Sample Genetics of Longevity Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Virtually every aspect of a person’s life and well being and, in turn, their survival and longevity is influenced by genes—early development and maturation, metabolic rate and body regulation, resistance and vulnerability to disease, and personality and behavior. Inasmuch as the influence of genes is inextricably intertwined with longevity, it is virtually impossible to consider the broad determinants of longevity without a basic understanding of the underlying genetics. Many of the questions asked by lay persons concerned with longevity are inherently genetic: do long-lived parents have long-lived children? Will it ever be possible to genetically alter the rate of aging? How do genes influence disease susceptibility?
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Conceptual Framework
1.1 Genetics
The influence of genes on the life span of organisms can be explained at several biological levels including: (a) a phylogenetic level where genes generate growth and form and thus determine the basic body plan, metabolic framework, and the broad details of a species’ life history. Thus from a phylogenetic perspective, insects are generally shorter lived than mammals, rodents are usually shorter lived than primates, and chimpanzees are generally shorter lived than humans, ceteris paribus; (b) a mechanistic level where genes regulate the basic mechanisms affecting the rate of aging and life span. For example, genes will influence the life span of individuals through their effects on oxidative damage or replicative senescence in cells; and (c) a disease vulnerability where genes determine the susceptibility of individuals to specific diseases. For example, an individual’s risk of contracting breast cancer or Huntington’s disease is linked to his or her genes.
1.2 Demographic
Longe ity is a shorthand term for a number of different actuarial constructs, the two most common of which are life expectancy which is an empirical concept referring to the average age of death in a cohort and life span (or maximum life span) which is a theoretical concept referring to the highest age attainable by any member of a population. Longevity is the actuarial outcome of individuals subject to the risk of death at each age expressed as age-specific mortality rate—the probability that an individual currently alive will die in the next time period (e.g., one year). Longevity can be altered by changing mortality in one of three ways, all of which are under genetic control: (a) shifting the age of maturity—this is one of the most common ways in which fruit fly longevity is increased in population genetics selection experiments; (b) reducing mortality over part of the life course—this can occur through disease prevention or cure and reductions in accidental deaths; and (c) reducing the actuarial rate of aging— this effectively reduces the rate of change (slope) of mortality with age.
2. Genetics of Longe ity in Model Systems
Genetic mechanisms that determine longevity and the rate of aging can be grouped into two categories: (a) private—those mechanisms that are idiosyncratic of certain lineages, populations, or species. Most mechanisms of aging associated with mutation accumulation are private mechanisms since the fate of these alleles is largely determined by genetic drift; and (b) public—mechanisms that are generally operational over a large diversity of organisms. By studying aging in different systems it is possible to begin to identify genes that appear in multiple species and thus likely control aging at the most fundamental level. In contrast, those genes that enhance longevity only in the species being studied may point toward genes that are less conserved and affect the mortality of smaller groups of related species.
2.1 Polygenic Approaches
The three main animal models used to study the polygenic (many genes) effects on longevity include:
(a) Mice. Studies on mouse strains congenic for the H-2 locus showed significant differences in longevity, that certain H-2 alleles were associated with increased life span, and that genes at the H-2 locus may affect the age of onset of reproductive cyclicity. Quantitative analysis suggests that immune responsiveness can be selected for in mice and that the mode of inheritance is polygenetic with 3–7 independent loci being involved in the response. The genetics of aging has also been studied through the use of senescence-accelerated mice which represents a polygenic progeroid model.
(b) Drosophila. Selection studies are polygenic by design—any number of genes which favor long life are retained in laboratory populations subjected to conditions favoring longevity such as allowing only the oldest individuals to breed. Selection studies on Drosophila have produced strains whose life spans differ by twofold. Associated with these life span differences include increased resistance to starvation, desiccation, and other environmental stresses and reduced ovary weight early in life.
(c) Nematodes. The primary approach for studying polygenic aspects of aging in the free-living soil nematode Caenorhabditis elegans has been quantitative trait loci (QTL) mapping. This technique is based on the concept that if one has a dense array of detectable genetic markers spread across each of the chromosomes, it is possible to make appropriate genetic crosses between nematode lines and determine which sets of markers are preferentially associated with the phenotype (i.e., longevity) of interest. The longevity genes thus lie somewhere between and are linked to the known markers. Nematode studies on crosses between two wild-type strains have identified QTLs that are responsible for increased life span located on chromosomes 2, 4, and the X. Estimates suggest that approximately seven QTLs are involved in determining life span of C. elegans.
