Sample Neural Basis Of Emotion Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Also, chech our custom research proposal writing service for professional assistance. We offer high-quality assignments for reasonable rates.
In order to provide a fundamental basis for understanding the neural basis of emotion, it is useful to have a clear view of the events that give rise to emotions and the functions of emotion, for these help to define the input systems in the brain involved in producing emotions, and the output systems in the brain that are involved in expressing emotion. With this basis, developed fully by Rolls (1999, 2000a) the neural systems that underlie emotions are then considered.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. The Events That Give Rise To Emotions
Emotions can usefully be defined as states produced by rewards and punishers. Rewards are stimuli for which an animal (including humans) will work, and punishers are stimuli that an animal will work to escape from or avoid. Rewards and punishers are reinforcers in that they alter the probability of behavior. Some stimuli are unlearned or primary reinforcers (e.g., the taste of food if the animal is hungry, or pain). Other stimuli may become reinforcing by learning, because of their association with such primary reinforcers, thereby becoming ‘secondary reinforcers.’ For example, fear is an emotional state which might be produced by a sound (the conditioned stimulus) that has previously been associated with a painful stimulus (the primary reinforcer.)
The different emotions can be described and classified according to whether the reinforcer is positive or negative, and by the reinforcement contingency. An outline of such a classification scheme is shown in Fig. 1.
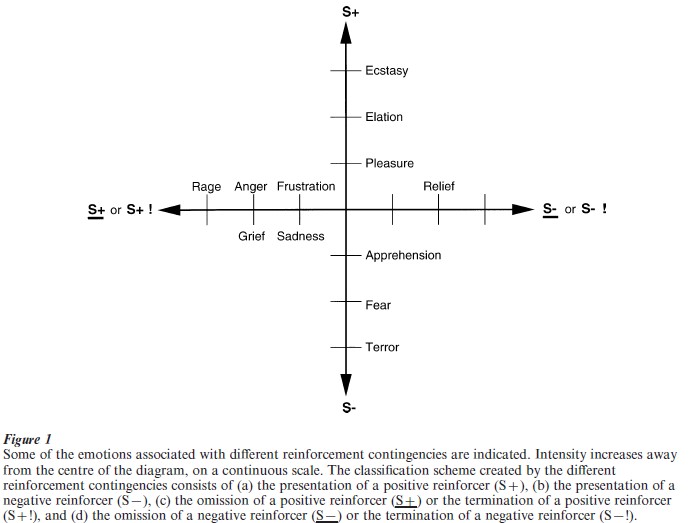
The operation of the following factors enables a very wide range of different emotions to described:
(a) The reinforcement contingency (see Fig. 1).
(b) The intensity of the reinforcer (see Fig. 1).
(c) Any environmental stimulus might have a number of different reinforcement associations. For example, a stimulus might be associated both with the presentation of a reward and of a punishment, allowing states such as conflict and guilt to arise.
(d) Emotions elicited by stimuli associated with different primary reinforcers will be different.
(e) Emotions elicited by different secondary reinforcing stimuli will be different from each other (even if the primary reinforcer is similar).
(f ) The emotion elicited can depend on whether an active or passive behavioral response is possible. For example, if an active behavioral response can occur to the omission of a previously rewarded stimulus, then anger might be produced, but if only passive behavior is possible, then sadness, depression, or grief might occur.
By combining these six factors, it is possible to account for a very wide range of emotions. It is also worth noting that
(a) emotions can be produced just as much by the recall of reinforcing events as by external reinforcing stimuli;
(b) cognitive processing (whether conscious or not) is important in many emotions, for very complex cognitive processing may be required to determine whether environmental events are reinforcing or not;
(c) emotions normally consist of cognitive processing which determines the reinforcing valence of the stimulus, and an elicited mood change if the valence is positive or negative; and
(d) and that stability of mood implies that absolute levels of reinforcement must be represented over moderately long time spans by the firing of mood- related neurons, a difficult operation which may contribute to ‘spontaneous’ mood swings, depression which occurs without a clear external cause, and the multiplicity of hormonal and transmitter systems which seem to be involved in the control of mood.
