Sample Motor Control Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. iResearchNet offers academic assignment help for students all over the world: writing from scratch, editing, proofreading, problem solving, from essays to dissertations, from humanities to STEM. We offer full confidentiality, safe payment, originality, and money-back guarantee. Secure your academic success with our risk-free services.
Motor control is a cross-disciplinary field of research in which the boundaries between established academic disciplines like psychology, physiology, neurology, engineering, and physical education are blurred. Within psychology, motor behavior tended to be a rather marginal topic for various reasons. When psychology is conceived as a science of the mind, movement is more or less beyond its scope. Less obviously, even when psychology is conceived as a science of behavior, issues of motor control do not become focal; for example, behaviorism was more concerned with “what is done” questions than with “how is it done” questions. Finally, although the first well-known psychology paper on motor control appeared at the end of the nineteenth century (Woodworth, 1899), and although James (1890, 1950) devoted a chapter to “The Production of Movement,” touching on the topic in several other chapters, the founding fathers of psychology did not stamp motor control as an essential ingredient of the emerging academic discipline.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
The field of motor control gains in importance as soon as one envisages that the human mind and brain may have evolved primarily to support action, not to contemplate the world. Then the question of how goals can be reached becomes critically important. This question alludes to problems of control, and motor control deals with particular goals that can be reached by moving one’s limbs.
In this chapter I first introduce the core problem of motor control and discuss different ways that it can be solved. Basically, there are two such ways: open-loop and closedloop control. Open-loop processes are initiated before a movement is actually executed, so they are described under the heading of motor preparation. The next section then deals with closed-loop processes, the exploitation of sensory feedback from an ongoing movement in the service of motor control, but also with other uses of sensory information. After the discussion of these rather fundamental issues, the perspective is enlarged somewhat. Many motor skills require coordinated movements of different limbs, which opens the topic of motor coordination. Finally, I shall address the flexibility of motor control which enables us to operate various tools and machines and to handle objects of various masses.
The Problem of Motor Control
An Outline of the Problem
Movements result from an interplay of passive and active forces.Passiveforcesareduetoourownmovementsaswellas to environmental factors like gravity. For example, in the swing phase of the walking cycle the thigh is rotated forward; initially the knee is flexed, followed by extension. This forward rotation of the shank results largely from passive forces of different origins. The deceleration of the knee extension, however,islargelyaresultofactivemuscularforces,withonly a small contribution of passive ones (Winter & Robertson, 1978).Thus, with the exception of a few very simple tasks, the production of movement requires not only the generation of appropriateactiveforces,butinadditionpassiveforceshaveto be taken into account.
Figure 12.1 illustrates a joint with two opposing muscles, a kind of minimal movement device. Muscles are designated as agonist and antagonist with respect to their function in a particular movement. For example, when the movement is a flexion of the joint, the flexor is the agonist and the extensor is the antagonist; for an extension, the functional roles of flexors and extensors are reversed. Of course, Figure 12.1 is extremely simplified, both with respect to the mechanical characteristics and with respect to the number of muscles acting on the joint.
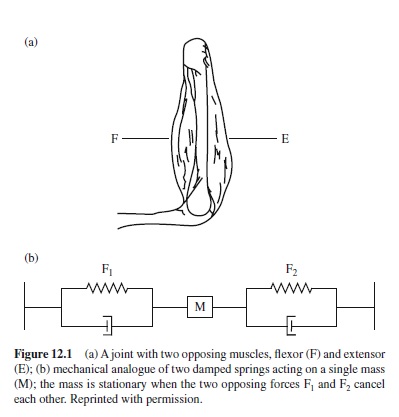
Muscles are complicated force generators. They contract when they are activated via the motor nerves. Each axon of a motor nerve innervates a smaller or larger bundle of muscle fibers; the axon together with its muscle fibers is called a motor unit. The activation can be recorded. Needle electrodes, which are inserted in the muscle tissue, allow one to record from single motor units, while surface electrodes pick up averaged and filtered electrical activity of motor units within a certain area below the electrodes. For isometric contractions, there is a systematic relation between electromyographically recorded muscle activity (EMG) and force. In particular, the relation between the integrated EMG signal and force is linear (Lippold, 1952). However, for movements for which phasic bursts of muscle activity are typical (at least when the movements are rapid), the relation is more complex.
Complications arise, first, from the temporal relations between bursts of muscle activity and forces, which can be fairly variable. In general, forces develop only with a delay when a muscle is activated, and after the end of the burst there is a gradual decay. Complications arise also from fatigue-induced changes, with fatigue being developed in the course of repeated or prolonged activity. In addition, for a given activation level, muscle force depends on the length of the muscle and on the rate of its contraction. In particular, the length-tension relation of muscle is important for models of motor control: Muscle force increases with increasing muscle length, and the slope becomes steeper the stronger the activation of the muscle is (e.g., Rack & Westbury, 1969). Although the length-tension relation is not really linear, a linear approximation is useful, at least for certain ranges of muscle length. Thus, one can think of a muscle as being mechanically similar to a damped spring (cf. Figure 12.1).
A muscle can actively contract, but not stretch. (A rubber band would perhaps be a better analogue than a spring.) Therefore at least two opposing muscles are needed for a simple joint. From Figure 12.1 it is apparent that, as the one muscle contracts, the other one will be stretched. This implies that, with given activations of the opposing muscles, the force of the contracting muscles declines while that of the stretched muscles increases. At a certain joint angle, and at a certain relation between the lengths of the opposing muscles, the forces developed by them will be equal, but in opposite directions, and thus cancel each other. The net force is zero, and the joint position at which this is the case is called the equilibrium position. There is considerable evidence that equilibrium positions are important for motor control (cf. Kelso & Holt, 1980; Polit & Bizzi, 1979). In the simplest version of a mass-spring model, movements come about simply by the specification of a new equilibrium position (e.g., Cooke, 1980), but experiments have revealed that the equilibrium position shifts continuously and not stepwise (Bizzi, Accornero, Chapple, & Hogan, 1984).
Movement results from the net force of opposing muscles (and, of course, from passive forces). Thus, at first glance there seems not to be much sense in cocontractions, in which opposing muscles are active simultaneously. Nevertheless, cocontractions can be observed in particular early during practice (e.g., Metz, 1970) and in tasks requiring high precision. Even when no net forces result from cocontractions, they modulate the mechanical characteristics of the joint like friction.
Saying that joint movement results from the net force of opposing muscles (in addition to passive forces) is not the whole story. More precisely, joint rotation results from the torque, which again is related to the net force in a fairly complicated way, with the relation being dependent on the joint angle. Even with the movement of the joint, the sequence of transformations from muscle activation to movement has not yet reached its end, because in general the goals for our movements are not defined in terms of joint angles.
Figure 12.2 illustrates a three-jointed arm with the endeffector pointing to a target. The goals of many movements are defined in terms of reaching for some spatial target; for other movements, as in catching a ball, there are temporal targets in addition; for still other movements, as in writing, goals are defined in terms of movement traces (or paths). From Figure 12.2 it is apparent that a particular configuration of joint angles is associated with a particular spatial position of the end-effector.
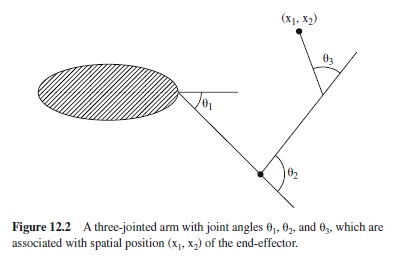
Thus far I have sketched the transformation of muscle activation to the spatial position of an end-effector like the tip of the index finger. The purpose was to give some impression of the complexity of this transformation without going into too much detail. Sometimes different components of the transformation are discussed separately, in particular the kinematic transformation (from joint angles to end-effector positions) and the dynamic transformation (from torques to movements of the joints). As a more general term, I shall use motor transformation to refer to the total transformation or some part of it.
Given the complexity and the time-varying characteristics of the motor transformation, one may wonder that humans— at least after the first few months of their life—are able to produce purposeful movements at all, and not only randomappearing ones. This requires that humans be able to determine the pattern of muscular activity that is required to produce a particular movement of a particular end-effector. The very fact that humans can produce purposeful movements indicates that nature has solved this core problem of motor control; what remains for the movement scientist is to gain an understanding of what the solution is.
An Outline of Possible Solutions
The core problem of motor control can be stated in a very simple and general way. Let T be a transformation of an input signal x into an output signal y. For example, y shall be a particular time-varying position of an end-effector, and x a vector that captures time-varying muscle activity. Then the general problem of control, and that of motor control in particular, is to determine an input signal x such that the output signal y becomes identical to the desired output signal y*. The problem is solved when the inverse of the transformation T can be determined, such that T–1T = 1. Thus, control requires the inversion of a transformation, and there are two fundamentally different ways to achieve this (see Jordan, 1996, for a detailed discussion).
Figure 12.3a illustrates an open-loop solution which requires an internal model T̂–1 of the transformation, or, more precisely, of its inverse. There are different ways of implementing such a model formally (e.g., Jordan, 1996). Of course, such an internal model is not necessarily a kind of entity, located in some part of the brain, but it can result from the activity of a network that is distributed widely across both central and more peripheral levels of the motor system (e.g., Kalveram, 1991).
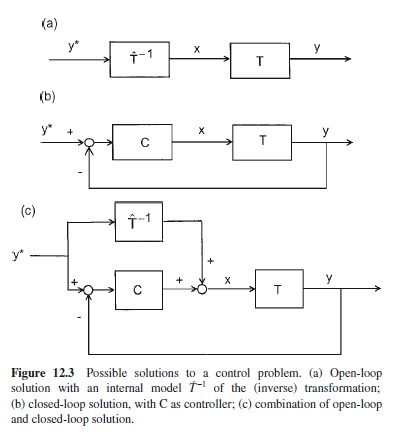
Figure 12.3b illustrates a closed-loop solution for which T̂ –1 no internal model T is required. Instead, the inversion of the transformation results from the structure of the loop. (This is shown formally by Jordan, 1996.) Intuitively this becomes clear from the following consideration. A closed-loop system can reduce the deviation between the output y and the desired output y*. To the extent that this is successful, y and y* become similar. This then implies, because y = T(x), that x approximates T–1(y*).
For some years, open-loop and closed-loop models of motor control were contrasted (cf. Stelmach, 1982). However, by now it is clear that nature combines both types of solution, roughly in a way illustrated in Figure 12.3c. This combination maintains the advantages of both types of solution and avoids the disadvantages of each of them. In addition, the combination exhibits some characteristics that match characteristics of human movements (Cruse, Dean, Heuer, & Schmidt, 1990).
The disadvantage of an open-loop solution is its limited precision. The motor transformation is complex, and it has time-varying characteristics. When we use tools or operate machines, there are additional transformations that must be taken into account, like the transformation of a steering-wheel rotation into a change of the direction in which a vehicle is heading.Thus, internal models of inverse transformations can only be approximations. The disadvantage of a closed-loop system is that it involves time delays and can become instable, in particular when the gain is high. On the other hand, a high gain is desirable to improve accuracy. When both systems are combined, open-loop control will serve to approximate the desired output; closed-loop control is suited to reducing the remaining deviation even when the gain is relatively small, which serves to avoid instabilities.
There are two different types of procedure to determine whether a control system is closed-loop or open-loop. The first is to cut the potential feedback loop, and the second is to distort the potential feedback signal. Both manipulations should have essentially no effect when the control system is open-loop, but strong effects when the control system is closed-loop; with eliminated feedback, the closed-loop system should produce no change of the output signal or only random changes, and with distorted feedback the output should be distorted. Human movements are often little affected by elimination of feedback, but strongly affected by its distortion. Such results do not give a clear answer with respect to the dichotomy of open-loop versus closed-loop control, but they conform to expectations based on the combined control modes (Cruse et al., 1990).
Indeterminateness of the Solutions
Typically movements are not fully determined by their goals. An example is reaching, with the goal being defined in terms of a spatial target position. Thus, only the endpoint of the movement is specified by the goal, but not its time-course. In spite of this indeterminateness a solution is reached, which takes additional task constraints as well as organismic constraints into account.
Perhaps the most extensively studied task constraint is the size of the spatial target, which affects movement duration and the shape of velocity-time curves (e.g., MacKenzie, Marteniuk, Dugas, Liske, & Eickmeier, 1987). Basically, for smaller targets humans choose to produce slower movements. The relation of movement time not only to target width, but also to the distance of the target from the start position, is of a particular kind known as Fitts’ law. The early 1950s, when Fitts (1954) first described the relation, saw the rise of information theory in psychology. Thus, the relation was formulated in terms of information measures, and the tradition has left it in that form. Fitts’ law states that movement time is a linear function of the index of difficulty, which is defined as log2(2A/W), A being the movement amplitude and W the width of the target.
Fitts’ law describes a particular kind of speed-accuracy trade-off: Faster movements have a larger scatter of their end-positions than slower movements, so when a small scatter is required because the target is small, slower movements have to be chosen. The law is astonishingly robust (cf. Keele, 1986), and it has given rise to various theoretical accounts (Crossman & Goodeve, 1963/1983; Fitts, 1954; Meyer, Abrams, Kornblum, Wright, & Smith, 1988), but also to alternative formulations (cf. Plamondon & Alimi, 1997) and to contrasting observations (e.g., Schmidt, Zelaznik, Hawkins, Frank, & Quinn, 1979), in particular for situations that require a certain movement duration, rather than reading a spatial target of a particular width. (Wright & Meyer, 1983; Zelaznik, Mone, McCabe, & Thaman, 1988).
Although they have received much less attention, other task constraints than target size affect the chosen movement trajectory. For example, it makes a difference whether the spatial target has to be hit or whether an object in the same position has to be grasped, and in the latter case it makes a difference whether the object is a tennis ball or a light bulb. The movement to the light bulb takes more time than the movement to the tennis ball; in particular, the deceleration of the movement toward the bulb is more gradual and extended in time (Marteniuk, MacKenzie, Jeannerod, Athènes, & Dugas, 1987). Another task constraint has been reported recently: The time it takes to move a mug to the mouth depends in a particular way on the diameter of the mug and the distance from the level of water to the edge (Latash & Jaric, in press). Such task constraints are at least to some degree reflected by our everyday experience.
A second type of constraints, which are taken into account when movement trajectories are indeterminate, is of a more organismic nature and related to the costs of movements. Although the general notion of cost minimization—as far as this is possible with the given task constraints—has a high degree of plausibility, it poses more of a problem than a solution. There are many different kinds of costs that can potentially be minimized. For example, Nelson (1983) analyzed the consequences of minimizing five different kinds of costs for the trajectories of movements aimed at a target. Other criteria have been added (e.g., Cruse, 1986; Cruse & Brüwer, 1987; Rosenbaum, Slotta, Vaughan, & Plamondon, 1991; Rosenbaum, Vaughan, Barnes, & Jorgensen, 1992; Uno, Kawato, & Suzuki, 1989), and perhaps any list will be incomplete.
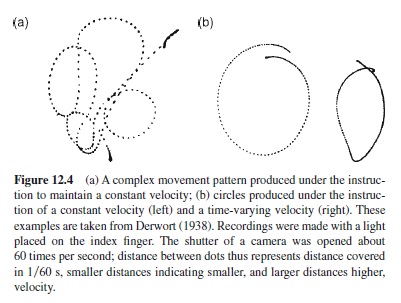
A fairly general principle seems to be that movement trajectories are selected by the criterion of smoothness. Although in principle smoothness can be defined in different ways, one of the possible criteria is minimization of jerk, that is, minimization of the integral of the squared third derivative of end-effector position with respect to time (Flash & Hogan, 1985). The principle can be extended and used to model complex movement patterns, as in handwriting (cf. Teulings, 1996). In addition, for drawing-like movements, it produces a particular relation between curvature and tangential velocity, which is known as the two-thirds power law (Viviani & Flash, 1995). Basically, with a larger radius of curvature, velocity tends to be higher than with a smaller radius of curvature even when the instruction is to maintain a constant velocity (Figure 12.4). The dependency of velocity on curvature is particularly conspicuous in drawing ellipses for which the radius of curvature varies continuously. Although the reverse relation has received less attention, variations of velocity do also induce variations of curvature; for example, when one attempts to draw circles with a pattern of smaller-highersmaller-higher velocity within each cycle, the result is likely to be ellipses (Derwort, 1938).
Indeterminateness does exist even when the goal of a movement specifies a trajectory of the end-effector in every detail. Of course, in such cases the movement trajectory is not indeterminate, but the input to the motor transformation is. The origin of the indeterminateness is apparent from Figure 12.2, where the target position is specified in terms of two spatial dimensions, but it can be reached with different configurations of three joints. More generally, the output of the motor transformation has a lower dimensionality than the input, so that the inversion of the motor transformation has no unique solution. The problem of how to deal with the many dimensions of the input is often called the degrees-offreedom problem. A consequence is motor equivalence: The same movement can be performed in many different ways.
Again, cost minimization can be considered as a way to reach a unique solution (cf. Cruse, 1986; Cruse & Brüwer, 1987; Rosenbaum et al., 1991). Another possibility is the freezing of degrees of freedom. For example, in handwriting adults mainly use the wrist and the fingers, and hardly or not at all the elbow and the shoulder joints. When one observes preschoolers at their first attempts to write (which might not be the appropriate term for the result, but perhaps for the intention), one can notice that the wrist and fingers are largely immobilized, and that mainly the more proximal joints, which are closer to the trunk, are used (Blöte & Dijkstra, 1989). This can also be observed when adult right-handers write with their left hand (Newell & van Emmerik, 1989). Finally, the high dimensionality of an input vector can be reduced to a small number of degrees of freedom by way of introducing covariations. A somewhat trivial example again can be seen in handwriting: With a normal tripod grip, thumb, index finger, and middle finger are mechanically coupled (because of holding the pen) and can no longer be moved independently.
Motor equivalence implies not only the existence of criteria for selecting one of the many options, but also that different options can be chosen in case that it is desirable or necessary. For example, when one asks people to tap with their index finger as rapidly as possible, and to do so as long as they can, several of them will gradually replace movements of the finger with movements of the wrist. Less incidentally, Lippold, Redfearn, and Vučo (1960) describe what they call “migration of activity” from one muscle to other ones during prolonged activity that induces muscular fatigue. More generally, the many-to-few mapping of the motor transformation leaves the option to select different subsets from the many input dimensions when some of them are functionally impaired, be it a fatigued muscle or an immobilized joint.
Motor Preparation
The initiation of a movement is a gradual and continuous process. In Figure 12.5 an example recording of a rapid index-finger flexion of about 20° amplitude is shown, as are in particular the position-time curve, the velocity-time curve, the acceleration-time curve, and the EMG of a finger flexor (agonist) and an extensor (antagonist). Faced with such recordings, it becomes somewhat difficult to answer the question of when the movement starts. Typically the start of a movement is defined in terms of a threshold for one of the kinematic signals. From Figure 12.5 it is apparent that definitions based on the acceleration signal generally lead to earlier initiation times than definitions based on the position signal: There can be a sizeable acceleration while position has hardly changed. Thus, any definition of the start of a movement is to some degree arbitrary.
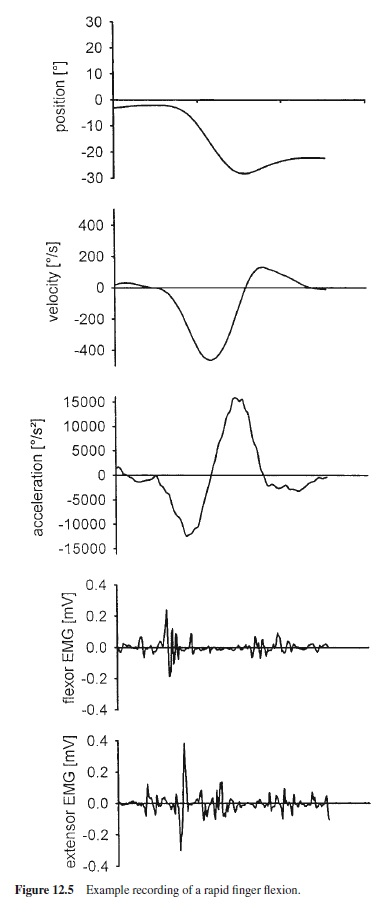
Muscle activity can be observed in advance of changes of kinematic signals, and the definition of the start of a movement can also be based on EMG traces. In many instances the agonist burst is over before a change of position can be seen.Thus,itis not too remarkable that the agonist burst is hardly or not at all affected when the overt movement is unexpectedly blocked. More remarkable is that the later bursts, which normally serve to decelerate the limb and to stabilize the end-position, still occur, although they serve no obvious purpose any more (Wadman, Denier van der Gon, Geuze, & Mol, 1979).
