or Anaplasma may occur in patients with LD. Ixodes scapularis is a vector for Anaplasma phagocytophilum, the bacterial agent of human granulocytic anaplasmosis (HGA, formerly ehrlichiosis), and Babesia mictroti, the parasitic agent of babesiosis. A high index of suspicion is needed in endemic areas or in patients who present with a more severe initial presentation. Symptoms such as high-grade fever >48 hours, despite LD-appropriate antibiotics or unexplained leukopenia, thrombocytopenia, or anemia should trigger a search for coinfection. Coinfection should be considered with persistence of symptoms despite resolution of the EM.
Lyme Disease Diagnosis
Diagnostic Techniques
The diagnosis is based on clinical findings in conjunction with laboratory confirmation. The EM rash is a de facto diagnosis in conjunction with a reliable history of a tick bite from an endemic area. IgM develops approximately 2 weeks after the EM appears; IgG develops 4 weeks after the EM rash and may persist indefinitely despite appropriate treatment.
Four diagnostic laboratory approaches have been employed: microscopy, culture, protein detection, and nucleic acid detection. Antigen detection assays and microscopy are limited by the paucity of recoverable Borrelia in disseminated disease. Culture is limited by the slow growth of Borrelia in the lab, with a doubling time of 12–24 h. They require highly specialized liquid culture media and may yield a rate of recovery less than 5% in patients with EM. The media are difficult to make, expensive to purchase, and easily contaminated with other microbes. Recently, larger-volume (9 ml) samples of plasma have improved culture yields over serum and whole blood samples.
For a diagnostic workup, a two-step approach is most definitive. The first step uses the enzyme-linked immunosorbent assay (ELISA) as a sensitive screen. ELISA measures antibodies against the organism in patient serum. If positive or indeterminate, these screens should be followed by a confirmatory Western blot. Immunoblots, which measure antibodies against specific components of the organism, have increased the sensitivity and standardization of diagnosis. Detection rates for serum antibodies are 20–50% in stage I, 70–90% in stage II, and nearly 100% in stage III Lyme disease. Therefore, if ELISA and western blot are negative in late LD, alternative diagnoses should be considered. An ELISA using the C6 epitope of VlsE may prove beneficial in assessing treatment response in early LD.
Lyme Disease Treatment
U.S. Guidelines
Updated treatment guidelines from the Infectious Disease Society of America (IDSA) from 2006 replace the previous treatment guidelines published in 2000 (Wormser et al., 2006). The following is an abbreviated excerpt from this informative guideline, which should serve as a template for diagnostic and treatment decisions. Doxycycline or cefuroxime axetil is recommended for early Lyme disease without cardiac or neurologic involvement in those over 8 years of age. IV ceftriaxone is recommended for patients with later disease affecting the joints or neurologic disease, but it is critical to emphasize that more details are provided in the IDSA guidelines, which should be consulted by any physician for both diagnostic and therapeutic guidance.
Postexposure Prophylaxis
According to the most recent IDSA guidelines (Wormser, 2006), for the prevention of LD after a documented tick bite, routine prophylaxis is not recommended unless three conditions are met: (1) the tick can be identified as a nymphal or adult I. scapularis attached >36 h (on the basis of engorgement or certainty of the time of the tick bite), (2) prophylaxis with doxycycline, if not contraindicated, can be started within 72 h of tick removal, and (3) ecologic information consistent with a local rate of Borrelia tick infection over 20%. A single dose of doxycycline 200 mg may be offered to adult patients, and a dose of 4 mg/kg may be offered to children over 8 years.
Prophylaxis after the bite of an I. pacifus is not necessary due to the low carriage rate of Borrelia burgdorferi.
