Sample Costs of Psychopharmacotherapy Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
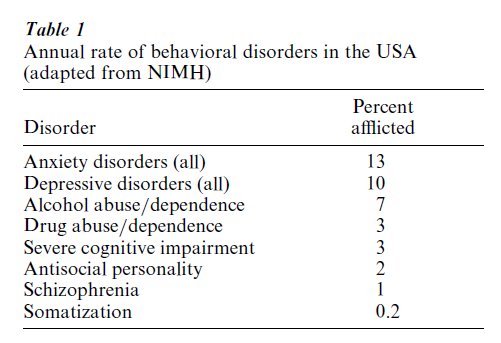
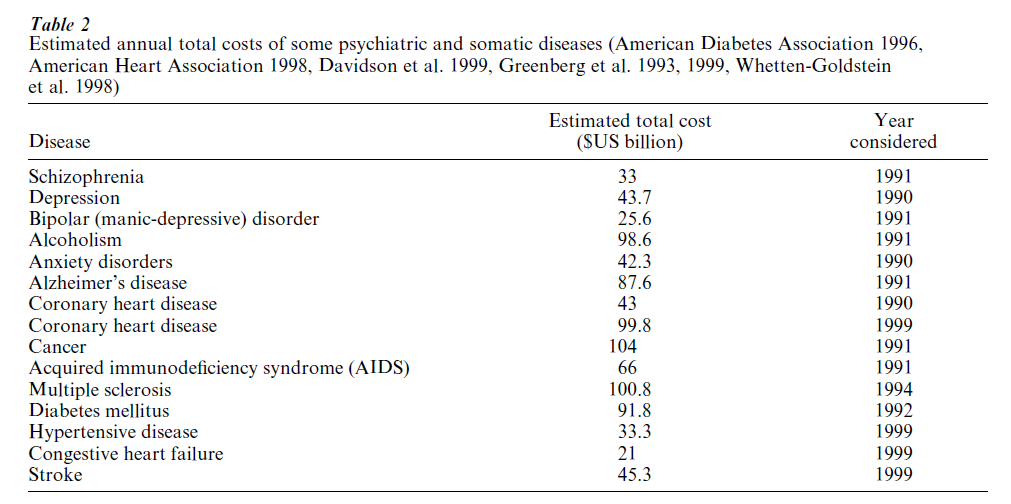
Psychopharmacotherapy is one of the mainstays of the treatment of psychiatric disorders besides social therapy and psychotherapy. Psychiatric disorders essentially comprise the affective disorders (depression, bipolar, i.e., manic-depressive disorder), the schizophrenias, the anxiety disorders (panic disorder, generalized anxiety, post-traumatic stress disorder, obsessive–compulsive disorder, social phobia), the eating disorders (bulimia and anorexia nervosa), the substance abuse and dependence disorders including alcoholism, the personality disorders, and the dementias (with Alzheimer-type dementia being the cause in about 70 percent of cases). They are highly prevalent (Table 1) and frequently take a chronic or chronic remittent course. Therefore, it is easily understandable that the total costs of psychiatric disorders compare very well with the costs of the leading somatic illnesses (Table 2). The prevalences of depression and of the eating disorders are increasing. The WHO burden of illness study (Murray and Lopez 1997) suggests that depression will be the leading cause of disability in the developed countries in 2020. Psychiatric disorders (except the dementias) manifest in early adulthood thus limiting the abilities of patients for social achievement.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Psychopharmacotherapy essentially comprises seven classes of drugs:
(a) neuroleptics for the treatment of psychotic states, especially the schizophrenias;
(b) antidepressants for the treatment of depression of whatever origin as well as for the long-term treatment of anxiety disorders;
(c) mood stabilizers (lithium, some anticonvulsants) for the short-term treatment of mania and for the long-term relapse and recurrence prevention of affective disorders;
(d) minor tranquilizers (essentially benzodiazepines) for the short-term treatment of anxiety and agitation of whatever origin and of alcohol withdrawal syndromes;
(e) hypnotics (essentially benzodiazepines and benzodiazepine-like drugs) for short-term treatment of insomnia;
(f ) anticraving compounds (bupropion for smoking cessation, acamprosate for alcohol abstinence, opiate antagonists for alcoholism and opiate dependence) which, however, are not yet approved in all countries; and
(g) cognition enhancers (nootropics such as piracetam, ginkgo biloba) for the nonspecific treatment of dementias and acetylcholinesterase inhibitors specifically for Alzheimer-type dementia.