The overt movement is preceded not only by muscle activity, but also by various kinds of preparatory processes which can be evidenced at different levels of the motor system (see Brunia, 1999, and Brunia, Haagh, & Scheirs, 1985, for overviews of psychophysiological findings). For example, in the electroencephalogram, movement-related activity can be seen when the start of the movement is used as a trigger for averaging. Even such simple voluntary movements as keypresses are preceded by a slowly increasing negativity that startsintheorderof1sbeforetheovertmovement.Thisreadiness potential or Bereitschaftspotential was first described by Kornhuber and Deecke (1965). Initially it is symmetrical, but in the last 100 or 200 ms it becomes asymmetrical, being stronger over the hemisphere contralateral to the responding hand. This kind of asymmetry can also be observed in reaction-time tasks. Called the lateralized readiness potential, it has become an important tool in information-processing research (see chapter by Proctor & Vu in this volume).
The Anticipatory Nature of Motor Preparation
The psychophysiological data indicate the existence of motor preparation, but they are more or less silent to the question of what goes on in functional terms. What they tell, of course, is that at least to some degree preparatory processes are specific for the forthcoming movement in that the data reflect some of its characteristics, like the hand used. Näätänen and Merisalo (1977) suggested that the essence of motor preparation is that everything is done in advance of the overt response that can be done, which amounts to activating the response up to a level close to the motor action limit. This characterization of motor preparation may be appropriate for simple movements like keypresses, but it falls short of capturing essential characteristics of motor preparation preceding a more complex movement.
Preparatory activities in general can anticipate the future to varying degrees. For example, in preparing for a vacation, one might book a hotel in advance for only the first night, or one might book hotels in different places for several nights to come. Activating a response close to the action limit means preparing only for movement initiation (like booking a hotel for the first night). However, motor preparation is also concerned with the future of the response (like booking hotels for several nights to come). There are at least three kinds of evidence for this.
The first kind of evidence is from reaction-time experiments. When the task is to perform a sequence of simple movements, simple reaction time increases with the length of the sequence. The seminal study was by Henry and Rogers (1960), who found increasing reaction times for (a) lifting a finger from a key, (b) lifting the finger from the key and grasping a tennis ball at a certain distance, and (c) lifting the finger from the key, touching the ball with the back of the hand, pressing another key, and hitting a second tennis ball. More systematic explorations of the sequence-length effect have been reported by Sternberg and coworkers (see Monsell, 1986, for an overview). With more homogeneous elements like keypresses, letter names, or words with a certain number of syllables, reaction time increases linearly with the number of sequence elements. At some length of about 6–12 elements, the increase of reaction time levels off, earlier for longer elements (like trisyllabic words) and later for shorter elements (like monosyllabic words).
The second kind of evidence is from studies of anticipatory postural adjustments (see Massion, 1992, for review).When a forthcoming movement threatens balance, the voluntary action is preceded by the appropriate postural adjustments. For example, Cordo and Nashner (1982) observed EMG activity of postural muscles in the leg of their standing subjects which preceded by about 40 ms the activity of arm muscles involved in the task of pulling a hand-held lever in response to an auditory signal. In a control condition with a passive support, the preparatory postural activity was absent, and arm-muscle activity had a shorter latency.Thus, anticipatory postural adjustments are not only specific with respect to the forthcoming voluntary movement (e.g., Zattara & Bouisset, 1986), but also with respect to context characteristics.
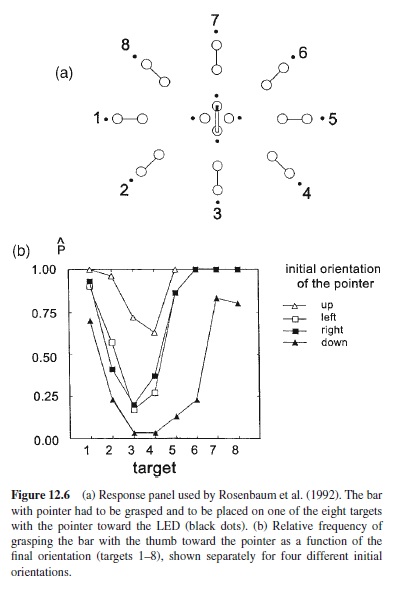
The third kind of evidence, finally, shows that earlier parts of a motor pattern are adapted to later parts. Evidence for this can be found in many skills (cf. Rosenbaum & Krist, 1996), but I shall focus here on a particularly basic kind of observation, the effect of end-state comfort (Rosenbaum & Jorgensen, 1992; Rosenbaum et al., 1992). Figure 12.6a illustrates the task of Rosenbaum et al. (1992, Exp. 1). The standing subject had to grasp a bar with a pointer, which had different initial orientations, the pointer pointing upward, downward, to the left or to the right. With the pointer upward or to the left, it is quite comfortable to grasp the bar with the thumb toward the pointer, but with the other two initial orientations this is less comfortable. Under speed instructions the subjects had not only to grasp the bar, but also to place it in one of eight target positions with the pointer toward the LED that signaled the target position in each trial; thus, there were differences in final orientation. For targets 6–8 and 1–2, holding the bar in the final orientation with the thumb toward the pointer is more or less comfortable, but for targets 3–5, holding the bar with the thumb away from the pointer is more comfortable. In Figure 12.6b the relative frequency of grasping the bar in its initial position with the thumb toward the pointer is shown. These data reveal not only an effect of the initial orientation of the bar, but also a clear effect of the final orientation. Thus, the effect of end-state comfort can be evidenced at the very start of the action, and it clearly indicates that motor preparation embraces anticipation.
The anticipatory nature of motor preparation implies that there is some kind of representation of the forthcoming movement before it begins. The existence of such a representation also implies that open-loop processes of motor control are of a particular nature in that they are predictive. In fact, the answer to the question of what goes on during motor preparation in functional terms may be largely that this kind of internal representation of the forthcoming movement is set up, which then allows for a more or less autonomous control.
Motor-Control Structures
There are different ways to conceptualize autonomous processes of motor control. In psychology it had been common to designate the anticipatory representation of a forthcoming movement as a motor program (and the process of setting it up as programming). However, this term has become associated with a particular conceptualization. Therefore, as a broader and more neutral term, Cruse et al. (1990) have suggested motor-control structures. There seem to be basically two different ways of modeling them, either in terms of prototypical functions or in terms of generative structures (Heuer, 1991).
Prototypical Functions
Movements vary qualitatively as well as quantitatively. One of the attempts to capture this basic observation is the notion of a generalized motor program, most explicitly introduced by Schmidt (1975). A generalized motor program is thought to control a set of movements that have certain characteristics in common. The specifics of each particular movement are thought to be determined by the program’s parameters. Thus, for a certain type of movement there should be invariant characteristics, which represent the signature of the program, and variable characteristics, which reflect the variable settings of its parameters. Of course, such a concept requires that the invariant characteristics of movements of a certain type be identified.
The theoretical problem of identifying invariant characteristics met with observations of an invariance of relative timing in different motor skills (see Gentner, 1987, for a review), which led Schmidt (1980, 1985) to propose that the relative timing is an invariant feature of movements that are controlled by a single generalized motor program. In addition, relative force was hypothesized to be a second invariant characteristic. With these assumptions, a generalized motor program can be described by way of a prototypical force-time function (), which can be scaled in time by a rate parameter and in amplitude by a force parameter.
The notion of a prototypical force-time function, which can be scaled in time as well as in amplitude, is reminiscent of the way we use coordinate systems to represent force-time curves. Thus one might suspect that the concept is related more to how we plot force as a function of time than to how the brain controls movement. Nevertheless, the notion is not biologically implausible. One can think of a spatially organized representation that is read at a certain rate and thus transformed into a temporally organized movement (cf. Lashley, 1951). The speed of reading would correspond to the rate parameter. Similarly, as the read signal is channeled to the muscles, it could be amplified to variable degrees (cf. von Holst, 1939). Thus, in principle, the notion of prototypical functions implies a certain degree of independence of temporal control and force control.
The most detailed application of prototypical force-time functions has been in models of the speed-accuracy tradeoff in rapid aimed movements. These so-called impulsevariability models account for the trade-off in terms of noise in the motor system (Meyer, Smith, & Wright, 1982; Schmidt, Sherwood, Zelaznik, & Leikind, 1985; Schmidt et al., 1979). However, it is not really necessary that prototypical curves specify forces; instead, they can also be thought of as specifying kinematic characteristics (e.g., Heuer, Schmidt, & Ghodsian, 1995; Kalveram, 1991). In fact, formal models of the autonomous processes of motor control are generally somewhat diverse or even indeterminate with respect to their output variables.
The motor transformation involves a number of different variables, and in principle any of these can be taken as output variable for models of motor-control structures. Ultimately, of course, muscles must be activated. In fact, the concept of a motor program has often been associated with a prestructured sequence of muscle commands (Keele, 1968). At the other extreme, motor-control structures can be modeled with the trajectory of the end-effector as the output. In the first case, the inversion of the motor transformation is assumed to be an integrated component of a motor-control structure. In the second case, it is left to additional and separate processes. Although the choice may be somewhat arbitrary, it implies an assumption about whether the internal model of the inverse motor transformation is specific for a particular type of movement governed by a particular motor-control structure, or whether it is generalized and thus applicable to different types of movement.
There are some considerations and data that favor the modeling of motor-control structures with end-effector kinematics as output. One consideration starts with the observation that both perception and action are externalized. For example, we do not see the image on the retina, but objects and their locations in the world. Similarly, awareness of our own movements is typically not in terms of muscular contractions and joint angles. Visual distances and movement amplitudes in the external world are commensurate, whereas proximal visual stimuli and patterns of muscular activity are not (cf. Prinz, 1992). Thus, to be compatible with how we perceive the world around us, movement should be represented in terms of world coordinates.
Another consideration starts with the assumption that the variables used in motor preparation or planning should reveal themselves by the possibility of describing them concisely as well as by their consistency. For example, for pointing in a two-dimensional plane as in Figure 12.2, the movement paths approximate straight lines, whereas the relations between joint-angles can be fairly complex. More specifically, plotting the y coordinates of the end-effector as a function of the x coordinates results in straight lines at least approximately, whereas plotting the elbow angle as a function of the shoulder angle results in strongly curved lines. This suggests that motor-control structures deal with the trajectory of the endeffector, and that the time-courses of joint angles are a consequence thereof (cf. Hollerbach & Atkeson, 1987). Similarly, kinematic characteristics of single-joint movements are highly similar for movements with and against gravity, whereas the patterns of muscular activity are grossly different (Virjii-Babul, Cooke, & Brown, 1994).
No matter for which kind of variable prototypical functions are defined, the notion is intimately related to the invariance of relative timing. The invariance is never really perfect, but often. It can be taken as a reasonable approximation. However, there are also clear deviations from invariance. For example, when the target size is reduced or accuracy rather than speed is emphasized, the relative duration of the deceleration phase of aimed movements tends to increase (Fisk & Goodale, 1989; MacKenzie et al., 1987). Moreover, the concept of a prototypical function takes a particular relative timing as a mandatory characteristic of a certain type of movement which cannot easily be changed; however, when after some practice in a particular temporal pattern the relative timing is changed, humans do not encounter particular difficulties (Heuer & Schmidt, 1988). Thus, prototypical functions do not represent a valid type of model for motor-control structures in general, but nevertheless they can capture important characteristics of some types of movement.
Generative Structures
Whereas a conceptualization of motor-control structures in terms of prototypical functions posits stored trajectories, conceptualizations in terms of generative structures posit networks that generate the trajectories. An example is a model by Saltzman and Kelso (1987) that belongs to a class they called the “task-dynamic approach.” For an aimed movement, Saltzman and Kelso defined a reach axis that runs through the target and the current position of the end-effector as well as an axis orthogonal to it. These axes define an abstract task space in which the end-effector is represented by a “task mass.” The target position is located in the origin of the task space and is assumed to have the characteristics of a point attractor. Thus, wherever the task mass is in task space, it will move toward the target governed by a set of simple equations of motion; for the reach axis x it is mTẍ + bTẋ + kTx = 0, with the index T designating parameters of the task space.
The task-dynamic approach goes beyond advance specifications of movements in task space. For example, joint movements are derived by way of coordinate transforms. However, for the present purpose only the highest level of the scheme is important. At first glance there does not seem to be much difference between describing a motor-control structure in terms of a differential equation that governs a generative structure or in terms of a solution of such an equation that could be stored as a prototypical function. However, there are differences. First, the parameterizations are different. Whereas the prototypical function has a rate and an amplitude parameter, the particular generative structure at hand has abstract mass, mT, friction, bT, and stiffness, kT, parameters. Variation of these parameters, for example, does not necessarily result in relative-timing invariance. Second, and perhaps more important, the generative structure is less susceptible to the effects of transient perturbations. It implements a movement characteristic called equifinality: Movements tend to reach their target even when they are transiently perturbed (Kelso & Holt, 1980; Polit & Bizzi, 1979; Schmidt & McGown, 1980).
Although the model of Saltzman and Kelso (1987) seems to be more mathematically than physiologically inspired, this is different with the VITE model of Bullock and Grossberg (1988). (VITE stands for vector-integration-to-endpoint.) The formal structure of an element of the model is illustrated in Figure 12.7. The variable P is an internal representation of the position of an effector, and T represents a target position. The variable V represents the (delayed) difference, and G the Go signal. In principle, the structure of Figure 12.7 is thought to be multiplied for different muscles that are involved in a voluntary movement, with V ≥ 0 for each particular muscle.
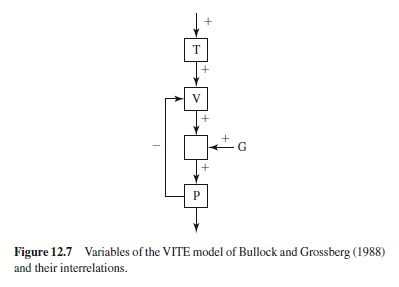
Without going into mathematical details, it is worth noting that the difference V in the case of aimed movements is again governed by a second-order differential equation (provided that G is a constant). In spite of this similarity, there are several basic differences from the model of Saltzman and Kelso (1987), in addition to the differences with respect to the role of physiological and psychological considerations in justifying the mathematics. The structure of Figure 12.7 is a kind of central closed-loop system. This system, however, is inoperative as long as the Go signal is zero; it is energized by the Go signal, which in addition can change across time so that the system is no longer linear. Bullock and Grossberg (1988) refer to a “factorization of pattern and energy.” Basically, the Go signal allows a separation of movement planning from movement initiation (cf. Gielen, van den Heuvel, & van Gisbergen, 1984), which implies that processes of motor preparation can be temporally separated from execution of the movement, but also that movements can be initiated before advance specification is finished.
Generative structures are not restricted to aimed movements. In fact, models of generative structures for periodic movements as they occur in locomotion are historically older. Network models of central pattern generators had already been proposed early in the twentieth century (Brown, 1911), and more elaborate versions continue to be developed (e.g., Grossberg, Pribe, & Cohen, 1997). In more abstract models, of course, point attractors can be replaced by limit-cycle attractors which produce stable oscillations (e.g., Kay, Kelso, Saltzman, & Schöner, 1987).
The Advance Specification of Movement Characteristics
During motor preparation an anticipatory representation of the forthcoming movement is constructed. This representation can be described as a motor-control structure, which allows (relatively) autonomous control of the movement independent of sensory feedback. In addition to being set up, the structure must be specified, with the appropriate parameters. This is a time-consuming process. Thus, variations in necessary preparatory activities are reflected in reaction times. In addition, when the available time is varied, it is possible to trace the time course of the specification of movement characteristics. Thus far, almost all studies on the advance specification of movement characteristics have employed aimed movements or isometric contractions with different quantitative characteristics, yet qualitatively different movements have hardly been used. Therefore, little can be said about setting up different motor-control structures, but more can be said about the advance specification of parameters.
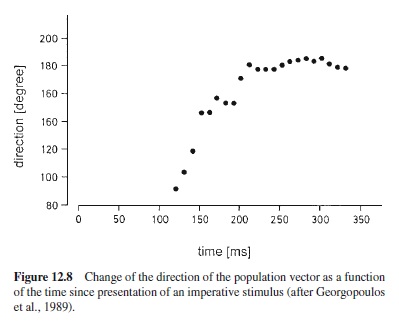
Figure 12.8 gives an example for the gradual specification of movement direction, adapted from Georgopoulos, Lurito, Petrides, Schwartz, and Massey (1989). These data are from a monkey who had been trained to perform a movement to one of eight potential targets arranged on a circle. When the target was dimly illuminated, the monkey had to reach for it directly, but when the luminance of the target was high, the monkey had to perform a movement that was rotated by 90° counterclockwise relative to the target. What is shown in Figure 12.8 is the gradual rotation of the population vector in such trials from the direction of the target (90°) to the direction of the movement (180°). The population vector is computed from the activity of directionally tuned neurons of the motor cortex and generally points in the direction of movement. Basically it is a weighted mean of the preferred directions of a sufficiently large sample of cortical units, with the weights being derived from the spike frequencies. The rotation of the population vector starts with a certain delay and proceeds with an almost constant slope until the target direction is reached. In human subjects this kind of rotation presumably gives rise to a systematic increase of reaction time when the angle between target and required direction of movement is increased (Georgopoulos & Massey, 1987).
The timed-response procedure allows one to trace the gradual specification of movement parameters from behavioral data. The method has been introduced for the study of the speed-accuracy trade-off in choice reaction time experiments (Schouten & Becker, 1967), and it has been adapted to the study of the advance specification of characteristics of isometric contractions and movements by Ghez and coworkers (Ghez et al., 1997; Hening, Favilla, & Ghez, 1988). Basically the method specifies a moment for the start of the movement; typically the movement has to be initiated in synchrony with the last of four tones which are presented in regular intervals. At a variable time before the last tone the target is presented, so the time available for motor specifications can be varied. The method is only suited for rapid movements or isometric contractions with short durations, so that the movement characteristics are largely determined in advance and little changed during execution.
Ghez and coworkers demonstrated the gradual specification of peak forces of isometric contractions as well as amplitudes and directions of movements with a time course similar to that of the neuronal population vector (cf. Figure 12.8). In addition, they showed that the gradual specifications break down when the differences between the alternative targets become too large (Ghez et al., 1997). When the difference between target directions is about 90° or larger, or the ratio of target amplitudes is about 12:1 or larger, the intermediate values between the two targets are no longer observed, and the choice between movement parameters becomes discrete. Thus, there seem to be two qualitatively different modes of parameter specification, namely gradual adjustments and discrete choices.
While the timed-response procedure provides a window into the gradual or discrete specification of movement characteristics, it has not been used as extensively as chronometric procedures. The latter type of studies is largely based on the movement precuing rationale of Rosenbaum (1980, 1983). Consider a set of four responses that differ on two dimensions like direction and amplitude. In a reaction time task, before presentation of the response signal, there is thus uncertainty with respect to both direction and amplitude, and after presentation of the response signal–during the reactiontime interval–both response characteristics have to be specified. When one of the dimensions is precued, it can be specified in advance of the response signal, and only one dimension remains to be specified after its presentation. Reaction time should be reduced by the time it takes to specify the precued dimension. When both dimensions are precued, both can be specified in advance, and reaction time should be reduced even more. In principle, if the rationale were fully valid, the times needed to specify various movement characteristics or combinations thereof could be estimated.