Borrelia Burgdorferi
Microbiology And Pathogenesis
Borrelia burgdorferi, the causative agent of Lyme disease, belongs to the phylum Spirochaetes. They are helical, irregularly coiled motile bacteria, 10–20 mm in length, 0.2–0.5 mm in width, containing 3–10 spirals. The ends are tapered, with 4–8 flagella at each end, inserted along the long axis. Strains were initially differentiated according to vector relationship, infectivity potential, less reliably cross-immunity, and now according to DNA sequences. The genome is small, containing approximately 1.5 megabases, which have been completely sequenced for one strain. The genome is composed of an approximately 910 kbp linear chromosome, and contains numerous circular and linear plasmids. It contains only a few biosynthetically active proteins, and is therefore dependent on its host for most nutrients.
Surface Proteins
The most well-known B. burgdorferi proteins are the ‘Osps’ (outer surface proteins), although most Osps currently have no known function. The most studied protein is OspA, which is expressed in unfed nymphal and adult ticks, and mediates adherence of spirochetes to the surface of tick midgut cells. OspA is also the major antigenic determinant in bacteria grown in vitro, constituted the human vaccine, and was the target of the original ELISA. OspC is a virulence factor correlated with spirochete transmission from tick to mammal. OspA and OspC are differentially expressed by the bacteria in the tick, responding to cues from the blood meal and changes in temperature, pH, and tick feeding as triggers. OspA is expressed in the unfed ticks, and OspC is expressed during transmission when OspA is down-regulated. OspC complexes with a tick salivary protein, Salp 15, which is thought to provide protection against the host immune system but is is not essential.
Borrelia species have developed several mechanisms of resistance to the host’s antimicrobial defenses. As few as 20 spirochetes are needed to establish infection in the host and disseminate. In addition, the B. burgdorferi life cycle requires persistent infection of immunocompetent wildlife. Complement activation destroys pathogens by coating them during opsonization, initiating a chain of events leading to eventual lysis. The spirochete may evade this arm of the host immune system by binding complement regulators, which inhibit microbial killing. Additionally, new proteins are expressed after the transmission of B. bugdorferi into the host. The variable major protein-like sequence, or VlsE, undergoes significant antigenic variation by recombining portions of multiple silent cassettes into the expression site, which also helps evade host immune responses.
Vector Of Lyme Disease
Ticks
Ticks are arthropods belonging to the class Arachnida, which includes spiders and scorpions. They are divided into two families: Ixodidae (hard ticks) and Argasidae (softbodied ticks). The head of the Ixodidae hard tick is visible from above and anterior in position, whereas in the Argasidae soft tick, the head is subterminal. Ticks are generally found in low-lying grass and shrubs, and are attracted to warmth and odor from byproducts of metabolism and respiration, such as carbon dioxide, ammonia, and lactic acid production. Ticks do not jump or fly; initial contact is made with the animal’s distal lower extremities. Ticks attach to a host while exhibiting questing behavior, in which contact with the host is made by the front legs while the back legs are attached to the vegetation. The tick climbs the host for up to 24 h to find a moist, dark, protected spot suitable for attachment. Frequent sites of attachment include the mouse’s ear, or at the level of the shoulder on a deer (hence the name I. scapularis). Species survival is predicated on the completion of a blood meal, and the tick is self-selected to attach in places where it will be less likely groomed or brushed off prior to repletion. The head and mouth of the tick are complex structures with many functional apparati assisting in piercing the skin, anchoring, and feeding. The barbed mouth part, the hypostome, assists in attachment during feeding. Ixodes tick saliva contains substances that seal the wound and anticoagulants and anti-inflammatory components that aid in engorgement. Borrelia burgdorferi takes advantage of at least two of the salivary components.
In the Northeastern and North Central United States, the black-legged or deer tick, Ixodes scapularis (formerly I. dammini) transmits LD. In Asia and Europe, I. ricinus (the sheep or castor bean tick) and I. persulcatus (the Taiga tick) are implicated in Borrelia carriage. The transmission cycle of B. burgdorferi to humans in the western United States involves a complex enzootic cycle. Ixodes pacificus functions as a tick vector to humans, but is not directly involved in maintenance. Dusky-footed woodrats (Neotoma fuscipes) and the non-I. ricinus complex tick, Ixodes neotomae, maintain this cycle. Ixodes pacificus ticks prefer to feed on a particular species of lizards that contain an antiborrelial factor in their blood, effectively eradicating the spirochete during feeding (Lane, 1988). I. pacificus will occasionally feed on other hosts, such as rodents and lagomorphs, allowing them to acquire and transmit infection.
