Sample Animal Memory and Cognition Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Animal cognition is of concern not only to psychologists but to numerous other scientists in diverse fields. It may be said that there has been an explosion of interest in animal cognition in recentyears.Two of the major but independent factors responsible for this increase in interest are a dissatisfaction with “simpler” associative approaches to animal behavior and the application of evolutionary thinking to an increasing number of problem areas. Rejecting associationism is not new (see, e.g., Lashley, 1951). Nor is applying evolution to cognition new, Darwin (1871) himself being a devotee of that approach. Increasingly, however, biologists and psychologists, among others, are turning to the study of animal behavior, if not animal cognition, within the context of evolution.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Behaviorism was an early dominant movement in American psychology. It suggested that the subject matter of psychology was behavior, and that behavior was best investigated employing animals, particularly in learning situations. Moreover, behavior was to be explained by eschewing mental states as explanatory devices while emphasizing learned associations, particularly associations between stimuli and responses. Two prominent exceptions to these more or less orthodox behavioristic views were those of Edward Chase Tolman (1932) and B. F. Skinner (1938). Tolman (1948) saw himself as a cognitive or purposive behaviorist and considered forms of explanations in addition to associations— for example, cognitive representations such as maps of spatial relations in the environment. For Skinner (a radical behaviorist), on the other hand, even associations were too mentalistic: Skinner rejected all forms of mentalistic explanation. Clark Hull’s (1943) form of behaviorism, developed from the 1930s to the 1950s, was quite popular. In Hull’s system, internal processes mediated between external stimuli and overt responses, but mediational events were not mental states. Rather, they were internal stimuli (e.g., stimuli arising from response feedback) and fractional forms of overt responses (e.g., small chewing movements).
Two basic learning processes were favored by the early behaviorists; these processes remain popular today. In one, Pavlovian conditioning, stimuli are presented without regard to the animal’s behavior. For example, a tone might be presented for a brief period, followed by food. Learning would be indexed by salivation, initially elicited by food, but later occurring to the tone. Interestingly, many Pavlovian phenomena obtained in birds and rats appear to take a similar form in humans (e.g., Wasserman & Berglan, 1998). In the other popular procedure, instrumental conditioning, reinforcement is contingent on responding in the presence of some stimulus. For example, a tone might signal a hungry rat to receive food by pressing a bar.
In the 1970s there arose within animal psychology a renewed concern with animal cognition (see, e.g., Hulse, Fowler, & Honig, 1978). This movement had several characteristics worth mentioning. It was concerned with problems not emphasized, or even recognized, within the broad conventional behavioristic approach to animal behavior—for example, how animals manage to get from one place to another (spatial learning). It was also concerned in considerable part with either augmenting, or in some cases replacing (see, e.g., Hulse & Dorsky, 1977), interpretations that stress associations between stimuli and responses with more cognitively slanted views. For example, in learning to go from one spatial location to another, do animals form a representation or map of the environment à la Tolman’s cognitive map? Finally, it was concerned with investigation in animals’ problems often investigated in people, for example, concept learning, list learning, numerical abilities, and so on.
Although the previously described approach to animal cognition has produced much in the way of useful data and theory, it can be said to be incomplete in some important respects. For one, the approach tended to emphasize behaviors acquired on the basis of an individual animal’s experience. Accordingly, it tended to ignore interesting behaviors shared by most (if not all) members of a particular species that appear to have relatively little in common in the way of a learning component. As we shall see, many such behaviors are controlled by internal mind-brain states normally associated with behaviors that are commonly classified as cognitive. In considering such species-characteristic behaviors, it would be well to keep in mind that all behaviors are the result of an interaction of environmental and genetic components. Progress in understanding animal cognition, if not cognition generally, may have much to gain by better understanding the processes controlling the behavior of sonar (for example) using bats, dancing bees, and bower-building birds, to mention only a few species that display interesting speciesspecific behaviors.
Other movements arising outside orthodox psychology have contributed substantially to our understanding of animal behavior and cognition. These include ethology, cognitive ethology, and evolutionary biology and psychology. Ethology, at its inception in the 1930s, was initially concerned with investigating the so-called “species-typical behavior” of animals in their normal environments in the wild. As ethology developed, it subsequently came to embrace laboratory studies as well, at least in some instances.An example of an initial concern in ethology would include filial imprinting in, say, ducklings, in which baby ducklings learn to follow their parents and parents learn to recognize their own progeny (e.g., Hoffman, 1978). As an example of the subsequent laboratory concern, we could mention lab studies of song acquisition in various species of birds (e.g., Marler, 1987; Marler & Peters, 1989). Both sorts of studies have contributed to our understanding of animal behavior. For example, the imprinting studies indicate that receptivity to certain classes of events has a developmental basis. The song-learning studies indicate, among other things, that some bird species can more easily learn the songs of their own species than those of some other species.
Cognitive ethology, influenced considerably by the work of the biologist Donald Griffin (1992), who pioneered work on echolocation in bats, emphasized (contrary to behaviorism) animal consciousness, awareness, and intentions. For example, when a plover leads a fox away from its nest and eggs by dragging a wing on the ground and then flies away vigorously when the fox is some distance from the nest, does the bird knowingly intend to deceive the fox? According to Griffin (1992), in stark disagreement with Skinner, a proper understanding of animal behavior necessarily entails inquiring into questions of subjective awareness. Consider the bat myotis. While cruising for food at night it emits ultrasonic vocalizations in particular directions. At cruising speed it emits about 10 clicks/s. On detecting an insect the bat homes in on its prey, raising its clicking rate to as many as 200 clicks/s. As the bat emits pulses at such high rates and considerable intensity, it in effect turns off its ears as the sound goes out— otherwise its ears would be injured. The bat’s ear muscles relax at the cessation of outputting the pulses so as to be sensitive to the returning echo. This process of send signal (tense muscles) and receive signal (relax muscles) can go as high as 50 cycles/s. A bat can determine the distance of its prey as well as its direction of movement, and can distinguish its own cries from those of its numerous hunting companions. Some insects have developed the capacity to take evasive action when detected by the bats, by going into dives and the like, yet they are often captured nevertheless. Clearly, the sonar system of some bats is extremely complicated, involving precise information processing on a split-second basis (see, e.g., Dawkins, 1996). As in the case of the plover, cognitive ethologists want to know how much of the complex information processing of the bat is under conscious, intentional control. As many have indicated, however, it may not be possible to determine what is going on in the mind of another species.