There are some broad conclusions that can be drawn from the results obtained, but there are also a number of problems that sometimes cast doubt on the general validity of the rationale (cf. Goodman & Kelso, 1980; Zelaznik, Shapiro, & Carter, 1982). Among the broad conclusions were that movement features are specified sequentially and in variable rather than fixed order (Rosenbaum, 1983). The first of these two broad conclusions can be doubted because the time needed to specify two dimensions can be smaller than the sum of the times needed to specify each of these dimensions (e.g., Lépine, Glencross, & Requin, 1989). In addition, timedresponse studies show essentially parallel specifications of amplitude and direction, perhaps accompanied by some slowing when two response characteristics are specified in parallel (Favilla & De Cecco, 1996; Favilla, Hening, & Ghez, 1989). Exceptions to the second broad conclusion seem to be rare. Fixedorderofspecificationsisindicatedbyashorteningofreaction time when a movement dimensionAis precued, which canbeobservedonlywhenmovementdimensionBisprecued as well, but not otherwise. This implies that the specification ofdimensionBisaprerequisiteforspecifyingA.Sucharesult would be expected when dimension B embraces qualitatively different movements, related to different motor-control structures rather than to different parameters of a single control structure. Qualitative variations of movement characteristics, however, have rarely been studied, but some results of Roth (1988) indeed suggest that precuing the direction and the force for throwing a ball does not result in systematic reaction time benefits as long as the type of throw is not known.
The Use of Sensory Information
The use of sensory information for the control of voluntary movement was among the historically early questions addressed by experimental psychology. Woodworth (1899) asked his subjects to produce reciprocal movements between two target lines in the pace of a metronome. With the participants’ eyes closed, accuracy was only little affected by frequency, but with the participants’ eyes open, accuracy increased relative to that found with closed eyes as soon as less than about two movements per second were produced. Anext major step was a study by Keele and Posner (1968) with discrete movements. Movement times were instructed, and the movements were performed with full vision or in the dark, with the room light being switched off at the start of the movements. Except for the shortest movement time of about 190 ms, the percentage of movements that hit the target was larger with than without vision. Subsequent studies showed that the minimal duration at which accuracy gains from the availability of vision becomes shorter—about 100 ms—when conditions with and without vision are blocked rather than randomized (Elliott & Allard, 1985; Zelaznik, Hawkins, & Kisselburgh, 1983). This minimal duration reflects processing delays, but it also reflects the time it takes until a change of the pattern of muscular activity has an effect on the movement.
Woodworth (1899) distinguished between two phases of a rapid aimed movement, an “initial adjustment” and a second phase of “current control.” This distinction seems to imply that accuracy should profit mainly when vision becomes available toward the end of aimed movements. However, even early vision can increase accuracy (Paillard, 1982), and accuracy increases when both initial and terminal periods of vision increase in duration (Spijkers, 1993). Thus, the view that vision is important only in the late parts of an aimed movement seems to be overly simplified.
From the basic findings it is clear that, in general, vision is not really necessary for the production of movements, but that it serves to improve accuracy. The same kind of generalization holds for the second important type of sensory information for motor control, proprioception. (For tasks that involve head movements, including stance and locomotion, the sensors of the inner ear also become important, although I shall neglect them here.) Regarding the role of proprioception for motor control, classic observations date back to Lashley (1917). Due to a spinal-cord lesion, the left knee joint of his patient was largely anesthetic and without cutaneous and tendon reflexes. In particular, the patient did not experience passive movements of the joint, nor could he reproduce them; only fairly rapid movements were noted, but the experienced direction of movement appeared random. However, when the patient was asked to move his foot by a certain distance specified in inches, the movements were surprisingly accurate, as were the reproductions of active movements; the latter reached the accuracy of a control subject. The basic finding that aimed movements are possible without proprioception (and, of course, without vision also) has been confirmed both in monkeys (e.g., Polit & Bizzi, 1979; Taub, Goldberg, & Taub, 1975) and—with local transient anesthesia—in humans (e.g., Kelso & Holt, 1980), although, of course, without proprioception there tends to be a reduction of accuracy.
The very fact that movements are possible without vision and proprioception proves that motor control is not just a closed-loop process but involves autonomous processes that do not depend on afferent information. The very fact that accuracy is generally increased when sensory information becomes available proves that motor-control structures also integrate this type of information. Beyond these basic generalizations, however, the use of sensory information becomes a highly complicated research issue because sensory information can be of various types and serves different purposes in motor control.

As a first example of some complexities, consider a task like writing or drawing. Normally we have no problems writing with our eyes closed, except that the positioning of the letters and words tends to become somewhat irregular in both dimensions of the plane. This is illustrated in Figure 12.9b. Figure 12.9a shows the writing of a deafferented patient both with and without vision (Teasdale et al., 1993). The patient had suffered a permanent loss of myelinated sensory fibers following episodes of sensory neuropathy, which resulted in a total loss of sensitivity to touch, vibration, pressure, and kinesthesia as well as an absence of tendon reflexes, although the motor nerve conduction velocities were normal. With vision, the writing of “Il fait tiède” seems rather normal, but without vision the placement of words, letters, and parts of letters is severely impaired, while individual letters remain largely intact. Similarly, in drawing ellipses with eyes closed, single ellipses appeared rather normal, but successive ellipses were displaced in space. Thus, absence of sensory information affects different aspects of the skill differently, and impairments are less severe when proprioception can serve as a substitute for absent vision.
Target Information
Vision and proprioception serve at least two different functions in motor control, which are not always clearly distinguished. First, they provide information about the desired movement or target information, and, second, they provide information about the actual movement or feedback information. In the typical case, target information is provided by vision only, and feedback information both by proprioception and by vision. Thus, vision provides both kinds of information, and the effects of absent vision can be attributed to either of them. The obvious question of whether target information or feedback information is more important for movement accuracy, as straightforward as it appears, cannot unequivocally be answered. In the literature, contrasting findings have been reported. For example, Carlton (1981) found vision of the hand to be more important, whereas Elliott and Madalena (1987) found vision of the target to be crucial for high levels of accuracy. Perhaps the results depend on subtle task characteristics. However, for throwing-like tasks, vision of the target seems to be critical in general (e.g., Whiting & Cockerill, 1974), and dissociating the direction of gaze from the direction of the throw or shot seems to be a critical element of successful penalties.
Specification of Spatial Targets
Targets for voluntary movements are typically defined in extrinsic or extrapersonal space, whereas movements are produced and proprioceptively sensed in personal space. Both kinds of space must be related to each other; they must be calibrated so that positions in extrinsic space can be assigned to positions in personal space and vice versa. When we move around, the calibration must be updated because personal space is shifted relative to extrinsic space. Even when we do not move around, the calibration tends to be labile. This lability can be evidenced from the examples of handwriting in Figure 12.9: With the writer’s eyes closed, calibration gets lost with the passage of time, so positions of letters or parts of them exhibit drift or random variation. This effect is much stronger when no proprioception is available.
An interesting example of failures that are at least partly caused by miscalibrations of extrinsic and personal space are unintended accelerations (cf. Schmidt, 1989). These occur in automatic-transmission cars when the transmission selector is shifted to the drive or reverse position, typically when the driver has just entered the car; when he or she is not familiar with the car, this is an additional risk factor. In manualtransmission cars, incidents of unintended acceleration are essentially absent. According to all that is known, unintended accelerations are caused by a misplacement of the right foot on the accelerator pedal rather than on the brake pedal without the driver’s being aware of this. Thus, when the car starts to move, he or she will press harder, which then has the unexpected effect of accelerating the car.
The position of the brake pedal is defined in the extrinsic space of the car, whereas the foot placement is defined in the personal space of the driver. In particular upon entering a car, and more so when it is an unfamiliar car, there is the risk of initial miscalibration. Thus, when extrinsic and personal space are not properly aligned, the correct placement of the foot in personal space might reach the wrong pedal in extrinsic space. Manual-transmission cars, in contrast, have a kind of built-in safeguard against such an initial miscalibration, because shifting gears requires that the clutch be operated beforehand. Thus, before the car is set into motion, the proper relation between foot placements and pedal positions is established.
Calibration, in principle, requires that objects, the locations of which are defined in world coordinates, be simultaneously located in personal space. Mostly it is vision that serves this purpose. However, personal space embraces not only vision: In addition to visual space, there are also a proprioceptive and a motor space, and these different spaces must be properly aligned with each other. For example, in order for us to reach to a visually located target, its location must be transformed into motor space, that is, into the appropriate parameters of a motor control structure. In addition, its location must be transformed into proprioceptive space, so that we can see and feel the limb in the same position. In a later section I shall discuss the plasticity of these relations; here I shall focus on the question of how a visually located spatial target is transformed into motor space.
An object can be localized visually both with respect to an observer (egocentrically) and with respect to another object (allocentrically or exocentrically; cf. the chapter by Proffitt & Caudek in this volume). Geometrically the location of the object can be described in terms of a vector. The length of the vector corresponds to the distance from the reference to the object; for egocentric location the reference is a point between the eyes (the cyclopean eye), and for allocentric location it is another object in the visual field. The direction is usually specified by angles both in a reference plane andorthogonal to it, but for the following its specification is of little importance. The available data suggest that both egocentric and allocentric localizations are used in the visual specification of targets. Which one dominates seems to depend on task characteristics.
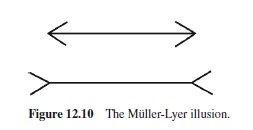
Figure 12.10 shows a well-known optical illusion, the Müller-Lyer illusion. Although the length of the shaft is the same in both figures, it appears longer in the figure with outgoing fins than in the figure with ingoing fins. Elliott and Lee (1995) used one of the intersections as the start position and the other intersection as the target position for aimed movements. Corresponding to the difference in perceived distance between the intersections in the two figures, movement amplitudes were longer with outgoing fins than with ingoing fins (cf. Gentilucci, Chiefi, Daprati, Saetti, & Toni, 1996). In contrast to this result, Mack, Heuer, Villardi, and Chambers (1985) found no effect or only a very small effect of the illusion on pointing responses.
Perhaps the critical difference to the study of Elliott and Lee (1995) was that the participants in the study of Mack et al. (1985) pointed not from one intersection to the other, but from a start position in their lap to one or the other of the two intersections. The difference between the two tasks suggests that the movements were based on allocentric (visual distance) and egocentric (visual location) information, respectively. In fact, when psychophysical judgments of the length of the shaft are replaced by judgments of the positions of the intersections, the illusion also disappears (Gillam & Chambers, 1985). Thus, although physically a distance is the difference between two positions on a line, this is not necessarily true for perceived distances and positions. This distinction between perception of location and perception of distance matches a distinction between different types of parameters for motor control structures, namely target positions versus distances (cf. Bock & Arnold, 1993; Nougier et al., 1996; Vindras & Viviani, 1998).
Specification of spatial targets in terms of distances implies a kind of relative reference system for a single movement: Wherever it starts, this position constitutes the origin. A visually registered distance (and direction) is then used to specify a movement in terms of distance (and direction) from the start position. This way of specifying movement characteristics has a straightforward consequence: Spatial errors should propagate across a sequence of movements. In contrast, with a fixed reference system as implied by the specification of target locations in terms of (egocentric) positions, spatial errors should not propagate. In studies based on this principle, Bock and Eckmiller (1986) and Bock and Arnold (1993) provided evidence for relative reference systems, that is, for amplitude specifications. The movements they studied were pointing movements with the invisible hand to a series of visual targets. However, Bock and Arnold also noted that error propagation was less than perfect. Heuer and Sangals (1998) used different analytical procedures, but these were based on the same principle of error propagation or the lack thereof. As would be expected, when only amplitudes and directions were indicated to the subjects, only a relative reference system was used. However, when sequences of target positions were shown, there was some influence of a fixed reference system, although the movements were performed on a digitizer and thus displaced from the target presentation in a manner similar to the way a computer mouse is used.
Gordon, Ghilardi, and Ghez (1994) provided evidence for a reference system with the origin in the start position based on a different rationale, again with a task in which targets were presented on a monitor and movements were performed on a digitizer. Targets were located on circles around the start position. The distribution of end-positions of movements to a single target typically has an elliptical shape. Under the assumption that the target position is specified in terms of direction and distance from the origin of the reference system, the axes of the elliptical error distributions, determined by principal component analysis, should be oriented in a particular way: The axes (one from each endpoint distribution) should cross in the origin. It turned out that the long axes of the error ellipses all pointed to the start position, as shown in Figure 12.11. Corresponding findings were reported by Vindras and Viviani (1998), who kept the target position constant but varied the start position.

Amplitude specifications allow accurate movements even when visual space and proprioceptive-motor space are not precisely aligned. Specifically, they do not require absolute calibration, but only relative calibration: It must be possible to map distances correctly from one space to another, but not positions. Of course, without absolute calibration, movements may drift away from that region of space where the targets are, as is typical with the use of a computer mouse. Without proprioception it seems that absolute calibration is essentially missing. In the case of the deafferented patient mentioned above, Nougier et al. (1996) found basically correct amplitude specifications in periodic movements between two targets, although there were gross errors in the actual end-positions relative to the targets.
Contrasting with the evidence for amplitude specifications or relative reference systems in tasks of the type “reaching from one object to another,” in tasks of the type “reaching out for an object” there is evidence for a reference system that is fixed, with the origin being at the shoulder or at a location intermediate between head and shoulder (Flanders, Helms Tillery, & Soechting, 1992). The analyses that led to this conclusion were again based on the assumption that errors of amplitude and direction should be essentially independent. However, when the start position of the hand is varied, an influence can again be seen, but not as dominant an influence as in the task of Gordon et al. (1994). Thus, McIntyre, Stratta, and Lacquaniti (1998) concluded that there is a mixture of different reference systems; in addition, errors of visual localization are added to errors of pointing.
Taken together, the evidence suggests that target information in general is specified both in terms of (egocentric) positions and in terms of (allocentric) distances and directions. Localization in terms of egocentric positions requires that, to perform a movement, the visual reference system be transformed to a proprioceptive-motor reference system, the first having its origin at the cyclopean eye, the latter having its origin at the shoulder, at least for certain types of arm movements. Localization in terms of allocentric distances and directions requires that the visual reference system be aligned with the proprioceptive-motor reference system in a way that the origin is in the current position of the endeffector. The relative importance of the two reference systems depends on task characteristics. In addition, there is also evidence that it can be modulated intentionally (Abrams & Landgraf, 1990).
Although spatial targets are mostly specified visually, they can also be specified proprioceptively, and again there is evidence for target specifications in terms of both position and amplitude, with the relative importance of these being affected both by task characteristics and intentions. In these experiments, participants produce a movement to a mechanical stop and thereafter reproduce this movement. When the start position is different for the second movement, participants can be instructed to reproduce either the amplitude of the first movement or its end-position. The general finding is a bias toward the target amplitude when the task is to reproduce the end-position, and a bias toward the end-position when the task is to reproduce the amplitude (Laabs, 1974). Although typically the reproduction of the end-position is more accurate than the reproduction of the amplitude, this is more so for longer movements, less so for shorter ones, and it may even be reversed for very short ones (Gundry, 1975; Stelmach, Kelso, & Wallace, 1975).
Specification of Temporal Targets
In tasks like catching, precisely timed movements are required: The hand must be in the proper place at the proper time and be closed with the proper timing to hold the ball. In very simple experimental tasks, finger taps have to be synchronized with pacing tones. Although the specification of temporal targets is fairly trivial in such tasks, the findings reveal to which aspects of the movements temporal goals are related. Acharacteristic finding is negative asynchrony, a systematic lead of the taps in the range of 20–50 ms, which, for example, is longer for tapping with the foot than for tapping with the finger (e.g., Aschersleben & Prinz, 1995).
The negative asynchrony is taken to indicate that the temporal target is not related to the physical movement itself, but rather to its sensory consequences, proprioceptive and tactile ones in particular, but also additional auditory ones if they are present. For example, because of the longer nerve-conduction times, sensory consequences of foot movements should be centrally available only later than sensory consequences of hand movements; thus negative asynchrony is larger in the former case than in the latter. When auditory feedback is added to the taps, negative asynchrony can be manipulated by varying the delay of the auditory feedback relative to the taps (Aschersleben & Prinz, 1997): Negative asynchrony declines when feedback tones are added without delay and increases as the delay becomes longer. With impaired tactile feedback, sensory consequences should also be delayed centrally, and negative asynchrony is increased (Aschersleben, Gehrke, & Prinz, 2001).
Synchronization of movements with discrete tones is necessarily anticipatory, provided that the interval between successive tones is sufficiently short (Engström, Kelso, & Holroyd, 1996). This is different in interceptive tasks. For example, when an object is approaching and one has to perform a frontoparallel movement that reaches the intersection of the object path and the movement path at the same time as the object does (cf. Figure 12.12a), it is possible in principle to continuously adjust the distance of the hand from the intersection to the distance of the object. In fact, this may actually happen if both the target object and the hand move slowly. At least, it is true that slower movements are adjusted more extensively to the approaching target after their start than rapid movements.

Let the start time be the time interval between the start of the interceptive movement and the time the target object reaches the intersection, and the temporal error be the time between the hand’s and the target object’s reaching the intersection (Figure 12.12b). Then, when the movement is started and runs off without further adjustments of its timing, the start time should be highly correlated with the temporal error. This strategy, in which the start time is selected according to the expected duration of a pre-selected movement pattern, is sometimes called operational timing (Tyldesley & Whiting, 1975). However, with temporal adjustments the correlation between start time and error should be reduced (Schmidt, 1972). This happens when the instructed movement duration is increased (Schmidt & Russell, 1972). Thus it seems that on the one hand the interceptive movement can be triggered by a particular state of the approaching object and then run off without further adjustments, and that on the other hand the time course of the interceptive movement can be guided by the approaching object, with mixtures of these two modes being possible.
In the simple task considered thus far the position of the intersection of object path and hand path is given. This is different for more natural tasks. Consider hitting a target that moves on a straight path in a frontoparallel plane like a spider on the wall. In principle, spiders can be hit in arbitrary places, but nevertheless the direction of the hitting movement has to be adjusted to an anticipated position of the moving target. A robust strategy is to adjust the lateral position of the hand to continuously updated estimates of the target position at the time the hand will reach the target plane; this requires an estimate of the time that remains until the hand reaches the plane and an estimate of the target’s velocity, which, however, need not really be correct (Smeets & Brenner, 1995).
The situation is somewhat different when balls have to be intercepted in a lateral position, either for catching them or for hitting them. According to Peper, Bootsma, Mestre, and Bakker (1994), the hand will be in the correct position in the plane of interception at the right time when its lateral velocity is continuously adjusted to the current difference between the lateral position of the hand and the approaching target, divided by the time that remains until the target reaches the plane of intersection. Proper lateral adjustments, which imply temporal adjustments as well, are evident even in high-speed skills like table tennis, although the relevant information is less clear (Bootsma & van Wieringen, 1990).
What is the basis for anticipations of temporal targets? For example, when we view an approaching ball, what allows us to predict when it will be in some position where we can intercept it (cf. the chapter by Proffitt & Caudek in this volume)? The time it takes until a moving object reaches a certain position is given by the distance of the object divided by its velocity. This ratio has time as unit, and it specifies time to contact with the position, provided the object moves on a straight path with constant velocity. As noted by Lee (1976), the information required to determine time to contact with an approaching object, or with an object the observer is approaching, is available even without determining distance and velocity, namely by the ratio of the size of the retinal image of the object and its rate of change. This variable, called , has become quite popular. There can be little doubt that it contributes both to temporal judgments (e.g., Schiff & Detwiler, 1979) and precisely timed actions (e.g., Savelsbergh, Whiting, & Bootsma, 1991). However, it is not the only relevant information; other kinds of information, for example binocular distance information, are used as well (Bennett, van der Kamp, Savelsbergh, & Davids, 1999; Heuer, 1993a). In a recent overview, Tresilian (1999) notes that the relation between rapid interceptive actions and the kind of information used is rather flexible and in no way invariant. There is a degree of task dependence that at present does not allow firm generalizations about how rapid interceptive actions are adjusted to their temporal targets.