The Tick Life Cycle
The black-legged or deer tick, Ixodes scapularis, has a 2-year, three-stage life cycle: larva, nymph, and adult. Ixodes scapularis is considered a three-host tick, in which feeding occurs once in each stage on each of three hosts. The adult female is dependent on a full blood meal for the proteins needed in egg production. Although some ticks are host-specific, most are opportunistic and feed on a variety of hosts, including mammals and birds.
Larvae hatch, rarely infected, from fertilized eggs, in late spring and feed once for two or more days. Transovarial transmission of Borrelia burgdorferi can occur, but less than 1% of field-collected larval ticks are infected, dismissing it as a significant contributor to the enzootic cycle. Borrelia transmission is transstadial (across stages; i.e., persisting from larva to nymph and from nymph to adult). In the northeastern and midwestern United States, larvae feed to repletion preferentially on the principal reservoir, the white-footed mouse, and occasionally on birds or other small mammals, such as chipmunks or voles. The white-footed field mouse provides a blood meal during which the larvae may obtain the Borrelia burgdorferi spirochete. The white-footed mouse infects 40–90% of the larval ticks that feed on it, according to a 1989 study by Mather published in the American Journal of Epidemiology. They drop to the ground and molt into nymphs and lie dormant over the winter period.

Most cases of LD are transmitted by the nymph (Figure 2a), which feeds actively from May through early July. If an infected nymph feeds from a noninfected mouse, the spirochete can be transmitted to the mouse, hence multiplying the opportunity to infect the next cohort of larvae. An infected mouse may provide blood meals with spirochetes to multiple feeding larvae or nymphs. Nymphs are not fastidious about which hosts on which they feed, and therefore account for the majority of human Lyme disease.
After this meal, the nymphs molt into adults. Adult females feed in April–June or October–November. Adult ticks (Figure 2b and 2c) may feed on humans and transmit the infection, but usually seek their blood meal from the white-tailed deer, a species that does not harbor Borrelia burgdorferi persistently, and is therefore considered an incompetent reservoir. The deer population serves to maintain the population of ticks. The white-tailed deer provides proteins that enable the tick to deposit a single large batch of 1000–18 000 eggs on the forest floor, propagating the next generation of vectors.
In specific geographic regions, certain birds may serve as primary hosts of vector ticks or as sources of spirochetal infection for uninfected vector ticks. In southern Connecticut, robins and wrens are reservoir-competent hosts of B. burgdorferi. However, in northwestern California, it has been demonstrated that birds contribute little to the enzootic nature of LD due to low tick parasitism and lack of detectable spirochete infection (Slowick and Lane, 2001).
Migrating birds are implicated in the spread of tick vectors and enzootic LD, especially with Borellia garinii species in Asia. In central Japan, nymphal ticks on 21 species of migratory birds carried Borellia garinii, not present in a survey of ticks in the same geographic area the previous season. Two of the 15 isolated Borrelia had rRNA sequences highly similar to B. garinii strains seen in Korea and Inner Mongolia. It was hypothesized the spirochete was introduced to a new geographical area by migratory birds from northeastern China via Korea (Ishigoro et al., 2000).
Lyme disease as a clinical entity has been known to occur occasionally in domesticated dogs. The two nonBorrelia vector species of ticks most commonly found on humans and companion animals in North America are the American dog tick (Dermacentor variabilis) and the groundhog tick (Ixodes cookei). They are frequent cause of unwarranted Lyme disease concern because neither of these species is an effective vector of Lyme borreliosis. Companion animals may bring ticks into the home or yard, but direct canine or feline-to-person transmission does not occur. Dogs may become infected, but are often asymptomatic. In dogs, antibiotic-resistant arthritis has not been described. However, rarely, canine Lyme nephritis has been associated with progressive and fatal disease (CDC, 2007).
