Sample Psychology of Face Recognition Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
There is currently much debate whether `face-specific’ neurons respond specifically to faces, or whether they are active when individuation of exemplars from other object categories with highly similar member items is required.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Behavioral Studies And Theoretical Models
Groucho Marx once said, `I never forget a face, but in your case I’ll make an exception.’ This statement is remarkable in that a person cannot actively choose to not recognize or remember a face. These processes proceed to completion without an apparently conscious effort on our part, and the complexity of this operation only becomes apparent when it breaks down, e.g., when a face appears familiar, but cannot be associated with a name or context of the original interaction. What is truly remarkable is that people can recognize faces that have not been seen for long periods of time.
After birth, probably one of the first objects seen repeatedly is a face. Infants actually attend more to faces than other stimulus categories (Morton and Johnson 1991). Being able to recognize the face of a parent is important the infant depends totally on them for nourishment and shelter. Research with children indicates that facial recognition develops fully by around 10 years of age (Carey 1992): at this time children no longer use a `piecemeal’ approach, but begin to identify faces more `holistically,’ as indicated by their impaired recognition performance when the faces are presented upside down. The inability of adults to successfully recognize inverted faces (Yin 1969) had been demonstrated previously.
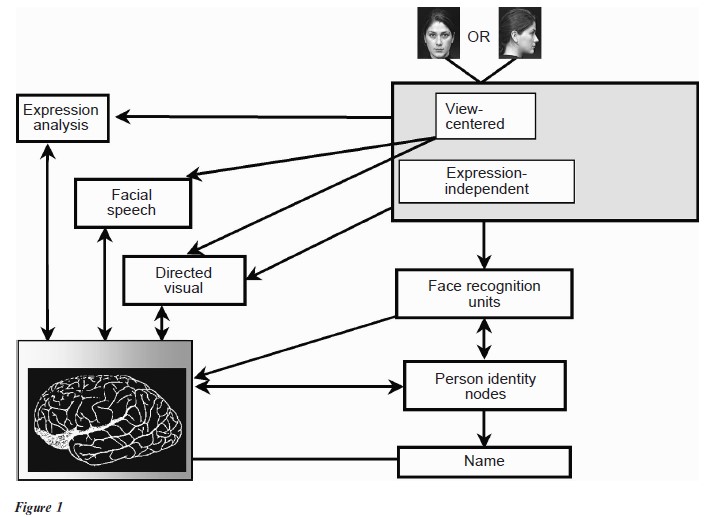
Face recognition studies in adults suggest that successful face recognition proceeds in a series of stages, based on behavioral studies of normal individuals and those with brain injury. This invuential model of face recognition (Fig. 1) was first proposed by Bruce and Young in 1986. Face perception, or detection, i.e., the ability to see a presented object as a face, and not a chair, forms the first stage of this process, the so-called structural encoding stage. The face and its features are processed holistically, and the output of the structural encoder feeds directly to so-called face recognition units (FRUs). At this stage, the familiarity judgment is made. Next, the FRU output activates so-called person identity nodes (PINs), allowing the information stored on that individual (e.g., gender, age, profession, relationship to observer, usual interaction congestive., work or home, specific details of pleasant or unpleasant previous contact with this person, etc.) to be accessed. Finally, the output from the PINs activates the name representation for that individual. The multi-part familiar facial recognition model described above can explain many errors in facial recognition that occur in everyday life (e.g., the face is familiar, but the person’s name cannot be accessed), and in cases of brain injury.
Prosopagnosia is the inability to recognize previously familiar faces. This condition can occur following a stroke or as a result of a brain tumor. The individual can no longer recognize even the faces of their spouse or children. Prosopagnosia can co-occur with other visual deficits, such as a loss of color vision (achromatopsia) and an inability to recognize everyday objects (object agnosia) (Meadows 1974). These visual deficits co-occur in brain injury, as brain regions selectively processing color, faces, and objects are located near one another. Apperceptive prosopagnosia is so named because the source of face recognition difficulty is largely due to disrupted basic visual perceptual mechanisms. This form of prosopagnosia can co-occur with object agnosia, and patients often describe a degraded, fragmented visual scene. Alternatively, basic visual perception may be fairly intact, and a face is seen as a `face,’ but the individual’s name or their personal details cannot be accessed, i.e., associative prosopagnosia. Interestingly, the ability to recognize facial expressions is dissociable from facial recognition (Humphreys et al. 1993), prompting the idea that the brain possesses parallel pathways that deal with facial identity and facial gesture, respectively (Allison et al. 2000).
Individuals with prosopagnosia do not appear to be able to recover the ability to recognize faces once the critical areas of the brain have been damaged. Remarkably, face recognition may be `hard-wired’ in the brain in the absence of postnatal experience with faces, as illustrated by a case of prosopagnosia in a 16-year old boy who sustained his brain injury at one day of age (Farah et al. 2000). This has led researchers to hunt for specialized brain circuitry that processes faces. Additionally, recordings from single nerve cells in visually sensitive regions of monkey brains show cells that respond specially to faces, and not to other object classes (for a review, see Desimone 1991, Milders and Perrett 1993). Given that humans and monkeys are both social animals, and that faces are an important stimulus in this context, it was thought likely that the human brain possesses nerve cells with similar response properties.
