Sample Neuromuscular System Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
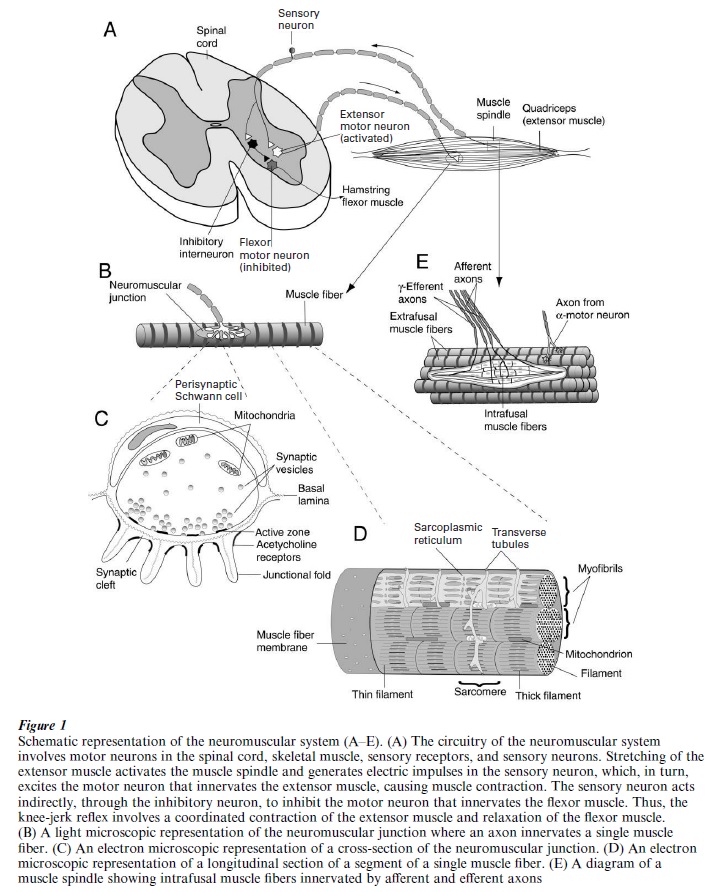
The neuromuscular system links the central nervous system to the peripheral nervous system and is composed of a neural circuit including motor neurons in the spinal cord, sensory neurons in the dorsal root ganglion, and skeletal muscle fibers (Fig. 1A). The neuromuscular system is essential to movements of the body, the control of posture and breathing. Electrical impulses triggered from the motor neuron propagate along the motor axon and result in the release of the neurotransmitter, acetylcholine, from the motor nerve terminal at the synaptic contact, called the neuromuscular junction (Fig. 1B, C). Acetylcholine molecules diffuse across a small gap and bind acetylcholine receptors on the muscle fiber. The binding results in the opening of ion channels, thereby changing the muscle membrane potential. The membrane depolarization triggers a release of internal calcium ions that leads to a cascade of events resulting in muscle contraction (Fig. 1D). The muscle spindles and Golgi tendon organs are sensory organs in the skeletal muscle that detect changes in the muscle fiber length and tension (Fig. 1E). The information is conveyed by electrical impulses propagating along sensory fibers of dorsal root ganglion neurons, which, in turn, innervate motor neurons in the spinal cord. The activation of motor neurons generates electrical impulses propagating along the motor axon and thereby completing the circuit of the knee-jerk reflex in the neuromuscular system (Fig. 1A). Motor neurons in the spinal cord also receive synaptic inputs from the brain to perform voluntary movements.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. The Neuromuscular Junction (NMJ)
The NMJ (Fig. 1B) plays an indispensable role in transmitting information from the motor neuron to the skeletal muscle (Salpeter 1987). The NMJ, also called the endplate, is a specialized region on the muscle surface, on which the motor nerve terminal makes a synaptic contact with a gap of approximately 50 nm, called the synaptic cleft (Fig. 1C), between these two cellular elements. Besides the nerve terminal and the muscle fiber, the third cellular element at the NMJ are glial cells, called perisynaptic Schwann cells (also known as terminal Schwann cells, Fig. 1C), which cap the nerve terminal. At the adult vertebrate NMJ, each skeletal muscle fiber is, in general, innervated by a single motor axon. Due to its relative simplicity and accessibility, the NMJ has served as a model system for investigating the structure, function, and development of chemical synapses (Sanes and Lichtman 1999).
