Sample Memory in the Fly Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
1. The Genetic Approach to Learning and Memory
The genetic approach to understand the molecular processes mediating learning and memory was founded upon the principles of genetics and molecular biology that were discovered in the first half of the twentieth century. Studies of mutant organisms with physical defects, along with knowledge of the structure of DNA, the hereditary material, led to the realization that a mutation in a single nucleotide of DNA offered the ultimate way of performing a biological dissection. In other words, a mutation that inactivates a single gene of an animal offers the biologist a way of studying the biological consequences of removing but a single building block from the animal. This genetic approach has been used to study many different questions in biology. Its application to the study of learning and memory and other behaviors can be traced to the laboratory of Seymour Benzer, beginning around 1970 (Benzer 1971).
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
This approach for dissecting behavior is reductionist in the sense that it reduces the problem to the molecular level. It begins with the idea that a mutation in an animal that produces a learning or memory deficiency identifies a gene and its protein product required for normal learning in nonmutant animals. With the identification of a sufficient number of genes and proteins that are involved, some mechanistic understanding of the molecular dynamics underlying the process can be gained. While this is true, it is now accepted that a genetic connection between gene and behavior is insufficient to gain the necessary depth of understanding. This is because the behavior of an animal emerges from complex interactions at levels other than the molecular. Molecules mediate learning through their biological functions and interactions but this occurs within the context of certain neurons. These neurons, in turn, can be part of complex neural networks that convey information or are involved in behavioral output. Thus, research in the late twentieth century revealed that a multilevel analysis of learning and memory is essential for a complete understanding of the process. In other words, it is necessary to understand learning and memory at the genetic level, the cellular level, the neuroanatomical level, and the behavioral level, since behavior emerges from biological processes at all of these functional levels. Thus, scientists now use the genetic method along with other approaches to gain a greater appreciation of the fundamental mechanisms underlying learning and memory.
2. Learning in the Fly
Adult Drosophila are able to learn many different types of information (Davis 1996). After walking into a chamber in which an odor cue is paired with an aversive stimulus of mild electric shock, the animals will tend to avoid the odor for many hours, indicating that they have learned this association (Quinn et al. 1974). They can also learn associations between the color of light and an aversive stimulus, or odors presented with a food reward. They can learn to avoid walking into one side of a small chamber after being punished by heat upon entering that side (Wustmann and Heisenberg 1997), and to avoid flying in the direction of a particular visual cue if that flight direction is punished by mild heat.
The characteristics of Drosophila learning are not unique, but reflect the learning principles established with other animals including mammals. For example, the memory of odors can be disrupted by anesthesia shortly after learning, like the amnestic effects produced by anesthesia, electroconvulsive shock therapy, or protein synthesis inhibitors when administered to other species shortly after learning (Squire 1987). Learning an association between an odor and an aversive stimulus requires that they be presented at the same time, reflecting the rule for simultaneous presentation of cues, or pairing, found with many forms of learning in vertebrates. Furthermore, giving Drosophila repeated learning trials that are separated in time (spaced training) is more effective than presenting the trials with no spacing (Tully et al. 1994). This greater effect of spaced training is also a principle of learning for other animals.
3. Genes Involved in Drosophila Learning
Although all of the aforementioned types of Drosophila learning could, in principle, be the focus of genetic studies, most research has been concentrated on odor learning. Many different mutants that disrupt odor learning have been isolated and the responsible genes have been cloned to identify the protein product of the gene. The best studied of these are listed in Table 1. Most of these mutants have pointed to the fact that intracellular signaling through the small molecule, cyclic AMP, is critical for Drosophila odor learning. The normal function of the dunce gene is required for normal odor learning and its product, as revealed by molecular cloning and expression, is the enzyme cyclic AMP phosphodiesterase (Chen et al. 1986). This enzyme removes excess cyclic AMP from the cell and in its absence, cyclic AMP levels become significantly elevated. It is thought that the high level of cyclic AMP found in dunce mutants prohibits the dynamic changes in cyclic AMP levels that occur during normal learning, or cause the desensitization of other signaling components (Fig. 1), producing a learning deficiency. The product of the rutabaga gene performs the opposite role. Its product, adenylyl cyclase, is responsible for synthesizing cyclic AMP in cells (Levin et al. 1992). Another protein that works to help adenylyl cyclase perform its function is neurofibromin (Guo et al. 2000). Cyclic AMP has its effects by activating a protein kinase known as protein kinase A. Mutations in the gene that codes for protein kinase A also disrupt odor learning (Drain et al. 1991, Skoulakis et al. 1993).
