Sample Motivation Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. iResearchNet offers academic assignment help for students all over the world: writing from scratch, editing, proofreading, problem solving, from essays to dissertations, from humanities to STEM. We offer full confidentiality, safe payment, originality, and money-back guarantee. Secure your academic success with our risk-free services.
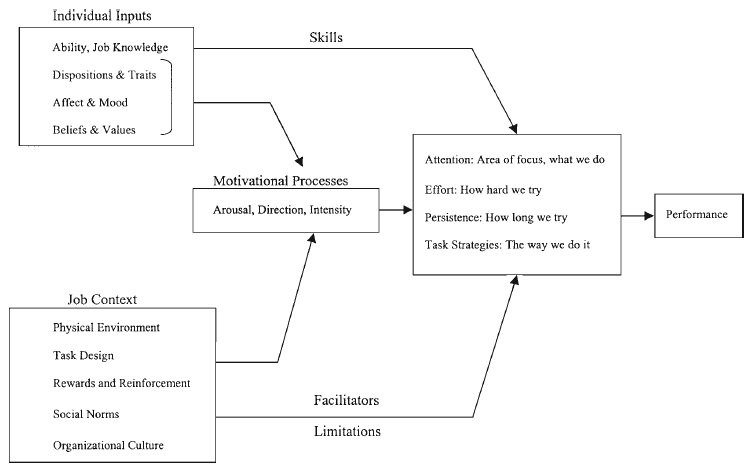
The first two questions that a research paper on motivation must confront may betray the current status of motivational constructs in much of psychology. The first is, Why do we need motivational concepts to explain behavior? The second is, How do we define motivation? The first goal of this research paper is to answer these questions in a general way by providing a framework with which to analyze basic motivational processes. We then apply this general framework to four motivated behavior systems: feeding, fear, sexual behavior, and temperature regulation. By so doing, we hope to illustrate the power of current thinking about motivation as an organizing and predictive structure for understanding behavior.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Why Do Theories of Behavior Need Motivational Constructs?
The goal of psychological theories is to explain and predict the variance in behavior. The two global factors to which this variance is most often attributed are genetic and learned influences. For instance, a particular species is genetically programmed to use certain sources of nourishment and not others. It is also clear that humans and other animals learn that some edible stimuli contain vital nutrients and others are toxic. Even complete knowledge of these factors and how they interact is probably not sufficient to understand all behavior; some variance is left over. Motivational constructs are invoked to explain this leftover variance. Genetically, humans need certain lipids, proteins, sugars, and vitamins to become reproductive individuals. We learn how to procure these commodities from our environment. Yet an individual may not always consume the perfect food when it is available, while at other times such food may be consumed to excess. The behavior is variable; learning and genetics alone cannot account for all of the behavior. Consequently, we invoke hunger, a motivational construct, to capture the remainder of the variance. For example, a theory of feeding might suggest that genes determine what we eat, and memory of past experiences tells us where to forage. Hunger activates foraging behavior and determines when we eat.
Any complete theory of behavior can be viewed as an analysis of variance with learning, genetics, and motivation configured to explain behavior as best as possible. Accordingly, any concept of motivation will be defined partly by the particular matrix of learning and genetics within which it is embedded. As a consequence, as our ideas about learning or behavior genetics change, so must our ideas about motivation. Indeed, our concept of motivation is dramatically different from the generalized need-based drive and the reciprocally inhibitory incentive motivation theories that characterized the earlier and later parts of the twentieth century. Although those theories have been very influential to the ideas developed here, we do not review them in this research paper. Instead, the reader is urged to consult Bolles (1975) for, arguably, the most authoritative review of those earlier approaches.
The analogy to analysis of variance highlights another important aspect of motivation, learning, and genetics. It is incorrect to think of these factors as independent “main” effects. Most of the variance in behavior is accounted for by the interactions between these factors. For example, research into constraints on learning demonstrated that even basic learning processes, such as Pavlovian and operant conditioning, have powerful and specific genetic influences that determine what information is readily acquired and what information is virtually impossible to assimilate (Seligman & Hager, 1972). Conversely, recent research on the neurobiology of learning suggests that the mechanism by which we encode information involves gene expression and that learning influences which genes are expressed (Bolhuis, Hetebrij, Den Boer-Visser, De Groot, & Zijlstra, 2001; Rosen, Fanselow, Young, Sitcoske, & Maren, 1998). Thus, learning and genetic factors affect behavior, and each other. We raise these examples to foreshadow that our explanation of motivation will also primarily reside within a description of these interactions.
A Definitional Framework for Motivation
The framework we advocate for understanding motivation is called functional behavior systems (Timberlake & Fanselow, 1994). Two aspects to defining a functional behavior system are common to the definition of any motivational construct: environmental cause and behavioral effect. These are the necessary components to any empirically tractable definition of an intervening variable. Afunctional behavior system must be anchored to objectively defined environmental causes. These are the antecedent conditions for activation of the behavior system and the things an experimenter can manipulate to turn on the system. The functional behavioral system must also have objectively observable behavioral consequences of activating the system.
Functional behavior systems have a third component to the definition that is unique to this approach. The naturally occurring problem that the system has evolved to solve is a component of the definition. This component is critical because modern views of motivation see behavior as being tightly organized around these functional concerns. Environmental causes and behavioral effects are grouped together about the naturally occurring problems that the successful organism is built to solve. This problem-oriented view focuses the analysis on how multiple behaviors relate to each other in a manner that is coordinated to solve a problem. Hunger and feeding are understood as a means to ensure that the necessary nutrients and calories are harvested from the environment. Hunger and feeding cannot be understood simply in terms of the amount eaten or the number of lever presses a rat makes for a food pellet. Nor can it be understood simply in terms of the postingestional consequences of food that satisfy some homeostatic requirement. Rather, for each species, food-related motivation is tailored to the niche that the animal occupies. An animal must search for appropriate items, procure them, prepare them, consume them, and digest them. The sequence is all-important, and a failure anywhere along the chain means that the organism fails to meet critical environmental demands. Different behaviors are necessary for each step; different rules apply to each component; and the analysis of behavior is a description of the path. A theory of motivation must capture the structure of this organization.
Impetus for the Development of Functional Behavior Systems
A metatheoretical concern in approaching motivation is how many separate motivations does a complex organism have? Freud (1915) voiced one extreme when he suggested that all motivation stemmed from a single unconscious source of energy. The instinct theorists of the early part of the twentieth century voiced another when they linked instincts directly to behaviors (e.g., Lorenz, 1937). Eventually, instinct theory crushed itself because there were no constraints on the number of instincts that could be generated. To avoid such problems, Hull (1943), like Freud (1915), argued for a single generalized source of motivation. The magnitude of this generalized drive was determined by summing all unsatisfied physiological needs; any perturbation of homeostasis resulted in an increase in a common source of behavioral energy. Empirically, Hull’s generalized drive theory failed because separate sources of motivation most often do not generalize. Thirsty animals tend not to eat, and frightened animals forsake eating and drinking. It also became clear that learning was at least as important a source of motivation as was homeostatic need. Often we eat because the situation tells us to. Past experience informs us that this is the proper time or place to eat.
To account for empirical challenges to Hull’s (1943) generalized drive principle, incentive motivational theories suggested that two types of motivation could be activated by Pavlovian means. Conditional stimuli (CSs) that predicted desirable outcomes, such as the occurrence of food or the absence of pain, activated an appetitive motivational system; CSs that predicted undesirable outcomes activated an aversive motivational system. Anything that activated the appetitive system stimulated appetitively related behaviors and suppressed aversively motivated behaviors. The opposite was true for stimuli that excited the aversive system. This explanation was an improvement because learning, in the form of Pavlovian conditioning, could provide a source of motivation. Additionally, the notion of two systems provides more selectivity than Hull’s (1943) generalized drive principle. The problem with this view is that it simply does not go far enough. As we shall see, cues associated with food do not simply cause an enhancement of food-associated behaviors. Rather, the cue signals that a particular class of food-related behavior is appropriate and that others are inappropriate. On the aversive side, fear and pain are organized in an antagonistic manner. Because fear inhibits pain-related behavior, how can fear, pain, and hunger simultaneously hold mutually reciprocal relationships? As we shall see, organizing these systems around their function makes sense of the relationships between classes of behavior. By combining function, antecedent cause, and behavioral effect into our definition of a motivational system, we are also successful in limiting the number of motivational systems that can be generated.
What Is Motivation?
The idea that we eat because we are hungry seems intuitively obvious. Both lay and several formal descriptions of behavior suggest that hunger is a response to food deprivation and that hunger engenders behaviors that correct the depletion. In this way, factors such as body weight or caloric intake are regulated about some set point. This homeostatic view has directed much research, and in many situations body weight appears to be held relatively constant. However, caloric intake and body weight are influenced by many variables, such as the type and quantity of food available, activity levels, season, and palatability.
Bolles (1980) has noted that if the experimenter holds several of these variables constant, the others will come to rest at some set of values. Thus, an observed set point or consistency may be an artifact of relatively static conditions.Additionally, because all these factors are variables in an equation, the experimenter is free to solve for any of them as a function of the others. In effect, body weight may appear to be regulated simply because you have kept the other variables constant. Alternatively, if you held body weight and the other variables constant, you could solve the equation for palatability and thereby conclude that palatability is regulated. From a functional perspective what is critical is that an animal ingests the necessary substances in sufficient quantities; how that is accomplished does not matter. Natural selection favors any scheme that satisfies the goal. In this regard, regulating palatability may make a lot of sense—and is a topic to which we will return later.
This idea is a general point about motivational terminology and motivational systems. We have to recognize that motivation is organized about the evolutionary requirement that the system needs to solve. Hunger, sexual arousal, and fear really refer to a behavioral organization that is imposed on an organism when the environment demands that a particular problem be solved. Motivation is no longer conceived of as a blind force that impels an animal forward. It is something that gives form, structure, and meaning to behavior, and it is from this vantage that we will begin to analyze some exemplars of specific motivational systems.
Feeding
The vast majority of animal species gain the nutrients they require to survive and grow by harvesting them from other living creatures. This strategy requires that animals have means to detect and capture these nutrients and that the behavioral systems governing these actions be sensitive to the availability of required nutrients and the physiological demands of the animal. Psychological examination of these requirements typically focuses on either the factors that initiate the behavior or the response topography of food-gathering behavior. We examine each of these aspects in turn.
Factors Governing Initiation of Feeding Behavior
Homeostasis
Richter (1927) observed that feeding behavior occurred in regular bouts that could be specified on the basis of their frequency, size, and temporal patterning. He suggested that finding the determinants of this regularity should be the goal of psychology and further indicated that homeostasis, the maintenance of a constant internal environment, could be one of these determinants. These observations have been supported by further research showing that animals frequently act as though defending a baseline level of intake, leading to the development of a depletion/repletion model of feeding initiation similar to homeostatic models developed to account for temperature regulation behavior (Satinoff, 1983). A great deal of evidence suggests that under relatively constant conditions, animals eat a regular amount each day and that the amount is sensitive to manipulations such as enforced deprivation or stomach preloading (Le Magnen, 1992). However, there are a number of problems with this analysis, and these problems become more intractable the more lifelike the experiment becomes. For example, initiation of feeding behavior has been demonstrated to be sensitive to a number of different factors including nutrient storage levels, food palatability, and circadian influences (Panksepp, 1974). The crucial factor in determining the influence of various manipulations on feeding behavior seems to be the nature of the experimental procedure used.
The Importance of Procedure
Collier (1987) described three different procedures that have been used to study feeding motivation. By far the most commonly used is the session procedure. Here, the animal is deprived of a required commodity for most of the day and is given repeated brief access to this commodity during a short, daily session. In such a procedure very few of the determinants of behavior are free to vary, placing most of the control of the animal’s behavior into the hands of the experimenter. Features of behavior including the number of trials, the intertrial interval, session length, portion size, response contingencies, and total intake are determined by the experimenter and not the animal (Collier & Johnson, 1997). This kind of procedure changes the response characteristics of the animals by placing a premium on rapid initiation and performance of the food-rewarded behavior and does not allow analysis of feeding initiation and termination because these are also determined by the experimenter, rather than the animal.
A second class of studies uses the free-feeding procedure in which animals are offered continuous access to the commodity and their pattern of feeding is recorded. Unlike the session procedure, there is no explicit deprivation, and the animal is free to control various parameters of food consumption, including meal initiation and termination. This procedure has led to the dominant depletion/repletion model of feeding motivation. This model hypothesizes that postingestive information about the nutrient content of the meal is compared against nutrient expenditure since the last meal to determine the nutrient preference and size/duration of the next meal (Le Magnen & Devos, 1980). Correlations between length of food deprivation and subsequent meal size or the rate of responding for subsequent feeding (Bolles, 1975; Le Magnen, 1992) provide support for this interpretation. However, these correlations are influenced by a number of other factors, including the availability of other behaviors (Collier, Johnson, & Mitchell, 1999), and do not provide a complete account of feeding initiation (Castonguay, Kaiser, & Stern, 1986). Even more important, the feeding initiation and subsequent meal patterning of free-feeding animals seem to be such that they never undergo nutrient depletion: Free-feeding animals never have empty stomachs (Collier, Hirsch, & Hamlin, 1972), meaning that a near-constant stream of nutrients enters the animal.This behavior suggests either that feeding initiation must be unrelated to depletion or that it must occur prior to, but not as a consequence of, nutrient depletion.
The Cost of Feeding
One major parametric influence on feeding behavior not included in the free-feeding procedure is the cost of procuring food. In the laboratory foraging procedure (Collier, 1983) the animal is not food deprived in the conventional sense—it has constant access to food resources—but food availability is restricted by making delivery contingent on the completion of a response contingency. Unlike the session procedure, the animal is free to control the various parameters of feeding behavior. Unlike the free-feeding procedure, the animal must not only work to gain access to the commodity, but it must balance the demands of gaining access to food with other biologically important activities such as drinking and sleeping. In these studies, the cost of food procurement, and not the repletion/depletion calculation, has been demonstrated to be the crucial determinant of feeding initiation (e.g., Collier et al., 1972). Experiments manipulating the cost of food procurement have demonstrated that the number of meals an animal takes in a day is directly related to the cost of initiating a meal. By varying the number of lever presses required to initiate a meal, Collier et al. (1972) demonstrated that the daily number of meals initiated by the animal is a linear function of the log of the response requirement. The number of small meals and the frequency of short intermeal intervals decreased as the response requirement increased, leading to a smaller number of larger meals and the conservation of total intake and body weight.