1.1 The Motor Nerve Terminal
Although the motor axon is wrapped by myelin sheath formed by Schwann cells, the terminal region is only capped by perisynaptic Schwann cells without myelin formation (Fig. 1B). In mammalian muscles, the nerve terminal branches into multiple grape-like varicosities, called synaptic boutons, which occupy a circumscribed region, approximately 50 µm in diameter, or less than 0.1 percent of the muscle surface area. At the frog NMJ, in contrast, the end of the motor fiber branches out into several elongated branches, each of which can extend several hundred µm long. The diameter of individual boutons or terminal branches is approximately 1–2 µm. Electron microscopy reveals mitochondria and conspicuous synaptic vesicles (Fig. 1C), approximately 20–50 nm in diameter, inside the nerve terminal. Synaptic vesicles tend to cluster around a thickened membrane specialization called the active zone (Fig. 1C) on the side of the nerve terminal facing the muscle fiber. The active zone is believed to be the site of transmitter release where the opening of synaptic vesicles during intense stimulation can be observed with electron microscopy (Heuser and Reese 1977). Freeze-fracture electron microscopy shows that the active zone is composed of two double-rows of large intramembrane particles (~ 10 nm in diameter), located precisely opposed to the opening of junctional folds where acetylcholine receptors are clustered on the muscle surface (Fig. 1C). These large intramembrane particles are thought to correspond to the location of voltage-gated calcium channels that allow Ca2+ entry to trigger exocytosis of synaptic vesicles and release of the transmitter. The precise alignment of voltage-gated calcium channels at the active zone and acetylcholine receptors at the junctional fold plays a strategic role in the speedy action of the transmitter-receptor interaction. The length of the active zone correlates with the efficacy of synaptic transmission.
1.2 Transmitter Release
The neurotransmitter, acetylcholine , is stored in synaptic vesicles. There are about 106 synaptic vesicles inside the nerve terminal at the frog NMJ. A variety of proteins on the synaptic vesicle and on the nerve terminal membrane govern the process of transmitter release. Following nerve stimulation, the conductance of voltage-gated calcium channels increases and the intracellular calcium concentration rises transiently. It is believed that some synaptic vesicle proteins serve as Ca2+ sensors and trigger the exocytosis of synaptic vesicles. Exocytosis involves binding between some integral proteins in the vesicle membrane, called vSNARES, and some in the nerve terminal membrane, called t-SNARES (Sudhof 1995). Their binding results in fusion between the synaptic vesicle membrane and the nerve terminal membrane at the active zone. Some of these SNARES are the targets of neurotoxins. For example, botulinum toxins cleave t-SNARES, causing transmitter release blockade and muscle paralysis.
The transmitter can be released spontaneously or evoked by nerve stimulation (Katz 1966). The spontaneous exocytosis of synaptic vesicles produces miniature endplate potentials (mepps), which occur randomly with an average frequency of about 1 Hz and amplitude of 0.5 mV. It is believed that the mepp is caused by the release of approximately 10,000 acetylcholine molecules stored in one synaptic vesicle. The nerve stimulation evokes nearly synchronous release of many synaptic vesicles and produces endplate potentials (epps). The amplitude of epps is dependent on Ca2+ influx in a non-linear manner such that a two-
fold increase in Ca2+ influx can increase the evoked transmitter release up to 16-fold. In a normal saline solution, each nerve impulse can trigger a synchronous release of about 200 synaptic vesicles and result in an epp that is large enough to trigger an action potential and muscle contraction. Following transmitter release, synaptic vesicle membranes are retrieved through a process called endocytosis that involves membrane invagination and formation of an endosome, a membrane compartment inside the nerve terminal (Heuser and Reese 1977). New synaptic vesicles bud off the endosome, refilled with acetylcholine. The local re- cycling of synaptic vesicles takes about 30 sec to 1 min to complete and provides the nerve terminal with a continual supply of synaptic vesicles even under intense stimulation.
1.3 Acetylcholine Receptors At The NMJ
The acetylcholine receptors (Unwin 1993) in skeletal muscles belong to the nicotinic type because nicotine derived from the tobacco plant activates these receptors. This is in contrast to the muscarinic type of acetylcholine receptors found in the brain that are activated by muscarine derived from a poisonous red mushroom. Both mepps and epps are caused by the binding of acetylcholine to nicotinic acetylcholine receptors on the muscle fiber. The acetylcholine receptors are densely packed, approximately 10,000 per µm2, at the endplate region, compared with fewer than 10 per µm2 on the rest of the muscle membrane.Each acetylcholine receptor at the adult NMJ consists of five subunits: two α-subunits, one β-, one δ-, andone ε-subunit. All of these five subunits contribute toform the receptor channel. As a consequence of two molecules of acetylcholine binding to the α-subunits,the receptor channel opens and allows ion flow thatresults in depolarization of the muscle membrane.