Feedback Information
Although movements can be performed in the absence of afferent information from the moving limb with an astonishing degree of accuracy, the use of feedback information is indicated by the effects of perturbations of feedback on performance. For example, proprioceptive information can be distorted by way of tendon vibration with a vibrator placed in the proper position on the skin. The effect is a tonic excitation of muscle spindles, which under normal conditions corresponds to a longer muscle and correspondingly different joint angle. If, for example, the biceps tendon is vibrated, the elbow angle is registered as being too large. When the elbow angle has to be matched to the elbow angle of the other arm, the matched angle is too small, corresponding to the distorted proprioceptive feedback on joint angles (Goodwin, McCloskey, & Matthews, 1972).
Regarding the effects of distorted visual feedback, a particularly striking example has been reported by Nielsen (1963). The participant’s task was to move one hand along a vertical line, but the visible gloved hand was that of the experimenter and followed a curved path rather than a straight one. Subjects attempted to correct the error so that they deviated from the target line in the opposite direction. In spite of the strong discrepancies between intended and felt movement on the one hand and visual feedback on the other hand it took several trials before participants came to realize that the visible gloved hand could not be their own.
In simple movements, feedback information is functionally of little importance because autonomous processes of motor control can operate on the basis of a sufficiently accurate internal model of the motor transformation, so that only little error remains for closed-loop control to operate on (except, of course, when feedback information is distorted). However, in tasks in which a sufficiently accurate internal model is not available, the availability of visual feedback gains critical importance. This is the case when we operate sufficiently complex machines or tools which effectively add to the normal motor transformation. Experimentally tracking tasks are suited to exploring the role of visual feedback (Poulton, 1957).
For example, when the movement of the hand is proportional to the motion of the cursor on a screen, tracking performance is rather robust against short periods of eliminated visual feedback. However, with velocity control–with which the position of the hand is proportional to the velocity of the cursor on the screen–even short periods of eliminated feedback can bring performance down to an almost chance level (e.g., Heuer, 1983, p. 54). Thus, visual feedback gains in importance the less accurate the internal model of the transformation by a machine is. Internal models of sufficiently complex transformations seem not to be developed, so that practice does not reduce the critical importance of visual feedback (Davidson, Jones, Sirisena, & Andreae, 2000).
Feedback information does not only serve to guide an ongoing movement; it is also required to learn and to maintain an internal model of a transformation (cf. Jordan, 1996), provided it is not too complex. For example, Sangals (1997) had his subjects practice a nonlinear relation between the amplitude of the movement of a computer mouse and the amplitude of the cursor movement. When visual feedback during each movement of a sequence was eliminated and only terminal feedback at the end of each movement was provided, the relation between (visual) target amplitudes and movement amplitudes remained nonlinear. However, when visual feedback was completely eliminated for a sequence of several movements, the relation between (visual) target amplitudes and movement amplitudes became linear, which is likely to be a kind of default relation (cf. Koh & Meyer, 1991).
Feedback information can be processed at various levels of control; that is, it can be integrated with autonomous control processes in different ways. Consider the simple task of sine tracking: Subjects control the motion of a cursor on the screen, with the target following a sinusoidal time course. In principle, subjects could function like a position servo, minimizing the deviation between the position of the cursor and the position of the target. In fact, with a low frequency of target motion this may actually be the case. However, with higher frequencies, which approach the range where performance breaks down, human subjects produce a sinusoidal movement and seem to adjust its frequency and phase (Noble, Fitts, & Warren, 1955). Similar indications for the processing of parametric feedback rather than positional feedback have been reported by Pew (1966), but in general the processing of parametric rather than positional feedback has received very little attention. In everyday tasks like driving a car it may be of critical importance; perhaps it is not the position of the car on the road that is controlled but, rather, parameters like the curvature of the path.
In tasks like tracking there is not only visual feedback, but also proprioceptive feedback, and both types of feedback are related to different objects: proprioceptive feedback to one’s own movements, but visual feedback to the motions of a controlled object.The relation between both types of feedback depends on the transformation implemented by the controlled machine. Only when the transformation is simple or well-learned, or both, can proprioceptive feedback replace visual feedback, but in general such intermodal matching is associated with some loss of accuracy as compared with intramodal matching of target and feedback information (e.g., Legge, 1965). On the other hand, when visual and proprioceptive feedback are different but nevertheless refer to the same object, as in the classical task of mirror drawing,the absenceof proprioceptive feedback can actually enhance performance (Lajoie et al., 1992).
Even when visual and proprioceptive feedback refer to the same object, they are not necessarily redundant. For example, when we use a knife, both vision and proprioception provide information about its current position with respect to the object to be cut. Of course, visual information is more accurate in this respect, and as far as the spatiotemporal characteristics of the movement are concerned, proprioceptive information is not really needed. However, it provides information that is not available visually, in particular about the resistance of the cut object. Thus, although vision is critical for registering spatiotemporal characteristics, proprioception is critical for registering force characteristics. The lack of this latter kind of information is a problem in remote control and other tasks that followed from recent technological developments (cf. the chapter by Klatzky & Lederman in this volume).
An example is minimally invasive surgery (cf. Tendick, Jennings, Tharp, & Stark, 1993). Such operations are performed by means of an endoscope and instruments that are pivoted roughly at their place of insertion into the tissue. Although the facts that movements of the hand result in movements of the tip of the instrument in the opposite direction and that the gain of lateral movements depends on translational movements seem not to pose severe practical problems, the lack of appropriate force feedback seems to be more critical. In particular, there is only poor proprioceptive information about reactive forces at the tip of the instrument, so there is the risk of damage to the tissue operated on.
Sensory Information for Motor Control and Perception
Much of the sensory information that is involved in the control of movements apparently has no access to consciousness. Folklore knows that one just has to do it without attending too much to how it is done. In fact, Wulff, Höß, and Prinz (1998) found better learning of gross motor skills when the attention of the learners was focused on the effects of the movements rather than on the movements themselves, for example on a stabilometer platform rather than on the feet (for review, see Wulf & Prinz, 2001). It is not only that we do not perceive our movements in all details—for example, in skills like the long jump we do not normally perceive the details of the movements of our extremities (Voigt, 1933)—but, in addition, our movements can be more precise than would be expected from the limits of our perceptual skills. This was not only one of the major claims of a motor branch of the so-called Ganzheitspsychologie (Klemm, 1938), but it has also been emphasized in more recent times. For example, McLeod, McLaughlin, and Nimmo-Smith (1985) ascribed the very small temporal variability in batting of only a few milliseconds to the functioning of a dedicated special-purpose mechanism. In any case, hitting a falling ball at a certain position of its path is more precise than pressing a key when the ball reaches the same position (Bootsma, 1989).
Clinical cases illustrate that humans can reach to visual targets that they do not perceive, provided that the blind areas of the visual field (scotoma) are caused by certain lesions (e.g., Campion, Latto, & Smith, 1983; Perenin & Jeannerod, 1978). This phenomenon has become known as blindsight. In addition, clinical data give evidence of double dissociations. For example, some patients can identify and describe objects, but they cannot use the information about size, form, and orientation of the objects to grasp them; other patients, in contrast, cannot perceive these features of objects, but nevertheless can grasp them (Goodale & Milner, 1992).
The dissociability of visual information for perception and for motor control supports a theoretical distinction that has received much attention during the last 20 years. Basing their idea mainly on lesion studies, Ungerleider and Mishkin (1982) proposed the distinction between two cortical visual systems, one involving the inferotemporal cortex and the other the posterior parietal cortex (ventral stream and dorsal stream, respectively). In functional terms, these two systems have been characterized as the what system and the where system, the former serving object identification and the latter space perception. Alternatively, they are characterized functionally as the what system and the how system, the former being in the service of perception and the latter in the service of motor control (e.g., Goodale & Humphrey, 1998; Goodale & Milner, 1992). This latter functional characterization does largely coincide with a distinction between a cognitive and a sensorimotor system (Bridgeman, Kirch, & Sperling, 1981; Bridgeman, Lewis, Heit, & Nagle, 1979).
The evidence for the functional separation of a cognitive and a sensorimotor system is based on differences between psychophysical judgments and motor responses to identical stimuli. For example, Bridgeman, Peery, and Anand (1997) exploited the long-known effect of asymmetric stimuli in the visual field on the perceived direction of a target. They presented targets within a frame which was centered or shifted to the left or to the right. The target could appear in five different positions, and participants had to give their judgments by pressing one of five keys immediately after the stimulus had disappeared. For these perceptual judgments there was a clear effect of the position of the frame: When the frame was shifted to the left, judgments were shifted to the right, and vice versa. In contrast, when participants had to rotate a pointer so that it pointed to the target just presented, about half the participants exhibited no effect of the position of the frame. This was so although the response mode varied randomly and was cued only after the target had disappeared.
When delays of a few seconds between the disappearance of the target and the response were introduced, all participants showed effects of the frame position on pointing. Bridgeman et al. (1997) took their findings to indicate that the sensorimotor representation of the target is short-lived and overridden by the cognitive representation when the delay between disappearance of the target and response becomes sufficiently long. In some subjects the sensorimotor representation might even be so short-lived that it hardly survives the target presentation.
Although the what versus how distinction currently has a dominant influence, it is most likely a simplification. Processing of visual information is widely distributed across the brain, and so is motor control. Thus, it is easy to conceive of a set of systems that for different kinds of responses make use of different combinations of the various neural representations of the visual world. From such a perspective, there would be a multiplicity of perception-action systems, for which there is indeed evidence in other primates than humans (cf. Goodale & Humphrey, 1998).
Motor Coordination
In a general sense, coordination is a characteristic of almost any skilled movement, in that skilled performance requires fairly precise relations between various kinematic, kinetic, and physiological variables. For example, in cranking (and related tasks like pedaling), force pulses need to be precisely timed to occur during a certain phase of the rotation of the crank or pedal (cf. Glencross, 1970); in rapid finger tapping, muscle activity of flexors and extensors must be timed to occur at certain phases of the movement cycle (Heuer, 1998); in reaching for an object, the opening of the fingers must be related to the movement of the hand toward the object (e.g., Jeannerod, 1984); and so on. With this broad meaning, the term coordination becomes almost equivalent to motor control. However, for this section I use a narrower meaning in that I focus on the coordinated movements of different effectors, mainly the two arms (interlimb coordination).
Task Constraints and Structural Constraints
Coordinated movements of the two hands are largely determined by the task constraints. For example, the coordination pattern for sweeping with a broom is different from that for bathing a baby. This certainly is not a fact that deserves elaboration. However, there are more subtle consequences of task constraints. Perhaps the most important of these is compensatory covariation.
As an example, consider the lip aperture in speaking. A particular lip aperture can be achieved by various combinations of the positions of the upper lip, the lower lip, and the jaw. These positions exhibit compensatory covariation such that, for example, a high position of the upper lip will be accompanied by higher positions of the lower lip and/or the jaw, and a low position of the upper lip by lower positions of the lower lip and/or the jaw (Abbs, Gracco, & Cole, 1984). Compensatory covariation can be observed not only when lip positions vary spontaneously, but also when they are perturbed by means of some mechanical device (Kelso, Tuller, Vatikiotis-Bateson, & Fowler, 1984). A task similar to reaching a certain lip aperture is that of grasping an object with a precision grip, wherein there is compensatory covariation of the positions of thumb and index finger (Darling, Cole, & Abbs, 1988).
Compensatory covariation can be seen as a way to reduce the degrees of freedom in motor control. In addition, compensatory covariation contributes to solving the problem of achieving a stable movement outcome in spite of variable components. For example, with the appropriate covariation of lip and jaw positions, lip aperture will hardly vary. In fact, the principle of compensatory covariation seems to be a general principle of stabilizing movement outcomes (Müller, 2001) which is not restricted to tasks in which different limbs are involved.
A highly illustrative task for compensatory covariations is throwing a ball a certain distance. For physical reasons, when the initial flight angle varies, the initial velocity of the ball has to covary to reach a certain target distance. In particular, with an initial angle of 45° the initial velocity has to be smallest, and it has to be increased as the initial flight angle deviates from 45°, provided that the height at which the ball is released is also the height at which the target is located. Conforming to these task constraints, Stimpel (1933) observed positive correlations across series of throws to a certain target position between initial velocity and the absolute deviation of the initial flight angle from 45°. It is not fully clear how the particular covariation is established, but there is the possibility that subjects produce equifinal trajectories of the hand such that across time initial flight angle and velocity covary in the proper way; thereby the proper relation is established independent of the precise time at which the ball is released (Müller & Loosch, 1999).
The plot of the relation between initial velocities and initial flight angles required for a certain outcome of the throws can be thought of as an equal-outcome curve (Heuer, 1989). Figure 12.13 gives an example for a particular dart-throwing task, which also shows that with a target of a certain width, deviations from the bull’s-eye-outcome curve have different consequences for accuracy depending on where on the curve subjects operate. In principle, equal-outcome curves can be determined for all sorts of tasks and for various sets of component variables. They specify how components of a skill must be related to each other in the service of satisfying the task constraints. In fact, this kind of analysis can become considerably more complex than what can be represented in terms of equal-outcome curves (or perhaps areas). An example is the analysis of juggling by Beek (1989).

The very fact that components of a motor pattern covary in the service of achieving particular outcomes has been taken as evidence for the existence of movement Gestalts (Bewegungsgestalten) by Stimpel (1933) and his advisor Klemm (1938), a notion that is analogous to perceptual Gestalts (see the chapter by Palmer in this volume). The core of the notion of a Bewegungsgestalt is the idea that the whole dominates its components and is more precise than expected from the components’ variabilities. Although these notions appear fairly outdated now, it cannot be overlooked that they anticipate synergetic concepts (Haken, 1982) that currently play an important role in the study of motor coordination (cf. Kelso, 1994; Schöner, 1994). One of the core concepts is that of an order parameter (or collective variable) that enslaves the component variables, so that higher level variables are not simply the result of lower level variables, but dominate the lower level variables instead. This general idea is captured by models like the task-dynamic model of Saltzman and Kelso (1987) and the knowledge model of Rosenbaum, Loukopoulos, Meulenbroek, Vaughan, and Engelbrecht (1995).
Coordination in the service of satisfying task constraints is flexible: That is, patterns of covariation between certain effectors that can be observed when one task is performed may be absent when a different task is performed (e.g., Kelso et al., 1984). Nevertheless, for biologically important tasks like standing, locomotion, eating, and so on, there may be more rigid coordination patterns that not only support these tasks, but may also impede performance of sufficiently different tasks. Although it is not certain that such more rigid coordination patterns for biologically important tasks are indeed the origin of structural constraints on coordination, it is certain that structural constraints do exist. Basically, they limit the range of task-specific coordination patterns; while they support the production of certain patterns, they tend to impede the production of deviating patterns.
Structural constraints support symmetrical movements of the two arms. Thus, mirror writing with the left hand becomes a fairly simple task when it is performed concurrently with normal writing of the right hand (Jung & Fach, 1984). The other side of the coin is the difficulty we encounter when we attempt to produce different spatiotemporal patterns concurrently with the two hands. Although it is certainly not true that both hands are constrained to act as a unit in the sense of having a common timing (Kelso, Southard, & Goodman, 1979; Schmidt et al., 1979), bimanual movements tend toward identical durations, and only with strictly required different target durations can this tendency be overcome (Spijkers, Tachmatzidis, Debus, Fischer, & Kausche, 1994). Other deviations from strict symmetry are easier to achieve, but nevertheless there is a widespread tendency for different movements with the two hands not to be as different as they should be; the systematic errors here point to the symmetric patterns that are the ones supported by structural constraints.
The nature of structural constraints on coordination and their combination with task constraints or task-related intentions is nicely captured by perhaps the most influential model of motor coordination and its developments (Haken, Kelso, & Bunz, 1985). This model applies to tasks that require concurrent oscillations of at least two effectors, for example, the two hands. When these oscillatory movements are produced symmetrically, there is essentially no difficulty in speeding them up as far as this is possible. However, when they are produced asymmetrically, the phase relation between the oscillations is less stable, and occasionally symmetric movement cycles intrude (Cohen, 1971). When the asymmetric movements are speeded up, stability is reduced even more, provided that subjects are instructed to maintain the asymmetric phase relation (Lee, Blandin, & Proteau, 1996). However, with a “let it go” instruction, subjects tend to switch to symmetric movements at a certain critical frequency (Kelso, 1984).
Haken et al. (1985) modeled these phenomena in terms of what came later to be called an intrinsic coordination dynamics. The model was formulated at two levels, the level of actual movements and the level of an order parameter that captures the relation between the periodic movements. Basically, at the kinematic level two nonlinear oscillators were posited, one for each effector, with a nonlinear coupling in addition. Relative phase was chosen as the order parameter (or collective variable); this is the phase difference between the two oscillatory movements. For this variable the dynamics were specified based mainly on formal considerations: φ = –a sinφ –2b sin 2φ. Better known is the formulation in terms of a po tential function V with = dV/dφ, V = –a cosφ – b cos 2φ. This potential, which is illustrated in Figure 12.14, has stable equilibria at φ = nπ, n = 0, ±1, ±2, . . . , provided the parameters a and b are within certain ranges. Stable equilibria are characterized by ’s being positive for smaller values of and negative for larger values, so that relative phase will drift back to the equilibrium angle whenever it deviates as a consequence of some perturbation; in the potential function, stable equilibria are characterized by minima.
The ratio of the parameters a and b is hypothesized to depend on movement frequency, b becoming relatively smaller as frequency increases. When it becomes sufficiently small, the stable equilibria at φ = mπ, m = ±1, ±3, ±5, . . . disappear (cf. Figure 12.14). This corresponds to the observation that, as the frequency increases, only symmetric oscillations (in-phase oscillations in formal terms) are maintained while asymmetric oscillations (anti-phase oscillations) tend to switch to symmetric ones.

This account of the switch is based pretty much on formal considerations, and other models are available with stronger reference to physiological or psychological considerations or both (Grossberg et al., 1997; Heuer, 1993b). In addition, the prediction that the switch should be associated with reduced movement amplitudes (Haken et al., 1985) is not necessarily correct (Peper & Beek, 1998). Nevertheless, the model captures nicely the soft nature of structural constraints, and an extension of it illustrates how structural constraints bias performance when they deviate from task constraints or taskrelated intentions.
Yamanishi, Kawato, and Suzuki (1980) asked their subjects to produce bimanual sequences of finger taps at various phase relations. The stability of phasing was highest with synchronous taps (relative phase of 0°), second highest with alternating taps (relative phase of 180°), and lower at all other relative phases. In addition the mean relative phases were biased toward the stable relative phases at 0° and 180°. Schöner and Kelso (1988) modeled these effects by way of adding a term to the intrinsic dynamics that reflects the “intention” to reach a target relative phase , so that the potential becomes
V = – a cosφ – b cos 2φ – c cos((φ –ψ )/2). When the intended relative phase differs from ψ = 0° and ψ = 180°, the minima of this potential are broader, corresponding to an increased variability, and shifted away from the intended relative phase, corresponding to the observed systematic biases.
Basic Structural Constraints on Coordination
Structural constraints on coordination are indicated by systematic errors. They have been studied mainly by means of two different types of task, the one involving sequences of movements with different effectors and the other discrete movements, mostly of short duration. By and large there seem to be no striking discrepancies between the conclusions based on the two types of experimental paradigm, although the precise relation between them is not fully clear. For example, one cannot exclude that certain constraints may evolve gradually so that they are effective for sequences of movements, but not for brief discrete ones.
A highly consistent finding is that periodic oscillations of the upper limbs are more stable when they are performed symmetrically than when they are performed asymmetrically. The same kind of observation has also been made for bimanual circle drawing (e.g., Carson, Thomas, Summers, Walkers, & Semjen, 1997). However, the symmetry constraint, which favors the concurrent activation of homologous muscle groups, is not universal; in addition, there is a bias toward identical movement directions (e.g., Serrien, Bogaerts, Suy, & Swinnen, 1999). This is particularly obvious for periodic movements with nonsymmetric effectors. For example, Baldissera, Cavallari, and Civaschi (1982) and Baldissera, Cavallari, Marini, and Tassone (1991) found that concurrent up-and-down movements of foot and hand in identical directions are more precisely coordinated than concurrent movements in opposite directions. Thus, although essentially there is always a preferred phase relation for periodic movements of different effectors, which phase relation this is depends on the particular effectors chosen and their plane of motion.
