Sample Neural Basis Of Motivation Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
This research paper will describe the organization of the neural systems and their interactions that are critical for the expression of motivated behaviors. Presented first is an influential model of how motivated behaviors are organized temporally that will provide the background for discussing the structure of underlying neural circuits. It will emphasize that any model or circuit analysis must ultimately account for the complexity of all behavioral components, and must explain how temporal sequences (ethograms) of behavioral episodes are organized and controlled. The neural systems thought to be critical for generating motivated behaviors are then discussed. At this point however, it is worth considering that, although the forebrain plays a critical role, there is probably no single neural system that puts the ‘motivation’ into ‘behavior.’
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Temporal Organization Of Motivated Behaviors
Motivated behavior is initiated by the neural interaction of interosensory information, exterosensory information, and arousal state. The outcome of this integration determines the value of the ‘drive’ associated with a particular behavior, and is used to select the most appropriate behavioral action, which is then expressed as a procurement (or appetitive) phase where the goal object is actively sought out (Toates 1986). Procurement behaviors include foraging, are individualized for the particular situation, can be quite complex, and are under cognitive control. When the goal object has been located, a consummatory phase allows the animal to interact directly with the goal object with more stereotypic rhythmic movements— licking, chewing, copulating, etc. Reward aversion functions, together with learning and memory processes are also critical during the procurement and consummatory phases; a previously rewarded or aversive experience of a particular goal object, remembering where it is located, and how to get there are important considerations. Finally, as the consummatory phase continues, a variety of interosensory feedback signals are generated that increase the probability that it will be terminated (commonly called satiety), presumably through the action of inhibitory networks. However, termination may also arise from separate exterosensory signals (e.g., the presence of a predator) that can override the ongoing behavior at any time, so allowing the animal to switch immediately to one that is more appropriate (McFarland and Sibly 1975).
2. Neural Systems
At the simplest level, four broad-ranging neural systems are concerned with generating motivated behaviors: those involved with the transduction and processing of sensory signals; those that control arousal state; those that process the types of information that neurally represents sensory objects; and those involved with motor control (Fig. 1). Using this scheme, four principal sets of inputs activate motor systems and generate motivated behaviors. The most complex motor control processes are those that generate anticipatory Behaviors and their accompanying autonomic and neuroendocrine motor events. In some instances, information derived from the systems controlling arousal state—for example, the circadian clock—may provide the predominant signals (input 1, Fig. 1). But in many instances this type of anticipatory control derives from the interactions between processed sensory information and those forebrain systems concerned with encoding object representation, particularly learning and memory, reward, and spatial orientation. The integrated output of these regions then influences the motor control systems (input 2, Fig. 1). However, increased drives for motivated behavior can also be produced by internally-generated deficit signals (e.g., the thirst arising from dehydration, or the hunger from starvation) that engage motor control networks more directly (input 3, Fig. 1). Finally, simple reflex actions (e.g., reflex rejection of unpalatable foods or fluids) are generated by more or less direct sensory inputs to the pre-motor and motor networks with little higher-order processing (input 4, Fig. 1). But of course, these reflex actions lack any motivated character.