All Borrelia species are vectorborne and require an arthropod able to acquire, maintain, and transmit infection. If the arthropod is not a competent vector, the spirochete will not survive, multiply, or propagate into a new host. Additionally, hard ticks, such as I. scapularis, must be attached for a significant period of time to permit transmission of pathogens. Des Vignes et al. demonstrated that Ixodes scapularis transmitted Borrelia burgdorferi only after 24, and optimally more than 48 h of attachment.
Epidemiology
Survey Techniques
Epidemiology of vectorborne disease mandates an understanding of the interrelationship between vector, host, etiologic agent, and environment. Three techniques have been employed to survey ticks. The CO2 trap is effective in sampling the Amblyomma (lone star tick) species, but this stationary strategy is less useful for ticks that exhibit questing behavior, such as I. ricinus. A ‘walking survey’ consists of collecting ticks on the surveyor after walking through vegetation. The third and most common technique consists of ‘dragging’ cloth behind or ‘flagging’ cloth in front of a researcher against vegetation to capture questing ticks.
Case Definition
The CDC surveillance case definition, updated in 1996, was developed for national reporting of LD and is not intended for clinical diagnosis. For surveillance purposes, a case of LD is defined as a physician-diagnosed erythema migrans >5 cm in diameter or at least one manifestation of late LD (i.e., musculoskeletal, cardiovascular, or neurologic) with laboratory confirmation. We discussed clinical findings and laboratory diagnosis of LD in earlier sections.
The Centers for Disease Control and Prevention began surveillance in 1982, and Lyme disease was made a notifiable condition in 1991 by the Council of State and Territorial Epidemiologists (CSTE). By 2000, the CDC recorded 17 730 cases, which increased in 2005 to a reported 23 305 cases. This yielded an average of 7.9 per 100 000 persons. In the 10 states where Lyme disease was most common, the average was 31.6 cases per 100 000 (CDC, 2007).
Distribution
North America
LD occurs primarily in three foci in the United States: in New England through the mid-Atlantic states (Maine to Virginia), in the Midwest (Wisconsin and Minnesota), and in the west (northern California and Oregon). The tick is found throughout the eastern United States to south central Texas; however, few Ixodes scapularis in that region harbor Borrelia burgdorferi.
Tick infection rates of Borrelia burgdorferi of 10–30% are found throughout parts of New England, parts of the mid-Atlantic states, and Wisconsin and Minnesota. Indirect epidemiological methods offer crude estimates based on passive reporting systems; active surveillance is employed in only a few endemic areas. This most likely represents an underestimation of true infection, as demonstrated by a deterministic mathematical model of LD incidence of and frequency of infected black-legged tick (Ixodes scapularis) bites in Westchester County, New York between 1991–94.
Cases are most frequently reported by the northeastern, mid-Atlantic, and north central states. LD has been reported in all states except Montana (through 2005); however, most sporadic cases are ‘imported.’ In 119 965 patients who reported at least one symptom between 1992–2004, erythema migrans was seen in 68% of the patients, arthritis in 33%, facial palsy in 8%, radiculopathy in 4%, and 1% each reported meningoencephalitis or heart block (sum is >100% due to multiple presenting symptoms reported). In 2005, Delaware and Connecticut reported the highest incidence (76.6 and 51.5 per 100 000, respectively), then New Jersey and Massachusetts (38.6 and 36.5 per 100 000), and Pennsylvania (34.5 per 100 000) (CDC, 2007).