A second highly consistent finding is related to the timing of bimanual response sequences. Such sequences are simple when they have the same frequency, and they are also fairly accurately produced when the frequencies are harmonically related, that is, by integer ratios. However, for polyrhythms performance deteriorates (e.g., Klapp, 1979). For this it is not essential that the polyrhythms are produced by the two hands, but poor or even chance performance can also be observed in vocal-manual tasks (Klapp, 1981). There seems to be a general rule that the variability of the temporal errors of individual responses increases as the product mn for m : n rhythms increases (Deutsch, 1983); for example, variability is higher with a 2 : 5 (mn = 20) than with a 2 : 3 (mn = 6) rhythm. When the overall rate of polyrhythms is increased, not only does performance become poorer, but in addition complex rhythms may switch to less complex ones, like 2 : 5 to 1 : 2 (e.g., Peper, 1995).
The observations on polyrhythms have been taken to suggest the existence of a unitary timing-control mechanism for movements of the two hands (Deutsch, 1983). In fact, formal analyses of polyrhythm production in terms of timer models (cf. Vorberg & Wing, 1996) generally reveal integrated control, in which the timing of a response with the one hand can be relative to a preceding response with the other hand (Jagacinski, Marshburn, Klapp, & Jones, 1988; Klapp et al., 1985; Summers, Rosenbaum, Burns, & Ford, 1993). The difficulty in the production of polyrhythms is then basically related to the complexity of the integrated timing control structure. Only recently evidence has been reported according to which professional pianists can exhibit parallel timing control for the two hands when they produce polyrhythms at rapid rates (Krampe, Kliegl, Mayr, Engbert, & Vorberg, 2000). Except for such a select population, however, temporal coupling appears to be so tight that tasks that apparently require decoupling are performed in a way that maintains a unitary timing control.
Relatively little research effort has been invested in the study of sequences of bimanual movements with different amplitudes. Franz, Zelaznik, and McCabe (1991) studied the concurrent production of periodic lines and circles with the two hands. Drawing circles with one hand requires periodic oscillations with the same amplitudes along both axes of the plane, while drawing lines with the other hand requires a periodic oscillation along only one axis; however, for the other axis, one can think of a periodic oscillation with zero amplitude. Franz et al. found that both the lines and the circles became elliptical (Figure 12.15). Thus, the different amplitudes of the oscillations became more similar in the bimanual task: The larger amplitude oscillations in drawing circles were reduced in amplitude, and the zero-amplitude oscillations in drawing lines were enhanced in amplitude. More straightforwardly, such an amplitude assimilation for periodic movements was shown by Spijkers and Heuer (1995) and by Franz (1997), both for lines (that is, one-dimensional oscillations) and for circles (that is, two-dimensional oscillations). In addition, Spijkers and Heuer (1995) found that the amplitude assimilation became stronger as the frequency of oscillations was increased.

Kelso, Tuller, and Harris (1983) had their subjects oscillate a finger and concurrently repeat the syllable stock. When every second syllable had to be stressed, there was an involuntary increase of the amplitude of the accompanying finger movement; similarly, voluntarily increased finger amplitudes were accompanied by involuntary stresses of the syllable. These findings, which have been confirmed by Chang and Hammond (1987), suggest that, in addition to amplitude assimilation, changes of amplitude might overflow to other effectors.
For a more systematic exploration of the contralateral effects of large-to-small or small-to-large amplitude changes, Spijkers and Heuer (1995; Heuer, Spijkers, Kleinsorge, & Steglich, 2000) studied conditions in which one hand had to produce constant-amplitude oscillations and the other hand oscillations with alternating short and long amplitudes. They found that the requirement to change the amplitude in the one hand produced a contralateral effect in addition to the one observed with constant-amplitude oscillations. Specifically, after a change from a short to a long amplitude, the amplitude of the contralateral hand was larger than when only long amplitudes were repeated, and after a change from a long to a short amplitude the amplitude of the contralateral hand was smaller than when only short amplitudes were repeated. These findings indicate that cross-manual effects result not only from concurrent execution of different amplitudes, but perhaps also from cross-talk between processes of amplitude specifications. This is also indicated by the observation that contralateral involuntary amplitude modulations can be produced not only by movements of alternating short and long amplitudes, but also by the imagery of such movements (Heuer, Spijkers, Kleinsorge, & van der Loo, 1998).
Discrete-movement studies give clear evidence of a tight temporal coupling in that movements of different amplitude and accuracy requirements tend to be of (almost) the same duration (Kelso et al., 1979; Marteniuk, MacKenzie, & Baba, 1984). However, amplitude assimilation is only weak and tends to be asymmetrical in that the amplitude of a shorter movement is increased, while the amplitude of the concurrent longer movement is only slightly or not at all reduced (Heuer, Spijkers, Kleinsorge, van der Loo, & Steglich, 1998; Marteniuk et al., 1984; Sherwood, 1991). The tighter temporal than spatial coupling is also indicated by the typical finding that movement durations are more strongly correlated across trials than movement amplitudes (e.g., Sherwood, 1991).
Structural constraints on coordination are double-faced: On the one hand, they support the performance of certain tasks, like the production of strictly symmetric movements of the upper limbs, and on the other hand they impede the performance of other tasks, like the production of asymmetric movements. Together with the basically soft nature of structural constraints, this suggests that their strength might be modulated depending on task requirements. In particular, their strength might be enhanced when this is appropriate for the task at hand, and it might be reduced when this is appropriate. Such a task-related modulation of structural constraints on coordination does indeed exist.
Sherwood (1991) found higher intermanual correlations between the amplitudes of discrete rapid reversal movements when same amplitudes rather than different amplitudes were required. The same result was reported by Heuer, Spijkers, Kleinsorge, van der Loo, and Steglich (1998), who in addition found aftereffects such that the intermanual amplitude correlation was higher subsequent to same-amplitude movements than following different-amplitude movements, and by Steglich, Heuer, Spijkers, and Kleinsorge (1999) for peak forces of isometric contractions with same and different target forces for the two hands.
Rinkenauer, Ulrich, and Wing (2001) showed for isometric contractions that the requirement to produce different peak forces is associated not only with a reduction of the intermanual correlation between peak forces, but also with a reduction of the intermanual correlation between rise times. Similarly, when different rise times were to be produced in another experiment, both the correlations between rise times and between amplitudes were reduced. These findings indicate that the decoupling, which can be observed when different movements or isometric contractions are to be produced with the two hands, is a generalized phenomenon which is not restricted to the movement characteristic, that is actually different. In addition, coupling with respect to peak forces can be more easily modulated than temporal coupling; in fact, peak forces can be fully decoupled, but timing cannot.
In right-handed people, the right and left hands take different roles in bimanual actions: The typical function assigned to the left hand is holding, while the right hand performs manipulations relative to the left one. Generalizing this typical assignment of functions, Guiard (1987) characterized the left and right hand as macrometric and micrometric, respectively. Whereas the left hand is specialized for largeamplitude and low-frequency movements, the right hand is specialized for accurate small-amplitude and high-frequency movements. This characterization of the two hands, together with the typical functions assigned to them in bimanual tasks, suggests that structural constraints on coordination may be asymmetric. Although the results on lateral asymmetries in bimanual tasks tend to be somewhat unreliable, there seem to be at least two consistent findings.
The first finding is that bimanual tasks are easier when lower-frequency movements are assigned to the left hand and higher-frequency movements to the right hand than with the opposite assignment of movements to hands. This is true for oscillatory movements (Gunkel, 1962) and discrete finger taps (Ibbotson & Morton, 1981; Peters, 1981). An example is tapping a steady beat with the left hand and a certain rhythm with the right hand. With the opposite assignment the task is harder, and when performance breaks down, it is typically in the right hand, which also starts to produce the rhythm assigned to the left hand. Thus, conforming to Guiard’s (1987) notion of macrometric and micrometric functional specializations of the two hands, task assignments that conform to these specializations are easier than task assignments that violate them. Also conforming to Guiard’s notion, Spijkers and Heuer (1995) observed stronger assimilations of movement amplitudes when large-amplitude oscillations were produced with the right hand and small-amplitude oscillations with the left hand than with the opposite assignment of amplitudes to hands.
A second rather consistent finding is a lead of the right hand in bimanual tasks like circle drawing (Stucchi & Viviani, 1993; Swinnen, Jardin, & Meulenbroek, 1996). Stucchi and Viviani (1993) hypothesized that in particular the timing of bimanual movements might originate from the hemisphere contralateral to the dominant hand. This hypothesis has received some support from a PET study (Viviani, Perani, Grassi, Bettinardi, & Fazio, 1998), and it is consistent with the evidence for tight temporal coupling (or unitary timing mechanisms) in bimanual tasks.
Levels of Coupling
Structural constraints on coordination can largely be understood as resulting from cross-talk between signals involved in motor control, specifically as a product of coupling, so that movements become more similar than intended. Formally, coupling terms are basic ingredients of dynamic models like the well-known model of Haken et al. (1985). However, these models collapse different kinds of coupling that may exist at different levels of motor control. Such levels can be distinguished both in functional and in anatomical terms. Here I shall focus on functionally defined levels. However, it may be worth mentioning that in anatomical terms there is some evidence for different origins of temporal and spatial coupling. For example, split-brain patients give no indication of a relaxed temporal coupling; if anything, temporal coupling becomes tighter (Preilowski, 1972; Tuller & Kelso, 1989). In contrast, spatial coupling seems to be relaxed in split-brain patients (Franz, Eliassen, Ivry, & Gazzaniga, 1996).
In functional terms, Marteniuk and MacKenzie (1980; Marteniuk et al., 1984) suggested a distinction between an execution level and a programming level, with cross-talk effects originating at both levels. There can be little doubt about the existence of cross-talk at the execution level. This kind of cross-talk reveals itself in the form of associated or mirror movements, that is, involuntary movements that accompany voluntary movements of the other hand. These can be observed in healthy adults (e.g., Durwen & Herzog, 1989, 1992; Todor & Lazarus, 1986), but they tend to be more conspicuous under a variety of neurological conditions or in children when inhibitory mechanisms, which serve to focus the basic bilateral innervation unilaterally, are impaired or not yet fully developed (McDowell & Wolff, 1997; Schott & Wyke, 1981). Models that rely on cross-talk at the execution level can account for several observations on, for example, bimanual circle drawing (Cattaert, Semjen, & Summers, 1999). Nevertheless, there are some results that strongly favor the notion of coupling during motor programming (or parametric cross-talk) in addition to cross-talk at the execution level.
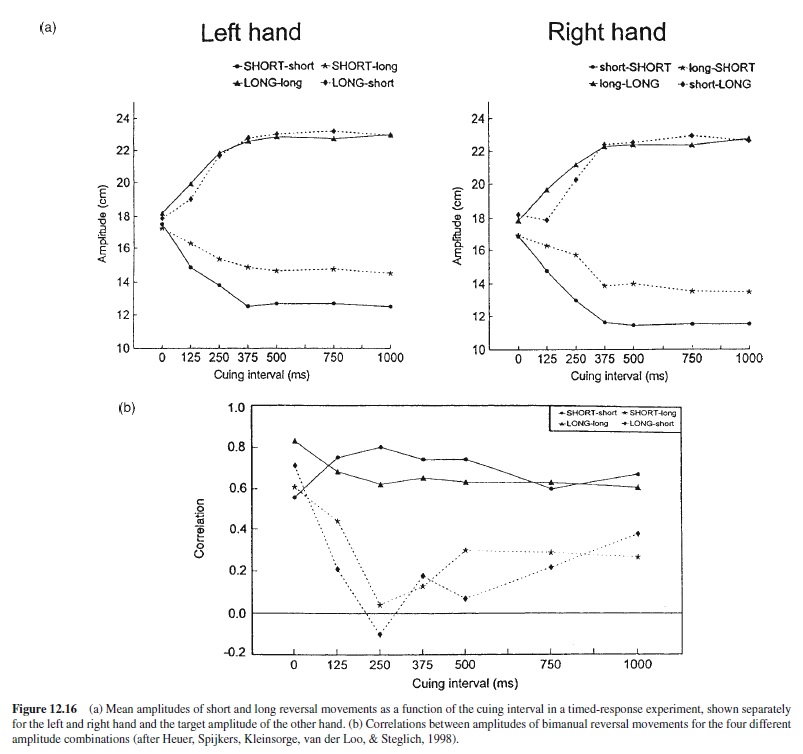
Figure 12.16 shows some data obtained with the timedresponse procedure (Heuer, Spijkers, Kleinsorge, van der Loo, & Steglich, 1998). The task of the participants was to produce bimanual reversal movements with short or long amplitudes. Movements were to be initiated in synchrony with the last of four pacing tones, and cues were presented at variable cuing intervals before the last tone. The cues indicated the amplitudes of the movements, which could be shortshort, long-long, short-long, and long-short. Participants had been instructed to prepare for movements with intermediate amplitudes and then to produce amplitudes according to the cues as far as this was possible.
The continuous lines in Figure 12.16a show how, with increasing preparation time, short and long movements reach their final amplitudes, beginning at an intermediate default amplitude. Broken lines show the temporal evolution of the short and long movement amplitudes when the target amplitude for the other hand was different, long and short, respectively. With long cuing intervals the already described asymmetric amplitude assimilation was found in that the amplitude of the short movement was enhanced by the concurrent requirement to produce a long-amplitude movement with the other hand, whereas the long-amplitude movement was not affected by the concurrent short-amplitude movement of the other hand. However, at short intervals there was also a transient reduction of the long amplitude, although the amplitude difference of the two hands was actually smaller than at long cuing intervals. In Figure 12.16b the correlations between the amplitudes of the two hands are shown: They stay at a high level for same-amplitude movements as the cuing interval increases, but they are rapidly reduced for different-amplitude movements. These basic findings have been replicated for different amplitude differences of the two hands (Heuer, Kleinsorge, Spijkers, & Steglich, 2001) and also for isometric contractions with same and different forces (Steglich et al., 1999).
The data of Figure 12.16a reflect the gradual specification of movement amplitudes, and they reveal a transient parametric coupling, that is, a coupling that is gradually relaxed when required by the task. This is also indicated by the intermanual correlations shown in Figure 12.16b. Parametric coupling does apply to concurrent specifications of movement parameters, but not to the time-varying force signals or other execution-related signals during actual performance of the movements. Thus it should also show up in reaction time, that is, before bimanual movements are actually initiated, and it should show up even in unimanual tasks, provided that parametric specifications for the movement executed have temporal overlap with parametric specifications for a movement with the other hand that is not produced concurrently. There is indeed some evidence for such effects with movements of the two hands with same and different amplitudes (Spijkers, Heuer, Kleinsorge, & van der Loo, 1997; Spijkers, Heuer, Kleinsorge, & Steglich, 2000).
Although parametric coupling seems to be transient as far as amplitude and peak-force specifications are concerned, this is different for temporal specifications. As reviewed above, for different target durations correlations between movement durations do not decline as strongly as correlations between peak forces do. Thus, there is a stronger static component to the parametric coupling, which accounts for the fact that it is extremely hard or even impossible to produce different temporal patterns with the two hands concurrently. If this is indeed the case, one would expect that reaction time for the choice between a left-hand and a righthand movement is longer when movements with different rather than same temporal characteristics are assigned to the two hands. The reason is that same temporal characteristics can be prepared concurrently in advance of the response signal (or perhaps immediately after presentation as long as the choice of the correct response is not yet finished), whereas this is impossible for different temporal characteristics. Such reaction-time differences do exist (see Heuer, 1990, for a review; Heuer, 1995).
Flexibility of Motor Control
The motor transformation, the relation between motor commands and resulting movements, is variable. On a short time scale, variations arise when we handle objects, tools, and machines, and when we move in different directions relative to gravity. On a longer time scale, variations result from body growth and other bodily changes. As a consequence of such variations, the internal model of the motor transformation, which captures the relations between motor commands, proprioceptive information, and visual information, must be flexible. To study this flexibility experimentally, the relations can be modified by way of transforming the normal visual input; in addition, they can be modified by way of adding external forces. I shall consider the flexibility of motor control in both respects in turn.
Adapting and Adjusting to New Visuo-Motor Transformations
Various kinds of optical transformations can be used to change the usual relation between movements (motor commands and proprioceptive movement information) and their visual effects (cf. Welch, 1978, for an overview). The history of such research dates back to the late nineteenth century, when Stratton (1896, 1897a, 1897b) used spectacles that served to turn the visual world upside down. Later Kohler (e.g., 1964) pursued this line of research with various sorts of distorting spectacles. All in all, the perceptual consequences of such severe transformations of the visual world are extremely complex and difficult, if not impossible, to understand. However, as far as motor behavior is concerned, this generally comes to appear fairly normal, even if adaptation can take several days.
A somewhat different and simpler type of transformation of the visual world was introduced by Helmholtz (1867), namely the use of wedge prisms, which serve to shift the visual world laterally. When no visual background (or only a homogeneous one) is available, the distorting effects of wedge prisms can be neglected and the consequence of the shifted egocentric visual direction can be studied. A typical lateral displacement is 11°. Thus, when participants are instructed to point to a target that is visually displaced to the right, their movements will end to the right of the physical target. When they receive feedback on the pointing errors, these will gradually disappear in the course of a series of movements. In principle the disappearance of the systematic pointing errors could be due to strategic corrections, that is, to simply pointing to the left of the perceived target. Alternatively, it can be due to a change of the internal model of the visuo-motor transformation. More revealing than the disappearance of the pointing error in the exposure phase is the negative aftereffect that can be observed after removal of the wedge prisms. Now, without visual feedback, subjects tend to point in the opposite direction, that is, to the left of the target when it had been visually displaced to the right. Negative aftereffects can also be observed when the prism strength is gradually increased in the exposure period with concurrent displacements of the physical target so that it remains in a constant egocentric direction; with this procedure, systematic pointing errors are masked by random errors and are not noticed by the subjects (Howard, 1968).
Prism adaptation implies some kind of change of the internal model of the motor transformation. What is the nature of this change? A first point to note is that it is generalized and not restricted to the particular movement performed during prism exposure. Instead, an exposure period with a single visual target results in negative aftereffects not only for this particular target, but for a range of targets in different directions, and when prismatic displacement is different for targets in different directions, aftereffects reveal a kind of linear interpolation for targets in between (Bedford, 1989). Beyond the generalization across the work space, the aftereffect is not even restricted to pointing at visual targets. In many cases it is approximately the sum of two components, a proprioceptive aftereffect and a visual aftereffect (Hay & Pick, 1966), with the relative size of the two components depending on exposure conditions (e.g., Kelso, Cook, Olson, & Epstein, 1975). A test of the proprioceptive aftereffect is pointing straight ahead, whereas a test for the visual aftereffect is to align a visual stimulus with the straight-ahead position. Thus, what is changed is not the specific relation between visual and proprioceptive direction, but rather the directional meaning of visual and/or proprioceptive signals.
Some findings suggest that the adaptation during prism exposure does not involve a modification of a single internal model of the motor transformation, but some kind of addition, so that the original model remains in existence. On the one hand, negative aftereffects decay even when participants remain in darkness (e.g., Dewar, 1971); on the other hand, even when participants are confronted with the normal visual world between experimental sessions, long-term effects of prismatic adaptation can be observed as soon as they are brought back to the experimental setup (McGonigle & Flook, 1978). In addition, with repeated alternations of periods with and without lateral displacement, or with different lateral displacements, aftereffects tend to disappear, and switching between different visuo-motor transformations becomes almost instantaneous (e.g., Kravitz, 1972; Welch, 1971). These and other results (cf. Welch, Bridgeman, Anand, & Browman, 1993) strongly suggest that multiple models of visuo-motor transformations can be learned and, when required by the task, selectively be put to use, although little is known about the nature of the cues that mediate the retrieval of stored internal models.