Ecologists are concerned with determining the interrelationship between an organism and its environment, often integrating experimental psychology with evolutionary biology to do so. An ecologist might investigate whether two closely related bird species have similar or different patterns of behaviors ranging from mating to food storage. In investigating such problems, ecologists pay close attention to such processes as perception, learning, and cognition, and in these respects have much in common with experimental psychologists.
Evolutionary biologists and psychologists are distinguished from some others concerned with animal cognition, most prominently by their particular conception of the mindbrain mechanisms controlling behavior. Their view is that the brain is composed of numerous specific mechanisms, often called modules, that are designed by evolution to solve specific problems. This theory of modularity is more or less universally accepted at the sensory level (e.g., eyes to solve the problem of sight) and at the level of organs (e.g., the heart to solve the problem of pumping blood), but is controversial at the level of higher order central processes (e.g., a module in the brain for the preference for one’s own kin) (see, e.g., Fodor, 1983).
One informative view of how various environmental factors interact with mental modules or specific problem-solving devices was proposed by Pinker (1994) (see Figure 14.1). The mental modules, which are built by heredity to solve some specific problem (e.g., speaking with others), are modified by the environment (e.g., hearing English rather than French) and by skills, knowledge, and values (e.g., knowing to speak when important information is to be conveyed). The approach shown in Figure 14.1 contrasts with a view of mind that is widespread in psychology in general and with a view of evolution held by many psychologists. Many psychologists tend to favor the idea that the mind is best conceptualized as a general problem-solving device, a device that can be applied to many different problems. As for evolution, many psychologists believe, implicitly if not explicitly, in what is known as continuity—for example, some process such as intelligence increases gradually and progressively from (say) birds to humans.
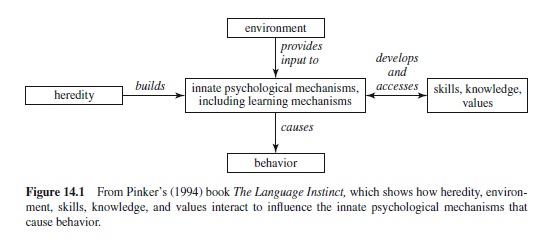
A compromise between the general computer versus specific models view is sometimes suggested. For example, Mithen (1996)—an archeologist—believes, on the basis of the fossil evidence and evidence from comparing various species of animals, that in humans the mental modules, rather than being completely independent or encapsulated, are capable of interacting with each other. In any event, evolutionary psychology rejects what has come to be known as the standard social science model, or SSSM. The SSSM, in brief, suggests that while animals may be controlled by biology, humans are responsive to culture. Dominated by learning, humans are molded by culture through a system of rewards and punishments, according to the SSSM. Whether some animals can be said to possess culture will be considered in the final section of this research paper.
The belief of evolutionary biologists and psychologists is that the mind consists (to use an analogy) of numerous specialized computers, each designed to solve some particular problem. This approach rules out, as is perhaps apparent, continuity in favor of the idea that animals that face particular problems evolve specialized learning and cognitive mechanisms to deal with those problems. To put the matter bluntly, a rat, a monkey, and a chimpanzee do not represent, only or necessarily, animals of increasing intelligence approaching that of a human being. There may indeed be some gain in learning ability over these species, but each at the same time has evolved specialized mechanisms to deal with the particular problems it faces in its own environment. For example, bees, which in some respects lack the learning abilities of rats, seem nevertheless to be better able to communicate the location of a food source to their conspecific than are rats. Bees, of course, communicate the distance and direction of a food source to their conspecifics by doing what is called a dance in the hive. To use another example closer to home, language, rather than having evolved slowly over many different species, may be a specialized ability in humans lacking in any significant respect in any other species. Most notably the much-investigated chimpanzee. If this is the case, then the considerable effort expended to teach dolphins, gorillas, and especially chimpanzees language may be less worthwhile, theoretically speaking, than the trainers of these animals might hope.
As the previous example may imply, evolutionary biologists and psychologists believe that at least some problems investigated by social scientists, who have an outdated conception of evolution, are a waste of time and effort. As Symons (1987, 1992) has noted, social scientists sometimes postulate explanatory mechanisms that could not possibly have survived if current evolutionary thinking is correct. As a more-or-less general example of what Symons has in mind, we might cite a belief that flows from the SSSM, that differences between individuals reared in different cultures are entirely due to culture itself—that is, to learning (see Tooby & Cosmides, 1992). According to this view, our species has a nature, but that nature, except for a few simple instincts, is entirely malleable. As indicated, evolutionary biologists and psychologists suggest, on the other hand, that brains, both human and animal, consist of many special-purpose devices, some of which may be widely shared over species, others of which may be common to only a few species, but that in any case constrain how experience (culture in humans) will affect the behavior of that species (see Figure 14.1).
What Is Animal Cognition?
