Sample Fear-Potentiated Startle Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
When people are afraid they tend to show a bigger startle response to a loud sound. Rats show a similar effect. This phenomenon, called ‘fear-potentiated startle,’ has been used to study the neural mechanisms involved in fear and anxiety in both rats and humans.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Fear-Potentiated Startle In Rats
Capitalizing on anecdotal evidence that people startle more when they are afraid, Brown et al. (1951) demonstrated that the amplitude of the acoustic startle reflex in the rat can be augmented by presenting the eliciting auditory startle stimulus shortly after onset (e.g., 3s) of a cue (e.g., a light) previously paired with a foot-shock. This fear-potentiated startle occurs with an auditory, visual, or tactile conditioned stimulus (CS), and when startle is elicited by either a loud sound or an airpuff in rats (Davis et al. 1993). In this test a central state of fear is considered to be the conditioned response. Conditioned fear is defined operationally by elevated startle amplitude in the presence of a cue previously paired with a shock. Thus, the CS does not elicit startle. Furthermore, the startle-eliciting stimulus is never paired with a shock. Instead, the CS is paired with a shock, and startle is elicited by another stimulus in the presence or absence of the CS. Fear-potentiated startle only occurs following paired rather than unpaired or random presentations of the CS and the shock, indicating that it is a measure of conditioned fear. Discriminations between visual and auditory conditioned stimuli, and between auditory cues and visual cues that differ in duration, have also been demonstrated with potentiated startle. Pairing one cue with shock does not lead to generalization of fear-potentiated startle in testing with other cues. Fear-potentiated startle still occurs reliably at least one month after original training, making it appropriate for the study of long-term memory as well.
It has been suggested, however, that potentiated startle may not reflect increased fear in the presence of a CS, but instead results from the animal making a postural adjustment (e.g., crouching) which is especially conducive to startle, in anticipation of the impending foot-shock. However, spinally transected rats that were held rigidly in a modified stereotaxic instrument, which prevented obvious postural adjustments, still showed fear-potentiated startle, measured with the pinna component of startle. Potentiation of startle measured electromyographically in neck muscles also occurs in the absence of any obvious postural adjustment. In addition, the magnitude of potentiated startle correlates highly with the degree of freezing, a very common measure of fear.
1.1 Effects Of Different Drugs
Drugs that reduce fear or anxiety in humans decrease potentiated startle in rats (Davis et al. 1993). Benzodiazepines, opiates, β-noradrenergic antagonists, drugs that decrease norepinephrine release, certain serotonin agonists, nicotine, and perhaps alcohol decrease or fully block fear-potentiated startle. In many cases these treatments do not depress startle levels in noise-alone trials, although this is not always the case. Conversely, drugs that increase norepinephrine release or decrease GABA (γ-aminobutyric acid) mediated neurotransmission and exaggerate anxiety in anxious people increase the magnitude of potentiated startle in rats.
1.2 Neural Systems Involved In Fear-Potentiated Startle
A major advantage of the fear-potentiated startle paradigm is that fear is measured by a change in a simple reflex. Hence, fear is expressed through some neural circuit that is activated by the CS and ultimately impinges on the startle circuit. Figure 1 shows a schematic summary diagram of the neural pathways we believe are required for both the acquisition and expression of fear-potentiated startle in rats using a visual CS. These pathways involve convergence of the CS and the unconditioned shock stimulus at the lateral and basolateral amygdala nuclei, which project to the central nucleus of the amygdala, and this in turn projects directly to the startle pathway.
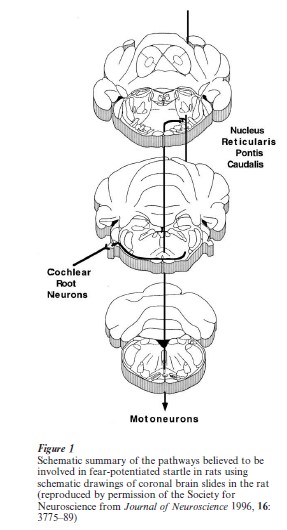
1.2.1 The Primary Acoustic Startle Pathway. Because the acoustic startle reflex has such a short latency (e.g., 8 ms measured electromyographically in the hindlegs), it must be mediated by a simple neural pathway. Using a variety of techniques, we have determined that the primary acoustic startle reflex in rats is probably mediated by three central synapses (Lee et al. 1996). The first involves terminals of the auditory nerve that synapse with large neurons embedded in the auditory nerve, called cochlear root neurons. These send exceedingly thick axons (sometimes as large as 7 µm in diameter) to neurons in the nucleus reticularis pontis caudalis (PnC). These cells project directly to motoneurons in the facial motor nucleus (pinna reflex and perhaps the eyeblink component of startle) and spinal cord (whole-body startle). Chemical lesions of these neurons or local infusion of glutamate antagonists in the vicinity of these synapses eliminated acoustic startle. Although other investigators have proposed that additional connections between the cochlear nucleus and the PnC may be important for startle (Koch and Schnitzler 1997), we believe that this simple, threesynapse pathway is the main acoustic startle pathway. For example, although the dorsal cochlear nucleus also projects directly to the PnC, lesions of the dorsal cochlear nucleus only decreased startle amplitude when high-intensity startle stimuli were used.
