Sample Prefrontal Cortex Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The prefrontal cortex is the cortex of association of the frontal lobes. It plays a major role in the temporal organization of behavior, reasoning and language. The underlying mechanisms are not fully understood, but are known to support at least two cognitive functions of temporal integration that are essential to that role: active short-term memory and preparatory set.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Anatomy
In evolution and in individual growth, the prefrontal cortex develops more slowly, yet further, than most other brain structures, in both size and weight (Fig. 1). Phylogenetically and ontogenetically, it is one of the last regions of the cortex (neocortex) to develop (Brodmann 1909). The human prefrontal cortex does not reach full structural maturation of cells and connections until young adulthood. In the adult human, the prefrontal cortex constitutes almost one-third of the totality of the neocortex. For reasons that are not well understood, this region of the cortex, which is one of the latest to develop, is also one of the most vulnerable to involution and pathological aging (Raz 2000).
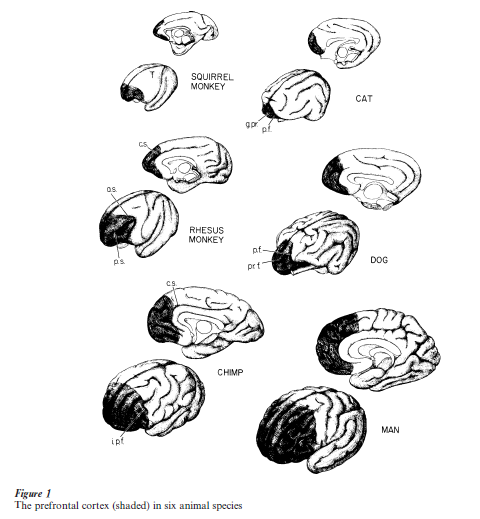
The primate prefrontal cortex (Petrides and Pandya 1994) is composed of three major parts or regions (Fig. 2): the dorsolateral prefrontal cortex, that is, the cortex of the dorsal and lateral convexity of the anterior portion of the frontal lobe (Brodmann cyto-architectonic area 46, and lateral parts of areas 8, 9, 10, and 11); the medial and cingulate prefrontal cortex, which is nearly flat and faces the medial surface of the contralateral frontal pole (areas 12, 24 and 32, and medial parts of 8, 9, 10, and 11); and the inferior orbital prefrontal cortex, slightly concave and located directly above the orbit (areas 13, 47, and inferior parts of 10, 11, and 13).
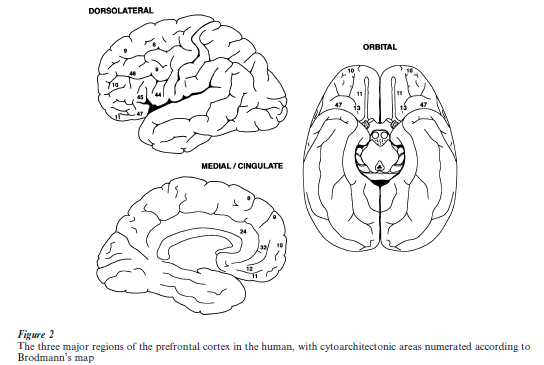
Of the three regions, the dorsolateral develops last and most. This region is bordered posteriorly by the premotor cortex (area 6); the medial and orbital prefrontal cortices (often considered together and designated orbitomedial prefrontal cortex) are anterior and adjacent to the corpus callosum and to limbic structures (piriform cortex and amygdala, cingulate cortex, septum, and hypothalamus). In terms of cellular architecture, the posterior orbitomedial cortex is transitional, with features of both the limbic cortex and neocortex.
2. Functional Connectivity
The functions of the prefrontal cortex can be best understood as components of a general role of frontal cortex in the representation and execution of the actions of the organism. In its totality, the frontal cortex can be considered motor cortex in the broadest sense. It represents and coordinates actions in all domains of adaptation of the organism to its internal and external environment. In order to coordinate such a wide gamut of activity, the prefrontal cortex receives inputs from, and sends outputs to, many other parts of the brain.