In a certain way, the situation when wearing laterally displacing prisms is similar to a situation that has become quite common during the last one or two decades, namely the operation of a computer mouse with concurrent movements of a cursor on a laterally displaced monitor. Although in the first case there are aftereffects, we encounter no difficulties in operating the mouse with different lateral displacements of the screen. There seem to be mainly two reasons for this difference: First, movements performed with the computer mouse are parameterized in terms of (allocentric) distances, whereas movements produced in experiments with laterally displacing prisms are parameterized in terms of (egocentric) locations. Second, and perhaps of less importance, is that with the computer mouse proprioceptive and visual information indicate different egocentric directions of different objects, the hand and the cursor, while in prism-adaptation studies they refer to the same object, the hand. Object identity is a factor that affects the size of negative aftereffects (Welch, 1972).
Visuo-motor transformations can also be changed such that the relations between (allocentric) visual distances and/or directions and (hand-centered) movement amplitudes and/or directions are modified. The basic findings seem to parallel those obtained in prism-adaptation studies to a remarkable degree. For example, aftereffects occur and multiple models of visuo-motor transformations can be learned and selectively accessed when appropriate (Cunningham & Welch, 1994). When a certain internal model has been learned, there seems to follow a kind of labile period in which the learning of a new transformation results in a modification of the model, but after a period of consolidation the learning of a new transformation results in the development of a new model rather than the overriding of the old one (Krakauer, Ghilardi, & Ghez, 1999). With sufficient delays between learning periods, it seems that repeated alternation between different transformations is not needed to acquire multiple internal models.
When discussing visual feedback, I have pointed to the limitations in acquiring internal models of additional transformations of one’s own movements. Whereas adjustments to changes of visuo-motor gains require only one or a few discrete movements (Young, 1969), adjustments to new relations between the directions of hand movements and cursor motions require more trials (e.g., Krakauer et al., 1999). Adjustments to nonlinear transformations require even longer experience, and for too complex transformations internal models can no longer be developed. Although the mastery of added transformations is typically not associated with their awareness (who could tell the gain factor of his or her computer mouse?), the difficulty of such transformations is affected by higher level cognitive processes. This is nicely illustrated by a little-known study of Merz, Kalveram, and Huber (1981), and additional evidence from reaction-time studies is reported in the chapter by Proctor and Vu in this volume.
In the experiment of Merz et al. (1981), participants controlled the movement of a cursor on an oscilloscope screen by means of lateral pressure on a knob. Their task was a tracking task in which they had to keep the cursor aligned with a moving target. For two groups of participants the cursor moved to the right when the knob was pressed to the right (compatible relation), and for two groups of participants the relation was reversed (incompatible). One group with each of the two levels of compatibility had the knob placed at the bottom of a steering wheel, while for the other two groups the steering wheel was covered by a piece of cardboard. In the incompatible conditions there was a strong practice effect. More important, however, is that the performance deficit in the incompatible conditions disappeared when the steering wheel was visible; performance in this condition was as good as in the compatible conditions. Thus, the visibility of the steering wheel enabled the subjects to change the incompatible relation to a compatible one in associating clockwise rotation of the wheel, which is consequent upon leftward pressure, with a rightward motion of the cursor on the screen.
Adjusting and Adapting to External Forces
When external forces vary, motor commands for intended movements have to be modulated accordingly. Except for movements with different directions relative to gravity, perhaps the most frequent adjustments are required when we deal with objects of different masses. Here, depending on the mass, the kinematic characteristics vary. Specifically, with increasing mass, peak acceleration and peak deceleration as well as peak velocity tend to decline, while movement duration tends to increase. This is true both for lifting objects (Gachoud, Mounoud, Hauert, & Viviani, 1983) and for moving them in a horizontal plane (Gottlieb, Corcos, & Agarwal, 1989). Thus, for objects with different masses, peak forces are not perfectly scaled, which would result in an invariant acceleration profile; instead, with increasing mass, acceleration and deceleration become smaller, but an increasing duration serves to avoid a shortening of the amplitude of the movements. In spite of these mass-dependent variations, the basic shape of the velocity profile remains invariant; that is, with the proper scaling of time and velocity, the profiles become identical (Bock, 1990; Ruitenbeek, 1984).
In lifting an object, it is not only the so-called load force which has to be adjusted to the mass (and weight) of the object, but also the grip force (cf. Johansson, 1996, for review). When the grip force is too weak, the object may slip; when it is too strong, the object may break; and, in addition, too high forces are uneconomical. Figure 12.17 shows the buildup of both types of force when an object is lifted. First, grip force starts to develop (preload phase), then load force (loading phase). When the load force is sufficiently strong, the object is lifted (transitional phase) and thereafter held in a certain position.
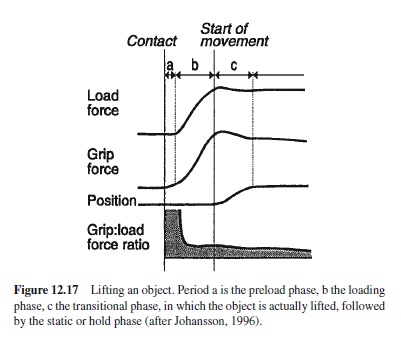
During the loading phase a certain relation between grip force and load force is established, which is generally somewhat higher than the minimal value required to prevent the object from slipping; this safety margin is typically in the range of 10–40%. Of course, the proper adjustment of the grip force depends not only on the object, but also on its surface characteristics. In fact, there is a delicate grip-force adjustment to the friction between fingers and object surface that depends not only on the surface characteristics of the object, but also on those of the skin, which change, for example, after washing one’s hands.
Adjustment of grip force is required not only when an object is lifted, but also when it is moved around, so that there is an inertial load in addition to the gravitational load. In a manner similar to the way load force and grip force increase in parallel when an object is lifted, grip force is modulated in parallel to inertial load while an object is moved (Flanagan, Tresilian, & Wing, 1993; Flanagan & Wing, 1995). In moving an object, there is an important difference between periodic horizontal and vertical movements. In horizontal movements, inertial load is orthogonal to gravitational load; inertial load reaches maxima both at the left and right movement reversals, and grip force reaches maxima at these points as well. Thus, there is a 1:2 ratio of the frequencies of periodic movements and grip-force modulations. In contrast, for vertical movements inertial and gravitational load add, so that total load is particularly strong at the lower movement reversal, but it can be close to zero at the upper movement reversal. In this case the frequency ratio becomes 1:1. This difference between horizontal and vertical movements disappears under conditions of microgravity because of the absence of gravitational load, and subjects adapt rapidly (during parabolic flights) to produce the appropriate 1:2 ratio also with vertical movements (Hermsdörfer et al., 2000).
Force adjustments are both predictive and reactive. This basic principle is evident already from the everyday observation that when someone hands an object to someone else, the latter can normally hold it without difficulties; only when the object is unexpectedly light or heavy, short-lived difficulties arise. Without knowing about the change of the mass of a moved object, the first movement is perturbed, but the next movement is properly adjusted to the new load (Bock, 1993). In fact, corrections for the unexpected mass set in as early as during the first movement, which, in the case of an unexpectedly high load, results mainly in a prolonged movement duration (Bock, 1993; Smeets, Erkelens, & Denier van der Gon, 1995). It seems that under conditions of microgravity, when objects are weightless but nevertheless have normal mass and thus inertial load, movements exhibit characteristics of movements with an unexpectedly high mass even for weeks (Sangals, Heuer, Manzey, & Lorenz, 1999).
Motor commands for active movements are most likely among the information that is involved in predictive force adjustments, as can be evidenced from the grip-force adjustments while moving a hand-held object. Of course, this kind of prediction can work only when force adjustments are required as a consequence of self-generated activity. When force adjustments are required to accommodate variations in load, which are independent of self-generated activity, predictions must rely on other kinds of information. Obviously, proper force adjustments depend on experience; the first movement after an unnoticed change of the load is performed with an initially maladjusted force, but not the second one. In addition, seen object size plays a role, although force adjustment is not necessarily related to estimates of weight. Gordon, Forssberg, Johansson, and Westling (1991) examined the lifting of boxes of identical weight but different sizes. Although subjects judged the smaller boxes to be heavier than the larger ones, peak grip and load forces were stronger for the larger ones. However, this difference was present only during lifting and disappeared during subsequent holding of the object, when force adjustments were perhaps related to the actual weight and no longer to the visually mediated predictive mechanisms.
Adjustments to objects of different masses seem to be comparatively simple achievements, similar to adjustments to different visuo-motor gains. When the external forces which act on a moving limb are transformed in a more complex way, similarities between adjustments to modified visuo-motor transformations and modified external force fields become more conspicuous. Shadmehr and MussaIvaldi (1994) introduced such forces while the participants moved a robot arm. For example, forces were proportional to hand velocity and nearly orthogonal to the direction of hand movement, so that initially the paths of the hand were strongly curved. With continued experience the paths became again approximately straight lines, which—as an aside—can be taken as additional evidence for the claim that motor planning refers to end-effector kinematics. After removal of the external forces there were negative aftereffects: The paths of the hand were curved again, but in the opposite direction. The aftereffects indicate that the adjustments were based on a new internal model of the dynamic transformation.
Similar to the findings with modified visuo-motor transformations, multiple internal models of dynamic transformations can be acquired (Shadmehr & Brashers-Krug, 1997). Again there seems to be a labile period after a new model has been learned, during which it will be unlearned when another dynamic transformation is experienced, but after a period of consolidation this is no longer the case. Once an internal model has survived the labile period, it can be put to efficient use even months later.
Adjustments to new dynamic transformations generalize across different types of movement (Condit, Gandolfo, & Mussa-Ivaldi, 1997) and also across the work space (Shadmehr & Mussa-Ivaldi, 1994). When movements are performed in a different region of the workspace from during the practice period, external forces can remain invariant either with respect to the movement of the end-effector in coordinates of extrinsic space or with respect to the joint movements. Generalizations across the work space turned out to be approximate in joint coordinates. This is consistent with a particularly intriguing parallel between adaptation to shifted visual directions and additional external forces.
Prism adaptation involves modified relations between proprioceptive and/or visual signals and their meaning in terms of egocentric directions. Adaptation to a modified external force field seems to involve a modified relation between muscle activations (or motor commands) and the directions of consequent movements (Shadmehr & Moussavi, 2000). For example, the EMG signal from an elbow flexor can be plotted as a function of movement direction (more precisely, it is the EMG signal integrated across a certain time interval around the start of the movement); this results in a directional tuning curve of a muscle. Of course, the peak of this curve is shifted when the shoulder joint is moved. However, there is also a shift induced by the adaptation to a new force field, and this shift is more or less additive to shifts associated with rotations at the shoulder. This adaptation-induced shift allows one to predict the generalization across the work space.
Moving On
Research on the adaptive capabilities of human motor control can serve to illustrate some fairly general characteristics of the field: First, different phases or waves can be distinguished; second, different lines of research come together and trigger new waves; third, research on applied problems often precedes more theoretically minded research; and fourth, new concepts enter the field, often coming from other academic disciplines. At present, research on adapting to visual distortions and to added transformations, as in tracking tasks, is combined; research on the latter has a strong applied history related, for example, to vehicle control. The new theoretical concepts come largely from modern control theory and robotics. On top of such developments are new measurement technologies, which have made the recording of movements easier and which open progressively wider windows onto the activity of the brain while it controls movement.
Science seems to be driven largely by practical needs and by the apparent human desire to have coherent ideas of oneself and the world one lives in. Perhaps some of the findings reported in this chapter challenge ideas humans tend to have about themselves, but as far as motor control is concerned, the more important driving forces seem to be practical, related to technical developments as in robotics, to the control of complex machines, and to new challenges for manual skills, as in minimally invasive surgery. Perhaps future developments will result in tighter links of the (functional) theoretical concepts of the field to the solution of applied problems on the one hand and to the neuronal substrates on the other hand.
Bibliography:
- Abbs, J. H., Gracco, V. L., & Cole, K. J. (1984). Control of multimovement coordination: Sensorimotor mechanisms in speech motor programming. Journal of Motor Behavior, 16, 195–232.
- Abrams, R. A., & Landgraf, J. Z. (1990). Differential use of distance and location information for spatial localization. Perception & Psychophysics, 47, 349–359.
- Aschersleben, G., Gehrke, J., & Prinz, W. (2001). Tapping with peripheral nerve block. A role for tactile feedback in the timing of movements. Experimental Brain Research, 136, 331–339.
- Aschersleben, G., & Prinz, W. (1995). Synchronizing actions with events: The role of sensory information. Perception & Psychophysics, 57, 305–317.
- Aschersleben, G., & Prinz, W. (1997). Delayed auditory feedback in synchronization. Journal of Motor Behavior, 29, 35–46.
- Baldissera, F., Cavallari, P., & Civaschi, P. (1982). Preferential coupling between voluntary movements of ipsilateral limbs. Neuroscience Letters, 34, 95–100.
- Baldissera, F., Cavallari, P., Marini, G., & Tassone, G. (1991). Differential control of in-phase and anti-phase coupling of rhythmic movements of ipsilateral hand and foot. Experimental Brain Research, 83, 375–380.
- Bedford, F. L. (1989). Constraints on learning new mappings between perceptual dimensions. Journal of Experimental Psychology: Human Perception and Performance, 15, 232–248.
- Beek, P. J. (1989). Juggling dynamics. Amsterdam: Free University Press.
- Bennett, S., van der Kamp, J., Savelsbergh, G. J., & Davids, K. (1999). Timing a one-handed catch. I. Effects of telestereoscopic viewing. Experimental Brain Research, 129, 362–368.
- Bizzi, E., Accornero, N., Chapple, W., & Hogan, N. (1984). Posture control and trajectory formation during arm movement. Journal of Neuroscience, 4, 2738–2744.
- Blöte, A. W., & Dijkstra, J. F. (1989). Task effects on young children’s performance in manipulating a pencil. Human Movement Science, 8, 515–528.
- Bock, O. (1990). Load compensation in human goal-directed movements. Behavioural Brain Research, 41, 167–177.
- Bock, O. (1993). Early stages of load compensation in human aimed arm movements. Behavioural Brain Research, 55, 61–68.
- Bock, O., & Arnold, K. (1993). Error accumulation and error correction in sequential pointing movements. Experimental Brain Research, 95, 111–117.
- Bock, O., & Eckmiller, R. (1986). Goal-directed arm movements in absence of visual guidance: Evidence for amplitude rather than position control. Experimental Brain Research, 62, 451–458.
- Bootsma, R. J. (1989). Accuracy of perceptual processes subserving different perception-action systems. Quarterly Journal of Experimental Psychology, 41A, 489–500.
- Bootsma, R. J., & van Wieringen, P. C. W. (1990). Timing an attacking forehand drive in table tennis. Journal of Experimental Psychology: Human Perception and Performance, 16, 21–29.
- Bridgeman, B., Kirch, M., & Sperling, A. (1981). Segregation of cognitive and motor aspects of visual function using induced motion. Perception & Psychophysics, 29, 336–342.
- Bridgeman, B., Lewis, S., Heit, G., & Nagle, M. (1979). Relation between cognitive and motor-oriented systems of visual position perception. Journal of Experimental Psychology: Human Perception and Performance, 5, 692–700.
- Bridgeman, B., Peery, S., & Anand, S. (1997). Interaction of cognitive and sensorimotor maps of visual space. Perception & Psychophysics, 59, 456–469.
- Brown, T. G. (1911). The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society, 84B, 308–319.
- Brunia, C. H. M. (1999). Neural aspects of anticipatory behavior. Acta Psychologica, 101, 213–242.
- Brunia, C. H. M., Haagh, S. A. V. M., & Scheirs, J. G. M. (1985). Waiting to respond: Electrophysiological measurements in man during preparation for a voluntary movement. In H. Heuer, U. Kleinbeck, & K.-H. Schmidt (Eds.), Motor behavior: Programming, control, and acquisition (pp. 35–78). Berlin, Germany: Springer.
- Bullock, , & Grossberg, S. (1988). Neural dynamics of planned arm movements: Emergent invariants and speed-accuracy properties during trajectory formation. Psychological Review, 95, 49–90.
- Campion, J., Latto, R., & Smith, Y. M. (1983). Is blindsight an effect of scattered light, spared cortex, and near-threshold vision? Behavioral and Brain Sciences, 6, 423–486.
- Carlton, L. G. (1981). Visual information: The control of aiming movements. Quarterly Journal of Experimental Psychology, 33A, 87–93.
- Carson, R. G.,Thomas, J., Summers, J. J.,Walters, M. R., & Semjen, A. (1997). The dynamics of bimanual circle drawing. Quarterly Journal of Experimental Psychology, 50A, 664–683.
- Cattaert, D., Semjen, A., & Summers, J. J. (1999). Simulating a neural cross-talk model for between-hand interference during bimanual circle drawing. Biological Cybernetics, 81, 343–358.
- Chang, P., & Hammond, G. R. (1987). Mutual interactions between speech and finger movements. Journal of Motor Behavior, 19, 265–274.
- Cohen, L. (1971). Synchronous bimanual movements performed by homologousandnonhomologousmuscles.PerceptualandMotor Skills, 32, 639–644.
- Condit, M. A., Gandolfo, F., & Mussa-Ivaldi, F. (1997). The motor system does not learn the dynamics of the arm by role memorizations of past experience. Journal of Neurophysiology, 78, 554–560.
- Cooke, J. D. (1980). The organization of simple, skilled movements. In G. E. Stelmach & J. Requin (Eds.), Tutorials in motor behavior (pp. 199–212). Amsterdam: North-Holland.
- Cordo, P. J., & Nashner, L. M. (1982). Properties of postural adjustments associated with rapid arm movements. Journal of Neurophysiology, 47, 287–302.
- Crossman, E. R. F. W., & Goodeve, P. J. (1963/1983). Feedback control of hand-movement and Fitts’ law. Quarterly Journal of ExperimentalPsychology,35A,251–278(originallypresentedatthe Meeting of the Experimental Psychology Society, Oxford, 1963).
- Cruse, H. (1986). Constraints for joint angle control of the human arm. Biological Cybernetics, 54, 125–132.
- Cruse, H., & Brüwer, M. (1987). The human arm as a redundant manipulator: The control of path and joint angles. Biological Cybernetics, 57, 137–144.
- Cruse, H., Dean, J., Heuer, H., & Schmidt, R. A. (1990). Utilization of sensory information for motor control. In O. Neumann & W. Prinz (Eds.), Relationships between perception and action: Current approaches (pp. 43–79). Berlin, Germany: Springer.
- Cunningham, H. A., & Welch, R. B. (1994). Multiple concurrent visual-motor mapping: implications for models of adaptation. Journal of Experimental Psychology: Human Perception and Performance, 20, 987–999.
- Darling, W. G., Cole, K. J., & Abbs, J. H. (1988). Kinematic variability in grasp movements as a function of practice and movement speed. Experimental Brain Research, 73, 225–235.
- Davidson, P. R., Jones, R. D., Sirisena, H. R., & Andreae, J. H. (2000). Detection of adaptive inverse models in the human motor system. Human Movement Science, 19, 761–795.
- Derwort, A. (1938). Untersuchungen über den Zeitablauf figurierter Bewegungen beim Menschen [Studies of the timing of figured movements in man]. Pflügers Archiv für die gesamte Physiologie, 240, 661–675.
- Dewar, R. (1971). Adaptation to displaced vision: Variations in the shaping technique. Perception & Psychophysics, 9, 155–157.
- Deutsch, D. (1983). The generation of two isochronous sequences in parallel. Perception & Psychophysics, 34, 331–337.
- Durwen, H. F., & Herzog, A. G. (1989). Electromyographic investigation of mirror movements in normal adults: Variation of frequency with side of movements, handedness, and dominance. Brain Dysfunction, 2, 84–92.
- Durwen, H. F., & Herzog, A. G. (1992). Electromyographic investigation of mirror movements in normal adults: Variation of frequency with site, effort, and repetition of movement. Brain Dysfunction, 5, 310–318.
- Elliott, D., & Allard, F. (1985). The utilization of visual feedback information during rapid pointing movements. Quarterly Journal of Experimental Psychology, 37A, 407–425.
- Elliott, D., & Lee, T. D. (1995). The role of target information on manual-aiming bias. Psychological Research, 58, 2–9.