The question What is animal cognition? has at least two answers. One is that it consists of all those topics treated in the last few chapters of animal-learning textbooks that are otherwise primarily concerned with Pavlovian and instrumental conditioning. This would include such topics as serial learning counting, language acquisition, concept learning, and the like. Another answer is that cognition may be identified with particular processes such as information processing, internal representations, attention, memory, and so on. Whatever one’s approach to animal cognition, it is the case (as we will attempt to demonstrate in this section) that distinguishing between the cognitive versus the noncognitive is difficult and in some cases perhaps even arbitrary.
Consider the idea that cognition involves the internal processing of information—a very reasonable suggestion. Keep in mind, however, that there are behaviors under the control of complex information processing that are not normally classified as cognitive. For example, the hunting behavior of bats, briefly described earlier, involves real-time computations of the prey’s distance, its speed of movement, its direction of movement, its moment-by-moment evasive actions, and the like. Surely some of the bat’s hunting behavior is learned: It may learn with experience to identify the prey’s species by the configuration of the returning echo. Yet equally surely, much of the bat’s complex, rapid information process is “hardwired” into its brain. Nor is the bat an exception. Bees, as indicated, after locating a food source must fly back to the hive where they communicate to their sisters the direction and distance of the desired commodity by doing what is called a dance that their sisters “comprehend”. Not only are the bees engaging in complex information processing, but the dance symbolizes or represents such parameters as the direction and distance of the food source. Essentially, the nervous systems of the watcher bees interpret particular dance movements as indicators of the distance and direction of the food sources.
Representation is involved when an isomorphism occurs between different events, say, between brain or nervoussystem states and aspects of the environment. The bees’ representations may be, relatively speaking, simple. Imagine, however, if you will, how complex the bat’s auditory representations of its prey must be. In real time it computes and updates its prey’s location, speed, direction of movement, and so on. As Dawkins (1976) has indicated, were bats able to do so they might find our species’ reliance on visual processing as strange and mysterious as we find their reliance on auditory processing. Many researchers may be reluctant to consider such behaviors as involving cognition, however, for the following reasons. A hallmark of cognition according to some, is that it allows animals to modify their behaviors to deal with a changing and unpredictable environment. Cognition, according to this view, allows animals to behave in a flexible manner in novel environments. Responses that are hardwired, so to speak, cannot, properly speaking, be considered cognitive. Consider language in people, however. According to some of the major authorities in the field the capacity to acquire language is innate in humans and can be described as an instinct (e.g., Bickerton, 1998; Pinker, 1994). Thus it is possible that understanding of, say, sonar use in bats may contribute to better understanding of language acquisition in humans, or, indeed, vice versa. On this basis, one may suggest that too sharp a distinction between hardwired behaviors, particularly complicated and elaborate ones, and cognition may not be useful.
Decision making and problem solving may properly be regarded as cognitive activities—but who can doubt that bats and bees (to use our familiar examples) are making numerous decisions (to dive when prey dives) and solving significant problems (to forage for food and return to hive to dance) when engaged in their normal activities? Consider another example of decision making. Male bower birds build large, elaborate nests that they decorate with brightly colored objects in the hope of attracting a female. If the nest fails in this regard, the male bird tears down the nest and builds a new one. Nest building by bower birds improves with experience, older birds building better nests than younger birds. Is bower building, therefore, an instance of flexible decision-making behavior in the face of novel circumstances, or is it merely hardwired? In any event, a cognitive ethologist might impute purpose and awareness to the bower birds’ nest building, perhaps more purpose and awareness than some might find reasonable. Yet, might we be equally unreasonable in going to the other extreme, dismissing the male bower bird’s nest-building activity as totally irrelevant to matters of animal cognition?
As indicated, current animal-learning textbooks often treat Pavlovian and instrumental conditioning separately from animal cognition. However, there are interpretations of Pavlovian conditioning in terms of attention (e.g., Mackintosh, 1975) and information processing (e.g., Pearce & Hall, 1980). To mention a final example, many orthodox instrumental learning phenomena, ranging from reward schedule effects to brightness discrimination learning, have been said to involve complex memorial processes (e.g., Capaldi, 1994). Thus, just as the distinction between hardwired behavior and cognition may be too sharply drawn, so too might the distinction between learning and cognition.
Cognitive Processes
Perception
Interestingly, built into the perceptual systems of animals are decision processes of the sort that could otherwise be mediated by learning or cognition. For example, the eye of a vertebrate is a complicated mechanism shaped by evolution to solve problems of importance to a given species in its particular environment. The senses, therefore, may be regarded as information-processing devices. Consider some examples. Frogs have retinal “bug detectors.” The retina of the rabbit contains several specialized mechanisms, including a “hawk detector.” Different species of birds have different retinal distributions of photoreceptors shaped by their particular environments. As one example, birds of prey, which tend to hunt from above, have the densest array of photoreceptors in the section of the retina that views the ground. Moreover, the placement of eyes in the head varies according to an animal’s lifestyle. In some animals, our species included, the eyes face toward the front. In other species, the eyes are placed more to the side of the head so as to better view stimuli from the sides and behind. To consider still another example, bees and some species of birds are able to detect ultraviolet light.
Some ant species send out foragers who follow more or less random paths in their explorations. On the way out the scouts lay down a train of scent molecules, or pheromones. When a scout finds food it returns to the colony. Ascout finding more nearby food returns to the nest sooner and thus lays down a stronger scent path. Other ants follow the stronger path. A longer path leading to food, discovered by any other scout, gets less traffic and its scent fades as the pheromones evaporate. This apparently simple sensory solution to a problem of importance to the survival of ants has, according to Peterson (2000), suggested to engineers and computer scientists “powerful computational methods for finding alternative traffic routes over congested telephone lines and novel algorithms for governing how robots operating independently would work together” (p. 314). Moreover, some computer scientists have devised software to solve complex problems by mimicking the pheromone-following behavior of ants.