Similar effects of meal-procurement cost have been demonstrated across a variety of animal species with a variety of evolutionary niches and foraging strategies (Collier & Johnson, 1990). The determination of meal cost appears to be calculated by the animal across a relatively long time window: Animals trained on alternating days of high and low cost learned to feed primarily on low-cost days (Morato, Johnson, & Collier, 1995). Animals also show a nonexclusive preference for feeding on low cost resources (Collier, 1982), on larger pellets where the cost is the same as for smaller pellets (Johnson & Collier, 1989), and on pellets with higher caloric density (Collier, Johnson, Borin, & Mathis, 1994). Animals also include risk of aversive events into the cost equation. Fanselow, Lester, and Helmstetter (1988) demonstrated that increased numbers of randomly occurring foot shocks led to changes in meal patterning similar to those induced by increased procurement costs. Characteristics of feeding demonstrated in session and free-feeding procedures, such as increased rates of responding or consumption or correlations between length of food deprivation and subsequent meal size, are not replicated in the laboratory feeding procedure (Collier & Johnson, 1997; Collier et al., 1999). This series of results has led Collier and his coworkers to suggest that the crucial determinants of feeding initiation are the costs associated with meal procurement and that physiological functions act to buffer the effects of variations in feeding initiation determined by procurement cost rather than as the instigators of feeding behavior (Collier, 1986).
The Behavioral Ecology of Feeding Cost
In the laboratory, costs are determined by the experimenter. In the real world these costs are determined by the animal’s ecological niche, placing feeding behavior under the direct controlofevolutionaryfactors.Feedingintensitycanbepredicted from relative predatory risk, as can be inferred from the study by Fanselow et al. (1988). For example, large predators could be expected to eat long-duration, low-intensity meals because they are not subject to threat from other animals. In contrast, small predators could be expected to eat short-duration, highintensity meals as they are themselves potential prey. These suggestions are consistent with ethological data (Estes, 1967a, 1967b; Schaller, 1966). Meal patterning and feeding initiation can be predicted from food type. Predators could be expected to sustain high procurement costs for their nutritionally rich meals, whereas herbivores—particularly small, monogastric herbivores—could be expected to take frequent meals because of the low quality and intensive processing required by their usual foods. These suggestions have been supported by experimental data indicating that cats can eat every three to four days when procurement costs are high and maintain bodyweight, whereas guinea pigs are unable to maintain their bodyweight with fewer than two to three meals per day and are unable to sustain high procurement costs (Hirsch & Collier, 1974; Kaufmann, Collier, Hill, & Collins, 1980).
Factors Governing Variety of Intake
Alliesthesia
Food selection must provide all the nutrients necessary for survival. This task is simple for a specialized feeder that eats very few foods. However, opportunistic omnivores such as rats and humans contend with a potentially bewildering array of choices. Traditional approaches have suggested that the body detects hunger when it is deprived of a particular commodity, and this homeostatic need sets in motion behaviors directed at correcting the deficit (e.g., Rodgers, 1967). Thus, intake of various nutrients could be regulated by set points for these nutrients. Food palatability had been suggested to be an alternative mechanism (Mook, 1987). Assume that an animal (or at least an opportunistic omnivore) eats because food tastes good. If that is combined with one other assumption, that food loses its incentive value when consumed, we have a mechanism that ensures intake of a variety of substances. This phenomenon is referred to as alliesthesia (Cabanac, 1971). Cabanac demonstrated that palatability ratings of sugar solution change from positive to negative following ingestion, but not simply the taste of, sucrose.
Sensory Satiety
Despite this evidence, it is also true that sensory, rather than postingestive, stimuli associated with food play an important role in inducing variety of intake. The clearest demonstrations of these effects are those demonstrating the effects of food variety in sated animals and people. When we sit down to our holiday meal, the turkey tastes exquisite, but after two or three helpings we can barely tolerate another bite. Yet despite our satiety, we proceed to eat a large dessert. The order of courses does not matter (Rolls, Laster, & Summerfelt, 1991); the critical determinant of renewed consumption is that the food has variety (Rolls, 1979). This variety effect has been demonstrated in humans and rats (see Raynor & Epstein, 2001, for a recent review), perhaps most dramatically by the obesity of rats given a variety of highly palatable foods (Sclafani & Springer, 1976). Rats under these conditions can more than double their weight and behave similarly to animals that have obesity-inducing brain lesions.
These findings do not undermine the alliesthesia model of food selection. Rather, they suggest that exposure to the sensory aspects of food, in the absence of ingestion, is sufficient to reduce the palatability, and therefore intake, of that food. A variety of studies demonstrated just such a result. Changes in the shape of the food have an effect on intake. Rolls, Rowe, and Rolls (1982) showed that subjects would consume more pasta if it were offered as spaghetti, half hoops, and bow ties than if it were offered as spaghetti alone. Guinard and Brun (1998) demonstrated that variation in another nonnutritive dimension, food texture, can similarly lead to increases in consumption. Rolls and Rolls (1997) have demonstrated that chewing or smelling food is sufficient to induce alliesthesialike reductions in the subsequent palatability of that food in the absence of eating that food. Thus, although ingestion may be sufficient to cause alliesthesia, it is not necessary: Sensory stimulation alone is sufficient to cause changes in palatability and to induce variety in food choice.
Factors Governing the Incentive Aspects of Foods
Cathexes
The regulation of feeding behavior through meal patterning and the regulation of food variety through alliesthesia assume that the animal knows which stimuli present in the environment are foods that will satisfy its nutritional requirements. In the case of opportunistic omnivores such as humans and rats, this knowledge must be learned. This process was described as the development of cathexes by Tolman (1949), who suggested that it involved affective, or emotional, learning that created positive affective reactions toward substances that fulfilled nutritional needs and negative affective reactions toward substances that did not or that caused unpleasant reactions such as nausea. Learning of negative cathexes has been the more fully explored of these processes through examination of conditioned taste (or flavor) aversion (CTA).
Exploration of CTA has demonstrated a distinction between aversive motivation caused by insults to the skin defense system, such as electric shock, and insults to the gut defense system caused by taste and emetic toxins (Garcia y Robertson & Garcia, 1985). This suggests that learning about the incentive value of food is based on selective associations between taste (and to a lesser extent olfactory stimuli) and postingestive consequences. However, in many cases the animal must make behavioral choices at a distance, before being in a position to taste the potentially aversive food. A great deal of research suggests that associations between the distal cues that guide behavior and the postingestive consequences of ingesting a food predicted by those cues require mediation by taste or olfactory cues (Garcia, 1989). This suggestion gives rise to a mediated-association view of food incentive learning: Postingestive consequences are associated with taste, and taste stimuli are associated with distal cues. Hence, feeding behavior is governed by a chain of distal cue–taste–postingestive consequence associations (Garcia, 1989).
The strongest evidence for this view comes from a variety of studies that emphasize the importance of taste in mediating CTA to distal cues. Rusiniak, Hankins, Garcia, and Brett (1979) demonstrated that although weak odor paired with nausea produces weak aversion to the odor, the same odor results in a much stronger aversion if presented in compound with a taste. Brett, Hankins, and Garcia (1976) demonstrated that after repeated trials, hawks rejected both black (poisoned) and white (safe) mice, but that following the addition of a distinctive taste to the black mice, the hawks began to reject the black mice and eat the white mice. Evidence also suggests that similar, though weaker, effects can be found by using the expectancy of a taste to mediate the CTA to distal cues. Holland (1981) paired a tone (distal) CS with a distinctive flavor before pairing the tone with a nausea-inducing lithium chloride injection. Subsequent testing showed decreased consumption of the tone-predicted food, indicating the development of an indirect, expectancy-based CTA. Taken together, these results indicate that learning about which foods in the environment to ingest is mediated by two different Pavlovian conditioning processes.
Incentive Learning
Although this system indicates to the animal in a general sense what is good to eat, it is not able to guide the animal’s day-to-day foraging behavior because the gustatory learning system proposed to underlie cathexes is purely affective; it encodes only positive or negative values. To the extent that an animal’s behavior reflects its current needs, the animal must be able to encode and act on the value of food given its current internal state. The evaluation of the incentive value of food given the animal’s current internal state is called incentive learning (Balleine, 1992).
The study of incentive learning is complicated by the fact that the effect of internal state on feeding responses seems to differ based on the associative procedure that is used to examine those behaviors. In Pavlovian conditioning procedures, internal state (e.g., deprivation) seems to act directly to increase the animal’s tendency to engage in foodreinforced behavior (Balleine, 1992). In contrast, in operant conditioning procedures, the effect of internal state on behavior depends on whether the animal has prior experience with the outcome of its behavior, the reinforcer, in that deprivation state (Dickinson & Balleine, 1994). In contrast to these effects, Davidson (1998) has shown in a Pavlovian conditioning procedure that the state of food deprivation on test had no effect on approach behavior unless the animals had had prior experience with the pellets in the undeprived state. Only rats that had previously eaten the pellets when undeprived and then tested undeprived showed a reduction in approach behavior. Just as Dickinson and Balleine (1994) interpreted their results, Davidson (1998) interpreted this as evidence that motivational control of Pavlovian food seeking by hunger has to be learned through experience of the reinforcer in both the deprived and undeprived states.
This analysis is further complicated by two additional findings. The first is that as experience with the instrumental action-outcome contingency increases, the motivational factors underlying performance also appear to shift. Increased training seems to result in a growing importance of Pavlovian incentive factors (i.e., deprivation state) and a decreasing importance of instrumental incentive learning (i.e., the incentive valuation of the outcome in the animal’s current deprivation state; Dickinson, Balleine, Watt, Gonzalez, & Boakes, 1995). The second is that different instrumental actions in a chain of responding required for reinforcement appear to be governed by different motivational factors. Instrumental actions that occur earlier in a chain of responses seem to be governed by the animal’s current evaluation of the reinforcer. In contrast, instrumental actions that occur immediately prior to reinforcer delivery appear to be directly regulated by the animal’s current deprivation state (Balleine, Garner, Gonzalez, & Dickinson, 1995). This latter finding—of a distinction in motivational control between proximal and distal responses—mirrors the common distinction between appetitive and consummatory responding (Craig, 1918; Konorski, 1967) that is also a component of ethological (Leyhausen, 1979; Tinbergen, 1951) and psychological theories of response organization (Domjan, 1994; Timberlake, 1983, 1994).
Feeding Response Organization
Appetitive and Consummatory Behavior
The last two sections have dealt with initiation of feeding and selection of food. Another important aspect of feeding motivation concerns the topography and organization of behaviors used to obtain food. The most influential view of feeding response organization is based on Craig’s (1918) distinction between appetitive and consummatory behavior. Consummatory behavior has typically been viewed as stereotyped responses that served as the endpoints of motivated sequences of behavior and could be defined by their quieting effect on the behaving animal. In contrast, appetitive behavior was conceived of as a sequence of variable but nonrandom behavior that served to increase the likelihood of the animal being able to perform the consummatory behavior by increasing the likelihood of interaction with the goal stimulus (Craig, 1918). Under this framework, specific examples of feeding consummatory behavior would include acts like chewing, swallowing, and stereotyped killing behavior such as the throat bite used by large cats. Appetitive behavior would include the typical behaviors of foraging such as motor search. These concepts were further refined by Lorenz’s (1937) analysis that redefined consummatory behavior as the fixed action pattern of an instinct and suggested that it was motivated by the buildup of action-specific energy.Appetitive behavior remained undirected behavior whose function was to increase the likelihood of the animal’s being able to perform the fixed action pattern by bringing it into contact with the releasing stimulus.
Parallels between the concept of the consummatory act and the reflex (Sherrington, 1906) and unconditioned response (Pavlov, 1927) led to the importation of the appetitive/ consummatorydistinctionfromethologicaltheorizingintothe realm of learning theory (e.g., Konorski, 1967). Whereas ethologists distinguished between consummatory and appetitive behaviors on the basis of response stereotypy, learning theorists distinguished them procedurally. Consummatory behavior was investigated in Pavlovian conditioning procedures, following Pavlov’s lead in examining the stimulus control of consummatory reflexes. Appetitive behavior was investigated in operant conditioning procedures that emphasized the flexibility of appetitive behavior by concentrating on arbitrary responses and arbitrary stimuli to control performance (Timberlake & Silva, 1995).
Although consummatory acts have been considered prototypically instinctive (Lorenz, 1937), careful research has demonstrated a role for learning in the development of consummatory behavior. The best demonstration of this influence comes from the work of Hogan (1973a, 1973b, 1977) on the development of feeding behavior in the Burmese red junglefowl, a close relative of the domestic chicken. Hogan (1973a) demonstrated that pecking behavior in newly hatched chicks did not discriminate between food and sand but that by 3 days of age, pecks were directed primarily at food. At that age, ingestion of food facilitated pecking, but not until 10 min to 1 hr after ingestion, and not specifically to food. Further studies (Hogan, 1973b) indicated that neither satiation nor hunger was responsible for this delayed increase and suggested instead that this effect was due to learning reinforced by the postingestive consequences of food consumption. Hogan (1977) demonstrated that only experience that involved pecking led to the development of discrimination between food and sand and that this required a postingestive delay of 2 min to 3 min, indicating that the discrimination is most likely based on short-term metabolic feedback. Hogan suggested that the behavioral control of pecking and the development of metabolic feedback develop independently, but experience is necessary for these two systems to become coordinated.
The Structure of Appetitive Behavior
The focus on using instrumental procedures to study appetitive behavior in psychology has, to a large extent, blinded it to the unlearned, underlying structure of appetitive behavior. Far frombeingundifferentiatedactivity,closeexaminationofmotivated behavior has demonstrated that appetitive behavior is organized into chains of behaviors that serve to increase the likelihood of the terminal act. The classic demonstration of this is Tinbergen’s (1951) analysis of the mating behavior of the stickleback, although similar demonstrations have been made for the organization of other appetitive behavior (e.g., Leyhausen, 1979). Despite the procedural difficulty in analyzing the underlying organization of appetitive behavior in arbitrary response operant procedures, this organization has made its presence felt through various phenomena variously described as constraints on learning, misbehavior, and adjunctive learning (Staddon & Simmelhag, 1970). The constraints on learning phenomena demonstrate the underlying behavioral organization of the animal through making some responses and stimuli easier to condition to various rewards than others. One example of many is the relative inability of animals to learn an instrumental response chain that requires bar pressing on a lever proximal to the feeder prior to pressing on a lever distal to the feeder in order to be reinforced, whereas the far-near sequence is learned rapidly (Silva, Timberlake, & Gont, 1998). Perhaps the classic examples of the intrusion of the underlying structure of appetitive behavior into operant responses are the reports of misbehavior made by the Brelands (Breland & Breland, 1961, 1966) in which the typical feeding behaviors of species began to intrude into well learned, arbitrary sequences of food-reinforced behavior.