Each of the three principal prefrontal regions has its own set of fiber connections mostly reciprocal and topologically organized—not only with the other two prefrontal regions, but with other cerebral structures, cortical and subcortical. Especially noteworthy for their number and functional implications are the connections of the three prefrontal regions with the thalamus, limbic structures, the basal ganglia, and other parts of the cortex. The orbital and medial prefrontal regions are well connected with the medial and anterior nuclei of the thalamus, the piriform cortex, the hippocampus, the amygdala, and the hypothalamus.
This connectivity with the thalamus and limbic structures falls into three major categories: (a) gustatory and olfactory inputs for the processing of information on taste and olfaction; (b) inputs from the internal milieu of the organism, notably from the hypothalamus, related to motivation, visceral functions, and affective states; and (c) efferent outputs to thalamic and limbic structures for the control of instinctual, emotional, and social behavior, as well as certain visceral functions.
The dorsolateral prefrontal cortex, on the other hand, is extensively interconnected with other areas of the frontal lobe, homolaterally and contralaterally (through corpus callosum), with the hippocampus, and with several areas of the neocortex of the temporal and parietal lobes, in the posterior aspects of the cerebral hemisphere (Pandya and Yeterian 1985). All this cortical connectivity is essential for cognition. It serves the integrative and associative functions that support the cardinal role of the prefrontal cortex in executive memory and in the temporal organization of behavior. In addition, the dorsolateral prefrontal cortex sends important efferent fibers to the basal ganglia, including caudate nucleus, and the cerebellum. This connectivity serves the motor output functions of the prefrontal cortex through structures that are involved in skeletal and ocular motilities.
3. Physiology
Drive, emotional behavior and visceral actions, as well as their affective connotations, are the physiological purview of medial and orbital prefrontal areas. The medial prefrontal cortex plays an important role in basic motivation, though this role is still poorly understood.
In the human, large lesions of this medial cortex lead to apathy, inattention, and lack of spontaneity in speech, behavior, and ideation (Cummings 1985). The orbital prefrontal cortex, on the other hand, seems essential for the inhibitory control of internal drives. Animals or humans with lesions of this cortex often manifest disinhibition of eating, sex, and aggression, in addition to related abnormalities of social and emotional behavior. The human subject with orbitofrontal damage commonly shows uninhibited instinctual behavior, impulsivity, reckless risk taking, coarse and tasteless humor, and poor moral judgement (Stuss and Benson 1986, Damasio et al. 1994). Oftentimes he or she runs into conflict with the law. Further, because internal impulse control is necessary for steady attention and normal goal directed behavior, the orbitomedial prefrontal cortex serves the general role of the prefrontal cortex in the organization of behavior and cognition.
The dorsolateral prefrontal cortex is involved in both the representation and the enactment of temporally organized behavior and of cognitive operations, which are required in language and logical reasoning (Luria 1966, Fuster 1997). These representations fall under the general category of motor or executive memory, which includes the representations of complex schemes of temporally organized action, such as plans, programs, or scripts of behavior and cognition. The qualifications of novelty and complexity should be emphasized because, after its acquisition and consolidation, routine behavior is no longer represented or enacted by the prefrontal cortex, but by neural structures at lower stages of the motor hierarchy (e.g., motor and premotor cortices, basal ganglia).
A subject of much debate is the functional specialization of areas within the dorsolateral prefrontal cortex. Given that this cortex can be anatomically subdivided into a number of cytoarchitectonically disctinct areas (Petrides and Pandya 1994), each with a separate set of connections (Pandya and Yeterian 1985), the functional specialization of its areas can be, in principle, reasonably assumed. Accordingly, some single-cell studies with monkeys support a degree of seemingly modular commitment of various dorsolateral areas to the representation of different categories of sensory information, such as spatial location or visual configuration (Goldman-Rakic 1995). Other single-unit studies, however, point to the essentially associative character of all dorsolateral prefrontal areas.
In the pursuit of behavioral goals, dorsolateral prefrontal cells associate sensory information across modalities (e.g., sight and sound) and across time (Fuster et al. 2000). Furthermore, some cells associate sensory stimuli with subsequent motor actions (Rainer et al. 1998). These associative properties of dorsolateral prefrontal cells point to their essential temporal integrative functions. Thus, the apparent sensory or motor specificity of certain dorsolateral areas may be related to the specificity of their predominant inputs from sensory systems or outputs to motor systems. In any case, that specificity of areas—thus far fragmentary, and far from established—seems secondary to their basic integrative functions at the cognitive level. Those functions (see later) are crucial for the temporal organization of behavior.