All of the previously cited examples, from ants to bees to frogs to birds to rabbits (not to mention echolocating in bats), indicate that sensory systems of animals have evolved to solve significant problems. Thus these systems, if not cognitive themselves, are at least in some instances the gateways to cognition, and they solve problems that would otherwise involve cognition. Moreover, a better understanding of these sensory systems, whether it be of pheromone-sensing ants, or of echolocation-using bats, may provide important clues to the operation of higher level cognitive processes.
Discrimination Learning and Categorization
In a discrimination learning study a hungry rat might be rewarded with food for responding to one stimulus, say, black (B), and nonrewarded for responding to another stimulus, say, white (W). The two stimuli may be presented separately on different trials (successive training) or together in the same trial (simultaneous training). In successive training, discrimination learning might be indexed by more vigorous responding to B (called the positive cue, in this case, B+ ) than to W (called the negative cue, W– ). In simultaneous training, B might appear irregularly on the left (B + W–) on half the trials and on the right (W – B+) on the remaining half. Discrimination learning might be indexed by the animal’s selection of B+ when it is either to the left or to the right of W– .
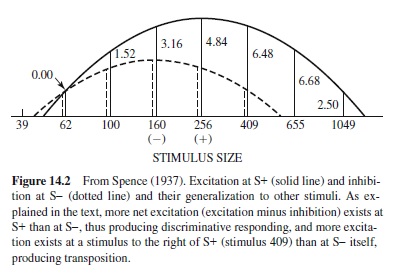
Discrimination learning has been and continues to be a major battleground between theories that stress associations and theories that stress other processes such as cognition or perception. Spence’s (1936, 1937) theory of discrimination learning is a good example of a more or less orthodox associative theory that has battled successfully with various nonassociative views. Spence’s theory suggests that all stimuli falling on the receptors when a response is made become excitatory when rewarded (i.e., such stimuli elicit responding) and that all stimuli falling on the receptors when a response is made become inhibitory when nonrewarded (e.g., such stimuli oppose responding). Both excitation and inhibition generalize to similar stimuli (stimulus generalization), and net excitation (excitation minus inhibition) regulates responding. This deceptively straightforward and simple theory is quite powerful. First, it can explain many discrimination learning phenomena. Second, the theory is general and can be used, for example, to explain the acquisition of concepts or categories, as will be explained shortly.
Spence’s theory, as indicated, suggests that a variety of stimuli simultaneously become excitatory when rewarded and inhibitory when nonrewarded . Lashley (1929) opposed Spence on this score, suggesting instead the more cognitive view that animals selectively attend to stimuli. Discrimination learning, according to Lashley, consists of successively eliminating, one by one, stimuli that fail to predict successfully, the animal ultimately fastening on the relevant stimulus dimension. Lashley’s attentional-cognitive view was not supported by experiments showing that reversal of the positive and negative cues (i.e., B +W – to W+ B–) while animals were still responding at a chance level (50% correct) on the original problem (B + W–) produced serious retardation in learning the reversal (W+B–). Such retardation should not occur, according to Lashley, because animals responding at a chance level have not (by definition) isolated the relevant stimulus dimension; thus, reversing the S+ and S – cues should not influence the final solution, which is contrary to fact. Attentional theories that assume animals can attend to two or more stimuli simultaneously are better able to deal with the reversal findings described previously (see, e.g., Sutherland & Mackintosh, 1971). Spence’s theory predicts, of course, that reversing the S+ and S– cues when the animal is responding at a 50% level will have a deleterious effect on discriminative responding. This is because animals responding at a 50% (or chance) level have nevertheless learned something about the S+ and S– cues, enough to retard reversal learning.
Gestalt psychology, which emphasized perception, explained discrimination learning in terms of learning relationships between stimuli. In a B + W– discrimination, for example, the animal would not learn that B is excitatory and W is inhibitory, as Spence suggested, but rather would learn to select the darker of the two stimuli. Offered as support for the Gestalt view was the phenomenon of transposition. For example, an animal that learned to select medium gray (positive) over light gray (negative) might, in a subsequent test phase, when given a choice between the medium gray and a newly introduced dark gray, actually select the novel dark gray because it is the darker of the two stimuli.
Spence’s arguments with Lashley and the Gestalt psychologists illustrate an important point suggested earlier: Discrimination learning has been and continues to be an important battleground for testing the adequacy of associative versus various nonassociative approaches to animal cognition and learning. Spence’s theory is able to explain transposition in associative terms without appealing to the learning of relationships. This is shown graphically in Figure 14.2. The figure shows inhibition and its generalization associated with the negative (S– ) cue (dotted line) and excitation and its generalization associated with the positive (S+ ) cue (solid line). Net excitation is shown by the length of the solid vertical lines above various stimulus points. Note that greater net excitation is associated with the S+ cue rather than with the S– cue, and so the animal will select the S+ cue. However, greater net excitation is associated with the cue to the right of the S+ cue, and so the animal will select that, novel untrained stimulus in preference to the S+ cue—the transposition phenomenon. In sum, Spence’s theory is able to explain transposition by employing rather orthodox associative concepts.