Explicit examination of the organization of appetitive behavior is a relatively recent phenomenon in learning situations and has largely taken place through the study of response topography in Pavlovian conditioning procedures and the subsequent development of behavior systems theories (Domjan, 1994; Fanselow & Lester, 1988; Timberlake, 1983).The behavioral organization of predatory foraging and feeding in the rat is the most extensively developed of these behavior systems and is presented as a specific example later. It is important to note that the precise behaviors and their organization would be expected to differ from species to species and within species based on local factors such as relative prey selection. In addition, as has been shown through operant conditioning, novel behaviors can readily be incorporated into the appetitive component of feeding behavior chains. This simple addition of new behaviors into an appetitively motivated chain of behavior can be contrasted with the relative inflexibility of aversively motivated behavior chains described in the section on aversively motivated response organization later.
A Feeding Response Organization: The Predatory Behavior System of the Rat
Timberlake (1983, 1990, 1993, 1997, 2001; Timberlake & Lucas, 1989; Timberlake & Silva, 1995) outlined a functional behavior system that describes the predatory foraging and feeding behavior of the rat in a hierarchical system that emphasizes the behavior-organizing role of motivational modes within the system. The behavior system includes selective stimulus processing mechanisms, timing and memory components, functional motor programs, and organizing motivational structures that interrelate to serve a particular function. Within that system, particular subsystems are defined by a collection of stimulus predispositions and motor outputs organized to achieve a particular goal (see Figures 2.1 and 2.2). In the case of the rat feeding system, activity in the predatory subsystem is indicated by heightened responsiveness to movement and the increased probability of predatory appetitive behaviors like chase and capture.
Timberlake (1993; Timberlake & Silva, 1995) suggested that within the predatory subsystem, functional behaviors are organized by motivational modes into response tendencies based on the temporal, spatial, and psychological distance to the prey. This view is complementary to the predatory imminence continuum developed by Fanselow (1989; Fanselow & Lester, 1988) in describing the functional behavior systems of defensive behavior that will be described more fully later. These modes describe the relative probability of particular responses given the appropriate environmental support stimuli and create the underlying organization of feeding behavior.
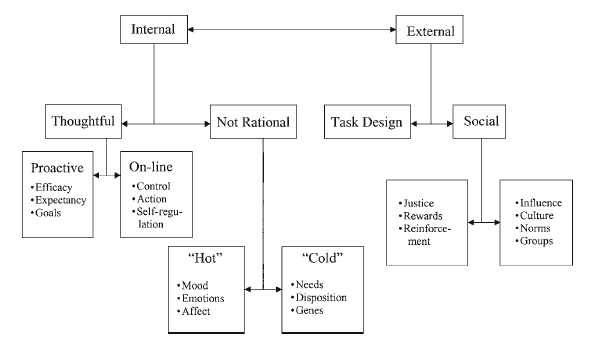
Following initiation of a predatory foraging sequence, behaviors such as motor search, visual search, target tracking, or substrate investigation are motivated by a general search mode that also specifies stimulus selectivities such as increased responding to novelty or movement. Environmental cues related to an increase in prey imminence cause a qualitative shift in stimulus and motor selectivity described as the focal search mode. Within the focal search mode, behavior patterns may shift to include responses such as chase and capture, stalking, or area-restricted search. Timberlake and Washburne (1989) investigated behavioral responses to artificial moving prey stimuli in seven different rodent species and noted that the topography of chase and capture behaviors directed toward the artificial prey stimulus were based on the subject’s species-typical predatory behavior. When food is present, the animal engages in behaviors directed toward the food item and again makes a qualitative shift to the stimulus selection and motor properties organized by the handling/ consuming mode. At this point, stimulus characteristics such as taste, odor, and orotactile stimulation are the predominant influences on behavior and motivation, as suggested by Garcia (1989) in his description of the factors involved in feeding cathexes, described earlier. Motor patterns are those typically described as consummatory behaviors, including the various kinds of ingestion and oral rejection behaviors.
The behavior systems model just outlined suggests that feeding response organization is governed by motivational, but not behavioral, modes. The exact nature of the behavior in any sequence is determined by the interaction of the animal’s motivational mode, its behavioral repertoire, and the affordances of the stimuli in the environment. Just as ethological theories of response organization suggest that chains of behavior are organized into relatively independent subunits with their own intermediate goals (Morris, 1958; Tinbergen, 1951), this behavior systems approach also separates behavior chains into functional subunits with related stimulus and motor preparedness and particular stimulus-response transactions that function as transitions between them.
Fear Motivation
Fear motivation reverses the perspective of feeding, as we focusonpreyandnotpredators.Becausethegoalofthepredator is to consume the prey, the selection pressure on defense is powerful because injured or dead individuals have infinitely diminished reproductive success. Thus it is not surprising that prey species have evolved elaborate behavioral strategies to deal with such threats. Fear is a motivational system that is provoked by danger signals in the environment, and when activated this system triggers defensive reactions that protect individuals from environmental dangers. In this section we examine fear from a behavioral systems perspective.
Because of this enormous selection pressure, species have several lines of defense. Some species rely on primary defensive strategies that “operate regardless of whether or not a predator is in the vicinity” (Edmunds, 1974, p. 1). Primary defense strategies include camouflage (the animal’s body color blends into environment) and Batesian mimicry (the animal’s body color and form resemble another species that has dangerous or unpleasant attributes). Although primary defenses contribute to survival, these strategies are relatively inflexible and insensitive to feedback. For example, green insects avoid wild bird predation more often when they are tethered to a green environment compared to a brown environment (Di Cesnola, 1904). Thus, the insect’s camouflage contributes to survival only when it rests in the matching green-colored environment, and the camouflage is ineffective elsewhere. In contrast to primary defense, secondary defensive strategies require that an animal respond to a threat with specific behaviors. Turtles withdraw into their hard shells; porcupines raise their sharp quills; and grasshoppers retreat a short distance and then become immobile when they are threatened. These behaviors can be inflexible, but they are often sensitive to feedback. Unlike primary defensive strategies, which are permanently employed, these defensive behaviors are triggered by a fear-driven motivational system.
The Pervasiveness of Fear in Motivated Behavior
Fear modulates other motivational systems. Animals that miss a meal or a mating opportunity usually live to eat or mate another day. Animals that fail to defend usually have no further reproductive chances. Therefore, fear takes precedence over other motivational systems. One of the first quantitative measures of fear was the ability to suppress food intake (Estes & Skinner, 1941). The effects of fear on feeding can also be subtle. As described earlier, Fanselow et al. (1988) demonstrated that rats adjust the size and frequency of meals in relation to shock density. Animals were housed in an environment that had a safe burrow. The burrow was attached to an area with a grid floor, and brief shock was delivered to this area on a random schedule. The rat could obtain food only if it risked venturing onto the grid floor area to eat. The results suggest that with increasing shock density, rats take fewer, but larger, meals. Thus, fear motivation seems to modulate foraging behaviors (i.e., feeding motivation). Similarly, rats cease foraging, retreat to a burrow, and delay further foraging for hours after they encounter a cat near the entrance of the burrow (Blanchard & Blanchard, 1989), and monkeys seem reluctant to reach over a snake to obtain food (Mineka & Cook, 1988). Fear also influences sexual motivation. For example, female stickleback fish produce few offspring with a male conspecific that displays inappropriate territorial aggression toward them (Hollis, Pharr, Dumas, Britton, & Field, 1997). During the aggressive act the female may be both injured and frightened by the male, and females often retreat from the vicinity when attacked. Thus, fear modulates sexual motivation by disrupting or delaying reproductive opportunities.
Factors Governing Initiation of Fear
An effective behavioral defensive strategy requires that animals identify threats with sufficient time to perform the appropriate defensive responses. Numerous types of stimuli can signal danger and activate fear motivational systems. These stimuli can be divided into three functional classes: learned fear stimuli, innate fear stimuli, and observational learning and fear stimuli.
Learned Fear Stimuli
Fear is rapidly learned and measured in the laboratory (Fanselow, 1994); it has direct clinical relevance (Bouton, Mineka, & Barlow, 2001); and it has become a standard method for exploring the behavioral processes and neural mechanisms of learning. In the prototypical laboratory experiment, a rat is placed in a chamber where it is presented with a tone that is followed by a brief aversive foot shock. Later during a test session, the rat is reexposed to either the conditioning chamber or the tone. During this reexposure the rat will engage in behaviors that are characteristic of fear. With this preparation the tone and the chamber, or context, serve as conditional stimuli (CSs). They were originally neutral stimuli, but after they were paired with an unconditional stimulus (US), the foot shock, the animal responded to the CS in a fearful manner. Such responses to the CSs are called conditional responses (CRs). These fear CRs occur specifically to the shock-paired stimuli, and these responses are used as measures of learning in Pavlovian experiments. To date, Pavlovian fear has been characterized with several CRs such as defensive freezing, reflex facilitation, heart rate, blood pressure, conditional suppression, conditional analgesia, and vocalizations (see Fendt & Fanselow, 1999, for review).
Animals can learn to associate a threat with numerous classes of CSs. Auditory cues, visual cues, olfactory cues, and tactile cues can all become fear CSs with the appropriate training regime. However, the nature of the CS is not arbitrary because animals are known to exhibit selective associations. This phenomenon is best exemplified by an experiment performed by Garcia and Koelling (1966) in which rats were presented with a compound CS. The compound CS consisted of auditory, visual, and flavor cues: a buzzing noise, a blinking light, and the taste of saccharin, respectively. During training trials the presentation of the compound CS was followed by the occurrence of footshock. During test sessions, rats exhibited fear reaction to the auditory and visual cue, and not to the flavor cue. Thus, this experiment suggests that in the rat visual and auditory cues are more readily associated with threat. Asymmetry in this sort of stimulus selection appears ubiquitous. Similar selective associations have been demonstrated in the pigeon (Foree & Lolordo, 1973). Further, tone onset is more readily associated with danger than light onset, which is more readily associated with safety (Jacobs & LoLordo, 1980). These findings suggest that stimulus selection in the laboratory reflects phylogenetic influences on stimulus selection in the species’natural niche.
Innate Fear Stimuli
Learned fear stimuli require that an animal have previous experience with the stimuli to recognize the potential threat. In contrast, innate fear stimuli are those stimuli that can be identified as potentially threatening without previous experience. Animals display these responses without any specific training experience.
It is difficult to develop unambiguous criteria that classify innate fear stimuli. For instance, an unlearned fear stimulus could be defined as a stimulus that elicits defensive behaviors during its first presentation. With this definition a cat may be considered an unlearned fear stimulus because laboratoryreared rats exhibit robust defensive behaviors during their first encounter with the predator. This behavior suggests that the rodent’s genome retains information to detect certain innate stimuli and provokes appropriate defensive reactions (Blanchard & Blanchard, 1972). However, defensive reactions to a cat could also be due to learning. In this alternative account some aspect of the cat’s movement is the aversive stimulus, and the rat exhibits defensive behaviors because it is in an environment that has been paired with an aversive stimulus. Thus, the rat freezes in the presence of the cat only because its movement has been paired with other features of the cat and not because the cat itself is an innately aversive stimulus. This interpretation is supported by the observation that a moving cat, dog, or inanimate card can trigger freezing in the rat, although the sound, smell, or sight of a dead cat does not (Blanchard, Mast, & Blanchard, 1975).
Also, the fact that a defensive response follows the first presentation of a stimulus is not sufficient to classify that stimulus as an innate releaser of fear. This is nicely illustrated by the analysis of electric shock. Fear responses such as freezing, defecation, and analgesia follow the first presentation of shock. However, shock per se does not unconditionally provoke these responses. Instead, it rapidly and immediately conditions fear to the contextual cues present before shock, and it is these conditional cues that elicit the behaviors. Removing these cues before shock (Fanselow, 1986) or after shock (Fanselow, 1980) eliminates the responses. Similar patterns appear to exist (Blanchard, Fukunaga, & Blanchard, 1976). Thus, we must exert considerable caution before concluding that something is an innate trigger of fear. This pattern also raises an important question about the motivational properties of something like shock, because although it supports conditioning of fear behavior, it does not provoke fear itself. This pattern may be similar to Balleine’s (1992) data, described earlier, suggesting that incentive properties of food must be learned.
Although prey species clearly react to predators in the wild with elaborate defensive responses (Coss & Owings, 1978), these studies cannot control for the ontogenetic history of the subject. Therefore, the best evidence for fear reactions to a predator comes from laboratory studies with rodents (Blanchard & Blanchard, 1972; Hirsch & Bolles, 1980; Lester & Fanselow, 1985). The strongest evidence for phylogenetic influences on defensive behavior comes from a study conducted by Hirsh and Bolles (1980). These investigators trapped two subspecies of wild deer mice that live in distinct regions of the state of Washington in the United States. Peromyscus maniculatus austerus comes from the moist forest regions in western Washington state, and Peromyscus maniculatus gambeli from an arid grassland region of eastern Washington state. These animals were bred in the laboratory, and their first generation of offspring were exposed to several predators selected from the eastern and western regions.
When tested, P. m. gambeli both survived more strikes and survived longer when exposed to a predatory snake from its niche compared to P. m. austerus. Thus, P. m. austerus was more vulnerable to attack by the predator alien to its niche. Moreover, P. m. gambeli exhibited more fear responses to the predator snake from its niche, compared to a nonpredatory snake. Thus, P. m. gambeli was able to discriminate between two types of snake. These results suggest that the probability of surviving an encounter with a predator is related to the evolutionary selection pressure that that predator exerts on the prey in their natural niche. Thus, animals adopt unlearned or innate defensive strategies that allow them to cope with predation in their niche.
Other observations suggest that a variety of species can innately identify predators from their own niche (see Hirsch & Bolles, 1980, for review). For example, rats exhibit robust fear reactions to cats during their first encounter with the predator, and this fear response does not seem to habituate rapidly (Blanchard et al., 1998). However, recall from our earlier discussion that cats are maximally fear provoking when they are moving. Thus, it is difficult to ascribe the fearprovoking ability to the cat “concept” when it is possible that cat-like movements are essential for provoking fear in the rat (Blanchard et al., 1975). Because a predator is a complex stimulus, research is needed to isolate what aspects of it have phylogenetic and ontogenetic fear-producing properties.
Bright light is another possible innate fear stimulus for rodents; rodents avoid it consistently. Presumably, light signals threat because rats are more visible in bright environments. Thus, negative phototaxis may be an example of defensive behavior. Walker and Davis (1997) reported that rats display enhanced startle after they have been exposed to bright light. These investigators suggested that bright light elicits fear and that this light-enhanced startle is a manifestation of that fear. Thus, this phenomenon resembles the fear-potentiated startle procedure in which startle behavior is enhanced by the presentation of learned fear stimuli (Davis, 1986).