Concerning the eating disorders, only one drug, the antidepressant fluoxetine, is approved for supportive treatment of bulimia.
Psychopharmacotherapy competes with psychotherapy in the treatment of mild to moderate de pression and the anxiety disorders. The relative merits of both alternatives are subject to debate which is fueled by a lack of scientific data, ideology, and economic interests of the health care providers involved. As a rule of thumb, pharmacotherapy and psychotherapy may be thought not to be alternatives but supplements whenever a psychiatric disorder is moderate to severe, although a synergistic action remains to be definitely proved.
Life expectancy increased dramatically in the twentieth century, and this was accompanied by an increase in the prevalence of age-associated diseases. In the field of psychiatry these are the dementias. Thus, although the number of healthy life years is generally increasing, the prevalence of chronic diseases is in- creasing in parallel. Consequently, the demand for health care services is increasing. Costs for drug therapy are increasing overproportionately because drug development has become increasingly complicated and costly. The mean investment necessary for developing a single new chemical entity to approval amounts to about US$500 million.
Worldwide, health care is one of the most rapidly growing markets with positive impacts on employment. Nevertheless, governments internationally tend to restrict the growth because at least part of it is publicly funded. The fundamental but unanswered question is what proportion of the gross domestic product society is willing to invest in health care. Obviously, however, most people value health and thus health care very highly. Thus, disorders, i.e., the patients suffering from them, compete for limited resources. Economic criteria have become a third dimension besides efficacy and effectiveness as well as tolerability and safety when selecting between treatment options. It is easily conceivable that psychiatric disorders, faced with persistent stigma, have a hard standing in this competition.
Pharmacoeconomics, a young scientific discipline, has developed a sound scientific basis for the evaluation of the economic dimension (Gold et al. 1996). This is indispensable in the interests of an ethically justifiable allocation of resources. The question is whether a new, more costly drug allows one to achieve better outcomes so that it is worth its higher costs. In principle, a new drug might even spare money in that the better outcome might help avoid costly, higher-level health care such as inpatient treatment and reduce indirect costs, e.g., by less absenteeism.
1. Basic Terms Of Pharmacoeconomics
Pharmacoeconomics is the science of measuring costs and outcomes associated with the use of pharmaceuticals in health-care delivery. Its objective is to improve public health through rational decision making when selecting among alternative therapies, e.g., for formularies and their impact on costs and outcomes. Several methodological guidelines have been published (e.g., CCOHTA 1994).
Outcomes are the possible end result of a particular treatment. Outcomes depend on the effectiveness of the treatment under the influence of all the confounding variables in real life. Therefore, costs have to be measured under real-life conditions. In contrast, the experimental efficacy trials are designed to eliminate as completely as possible all confounding influences. The outcome parameter chosen should have high everyday relevance, e.g., the rate of full remissions of depression.
Costs are the resources consumed by the illness and by its treatment. Direct costs are those directly related to treatment such as prescribed medication, self-medication, consulting the physician, blood tests, transportation, psychotherapy, occupational therapy, informal care by relatives, etc., as well as costs for hospital and nursing-home treatment. Direct costs include those for diagnosis and treatment of side effects and complications of the initial therapy. There is methodological controversy as to whether the costs of social support (welfare, unemployment benefit, disability pension, housing benefit, etc.) should be included in the direct costs. A complete evaluation of direct costs should consider the opportunity costs, i.e., the lost potential of the resources invested to provide more efficient benefits in other fields.
Indirect or future costs are those related to lost productivity due to illness, i.e., due to absenteeism, premature pensioning because of disability, and premature death (e.g., by suicide) as well as those related to health care consumption during the years of life gained owing to the intervention. Concerning psychiatric disorders, costs of violence and crime also have to be considered. Intangible costs comprise those attributable to impairments of the quality of life, e.g., of the patients themselves and their relatives and acquaintances involved in the care.
The economic impact of treatment extends into the future. If costs and benefits accrue during different periods, costs and savings have to be discounted for the remaining life-span of the patient sample in question. Discount rates are supposed to reflect the rates of return on private-sector investments and are generally set at 3–5 percent.
If the costs are low and the outcomes excellent the decision in favor of the treatment is obvious as is its rejection if the costs for poor outcomes are high. Therefore, pharmacoeconomic analyses make sense only when both the costs and the quality of treatment are high or low. The economic efficiency then is the ratio of costs divided by the outcomes. When allocating resources the drug yielding the most favorable outcomes at the lowest cost will be favored.