2.2 Mutants
Inasmuch as mutations modify the life span of animals through mechanisms that may be shared by common effects on metabolism and gene expression, the analysis of mutations is the most effective means of identifying genes involved in a biological process because the physiological activity between the mutant and the wild type can be compared. However, one of the problems with this approach in aging research is that it is sometimes difficult to identify mutant strains with altered aging processes.
(a) Yeast (Saccharomyces cere isiae). Mutation analysis of aging in yeast has yielded a number of candidate genes including LAG1 that determines both mean and maximum life span, LAC1, a virtual copy of LAG1 on a different chromosome, and RAS1 and RAS2 which have life shortening and life extending effects, respectively. One of the major distinctions between aging in yeast and aging in other model organisms is that in yeast the scale on which aging is measured in yeast is reproduction (i.e., budding) but in other organisms aging is measured in chronological time.
(b) Nematodes (C. elegans). Mutational analysis in nematodes has yielded much physiological information on aging and has identified regions of the genome that contain longevity genes including daf genes which control a program that results in the formation of a dispersal form, the dauer larva—an alternative waiting stage that worms may enter in response to unfavorable periods (e.g., insufficient food), the gene age-1 which has been assigned to the daf pathway for adult longevity but which increases the resistance to stresses, and clk-1 which appears to control metabolic adjustments, particularly in response to temperature. Virtually all of the increases in longevity in mutant strains of nematodes have occurred at the postreproductive ages.
(c) Drosophila. The effects of single genes in Drosophila that extend longevity have been difficult to find perhaps because of the lack of a waiting stage similar to the nematode dauer or yeast spore stages. However, flies which carry the ‘Methuselah gene,’ mth, displayed a 35 percent increase in life span and exhibited enhanced resistance to stress.
2.3 Transgenics
Transgenics refers to the transfer of genes from one organism to another which, in the context of aging, means transferring into a model organism a gene that extends longevity in another organism. If this so-called ‘transgenic’ organism then lives longer than the wild type, this then provides the ultimate demonstration that the gene affects the aging process either directly or indirectly. The best example of the use of this concept in aging research is in Drosophila using transgenic strains that overexpress several different enzymes and exhibit greater longevity and lower mortality than the wild type. Transgenic strains of both C. elegans and mice have been produced but have not yet produced definitive results.
2.4 Gene Expression and Regulation
Although the DNA in all somatic cells is the same and thus contains the same information, each cell expresses only a distinct subset of its genes at any one time. Which genes are expressed differs between cell types and also differs slightly as organisms age. For example, blood cells only expresses the genes needed to produce hemoglobin and other proteins associated with blood; genes capable of producing hair are not expressed in tongue cells. Altered gene expression has been suggested as a central mechanism for causing aging and senescence in which regulatory elements act like on (up-regulated), off (down-regulated), and dimmer switches. Experimental manipulations of life span in both flies and rodents have been shown to cause changes in gene activity including: (a) during temperature-induced shifts in fruit fly life spans, the age schedule for decreased expression of genes in the adult antenna and muscle is shifted in proportion to life span over a threefold range; (b) the potential influence of physiological factors in rat brain aging is demonstrated by the impact of food restriction which slows the age-related increases in transcription; and (c) gene expression is altered in bakers’ yeast (Saccharomyces cere isiae) through underor overexpression of some wild-type genes, a gene that is homologous to the human gene for Werner progeria, and of genes causing a redistribution of telomeric silencing proteins.
2.5 Mitotic Clock
The majority of cell types grown in laboratory cultures have a finite ability to proliferate. After a number of population doublings the cell cultures enter the terminally nondividing state referred to as replicative senescence (so-called Hayflick limit). Many investigations have established a link between aging in i o (live animals) and the proliferative potential of cells in culture. For example, cell cultures derived from one of the longest-lived species of animals, the Galapagos tortoise, doubled up to 130 times, whereas cultures derived from mice with maximum life spans of three years were capable of doubling only 10 or fewer times. One of the most compelling theories for explaining replicative senescence is that incomplete replication of specialized structures at the ends of chromosomes called telomeres account for the gradual loss of proliferation potential. Telomeres are essential for proper chromosome structure and function including complete replication of the genome—if organisms could not overcome the ‘end-replication’ problem, they would fail to pass their complete genetic complement from generation to generation. The simplest theory accounting for the Hayflick limit is one in which permanent cell-cycle arrest is due to a checkpoint mechanism that interprets a critically short telomere length as damaged DNA and causes cells to exit the cell cycle. This telomere hypothesis of aging provides a molecular mechanism for counting cell divisions in the normal somatic cell. According to the telomere shortening model of cell senescence, to avoid telomere loss and eventual cell cycle arrest it is necessary for cells to synthesize telomeric DNA by expressing the enzyme telomerase. Therefore, a prediction of this model and one that is borne out in recent studies is that telomerase activity will be absent in normal somatic cells but present in germline cells and carcinoma cells.