Recent evidence has also suggested that predator odors may act as innate releasers of defensive behavior. For example, Wallace and Rosen (2000) reported that exposure to a component of fox feces, trimethylthiazoline (TMT), elicits freezing behavior in the rat. However, these results may be related to the intensity of the odor and to the test chamber’s small dimensions. What is needed in all these cases is a set of criteria that unambiguously indicate that a stimulus is an innate fear stimulus. We do not have these criteria yet, but we know from the research with shock that a defensive response following the first occurrence of a stimulus is not sufficient.
Observational Learning and Fear Stimuli
This third class of fear stimuli has been developed from studies on social interactions in monkeys. Lab-reared monkeys normally do not exhibit fear reactions in the presence of a snake, whereas wild-reared monkeys do (Mineka & Cook, 1988). However, the fear of snakes can be socially transmitted by a phenomenon called observational learning.
In these experiments a lab-reared observer monkey can view a wild-reared cohort as it interacts with an object. The object may be a snake, a toy snake, or a flower. If the cohort is interacting with a toy snake or a flower, the animal does not exhibit any fear responses, such as fear grimacing or walking away. When this same monkey interacts with the snake, it will exhibit fear reactions. Interestingly, when an observer monkey sees its cohort engaging in fear behaviors when it encounters the snake, the observer monkey will later display fear responses to the snake. Mineka suggests that monkeys can learn about threats by observing conspecifics interact with threatening stimuli.
This phenomenon demonstrates a sophisticated means to learn about threats. Notice that the monkey can learn to fear the snake without direct experience with the snake. This phenomenon is distinct from a typical Pavlovian fearconditioning session because the animal does not experience theUSdirectly.Itlearnsfearofthesnakethroughobservation. Regardless, observational learning shares selection processes that are similar to standard Pavlovian learned fear, and monkeys readily learned fear to snakes, but not to flowers, through observation. Thus, this type of fear may actually be a phylogenetically predisposed form of learning as well.
Functional Behavior Systems Analysis of Defensive Behavior
Fear elicits defensive behavior in a myriad of species (Edmunds, 1974). Each species has its own repertoire of defensive behaviors, and similar species such as the rat and hamster may react to a similar threat in very different ways. But if a species has a number of defensive behaviors in its repertoire, how does it select among them?
Throughout much of the twentieth century, the selection of fear-motivated behavior was most commonly explained with reinforcement principles. For example, Mowrer and Lamoreaux (1946) suggested that animals learn to avoid fearprovoking stimuli because the event of not receiving an aversive stimulus is reinforcing. Thus, rats learn to flee from predators because the tendency to flee is strengthened by negative reinforcement when they successfully avoid predation. Despite their popularity, however, theories like these provide an inadequate account of fear-motivated behavior (summarized in Bolles, 1975). Consequently, alternative accounts that use a behavioral systems approach to explain these behaviors have been developed. These explanations acknowledge that different species may use distinct defensive responses. These explanations of defensive behavior also deemphasize the importance of reinforcement in response production and emphasize the primacy of innate defensive behaviors.
The first data that led to these behavioral systems explanations came from Gibson (1952), who studied defensive behavior in the goat. She demonstrated Pavlovian conditioning of the goat’s leg flexion response and noted that goats performed many different behaviors such as running away, turning around, and backing up after the shock was delivered. Gibson concluded that leg flexion itself was not a defensive reaction but that it was simply a common component of the other behaviors that she observed. Thus, leg flexion in the goat appears to be a component of several defensive responses.
Akin to Gibson’s findings, Bolles (1970) proposed an explanation of avoidance behavior known as the speciesspecific defensive reaction (SSDR) hypothesis. This hypothesis suggests that every species has its own repertoire of innate defensive behaviors and that animals perform these behaviors unconditionally when they become afraid. For example, a rat’s SSDRs include fleeing, freezing, fighting, and dark preference. Thus, when a rat becomes afraid, it will perform these defensive behaviors unconditionally; it does not learn to perform these responses via reinforcement. Bolles included a response selection rule in the original formulation of SSDR theory. He suggested that SSDRs were organized in a hierarchy but that the hierarchy could be rearranged by experience. If fleeing is ineffective in avoiding shock, that SSDR will be suppressed by punishment, and as a result the animal will switch to the next SSDR in the hierarchy. Upon further examination of this idea, however, Bolles and Riley (1973) concluded that freezing could not be punished by shock, and as a result the punishment rule could not explain how an animal switched between different SSDRs when threatened.
The Organization of Defensive Behavior: Predatory Imminence Theory
As an alternative to Bolles’ explanation of defensive behavior, Fanselow (1989) developed the theory of the predatory imminence continuum. In this theory, Fanselow retains the basic tenets of the SSDR theory: Animals use innate SSDRs in defensive situations. However, Fanselow proposed a different response selection rule that determines which SSDR an animal will perform at any given moment. This rule suggests that the selection of specific defensive responses is related to a continuum of the physical and psychological distances between the predator and prey. Thus, given that danger signals elicit fear, response selection is mediated by fear directly. Specifically, high levels of imminence vigorously activate the fear motivational system, whereas low levels of imminence activate the fear system weakly.The relative activation of the fear motivational system thereby determines the selection of defensive behaviors.
Just as there are responses that are particular to each stage of predatory imminence, there are sets of stimuli that tend to be correlated with each stage. These relationships can be illustrated by considering four situations from the rat’s natural environment that differ in predatory imminence.
- A safe burrow. When a rat rests in a safe environment such as a burrow, predatory imminence is relatively low. In this environment the animal may not exhibit any sort of defensive behaviors because none are needed. Alternatively, the act of remaining in the burrow could itself be classified as a defensive behavior because it significantly reduces the threat of predation.
- A preencounter environment. As a rat leaves its burrow to forage for food, predatory imminence increases because the probability of encountering a predator increases. Rats engage in preencounter defensive behaviors when their circumstances might lead to an encounter with a predator, but the predator has not yet been detected.These behaviors include changes in meal pattern foraging, thigmotaxis, dark preference, defensive burying, retreating to a burrow, and leaving the burrow via investigative, stretch-approach behavior.
- A postencounter environment. Predatory imminence increases further when a rat encounters a threat, and it will engage in postencounter defensive behaviors. The rat’s prominent postencounter defensive behavior is freezing. Rats freeze when they encounter predators, and also when they encounter aversive stimuli. Other postencounter defensive behaviors include conditional analgesia.
- A circa-strike situation. When the rat’s postencounter defensive behaviors have failed, a predator will typically attack. As the predator makes contact with the prey, the rat switches to circa-strike defensive behaviors. These behaviors seek to reduce predatory imminence by either escaping the attack or fending off the predator. When attacked, the rat engages in a rapid bout of flight called the activity burst, and it may also engage in defensive fighting.
Notice that two factors change across the predatory imminence continuum. First, the physical distance between predator and prey typically decreases as predatory imminence increases. Second, the psychological distance decreases as the perceived danger of the threat increases. This feature accounts for situations where the prey may fail to detect the threat, although the absolute physical distance between them is small. Thus, if a rat does not notice a cat, it may not freeze or flee despite the close proximity of the predator.
The utility of predatory imminence theory lies in its ability to predict the form of defensive behavior based on these two selection principles. One challenge of the theory lies in discoveringthespecificdefensivebehaviorsforeachspecies.Itis entirely possible that similar species use different SSDRs and thattheseSSDRsmaybeorganizedalongthepredatoryimminence continuum is different ways. For example, although the dominant postencounter defensive behavior for a rat is freezing, hamsters may exhibit flight when threatened (Potegal, Huhman, Moore, & Meyerhoff, 1993).
Defensive Behaviors on the Predatory Imminence Continuum
In the last section we explained the predatory imminence continuum, the basis of a functional behavior systems approach to defense. This continuum is divided into three functional classes of defensive behavior: preencounter, postencounter, and circa-strike defensive behaviors. In this section we describe and organize these behaviors according to the predatory imminence continuum. In many cases, a particular defensive behavior may fall into a single category of predatory imminence (e.g., freezing). However, the expression of some behaviors (e.g., flight) may actually reflect several different components of defensive behavior that fall into different categories.
Preencounter Defensive Behaviors
Animals display preencounter defensive behaviors in situations where a predator may be present but that predator has not yet been detected.
Meal-Pattern Adjustment. A rat may be at higher risk from predators when it leaves its burrow to forage for food. One strategy that diminishes this threat is to reduce the number of foraging excursions by increasing the size of the meal consumed on each trip. Indeed, when rats are housed in an environment that requires them to traverse a shock grid to forage for food, they modify the size and frequency of meals taken in relation to shock density. Specifically, with increasing shock density, rats take fewer, but larger, meals (Fanselow et al., 1988).
Dark Preference. Rodents have a preference for dark places. This behavior presumably has a defensive purpose because rodents are less likely to be detected by predators when they occupy a dark location (e.g., Valle, 1970). Rodents may engage in this behavior in both preencounter and postencounter defensive situations.
Thigmotaxis. Rodents have a tendency to stay near walls. This behavior contributes to successful defense because it limits the threat of attack from behind and because it may also reduce the animal’s visibility (e.g., Valle, 1970). Rodents may engage in this behavior in both preencounter and postencounter defensive situations.
Burying. Rodents bury threatening objects when materials such as wood chip bedding or wooden blocks are available. For example, rats bury a metal rod that delivers shock to the animal (Pinel & Treit, 1978). The specific purpose of this behavior is disputed. Some investigators suggest that burying is fear response akin to defensive attack of the shock prod (Pinel & Treit, 1978). Other investigators have offered alternative explanations that describe burying as a manifestation of preemptive nest maintenance directed at protecting the animal from further attack (Fanselow, Sigmundi, & Williams, 1987). An interesting property of burying is that this behavior typically emerges only after rats have engaged in other defensive behaviors: Most rats freeze and flee before engaging in burying. Thus, burying is not prominent when predatory imminence is relatively high. It is also often directed at exits as much as the shock source (Modaresi, 1982). Thus, it seems likely that burying is a preencounter nest-maintenance behavior in rats. However, in some species, such as ground squirrels, it represents a higher imminence nest-defense behavior (Coss & Owings, 1978).
Stretch Approach. Stretch-approach behavior is prominent when a rodent encounters a localizable noxious object, such as a shock prod. In this situation, the level of predatory imminence is ambiguous, and this behavior may be thought of as a cautious exploratory behavior employed to collect information about potential threats. This elaborate behavioral sequence
—–begins with the rat advancing slowly towards the aversive object in a low, stretched posture. As it advances, the rat periodically stops and leans forward towards the object [in a manner that] carries the rat into the vicinity of the aversive test object, from where it is able to sniff it, palpate it with its vibrissae, and occasionally contact it with its nose. (Pinel & Mana, 1989, p. 143)—–
Rodents exhibit stretch-attend to potential predators (Goldthwaite, Coss, & Owings, 1990), to areas of the test apparatus in which they have received shock (Van der Poel, 1979), and to objects that have been the source of an electric shock (Pinel, Mana, & Ward 1989). Pinel and Mana (1989) suggested that this behavior functions to provide information about the potentially hazardous object or location and that olfactory and tactile information via the vibrissae are important elements of this information gathering.
Leaving and Entering the Burrow. Rats often display stretch-approach behavior if there is some potential danger in the environment. Alternatively, if the rat has already left the burrow but remains nearby, a slight increase in predatory imminence will cause retreat to the burrow. This action is one form of flight. Such retreats to the burrow may be accompanied by freezing within the burrow (Blanchard & Blanchard, 1989). However, if the animal is far from the burrow, or the increase in predatory imminence is greater, the animal will enter a different stage of behavior, postencounter defense.
Postencounter Defensive Behaviors
Rodents engage in postencounter defensive behaviors when preencounter defenses have failed and a threat has been detected in the environment.
Freezing. Frightened rats display freezing behavior. This defensive behavior is prominent in but not exclusive to rodent species, and it is characterized by the absence of all movement except for breathing. In the wild, rodents often freeze when they encounter a predator. This behavior is an effective defensive strategy because many predators have difficulty detecting an immobile target, and movement can act as a releasing stimulus for predatory attack (Fanselow & Lester, 1988). In the laboratory this behavior is prevalent when rodents are presented with a CS that has been paired with foot shock (e.g., Fanselow, 1980). Rats usually freeze next to an object (thigmotaxis) such as a wall or corner. This behavior occurs even when the fear stimulus is present and the rat is not next to the object. Thus, part of the freezing response may be withdrawal to a rapidly and easily accessible location to freeze (Sigmundi, 1997). Thus, the freezing sequence contains a component of flight.
Conditional Analgesia. Rodents become analgesic when they encounter learned fear stimuli. Although triggered by fear stimuli, this analgesia becomes useful if the animal suffers injury from a predatory attack. Reduced pain sensitivity permits the animal to express defensive behaviors and forego recuperative behaviors when predatory imminence is high (Bolles & Fanselow, 1980).
Circa-Strike Defensive Behaviors
Rodents engage in circa-strike defensive behaviors when all other defensive strategies have failed. Thus, these behaviors are prominent when predatory imminence is relatively high.
Flight. Another defensive behavior that is common to rodents and many species is flight. In circa strike, flight consists of a rapid burst of activity away from the predator. If cornered, a rat will vocalize, bare its teeth, or jump beyond or at the predator (Blanchard & Blanchard, 1989). The activity burst to electric shock and the potentiated startle response of an already frightened rat to a loud noise are other examples of this behavior.
Fighting. When other defensive behaviors have failed, rodents often resort to defensive fighting when the predator attacks. In the laboratory this behavior emerges when two cohorts receive a series of inescapable foot shocks (Fanselow & Sigmundi, 1982). Fighting emerges only after many presentations of foot shock. Presumably, the attacks are an attempt to halt shock delivery, and rats attribute the delivery of shock to their cohort.
In the analysis of defense it may be important to distinguish between immediate and subsequent behaviors. Let us consider a hypothetical situation that involves a rat encountering a threat. When a rat receives a shock via a shock prod, the animal’s initial response is to retreat from the shock source and then exhibit freezing behavior. Later the animal may return to the shock source’s vicinity, and then it may exhibit freezing, stretch-attend, and defensive burying behaviors. The animal may also move away from the shock prod in a manner that resembles retreat to a burrow.