More recently, individuals concerned with evaluating the role of cognition have employed categorization experiments that are, essentially, elaborate discrimination learning investigations. In these, pigeons might be shown numerous photographic slides containing (say) trees and numerous other slides lacking trees (e.g., Herrnstein, 1979). The pigeon might be rewarded for pecking the “tree” slides (S+ cue) but not reinforced for pecking the non-tree slides (S– cue). The pigeons readily learn this sort of discrimination, which they transfer well to novel stimuli. The meaning of results of this sort remains unclear. For example, one might think it is easier to learn a category (trees vs. non-trees) than to learn 80 unrelated slides (40 + and 40– ). Vaughan and Greene (1984), however, employing 160 + slides and 160 – slides, uncategorized, showed that pigeons more or less easily came to master the discrimination and even performed well after a 2-year rest. Although pigeons perform better with categorical than with noncategorical grouping of stimuli, this may not indicate that they learned a concept (see Watanabe, Lea, & Dittrich, 1993). It is the case that category slides will have more in common with each other than will noncategory slides. Thus more excitatory stimulus generalization will occur between category slides than between noncategory slides. An associative approach can explain much of the available category data (Wasserman & Astley, 1994). Feature theory, the view that a set of conjoined features separates category members from nonmembers, has been applied to category data (Watanabe et al., 1993). Feature theory, too, is compatible with an associative analysis.
In categorization experiments animals may come to form a prototype, an exemplar representing the central tendency of all of the individual exemplars. For example, a robin is a better prototype of bird than is, say, a penguin. There is no compelling evidence that animals form prototypes (see, e.g., Mackintosh, 1995). Rather, available data in animals can be interpreted in terms of exemplars.
Serial Learning
The specific experiments cited in the previous section, as well as many other types, are explicitly recognized instances of discrimination learning. However, there are many other types of investigations that are clear instances of discrimination learning but are not generally considered under that heading. A case in point is serial learning, a procedure popular in animal and human learning alike. In one type of serial learning task, items are presented successively in a particular order (e.g., A-B-C-D) and the animal’s task is to learn both the items and the specific order in which they occur. People master many sorts of successive serial tasks: days of the week, months of the year, the alphabet, and so on. In a serial learning task in which items are presented successively, the animal must learn to respond differentially to different stimuli; thus serial learning is a variety of discrimination learning.
Not surprisingly, the issues raised in animal serial learning are quite similar to those raised in connection with explicitly recognized instances of discrimination learning. In some respects, however, contrary to popular opinion, serial learning data are more germane to the issue of animal cognition than are currently available, explicitly recognized instances of discrimination learning, including category learning. For one thing, there can be little doubt that serial learning, as investigated using animals, involves cognition of some sort, particularly memory, as we shall see. For another, it is clear that categorization (called chunking) is involved in serial learning, and it apparently cannot be explained in terms of stimulus generalization.
Consider an animal that learned to respond appropriately to a progressively decreasing series of reward magnitudes terminating in nonreward (e.g., large reward, medium reward, small reward, nonreward). Appropriate responding might consist of progressively weaker responding over the series. What has an animal learned in such a case? Consider three different interpretations. The animal may, as the Gestalt psychologists have suggested, have learned a relationship among the items; that is, it may be that reward magnitude decreases monotonically over trials (e.g., Hulse & Dorsky, 1977). The animal may learn an association between the item and its position in the series—that is, that Position 1 signals large reward, Position 2 signals medium reward, and so on (Burns, Dunkman, & Detloff, 1999). The animal may also learn an association between the memory of one or more prior items and the current item; that is, the memory of Item A(large reward) signals B (medium reward), the memories of Items A and B signal C (small reward), and so on (see Capaldi, 1994). Recent evidence suggests that rats are able to employ either item cues or position cues in learning a successively presented series of food items (Burns et al., 1999; Capaldi & Miller, 2001a). The conditions under which rats may tend to employ either position cues or item cues or both have yet to be isolated and identified clearly.
In the sort of serial learning task examined in this section, items are separated by a retention interval. For example, a given item, say, A, may be presented and removed minutes or hours before the participant is asked to respond to the next item, B. Appropriate serial responding under retention interval conditions necessarily involves employing the memory or representation of some prior event (item memory, position memory, or both) in order to anticipate the current event correctly. Series may be employed such that the memory or representation involved is necessarily that of one or more prior items. As an example, consider rats that have received two slightly different series in irregular order: XNYand ZNN (Capaldi & Miller, 1988a). X, Y, and Z are discriminably different food items; N is nonreward. Items of each series were separated by shorter intervals than that separating the series themselves. Rats trained XNYand ZNN learn to respond correctly to the third item of the series—that is, to respond more vigorously to Y than to N. Trial 3 running cannot be mediated by the Trial 2 event because it is the same in both series, N. Whatever else may be the case, therefore, discriminative responding on Trial 3 requires that the rat remember on Trial 3 the item presented on Trial 1; that is, the rat must respond more vigorously when X occurred on Trial 1 than when Z occurred on Trial 1. Further implicating memory, rats have responded more vigorously on rewarded (R) than nonrewarded (N) trials when the R and N trials were alternated (R, N, R, N, etc.) and the retention interval was 24 hr (e.g., Capaldi & Lynch, 1966; Capaldi & Minkoff, 1966).
A chunk consists of lower order functional elements (e.g., letters of the alphabet) combined to form higher order elements (e.g., words). If items of a series are grouped—say some on the left, others on the right—rats may tend to chunk items similarly grouped (see Capaldi, 1992). Chunking is clearly a form of categorization. Grouping cues that lead to chunking in people have been used with rats and shown to be similarly effective. These include the presentation of items under different brightness conditions, in different spatial locations, and at different temporal intervals (see, e.g., Capaldi, 1992, 1994).
Another method employed in investigating serial learning is the displaying of all items simultaneously rather than successively. The participants’ task is to respond to the items in a particular order. One of the more interesting findings obtained employing the simultaneous presentation of items is that monkeys appear to have a better grasp of an overall sequence of events than do pigeons. For example, pigeons make more errors than monkeys to interior items of, say, a five-item series (see D’Amato & Colombo, 1988; Terrace, 1986).