3. Genetics of Human Longevity
3.1 Gerontogenes in Humans
The term gerontogenes is used to describe genes that are involved in aging and usually involves genes whose normal function shortens rather than lengthens life. One of the approaches that researchers use in an attempt to identify genes that influence aging in humans is to study the prevalence of different genes in centenarians relative to younger people (e.g., 20–60 years). One of the most studied genetic polymorphisms involved in human longevity is the apolopoprotein E (APO-E) gene which encodes a protein that is synthesized in the liver, brain, spleen, kidneys, and macrophages and in human populations exists as three common alleles—E2, E3, and E4. Frequencies of these three alleles in centenarians and in noncentenarians revealed that: (a) there was a shortage of the APO-E4 allele among centenarians (5.2 percent) compared to controls (11.2 percent) which suggests a negative effect of this allele on longevity. In fact, individuals who carry the APO-E4 allele are strongly predisposed to developing late-onset Alzheimer’s disease; and (b) an excess of APO-E2 allele among centenarians (12.8 percent) relative to the normal controls (6.8 percent) which suggests a positive effect of this allele on longevity.
3.2 Age-related Genetic Diseases
3.2.1 Progeroid mutations.
Progeroid mutations refer to one of several human genetic syndromes that accelerate some aspects of aging. Although all of the progeroid-like diseases are rare, their effects on aging in young persons are so striking that they have received considerable attention in gerontology and medicine. In this section two of the best known progeroid diseases—Hutchinson–Gilford syndrome and Werner’s syndrome—will be described. The outward signs of premature aging are similar in both of these diseases but they differ in their ages of onset and ultimately in the ages at which patients succumb to the disease.
Hutchinson–Gilford (HG) syndrome is one of the rarest genetic syndromes in humans with only 17 cases reported in the world by 1990. It is caused by a de no o gene mutation that is expressed in a dominant manner. At birth HG patients appear normal, but by about one year severe growth retardation is seen followed by rapid loss of subcutaneous fat and hair resulting in a prominent display of the scalp veins. Median age of death in HG patients is 12 years. The most prevalent progeriod disease is Werner’s syndrome which is an autosomal (i.e., all chromosomes except sex chromosome) recessive disease that is first manifested as a failure to undergo the usual adolescent growth spurt. By the early twenties persons with this disease suffer substantial hair loss, graying of hair, atrophy of the skin, and cataracts as well as an increase in the likelihood of diabetes and cancers. Cytogenetic and molecular genetic studies have suggested that Werner’s syndrome is due to a loss in the maintenance of genomic stability due to an unusually high frequency of reciprocal chromosome translocation and of singlegene mutations referred to as a mutator phenotype.
3.2.2 Adult-onset diseases.
This section contains a description of three of the best-known adult-onset genetic diseases. The first is Lou Gehrig’s disease which is formally referred to in the medical literature as amyotrophic lateral sclerosis (ALS). It is a progressive paralytic disorder that strikes middle-aged adults and is usually fatal within five years after diagnosis. Paralysis is due to degeneration of the large motor neurons of the brain and spinal cord. Approximately 10 percent of the ALS cases are inherited as an autosomal dominant disorder while the remaining 90 percent are sporadic. The second of the adult-onset genetic diseases is Huntington’s disease (HD) which is due to an autosomal dominant gene that typically causes degeneration of the nervous system. HD disease causes memory loss and dementia as well as wrinkled skin, weight loss, and uncontrollable jerky movements. It differs from Alzheimer’s disease inasmuch as the average age of onset for this disease occurs at 35–42 years with death occurring within about 15 years after diagnosis. The third and most extensively studied age-related genetic disease in humans is Alzheimer’s disease (AD). Approximately 2–6 percent of people over 65 show symptoms of AD with the prevalence of symptoms increasing with age—as many as 40 percent of persons over 65 show symptoms of AD. Although the majority of cases of AD appear to be sporadic, several hundred families have been identified in which AD is inherited as an autosomal dominant mode of transmission. Analysis of the familial AD (FAD) has allowed the localization of three distinct genes that are involved in the specification, including: (a) rare mutations in the genes encoding the precursor protein of Alzheimerassociated amyloid on chromosome 21 (2–3 percent); (b) genes on chromosome 14 account for late-onset forms of AD (after age 65); and (c) genes on chromosome 19 are responsible for early-onset forms of AD. One of the major complications of studies of the disease is determining the degree to which FAD is genetic—estimates range from 10 to 100 percent. Individuals homozygous for APOE-ε4 have an almost eightfold increase in the probability of getting AD at almost any age when compared with people carrying other alleles of APOE.