In the previous section we described the functional behavior systems view of defensive behavior. This view suggests that defensive behavior is organized by a continuum of perceived danger: When the threat is perceived, rats express specific sets of defensive behaviors that are qualitatively different from those expressed when the threat has not been detected. This discrimination may also vary with time if animals continually update their concept of perceived danger. This updating process may then contribute to the selection of defensive behaviors in the shock prod scenario: Initially, rats move away from the shock source and freeze, and later on they freeze, bury, and stretch-attend. Notice that the movement away from the shock prod expressed immediately differs from the flight expressed later. Thus, the immediate response to shock delivery may differ qualitatively from subsequent responses to the environment because the animal has updated its concept of perceived danger. Such updating likely depends on the basic principles of extinction, or possibly the reconsolidation phenomenon that has recently received attention (Nader, Schafe, & LeDoux, 2000).
Neural Substrates of Learned Defensive Behavior
Mammalian species share fundamentally similar brain circuits that underlie fear behavior. Indeed, in humans, rats, mice, rabbits, and monkeys the amygdala is a prominent component of the fear circuit. To date, more is known about the brain circuits that support learned fear owing to the popularity of Pavlovian fear conditioning as a model for experimental analysis. Less is known about innate fear circuitry, although evidence seems to suggest that these circuits overlap (e.g., Walker & Davis, 1997). Fendt and Fanselow (1999) have provided a comprehensive review of the neural structures of defensive behavior. Numerous brain structures mediate the acquisition and expression of Pavlovian learned fear.
The Amygdala
The amygdala consists of a cluster of interconnected nuclei that reside in the medial temporal lobe. Brown and Schaffer (1886) provided the first evidence that implicated the amygdala in emotional processing. They demonstrated that large lesions of the temporal lobe tamed previously fierce monkeys. Similarly, Kluver and Bucy (1939) described the emotional disturbances triggered by these large lesions, and Weiskrantz (1956) reported that many features of the disturbance were generated by more selective damage to the amygdala. Based on work done primarily with the Pavlovian fear conditioning paradigm, three nuclei within the amygdala are known to make major contributions to fear behavior: the lateral (LA), basal (BA), and central nuclei (CEA).
The lateral and basal nuclei comprise the frontotemporal complex (FTC; Swanson & Petrovich, 1998). This complex communicates most closely with the frontal and temporal lobes, and it is important in the acquisition of learned fear. Moreover, the FTC has characteristics that make it a plausible site of encoding for the learned association that is established during fear conditioning (Fanselow & LeDoux, 1999). First, the FTC receives inputs from all sensory modalities, including brain regions that are involved with nociception (Fendt & Fanselow, 1999). Thus, sensory information of the CS and pain information of the US converge in the FTC. Second, Pavlovian fear conditioning enhances the response of cells in the FTC that respond to tone CSs (Quirk, Repa, & LeDoux, 1995). Third, lesions of the FTC produce a pronounced and often total loss of many Pavlovian fear responses (e.g, Maren, 1998); fourth, chemical inactivation of this structure is similarly disruptive to fear learning (e.g., Gewirtz & Davis, 1997). Thus, the FTC is critical for the acquisition of Pavlovian fear conditioning and is a plausible site for the encoding and storage of the learned association.
The CEA may be conceived of as the output of the amygdala. It is closely tied with the striatum and is specialized to modulate motor outflow (Swanson & Petrovich, 1998). The CEA projects to a variety of structures, including the periaqueductal gray (PAG), the reticular formation, and the lateral hypothalamus. Both the lateral and basal nuclei of the amygdala project to the CEA. Lesions to the CEA disrupt the expression of a wide range of defensive behaviors (e.g., Kapp, Frysinger, Gallagher, & Haselton, 1979).
The Periaqueductal Gray
ThePAGishighlyinterconnectedwiththeCEA(Rizvi,Ennis, Behbehani, & Shipley, 1991). This region seems to act as a coordinator of defensive behaviors, and expression of defensive behaviors can be dissociated within the PAG. For example, electrical stimulation of the dorsal-lateral PAG (dlPAG) triggers robust activity burst–like behavior (Fanselow, 1994), whereas damage to this structure disrupts the shock-induced activity burst (Fanselow, 1994). Similarly, chemical stimulation of the caudal third of the dlPAG triggers “bursts of forward locomotion” that alternate with periods of immobility (Bandler & Depaulis, 1991, p. 183). Consequently, the dlPAG seems to coordinate overt defensive reactions, such as flight.
In contrast, similar treatments to the ventral PAG (vPAG) have very different effects. Chemical or electrical stimulation of the vPAG triggers freezing behavior, and lesions to this structure disrupt conditional freezing to aversive CSs (Fanselow, 1991). Other fear responses can also be dissociated within the vPAG. For example, the infusion of an opiate antagonist will disrupt fear-induced analgesia but spare conditioned freezing (Fanselow, 1991). Thus, the vPAG seems to coordinate conditional freezing and opiate analgesia. Based on these results, Fanselow (1994) suggested that postencounter defenses are related to the vPAG and its inputs from the amygdala, whereas circa-strike behaviors are related to the dlPAG and its inputs from the superior colliculus.At this time, little is known about the neural substrates of preencounter defenses.
Neural Substrates of Unlearned Defensive Behavior
Much less is known about the neural substrates of innate fear behavior. Walker and Davis (1997) reported that chemical inactivation of the bed nucleus of the stria terminalis (BNST) disrupts light-potentiated startle, but chemical inactivation of the CEAdisrupts only fear-potentiated startle. Inactivation of the FTC disrupts both behaviors. Thus, available evidence suggests that learned and unlearned fear responses can be dissociated within a region described as the extended amygdala (Swanson & Petrovich, 1998). Wallace and Rosen (2001) reported that electrolytic lesions to the LA disrupt freezing to a predator’s odor, whereas excitotoxic lesions did not. Both these lesions disrupt freezing to learned fear stimuli. This result suggests that innate and learned fear can also be dissociated within the amygdala.
Sexual Motivation
Nothing is more closely tied to evolutionary fitness than reproductive success. The most direct measure of reproductive success is the number of offspring that survive, and therefore the terminal goal of a sexual behavior system is successful production of offspring. Animal species display a wide variety of reproductive strategies to produce offspring. Monogamy involves the pairing of a single male and female for the duration of the reproductive cycle. This strategy occurs mostly in species that split the burden of parental care across both parents. Polygyny involves the association of a single male with multiple females, and polyandry involves the association of a single female with multiple males. These polygamous strategies are common in species that distribute the burden of parental care unequally. These mating strategies often influence sexual motivation. Monogamous animals very often display biparental care of offspring, and sexual learning does not typically influence male competition in these species. Accordingly, sexual motivation in monogamous species is relatively similar across sexes. In contrast, species that display intense male competition typically adopt polygamy, and sexual learning and motivation vary greatly across sex (Domjan & Hollis, 1988).
Cues That Signal Reproductive Opportunity
Many species display cues that connote reproductive availability. These cues frequently are shaped by the genotype of the animal. For example, in rodent species olfaction is the primary sensory modality; rodents smell much better than they see. Accordingly, olfactory cues such as pheromones often signal a sexual opportunity in rodent species (Pfaff & Pfaffman, 1969). In contrast, birds see better than they smell, and visual cues ordinarily provide mating signals (Domjan & Hall, 1986). Females of species that undergo estrus often display overt cues that signal reproductive availability. For example, in primate species, such as the chimpanzee, females display swelling of the vaginal lips during estrus, and this cue signals reproductive availability (Mook, 1987).
Sign Stimuli
In some species the appearance of a member of the opposite gender is the dominant cue for a mating opportunity. However, often the essential cue can be reduced to an element or component of the mating partner. These components, called sign stimuli (Tinbergen, 1951), are sufficient to elicit sexual behaviors. For example, male chickens attempt to copulate with models of the torso of female conspecifics (Carbaugh, Schein, & Hale, 1962), and male quails attempt to mate with models including a female quail’s head and neck (Domjan, Lyons, North, & Bruell, 1986). Thus, mere components of a whole animal are sufficient cues to elicit reproductive behavior.
Learned Cues
Learning certainly contributes to the recognition of reproductive opportunity. For instance, male blue gourami fish (Trichogaster trichopterus) normally display aggressive territorial behavior. These fish compete with other males for nest sites, and they attack intruders because the control of territory confers reproductive advantage. This aggressive tendency is so pronounced that males often spoil mating opportunities by mistakenly attacking female gouramis. However, male gouramis can learn to anticipate the approach of a female gourami when a cue reliably precedes her appearance during conditioning sessions (Hollis, Cadieux, & Colbert, 1989; Hollis et al., 1997). As a result of such Pavlovian conditioning, the cue acts as a CS that signals the appearance of the female. Males trained with this contingency both display less aggression toward females and spawn more offspring (Hollis et al., 1997). Thus, learning contributes to the recognition of a reproductive opportunity. Moreover, it contributes to evolutionary fitness by increasing fecundity. This result by Hollis et al. stands as the single most direct and unequivocal evidence that Pavlovian conditioning, indeed any form of learning, has a direct influence on evolutionary success.
Learning also contributes to the mating success of male Japanese quails (Coturnix japonica). For instance, neutral cues previously paired with a sexual encounter elicit CRs, suchasapproachbehavior.Thesecuesalsoshortencopulatory latencies (Domjan, etal., 1986). Thus, discrete Pavlovian CSs, such as red lights, buzzers, or inanimate objects, can elicit responses that facilitate reproductive behaviors in the quail.
Contextual cues may also contribute to reproductive signaling. Domjan et al. (1989) reported that male quails attempt to mate with models of a female quail only if they have previously copulated with a live quail in the test chamber. Thus, the location or context of previous sexual experience can act as a signal that facilitates the occurrence of sexual behavior. Additionally, contextual cues increase the male quail’s sperm production (Domjan, Blesbois, & Williams, 1998). Notably, this demonstrates that Pavlovian learning may directly enhance reproductive success by facilitating the bird’s ability to fertilize multiple eggs and produce offspring.
Sexual learning also directly influences mate selection. For example, when an orange feather is repeatedly paired with a sexual encounter, male quails display a preference for birds adorned with this cue. Males both spend more time near and display more copulatory behaviors toward these females compared to controls (Domjan, O’Vary, & Greene, 1988). Thus, Pavlovian conditioning sways attractiveness, thereby influencing mate selection.
Along with neutral cues, learning also facilitates the sexual efficacy of sign stimuli. For example, the model of a female’s head and neck elicits copulatory behavior in experienced, but not in sexually naive, male quails (Domjan et al., 1989). Thus, during sexual encounters these birds may learn to identify species-typical cues, such as the plumage of female conspecifics.
Organization of the Sexual Behavior System
Sexual behavior does not begin and end with the act of copulation. Instead, species exhibit numerous behaviors that contribute to reproductive success that are not directly connected to the sex act. For example, male blue gouramis build nests used for spawning prior to contact with female conspecifics. This behavior improves reproductive success because nest occupancy increases the probability that these fish will attract a mate. Concurrently, these fish compete with male conspecifics to secure suitable nesting areas, and they display aggressive territorial behavior to defend or take control of a nest site. Thus, because these behaviors can greatly increase reproductive opportunities, sexual behavior can be linked to activities that are temporally distant from the sex act.
Domjan and associates (e.g., Domjan & Hall, 1986) described a set of behaviors that contribute to the reproductive success of Japanese quails. Males engage in general search behavior when they encounter cues distal to the female. For example, birds pace around the test chamber when they encounter a cue that has been conditioned with a long CS-US interval. This cue is relatively distal to the female because it signals that a female will appear only after a long time period elapses (Akins, Domjan, & Gutierrez, 1994). In contrast, cues conditioned with a short CS-US interval elicit focal search behavior. For instance, birds approach a red light that has previously been paired with a sexual encounter (Akins et al., 1994). This cue is relatively proximal because it signals that the female will appear after a short time period elapses. Male quails also engage in copulatory or consummatory sexual responses. These responses are elicited by cues signaling that a sexual encounter is imminent. Thus, female conspecifics or sign stimuli elicit copulatory behavior.
Domjan and his colleagues have characterized a range of stimuli that elicit an array of sexual responses in the Japanese quail. With these observations Domjan has articulated a behavioral systems account of sexual behavior that contains both a stimulus and a response dimension. Each dimension includes three categories. The response dimension includes general search behavior, focal search behavior, and copulatory behavior. The stimulus dimension includes contextual cues, local cues, and species-typical cues.
In the model, stimuli are arranged on a temporal and spatial continuum that varies by the cue’s proximity to the female quail. This continuum is similar to the spatiotemporal organization hypothesized by Timberlake (1983) in his feeding behavior system and by Fanselow (1989) in his description of defensive behavior, both discussed earlier. Prior to sexual conditioning, contextual and local cues are distal from the female and do not activate sexual behavior, whereas species-typical cues are more proximal and can elicit sexual behavior unconditionally. After a sexual conditioning event, contextual and local cues may elicit sexual behavior, and responding to species-typical cues is facilitated. Thus, according to Domjan’s view, “conditioning serves to increase the range of stimuli that are effective in eliciting sexual behavior” (Domjan, 1994, p. 426). That is, learning shifts the position of cues on the continuum by increasing their proximity to the female and thereby enhancing the cues’ability to release sexual responses.
This shift on the continuum is manifested also by the change in repertoire of responses that stimuli come to elicit. Prior to conditioning, local cues elicit weak general search behavior. After conditioning they may trigger both focal search and copulatory behavior. Additionally, the strength of general search behavior is enhanced. For example, approach behavior is a form of local search behavior. Quails display approach behavior to a red light only after the cue has been paired with a sexual encounter (Domjan et al., 1986).
In the introduction we made the point that behavior is a bidirectional interaction among motivation, learning, and genetics. Perhaps nowhere is this clearer than in sexual motivation. The work of Domjan and Hollis indicates that experience strongly influences with which members of our species we prefer to mate. Because Pavlovian conditioning determines attractiveness, it also determines which sets of genes recombine. Because conditioning determines reproductive success, measured rather directly by sperm and offspring production, it also determines what genes are best represented in the next generation of many vertebrate species. Not only does the reproductive success that drives evolution influence our learning abilities, but our learning abilities drive that reproductive success as well.
Temperature Motivation
Body temperature regulation is essential for the survival of animal species. Most species are adapted to the temperature range of their niche, and they can only maintain normal activity within a relatively narrow window of body temperature imposed by their genetic makeup. At extreme body temperatures critical enzymes cannot function, energy metabolism is compromised, and body systems fail. Thus, animals that fail to maintain body temperature within the critical range of their species die. Because of this stringent evolutionary selective pressure, species have adapted multiple strategies to cope with the problem of body temperature regulation.