2. The Functions Of Emotion
The functions of emotion can be summarized as follows.
(a) The elicitation of autonomic responses (e.g., a change in heart rate) and endocrine responses (e.g., the release of adrenaline). These prepare the body for action.
(b) Flexibility of behavioral responses to reinforcing stimuli. Emotional (and motivational) states allow a simple interface between sensory inputs and motor outputs. The new understanding is that some genes specify which stimuli are rewarding and punishing, and these stimuli are the goals for flexible actions. For example, genes specify that strong somatosensory stimuli produce a state of pain which animals (including humans) are built to perform actions to avoid or escape. The state produced by the painful stimulus is an emotional state which has the adaptive function of identifying and maintaining the goal for an action. This is an efficient design, for the genes need only specify the goals for actions, and not the actions themselves, so that the actions can be selected flexibly to meet the goals. It would be very inflexible and inefficient for the genes to specify actions themselves. In this way, emotions provide an efficient interface between sensory systems and actions. The hypothesis is that our emotions correspond to the goal-specifying states arising from stimuli that genes have found it adaptive and possible to specify. Rolls (1999) provides in Table 10.1 the start of a list of some of the primary reinforcers that genes appear to specify, and thus an idea of how a wide range of different emotions have evolved because of their adaptive implications.
(c) Emotion is motivating. For example, fear learned by stimulus-reinforcement association formation provides the motivation for actions performed to avoid noxious stimuli.
(d) Communication. For example, monkeys may communicate their emotional state to others, by making an open-mouth threat to indicate the extent to which they are willing to compete for resources, and this may influence the behavior of other animals. This aspect of emotion was emphasized by Darwin (1872).
(e) Social bonding. Examples are the emotions associated with the attachment of the parents to their young, and the attachment of the young to their parents.
(f ) The current mood state can affect the cognitive evaluation of events or memories (see Oatley and Jenkins 1996), and this may have the function of facilitating continuity in the interpretation of the reinforcing value of events in the environment.
(g) Emotion may facilitate the storage of memories. One way in which this occurs is that episodic memory (i.e., one’s memory of particular episodes) is facilitated by emotional states. This may be advantageous in that storing many details of the prevailing situation when a strong reinforcer is delivered may be useful in generating appropriate behavior in situations with some similarities in the future. A second way is that the current emotional state may be stored with episodic memories, providing a mechanism for the current emotional state to affect which memories are recalled. A third way in which emotion may affect the storage of memories is by guiding the cerebral cortex in the representations of the world which are set up. For example, in the visual system, it may be useful to build perceptual representations or analyzers which are different from each other if they are associated with different reinforcers, and it may be less likely to build them if they have no association with reinforcement. (h) Another function of emotion is that by enduring for minutes or longer after a reinforcing stimulus has occurred, it may help to produce persistent and continuing motivation and direction of behavior, to help achieve a goal or goals.
(i) Emotion may trigger the recall of memories stored in neocortical representations. Amygdala back- projections to the cortex could perform this for emotion in a way analogous to that in which the hippocampus could implement the retrieval in the neocortex of recent (episodic) memories (Rolls and Treves 1998).
( j) Rewards and punishers, and the emotional states they produce, provide a common currency for the behavior selection process between competing alternative actions.
3. Brain Mechanisms Of Emotion
3.1 Overview
Animals are built with neural systems that enable them to evaluate which environmental stimuli, whether learned or not, are rewarding and punishing, that is will produce emotions and will be worked for or avoided. Sensory stimuli are normally processed through several stages of cortical processing to produce a sensory representation of the object before emotional valence is decoded. For example, in the taste system, taste is analyzed in primates to provide a representation of what the taste is in the primary taste cortex, and this representation is independent of the reward value of the taste in that it is not affected by hunger. In the secondary taste cortex, in the orbitofrontal region (see Fig. 2), the reward value of the taste is represented, in that neurons only respond to the taste if the primate is hungry. In another example, in the visual system representations of objects which are view-, positionand size-invariant are produced in the inferior temporal visual cortex after many stages of cortical processing, and these representations are independent of the emotional valence of the object.