2. Types Of Pharmacoeconomic Analysis
The basic requirement of a pharmacoeconomic analysis is the definition of the perspective, e.g., that of the patient, the health care provider, the health insurance as the payer, or the societal perspective of government. The categories of costs to be considered depend on this perspective. For example, from a provider’s perspective, the value of an antidepressant drug under capitation may be expressed in terms of cost per reduction in some depression scale score. From the patient’s perspective, the value may be in cost per full treatment response with least side effects. From the societal perspective, the value may be in cost per patient returning to continuous employment at or above the level of previous education. Simply to compare the daily costs of medication does not fulfill the criteria of pharmacoeconomic analysis.
The principle types of analysis include costminimization analysis, cost-effectiveness analysis, cost-utility analysis and cost-benefit analysis. Costminimization analysis (CMA) assumes that the interventions in question yield identical outcomes; this is rarely valid. Cost-effectiveness analysis (CEA) compares the costs of alternative treatments in terms of a specific health outcome such as life-years gained, e.g., by reducing mortality from suicide, or responder rates. Cost-utility analysis (CUA) adjusts the life-years gained by some measure for quality of life. Thus, the results of CUA are typically expressed as costs per quality adjusted life years (QALYs). Quality of life is assessed either by some rating scale for health-related quality of life or, more typically, by determining the preferences of patients suffering from specific diseases for one or the other disease state as opposed to perfect health where perfect health is set as ‘1’ and death as ‘0.’ In cost-benefit analysis (CBA), the QALYs gained are transformed into monetary terms using the human capital approach or willingness-to-pay approach.
As there is no one-to-one relationship between therapeutic intervention and outcome and its financial sequelae, the impact of these uncertainties has to be estimated. This is done by sensitivity analysis, where key variables are systematically modified and the economic consequences calculated yielding bestversus worst-case scenarios.
3. Pharmacoeconomic Study Designs
There are two principle approaches to pharmacoeconomic analysis: the clinical trial approach (experimental or observational), and the modeling or systems approach. The former means gathering cost data (retrospectively or, preferably, prospectively) in clinical trials. The latter ‘in vitro’ approach applies decision-tree analysis to data available from published literature and databases.
Current guidelines favor the clinical trial approach as the ‘gold standard.’ Modeling, however, can overcome limitations implicit in clinical trials. These include the selection bias concerning the patients recruited in randomized, strictly controlled, and therefore artificial efficacy trials, thus limiting generalizability. Furthermore, their observation period rarely exceeds a few months whereas economic consequences may extend far into the future. Efficacy trials necessarily are underpowered for economic analysis because sample size estimation is based on the critical difference and variability of the efficacy parameter. Obviously, the critical difference and variability of economic data will fundamentally differ. Clinical trials using the mirror image design, i.e., comparing costs and benefits in a defined period before and after administering a specific drug, are of limited value because at least in psychiatric disorders there is a spontaneous decrease of the costs per outcome during the course of illness irrespective of the mode of treatment.
The putatively best experimental design is a prospective, randomized, ‘naturalistic,’ i.e., non-blind, adequately powered clinical trial. Both principle approaches, the clinical trial approach and modeling, have their weaknesses and strengths so that they are not really exclusive alternatives but complementary.
Friedberg et al. (1999) found that studies sponsored by drug companies were less likely to report unfavorable results than studies funded by nonprofit organizations. This finding could be due to various sources of bias which, however, if present, still have to be identified.
The organization of health-care supply, the healthcare utilization patterns, and the prices differ considerably between and even within cultures. Therefore, the results of pharmacoeconomic analyses cannot simply be transferred from one health care market to another.
4. Pharmacoeconomic Findings With Neuroleptics
Since the 1990s new neuroleptics have been introduced (amisulpride, clozapine, olanzapine, quetiapine, risperidone, sertindole, zotepine), although not yet approved in all countries. These compounds are called ‘atypical’ because they have a lower incidence of extrapyramidal side effects (e.g., Parkinsonism) than conventional neuroleptics (e.g., haloperidol, fluphena-zine). Moreover, they possibly have superior efficacy concerning cognitive dysfunction and so-called negative symptoms as well as quality of life in schizophrenia. These advantages might improve compliance, which is most important in long-term relapse and recurrence prevention. The costs of daily treatment with atypical neuroleptics exceed those of conventional neuroleptics by a factor of 2–8 depending on the compound and the country considered. On the other hand, neuroleptic medication costs generally make only about 4–8 percent of direct costs.