Thermoregulatory Responses
Species utilize both physiological and behavioral means to cope with the environmental demands of body temperature regulation. These two categories of processes interact to provide an adequate temperature regulation strategy in each species and individual. Specific body temperature regulation strategies abound in the animal kingdom (e.g., Prosser & Nelson, 1981; Bartholomew, 1982). In this section we describe several strategies of thermoregulation that have evolved. Two broad categories of these strategies are ectothermy and endothermy. Ectothermic animals rely on environmental heat for body warming. Endothermic animals use metabolic heat for body warming. Animals belonging to these broad groups often display distinct behavioral tendencies because these strategies impose different thermoregulatory needs.
The Mountain Lizard
The South American mountain lizard (Liolamus) is both an ectotherm and a poikilotherm. Poikilotherms are ectothermic animals whose body temperature may vary widely at different times of the day or year. These animals often maintain body temperatures that exceed the environmental temperature during periods of activity, whereas they display relatively cold body temperatures during periods of inactivity. To accomplish these extremes, poikilotherms rely heavily on behavioral means to regulate body temperature. For example, Liolamus avoids freezing Andes temperatures by staying in its burrow during the night. Just after sunrise the animal emerges and moves to a position exposed to direct sunlight to absorb solar energy until its body temperature shifts from approximately 5°C to upward of 30°C. Throughout the day this lizard shuttles between sunlit and shaded microenvironments to maintain this body temperature (Bartholomew, 1982).
The Polar Bear
Polar bears live in and near the Arctic Circle. These large mammals are endotherms, and they commonly sustain activity in extreme thermal conditions that range from approximately 15°C in summer months to –30°C in winter months. Because of these drastic seasonal environmental demands, polar bears have adapted strategies that permit the animal to maintain its body temperature across the full range of environmental temperatures in its habitat.
Polar bears are genetically organized to cope with the temperature demands of their niche, and this organization is manifested in physiological adaptations. First, polar bears have a layer of blubber and fur over much of their bodies. This tissue helps insulate the animal and maintain its body temperatureinwintermonths.Second,apolarbear’ssnout,ears,nose, footpads, and inner thighs dissipate heat efficiently because they have limited insulation (Stirling, 1988). As we shall see, these physiological adaptations contribute to an effective behavioral thermoregulation scheme useful in both hot and cold environments.
As mentioned earlier, polar bears have several poorly insulated body areas, or hot spots. These hot spots are useful for behavioral thermoregulation because bears can adopt distinct postures depending on whether they need to expel or conserve heat. In warm environments, bears dissipate heat by exposing these hot spots, and in colder environments they conceal these areas (Stirling, 1988). Notice that the form of the bear’s response is sensitive to environmental temperature. This thermoregulatory scheme is fairly common among endotherms.
The Rat
Rats are small mammals that live commensally with humans. These animals populate temperate zones and also live inside burrows and buildings in cold climates. Rats are endotherms that exhibit a variety of thermoregulatory behaviors (Hainsworth & Stricker, 1970). The rat’s body temperature typically varies between 37°C and 38°C at neutral environmental temperatures (approximately 28°C). When environmental temperatures rise above this level, rats display a constellation of responses that promote metabolic efficiency and survival. For example, when environmental temperatures range between 36°C and 41°C, rats exhibit a sustained hyperthermia with a magnitude that exceeds the environmental temperature. This phenomenon is an adaptive and regulated response. Rats benefit from this increase in body temperature because it permits them to lose metabolic heat to the environment via conduction (Hainsworth & Stricker, 1970). Above 41°C rats are unable to sustain hyperthermia relative to the environment.
Rats also exhibit two behavioral responses to heat stress within the range that provokes hyperthermia (36°C to 41°C). At moderate levels of heat stress, rats frequently lay with a relaxed body posture often called prone extension. Much like the polar bear, the rat uses this behavior to dissipate heat by exposing body regions that conduct heat efficiently. In this case the rat’s tail acts as a thermal radiator because it is both vascularized and lacking in insulation. Thus, excess body heat is readily dissipated through the tail (Rand, Burton, & Ing, 1965). Along with prone extension, rats display saliva spreading in response to moderate heat stress. This behavior exploits evaporative cooling as a means to regulate body temperature (Hainsworth, 1967), and it is characterized by the active distribution of saliva from the mouth with the forelimbs. The spreading initially focuses on the head, neck, and paw regions and later targets the ventral regions with emphasis on the scrotum and tail. Saliva spreading is prevalent in animals that lack sweat glands, such as rats, opossums, and desert rodents. Other terrestrial animals, such as humans, exploit evaporative cooling by sweating.
Above approximately 41°C, rats can no longer regulate heat exchange with controlled hyperthermia. Also, the expression of a relaxed body posture gives way to a pronounced increase in activity that is probably a manifestation of escape behavior (Hainsworth, 1967). At higher temperatures, rats also exhibit saliva spreading. The adaptive advantage of this behavior is demonstrated by the observation that desalivated rats die within 1 hr to 2 hr of high heat stress, although normal rats survive for at least 5 hr of exposure (Hainsworth, 1967).
When a pregnant rat encounters inescapable heat stress, it responds with the array of thermoregulatory responses that are typical in her species. For example, the rat will engage in both body extension and saliva spreading when heat stressed (Wilson & Stricker, 1979). However, these animals face amplified thermal demands because their body mass increases relative to the size of the available thermal windows that expel body heat via conduction. Consequently, to regulate body temperature these mothers compensate by lowering their threshold for saliva spreading, and pregnant mothers display saliva spreading at 30°C (Wilson & Stricker, 1979). Similarly, the animal’s threshold for salivary secretion from the submaxillary gland decreases, thereby providing an increased saliva reservoir (Wilson & Stricker, 1979). These measures contribute to successful thermoregulation for both the mother and her offspring.
Rat mothers bear sizable litters that remain together until weaning. These pups are particularly susceptible to hypothermia because they produce little metabolic heat that is quickly lost to the environment. Moreover, pups are born with no fur and little insulation, and they do not exhibit thermogenesis via shivering behavior (Hull, 1973). Given these obstacles, rat pups may seem reliant on parental care for thermal regulation. However, when exposed to a cold environment, rat pups clump together in a manner that reduces each pups exposed body surface area. This huddling provides behavioral thermoregulation because it lessens the heat lost to the environment via conduction (Alberts, 1978).
Huddling behavior is modulated by environmental temperature. Specifically, with decreasing environmental temperature, the total surface area of the huddle diminishes. Conversely, the total surface area of the huddle increases as the environmental temperature rises (Alberts, 1978). Thus, pups act as a unit by adjusting their group’s exposed surface area in a manner that defends body temperature against environmental changes.
Individual pups follow a typical movement pattern through the huddle that contributes to the changes in the whole litter’s exposed surface area. These movements are competitive adjustments that position a pup in a thermally desirable location. In colder environments pups move toward the middle of the huddle, and in warm environments they shift to the periphery (Alberts, 1978). Collectively, these adjustments make the litter behave as an organized unit sensitive to the environmental temperature.
Fever
When mammals are infected by pathogens, they display an array of nonspecific “sickness” responses that include fever and fatigue. Traditionally, these symptoms were thought to result from an inability to perform normal activities because of the compromised physiological state of the sick individual. As an alternative, Bolles and Fanselow (1980) suggested that illness involving fever might be a particularly strong activator of the recuperative motivational system. Consistent with this speculation, investigators have recently suggested that sickness is an adaptive motivational response that aids recuperation (Aubert, 1999; Watkins & Maier, 2000). Importantly, part of the sickness response involves fever: a sustained hyperthermia. Thus, mammals actively modulate their body temperature as an adaptive response to pathogens. Fever and recuperation therefore may have some degree of positive feedback between them.
Learning and Thermoregulatory Responses
Earlier we described how animals learn to anticipate things like danger or to expect the appearance of a potential mating partner. What evidence exists that animals learn to anticipate thermal conditions? Most investigations in this realm have focused on escape behavior (e.g., Howard, 1962) or on the effects that environmental temperatures have on learning acquisition (e.g., Hack, 1933). In a typical escape procedure an animal is exposed to an aversive stimulus until it performs a response. For example, rats exposed to cold temperatures will press a bar to gain access to a heat lamp. Over trials, rats become very efficient at this response, and they often drive the ambient temperature up to room temperature. But what do the animals learn during these conditioning trials? Animals may learn that the bar pressing makes the chamber warm, but these studies provide little evidence for the notion that rats perform thermoregulatory responses because they anticipate the problem.
Very few studies demonstrate that animals will learn to perform a response that avoids hot or cold stress. Nor do many studies demonstrate that thermal cues can elicit learned CRs. Interestingly, studies that demonstrate these responses to thermal reinforcers have frequently used infant animals as subjects. For example, newborn chicks can be autoshaped to peck a bar for food (Wasserman, 1973). Newborn dogs will perform an avoidance response to avoid a cold reinforcer (Stanley, Barrett, & Bacon, 1974), and newborn rat pups exhibit tachycardia as a CR when an odor is paired with cold temperature (Martin & Alberts, 1982).
Recall that newborn animals, such as the rat pup, have little insulation and that thermoregulation requires more elaborate behavioral strategies. Perhaps we more readily observe thermal Pavlovian conditioning in the rat pup because its niche requires such learning. This suggestion may have implications for how we view thermoregulatory behavior, and it is further developed in the next section.
A Thermoregulatory Behavior System?
We have described how animals regulate body temperature with both physiological and behavioral means. Conspicuously, we have not yet provided substantial analysis of these responses. Why then would they be included in a research paper on the topic of motivation? Let us consider the traditional account of thermoregulatory behavior before we answer this question.
The Homeostatic Explanation
The concept of homeostasis has been the fundamental principle employed by traditional explanations of thermoregulatory behavior. This idea, first applied by Cannon (1932), assumes that each animal has a body temperature set point, and that thermoregulatory behavior is activated whenever the animal is perturbed from this reference. Thus, if an animal is cold, it automatically performs a series of responses to return to its set point. This explanation implies that the animal uses a “comparator” to assess the difference between its actual body temperature and its set point temperature and that whenever there is a discrepancy between these values, the system activates behaviors to remedy the discrepancy.
Santinoff (1983) provided both an eloquent review of the neural circuitry of thermoregulation and an explanation of homeostasis. The reader is advised to consult the work for both a useful historical perspective and a comprehensive analysis of the subject. Available evidence suggests that the anterior hypothalamus (AH) and the preoptic (POA) provide a significant contribution to the neural control of thermoregulatory behavior in mammals. For example, body temperature in animals with lesions to these areas has been shown to drop sharply in cold environments (e.g., Satinoff & Rutstein, 1970). Similarly, appropriate thermoregulatory responses are activated when this structure is either cooled or heated (e.g., Fusco, Hardy, & Hammel, 1961), and electrical stimulation of this region elicits prone extension (Roberts & Mooney, 1974). Additionally, the POA and AH also contain neurons that are sensitive to temperature change (Nakayama, Hammel, Hardy, & Eisenman, 1963). Thus, the AH and POA have the capacity to detect changes in temperatures; damage to this region disrupts thermoregulation; and stimulation of this region elicits appropriate responding. Together, these observations suggest that the AH and POA complex might be the neural manifestation of the comparator that detects deviance from thermal homeostasis. However, lesions to this complex do not disrupt some forms of behavioral thermoregulation. For example, rats with AH lesions are able to bar press to obtain access to a warm heat lamp in a cold environment (Satinoff & Rutstein, 1970). Thus, animals with AH lesions can both detect perturbations from their normal body temperature and perform an appropriate response to hypothermia. These and other observations argue against the hypothesis that suggests the AH and POAare the neural locus for the thermoregulatory comparator. Satinoff (1983) has developed a more sophisticated theory of thermoregulation that suggests multiple comparators linked to separate thermoregulatory behaviors and these units are organized in a hierarchical manner.
The principle of homeostatic thermoregulation suggests that regulatory responses occur whenever body temperature deviates from the set point. This homeostatic explanation does not require a motivational system, but we suggest that thermoregulation does. That is, perhaps a behavioral systems approach to thermoregulatory behavior is warranted. Let us consider several points. First, the cost of ineffective thermoregulation is significant, so there is evolutionary pressure to develop sophisticated thermoregulatory schemes. Second, numerous animal species have adapted elaborate behavioral strategies that assist in thermoregulation. Ectotherms rely almost entirely on behavioral means. Other animals, such as the rat, display an array of thermoregulatory behaviors that could be organized on a continuum of relative heat stress. Indeed, these behaviors seem to vary with the rat’s niche, as neonates display a different repertoire than do adults. Third, some responses to heat stress are incompatible with the “homeostatic” account of thermoregulation. For example, rats display a controlled hyperthermia response under conditions of heat stress, and mammals exhibit fever when they are infected by pathogens. These responses actively increase the discrepancy in body temperature from the animal’s set point. Thus, these responses are incompatible with the concept of a homeostasis unless resetting the reference temperature is a valid means at achieving homeostasis. Fourth, infant animals provide the best examples of learning in relation to thermal cues. These animals must cope with thermal challenge in their niche. Perhaps we detect their ability to learn about thermal cues because learning about these cues is critical to their survival. Conceivably, many animals in many systems can learn about thermal cues, and we have not detected them only because the homeostatic thermoregulatory explanation ignores the relevance of learning.
In summary, thermoregulation is crucial to survival in perhaps every niche, and many behavioral responses have been developed to cope with the problem. Given the cost of poor thermoregulation and the propensity for animals to learn and adapt, we propose that the study of thermoregulatory behavior may profit by adopting a behavior systems approach.
Conclusions
We began this reseach paper by suggesting that motivation accounts for that proportion of the variation in behavior not accounted for by learned and genetic influences. Why is it that an animal in the same environment presented with the same food will eat on one occasion and not on another? Given that genetic influences have been held constant and that no new information has been learned about the food or the environment, this variation must be due to changes in motivation manifested through changes in behavior. The challenge with defining motivation is to avoid merely redescribing the behavior in new and empirically intractable terms. The method we have suggested for avoiding this problem is to specify the environmental cause and behavioral effect of any changes in the hypothesized motivational construct. By defining these antecedents and consequences in terms of the ecological and evolutionary problems the animal must solve, we protect ourselves from explanations that assume an unlimited number of “motivations,” as did the old theories of instinct. In addition, this focus on the functional aspects of motivational processes forces us to consider both the ecological niche that the animal occupies and the organization of the behaviors it uses to cope with the problems of the niche.
This explicitly ecological view allows the concept of motivation to make contact with behavioral ecology and evolution. Learning and genetics are not the sole determinants of behavior; an animal’s ecological niche must also be considered. Animals have evolved solutions to specific environmental problems, and an understanding of these relationships can inform psychological theories of motivation and learning. Collier and Johnson (1990) suggested that appreciating that small predators are themselves potential prey gives insight into the differences in feeding rate between small and large predators. Indeed, Fanselow et al. (1988) have demonstrated that predatory risk is an important determinant in the initiation of feeding behavior. Traditional homeostatic perspectives could not contribute this insight.