1.2.2 The Role Of The Thalamus. Visual information uses parallel pathways that involve either (a) direct retinal inputs to the lateral posterior nucleus of the thalamus, which projects directly to the perirhinal cortex, or (b) retinal inputs to the dorsal lateral geniculate nucleus, which projects indirectly to the perirhinal cortex via the visual cortex. Lesions of both of these areas of the visual thalamus together, but not either one alone, blocked fear-potentiated startle using a visual CS (Shi and Davis 1996).
If an auditory CS is used, this involves parallel inputs from the auditory thalamus to the perirhinal cortex, either directly or indirectly via the auditory cortex (Campeau and Davis 1995a). Pre or posttraining lesions of the entire auditory thalamus completely blocked fear-potentiated startle to an auditory CS but not to a visual CS. Post-training lesions restricted to the main body of the medial geniculate, which projects to the perirhinal cortex via the auditory cortex, also specifically blocked fear-potentiated startle to the auditory CS.
Pain information reaches the amygdala via parallel pathways that include the caudal granular dysgranular insular cortex and the posterior intralaminar nuclei of the thalamus. Pretraining lesions of both the insular cortex and the posterior intralaminar nuclei of the thalamus, but not lesions of either structure alone, blocked the acquisition of fear-potentiated startle (Campeau and Davis 1995a, Shi and Davis 1999). However, post-training lesions of both of these areas together did not prevent expression of conditioned fear. These results suggest that parallel cortical and subcortical pathways are involved in relaying shock information during fear conditioning.
1.2.3 The Role Of The Anterior Perirhinal And Insular Cortex. The perirhinal cortex, which receives either visual or auditory CS information, projects directly to the lateral and basolateral amygdala. Post-training lesions of the anterior perirhinal cortex completely blocked the expression of fear-potentiated startle using a visual CS (Rosen et al. 1992), provided the lesion destroyed both the dysgranular and agranular portions of the perirhinal cortex. Post-training lesions of the perirhinal area (including secondary auditory cortices) blocked fear-potentiated startle to both auditory and visual CS (Campeau and Davis 1995a). However, reliable potentiated startle was observed after retraining in animals that had sustained main geniculate body lesions (which would destroy cortical connections between the thalamus and perirhinal cortex), or following pretraining lesions of the perirhinal area. These data suggest that cortical areas are normally used for the expression of fear conditioning, but that subcortical areas can take over if the cortex is damaged. Finally, as mentioned before, shock information seems to require the insular cortex, which in turn projects directly to the lateral nucleus of the amygdala.
1.2.4 The Role Of The Amygdala. The perirhinal cortex projects directly to the lateral and basolateral amygdala. Selective destruction of cell bodies in the lateral and basolateral nuclei by local infusion of neurotoxins caused a complete blockade of fearpotentiated startle when the lesions were made, whether before or after training (Sananes and Davis 1992). This blockade did not seem to result from a disruption of vision, and other studies found that neurotoxic lesions of these amygdaloid nuclei also blocked fear-potentiated startle using an auditory CS. In contrast, lesions of a variety of other subcortical areas, such as the hippocampus, the septal area, the cerebellum, or the bed nucleus of the stria terminalis, had no consistent effect. The lateral, and especially the basolateral, nuclei of the amygdala project directly to the central nucleus of the amygdala, lesions of which blocked the expression of fearpotentiated startle using either a visual (Hitchcock and Davis 1986) or an auditory CS (Campeau and Davis 1995b).
Because CS and shock information converge neurally at the lateral and basolateral amygdala nuclei, this could be the site of plasticity for fear-potentiated startle. In fact, local infusion of NMDA (N-methyl-D aspartate) antagonists into the amygdala blocked the acquisition, but not the expression, of fear-potentiated startle using either a visual or an auditory CS (Davis et al. 1993). This was probably not a result of shock information failing to reach the amygdala, because this treatment also blocked the acquisition of secondorder fear-potentiated startle, which does not involve shock during second-order training.