The pharmacoeconomic analyses actually available for neuroleptics are limited to amisulpride, clozapine, olanzapine, risperidone, and sertindole in schizophrenia. The majority of studies address clozapine, olanzapine, and risperidone. The costs are mostly considered from the payer’s perspective, i.e., addressing direct costs. Most are cost-effectiveness analyses, some cost-utility analyses. The majority follow the modeling approach. The economic analyses based on clinical efficacy trials have the limitations discussed above, especially those restricting the analysis to study completers. This is far from the naturalistic, real-world approach necessary. There is only one randomized, naturalistic, adequately powered trial comparing clozapine with conventional neuroleptics, where clozapine significantly reduced the frequency and duration of inpatient treatment; the cost data are to be reported. All in all, the current evidence suggests that clozapine is cost-effective for neuroleptic-refractory schizophrenia and that olanzapine and risperidone therapy may be cost-neutral despite higher medication costs (Revicki 1999). Nevertheless, the access of patients to the new therapeutic chances is restricted on economic grounds, although this varies internationally.
5. Pharmacoeconomic Findings With Antidepressants
Since the 1980s, a number of new antidepressants have been introduced (selective serotonin reuptake inhibitors (SSRI): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline; the selective norepinephrine reuptake inhibitor reboxetine; the serotonin–norepinephrine reuptake inhibitor venlafaxine; the norepinephrine autoand heteroreceptor blocker mirtazapine; the reversible and selective inhibitor of monoamine oxidase moclobemide), although not yet approved in all countries. Their efficacy is comparable to that of traditional tricyclic antidepressants (TCAs such as amitriptyline, imipramine, desipramine, nortriptyline), but owing to selectivity their tolerability and safety are superior. Safety is of specific concern in view of the suicide risk, although antidepressants are involved only in the minority ( < 10 percent) of suicides. These advantages help reduce drop-out rates although to a limited degree (about 25 percent versus 29 percent). Nevertheless, this might be particularly relevant in long-term recurrence prevention which is necessary in about 60 percent of patients in whom antidepressants are indicated. There is a dramatic underrecognition and undertreatment worldwide (Davidson et al. 1999, Tylee et al. 1999). Undertreatment causes a doubling of direct costs due to increased utilization of other medical services (Manning and Wells 1995) and increases indirect costs due to absenteeism. The advantages of innovative antidepressants might increase acceptability and thus utilization of antidepressants. The costs of daily treatment with selective antidepressants, however, exceed those of TCAs by a factor of 2–20 depending on the compound and the country considered. On the other hand, antidepressant medication costs generally constitute only about 3–6 percent of direct costs.
Pharmacoeconomic analyses published cover all innovative antidepressants except reboxetine. All but one (Gould et al. 1995) are restricted to depression. The costs are mostly considered from the payer’s perspective. Most are cost-effectiveness analyses, only a few cost-utility and cost-benefit analyses. There are no randomized, open-label, naturalistic, adequately powered trials but some studies analyzed large, complete samples of HMO participants. By far the majority follow the modeling approach. There are some outstanding analyses in which classes of compounds are compared (e.g., CCOHTA 1997, Casciano et al. 2000). Overall, the current evidence suggests that extended-release venlafaxine might be more costeffective than SSRIs and that the cost-effectiveness of SSRIs might equal or even exceed that of TCAs, despite higher medication costs (Conner et al. 1999, Casciano et al. 2000). However, more research is needed before this can be taken for granted. Accordingly, there is debate as to whether the access of patients to innovative antidepressants should be restricted or whether they should be recommended as first-choice drugs (CCOHTA 1997).
The limited evidence available suggests that psychotherapy is more likely to be cost-effective the more severely afflicted the patients are (Gabbard et al. 1997). The cost of psychotherapy is about 3–6 times higher than that of antidepressant drug therapy. One modeling study (Antonuccio et al. 1997) suggests, however, that cognitive behavioral therapy (CBT) might be more cost-efficient than fluoxetine treatment of depression owing to lower recurrence rates after psychotherapy. In a CUA based on a randomized controlled trial (Lave et al. 1998), nortriptyline was slightly superior to interpersonal psychotherapy (IPT). One meta-analysis (Gould et al. 1995) suggests that CBT is superior to imipramine and cost-effective in panic disorder. Economic analyses addressing the relative merits of pharmacotherapy and psychotherapy are completely lacking for prominent diseases such as obsessive–compulsive disorder, generalized anxiety, and social phobia.