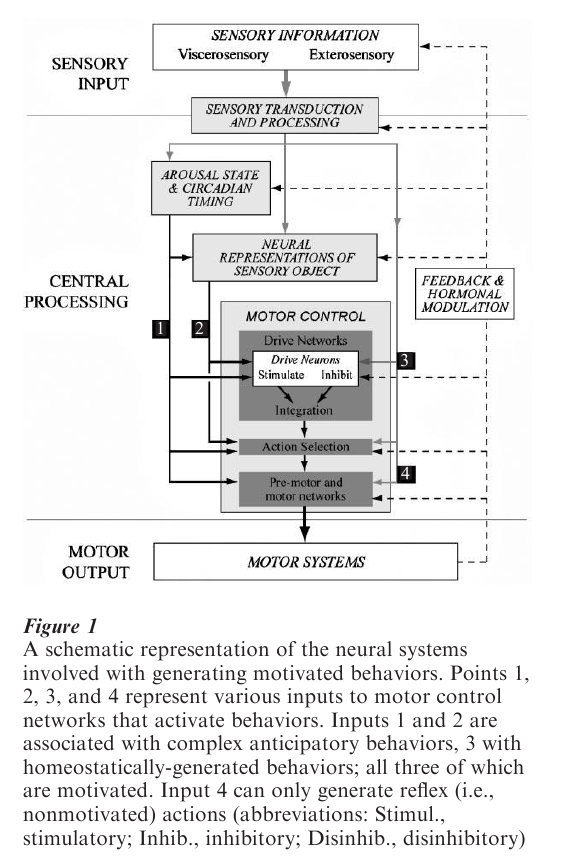
The notion of drive and the reduction in the level of specific drive states following particular behaviors has been a very influential if somewhat controversial concept in neuroscience. The idea that some neural mechanism selects the behavior that is the most appropriate to reduce the drive state that has the highest value is a useful one from the perspective of delineating neural systems. In this way, drive is determined by an interaction between arousal state, those interosensory signals that encode internal state, and sensory object representations that effectively encode the current incentive value of a particular goal object (see Toates 1986). In this way, inputs 1, 2, and 3 to motor control networks (Fig. 1) can be thought of as ‘drive-determining’ interactions. Finally, the integration of this information within motor control systems governs which action is selected, and thereby which particular behavior is expressed.
2.1 Sensory Information
The neural systems that control motivated behaviors are regulated by a host of sensory inputs, which can be categorized either as interosensory signals that encode internal state or exterosensory inputs that encode features of the goal object such as smell, taste, temperature, tactile properties, and appearance. Each of these sensory signals has specific transduction mechanisms and ‘labeled line’ access to central processing networks found throughout the brain. Although a great deal of important sensory processing occurs within the telencephalon—particularly sensory cortex—the preliminary sensory processing that occurs subcortically can have important implications for the control of motivated behaviors, for example the altered gustatory processing that occurs in the hindbrain of hyponatremic animals is an important adjunct to increased sodium appetite.
Some sensory signals can access command networks directly, as typified by the drinking initiated by the effects of increasing plasma osmolality or angiotensin II (A-II; see Thirst and Drinking), or the deficitinduced feeding activated by those adiposity signals (primarily leptin and insulin) that have direct hypothalamic actions (Elmquist et al. 1999). In both these cases, it is not clear whether the initial sensory processing of deficit signals utilize the sensory object representation networks, but the fact that hypothalamic regions involved with this type of sensory transduction project directly to regions concerned with ingestive motor control, suggests that they may not.
2.2 Arousal State Control And Behavioral Timing
Parts of the brain provide critical timing information and control arousal state so enabling motor command networks to generate the types of actions that are anticipatory. This system includes the suprachiasmatic nucleus in the hypothalamus, which generates the circadian timing signal that entrains virtually all neural activity within limits determined by the prevailing photoperiod. In terms of arousal state, catecholamine cell groups in the hindbrain (e.g., the locus coeruleus), histaminergic neurons in the tuberomammillary nucleus, the ventrolateral preoptic nucleus, and the recently identified hypocretin orexin neurons in the LHA also supply information that is of critical importance for controlling arousal state.
2.3 Neural Representation Of Sensory Objects
Some processes important for motivational motor control generate neural representations of sensory objects. These include the following: learning and memory mechanisms in the telencephalon and cerebellum; reward aversion systems in the midbrain ventral tegmentum, parts of the basal forebrain (particularly the nucleus accumbens), amygdala, and parts of the cortex, particularly prefrontal regions; and systems in the hippocampus and parts of the cortex responsible for allocentric and egocentric spatial representation. A great deal of exterosensory information is processed through these networks, which collectively assign what has been called ‘incentive value’ to a particular goal object (Toates 1986). The neural pathways mediating the interactions between the object representation and motor networks are not fully understood, but sets of bidirectional connections between the hypothalamus and cortical structures such as the prefrontal cortex and hippocampus, as well as subcortical regions such as the amygdala, septal nuclei, bed nuclei of the stria terminalis, and basal ganglia are all likely to be critical in the integrative operations that designate and coordinate the full spectrum of motor events associated with motivated behaviors (Saper 1985, Risold et al. 1997, Swanson and Petrovich 1998).