By eliciting startle-like responses electrically from various points along the startle pathway before and after presentation of a light previously paired with a shock, we concluded that fear ultimately alters transmission at the PnC. Consistent with this, local infusion into the PnC of several compounds, such as glutamate antagonists and corticotropin-releasing hormone antagonists, blocked fear-potentiated startle at doses that have no effect on baseline startle (Koch and Schnitzler 1997).
The central nucleus of the amygdala projects directly to the PnC, and lesions at several points along this pathway blocked the expression of fearpotentiated startle. Low-level electrical stimulation of the amygdala markedly increased the acoustic startle amplitude (Rosen and Davis 1988) at currents and durations that did not produce any other signs of behavioral activation. Transit times from the amygdala to the startle circuit appear to be about 4–5 ms or even less. Electrical stimulation of the amygdala in anesthetized rats facilitated tone-evoked activation of cells in the PnC (Koch and Ebert 1993).
However, it is now clear that there is at least one critical synapse between the amygdala and the startle pathway, located in an area in or very close to the mesencephalic reticular formation, dorsal periaqueductal gray or deep white layers of the superior colliculus (Koch and Schnitzler 1997). Neurotoxic lesions of this area or local infusion of muscimol blocked the expression, but not the acquisition, of fear-potentiated startle.
2. Fear-Potentiated Startle In Humans
Fear-potentiated startle can also be measured in humans by using the eyeblink component of the startle reflex. In one test, people are told that when a certain colored light comes on they might get a shock on the wrist, whereas they will not get a shock when a different colored light comes on. Startle is elicited with bursts of noise through earphones, and the eyeblink component of startle is measured electromyographically from the orbicularis oculi muscles. Startle amplitude was consistently higher in the presence of the light that signaled shock (Grillon et al. 1991). The size of this increase depended on the time when the subject expected the shock on the basis of verbal instructions, and also occurred when conditioning procedures were used.
Thus far, there is a close correspondence between results gathered in rats and humans. Brain imaging studies show that the amygdala is activated during this verbally mediated fear-potentiated startle test, and that patients with lesions of the amygdala fail to display fear-potentiated startle. People also show an increase in startle when they see scary pictures—for example, of a gun in their face, a dog about to bite them, or mutilated bodies (Lang et al. 1990). The size of the increase in startle was related directly to how the subjects rated the pictures in terms of negative valence and arousal.
Bibliography:
- Brown J S, Kalish H I, Farber I E 1951 Conditional fear as revealed by magnitude of startle response to an auditory stimulus. Journal of Experimental Psychology 41: 317–28
- Campeau S, Davis M 1995a Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience 15: 2312–27
- Campeau S, Davis M 1995b Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience 15: 2301–11
- Davis M, Falls W A, Campeau S, Kim M 1993 Fear-potentiated startle: A neural and pharmacological analysis. Behavioural Brain Research 58: 175–98
- Grillon C, Ameli R, Woods S W, Merikangas K, Davis M 1991 Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28: 588–95
- Hitchcock J M, Davis M 1986 Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience 100: 11–22
- Koch M, Ebert U 1993 Enhancement of the acoustic startle response by stimulation of an excitatory pathway from the central amygdala basal nucleus of Meynert to the pontine reticular formation. Experimental Brain Research 93: 231–41
- Koch M, Schnitzler H-U 1997 The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behavioural Brain Research 89: 35–49
- Lang P J, Bradley M M, Cuthbert B N 1990 Emotion, attention, and the startle reflex. Psychological Review 97: 377–95
- Lee Y L, Lopez D E, Meloni E G, Davis M 1996 A primary acoustic startle pathway: Obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. Journal of Neuroscience 16: 3775–89
- Rosen J B, Davis M 1988 Enhancement of acoustic startle by electrical stimulation of the amygdala. Behavioral Neuroscience 102: 195–202
- Rosen J B, Hitchcock J M, Miserendino M J D, Falls W A, Campeau S, Davis M 1992 Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. Journal of Neuroscience 12: 4624–33
- Sananes C B, Davis M 1992 N-Methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fearpotentiated startle and shock sensitization of startle. Behavioral Neuroscience 106: 72–80
- Shi C, Davis M 1996 Anatomical tracing and lesion studies of visual pathways involved in fear conditioning measured with fear potentiated startle. Society for Neuroscience Abstracts 22: 1115
- Shi C-J, Davis M 1999 Pain pathways involved in fear conditioning measured with fear-potentiated startle: Lesion studies. Journal of Neuroscience 19: 420–30