3.3 Genealogical Studies
Despite studies demonstrating that genetics account for only 25–35 percent of the total variance in longevity, one of the most enduring and widespread notions in gerontology is the longevity resemblance of kin, particularly between parents and offspring and among siblings. The findings on kinship resemblance of longevity can be grouped into three categories.
(a) Parent–offspring ( familial) resemblance. Although a modest familial component has also been established in several studies, they are generally plagued by the problem of conflation of shared genetics and environments and so cannot provide conclusive evidence for a genetic component of longevity.
(b) Twin registries. This use of twin registries has allowed more quantitative analyses of familial resemblance. Comparing the correlations between monozygotic (genetically identical) twins, fraternal twins, and nontwin siblings begins to disentangle the effects of shared genes from those of shared family environments. Investigations of the relationship between age of death between like-sex fraternal (dizygotic) twins and between identical (monozygotic) twins both reared together and reared apart reveal that longevity is moderately heritable. One twin study of longevity reported that the average absolute difference in age at death between two members of a monozygotic twin pair, two members of a dizygotic twin pair, and two randomly selected same-sex individuals was 14.1, 18.5, and 19.2 years, respectively.
(c) Comparati e ancestry of centenarians and shorterli ed groups. This comparative approach can elucidate the familial resemblance in extreme longevity. Siblings of centenarians in one study had a four times greater chance of surviving to their early nineties than siblings of a comparison group that died at age 73. Twenty- four percent (13 55) of the immediate ancestors of the oldest person on record, Jeanne Calment from France (died at 122 years 164 days) longer than 80 years, vs. 2 percent in a reference family (1 50). Her immediate ancestors lived an average of 68.2 years vs. 57.7 years for controls in the reference family. An implication of the twin and centenarian ancestry studies is that whereas life span in general is under moderate genetic control, genes play a somewhat larger role for persons attaining the more extreme ages.
4. Evolutionary Biology of Aging
4.1 Theoretical Foundation
Whereas gerontology is concerned with questions concerning the mechanisms of aging, evolutionary biology is concerned with questions in a different biological domain concerned with how senescence evolved. Evolutionary theory of aging postulates that the force of natural selection will always decrease with age with either replacement-level or positive population growth. This provides the conceptual foundation for two population genetic hypotheses of aging. The first is termed negati e pleiotropy and is based on the concept that alleles that have beneficial effects on one set of components of fitness also have deleterious effects on other components of fitness. The underlying concept is one of tradeoffs—a beneficial effect at young ages may have a deleterious effect at older ages. The declining force of natural selection leads to a tendency for selection to fix alleles that have early beneficial effects, but later deleterious effects. This biases evolution toward the production of vigorous young organisms and decrepit old organisms. Although antagonistic pleiotropy is an important possible mechanism for the evolution of aging, it probably plays a limited role in explaining the persistence of genetic variation in fitness components. The second population genetic hypothesis concerning the evolution of senescence is mutation accumulation which arises when the force of natural selection has declined to a point where it has little impact on recurrent deleterious mutations with effects confined to late life.
4.2 Empirical Foundation
A number of empirical studies concerned with understanding the extent to which genes control longevity have been conducted on Drosophila in which the ages of reproduction are carefully controlled. A typical design involves the young–old dichotomy of fly strains. Specifically, ‘young’ lines are created by allowing females to lay eggs at two weeks of age and rearing their offspring whose eggs, in turn, are collected at 14 days. This is repeated for a number of generations. The ‘old’ lines of flies are created using the same concept except that eggs are collected only from the females who have survived to much older ages. The results of these types of selection studies have revealed that the mean and maximum life spans of cultures selected for early reproduction are lower than those for cultures selected for later reproduction.