2.4 Motor Control
Fig. 1 shows that motor control systems operate at three levels: drive networks that set up and coordinate specific motor events; action selection systems concerned with the planning, selection, and the moment-to-moment execution of particular motor actions; and the premotor/motor neuron networks that actually execute them motor actions. Furthermore, Fig. 1 shows a clear distinction between drive networks and object recognition systems. This derives from the fact that object representation systems are goal nonspecific, whereas drive networks are explicitly concerned with specific behaviors. For example, an animal uses the same networks in the telencephalon for spatial navigation whether it is looking for food, a mate, or shelter, whereas parts of the hypothalamus are concerned exclusively with feeding or sexual behavior. However, in terms of the organization of other neural structures (e.g., cell groups in the basal ganglia), this distinction may not be so clear-cut, and it may prove difficult to determine experimentally within which system a particular set of neurons belongs.
2.4.1 Drive Networks. The concept of drive networks evolved from the idea of discretely localized hypothalamic satiety and hunger ‘centers’ popular during the 1950s and 1960s. The center concept was synthesized by Eliot Stellar into a scheme for explaining the role of the hypothalamus in controlling motivated behaviors (Stellar 1954). Elaborating these pioneering studies since has replaced the idea of isolated ‘centers’ with a scheme where sets of more widely distributed but highly interconnected motor drive networks direct the somatic, autonomic, and neuroendocrine motor responses that accompany particular behaviors. In accord with Stellar’s original idea, each individual drive network contains neurons that either stimulates or inhibits a particular motor event. The exact nature of the expressed behavior, or whether it is expressed at all, is then determined by the integrated output of these neurons. Furthermore, it is likely that a separate series of circuits is concerned with disinhibiting particular motor actions. Although the location of these disinhibitory circuits is unclear, Swanson (2000) has suggested that neurons in the pallidum may function in this way.
Determining the precise anatomical substrates of the drive networks has proved quite difficult. However, a wealth of data cataloging the stimulatory or inhibitory effects of neuropeptides on particular motor functions clearly concurs with earlier lesion and stimulation experiments that have identified the hypothalamus as a key locus for the components of specific drive networks. Many groups have identified which hypothalamic neurons synthesize these neuropeptides and have determined where they project. One critical point that has emerged from these studies is that placing individual hypothalamic cell groups within one or other of drive network is rather difficult. This is because traditionally-defined cell groups such as the lateral hypothalamic area (LHA), arcuate (ARH), or paraventricular (PVH) nuclei appear to contain elements that, in terms of function, belong to more than one type of drive network; it seems unlikely that there is a tight ‘one cell group–one network’ relationship in the hypothalamus.
A well-documented example of a stimulatory network is the one activated by circulating A-II to stimulate drinking. Results from many studies have shown that A-II is detected by a central sensory transducer, the subfornical organ (SFO), which then directly and specifically stimulates water intake. The SFO provides efferents—most of which also contain A-II (Swanson 1987)—to a relatively limited set of structures, including parts of the prefrontal cortex, substantia innominata, medial preoptic area, bed nuclei of the stria terminalis, zona incerta, PVH, supraoptic nucleus, and LHA (Swanson 1987); presumably it is these regions that constitute part of the stimulatory network that initiates the motor aspects of drinking. Neuropeptide Y (NPY) neurons in the ARH contribute to a well-known example of a stimulatory eating mechanism; results from many studies show that NPY contributes to a circuit that directly stimulates food intake.