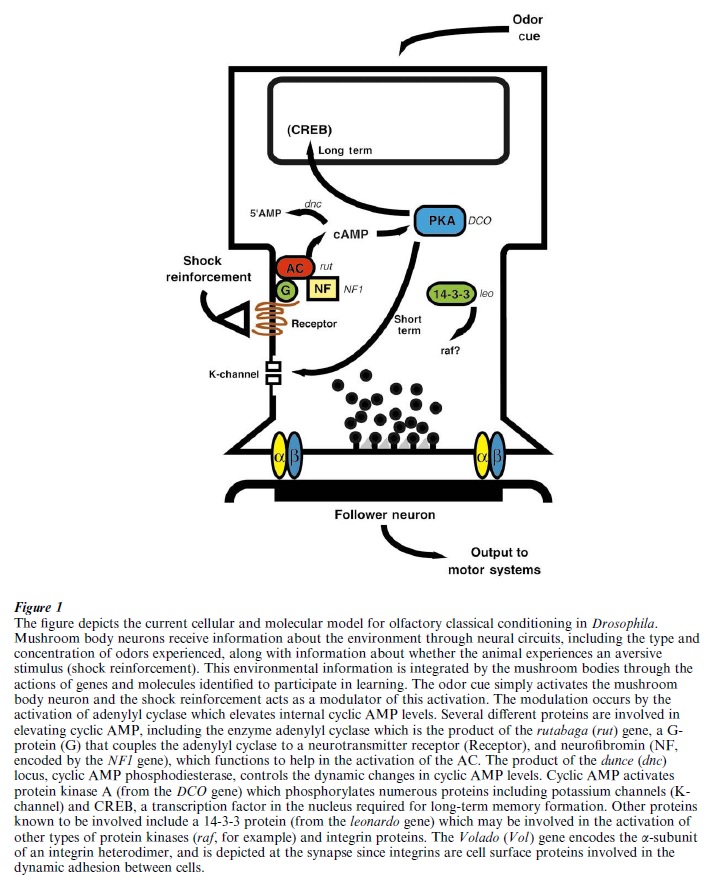
Protein kinase A, in turn, is known to phosphorylate and activate numerous proteins. Some of these phosphorylations result in the activation of proteins involved in short-term memory. One protein, however, known as CREB, is activated by protein kinase A and is required specifically for long-term memory. Inactivation of the CREB gene in Drosophila blocks the formation of long-term, but not short-term, odor memories (Yin et al. 1994). The role of CREB is that of a transcription factor, that is, to turn on or off the expression of other genes. Thus, CREB functions in long-term memory by regulating the expression of other genes.
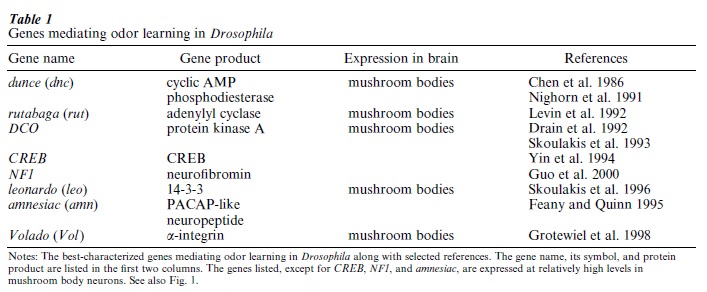
Although cyclic AMP signaling has emerged as a dominant theme for Drosophila odor learning, other genes that may be part of other signaling pathways and cell adhesion proteins have also been found to be essential (Table 1). The amnesiac (amn) gene codes for a neuropeptide with similarities to the vertebrate neuropeptide, pituitary adenylyl cyclase activating peptide (PACAP) (Feany and Quinn 1995). It is also likely involved in the cyclic AMP signaling system (Fig. 1) but its relationship to other components of the pathway is not yet established. The leonardo (leo) gene encodes a protein known as 14-3-3, which can function in the activation of other types of protein kinase, including the raf protein kinase and protein kinase C (Skoulakis and Davis 1996). Cell adhesion molecules of the integrin family have been implicated in shortterm odor memory through the discovery of the Volado (Vol ) gene, a gene required for flies to establish short-term odor memory and one that codes for an αintegrin (Grotewiel et al. 1998). These cell adhesion molecules function in a dynamic way, in that they are modulated by intracellular signaling pathways to rapidly form and break contacts with neighboring cells. It is currently thought that integrins work at the synapse to alter the adhesion of components at the synapse and to alter intercellular signaling.