Prior to innervation, acetylcholine receptors are distributed throughout the muscle membrane at a density of approximately 1000 per µm2 during development. A protein, called agrin, triggers the aggregation of acetylcholine receptors at the developing NMJ (McMahan 1990). Agrin is synthesized by the motor neuron, released from the motor nerve terminal, and incorporated into the extracellular matrix (see Section 1.5) in the synaptic cleft. Agrin interacts with several molecules, including dystroglycan and a muscle-specific tyrosine kinase, located on the muscle membrane, and stimulates the aggregation of acetyl- choline receptors, which are also associated with a cytoplasmic protein termed rapsyn. Motor neurons also enhance the synthesis of acetylcholine receptors through a molecule named neuregulin, which interacts with a set of receptor tyrosine kinases in the muscle membrane (Fischbach and Rosen 1997). After nerve injury, the density of acetylcholine receptors in the muscle membrane outside the NMJ increases markedly, a phenomenon termed denervation super-sensitivity. Direct muscle stimulation can prevent or reverse denervation supersensitivity. Thus, the motor nerve plays a role not only in the aggregation and synthesis of acetylcholine receptors, mediated by agrin and neuregulin, respectively, but also in the nerve activity-mediated suppression of acetylcholine receptor expression in the extrajunctional region of the muscle (Sanes and Lichtman 1999). As a result of these effects of the motor nerve terminal, acetylcholine receptors are concentrated at the endplate region where they can readily bind acetylcholine molecules released from the terminal.
1.4 The Perisynaptic Schwann Cell (PSC)
The PSC is a glial cell that overlays the nerve terminal (Fig. 1C). The role of the PSC had not been studied extensively until the 1990s. Neural activity can cause an elevation of internal Ca2+ concentration and regulate gene expression in the PSC. Conversely, activation of the PSC can regulate the amount of transmitter released. Thus, modulation of the efficacy of neuromuscular transmission likely involves reciprocal interactions between the nerve terminal and the PSC. Complex neuron-glia interactions have also been found at the NMJ after nerve injury. PSC processes sprout profusely beyond the original endplate region following nerve injury. As reinnervation progresses, nerve terminals closely follow the preceding PSC sprouts. It is believed that these PSC sprouts play a role in guiding and leading nerve regeneration and sprouting after nerve injury (Son et al. 1996). Schwann cells express many trophic factors that may be important for neuronal survival and axonal growth. Thus, it is likely that the PSC plays a role in the modulation of synaptic function, as well as in the formation and maintenance of synaptic connections.
1.5 The Extracellular Matrix At The NMJ
Besides agrin, there are many important molecules that reside in the synaptic cleft, which is filled with amorphous material called the extracellular matrix or basal lamina (Fig. 1C). Some of these molecules are large glycoproteins, such as laminins, which may promote nerve growth, adhesion between nerve and muscle, or differentiation of the nerve terminal (Sanes and Lichtman 1999). An important enzyme located in the extracellular matrix is acetylcholinesterase, which hydrolyzes acetylcholine molecules that do not bind to acetylcholine receptors. The prompt removal of acetylcholine from the synaptic cleft by acetylcholinesterase prevents desensitization of acetylcholine receptors caused by the continuous presence of acetylcholine. Thus, acetylcholinesterase plays an essential role in ensuring proper transmission between nerve and muscle (Salpeter 1987).
2. Skeletal Muscle Contraction
A typical mammalian skeletal muscle is composed of hundreds of individual muscle fibers that are approximately 50–100 µm in diameter and 2–6 cm in length. Each muscle fiber is a multi-nucleated muscle cell and is made up of myofibrils, the contractile elements that carry out the work of contraction. Each myofibril consists of a chain of repeated contractile units called sarcomeres (Fig. 1D). In each sarcomere, thin and thick filaments run parallel to one another with varying degrees of overlap depending on the contractile state. The thin filament is made primarily of actin molecules and the thick filament of myosin molecules. According to the sliding filament model, Ca2+ triggers interactions between actin and myosin molecules and results in the sliding of thin and thick filaments past one another, thereby causing muscle contraction. Following nerve stimulation and synaptic transmission, electrical impulses generated in the muscle fiber spread rapidly into membrane invaginations, called transverse tubules, which extend into sarcomeres and form contacts with sarcoplasmic reticulum, a modified en doplasmic reticulum that sequesters and releases Ca2+. It is thought that depolarization of the transverse tubule results in the opening of Ca2+ release channels located on the sarcoplasmic reticulum membrane. As a result, Ca2+ is released from the sarcoplasmic reticulum into the cytosol and initiates muscle contraction. Muscle relaxation occurs when Ca2+ ions are pumped back into the sarcoplasmic reticulum. Thus, Ca2+ plays important roles not only in transmitter release but also in excitation-contraction coupling in the neuromuscular system (Engel and Franzini-Armstrong 1994).