2. Neuroimaging And Neurophysiological Studies
In the latter part of the twentieth century many human physiological studies were dedicated to investigating the neural mechanisms underlying facial recognition (recently reviewed by Haxby et al. 2000). This was prompted, in part, by the development of neuroimaging techniques such as positron emission tomography (PET) and, more recently, functional magnetic resonance imaging (fMRI). Both methods effectively measure focal changes in brain blood how during perception and cognition. One of the firest investigations of face perception and recognition was performed by Justine Sergent working at the Montreal Neurological Institute in 1992 (Sergent et al. 1992). Sergent and her colleagues performed a PET study examining differences in cerebral blood How when normal adult subjects viewed pictures of faces and discriminated between various facial attributes. For example, subjects made gender discriminations (deciding whether a face was male or female), remembering if a particular face had been shown to the subject previously, and so on. The blood how patterns seen in these conditions were contrasted relative to conditions where subjects viewed visual material such as gratings (grids of black and white lines). These studies indented regions of the occipital and temporal lobe on the underside of the brain as being selectively active when subjects viewed and discriminated between faces. Since then many investigators have followed suit and studied other aspects of facial processing (reviewed by Haxby et al. 2000), and the studies show concordance with this initial investigation. Additionally, it is now thought that while `face-selective’ regions in both hemispheres possess the capability to process faces, it is the right hemisphere that is more important for this process. In prosopagnosia, for example, if the lesion occurs on one side of the brain it is usually on the right side (De Renzi et al. 1994).
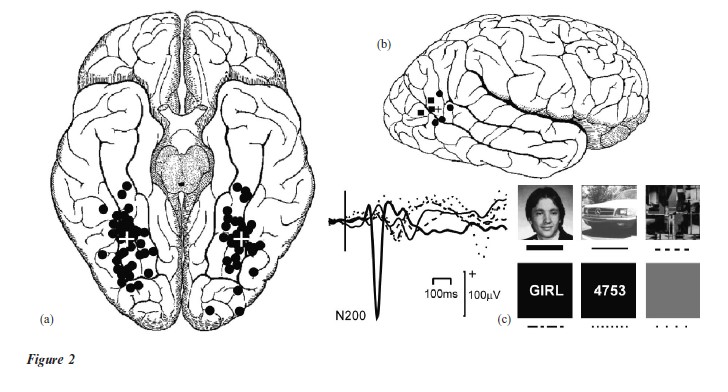
Blood how studies show what is active in the brain; however, these methods cannot examine these changes over a fine time window. Recording the electrical at around one fifth of a second (200 ms) after facial onset, known as an N200. (Modified from Puce et al. 1999)
A large voltage negative (down) wave is seen activity of the brain (EEG) can resolve when this activity occurs to thousandths of a second (millisecond). If the EEG is recorded from the scalp it may be difficult to identify where these active structures are in the brain. One potential way around this problem is to perform recordings of the electrical activity directly from the surface of the brain. This occurs in the routine assessment of patients who are being considered for epilepsy surgery. This method has allowed the `what’ and `when’ of the face recognition process to be mapped accurately in both space and time. Faceselective regions of brain on the underside (Fig. 2(a)), and side of the brain (Fig. 2(b)) have been mapped using this method. Face-specific areas in these studies overlap those seen in neuroimaging studies in healthy subjects, and the sites of injury in prosopagnosia. After a face is presented, the brain generates a large wave (N200) at around 200 milliseconds, which is negative in voltage and is around 2¬ 10− of a volt, or 200 microvolts, in size (Fig. 2(c)). The N200 event related potential (ERP) occurs irrespective of whether the observer attempts to recognize the face or not, and does not depend on the lighting conditions, size, orientation of the face, gender, or familiarity of the face (Puce et al. 1999).
The robustness of the N200 in the large number of perceptual manipulations and the seemingly automatic way in which the response is generated suggests that this may be a neural correlate of the structural encoder of Bruce and Young’s (1986) model. These data are consistent with behavioral studies of face perception, where healthy subjects can readily detect faces relative to other object categories, despite stimulus degradation, fragmentation, rotation, inversion, manipulations of light and shade, and so on (Bruce and Young 1998).
Under these same perceptual manipulations, facial recognition can be impaired. Individuating one person’s face from another requires that the features that are unique to that particular (familiar) individual are extracted and matched to a pre-existing `template.’ Manipulations that impair our ability to extract subtle spatial differences will affect successful facial recognition. For example, inverted familiar faces are difficult to recognize (compare Fig. 3(a) with Fig. 3(b)). Similarly, a negative image may make the face unrecognizable (Fig. 3(c)). We are forced to rely on idiosyncratic, incidental details like the cigar and moustache so that we can infer that we are looking at Groucho Marx’s face in Fig. 3(c). Similarly, manipulations of spatial frequency content or amount of detail of the face can also impair facial recognition (Fig. 3(d), (e)).