Europe
In Europe, approaches to collecting data vary, which makes it difficult to compare and contrast incidence and prevalence. In the majority of countries, data are collected through diagnostic laboratories. Based on 2006 available data, the highest reported incidence is in Central Europe, with 206 cases per 100 000 persons in Slovenia and 135 per 100 000 in Austria. In southern Europe, such as Italy and Portugal, the incidence appears to be much lower, with an incidence of 1 per 100 000 (Smith et al., 2006).
The rising incidence of Lyme disease is multifactorial. Although deer are irrelevant as reservoirs of B. burgdorferi, they are principal maintenance hosts for adult blacklegged ticks, and the presence of deer appears to be mandatory for the establishment of I. scapularis in any area. The proliferation of the white-tailed deer was a major factor of endemic LD and linked to the spread of the I. scapularis ticks (Steere et al., 2001). Increased tick abundance in conjunction with increased human contact with wildlife and their habitats during outdoor recreational activities facilitates transmission.
Prevention
The best method for preventing infection with B. burgdorferi and other tickborne illnesses is via primary prevention by exposure reduction. As with all primary prevention techniques, counseling and education programs are important, and should be targeted to those in endemic regions who spend a lot of time outdoors. Parents should be instructed on tick prevention strategies and full-body tick check after time outdoors. Landscaping techniques such as placing wood tiles or chips around the backyard’s outer border may prevent tick encroachment into the yard. Yearly application of acaracides (a pesticide that kills ticks and mites as well as insects) to dense vegetations may help reduce tick exposure as well.
If exposure is unavoidable, recommendations to reduce the risk of infection include the use of personal protective equipment, such as long-sleeved shirts buttoned at the cuffs and tucked into pants, along with long pants tucked into socks, in combination with tick repellents containing DEET (N,N,-diethyl-m-toluamide). Long clothing is generally not favored during warmer weather; therefore, daily tick checks are recommended for prompt tick removal prior to transmission. Ticks should be removed with tweezers directed at the mouth parts. Pinching with fingers, suffocation with oil or petrolatum, and scraping or burning should be avoided due to increased risk of transmission by increasing salivary secretions.
Permethrin, a synthetic pyrethroid acaricide, can be applied to clothing and when used in combination with DEET is effective in preventing a tick bite. This combination in conjunction with appropriate battle dress uniform is considered by the U.S. Department of Defense as the Insect Repellent System. When possible, lightcolored clothing allows for easier tick detection.
Vaccines
Two human vaccines were developed, and in 1998 the antigenic OspA protein with an aluminum hydroxide adjuvant, Lymerix, was approved, but pulled during postmarket phase IV trials in 2002 due to low sales and questionable reports of postvaccine arthritis and neurologic changes. There is currently no human vaccine on the market, but second-generation vaccines may be pursued using customized OspA or multiple protein combinations.
The Vaccine Adverse Events Reporting System (VAERS), established in 1990 as a cooperative program between the CDC and the Food and Drug Administration, requires reporting of all adverse events temporally related to vaccine administration. This provides post marketing vaccine safety surveillance for all U.S. licensed vaccines. By 2001, with over 1.4 million Lyme vaccine doses administered, 905 reports of mild, self-limited reactions and 59 reports of arthritis were reported to VAERS. The incidence pattern occurred at the same rate as background, and in unvaccinated individuals, and the FDA found no conclusive evidence that the Lyme vaccine was related to the reported adverse events. A 4-year postlicense vaccine safety and efficacy case control study was planned; however, vaccine sales fell dramatically and GlaxoSmithKline withdrew Lymerix from the market, citing poor sales.
A second approach in eradicating Lyme disease is to target the principal reservoir, the white-footed mouse. In a proof-of-principle study, over 1000 mice located in a southern Connecticut study site were randomized to receive either OspA recombinant vaccine or placebo; the study found approximately 16% reduction of nymphal infection prevalence in the vaccinated group (Tsao et al., 2004).