It is likely, though not proven, that the connectivity of the prefrontal cortex with the hippocampus is essential for the formation of executive memory, including the schemas of behavioral and cognitive action. Once they have been formed, the executive memory networks of the cortex of the frontal lobe appear hierarchically organized. Represented in primary motor cortex, the lowest stage of that organization, are the simplest motor memories, elements of motor action defined by specific movement and muscle group (i.e., innate or phyletic motor memory). Immediately above, in the premotor cortex, are representations defined by trajectory and goal of movement. At the summit, in the dorsolateral prefrontal cortex, are representations of complex schemas and sequences of goal-directed action. In a manner still unknown, this cortical region represents the broad schemas of action and, in addition, is critically involved in the enactment of those schemas. A consistent component of the dorsolateral frontal-lobe syndrome in the human is the inability to formulate and to enact plans of action.
4. Temporal Structuring Of Action
The main function of the dorsolateral prefrontal cortex is the temporal organization of behavior, language and reasoning. This cortical region appears to secure the orderly and timely execution of all new goal-directed sequences in those three domains of activity. As a precondition for its enactment, however, a new or recently learned sequence of acts is presumably represented in prefrontal networks in the form of a schema or plan of action (executive memory). The execution of that sequence is a highly complex process of temporal integration that probably engages many cortical and subcortical brain regions. The dorsolateral prefrontal cortex seems to orchestrate the functions of many other parts of the brain. It does so with the purpose and end-result of structuring behavior and cognition in time, of forming temporal gestalts (or melodies of action) toward the attainment of biological and cognitive goals.
In as much as intelligence is manifested and tested by performance of logical goal-directed directed tasks and sequences of behavior and language, it is reasonable to assume that the dorsolateral prefrontal cortex is an important component of the neural substrate of intelligence. Indeed, it has been argued on the basis of empirical data from the human, that this cortex is the foundation of general intelligence, as measured by Spearman (Duncan et al. 1996). This psychological metric reflects the proficiency of a subject in performance of a large variety of tests, all of which require the temporal integration of behavioral, verbal or cognitive contents.
Towards the end of the twentieth century, evidence has been accumulating for two temporally integrative functions of dorsolateral prefrontal cortex providing the neural and cognitive support to the process of temporal organization. Those two functions are temporally symmetrical and mutually complementary. The first is a temporally retrospective function of short-term memory; the second, a temporally prospective function of preparatory set. Both together help the organism to bridge time between sensory and motor events, and thus to mediate cross-temporal contingencies of behavior. Both seem to share the same or overlapping networks of dorsolateral prefrontal cortex. Both depend on the coordination of neural activity between this cortex and the association cortices of the posterior regions of the cerebral hemispheres.
4.1 Short-Term Memory
Short-term memory is commonly conceptualized as the temporary retention of information before it becomes permanent or long-term memory. Recently, however, cognitive psychology has developed a different concept of short-term memory: the temporary retention of information, old or new, for the execution of an action in the short term, as is required for the performance of complex behavior or the solution of problems. This kind of short-term memory has been called working memory (Baddeley 1986). There is considerable evidence that both types of short-term memory share the same cortical neurons and networks, and that working memory consists in the sustained activation of a wide cortical network representing information to be used prospectively in the near term. That information may be newly acquired or may be an item of long-term memory reactivated for the short term as the occasion demands. As that memory contributes to form a temporal structure of behavioral or cognitive action, the neurons of dorsolateral prefrontal cortex are part of the activated memory network. There is conclusive evidence that the dorsolateral prefrontal region is critically involved in all forms of active short-term memory toward a goal, or working memory (Fuster 1997).
The evidence comes chiefly from two methodologies, selective cortical lesion (or inactivation) and single-cell recording, in the nonhuman primate performing short-term memory or delay tasks (e.g., delayed response, delayed matching). In these tasks, the animal is presented on successive trials with an item of sensory information—which varies from trial to trial—and is required to retain it in memory for electing and executing the appropriate motor response a few seconds or minutes later. Each trial amounts to a specific selection from a set repertoire of actions, a selection that depends on the sensory cue that has preceded it by seconds or minutes. The relative novelty of the relevant sensory–motor association for that particular trial, and the need to retain a sensory item temporarily in memory, place both the trial and the memory under prefrontal control. Monkeys with lesions of dorsolateral prefrontal cortex perform poorly in delay tasks. They seem unable to keep the working memory of the stimulus for each trial. In a normal animal performing a delay task, neurons of that cortex fire persistently—and often in stimulusspecific manner—during the delay (memory period) of every trial.