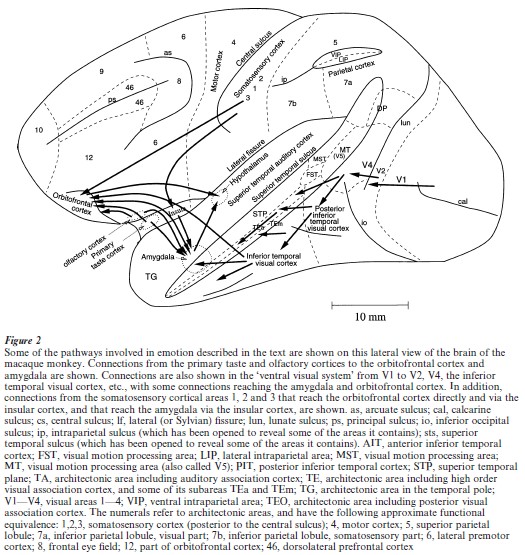
Then in structures such as the orbitofrontal cortex and amygdala, which receive from the inferior temporal visual cortex, associations are learned between the objects and the primary reinforcers associated with them, by the process of stimulus-reinforcement association learning. In the orbitofrontal cortex and amygdala emotional states are thus represented.
Consistent with this, electrical stimulation of the orbitofrontal cortex and amygdala is rewarding, and damage to these structures affects emotional behavior by affecting stimulus-reinforcement association learning. These brain regions influence the selection of behavioral actions through brain systems such as the ventral striatum and other parts of the basal ganglia.
3.2 The Amygdala
The amygdala receives information about primary reinforcers (such as taste and touch), and also information about visual and auditory stimuli from higher cortical areas (such as the inferior temporal cortex) that can be associated by learning with primary reinforcers (Fig. 2). Bilateral removal of the amygdala in monkeys produces tameness, a lack of emotional responsiveness, the excessive examination of objects, often with the mouth, and the eating of previously rejected items such as meat (the Kluver-Bucy syndrome). In analyses of the bases of these behavioral changes, it has been observed that there are deficits in learning to associate stimuli with primary reinforcement, including both punishments and rewards. The association learning deficit is present when the associations must be learned from a previously neutral stimulus (e.g., the sight of an object) to a primary reinforcing stimulus (such as the taste of food).
Further evidence linking the amygdala to reinforcement mechanisms is that monkeys will work in order to obtain electrical stimulation of the amygdala, that single neurons in the amygdala are activated by brainstimulation reward of a number of different sites, and that some amygdala neurons respond mainly to rewarding stimuli, and others to punishing stimuli. The association learning in the amygdala may be implemented by associatively modifiable synapses from visual and auditory neurons on to neurons receiving inputs from taste, olfactory or somatosensory primary reinforcers (LeDoux 1996). Consistent with this, Davis (2000) has found that at least one type of associative learning in the amygdala can be blocked by local application to the amygdala of a NMDA receptor blocker, which blocks long-term potentiation (LTP), which is a model of the synaptic changes that underlie learning (see Rolls and Treves 1998). Consistent with the hypothesis that the learned incentive (conditioned reinforcing) effects of previously neutral stimuli paired with rewards are mediated by the amygdala acting through the ventral striatum, amphetamine injections into the ventral striatum enhanced the effects of a conditioned reinforcing stimulus only if the amygdala was intact (see also Everitt et al. 2000).
An interesting group of neurons in the amygdala (in, for example, the basal accessory nucleus) responds primarily to faces. They are probably part of a system which has evolved for the rapid and reliable identification of individuals from their faces, and of facial expressions, because of the importance of this in primate social behavior. Consistent with this, activation of the human amygdala can be produced in neuroimaging studies by some face expressions, and lesions of the human amygdala may cause difficulty in the identification of some face expressions.