4. Brain Neurons Mediating Insect Learning
A major refocus of the Drosophila learning field began in 1991 with the discovery of the neurons clearly required for odor learning (Nighorn et al. 1991). There are about 5,000 mushroom body neurons in Drosophila and these neurons are similar to neurons in the primary olfactory cortex of the human brain. The primary olfactory cortex is known to be important for odor learning in vertebrate species. The fact that mushroom body neurons are largely responsible for odor learning by insects is supported by many different types of research, among which is the discovery that many of the genes required for learning are highly expressed in mushroom body neurons relative to other neurons (Davis 1993). The product of dunce is highly expressed in mushroom body neurons as are protein kinase A and the products of rutabaga, leonardo, and Volado (Table 1). It is not yet known whether CREB, neurofibromin, or amnesic is highly expressed in these neurons. These observations have led to a cellular model for Drosophila odor learning (Nighorn et al. 1991, Fig. 1). This model envisions mushroom body neurons as integrators of the information presented during training, that being specific odors and electric shock. This integration changes the physiology of mushroom body neurons using the cyclic AMP signaling system such that they activate neural circuits for avoidance behavior after learning.
Drosophila is a powerful biological system for the discovery of genes involved in learning and memory, and for elucidating principles for the molecular events underlying learning. It is of additional interest that many of the genes identified as participating in Drosophila learning have been implicated in learning in other species (Davis 2000). And the field blossomed in the 1990s with the discovery of the neurons that mediate odor learning. Nevertheless, the physiological changes that occur within mushroom body neurons during odor learning remain speculative and the subject of models (Fig. 1). To use genetics to make that final link between changes in cellular physiology and learning remains a challenge for the future.
Bibliography:
- Benzer S 1971 From the gene to behavior. Journal of the American Medical Association 281: 24–37
- Chen C-N, Denome S, Davis R L 1986 Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce⁺ gene, the structural gene for cAMP phosphodiesterase. Proceedings of the National Academy of Sciences of the United States of America 83: 9313–17
- Davis R L 1993 Mushroom bodies and Drosophila learning. Neuron 11: 1–4
- Davis R L 1996 Biochemistry and physiology of Drosophila learning mutants. Physiological Re iews 76: 299–317
- Davis R L 2000 Neurofibromin progress in the fly. Nature News & Views 403: 846–47
- Drain P, Folkers E, Quinn W G 1991 cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron 6: 71–72
- Feany M, Quinn W 1995 A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 268: 825–26
- Grotewiel M S, Beck C D O, Wu K-H, Zhu X-R, Davis R L 1998 Integrin-mediated short-term memory in Drosophila. Nature 391: 455–60
- Guo H-F, Tong J, Hannan F, Luo L, Zhong Y 2000 A neurofibromastosis-1-regulated pathway is required for learning in Drosophila. Nature 403: 895–98
- Levin L, Han P-L, Hwang P M, Feinstein P G, Davis R L, Reed R R 1992 The Drosophila learning and memory gene rutabaga encodes a Ca²⁺ /calmodulin-responsive adenylyl cyclase. Cell 68: 479–89
- Nighorn A, Healy M, Davis R L 1991 The cAMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in mushroom body neuropil. Neuron 6: 455–67
- Quinn W G, Harris W A, Benzer S 1974 Conditioned behavior in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America 71: 708–12
- Skoulakis E M C, Davis R L 1996 Olfactory learning deficits in mutants for Leonardo, a Drosophila gene encoding a 14-3-3 protein. Neuron 17: 931–44
- Skoulakis E M C, Kalderon D, Davis R L 1993 Preferential expression of the catalytic subunit of PKA in the mushroom bodies and its role in learning and memory. Neuron 11: 197–208
- Squire L R 1987 Memory and Brain. Oxford University Press, New York
- Tully T, Preat T, Boynton S C, Del Vecchio M 1994 Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47
- Wustmann G, Heisenberg M 1997 Behavioral manipulation of retrieval in a spatial memory task for Drosophila melanogaster. Learning & Memory 4: 328–36
- Yin J C P, Wallach J S, Del Vecchio M, Wilder E L, Zhou H, Quinn W G, Tully T 1994 Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49–58