3. Sensory And Motor Controls Of The Neuromuscular System
The neuromuscular system is under both voluntary controls and reflex controls. The motor neurons in the spinal cord receive synaptic connections from other neurons in the central nervous system for voluntary control of body movements. The spinal motor neurons are also involved in reflex controls to maintain proper posture and to prevent muscle injury from excessive contraction. Two sensory receptors, the muscle spindle and the Golgi tendon organ, provide information on muscle length and tension, respectively, to motor neurons for reflex controls (Engel and FranziniArmstrong 1994). The message may excite or inhibit motor neurons depending on the extent of the stretch or the tension, and on the type of muscle involved.
Muscle spindles are embedded in, and run parallel to the long axis of, the skeletal muscle (Fig. 1E). These spindle-shaped sensory organs consist of a group of intrafusal muscle fibers, which are modified muscle fibers lacking myofibrils in the central region. Thus, the centers of the intrafusal fibers are not contractile, in contrast to the regular skeletal muscle fibers, which are also called the extrafusal fibers. The ends of the intrafusal fibers are innervated by nerve terminals of small-diameter motor neurons, called γ motor neurons. This is in contrast to the regular extrafusal fibers, which are innervated by the large α motor neurons. Activation of γ motor neurons causes shortening of the polar regions of the intrafusal fiber and thus enhances the sensitivity of the muscle spindle to stretch. In addition to motor innervation, intrafusal fibers are also wrapped by nerve endings of sensory (afferent) fibers. The cell bodies of sensory fibers reside in the dorsal root ganglion.
The role of the muscle spindle is exemplified in the knee-jerk reflex (Kandel et al. 2000). When the quadriceps (extensor) muscle in the thigh is stretched, for example, by tapping the kneecap with a small rubber hammer, the stretching activates the sensory nerve endings in the muscle spindle. This stimulation generates receptor potentials and activates the sensory neuron, which propagates electrical impulses towards the spinal cord. The sensory axon makes excitatory synapses directly onto the α motor neuron that innervates the extrafusal fibers of the quadriceps muscle (Fig. 1A). Thus, the stretch of the muscle spindle in the quadriceps muscle activates the α motor neuron, which, in turn, causes the quadriceps to contract. As a result, the lower leg swings forward. This simple reflex, which involves only one synapse between the sensory neuron and the motor neuron, is called the monosynaptic stretch reflex. In addition to the monosynaptic pathway, the sensory neuron acts indirectly, through an inhibitory neuron, to inhibit the motor neuron that innervates the opposing hamstring (flexor) muscle (Fig. 1A). The knee-jerk reflex involves a simultaneous activation of both pathways and results in a coordinated contraction of the extensor quadriceps muscle and relaxation of the flexor hamstring muscle.
Whereas muscle spindles are most sensitive to stretch, Golgi tendon organs are most sensitive to tension. Golgi tendon organs reside at the junction between the tendons and muscle fibers, and are thus arranged in series to the skeletal muscle. Each tendon organ, about 1 mm long and 0.1 mm in diameter, consists of unmyelinated nerve endings that intertwine among collagen fibers encapsulated by connective tissue. Excessive muscle contraction activates Golgi tendon organs, which inhibit α motor neurons that innervate the activated muscle. Thus, the Golgi tendon organ provides negative-feedback control, which prevents extreme muscle contraction that might damage the muscle fibers.