These observations denote the importance of cellular dynamics in dorsolateral prefrontal cortex for working memory and the temporal organization of behavior. Recent neuroimaging methods have provided further evidence, at a more global level, of the involvement of the dorsolateral prefrontal cortex in working memory (Smith and Jonides 1999). Unlike microelectrode studies, however, and because of its constraints of temporal and spatial resolution, neuroimaging does not yet reveal the dynamics of neuronal populations of this cortex in working memory. What defines prefrontal memory in the active state, or working memory, is the teleological quality of that memory, which is mobilized for the construction of future action. As noted in Sect. 3, one source of apparent areal specialization in dorsolateral prefrontal cortex is the nature of sensory inputs leading to associated action (visual, spatial, etc.). The other is the nature of the associated actions themselves (skeletal movements, ocular movements, etc.). Sensory or motor-specific cell groupings and areas within that cortex are probably components of teleological associations, and thus the paths to prospective action. In any case, the integrative functions of dorsolateral cortex, namely memory and set, are supramodal and transcend the sensory or motor properties of any given prefrontal area.
4.2 Set
Also from the teleological character of the temporal structuring of action derives the prefrontal function of preparatory set. Setting receptors and effectors for prospective actions is the temporal mirror image of active short-term memory. This second integrative function, preparatory set, is the other side of the coin of the superordinate prefrontal function of temporal organization. It prepares the organism for anticipated percepts and actions (memory of the future) that will complete the bridging of a cross-temporal contingency. Although the mechanisms of set are not yet known, they probably include the priming of sensory and motor structures via efferent prefrontal connections. In the monkey and the human, the activation of dorsolateral prefrontal cortex in preparatory set is manifested by a progressive increase of cell discharge and slow surface-negative potentials before a stimuluscontingent act. The cellular evidence indicates that memory and set occur largely at the same time. Both seem to be processed simultaneously in the period between two mutually contingent events.
While some prefrontal cells engage in working memory of the cue, others in the same region do it in the preparation of the upcoming behavioral response. The activity of those cells shows that, while the representation of sensory information tends to wane over a delay, the representation of the prepared response tends to increase (Quintana and Fuster 1999). The choice of one motor act among alternatives is, on the motor side, the equivalent of focusing sensory attention. Thus, preparatory set, the second cognitive function of the dorsolateral prefrontal cortex, can be rightly considered attention, the focusing of attention on a motor act to ensure its prompt and efficient execution.
In summary, the symmetry of the two temporal integrative functions of prefrontal cortex, memory and set, is paralleled by a symmetry of attentive processes, one sensory and the other motor. Active short-term memory is attention focused on the representation of a recent sensory event, whereas preparatory set is attention focused on a consequent and subsequent act. Although attention—especially perceptual attention in the human—is usually associated with consciousness, neither of the two temporal integrative functions of the prefrontal cortex—active memory or set—need be conscious.
5. Perception–Action Cycle
The organization of new and complex behavior commonly requires a continuous succession of temporal integrations of percepts and actions. In this succession, each integration depends to some degree on previous ones. Each act produces changes in the environment that will determine and modify subsequent perceptions, and these will determine and modify subsequent acts. The continuous operation of this cybernetic cycle of interactions of the organism with its environment is a basic principle of biology, the so-called perception–action cycle.
As complex new behavior evolves toward a goal, percepts and acts that are mutually contingent are often separated by time and by other intervening acts and percepts. Further, the pursuit of that goal requires the intervening attainment of lesser or subordinate goals. Thus, the temporal organization of the behavior requires a continuous mediation of contingencies across time. To mediate those cross-temporal contingencies is a function of the biological substrate of the perception–action cycle. The dorsolateral prefrontal cortex is at the apex of that substrate, which basically consists of two parallel hierarchies of neural structures, one sensory and the other motor, extending from the spinal cord to the cerebral cortex. Corresponding levels of each hierarchy are reciprocally interconnected, while each structure in either hierarchy is reciprocally interconnected with the one above and the one below. Figure 3 shows the cortical stages of those hierarchies. The contingencies of automatic and routine behavior (e.g., walking, reflex acts) are mediated at lower, subcortical, stages of the neural organization of the perception action cycle.