A reward schedule investigation consists of presenting rewards according to some rule. For example, food reward might occur on a random half of all trials, nonreward on the other half. A reward schedule of this sort, called a 50% irregular partial reward schedule, is clearly of major concern to various orthodox theories of animal learning. We might ask what, theoretically speaking, the difference is between a 50% irregular schedule of partial reward and a serial learning task in which, say, reward magnitudes become progressively smaller over successive trials (a decreasing monotonic schedule). It is the case that the two sorts of situation have been treated differently in that many theories that attempt to deal with 50% irregular schedules do not attempt to deal with monotonic schedules, and vice versa. Recent evidence suggests that treating the two sorts of schedules differently appears to be unjustified (Capaldi & Miller, in 2001b). That is, memory, which is clearly a major factor controlling performance in orthodox cases of serial learning (e.g., the monotonic schedule) is also a major factor in controlling performance in orthodox reward schedule cases (e.g., the 50% irregular schedule). Capaldi and Miller (2001b) demonstrated, essentially, that similar variables, such as the number of nonrewarded trials that occur in succession, have identical effects in the two situations. The general implication of such findings is as follows. If a clearly cognitive process such as memory is intimately involved in regulating performance under 50% irregular schedules, it is probably a factor in regulating instrumental learning generally. Put somewhat differently, the usual distinction between orthodox learning tasks (e.g., varieties of instrumental conditioning) and orthodox cognitive tasks (e.g., complex serial learning) may be artificial, cognition being involved in both.
The investigation of chunking in serial learning provides a window into the ability of an animal such as the rat to organize separately presented items into wholes. Evidence has been presented indicating that rats can form three different sorts of chunks of varying degrees of complexity (see Capaldi, 1992; Haggbloom, Birmingham, & Scranton, 1992). Consider a rat trained in a runway—an apparatus in which the animal must run from one end of a confined path to the other end to obtain food. The first (and lowest order) chunk formed is what is called the trial chunk. Atrial chunk consists of the animal’s combining into a single whole the separate events of the trial—for example the opening of a door to allow the animal access to the runway, to the animal’s running in the middle section of the runway, to its entering the goal box at the end of the runway. The next highest chunk is called a series chunk, which consists of the animal’s combining trial chunks into a higher level chunk. For example, a rat trained under four nonrewarded trials followed by a rewarded trial responds as follows: It begins by running slowly to the initial nonrewarded trial, the progressively increases its running speed over the successive nonreward trials, until by the terminal nonrewarded trial, the animal runs about as fast as it is able. Such responding indicates that the rat is treating the five trials, four nonrewarded followed by a reward, as a single organized whole or a chunk. The third chunk, a list chunk, consists of the animal’s using a series chunk as a discriminative stimulus or signal for a subsequent series chunk. For example, rats have learned that a particular series of perhaps three trials (say, two rewards followed by a nonreward) will signal, some 1 to 20 min later, another distinctive subsequent series of, say, three trials.This only reliable signal of the subsequent series is the initial series. Under these conditions, rats have correctly anticipated the trials of the subsequent series (e.g., running fast, fast, slow, respectively, over the three trials consisting of two rewards followed by a nonreward). List chunks indicate that rats possess a fairly high capacity to organize discrete events into wholes (see Haggbloom et al., 1992).
An explanation of how chunks are formed in serial tasks stresses overshadowing (Capaldi, Birmingham, & Miller, 1999). Overshadowing may occur when two stimuli,Aand B, signal some event, X, when one of the stimuli is a more valid or reliable signal than the other. In a case of this sort the more valid or reliable signal becomes the stronger signal for X. In a serial task, item validity may be reduced when similar or identical items signal different items. For example, in the series A-B-C-B-D-E, the validity of B is reduced because it signals both C and D. Thus, if some novel cue were to signal D exclusively, it would be more valid than B, and it (rather than B) would become the better signal for D. This would result in the formation of the series A-B-C-B-D-E into two chunks,A-B-C-B and D-E.
Numerical Abilities
In recent years, much experimental effort has been invested in detecting numerical abilities in animals, most notably birds, rats, monkeys, and chimpanzees (see, e.g., Boysen & Capaldi, 1993). At a relatively simple level, animals may be asked to discriminate between two quantities, more versus less—a relative numerousness discrimination. At a more complicated level, animals may be asked to perform some operation on numbers, such as addition or subtraction. In between these extremes animals may be asked to count—that is, to enumerate items explicitly. The accumulating evidence reveals that animals may be able to do each of these things, although operating on numbers has as yet been demonstrated only in the chimpanzee (Boysen & Berntson, 1989). In that study a chimpanzee that visited a number of food sites, each containing a different number of food items, was able at the end of the circuit to select an Arabic numeral corresponding to the total number of items seen. The animal had not been explicitly trained to add items, only to enumerate them.
In explicit counting studies, items have been presented either simultaneously or successively. In such investigations a variety of stimuli are confounded with number of items, and these confounds must be removed. For example, all else being equal, it takes longer to present three items than two items. Contemporary counting studies (e.g., Capaldi, 1998) have gone to great lengths to eliminate these confounds successively. Those studies and others have found that animals such as birds, rats, and monkeys can make discriminations based upon the number of items. One of the major issues in counting studies is whether animals count reluctantly and only when the number of items is the only discriminative cue available (Davis & Pérusse, 1988). Furthermore, do animals count routinely and rather easily, employing the number of items as a discriminative cue even when number is confounded with other variables (e.g., Capaldi & Miller, 1988b)?