4. Toxins That Affect The Neuromuscular System
Because of the importance of the neuromuscular system to vital functions, such as respiration and escape from predators, it is not surprising that nature has evolved animals and plants that produce a variety of poisons to block neuromuscular transmission and thereby paralyze their prey. For example, αbungarotoxin, a peptide found in the venom of the banded krait Bungarus multicinctus, blocks neuromuscular transmission by irreversibly binding to nicotinic acetylcholine receptors (Chang 1979). Because of its irreversible binding, α-bungarotoxin has been a useful tool to study the function and distribution of acetylcholine receptors at the NMJ. Another acetylcholine receptor blocker is curare, a mixture of plant toxins used for poison darts by South American Indians. Other toxins block neuromuscular transmission by affecting transmitter release. For example, fish-hunting marine cone snails disable their prey by injecting an array of toxins, known as conotoxins, some of which block voltage-gated calcium channels (Olivera et al. 1994). The venom in the female black widow spider contains α-latrotoxin that causes a massive release of acetylcholine at the NMJ. Botulinum toxin and tetanus toxin from bacterial growth in infected food or tissues cleave some proteins associated with synaptic vesicle release and thus disrupt neuromuscular function. Another mode of interfering with neuromuscular transmission is to inactivate acetylcholinesterase. For example, many deadly nerve gases are inhibitors of acetyl-cholinesterase and cause paralysis of the respiratory muscle through the accumulation of acetylcholine molecules, thereby desensitizing and inactivating acetylcholine receptors.
5. Diseases Of The Neuromuscular System
Diseases of the neuromuscular system (Engel and Franzini-Armstrong 1994) include disorders of the NMJ, degeneration of motor neurons in the spinal cord, and muscular dystrophies. The most common disorder affecting transmission at the NMJ is myasthenia gravis. This disease is characterized by weakness of limb muscles and cranial muscles. Myasthenia gravis is an autoimmune disease in which patients produce antibodies against their own nicotinic acetylcholine receptors at the NMJ. These antibodies inhibit neuromuscular function by reducing the number of functional receptors, or by interfering with the binding of acetylcholine to the receptors. Another neuromuscular disorder is the Lambert-Eaton myasthenia syndrome, also an autoimmune disease in which patients generate antibodies against voltage-gated calcium channels at the motor nerve terminal, thereby disrupting transmitter release. The best-known motor neuron disorder is amyotrophic lateral sclerosis, also known as Lou Gehrig disease. It is not known why only motor neurons, but not sensory neurons, die in this devastating disease. As a result of the motor neuron degeneration, patients experience weakness of the arms and legs, and eventually suffer fatal failure of respiratory muscles. Muscular dystrophies, characterized by muscle weakness and loss of muscle fibers, are myopathic diseases. One severe form of these diseases is Duchenne muscular dystrophy, which is an X-linked inherited disease causing symptoms only in boys. The disease is attributed to a deficiency in dystrophin, a large protein located on the inner surface of the muscle membrane. Dystrophin links actin molecules and an array of glycoproteins that form a complex near the muscle membrane. Absence of dystrophin or some of these dystrophin-associated glycoproteins causes a disruption of the muscle membrane and thereby muscle degeneration.
Bibliography:
- Chang C C 1979 The action of snake venoms on nerve and muscle. In: Lee C Y (ed.) Snake Venoms: Handbook Experimental Pharmacology, Vol. 52. Springer, Berlin, pp. 309–76
- Engel A G, Franzini-Armstrong C 1994 Myology. McGrawHill, New York
- Fischbach G D, Rosen K M 1997 ARIA: a neuromuscular junction neuregulin. Annual Review of Neuroscience 20: 429–58
- Heuser J E, Reese T S 1977 Structure of the synapse. In: Kandel E R (ed.) Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts, Sect. 1, The Nervous System. Vol. 1, Cellular Biology of Neurons, Part 1, American Physiological Society, Bethesda, MD, pp. 261–94
- Kandel E R, Schwartz J H, Jessell T M 2000 Principles of Neural Science, 4th edn. McGraw-Hill, New York
- Katz B 1966 Nerve, Muscle and Synapse. McGraw-Hill, New York
- McMahan U J 1990 The agrin hypothesis. Cold Spring Harbor Symposium Quantitati e Biology 5: 407–18
- Olivera B M, Miljanich G P, Ramachandran J, Adams M E 1994 Calcium channel diversity and neurotransmitter release: The ω-conotoxins and ω-agatoxins. Annual Review of Biochemistry 63: 823–67
- Salpeter M M 1987 The Vertebrate Neuromuscular Junction. Alan R. Liss, Inc, New York
- Sanes J R, Lichtman J W 1999 Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience 22: 389–442
- Son Y J, Trachtenberg J T, Thompson W J 1996 Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends in Neurosciences 19: 280–5
- Sudhof T C 1995 The synaptic vesicle cycle: a cascade of proteinprotein interactions. Nature 375: 645–53
- Unwin N 1993 Neurotransmitter action: opening of ligand gated ion channels. Cell 72(Suppl.): 31–41