The ability to discriminate between individual faces is based on detecting changes in subtle spatial configurations in a homogeneous object category, unlike any other object category dealt with on a daily basis. Our-specialized facial recognition skills are so honed that behavioral studies have repeatedly demonstrated an own-race advantage for facial recognition across different ethnic groups, i.e., Caucasian, Asian (Brigham 1986). These data suggest that there really might be a basis for the often-heard comment from travelers that the faces of people of other races look alike. Different ethnic groups have idiosyncrasies in their facial features that an individual member of that particular group learns to differentiate. The expertise that develops with the individuals own ethnic group may hence not necessarily be generalizable to another ethnic group.
3. Does `Face-Specific’ Cortex Participate Only In Face Processing?
Are faces a special stimulus category? There is no doubt that we are experts with faces. However, there is debate about the nature of this expertise, and there are currently many unanswered questions regarding these issues. For example, do `face-specific’ regions of the brain deal only with faces, or are they also active in individuals who are experts with other object categories? i.e., are these neurons functioning specifically for detecting and recognizing face, or are they a more general expert `individuator’ of categories of objects with highly similar member items? (Gauthier and Logothetis 2000) Is the expertise with faces, and associated brain circuitry, that develops with the developing brain throughout childhood, different to expertise acquired with other stimulus categories in adult life?
How can these various inter-related processes be disentangled, given that we cannot test people who do not have a lifetime’s exposure to faces? There are a number of approaches that are currently being undertaken in order to try and unravel these issues. First, some insight may come from studying patients with developmental prosopagnosia (Duchaine 2000). This is an extremely rare disorder, where the individual has never developed the ability to recognize faces. Physiological and behavioral studies of face and object recognition in these individuals relative to both healthy and brain-injured subjects may shed some light on these questions. Second, face perception and recognition studies using cutting-edge neuroimaging techniques may be helpful. Direct recordings of electrical activity from the brain indicate that face sensitive regions exist in a patchy mosaic with regions responsive to objects, and to words, for example. The relatively coarse spatial resolution in most neuroimaging studies to date could produce blood-Їow measures containing contributions from different kinds of category-specific regions, making it difficult to evaluate exactly how these brain regions deal with facial information. Studies of facial recognition performed with (high field strength) functional MRI combined with recordings of the electrical activity of the brain in the same subject may finally shed some light on why it is impossible to forget a face, despite our best attempts to do so.
Bibliography:
- Allison T, Puce A, McCarthy G 2000 Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences 4: 267±78
- Brigham J C 1986 The impudence of race on face recognition. In: Ellis H D, Jeeves M A, Newcombe F, Young A (eds.) Aspects of Face Processing. Martinus Nijhoff, Dordrecht, The Netherlands, pp. 170±77
- Bruce V, Young A 1986 Understanding face recognition. British Journal of Psychology 77: 305±27
- Bruce V, Young A 1998 In the Eye of the Beholder. The Science of Face Perception. Oxford University Press, Oxford, UK
- Carey S 1992 Becoming a face expert. Philosophical Transactions of the Royal Society London: Biology 335: 95±103
- De Renzi E, Perani D, Carlesimo G A, Silveri M C, Fazio F 1994 Prosopagnosia can be associated with damage confined to the right hemisphere an MRI and PET study and a review of the literature. Neuropsychologia 32: 893±902
- Desimone R 1991 Face-selective cells in the temporal cortex of monkeys. Journal of Cognitive Neuroscience 3: 1±8
- Duchaine B C 2000 Developmental prosopagnosia with normal configural processing. NeuroReport 11: 79±83
- Farah M J, Rabiowitz C, Quinn G E, Liu G T 2000 Early commitment of neural substrates for face recognition. Cogniti e Neuropsychology 17: 117–23
- Gauthier I, Logothetis N K 2000 Is face recognition not so unique after all? Cognitive Neuropsychology 17: 125–142
- Haxby J V, Hoffman E A, Gobbini M I 2000 The distributed human neural system for face perception. Trends in Cognitive Science 4: 223–33
- Humphreys G W, Donnelly N, Riddoch M J 1993 Expression is computed separately from facial identity, and it is computed separately for moving and static faces: neuropsychological evidence. Neuropsychologia 31: 173–181
- Meadows J C 1974 The anatomical basis of prosopagnosia. Journal of Neurology, Neurosurgery and Psychiatry 37: 489–501
- Milders M V, Perrett D I 1993 Recent developments in the neuropsychology and physiology of face processing. Baillieres Clinical Neurology 2: 361–88
- Morton J, Johnson M 1991 CONSPEC and CONLEARN: A two-process theory of infant face recognition. Psychological Reviews 98: 164–81
- Puce A, Allison T, McCarthy G 1999 Electrophysiological studies of human face perception, III. Effects of top-down processing on face-specific potentials. Cerebral Cortex 9: 445–58
- Sergent J, Ohta X, McDonald X 1992 Functional neuroanatomy of face and object processing. Brain 115: 15–36
- Yin R K 1969 Looking at upside-down faces. Journal of Experimental Psychology 81: 141–45