3.3 The Orbitofrontal Cortex
The orbitofrontal cortex receives inputs from the inferior temporal visual cortex and superior temporal auditory cortex; from the primary taste cortex and the primary olfactory (pyriform) cortex (see Fig. 2); from the amygdala, and from the midbrain dopamine neurons. Damage to the caudal orbitofrontal cortex in the monkey produces emotional changes. These include decreased aggression to humans and to stimuli such as a snake and a doll, and a reduced tendency to reject foods such as meat. These changes may be related to a failure to react normally to and learn from nonreward in a number of different situations. This failure is evident as a tendency to respond when responses are inappropriate, for example no longer rewarded.
For example, monkeys with orbitofrontal damage are impaired on Go NoGo task performance (in which they should make a response to one stimulus to obtain a reward, and should not make a response to another stimulus in order to avoid a punishment), in that they Go on the NoGo trials. They are also impaired in an object-reversal task in that they respond to the object which was formerly rewarded with food. They are also impaired in extinction in that they continue to respond to an object which is no longer rewarded. Further, the visual discrimination learning deficit shown by monkeys with orbitofrontal cortex damage, may be due to the tendency of these monkeys not to withhold responses to nonrewarded stimuli.
The primate orbitofrontal cortex contains neurons which respond to the reward value of taste (a primary reinforcer), in that they only respond to the taste of food when hunger is present (which is when food is rewarding). It also contains neurons which learn to respond to visual stimuli associated with a primary reward such as taste, and which reverse their responses to another visual stimulus in one trial when the rewards and punishers available from those visual stimuli reverse. Further, these visual responses reflect reward, in that feeding the monkey to satiety reduces the responses of these neurons to zero. Moreover, in part of this orbitofrontal region, some neurons combine taste and olfactory inputs, in that they are bimodal, and are in 40 percent of cases affected by olfactory-to-taste association learning and by feeding the monkey to satiety, which reduces the reward value. In addition, some neurons in the primate orbitofrontal cortex respond to the sight of faces. These neurons are likely to be involved in learning which emotional responses are currently appropriate to particular individuals, and in making appropriate emotional responses given the face expression.
It has been found that another class of neurons in the orbitofrontal cortex of the monkey responds in certain nonreward situations. For example, some neurons responded in extinction, immediately after a lick had been made to a visual stimulus which had previously been associated with fruit juice reward, and other neurons responded in a reversal task, immediately after the monkey had responded to the previously-rewarded visual stimulus, but had obtained punishment rather than reward. Another class of orbitofrontal neuron responded to particular visual stimuli only if they were associated with reward, and these neurons showed one trial stimulus-reinforcement association reversal. Another class of neuron conveyed information about whether a reward had been given, responding, for example, to the taste of sucrose, or for other neurons of saline.
These types of information may be represented in the responses of orbitofrontal neurons because they are part of a mechanism which evaluates whether a reward is expected, and generates a mismatch (evident as a firing of the nonreward neurons) if reward is not obtained when it is expected (see Rolls 1999, 2000a, 2000b). These neuronal responses provide further evidence that the orbitofrontal cortex is involved in emotional responses, particularly when these involve correcting previously learned reinforcement contingencies, in situations which include those usually described as involving frustration.
It is of interest and potential clinical importance that a number of the symptoms of frontal lobe damage in humans appear to be related to this type of function; altering behavior when stimulus-reinforcement associations alter, as described next. Thus, humans with frontal lobe damage can show impairments in a number of tasks in which an alteration of behavioral strategy is required in response to a change in environmental reinforcement contingencies (Rolls et al. 1994; Damasio 1994). Some of the personality changes that can follow frontal lobe damage may be related to a similar type of dysfunction. For example, the euphoria, irresponsibility, lack of affect, and lack of concern for the present or future which can follow frontal lobe damage may also be related to a dysfunction in altering behavior appropriately in response to a change in reinforcement contingencies. At one time, following a report by Moniz (1936), prefrontal lobotomies or leucotomies (cutting white matter) were performed in humans to attempt to alleviate a variety of problems, and although irrational anxiety or emotional outbursts were sometimes controlled, intellectual deficits and other side effects were often apparent (see Valenstein 1974). Thus these operations have been essentially discontinued.