In addition to highlighting the importance of ecological variables in determining motivational influences on behavior, the analyses presented in this research paper can also be used to examine similarities and differences between motivational systems. A persistent theoretical problem in theories of motivation has been specifying the number and form of motivational processes with which an animal is equipped. We have suggested that the animal is equipped with as many motivational systems as there are classes of problems in the environment for it to solve. We expect that the reader has been struck by the amount of similarity between the response organizations proposed to account for feeding and sexual behavior, and to a lesser extent between those structures and that proposed to account for the organization of defensive behavior. Each consists of a collection of motivational modes organized by some kind of imminence continuum. Each includes a set of preexisting stimulus processing and response production tendencies. The extent to which these similarities are valid remains to be determined, and this question deserves study. Just as interesting are those disparities between the response organizations. Appetitive behavior in the feeding behavior system is extremely flexible. Flexibility in sexually motivated appetitive behavior has also been demonstrated but is much less well investigated. In contrast, defensive behavior seems more rigid, perhaps due to the inherently conservative nature of defense.
The behavioral systems view suggests that motivation is a much more complex phenomenon than that described by theories of drive, incentive motivation, or opposing affective states. Any complete conception must include physiological, psychological, ecological, and evolutionary factors. Our approach attempts to address these requirements.
Bibliography:
- Akins, C. K., Domjan, M., & Gutierrez, G. (1994). Topography of sexually conditioned behavior in male Japanese quail (Coturnix japonica) depends on the CS-US interval. Journal of Experimental Psychology: Animal Behavior Processes, 20, 199–209.
- Alberts, J. R. (1978). Huddling by rat pups: Group behavioral mechanisms of temperature regulation and energy conservation. Journal of Comparative & Physiological Psychology, 92, 231–245.
- Aubert, A. (1999). Sickness and behaviour in animals: A motivational perspective. Neuroscience & Biobehavioral Reviews, 23, 1029–1036.
- Balleine, B. (1992). Instrumental performance following a shift in primary motivation depends upon incentive learning. Journal of Experimental Psychology: Animal Behavior Processes, 18, 236–250.
- Balleine, B., Garner, C., Gonzalez, F. & Dickinson, A. (1995). Motivational control of heterogeneous instrumental chains. Journal of Experimental Psychology: Animal Behavior Processes, 21, 203–217.
- Bandler, R., & Depaulis, A. (1991). Midbrain periaqueductal gray control of defensive behavior in the cat and rat. In A. Depauilis & R. Bandler (Eds.), The midbrain periaqueductal gray matter: Functional, anatomical and immunohistochemical organization (pp. 175–199). New York: Plenum Press.
- Bartholomew, G. A. (1982). Body temperature and energy metabolism. In M. A. Gordon (Ed.), Animal physiology (pp. 333–406). New York: Macmillan.
- Blanchard, R. J., & Blanchard, D. C. (1972). Effects of hippocampal lesions on the rat’s reaction to a cat. Journal of Comparative & Physiological Psychology, 78, 77–82.
- Blanchard, R. J., & Blanchard, D. C. (1989). Antipredator defensive behaviors in a visible burrow system. Journal of Comparative Psychology, 103, 70–82.
- Blanchard, R. J., Fukunaga, K. K., & Blanchard, D. C. (1976). Environmental control of defensive reactions to a cat. Bulletin of the Psychonomic Society, 8, 179–181.
- Blanchard, R. J., Mast, M., & Blanchard, D. C. (1975). Stimulus control of defensive reactions in the albino rat. Journal of Comparative & Physiological Psychology, 88, 81–88.
- Blanchard, R. J., Nikulina, J. N., Sakai, R. R., McKittrick, C., McEwen, B., & Blanchard, D. C. (1998). Behavioral and endocrine change following chronic predatory stress. Physiology & Behavior, 63, 561–569.
- Bolhuis, J. J., Hetebrij, E., Den Boer-Visser, A. M., De Groot, H., & Zijlstra, G. G. (2001). Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. European Journal of Neuroscience, 13, 2165–2170.
- Bolles, R. C. (1970). Species-specific defense reactions and avoidance learning. Psychological Review, 77, 32–48.
- Bolles, R. C. (1975). Theory of motivation. New York: Harper and Row.
- Bolles, R. C. (1980). Some functional thoughts about regulation. In F. M. Toates & T. R. Halliday (Eds.), Analysis of motivational processes, (pp. 62–76). London: Academic Press.
- Bolles, R. C., & Fanselow, M. S. (1980). A perceptual-defensiverecuperative model of fear and pain. Behavioral & Brain Sciences, 3, 291–323.
- Bolles, R. C., & Riley, A. L. (1973). Freezing as an avoidance response: Another look at the operant-respondent distinction. Learning & Motivation, 4, 268–275.
- Bouton, M. E., Mineka, S., & Barlow, D. H. (2001). Amodern learning theory perspective on the etiology of panic disorder. Psychological Review, 108, 4–32.
- Breland, K., & Breland, M. (1961). The misbehavior of organisms. American Psychologist, 16, 681–684.
- Breland, K., & Breland, M. (1966). Animal behavior. New York: Academic Press.
- Brett, L. P., Hankins, W. G., & Garcia, J. (1976). Prey-lithium aversions: Vol. 3. Buteo hawks. Behavioral Biology, 17, 87–98.
- Brown, S., & Schaffer, A. (1886). An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society of London, 179, 303–327.
- Cabanac, M. (1971). Physiological role of pleasure. Science, 173, 1103–1107.
- Cannon, W. B. (1932). The wisdom of the body. New York: W. W. Norton.
- Carbaugh, B. T., Schein, M. W., & Hale, E. B. (1962). Effects of morphological variations of chicken models on sexual responses of cocks. Animal Behaviour, 10, 235–238.
- Castonguay, T. W., Kaiser, L., & Stern, J. S. (1986). Meal pattern analysis:Artifacts,assumptionsandimplications.BrainResearch Bulletin, 17, 439–443.
- Collier, G. H. (1982). Determinants of choice. In D. J. Bernstein (Ed.), Nebraska symposium on motivation: Vol. 3. Response structure and motivation (pp. 69–127). Lincoln: University of Nebraska Press.
- Collier, G. H. (1983). Life in closed economy: The ecology of learningandmotivation.InM.D.Zeiler&P.Harzem(Eds.),Advances in the analysis of behavior (Vol. 3, pp. 223–274). Chichester, UK: Wiley.
- Collier, G. H. (1986). The dialogue between the house economist and the resident physiologist. Nutrition and Behavior, 3, 9–26.
- Collier, G. H. (1987). Operant methodologies for studying feeding and drinking. In F. Toates & N. Rowland (Eds.), Methods and techniques for studying feeding and drinking behavior (pp. 37–76). Amsterdam: Elsevier.
- Collier, G. H., Hirsch, E., & Hamlin, P. H. (1972). The ecological determinants of reinforcement in the rat. Physiology & Behavior, 9, 705–716.
- Collier, G. H., & Johnson, D. F. (1990). The time window of feeding. Physiology & Behavior, 48, 771–777.
- Collier, G. H., & Johnson, D. F. (1997). Who is in charge? Animal versus experimenter control. Appetite, 29, 159–180.
- Collier, G. H., Johnson, D. F., Borin, G., & Mathis, C. E. (1994). Drinkinginapatchyenvironment:Theeffectofthepriceofwater. Journal of the Experimental Analysis of Behavior, 63, 169–184.
- Collier, G. H., Johnson, D. F., & Mitchell, C. (1999). The relation between meal size and the time between meals: effects of cage complexity and food cost. Physiology & Behavior, 67, 339–346.
- Coss, R. G., & Owings, D. H. (1978). Snake-directed behavior by snake naive and experienced California ground squirrels in a simulated burrow. Zeitschrift fuer Tierpsychologie, 48, 421–435.
- Craig, W. (1918). Appetites and aversions as the constituents of instincts. Biological Bulletin of Marine Biology, Woods Hole, MA, 34, 91–107.
- Davidson, T. L. (1998). Hunger cues as modulatory stimuli. In N. A. Schmajuk & P. C. Hollands (Eds.), Occasion setting: Associative learning and cognition in animals (pp. 223–248). Washington, DC: American Psychological Association.
- Davis, M. (1986). Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behavioral Neuroscience, 100, 814–824.
- Di Cesnola, A. P. (1904). Preliminary note on the protective value of colour in Mantis religiosa. Biometrika, 3, 58–59.
- Dickinson, A., & Balleine, B. (1994). Motivational control of goaldirected action. Animal Learning & Behavior, 22, 1–18.
- Dickinson, A., Balleine, B., Watt, A., Gonzalez, F., & Boakes, R. A. (1995). Motivational control after extended instrumental training. Animal Learning & Behavior, 23, 197–206.
- Domjan, M. (1994). Formulation of a behavior system for sexual conditioning. Psychonomic Bulletin & Review, 1, 421–428.
- Domjan, M., Blesbois, E., & Williams, J. (1998). The adaptive significance of sexual conditioning: Pavlovian control of sperm release. Psychological Science, 9, 411–415.
- Domjan, M., Greene, P., & North, N. C. (1989). Contextual conditioning and the control of copulatory behavior by species-specific sign stimuli in male Japanese quail. Journal of Experimental Psychology: Animal Behavior Processes, 15, 147–153.
- Domjan, M., & Hall, S. (1986). Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): Male behavior. Journal of Comparative Psychology, 100, 59–67.
- Domjan, M., & Hollis, K. L. (1988). Reproductive behavior: A potential model system for adaptive specializations in learning. In R. C. Bolles & M. D. Beecher (Eds.), Evolution and learning (pp. 213–237). Hillsdale, NJ: Erlbaum.
- Domjan, M., Lyons, R., North, N. C., & Bruell, J. (1986). Sexual Pavlovian conditioned approach behavior in male Japanese quail (Coturnix coturnix japonica). Journal of Comparative Psychology, 100, 413–421.
- Domjan, M., O’Vary, D., & Greene, P. (1988). Conditioning of appetitive and consummatory sexual behavior in male Japanese Quail. Journal of the Experimental Analysis of Behavior, 50, 505–519.
- Edmunds, M. (1974). Defence in animals: A survey of anti-predator defences. Burnt Mill, UK: Longman.
- Estes, R. D. (1967a). Predators and scavengers: Part 1. Natural History N. Y., 76, 20–29.
- Estes, R. D. (1967b). Predators and scavengers: Part 2. Natural History N. Y., 76, 38–47.
- Estes, W. K., & Skinner, B. F. (1941). Some quantitative properties of anxiety. Journal of Experimental Psychology, 29, 390–400.
- Fanselow, M. S. (1980). Conditional and unconditional components of post-shock freezing in rats. Pavlovian Journal of Biological Sciences, 15, 177–182.
- Fanselow, M. S. (1986). Associative vs. topographical accounts of the immediate-shock freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning & Motivation, 17, 16–39.
- Fanselow, M. S. (1989). The adaptive function of conditioned defensive behavior: An ecological approach to Pavlovian stimulus-substitution theory. In R. J. Blanchard & P. F. Brain (Eds.), Ethoexperimental approaches to the study of behavior (pp. 151–166). Norwell, MA: Kluwer.
- Fanselow, M. S. (1991). The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In A. Depaulis & R. Bandler (Eds.), The midbrain periaqueductal gray matter: Functional, anatomical and immunohistochemical organization (pp. 151–173). New York: Plenum.
- Fanselow, M. S. (1994). Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review, 1, 429–438.
- Fanselow, M. S., & LeDoux, J. E. (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron, 23, 229–232.
- Fanselow, M., & Lester, L. (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In R. C. Bolles & M. D. Beecher (Eds.), Evolution and learning (pp.185–211). Hillsdale, NJ: Erlbaum.
- Fanselow, M. S., Lester, L. S., & Helmstetter, F. J. (1988). Changes in feeding and foraging patterns as an antipredator defensive strategy: A laboratory simulation using aversive stimulation in a closed economy. Journal of the Experimental Analysis of Behavior, 50, 361–374.
- Fanselow, M. S., & Sigmundi, R. A. (1982). The enhancement and reduction of defensive fighting by naloxone pretreatment. Physiological Psychology, 10, 313–316.
- Fanselow, M. S., Sigmundi, R. A., & Williams, J. L. (1987). Response selection and the hierarchical organization of speciesspecific defense reactions: The relationship between freezing, flight, and defensive burying. Psychological Record, 37, 381– 386.
- Fendt, M., & Fanselow, M. S. (1999). The neuroanatomical basis of conditioned fear. Neuroscience & Biobehavioral Reviews, 23, 743–760.
- Foree, D. D., & Lolordo, V. M. (1973). Attention in the pigeon: differential effects of food-getting versus shock-avoidance procedures. Journal of Comparative & Physiological Psychology, 85, 551–558.
- Freud, S. (1953). Instincts and their vicissitudes. In J. Strachey (Ed.), The Standard edition of the complete psychological works of Sigmund Freud (Vol. 14, pp. 109–140). London: Hogarth Press.
- Fusco, M. M., Hardy, J. D., & Hammel, H. T. (1961). Interaction of central and peripheral factors in physiological temperature regulation. American Journal of Physiology, 200, 572–580.
- Garcia, J. (1989). Food for Tolman: Cognition and cathexis in concert. In T. Archer & L.-G. Nilsson (Eds.), Aversion, avoidance, and anxiety: Perspectives on aversively motivated behavior (pp. 45–85). Hillsdale NJ: Erlbaum.
- Garcia, J., & Koelling, R. A. (1966). Relation of cue to consequence in avoidance learning. Psychonomic Science, 4, 123–124.
- Garcia y Robertson, R., & Garcia, J. (1985). X-rays and learned taste aversions: Historical and psychological ramifications. In T. G. Burish, S. M. Levy, & B. E. Meyerowitz (Eds.), Cancer, nutrition and eating behavior: A biobehavioral perspective (pp. 11–41). Hillsdale NJ: Erlbaum.
- Gewirtz, J. C., & Davis, M. (1997). Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature, 388, 471–474.
- Gibson, E. J. (1952). The role of shock in reinforcement. Journal of Comparative & Physiological Psychology, 45, 18–30.
- Goldthwaite, O., Coss, R. G., & Owings, D. H. (1990). Evolutionary dissipation of an antisnake system: Differential behavior by California and Arctic ground squirrels in above- and belowground contexts. Behaviour, 112, 246–269.
- Guinard, J., & Brun, P. (1998). Sensory-specific satiety: Comparison of taste and texture effects. Appetite, 31, 141–157.
- Hack, E. R. (1933). Learning as a function of water temperature. Journal of Experimental Psychology, 16, 442–445.