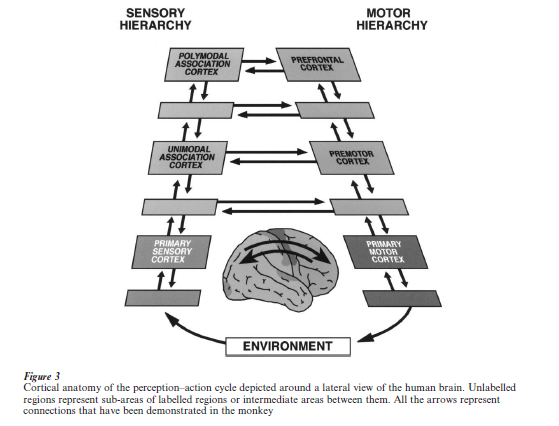
Complex new behavior, however, engages the cortical stages of the cycle neural hierarchies. Because this kind of behavior usually contains multiple and complex contingencies across time, the highest neocortical stages are called into action for its implementation, in particular the dorsolateral prefrontal cortex. This cortex, in functional coordination with the sensory association cortices of the posterior hemisphere, mediates cross-temporal contingencies at the top of the cycle. The prefrontal cortex supports that mediation across time through its two temporal integrative functions of active memory and preparatory set. Both require the functional interaction of prefrontal with posterior cortices, as suggested by combined use of behavioral, cortical-cooling and cell-recording methods (Fuster et al. 1985).
The understanding of the mechanisms of that interaction will lead to more precise knowledge than we now have of the functions of the prefrontal cortex in the temporal organization of behavior. For a number of empirical and theoretical reasons, the role of this cortex in the organization of language and reasoning is probably based on similar functions and mechanisms.
Bibliography:
- Baddeley A 1986 Working Memory. Clarendon Press, Oxford, UK
- Brodmann K 1909 Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig, Germany
- Cummings J L 1985 Clinical Neuropsychiatry. Grune and Stratton, Orlando, FL
- Damasio H, Grabowski T, Frank R, Galaburda A M, Damasio A R 1994 The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science 264: 1102–5
- Duncan J, Emslie H, Williams P 1996 Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogniti e Psychology 30: 257–303
- Fuster J M 1997 The Prefrontal Cortex: Anatomy Physiology, and Neuropsychology of the Frontal Lobe, 3rd edn. LippincottRaven, Philadelphia, PA
- Fuster J M, Bauer R H, Jervey J P 1985 Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Research 330: 299–307
- Fuster J M, Bodner M, Kroger J 2000 Cross-modal and crosstemporal association in neurons of frontal cortex. Nature 405: 347–51
- Goldman-Rakic P S 1995 Architecture of the prefrontal cortex and the central executive. Proceedings of the National Academy of Science 769: 71–83
- Luria A R 1966 Higher Cortical Functions in Man. Basic Books, New York
- Pandya D N, Yeterian E H 1985 Architecture and connections of cortical association areas. In: Peters A, Jones E G (eds.) Cerebral Cortex. Plenum Press, New York, Vol. 4, pp. 3–61
- Petrides M, Pandya D N 1994 Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J (eds.) Handbook of Neuropsychology. Elsevier, Amsterdam, pp. 17–58
- Quintana J, Fuster J M 1999 From perception to action: Temporal integrative functions of prefrontal and parietal neurons. Cerebral Cortex 9: 213–21
- Rainer G, Assad W F, Miller E 1998 Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393: 577–9
- Raz N 2000 Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik F I M, Salthouse T A (eds.) Handbook of Aging and Cognition. Erlbaum, Mahwah, NJ, pp. 1–90
- Smith E E, Jonides J 1999 Storage and executive processes in the frontal lobes. Science 283: 1657–61
- Stuss D T, Benson D F 1986 The Frontal Lobes. Raven Press, New York