6. Pharmacoeconomic Findings With Mood Stabilizers
Mood stabilizers by definition reduce the risk of relapses and recurrences and thus putatively hospitalization. Therefore, they may be expected to reduce direct costs (about 60 percent for hospitalization). Lithium is the only drug reducing mortality essentially by reducing the suicide rate (Ahrens 1997). Only a few pharmacoeconomic analyses have been published. Lithium was more cost-effective than carbamazepine from the payer’s perspective in a modeling approach based on retrospectively gathered data from a randomized controlled clinical trial (Dardennes et al. 1999). Valproate was more cost-effective than lithium over 1 year from the payer’s perspective in a modeling approach (Keck et al. 1996); the prophylactic efficacy of valproate, however, remains to be established.
7. Pharmacoeconomic Findings With Tranquilizers And Hypnotics
There are almost no economic analyses published on tranquilizers or hypnotics. Yuen et al. (1997) found that hospital stay was longer and costs were higher in elderly people if sedative–hypnotic medications were prescribed, even if prescription adhered to established guidelines. Research is needed especially to clarify whether the higher costs of the newer hypnotics (zaleplon, zolpidem, zopiclone) compared with benzodiazepines are economically justified.
8. Pharmacoeconomic Findings With Anticraving Drugs
Long-term acamprosate adjunctive to conventional therapy was found to be cost-effective in the treatment of alcoholism in the German setting in a single, retrospective modeling study (Schadlich and Brecht 1998). Opiate antagonists (Howard et al. 1996), opiate agonist (methadone) substitution, and bupropion have not been evaluated economically.
9. Pharmacoeconomic Findings With Cognitive Enhancers
There are no economic analyses published on nootropics. The acetylcholinesterase inhibitors donepezil (Foster and Plosker 1999) and rivastigmine (Fenn and Gray 1999) seem to be at least cost-neutral, especially by delaying the need for institutionalization. The current evidence is restricted, however, to a few observational studies and modeling analyses using the cost-effectiveness approach from the payer’s perspective. Economic analyses on galanthamine, an acetylcholinesterase inhibitor and nicotinic agonist expected to be approved, remain to be published.
10. Conclusions And Some Precautions
The innovative neuroleptics and antidepressants not only have the potential for a balance of costs despite higher costs of medication, but might even save direct costs essentially by reducing the risk of institutionalization owing to lower drop-out rates in comparison with traditional compounds. This conclusion, however, is based essentially on decision analyses, i.e., modeling. More research, particularly large-scale, randomized trials under naturalistic conditions, is needed. From a systemic perspective, the potential cost savings can only be realized if the institutions concerned, e.g., hospitals, reduce their capacities accordingly. About 80 percent of the costs of hospitals are common costs, mostly wages and salaries. Whereas in a free health-care market the savings might be realized by market forces, in systems bound in economic welfare a political will is needed to enforce the inevitable dismissal of staff.
Bibliography:
- Ahrens B 1997 Mortality studies and the effectiveness of drugs in long-term treatment. Pharmacopsychiatry 30 (Suppl. 1): 57–61
- Antonuccio D O, Thomas M, Danton W G 1997 A cost-effectiveness analysis of cognitive behavior therapy and fluoxetine (Prozac) in the treatment of depression. Behavior Therapy 28: 187–210
- Bacaltchuk J, Trefiglio R P, Oliveira I R, Hay P, Lima M S, Mari J J 2000 Combination of antidepressants and psychological treatments for bulimia nervosa: a systematic review. Acta Psychiatrica Scandinavica 101: 256–64
- CCOHTA 1994 Canadian Coordinating Office for Health Technology Assessment 1994. Guidelines for the Economic Evaluation of Pharmaceuticals: Canada. Canadian Coordinating Office for Health Technology Assessment (CCOHTA), Ottawa, ON
- CCOHTA 1997. Canadian Coordinating Office for Health Technology Assessment 1997. Selective Serotonin Reuptake Inhibitors (SSRIs) for Major Depression. Part II. The Costeffectiveness of SSRIs in Treatment of Depression: Canada.