- Hainsworth, F. R. (1967). Saliva spreading, activity, and body temperature regulation in the rat. American Journal of Physiology, 212, 1288–1292.
- Hainsworth, F. R., & Stricker, E. M. (1970). Salivary cooling in rats in the heat. In A. P. Gagge & J. A. Stolwijk (Eds.), Physiological and behavioral temperature regulation (pp. 611–626). Springfield, IL: Charles C. Thomas.
- Hirsch, S. M., & Bolles, R. C. (1980). On the ability of prey to recognize predators. Zeitschrift fuer Tierpsychologie, 54, 71–84.
- Hirsch, E., & Collier, G. (1974). The ecological determinants of reinforcement in the guinea pig. Physiology & Behavior, 12, 239–249.
- Hogan, J. A. (1973a). Development of food recognition in young chicks: Vol. 1. Maturation and nutrition. Journal of Comparative & Physiological Psychology, 83, 355–366.
- Hogan, J. A. (1973b). Development of food recognition in young chicks: Vol. 2. Learned association over long delays. Journal of Comparative & Physiological Psychology, 83, 367–373.
- Hogan, J. A. (1977). Development of food recognition in young chicks: Vol. 4. Associative and nonassociative effects of experience. Journal of Comparative & Physiological Psychology, 91, 839–850.
- Holland, P. C. (1981). Acquisition of representation-mediated conditioned food aversions. Learning & Motivation, 12, 1–18.
- Hollis, K. L., Cadieux, E. L., & Colbert, M. M. (1989). The biological function of Pavlovian conditioning: Amechanism for mating success in the blue gourami (Trichogaster trichopterus). Journal of Comparative Psychology, 103, 115–121.
- Hollis, K. L., Pharr, V. L., Dumas, M. J., Britton, G.B., & Field, J. (1997). Classical conditioning provides paternity advantage for territorial male blue gouramis (Trichogaster trichopterus). Journal of Comparative Psychology, 111, 219–225.
- Howard, T. C. (1962). Conditioned temperature drive in rats. Psychological Reports, 10, 371–373.
- Hull, C. L. (1943). Principles of behavior: An introduction to behavior theory. New York: Appleton-Century-Crofts.
- Hull, D. (1973). Thermoregulation in young mammals. In C. G. Whittow (Ed.), Comparative physiology of thermoregulation. (Vol. 3, pp. 167–200). New York: Academic Press.
- Jacobs, W. J., & LoLordo, V. M. (1980). Constraints on Pavlovian aversive conditioning: Implications for avoidance learning in the rat. Learning & Motivation, 11, 427–455.
- Johnson, D. F., & Collier, G. (1989). Patch choice and meal size of foraging rats as a function of the profitability of food. Animal Behaviour, 38, 285–297.
- Kapp, B. S., Frysinger, R. C., Gallagher, M., & Haselton, J. R. (1979) Amygdala central nucleus lesions: Effect on heart rate conditioning in the rabbit. Physiology & Behavior, 23, 1109– 1117.
- Kaufmann, L. W., Collier, G., Hill, W., & Collins, K. (1980). Meal cost and meal patterns in an uncaged domestic cat. Physiology & Behavior, 25, 135–137.
- Kluver, H., & Bucy, P. C. (1939). Preliminary analysis of the temporal lobes in monkeys. Biological Psychiatry, 42, 461–471.
- Konorski, J. (1967). Integrative activity of the brain: An interdisciplinary approach. Chicago: University of Chicago Press.
- Le Magnen, J. (1992). Neurobiology of feeding and nutrition. New York: Academic Press.
- Le Magnen, J., & Devos, M. (1980). Parameters of the meal pattern in rats: their assessment and physiological significance. Neuroscience & Biobehavioral Reviews, 4, 1–11.
- Lester, L. S., & Fanselow, M. S. (1985). Exposure to a cat produces opioid analgesia in rats. Behavioral Neuroscience, 99, 756–759.
- Leyhausen, P. (1979). Cat behavior: The predatory and social behavior of domestic and wild cats. New York: Garland.
- Lorenz, K. (1937). The establishment of the instinct concept. Die Naturwissenschaften, 25, 280–300.
- Maren, S. (1998). Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. Journal of Neuroscience, 18, 3088–3097.
- Martin, L. T., & Alberts, J. R. (1982). Associative learning in neonatal rats revealed by cardiac response patterns. Journal of Comparative & Physiological Psychology, 96, 668–675.
- Mineka, S., & Cook, M. (1988). Social learning and the acquisition of snake fear in monkeys. In T. R. Zentall & B. G. Galef, Jr. (Eds.), Social learning: Psychological and biological perspectives (pp. 51–73). Hillsdale, NJ: Erlbaum.
- Modaresi, H. A. (1982). Defensive behavior of the rat in a shock prod situation: Effects of the subjects location on preference. Animal Learning and Behavior, 10, 97–102.
- Mook, D. G. (1987). Motivation: the organization of action. New York: W. W. Norton.
- Morato, S., Johnson, D. F., & Collier, G. (1995). Feeding patterns of rats when food-access cost is alternately low and high. Physiology & Behavior, 57, 21–26.
- Morris, D. (1958). The reproductive behavior of the ten-spined stickleback (Pygosteus pungitius L.). Behaviour, 61, 1–154.
- Mowrer, O. H., & Lamoreaux, R. R. (1946). Fear as an intervening variable in avoidance conditioning. Journal of Comparative Psychology, 39, 29–50.
- Nader, K., Schafe, G. E., & LeDoux, J. E. (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature, 406, 722–726.
- Nakayama, T., Hammel, H. T., Hardy, J. D., & Eisenman, J. S. (1963). Thermal stimulation of electrical single unit of the preoptic region. American Journal of Physiology, 204,
- Panksepp, J. (1974). Hypothalamic regulation of energy balance and feeding behavior. Federation Proceedings, 33, 1150–1165.
- Pavlov, I. P. (1927). Conditioned reflexes. Oxford: Oxford University Press.
- Pfaff, D. W., & Pfaffman, C. (1969). Behavioral and electrophysiological responses of male rats to female rat urine odors. In C. Pfaffmann (Ed.), Olfaction and taste (pp. 258–267). New York: Rockefeller University Press.
- Pinel, J. P., & Mana, M. (1989). Adaptive interactions of rats with dangerous inanimate objects: Support for a cognitive theory of defensive behavior. In R. J. Blanchard & P. F. Brain (Eds.), Ethoexperimental approaches to the study of behavior (pp. 137–150). Norwell, MA: Kluwer.
- Pinel, J. P., Mana, M. J., & Ward, J. A. (1989). Stretched-approach sequences directed at a localized shock source by Rattus norvegicus. Journal of Comparative Psychology, 103, 140–148.
- Pinel, J. P., & Treit, D. (1978). Burying as a defensive response in rats. Journal of Comparative & Physiological Psychology, 92, 708–712.
- Potegal, M., Huhman, K., Moore, T., & Meyerhoff, J. (1993). Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus). Behavioral & Neural Biology, 60, 93–102.
- Prosser, C. L., & Nelson, D. O. (1981). The role of nervous systems in temperature adaptation to poikilotherms. Annual Review of Physiology, 3, 281–300.
- Quirk, G. J., Repa, C., & LeDoux, J. E. (1995). Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron, 15, 1029–39.
- Rand, R. P., Burton, A. C., & Ing, T. (1965). The rail of the rat in temperature regulation and acclimatization. Canadian Journal of Physiology and Pharmacology, 43, 257–267.
- Raynor, H. A., & Epstein, L. H. (2001). Dietary variety, energy regulation, and obesity. Psychological Bulletin, 127, 325–341.
- Richter, C. P. (1927). Animal behavior and internal drives. Quarterly Review of Biology, 2, 307–343.
- Rizvi, T. A., Ennis, M., Behbehani, M. M., & Shipley, M. T. (1991). Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. Journal of Comparative Neurology, 303, 121–31.
- Roberts, W. W., & Mooney, R. D. (1974). Brain areas controlling thermoregulatory grooming prone extension, locomotion, and tail vasodilation in rats. Journal of Comparative & Physiological Psychology, 86, 470–480.
- Rodgers, W. L. (1967). Specificity of specific hungers. Journal of Comparative and Physiological Psychology, 64, 49–58.
- Rolls, B. J. (1979). How variety and palatability can stimulate appetite. Nutrition Bulletin, 5, 78–86.
- Rolls, B. J., Laster, L. J., & Summerfelt, A. T. (1991). Meal order reversal: Effects of eating a sweet course first or last. Appetite, 16, 141–148.
- Rolls, E. T., & Rolls, J. H. (1997). Olfactory sensory-specific satiety in humans. Physiology & Behavior, 61, 461–473.
- Rolls, B. J., Rowe, E. T., & Rolls, E. T. (1982). How sensory properties of food affect human feeding behavior. Physiology & Behavior, 29, 409–417.
- Rosen, J. B., Fanselow, M. S., Young, S. L., Sitcoske, M., & Maren, S. (1998). Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research, 796, 132–142.
- Rusiniak, K. W., Hankins, W. G., Garcia, J., & Brett, L. P. (1979). Flavor-illness aversions: Potentiation of odor by taste in rats. Behavioral & Neural Biology, 25, 1–17.
- Satinoff, E. (1983). A reevaluation of the concept of the homeostatic organization of temperature regulation. In E. Satinoff & P. Teitelbaum (Eds.), Handbook of behavioral neurobiology: Vol. 6. Motivation (pp. 443–472). New York: Plenum Press.
- Satinoff, E., & Rutstein, J. (1970). Behavioral thermoregulation in rats with anterior hypothalamic lesions. Journal of Comparative & Physiological Psychology, 71, 77–82.
- Schaller, G. B. (1966). The tiger and its prey. Natural History N. Y., 75, 30–37.
- Sclafani, A., & Springer, D. (1976). Dietary obesity in adult rats: Similarities to hypothalamic and human obesity syndromes. Physiology & Behavior, 17, 461–471.
- Seligman, M. E., & Hager, J. L. (1972). Biological boundaries of learning. East Norwalk, CT: Appleton-Century-Crofts.
- Sherrington, C. S. (1906). The integrative action of the nervous system. New York: Scribner.
- Sigmundi, R. A. (1997). Performance rules for problem-specific defense reactions. In M. E. Bouton & M. S. Fanselow (Eds.), Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles (pp. 305–319). Washington, DC: American Psychological Association.
- Silva, F. J., Timberlake, W., & Gont, R. S. (1998). Spatiotemporal characteristics of serial CSs and their relation to search modes and response form. Animal Learning & Behavior, 26, 299–312.
- Staddon, J. E., & Simmelhag, V. L. (1970). The “supersitition” experiment: A reexamination of its implications for the principles of adaptive behavior. Psychological Review, 78, 3–43.
- Stanley, W. C., Barrett, J. E., & Bacon, W. E. (1974). Conditioning and extinction of avoidance and escape behavior in neonatal dogs. Journal of Comparative & Physiological Psychology, 87, 163–172.
- Stirling, I. (1988). Polar bears. Ann Arbor: University of Michigan Press.
- Swanson, L. W., & Petrovich, G. D. (1998). What is the amygdala? Trends in Neurosciences, 21, 323–331.
- Timberlake, W. (1983). The functional organization of appetitive behavior: Behavior systems and learning. In M. D. Zeiler & P. Harzem (Eds.), Advances in the analysis of behavior: Vol. 3. Biological factors in learning (pp. 177–221). Chichester, UK: Wiley.
- Timberlake, W. (1990). Natural learning in laboratory paradigms. In D. A. Dewsbury (Ed.), Contemporary issues in comparative psychology (pp. 31–54). Sunderland, MA: Sinauer.
- Timberlake, W. (1993). Behavior systems and reinforcement: An integrative approach. Journal of the Experimental Analysis of Behavior, 60, 105–128.
- Timberlake, W. (1994). Behavior systems, associationism and Pavlovian conditioning. Psychonomic Bulletin & Review, 1, 405–420.
- Timberlake, W. (1997). An animal-centered, causal-system approach to the understanding and control of behavior. Applied Animal Behavior Science, 53, 107–129.
- Timberlake, W. (2001). Integrating niche-related and general process approaches in the study of learning. Behavioural Processes, 54, 79–94.
- Timberlake, W., & Fanselow, M. S. (1994). Symposium on behavior systems: Learning, neurophysiology, and development. Psychonomic Bulletin & Review, 1, 403–404.
- Timberlake, W., & Lucas, G. A. (1989). Behavior systems and learning: From misbehavior to general principles. In S. B. Klein & R. R. Mowrer (Eds.), Contemporary learning theory and the impact of biological constraints on learning (pp. 237–275). Hillsdale, NJ: Erlbaum.
- Timberlake, W., & Silva, K. M. (1995).Appetitive behavior in ethology, psychology and behavior systems. In N. Thompson (Ed.), Perspectivesinethology(pp.211–253).NewYork:PlenumPress.
- Timberlake, W., & Washburne, D. L. (1989). Feeding ecology and laboratory predatory behavior toward live and artificial moving prey in seven rodent species. Animal Learning & Behavior, 17, 1–10.
- Tinbergen, N. (1951). The study of instinct. Oxford, UK: Clarendon Press.
- Tolman, E. C. (1949). The nature and function of wants. Psychological Review, 56, 357–369.
- Valle, F. P. (1970). Effects of strain, sex, and illumination on openfield behavior of rats. American Journal of Psychology, 83, 103–111.
- Van der Poel, A. M. (1979). A note on “stretched attention,” a behavioural element indicative of an approach-avoidance conflict in rats. Animal Behaviour, 27, 446–450.
- Walker, D. L., & Davis, M. (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience, 17, 9375–9383.
- Wallace, K. J., & Rosen, J. B. (2000). Predator odor as an unconditioned fear stimulus in rats: Elicitation of freezing by trimethylthiazoline, a component of fox feces. Behavioral Neuroscience, 114, 912–922.
- Wallace, K. J., & Rosen, J. B. (2001). Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned but not unconditioned fear of a predator odor: Comparison with electrolytic lesions. Journal of Neuroscience, 21, 3619–3627.
- Wasserman, E. A. (1973). Pavlovian conditioning with heat reinforcement produces stimulus-directed pecking in chicks. Science, 181, 875–877.
- Watkins, L. R., & Maier, S. F. (2000). The pain of being sick: Implications of immune-to-brain communication for understanding pain. Annual Review of Psychology, 51, 29–57.
- Weiskrantz, L. (1956). Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative & Physiological Psychology, 49, 381–391.
- Wilson, N. E., & Stricker, E. M. (1979). Thermal homeostasis in pregnant rats during heat stress. Journal of Comparative & Physiological Psychology, 93, 585–594.