- Elliott, D., & Madalena, J. (1987). The influence of premovement visual information on manual aiming. Quarterly Journal of Experimental Psychology, 39A, 541–559.
- Engström, D. A., Kelso, J. A. S., & Holroyd, T. (1996). Reactionanticipation transitions in human perception-action patterns. Human Movement Science, 15, 809–832.
- Favilla, M., & De Cecco, E. (1996). Parallel direction and extent specification of planar reaching arm movements in humans. Neuropsychology, 34, 609–613.
- Favilla, M., Hening, W., & Ghez, C. (1989). Trajectory control in targeted force impulses. VI. Independent specification of response amplitude and direction. Experimental Brain Research, 75, 280–294.
- Fisk, J. D., & Goodale, M. A. (1989). The effects of instructions to subjects on the programming of visually directed reaching movements. Journal of Motor Behavior, 21, 5–19.
- Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47, 381–391.
- Flanagan, J. R., Tresilian, J., & Wing, A. M. (1993). Coupling of grip force and load force during arm movements with grasped objects. Neuroscience Letters, 152, 53–56.
- Flanagan, J. R., & Wing, A. M. (1995). The stability of precision grip forces during cyclic arm movements with a hand-held load. Experimental Brain Research, 105, 455–464.
- Flanders, M., Helms Tillery, S. I., & Soechting, J. F. (1992). Early stages in a sensorimotor transformation. Behavioral and Brain Sciences, 15, 309–362.
- Flash, T., & Hogan, N. (1985). The coordination of arm movements: An experimentally confirmed mathematical model. Journal of Neuroscience, 5, 1688–1703.
- Franz, E. A. (1997). Spatial coupling in the coordination of complex actions. Quarterly Journal of Experimental Psychology, 50A, 684–704.
- Franz, E. A., Eliassen, J. C., Ivry, R. B., & Gazzaniga, M. S. (1996). Dissociation of spatial and temporal coupling in the bimanual movements of callosotomy patients. Psychological Science, 7, 306–310.
- Franz, E. A., Zelaznik, H. N., & McCabe, G. (1991). Spatial topological constraints in a bimanual task. Acta Psychologica, 77, 137–151.
- Gachoud, J. P., Mounoud, P., Hauert, C. A., & Viviani, P. (1983). Motor strategies in lifting movements: A comparison of adult and child performance. Journal of Motor Behavior, 15, 202–216.
- Gentilucci,M.,Chiefi,S.,Daprati,E.,Saetti,M.C.,&Toni,I.(1996). Visual illusion and action. Neuropsychologia, 34, 369–376.
- Gentner, D. R. (1987). Timing of skilled motor performance: Tests of the proportional duration model. Psychological Review, 94, 255–276.
- Georgopoulos, A. P., Lurito, J. T., Petrides, M., Schwartz, A. B., & Massey, J. T. (1989). Mental rotation of the neuronal population vector. Science, 243, 234–236.
- Georgopoulos, A. P., & Massey, J. T. (1987). Cognitive spatialmotor processes. 1. The making of movements at various angles from a stimulus direction. Experimental Brain Research, 65, 361–370.
- Ghez, C., Favilla, M., Ghilardi, M. F., Gordon, J., Bermejo, R., & Pullman, S. (1997). Discrete and continuous planning of hand movements and isometric force trajectories. Experimental Brain Research, 115, 217–233.
- Gielen, S. C. A. M., van den Heuvel, P. J. M., & van Gisbergen, J. A. M. (1984). Coordination of fast eye and arm movements in a tracking task. Experimental Brain Research, 56, 154–161.
- Gillam, B., & Chambers, D. (1985). Size and position are incongruous: Measurements on the Müller-Lyer figure. Perception & Psychophysics, 37, 549–556.
- Glencross, D. J. (1970). Serial organization and timing in a motor skill. Journal of Motor Behavior, 2, 229–237.
- Goodale, M. A., & Humphrey, G. K. (1998). The objects of action and perception. Cognition, 67, 181–207.
- Goodale, M. A., & Milner, A. D. (1992). Separate visual pathways for perception and action. Trends in Neuroscience, 15, 20–25.
- Goodman, D., & Kelso, J. A. S. (1980). Are movements preparated in parts? Not under compatible (naturalized) conditions. Journal of Experimental Psychology: General, 109, 475–495.
- Goodwin, G. M., McCloskey, D. I., & Matthews, P. B. C. (1972). The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain, 95, 705–748.
- Gordon, M., Forssberg, H., Johansson, R. S., & Westling, G. (1991). Visual size cues in the programming of manipulative forces during precision grip. Experimental Brain Research, 83, 477–482.
- Gordon, J., Ghilardi, M. F., & Ghez, C. (1994). Accuracy of planar reaching movements. I. Independence of direction and extent variability. Experimental Brain Research, 99, 97–111.
- Gottlieb, G. L., Corcos, D. M., & Agarwal, G. C. (1989). Organizing principles for single joint movements. I: A speed-insensitive strategy. Journal of Neurophysiology, 62, 342–357.
- Grossberg, S., Pribe, C., & Cohen, M. A. (1997). Neural control of interlimb oscillations: I. Human bimanual coordination. Biological Cybernetics, 77, 131–140.
- Guiard, Y. (1987). Asymmetric division of labor in human skilled bimanual action: The kinematic chain as a model. Journal of Motor Behavior, 19, 486–517.
- Gundry, J. (1975). The use of location and distance in reproducing different amplitudes of movement. Journal of Motor Behavior, 7, 91–100.
- Gunkel, M. (1962). Über relative Koordination bei willkürlichen menschlichen Gliederbewegungen [On relative coordination in voluntary human limb movements]. Pflügers Archiv für die gesamte Physiologie, 275, 472–477.
- Haken, H. (1982). Synergetik: Eine Einführung [Synergetics: An introduction]. Berlin, Germany: Springer.
- Haken, H., Kelso, J. A. S., & Bunz, H. (1985). A theoretical model of phase transitions in human hand movements. Biological Cybernetics, 51, 347–356.
- Hay, J. C., & Pick, H. (1966). Visual and proprioceptive adaptation to optical displacement of the visual stimulus. Journal of Experimental Psychology, 71, 150–158.
- Helmholtz, H. von. (1867). Handbuch der Physiologischen Optik [Handbook of Physiological Optics]. Leipzig, Germany: Voss.
- Hening, W., Favilla, M., & Ghez, C. (1988). Trajectory control in targeted force impulses. V. Gradual specification of response amplitude. Experimental Brain Research, 71, 116–128.
- Henry, F. M., & Rogers, D. E. (1960). Increased response latency for complicated movements and a “memory drum” theory of neuromotor reaction. Research Quarterly, 31, 448–458.
- Hermsdörfer, J., Marquardt, C., Philipp, J., Zierdt, A., Nowak, D., Glasauer, S., & Mai, N. (2000). Moving weightless objects. Grip force control during microgravity. Experimental Brain Research, 132, 52–64.
- Heuer, H. (1983). Bewegungslernen [Motor learning]. Stuttgart, Germany: Kohlhammer.
- Heuer, H. (1989). Movement strategies as points on equal-outcome curves. Behavioral and Brain Sciences, 12, 220–221.
- Heuer, H. (1990). Rapid responses with the left or right hand: Response-response compatibility effects due to intermanual interactions. In R. W. Proctor & T. G. Reeve (Eds.), Stimulusresponse compatibility: An integrated perspective (pp. 311– 342). Amsterdam: North-Holland.
- Heuer, H. (1991). Invariant relative timing in motor-program theory. In J. Fagard & P. H. Wolff (Eds.), The development of timing control and temporal organization in coordinated action: Invariant relative timing, rhythms, and coordination (pp. 37–68). Amsterdam: North-Holland.
- Heuer, H. (1993a). Estimates of time to contact based on changing size and changing target vergence. Perception, 22, 549–563.
- Heuer, H. (1993b). Structural constraints on bimanual movements. Psychological Research, 55, 83–98.
- Heuer, H. (1995). Models for response-response compatibility: The effects of the relation between responses in a choice task. Acta Psychologica, 90, 315–332.
- Heuer, H. (1998). Blocking in rapid finger tapping: The role of variability in proximo-distal coordination. Journal of Motor Behavior, 30, 130–142.
- Heuer, H., Kleinsorge, T., Spijkers, W., & Steglich, C. (2001). Static and phasic cross-talk effects in discrete bimanual reversal movements. Journal of Motor Behavior, 33, 67–85.
- Heuer, H., & Sangals, J. (1998). Task-dependent mixtures of coordinate systems in visuomotor transformations. Experimental Brain Research, 119, 224–236.
- Heuer, H., & Schmidt, R. A. (1988). Transfer of learning among motor patterns with different relative timing. Journal of Experimental Psychology: Human Perception and Performance, 14, 241–252.
- Heuer, H., Schmidt, R. A., & Ghodsian, D. (1995). Generalized motor programs for rapid bimanual tasks: A two-level multiplicative-rate model. Biological Cybernetics, 73, 343–356.
- Heuer, H., Spijkers, W., Kleinsorge, T., & Steglich, C. (2000). Parametrische Kopplung bei Folgen beidhändiger Umkehrbewegungen mit gleichen und unterschiedlichen Weiten [Parametric coupling in sequences of bimanual reversal movements with same and different amplitudes]. Zeitschrift für experimentelle Psychologie, 47, 34–49.
- Heuer, H., Spijkers, W., Kleinsorge, T., & van der Loo, H. (1998a). Period duration of physical and imaginary movement sequences affects contralateral amplitude modulation. Quarterly Journal of Experimental Psychology, 51A, 755–779.
- Heuer, H., Spijkers, W., Kleinsorge, T., van der Loo, H., & Steglich, C. (1998b). The time course of cross-talk during the simultaneous specification of bimanual movement amplitudes. Experimental Brain Research, 118, 381–392.
- Hollerbach, J. M., & Atkeson, C. G. (1987). Deducing planning variables from experimental arm trajectories: Pitfalls and possibilities. Biological Cybernetics, 56, 279–292.
- Holst, E. von (1939). Die relative Koordination als Phänomen und als Methode zentralnervöser Funktionsanalyse [Relative coordination as phenomenon and as a method for the functional analysis of the central nervous system]. Ergebnisse der Physiologie, 42, 228–306.
- Howard, I. P. (1968). Displacing the optical array. In S. J. Freedman (Ed.), The neuropsychology of spatially oriented behavior (pp. 19–36). Homewood, IL: Dorsey Press.
- Ibbotson, N. R., & Morton, J. (1981). Rhythm and dominance. Cognition, 9, 125–138.
- Jagacinski, R. J., Marshburn, E., Klapp, S. T., & Jones, M. R. (1988). Tests of parallel versus integrated structure in polyrhythmic tapping. Journal of Motor Behavior, 20, 416–442.
- James, W. (1950). The principles of psychology. New York: Dover. (Original work published 1890)
- Jeannerod, M. (1984). The timing of natural prehension movements. Journal of Motor Behavior, 16, 235–254.
- Johansson, R. S. (1996). Sensory and memory information in the control of dexterous manipulation. In F. Lacquaniti & P. Viviani (Eds.), Neural bases of motor behaviour (pp. 205–260). Dordrecht, The Netherlands: Kluwer.
- Jordan, M. I. (1996). Computational aspects of motor control and motor learning. In H. Heuer & S. W. Keele (Eds.), Handbook of perception and action: Vol. 2. Motor skills (pp. 71–120). London: Academic Press.
- Jung, R., & Fach, C. (1984). Spiegelschrift und Umkehrschrift bei Linkshändern und Rechtshändern: Ein Beitrag zum Balkentransfer und Umkehrlernen [Mirrorscript and invalid script in lefthanders and righthanders: A contribution to colossal transfer and learned inversion]. In L. Spillmann & B. R. Wooten (Eds.), Sensory experience, adaptation, and perception: Festschrift for Ivo Kohler (pp. 377–399). Hillsdale, NJ: Erlbaum.
- Kalveram, K.-T. (1991). Pattern generating and reflex-like processes controlling aiming movements in the presence of inertia, damping and gravity. Biological Cybernetics, 64, 413–419.
- Kay, B. A., Kelso, J. A. S., Saltzman, E., & Schöner, G. (1987). Space-time behavior of single and bimanual rhythmical movements: Data and limit cycle model. Journal of Experimental Psychology: Human Perception and Performance, 13, 178–192.
- Keele, S. W. (1968). Movement control in skilled motor performance. Psychological Bulletin, 70, 387–403.
- Keele, S. W. (1986). Motor control. In K. R. Boff, L. Kaufman, & J. P. Thomas (Eds.), Handbook of perception and performance: Vol. 2. Cognitive processes and performance (pp. 30-1–30-60). New York: Wiley.
- Keele, S. W., & Posner, M. I. (1968). Processing of visual feedback in rapid movements. Journal of Experimental Psychology, 77, 155–158.
- Kelso, J. A. S. (1984). Phase transitions and critical behavior in human bimanual coordination. American Journal of Physiology: Regulatory, Integrative, and Comparative, 246, R1000– R1004.
- Kelso, J. A. S. (1994). Elementary coordination dynamics. In S. P. Swinnen, H. Heuer, J. Massion, & P. Casaer (Eds.), Interlimb coordination: Neural, dynamical, and cognitive constraints (pp. 301–318). San Diego, CA: Academic Press.
- Kelso, J. A. S., Cook, E., Olson, M. E., & Epstein, W. (1975). Allocation of attention and the locus of adaptation to displaced vision. Journal of Experimental Psychology: Human Perception and Performance, 1, 237–245.
- Kelso, J. A. S., & Holt, K. G. (1980). Exploring a vibratory system analysis of human movement production. Journal of Neurophysiology, 43, 1183–1196.
- Kelso, J. A. S., Southard, D. L., & Goodman, D. (1979). On the coordination of two-handed movements. Journal of Experimental Psychology: Human Perception and Performance, 5, 229– 238.
- Kelso, J. A. S., Tuller, B., & Harris, K. S. (1983). A “dynamic pattern” perspective on the control and coordination of movement. In P. F. MacNeilage (Ed.), The production of speech (pp. 137–173). Berlin, Germany: Springer.
- Kelso, J. A. S., Tuller, B., Vatikiotis-Bateson, E., & Fowler, C. A. (1984). Functionally specific articulatory cooperation following jaw perturbation during speech: Evidence for coordinative structures. Journal of Experimental Psychology: Human Perception and Performance, 10, 812–832.
- Klapp, S. T. (1979). Doing two things at once: The role of temporal compatibility. Memory and Cognition, 7, 375–381.
- Klapp, S. T. (1981). Temporal compatibility in dual motor tasks II: simultaneous articulation and hand movements. Memory and Cognition, 9, 398–401.
- Klapp, S. T., Hill, M., Tyler, J., Martin, Z., Jagacinski, R., & Jones, M. (1985). On marching to two different drummers: Perceptual aspects of the difficulties. Journal of Experimental Psychology: Human Perception and Performance, 11, 814–828.
- Klemm, O. (1938). Zwölf Leitsätze zu einer Psychologie der Leibesübungen [Twelve guiding principles for a psychology of gymnastics]. Neue Psychologische Studien, 9, 351–382.
- Koh,K.,&Meyer,D.E.(1991).Functionlearning:Inductionofcontinuous stimulus-response relations. Journal of Experimental Psychology: Learning, Memory and Cognition, 17, 811–836.
- Kohler, I. (1964). The formation and transformation of the perceptual world. Psychological Issues, 3, 1–173.
- Kornhuber, H. H., & Deecke, L. (1965). Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale [Changes of brain potentials in voluntary and passive movements of man: Bereitschaftspotential and reafferente potentiale]. Pflügers Archiv für die gesamte Physiologie, 284, 1–17.
- Krakauer, J. W., Ghilardi, M. F., & Ghez, C. (1999). Independent learning of internal models for kinematic and dynamic control of reaching. Nature Neuroscience, 2, 1026–1031.
- Krampe, R. T., Kliegl, R., Mayr, U., Engbert, R., & Vorberg, D. (2000). The fast and the slow of skilled bimanual rhythm production: Parallel versus integrated timing. Journal of Experimental Psychology: Human Perception and Performance, 26, 206–233.
- Kravitz, J. H. (1972). Conditioned adaptation to prismatic displacement. Perception & Psychophysics, 11, 38–42.
- Laabs, G. J. (1974). The effect of interpolated motor activity on the short-term retention of movement distance and end-location. Journal of Motor Behavior, 6, 279–288.
- Lajoie, Y., Paillard, J., Teasdale, N., Bard, C., Fleury, M., Forget, R., & Lamarre, Y. (1992). Mirror drawing in a deafferented patient and normal subjects: Visuoproprioceptive conflict. Neurology, 42, 1104–1106.
- Lashley, K. S. (1917). The accuracy of movement in the absence of excitation from the moving organ. American Journal of Physiology, 43, 169–194.
- Lashley, K. S. (1951). The problem of serial order in behavior. In L. A. Jeffress (Ed.), Cerebral mechanism in behavior (pp. 112– 131). New York: Wiley.
- Latash, M. L., & Jaric, S. (in press). The organization of drinking: Postural characteristics of the arm-head coordination. Journal of Motor Behavior.
- Lee, D. N. (1976). A theory of visual control of braking based on information about time-to-collision. Perception, 5, 437–459.
- Lee, T. D., Blandin, Y., & Proteau, L. (1996). Effects of task instructions and oscillation frequency on bimanual coordination. Psychological Research, 59, 100–106.
- Legge, D. (1965). Analysis of visual and proprioceptive components of motor skill by means of a drug. British Journal of Psychology, 56, 243–254.
- Lépine, D., Glencross, D., & Requin, J. (1989). Some experimental evidence for and against a parametric conception of movement programming. Journal of Experimental Psychology: Human Perception and Performance, 15, 347–362.
- Lippold, O. C. J. (1952). The relation between integrated action potentials in a human muscle and its isometric tension. Journal of Physiology, 117, 492–499.
- Lippold, O. C. J., Redfearn, J. W. T., & Vucˇo, j. (1960). The electromyography of fatigue. Ergonomics, 3, 121–131.
- Mack, A., Heuer, F., Villardi, K., & Chambers, D. (1985). The dissociation of position and extent in Müller-Lyer figures. Perception & Psychophysics, 37, 335–344.
- MacKenzie, C. L., Marteniuk, R. G., Dugas, C., Liske, D., & Eickmeier, B. (1987). Three-dimensional movement trajectories in Fitts’ task: Implications for control. Quarterly Journal of Experimental Psychology, 39A, 629–647.
- Marteniuk, R. G., & MacKenzie, C. L. (1980). Apreliminary theory of two-hand co-ordinated control. In G. E. Stelmach & J. Requin (Eds.), Tutorials in motor behavior (pp. 185–197). Amsterdam: North-Holland.
- Marteniuk, R. G., MacKenzie, C. L., & Baba, D. M. (1984). Bimanual movement control: Information processing and interaction effects. Quarterly Journal of Experimental Psychology, 36A, 335–365.
- Marteniuk, R. G., MacKenzie, C. L., Jeannerod, M., Athènes, S., & Dugas, C. (1987). Constraints on human arm movement trajectories. Canadian Journal of Psychology, 41, 365–378.
- Massion, J. (1992). Movement, posture, and equilibrium: Interactions and coordination. Progress in Neurobiology, 38, 35–56.
- McDowell, M. J., & Wolff, P. H. (1997). A functional analysis of human mirror movements. Journal of Motor Behavior, 29, 85–96.
- McGonigle, B. O., & Flook, J. (1978). Long-term retention of single and multistate prismatic adaptation by human. Nature, 272, 364–366.
- McIntyre, J., Stratta, F., & Lacquaniti, F. (1998). Short-term memory for reaching to visual targets: Psychophysical evidence for body-centered reference frames. Journal of Neuroscience, 18, 8423–8435.
- McLeod, P., McLaughlin, C., & Nimmo-Smith, I. (1985). Information encapsulation and automaticity: Evidence from the visual control of finely timed actions. In M. I. Posner & O. S. M. Marin (Eds.), Attention and performance XI (pp. 391–406). Hillsdale, NJ: Erlbaum.