An animal may be said to be counting if its behavior suggests conformance with three principles. The items to be counted or enumerated should be arrayed in one-to-one correspondence to internal number tags, which in the case of people are conventional symbols such as one, two, three, and so on. The tags should be applied to events in a stable order. Thus we may not enumerate items one, two, three in one occasion and one, three, two on another occasion. The order irrelevance principle suggests that items may be enumerated in any order. For example, in enumerating three different items X, Y, and Z we may do so in any order: X first and Z last, or Z first and X last, and so on. In experiments reported by Capaldi and collaborators (see, e.g., Capaldi, 1993; Capaldi & Miller, 1988b), rats were shown to be able to enumerate successively presented food items according to the three principles just outlined. In those experiments, control was exercised over variables confounded with number of successively presented food items, such as amount eaten, time spent eating, response effort expended in obtaining food items, and so on (see Capaldi, 1993, 1998).
Gelman and Gallistel (1978) suggested that children count effortlessly and as a matter of course. One might say that counting is an instinct in humans (see, e.g., Spelke, 2001). Capaldi and Miller (1988b; see also Capaldi, 1993) suggested that rats also enumerate items as a matter of course and will do so even when number of items is confounded with other variables. In one experiment, Capaldi and Miller (1988b) trained rats such that number of food items was confounded with a number of other variables, among them, time and effort. When the confounds were removed and only number of food items was a valid cue, the rats continued to behave appropriately, indicating that counting occurred even when other valid cues were simultaneously available.
Highly interesting data relevant to this important issue were recently reported by Brannon and Terrace (2000). In that investigation, rhesus monkeys were trained to enumerate items (such as geometrical forms) that were presented visually. In initial training sessions the animals enumerated 1 to 4 items. Having mastered 1 to 4 items, the monkeys were now asked to enumerate 5 to 9 items without explicit training. The monkeys quickly did so. Brannon and Terrace suggested that these findings indicated that monkeys count even when not forced to do so.
The interests of investigators concerned with counting in animals vary. Some seem interested in animal counting for its own sake. Some seem interested in similarities and differences between human and animal cognition (see, e.g., Brannon & Terrace, 2000). Still others have suggested that counting is of interest because it is routinely involved in many learning situations ranging from irregular reward schedule to serial learning (e.g., Capaldi, 1994; Capaldi & Miller, 1988b).
Theory of Mind
As applied to humans, having a theory of mind means that we attribute behavior—our own as well as that of others—to beliefs and desires. Baron-Cohen (1995) has suggested that humans have an innate “theory of mind module.” According to Baron-Cohen, autistic children, some of whom seem not to be aware of others (as evidenced by their sitting alone, rocking back and forth, and in other respects living in a private world), lack a theory of mind.
Premack and Woodruff (1978) asked whether the chimpanzee has a theory of mind. This question has sparked considerable research and much controversy over the last 20 yearsorso.C.M.Heyes(1998)concludedthatnoconvincing evidence has been produced to suggest that chimpanzees have a theory of mind. Reaction to her criticisms has been varied. At one extreme, Gordon (1998) suggested that the question itself is ill conceived and thus not worth asking. Byrne (1998) suggests, on the other hand, that Hayes misrepresents findings and that the theory-of-mind approach is a useful one.
Heyes (1998) identified six areas of investigations emphasized in theory-of-mind research: imitation, self-recognition, social relationships, rule taking, deception, and perspective taking. In this research paper, two of these areas will be discussed in enough detail to give, hopefully, an adequate idea of what is intended by the term theory of mind. These are self-recognition and imitation.
Self-Recognition
Gordon Gallup (1970) presented chimpanzees with mirrors. Initially the chimpanzees reacted to the mirror images as though they were other chimpanzees. Following hours of experience with mirrors, the chimpanzees dropped otherdirected behavior in favor of what Gallup termed self-directed behavior. These self-directed behaviors were interpreted by Gallup to indicate that a chimpanzee recognized the image in the mirror as itself. To provide better evidence for selfrecognition, Gallup devised the mark test: He anesthetized the animals and marked then with an undetectable (i.e., odorless) dye over one eye and the opposite ear—areas that could not be seen without the aid of the mirror. The basic finding was that chimpanzees that had mirror experience showed markdirected behavior, whereas control chimpanzees lacking mirror experience did not.
Gallup’s (1970) initial conclusion was that chimpanzees are capable of recognizing themselves and therefore have a sense of self-awareness. Subsequently, Gallup (1977) extended his conclusions. The ability to self-recognize, Gallup suggested, implied consciousness and self-consciousness, the latter encompassing the ability to think about thinking and to be aware of one’s own state. In 1982, Gallup went still further. An animal that is self-aware, he suggested, has a mind, and having a mind includes having empathy and the ability to deceive.
Human children, of course, have passed the mark test, as have some orangutans. After several failures, gorillas have been shown to pass the mark test. In general, monkeys fail the mark test, and even in instances in which behavior has been directed at the mark, the observation is equivocal. A variety of additional findings have been reported: Not all chimpanzees pass the mark test; young chimpanzees (below age 3 years, 6 months) may prove likely to fail the mark test. Epstein, Lanza, and Skinner (1981) claim to have trained a pigeon to pass the mark test, a claim that has been disputed (e.g., Gallup, 1982).
Criticism of the self-recognition claim ranges from the observation that failing the mark test may not imply a diminished mental capacity, to the observation that passing the mark test may not indicate advanced mental capacity. As an example of the former, it has been observed that monkeys may not look in the mirror because eye-contact is a threatening gesture. As an example of the latter, mirror recognition may imply no more than that the animal has a “body concept,” one that is used in, say, ordinary locomotion.
A test similar to the mark test has been employed with children; it is called the rouge test. Children are given a small rouge mark below the right eye that can be recognized only by using a mirror. By about 19 months of age, 52% of children immediately direct behavior to the rouge, indicting selfrecognition (Asendorpf, Warkentin, & Baudonniere, 1996).