5. Longevity Implications of Molecular Medicine
One of the most profound changes in biological research including biomedical and gerontological studies has been the emergence of molecular biology and genomics. These two disciplines are leading to the creation of a universal periodic table of life that will reflect common genetic properties and patterns of ancestral and functional affinities among the genes of both plants and animals. Comparison of related organisms will reveal regulatory regions and key architectural features of proteins that can be used as Rosetta stones for translating and understanding informational pathways and for deciphering biological complexity.
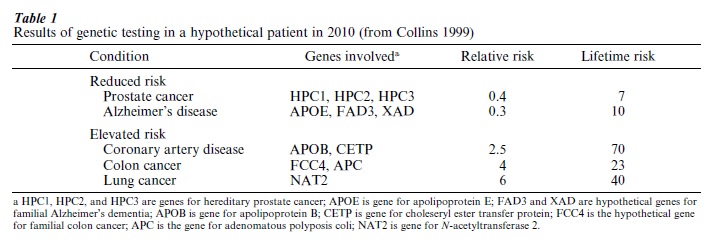
In the very near future, molecular biology and genomics research will have a profound impact on the quantity and quality of human life through disease prevention. One of the best examples of how this emerging science and technology might be applied in the future is provided by the hypothetical example given in a paper by Francis Collins—the Director of the Human Genome Project. The human genome project will provide a baseline of an individual’s genome to shed light on the person’s risk profile and thus point to prevention strategies if available. The example uses a 23-year-old male college graduate who has smoked for six years and has a strong paternal history of heart disease. This individual agrees to undergo 15 genetic tests that provide risk information (both relative and absolute) for illnesses for which preventive strategies are available. The results of the genetic testing in this hypothetical patient are given in Table 1. The good news is that his risks of contracting prostate cancer and Alzheimer’s disease are reduced because he carries low-risk variants of the several genes that contribute to the illnesses. The bad news is the genetic evidence of his increased risks of contracting coronary artery disease, colon cancer, and lung cancer. Confronted with this information, the patient arrives at that ‘teachable moment’ when a lifelong change in health-related behavior is possible by focusing on reducing specific risks including quitting smoking, reducing his cholesterol level, and having annual colonoscopies starting at age 45.
Bibliography:
- Abbott J H, Abbey H, Bolling D R, Murphy E A 1978 The familial component in longevity—a study of offspring of nonagenarians: III. Intrafamilial studies. American Journal of Medical Genetics 2: 105–20
- Bocquet-Appel J-P, Jakobi L 1990 Familial transmission of longevity. Annals of Human Biology 17: 81–95
- Chippindale A K, Leroi A M, Kim S B, Rose M R 1993 Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. Journal of Evolutionary Biology 6: 171–93
- Collins F S 1999 Shattuck Lecture—Medical and societal consequences of the human genome project. New England Journal of Medicine 341: 28–37
- Curtsinger J W, Service P M, Prout T 1994 Antagonistic pleiotropy, reversal of dominance, and genetic polymorphism. The American Naturalist 144: 210–28
- Hamilton W D 1966 The moulding of senescence by natural selection. Journal of Theoretical Biology 12: 12–45
- Lee C-K, Klopp R G, Weindruch R, Prolla T A 1999 Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–3
- Lin Y-J, Seroude L, Benzer S 1998 Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282: 943–6
- Ljungquist B, Berg S, Lanke J, McClearn G E, Pedersen N L 1998 The effect of genetic factors for longevity: A comparison of identical and fraternal twins in the Swedish twin registry. Journal of Gerontology 53A: M441–6
- Masoro E J 1988 Minireview: Food restriction in rodents: An evaluation of its role in the study of aging. Journal of Gerontology 43: B59–64
- McGue M, Vaupel J W, Holm N, Harvald B 1993 Longevity is moderately heritable in a sample of Danish twins born 1870–1880. Journal of Gerontology 48: B237–44
- Pearl R 1931 Studies on human longevity. IV. The inheritance of longevity. Preliminary report. Human Biology 3: 245–69
- Perls T T, Alpert L, Fretts R C 1997 Middle-aged mothers live longer. Nature 389: 133
- Perls T T, Bubrick E, Wager C G, Vijg J, Kruglyak L 1998 Siblings of centenarians live longer. Lancet 351: 1560
- Ridley M 1999 Genome. Harper-Collins Publishers, New York
- Rose M R 1991 The Evolutionary Biology of Aging. Oxford University Press, New York