- Canadian Coordinating Office for Health Technology Assessment (CCOHTA), Ottawa, ON
- Casciano R, Arikian S R, Tarride J-E, Casciano J, Doyle J J 2000 Antidepressant selection for major depressive disorder: the budgetary impact on managed care. Drug Benefit Trends 12: 6–16
- Conner T M, Crismon M L, Still D J 1999 A critical review of selected pharmacoeconomic analyses of antidepressant therapy. Annals of Pharmacotherapy 33: 364–72
- Dardennes R, Lafuma A, Watkins S 1999 Prophylactic treatment of mood disorders: a cost-effectiveness analysis comparing lithium and carbamazepine. Encephale 25: 391–400
- Davidson J R T, Meltzer-Brody S E, Goldberg D, Wittchen HU, Magruder K M, Lecrubier Y, Ballenger J C, Tylee A, Schulberg H C, Nutt D J 1999 The underrecognition and undertreatment of depression: what is the breadth and depth of the problem? Discussion. Journal of Clinical Psychiatry 60 (Suppl. 7): 10–11
- Fenn P, Gray A 1999 Estimating long term cost savings from treatment of Alzheimer’s disease: a modelling approach. PharmacoEconomics 16: 165–74
- Foster R H, Plosker G L 1999 Donepezil: pharmacoeconomic implications of therapy. PharmacoEconomics 16: 99–114
- Friedberg M, Saffran B, Stinson T J, Nelson W, Bennett C L 1999 Evaluation of conflict of interest in economic analyses of new drugs used in oncology. Journal of the American Medical Association 282: 1453–7
- Gabbard G O, Lazar S G, Hornberger J, Spiegel D 1997 The economic impact of psychotherapy: a review. American Journal of Psychiatry 154: 147–55
- Gold M R, Russell L B, Siegel J E, Weinstein M C (eds.) 1996 Cost-effectiveness in Health and Medicine. Oxford University Press, Oxford, UK
- Gould R A, Otto M W, Pollack M H 1995 A meta-analysis of treatment outcome for panic disorder. Clinical Psychological Review 15: 819–44
- Greenberg P E, Sisitsky T, Kessler R C, Finkelstein S N, Berndt E R, Davidson J R T, Ballenger J C, Fyer A J 1999 The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry 60: 427–35
- Greenberg P E, Stiglin L E, Finkelstein S N, Berndt E R 1993 The economic burden of depression in 1990. Journal of Clinical Psychiatry 54: 405–18
- Howard M O, McGuffin R W, Saxon A J, Sloan K L, Walker R D 1996 Clinical issues related to the costs of alcoholism. PharmacoEconomics 9: 134–45
- Keck P E Jr, Nabulsi A A, Taylor J L, Henke C J, Chmiel J J, Stanton S P, Bennett J A 1996 A pharmacoeconomic model of divalproex vs. lithium in the acute and prophylactic treatment of bipolar I disorder. Journal of Clinical Psychiatry 57: 213–22
- Lave J R, Frank R G, Schulberg H C, Kamlet M S 1998 Costeffectiveness of treatments for major depression in primary care practice. Archives of General Psychiatry 55: 645–51
- Manning W, Wells K B 1995 How can care for depression become more cost-effective? Journal of the American Medical Association 274: 1931–4
- Murray C J L, Lopez A D 1997 Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 349: 1498–504
- Revicki D A 1999 Pharmacoeconomic studies of atypical antipsychotic drugs for the treatment of schizophrenia. Schizo-phrenia Research 35 (Suppl.): S101–9
- Schadlich P K, Brecht J G 1998 The cost effectiveness of acamprosate in the treatment of alcoholism in Germany: economic evaluation of the prevention of relapse with acamprosate in the management of alcoholism (PRAMA) study. PharmacoEconomics 13: 719–30
- Tylee A, Gastpar M, Lepine J-P, Mendlewicz J 1999 DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. International Clinical Psychopharmacology 14: 139–51
- Whetten-Goldstein K, Sloan F A, Goldstein L B, Kulas E D 1998 A comprehensive assessment of the cost of multiple sclerosis in the United States. Multiple Sclerosis 4: 419–25
- Yuen E J, Zisselman M H, Louis D Z, Rovner B W 1997 Sedative–hypnotic use by the elderly: effects on hospital length of stay and costs. Journal of Mental Health Administration 24: 90–7