To investigate the possible significance of face-related inputs to orbitofrontal visual neurons described above, the responses to faces that were made by patients with orbitofrontal damage produced by pathology or trauma were tested. Impairments in the identification of facial and vocal emotional expression were demonstrated in a group of patients with ventral frontal lobe damage who had socially inappropriate behavior (Hornak et al. 1996). The expression identification impairments could occur independently of perceptual impairments in facial recognition, voice discrimination, or environmental sound recognition. Thus the orbitofrontal cortex in humans appears to be important not only in the rapid relearning of stimulus- reinforcement associations, but also in representing some of the stimuli, such as face expression, which provide reinforcing information. Consistent with this, neuroimaging studies in humans are showing representations which reflect the pleasantness of the taste and smell of food, the pleasantness of touch, and also of quite abstract rewards and punishers such as winning or losing money (O’Doherty et al. 2001.)
The behavioral selection system must deal with many competing rewards, goals, and priorities. This selection process must be capable of responding to many different types of reward decoded in different brain systems that have evolved at different times, even including the use, in humans, of a language system to enable long-term plans to be made. These many different brain systems, some involving the implicit (unconscious) evaluation of rewards, and others explicit, verbal, conscious, evaluation of rewards and planned long-term goals, must all enter into the selector of behavior. Although poorly understood, the issue of emotional feelings are part of the much larger problem of consciousness, and may involve the capacity to have thoughts about thoughts, that is higher order thoughts (see Rolls 1999, 2000a).
Bibliography:
- Darwin C 1872 The Expression of the Emotions in Man and Animals 3rd edn. University of Chicago Press, Chicago
- Damasio A R 1994 Descartes’ Error. Putnam
- Davis M 2000 The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton J P (ed.) The Amygdala: Second Edition. A Functional Analysis. Oxford University Press, Oxford, UK, Chap. 6, pp. 213–87
- Everitt B J, Cardinal R N, Hall J, Parkinson J A, Robbins T W 2000 Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton J P (ed.) The Amygdala: Second Edition. A Functional Analysis. Oxford University Press, Oxford, UK, Chap. 10, pp. 353–90
- Hornak J, Rolls E T, Wade D 1996 Face and voice expression identification in patients with emotional and behavioral changes following ventral frontal lobe damage. Neuropsychologia 34: 247–61
- LeDoux J E 1996 The Emotional Brain. Simon and Schuster, New York
- Moniz E 1936 Tentatives operatoires dans le traitement de certaines psychoses. Masson, Paris
- Oatley K, Jenkins J M 1996 Understanding Emotions. Blackwell, Oxford, UK
- O’Doherty J, Kringelbach M L, Rolls E T, Hornak J, Andrews C 2001 Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience 4: 95–102
- Rolls E T 1999 The Brain and Emotion. Oxford University Press, Oxford, UK
- Rolls E T 2000a Precis of The Brain and Emotion. Behavioral and Brain Sciences 23: 177–233
- Rolls E T 2000b Neurophysiology and functions of the primate amygdala, and the neural basis of emotion. In: Aggleton J P (ed.) The Amygdala: Second Edition. A Functional Analysis. Oxford University Press, Oxford, UK, Chap. 13, pp. 447–78
- Rolls E T, Hornak J, Wade D, McGrath J 1994 Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry 57: 1518–24
- Rolls E T, Treves A 1998 Neural Networks and Brain Function. Oxford University Press, Oxford, UK
- Valenstein E S 1974 Brain Control. A Critical Examination of Brain Stimulation and Psychosurgery. Wiley, New York
- Young A W, Hellawell D J, Van de Wal C, Johnson M 1996 Facial expression processing after amygdalotomy. Neuropsychologia 34: 31–9