Conclusion
Lyme disease has emerged as a significant cause of morbidity since its characterization in the mid-late 1970s. It illustrates the interplay of ecology with dynamic interspecies relationships in the establishment and amplification of a vectorborne infectious disease. Attempt at control should be multifaceted, with primary prevention at the outset, aimed at the vector, reservoir, etiologic agent, and, when possible, the host.
Bibliography:
- Burgdorfer W, Barbour A, Hayes SF, et al. (1982) Lyme disease: A tick-borne spirochetosis? Science 216: 1317–1319.
- Feder HM, Johnson BJB, O’Connel S, et al. (2007) A critical appraisal of ‘Chronic Lyme Disease’. New England Journal of Medicine 357: 1422–1430.
- Ishigoro F, Takada N, Masuzawa T, and Fukui T (2000) Prevalence of Lyme disease Borrelia spp. in ticks from migratory birds on the Japanese mainland. Applied and Environmental Microbiology 66: 982–986.
- Klempner MS, Hu LT, Evans J, et al. (2001) Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. New England Journal of Medicine 345: 85–92.
- Lane RS (1996) Risk of human exposure to vector ticks in heavily used recreational area in Northern California. American Journal of Tropical Medicine and Hygeine 55: 165–173.
- Scrimenti RJ (1970) Erythema chronica migrans. Archives of Dermatology 102: 104–105.
- Slowik TJ and Lane RS (2001) Birds and their ticks in northwestern California: Minimal contribution to Borrelia Burgdorferi enzootiology. Journal of Parasitology 87: 755–761.
- Smith R, Takkinen J, and Editorial Team (2006) Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Eurosurveillance 11(6): E060622.1. www.eurosurveillance.org (accessed November 2007).
- Steere AC (2001) Lyme disease. New England Journal of Medicine 345: 115–125.
- Steere AC, Malawista SE, Snydman DR, et al. (1977) Lyme arthritis: An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis and Rheumatism 20: 7–17.
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, and Barbour AG (2004) An ecological approach to preventing human infection: Vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proceedings of the National Academy of Sciences 101: 18159–18164.
- Wormser GP (2006) Hematogenous dissemination in early Lyme disease. Wiener Klinische Wochenschrift 118: 634–637.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. (2006) The clinical assessment, treatment and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Disease Society of America. Clinical Infectious Diseases 43: 1089–1134.
- Aguero-Rosenfeld M, Wang G, Schwartz I, and Wormser G (2005) Diagnosis of Lyme borreliosis. Clinical Microbiology Reviews 18: 484–509.
- Barbour AG and Hayes SF (1998) Biology of Borrelia species. Microbiological Reviews 50: 381–400.
- Brown RN and Lane RS (1992) Lyme disease in California: A novel enzootic transmission cycle of Borrelia burgdorferi. Science 256: 1439–1442.
- CDC (2007) Learn About Lyme Disease. www.cdc.gov/ncidod/dvbid/lyme (accessed November 2007).
- Dennis DT (1998) Epidemiology, ecology and prevention of Lyme disease. In: Rahn DW and Evans JE (eds.) Lyme Disease, pp. 7–48. Philadelphia, PA: American College of Physicians.
- Nigrovic LE and Thompson KM (2006) Editorial review: The Lyme vaccine: A cautionary tale. Epidemiology and Infection 135: 1–8.
- Piesman J, Donahue JG, Mather TN, et al. (1986) Transovarially acquired Lyme disease spirochetes (Borrelia burgdorferi) in fieldcollected larval Ixodes dammini (Acari: Ixodidae). Journal of Medical Entomology 23: 219.
- Ramamoorthi N, Narasimhan S, Pal U, et al. (2005) The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436: 573–577.
- Schwan TG, Peisman J, Golde WT, Dolan MC, and Rosa PA (1995) Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proceedings of the National Academy of Sciences 92: 2909–2913.
- Wilske B (2005) Epidemiology and diagnosis of Lyme borreliosis. Annals of Medicine 37: 568–579.
- Zhang JR and Norris SJ (1998) Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversation. Infection and Immunity 66: 3698–3704.