In terms of inhibitory networks, feeding again provides good examples of hypothalamic components. For example, the peptide α-MSH is synthesized in ARH neurons from the proopiomelanocortin (POMC) gene and provides an inhibitory signal to feeding that acts at melanocortin 4 receptors ex- pressed by neurons in the LHA and PVH (Elmquist et al. 1999). POMC neurons in the ARH and retro-chiasmatic area also express cocaine and amphetamine-regulated transcript. This widely expressed neuropeptide will inhibit eating without affecting spontaneous drinking when injected introcerebroventricularly.
Finally, interactions between different motivational drive systems, particularly in the hypothalamus are of paramount importance. For example, the effects of starvation are not limited just to increasing the drive to eat, they also reduce reproductive capacity. Similarly, dehydration leads to severe anorexia as well as increasing the drive to drink (Watts 2001). This crossbehavioral coordination is achieved by hormonal modulation acting together with the divergent neuroanatomic outputs from individual drive networks.
2.4.2 Premotor And Motor Networks. The brain has three sets of motor neurons with which to effect both motivated behavioral action and the internallydirected motor actions that accompany it: α-motor neurons in the ventral horn of the spinal cord that control the striate musculature and hence the expression of all behavior; postganglionic sympathetic and parasympathetic motor neurons that control autonomic motor function; and sets of parvicellular and magnocellular neuroendocrine motor neurons in the hypothalamus that control pituitary function. Motivated behaviors involve the coordinated activation, to a greater or lesser degree, of all three motor systems.
Sets of premotor networks directly control oscillatory and more complex patterns of motor neuron firing. Simple rhythmic movement patterns develop from an interaction between oscillatory rhythm generators, which directly involve the motor neurons, and networks of premotor central pattern generators located somewhat more distally in the spinal cord and hindbrain. A critical feature of these pattern generators is that they are capable of producing rhythmic output without sensory input. In turn, pattern generator output is modulated further by afferents from those parts of the appropriate drive networks in the diencephalon and telencephalon. These often highly varied inputs provide the critical command and contextual information from object representational networks for selecting the most appropriate motor program at any particular time. Collectively, all these components begin to account for how motivated behavior is organized.
2.5 Feedback And Humoral Modulation
Feedback is a critical feature of behavioral control, and sensory signals encoding the magnitude and consequences of generated motor actions can control the length of a motivated behavioral episode. For example, postabsorptive humoral feedback (e.g., increasing CCK or decreasing plasma osmolality) and interosensory signals (e.g., gastric distension, oropharyngeal metering) lead to the termination of ingestive behaviors and subsequent behavioral refractoriness. Finally, rather than acting as feedback control a further set of regulatory signals are more modulatory and can influence a variety of neural structures at all brain levels. Steroid hormones, particularly gonadal steroids, are important components of this type, and many familiar motivated behaviors are critically regulated by this type of signal.
Bibliography:
- Elmquist J K, Elias C F, Saper C B 1999 From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron 22: 221–32
- McFarland D J, Sibly R M 1975 The behavioural final common path. Philosophical Transactions of the Royal Society of London, Series B 270: 265–93
- Risold P Y, Thompson R H, Swanson L W 1997 The structural organization of connections between hypothalamus and cerebral cortex. Brain Research Reviews 24: 197–254
- Saper C B 1985 Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. Journal of Comparative Neurology 237: 21–46
- Stellar E 1954 The physiology of emotion. Psychological Reviews 61: 5–22
- Swanson L W 1987 The hypothalamus. In: Bjorklund A, Hokfelt T, Swanson L W (eds.) Handbook of Chemical Neuroanatomy. Elsevier, Amsterdam, pp. 1–124
- Swanson L W 2000 Cerebral hemisphere regulation of motivated behavior. Brain Research 886: 113–64
- Swanson L W, Petrovich G D 1998 What is the amygdala? Trends in Neuroscience 21: 323–31
- Toates F 1986 Motivational Systems. Cambridge University Press, Cambridge, UK
- Watts A G 2001 Neuropeptides and the integration of motor responses to dehydration. Annual Review of Neuroscience 24: 357–84