- Merz, F., Kalveram, K.-T., & Huber, K. (1981). Der Einfluß kognitiver Faktoren auf Steuerleistungen [The role of cognitive factors in manual tracking]. In L. Tent (Ed.), Erkennen–Wollen– Handeln (pp. 327–335). Göttingen, Germany: Hogrefe.
- Metz, A. M. (1970). Änderungen der myoelektrischen Aktivität während eines sensomotorischen Lernprozesses [Changes of myoelectric activity during motor learning]. Zeitschrift für Psychologie, 178, 51–88.
- Meyer, D. E., Abrams, R. A., Kornblum, S., Wright, C. E., & Smith, J. E. K. (1988). Optimality in human motor performance: Ideal control of rapid aimed movements. Psychological Review, 95, 340–370.
- Meyer, D. E., Smith, J. E. K., & Wright, C. E. (1982). Models for the speed and accuracy of aimed movements. Psychological Review, 89, 449–482.
- Monsell, S. (1986). Programming of complex sequences: Evidence from the timing of rapid speech and other productions. In H. Heuer & C. Fromm (Eds.), Generation and modulation of action patterns (pp. 72–86). Berlin, Germany: Springer.
- Müller, H. (2001). Ausführungsvariabilität und Ergebniskonstanz [Variability of execution and constancy of outcomes]. Lengerich: Pabst Science Publishers.
- Müller, H., & Loosch, E. (1999). Functional variability and an equifinal path of movement during targeted throwing. Journal of Human Movement Studies, 36, 103–126.
- Näätänen, R., & Merisalo, A. (1977). Expectancy and preparation in simple reaction time. In S. Dornic (Ed.), Attention and performance VI (pp. 115–138) Hillsdale, NJ: Erlbaum.
- Nelson, W. L. (1983). Physical principles for economies of skilled movements. Biological Cybernetics, 46, 135–147.
- Newell, K. M., & van Emmerik, R. E. A. (1989). The acquisition of coordination: Preliminary analysis of learning to write. Human Movement Science, 8, 17–32.
- Nielsen, T. I. (1963). Volition: A new experimental approach. Scandinavian Journal of Psychology, 4, 225–230.
- Noble, M., Fitts, P. M., & Warren, C. E. (1955). The frequency response of skilled subjects in a pursuit tracking task. Journal of Experimental Psychology, 49, 249–256.
- Nougier, V., Bard, C., Fleury, M., Teasdale, N., Cole, J., Forget, R., Paillard, J., & Lamarre, Y. (1996). Control of single-joint movements in deafferented patients: Evidence for amplitude coding rather than position control. Experimental Brain Research, 109, 473–482.
- Paillard, J. (1982). The contribution of peripheral and central vision to visually guided reaching. In D. J. Ingle, M. A. Goodale, & R. J. W. Mansfield (Eds.), Analysis of visual behavior (pp. 367–385). Cambridge, MA: MIT Press.
- Peper, C. E. (1995). Tapping dynamics. Unpublished doctoral dissertation, Free University Amsterdam.
- Peper, C. E., & Beek, P. J. (1998). Are frequency-induced transitions in rhythmic coordination mediated by a drop in amplitude? Biological Cybernetics, 79, 291–300.
- Peper, L., Bootsma, R. J., Mestre, D. R., & Bakker, F. C. (1994). Catching balls: How to get the hand to the right place at the right time. Journal of Experimental Psychology: Human Perception and Performance, 20, 591–612.
- Perenin, M. T., & Jeannerod, M. (1978). Visual function within the hemianopic field following early cerebral hemidecortication in man: I. Spatial localization. Neuropsychologia, 16, 1–13.
- Peters, M. (1981). Attentional asymmetries during concurrent bimanual performance. Quarterly Journal of Experimental Psychology, 33A, 95–103.
- Pew, R.W. (1966). Acquisition of hierarchical control over the temporal organization of skill. Journal of Experimental Psychology, 71, 764–771.
- Plamondon, R., & Alimi, A. M. (1997). Speed/accuracy trade-offs in target-directed movements. Behavioral and Brain Sciences, 20, 279–349.
- Polit, A., & Bizzi, E. (1979). Characteristics of motor programs underlying arm movements in monkeys. Journal of Neurophysiology, 42, 183–194.
- Poulton, E. C. (1957). On the stimulus and response in pursuit tracking. Journal of Experimental Psychology, 53, 189–194.
- Preilowski, B. (1972). Possible contribution of the anterior forebrain commissures to bilateral motor coordination. Neuropsychologia, 10, 267–277.
- Prinz, W. (1992). Why don’t we perceive our brain states? European Journal of Cognitive Psychology, 4, 1–20.
- Rack, P. M. H., & Westbury, D. R. (1969). The effects of length and stimulus rate on tension in the isometric cat soleus muscle. Journal of Physiology, 204, 443–460.
- Rinkenauer, G., Ulrich, R., & Wing, A. (2001). Brief bimanual force phases: Correlations between the hands in force and time. Journal of Experimental Psychology: General, Human Perception and Performance, 27, 1485–1497.
- Rosenbaum, D. A. (1980). Human movement initiation: Specification of arm, direction, and extent. Journal of Experimental Psychology: General, 109, 444–474.
- Rosenbaum, D. A. (1983). The movement precuing technique: Assumptions, applications, and extensions. In R.A. Magill (Ed.), Memory and control of action (pp. 231–274).Amsterdam: NorthHolland.
- Rosenbaum, D. A., & Jorgensen, M. J. (1992). Planning macroscopic aspects of manual control. Human Movement Science, 11, 61–69.
- Rosenbaum, D. A., & Krist, H. (1996). Antecedents of action. In H. Heuer & S. W. Keele (Eds.), Handbook of perception and action: Vol. 2. Motor skills (pp. 3–69). London: Academic Press.
- Rosenbaum, D. A., Loukopoulos, L. D., Meulenbroek, R. G. J., Vaughan, J., & Engelbrecht, S. E. (1995). Planning reaches by evaluating stored postures. Psychological Review, 102, 28–67.
- Rosenbaum, D. A., Slotta, J. D., Vaughan, J., & Plamondon, R. (1991). Optimal movement selection. Psychological Science, 2, 86–91.
- Rosenbaum, D. A., Vaughan, J., Barnes, H. J., & Jorgensen, M. J. (1992). Time course of movement planning: Selection of handgrips for object manipulation. Journal of Experimental Psychology: Learning, Memory, and Cognition, 18, 1058–1073.
- Roth, K. (1988). Investigations on the basis of the generalized motor programme hypothesis. In O. G. Meijer & K. Roth (Eds.), Complex movement behaviour: “The” motor-action controversy (pp. 261–288). Amsterdam: North-Holland.
- Ruitenbeek, J. C. (1984). Invariants in loaded goal directed movements. Biological Cybernetics, 51, 11–20.
- Saltzman, E., & Kelso, J. A. S. (1987). Skilled actions: A taskdynamic approach. Psychological Review, 94, 84–106.
- Sangals, J. (1997). Der Einfluß der Bewegungsrückmeldung auf das Erlernen nichtlinearer Werkzeugtransformationen [The effect of movement feedback on the learning of nonlinear transformations]. Unpublished doctoral dissertation, Philipps-Universitat, Marburg, Marburg, Germany.
- Sangals, J., Heuer, H., Manzey, D., & Lorenz, B. (1999). Changed visuomotor transformations during and after prolonged microgravity. Experimental Brain Research, 129, 378–390.
- Savelsbergh, G. J. P., Whiting, H. T. A., & Bootsma, R. J. (1991). Grasping tau. Journal of Experimental Psychology: Human Perception and Performance, 17, 315–322.
- Schiff, W., & Detwiler, M. L. (1979). Information used in judging impending collision. Perception, 8, 647–658.
- Schmidt, R. A. (1972). The Index of Preprogramming (IP): A statistical method for evaluating the role of feedback in simple movements. Psychonomic Science, 27, 83–85.
- Schmidt, R. A. (1975). A schema theory of discrete motor skill learning. Psychological Review, 82, 225–260.
- Schmidt, R. A. (1980). On the theoretical status of time in motorprogram representations. In G. E. Stelmach & J. Requin (Eds.), Tutorials in motor behavior (pp. 145–165). Amsterdam: NorthHolland.
- Schmidt, R. A. (1985). The search for invariance in skilled movement behavior. Research Quarterly for Exercise and Sport, 56, 188–200.
- Schmidt, R. A. (1989). Unintended acceleration: Areview of human factors contributions. Human Factors, 31, 345–364.
- Schmidt, R. A., & McGown, C. (1980). Terminal accuracy of unexpectedly loaded rapid movements: Evidence for a mass-spring mechanism in programming. Journal of Motor Behavior, 12, 149–161.
- Schmidt, R. A., & Russell, D. G. (1972). Movement velocity and movement time as determinants of degree of preprogramming in simple movements. Journal of Experimental Psychology, 96, 315–320.
- Schmidt, R. A., Sherwood, D. E., Zelaznik, H. N., & Leikind, B. J. (1985). Speed-accuracy tradeoffs in motor behavior: Theories of impulse variability. In H. Heuer, U. Kleinbeck, & K.-H. Schmidt (Eds.), Motor behavior: Programming, control, and acquisition (pp. 79–123). Berlin, Germany: Springer.
- Schmidt, R. A., Zelaznik, H. N., Hawkins, B., Frank, J. S., & Quinn, J. T. (1979). Motor-output variability: A theory for the accuracy of rapid motor acts. Psychological Review, 86, 415–451.
- Schöner, G. (1994). From interlimb coordination to trajectory formation: Common dynamical principles. In S. P. Swinnen, H. Heuer, J. Massion, & P. Casaer (Eds.), Interlimb coordination. Neural, dynamical, and cognitive constraints (pp. 339– 368). San Diego, CA: Academic Press.
- Schöner, G., & Kelso, J. A. S. (1988). A synergetic theory of environmentally-specified and learned patterns of movement coordination. I: Relative phase dynamics. Biological Cybernetics, 58, 71–80.
- Schott, G. D., & Wyke, M. A. (1981). Congenital mirror movements. Journal of Neurology, Neurosurgery, & Psychiatry, 44, 586–599.
- Schouten, J. F., & Becker, J. A. M. (1967). Reaction time and accuracy. Acta Psychologica, 27, 143–153.
- Serrien, D. J., Bogaerts, H., Suy, E., & Swinnen, S.P. (1999). The identification of coordination constraints across planes of motion. Experimental Brain Research, 128, 250–255.
- Shadmehr, R., & Brashers-Krug, T. (1997). Functional stages in the formation of human long-term motor memory. Journal of Neuroscience, 17, 409–419.
- Shadmehr, R., & Moussavi, Z. M. K. (2000). Spatial generalization from learning dynamics of reaching movements. Journal of Neuroscience, 20, 7807–7815.
- Shadmehr, R., & Mussa-Ivaldi, F. A. (1994). Adaptive representation of dynamics during learning of a motor task. Journal of Neuroscience, 14, 3208–3224.
- Sherwood, D. E. (1991). Distance and location assimilation in rapid bimanual movement. Research Quarterly for Exercise and Sport, 62, 302–308.
- Smeets, J. B. J., & Brenner, E. (1995). Perception and action are based on the same visual information: Distinction between position and velocity. Journal of Experimental Psychology: Human Perception and Performance, 21, 19–31.
- Smeets, J. B. J., Erkelens, C. J., & Denier van der Gon, J. J. (1995). Perturbations of fast goal-directed arm movements: Different behavior of early and late EMG responses. Journal of Motor Behavior, 27, 77–88.
- Spijkers, W. (1993). Sehen und Handeln: Die Rolle visueller Information bei zielgerichteten Bewegungen [Perception and action: The role of visual information in aimed movements]. Aachen, Germany: Shaker.
- Spijkers, W., & Heuer, H. (1995). Structural constraints on the performance of symmetrical bimanual movements with different amplitudes. Quarterly Journal of Experimental Psychology, 48A, 716–740.
- Spijkers, W., Heuer, H., Kleinsorge, T., & Steglich, C. (2000). The specification of movement amplitudes for the left and right hand: Evidence for transient parametric coupling from overlappingtask performance. Journal of Experimental Psychology: Human Perception and Performance, 26, 1091–1105.
- Spijkers, W., Heuer, H., Kleinsorge, T., & van der Loo, H. (1997). Preparation of bimanual movements with same and different amplitudes: Specification interference as revealed by reaction time. Acta Psychologica, 96, 207–227.
- Spijkers, W., Tachmatzidis, K., Debus, G., Fischer, M., & Kausche, I. (1994). Temporal coordination of alternative and simultaneous aiming movements of constrained timing structure. Psychological Research, 57, 20–29.
- Steglich, C., Heuer, H., Spijkers, W., & Kleinsorge, T. (1999). Bimanual coupling during the specification of isometric forces. Experimental Brain Research, 129, 302–316.
- Stelmach, G. E. (1982). Motor control and motor learning: Theclosed-loopperspective.InJ.A.S.Kelso(Ed.),Humanmotor behavior: An introduction (pp. 93–115). Hillsdale, NJ: Erlbaum.
- Stelmach, G. E., Kelso, J. A. S., & Wallace, S. A. (1975). Preselection in short-term motor memory. Journal of Experimental Psychology: Human Learning and Memory, 1, 745–755.
- Stimpel, E. (1933). Der Wurf [The throw]. Neue Psychologische Studien, 9, 105–138.
- Stratton, G. M. (1896). Some preliminary experiments in vision without inversion of the retinal image. Psychological Review, 3, 611–617.
- Stratton, G. M. (1897a). Upright vision and the retinal image. Psychological Review, 4, 182–187.
- Stratton, G. M. (1897b). Vision without inversion of the retinal image. Psychological Review, 4, 341–360, 463–481.
- Stucchi, N., & Viviani, P. (1993). Cerebral dominance and asynchrony between bimanual two-dimensional movements. Journal of Experimental Psychology: Human Perception and Performance, 19, 1200–1220.
- Summers, J. J., Rosenbaum, D. A., Burns, B. D., & Ford, S. K. (1993). Production of polyrhythms. Journal of Experimental Psychology: Human Perception and Performance, 19, 416– 428.
- Swinnen, S. P., Jardin, K., & Meulenbroek, R. (1996). Betweenlimb asynchronies during bimanual coordination: Effects of manual dominance and attentional cueing. Neuropsychologica, 34, 1203–1213.
- Taub, E., Goldberg, I. A., & Taub, P. (1975). Deafferentation in monkeys: Pointing at a target without visual feedback. Experimental Neurology, 46, 178–186.
- Teasdale, N., Forget, R., Bard, C., Paillard, J., Fleury, M., & Lamarre, Y. (1993). The role of proprioceptive information for the production of isometric forces and for handwriting tasks. Acta Psychologica, 82, 179–191.
- Tendick, F., Jennings, R. W., Tharp, G., & Stark, L. (1993). Sensing and manipulation problems in endoscopic surgery: experiment, analysis, and observation. Presence, 2, 66–81.
- Teulings, H.-L. (1996). Handwriting movement control. In H. Heuer & S. W. Keele (Eds.), Handbook of perception and action: Vol. 2. Motor skills (pp. 561–613). London: Academic Press.
- Todor, J. I., & Lazarus, J. C. (1986). Exertion level and the intensity of associated movements. Developmental Medicine & Child Neurology, 28, 205–212.
- Tresilian, J. R. (1999). Analysis of recent empirical challenges to an account of interceptive timing. Perception & Psychophysics, 61, 515–528.
- Tuller, B., & Kelso, J. A. S. (1989). Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Experimental Brain Research, 75, 306–316.
- Tyldesley, D. A., & Whiting, H. T. A. (1975). Operational timing. Journal of Human Movement Studies, 1, 172–177.
- Ungerleider, L. G., & Mishkin, M. (1982). Two cortical visual systems. In D. J. Ingle, M. A. Goodale, & R. J. W. Mansfield (Eds.), Analysis of visual behavior (pp. 549–586). Cambridge, MA: MIT
- Uno, Y., Kawato, M., & Suzuki, R. (1989). Formation and control of optimal trajectory in human multijoint arm movement: Minimum torque-change model. Biological Cybernetics, 61, 89–101.
- Vindras, P., & Viviani, P. (1998). Frames of reference and control parameters in visuo manual pointint. Journal of Experimental Psychology: Human Perception and Performance, 24, 569–591.
- Virjii-Babul, N., Cooke, J. D., & Brown, S. H. (1994). Effects of gravitational forces on single joint arm movements in humans. Experimental Brain Research, 99, 338–346.
- Viviani, P., & Flash, T. (1995). Minimum-jerk, two-thirds power law, and isochrony: Converging approaches to movement planning. Journal of Experimental Psychology: Human Perception and Performance, 21, 32–53.
- Viviani, P., Perani, D., Grassi, F., Bettinardi, V., & Fazio, F. (1998). Hemispheric asymmetries and bimanual asynchrony in left- and right-handers. Experimental Brain Research, 120, 531–536.
- Voigt, E. (1933). Über den Aufbau von Bewegungsgestalten [On the composition of movement Gestalts]. Neue Psychologische Studien, 9, 1–32.
- Vorberg, D., & Wing, A. M. (1996). Modeling variability and dependence in timing. In H. Heuer & S. W. Keele (Eds.), Handbook of perception and action: Vol. 2. Motor skills (pp. 181– 262). London: Academic Press.
- Wadman, W. J., Denier van der Gon, J. J., Geuze, R. H., & Mol, C. R. (1979). Control of fast goal-directed arm movements. Journal of Human Movement Studies, 5, 3–17.
- Welch, R. B. (1971). Discriminative conditioning of prism adaptation. Perception & Psychophysics, 10, 90–92.
- Welch, R. B. (1972). The effect of experienced limb identity upon adaptation to simulated displacement of the visual field. Perception & Psychophysics, 12, 453–456.
- Welch, R. B. (1978). Perceptual modification. Adapting to altered sensory environments. New York: Academic Press.
- Welch, R. B., Bridgeman, B., Anand, S., & Browman, K. E. (1993). Alternating prism exposure causes dual adaptation and generalization to a novel displacement. Perception & Psychophysics, 54, 195–204.
- Whiting, H. T. A., & Cockerill, I. M. (1974). Eyes on hand—eyes on target? Journal of Motor Behavior, 6, 27–32.
- Winter, D.A., & Robertson, D. G. E. (1978). Joint torque and energy patterns in normal gait. Biological Cybernetics, 29, 137–142.
- Woodworth, R. S. (1899). The accuracy of voluntary movement. Psychological Review, 3 (Monograph Suppl. 3).
- Wright, C. E., & Meyer, D. E. (1983). Conditions for a linear speedaccuracy trade-off in aimed movements. Quarterly Journal of Experimental Psychology, 35A, 279–296.
- Wulf, G., Höß, M., & Prinz, W. (1998). Instructions for motor learning: differential effects of internal vs. external focus of attention. Journal of Motor Behavior, 30, 169–179.
- Wulf, G., & Prinz, W. (2001). Directing attention to movement effects enhances learning: A review. Psychonomic Bulletin and Review, 8, 648–660.
- Yamanishi, J., Kawato, M., & Suzuki, R. (1980). Two coupled oscillators as a model of the coordinated finger tapping by both hands. Biological Cybernetics, 37, 219–225.
- Young, L. R. (1969). On adaptive manual control. Ergonomics, 12, 635–675.
- Zattara, M., & Bouisset, S. (1986). Chronometric analysis of the posturo-kinetic programming of voluntary movement. Journal of Motor Behavior, 18, 215–223.
- Zelaznik, H. N., Hawkins, B., & Kisselburgh, L. (1983). Rapid visual feedback processing in single-aiming movements. Journal of Motor Behavior, 15, 217–236.
- Zelaznik, H. N., Mone, S., McCabe, G. P., & Thaman, C. (1988). Role of temporal and spatial precision in determining the nature of the speed-accuracy trade-off in aimed hand movements. Journal of Experimental Psychology: Human Perception and Performance, 14, 221–230.
- Zelaznik, H. N., Shapiro, D. C., & Carter, M. C. (1982). The specification of digit and duration during motor programming: A new method of precueing. Journal of Motor Behavior, 14, 57–68.