Imitation and Social Learning
By observing another engage in some extended act that involves a number of different and discrete steps, a person may learn in a matter of minutes what might otherwise require hours or days of individual effort without guidance. Any number of such activities comes to mind, ranging from changing a tire to setting a VCR. Perhaps because learning by observing others is so important and widespread in the human species, some have taken it to be a hallmark of intelligence in other species. For example, Romanes (1882) provided a number of rich examples of animals’ engaging in quite complex behaviors established by imitation. The problem is that Romanes’s examples were based on anecdotes and thus his data by modern and entirely reasonable standards are deficient.
Determining the extent to which other species learn by imitation is a more difficult problem than it appears to be at first blush. For example, what sort of behaviors should be selected for analysis? Well-fed chickens can be induced to continue to eat by watching other chickens feed. It would seem that species-typical behavior, such as chickens’ pecking for food, would provide relatively unconvincing evidence for true imitation. The idea of learning by imitation would seem to require three things to be entirely convincing: The behavior to be imitated should be stored as a representation by the observer; the observer’s behavior should be a more or less faithful copy of the demonstrator’s behavior; and the behavior imitated should be reasonably complex and not a speciestypical behavior such as pecking by a chicken. A useful experimental design to study imitation is called the two-action test. In this design, a given result may be accomplished in two different ways. Observers should be shown to engage in the particular behavior that they were allowed to observe rather than the one not observed (Heyes, 1996).
Rats prefer foods their mothers ingested during pregnancy and after birth. Nursing rats come to prefer foods ingested by their mothers. Rats also come to prefer foods eaten by their conspecific; one way they determine what this is food is is by smelling the breath of their conspecific. It has been shown that a group of rats that has come to prefer a specific flavor will pass on that preference to new members of the group.
Roof rats have come to occupy the pine forests of Israel, where they subsist on pine seeds, which are rendered difficult to extract because of the tough scales that must be removed. To remove the scales, the rat must begin chewing on them at the base of the cone, removing them scale by scale by following the spiral pattern that goes to the top of the cone. Rats have failed to learn this if left to their own devices. If raised with a mother who is an efficient stripper they do acquire the knack of getting to the seeds—but not, apparently, through imitation. They acquire the behavior by getting access to cones that have already been partially stripped, even if they have been partially stripped by the experimenter.
In a noteworthy case of observational conditioning, monkeyshaveacquiredfearofsnakesbyobservingademonstrator monkey exhibiting such fear (Mineka & Cook, 1988). Fear is acquired even by merely observing a demonstrator on video showing fear to a toy snake. Monkeys have observed demonstrators on video showing an apparent fear of flowers that had been spliced into the film in place of the snake. Fear of snakes is acquired more readily by naive monkeys than fear of flowers.
In addition to observational conditioning, three others categories of learning have been distinguished: stimulus enhancement, emulation, and imitation. In stimulus enhancement, the demonstrator’s behavior simply directs the observer to stimuli that makes copying the demonstrated behavior more likely. Emulation occurs when behavior is copied in a more or less general way by employing techniques different from that of the demonstrator. Imitation involves faithful copying of the demonstrator’s behavior. Children appear to imitate behavior more faithfully than chimpanzees (see, e.g., Whiten & Boesch, 1999).
Interest in imitation or learning from others has a long history (e.g., Romanes, 1882; Thorndike, 1911), and over that long period of time scores of observational and experimental reports have been published. Nevertheless, we know little about imitation. One problem is the lack of a useful theory of imitation that can direct our efforts into useful channels. Another is that only in relatively recent times have we developed useful experimental procedures for investigating learning by observation. Two examples here would include the video techniques mentioned earlier for examining fear acquisition in monkeys, and the two-action experimental design. It may not be too optimistic to conclude that our understanding of imitation may undergo rapid and significant development over the next few years.
Interval Timing
The ability of animals to time arbitrary events has been intensively investigated in recent years through a variety of procedures. How animals time intervals is seen as important for understanding learning and cognition generally. For example, according to one view, animals employ the same mechanisms to time events as to count them. Even more generally, timing events has been seen as fundamental to understanding all varieties of learning and cognition (Gallistel & Gibbon, 2000). That is, both Pavlovian and instrumental procedures are seen as involving the learning of temporal intervals. For example, in the Pavlovian preparation the animal responds most vigorously at the termination of the conditioned stimulus in delayed conditioning. This finding suggests that the animals are sensitive to the time elapsed since the stimulus was presented. In instrumental conditioning, it has been suggested that the animals compared the time to reinforcement in the trial to the overall time between reinforcements (but see Capaldi, Alptekin, & Birmingham, 1996).
Most studies explicitly concerned with timing have used instrumental conditioning. In the peak procedure, animals receive many daily trials in which reinforcement occurs after a fixed time following the onset of a signal (Roberts, 1981). A major finding is that animals respond most at approximately the time that reinforcement is due. For example, if the interval is 20 s, most responding occurs at about 20 s. Another major finding using the peak procedure is that maximum response rate is reached at a certain proportion of the way into this interval regardless of the interval’s length.
In tests of temporal generalization, an animal is reinforced for responding to one signal duration but not others. A major finding using this procedure is that a typical generalization gradient is obtained with maximum responding confined to the reinforced duration (Church & Gibbon, 1982).
In the bisection procedure, two levers may be inserted into an operant box; the rat is reinforced for pressing the left lever after a tone of one duration and the right lever after a tone of another duration (Church & Deluty, 1977). In the test condition, the rat is presented with tones of intermediate duration. Of special interest is the duration at which they choose each lever half the time (50% responding). It develops that the interval used by rats is the geometric mean of the two intervals not the arithmetic mean. For example, if the intervals are 4 s and 16 s, respectively, the arithmetic mean is 10 ([4+16]/2), whereas the geometric mean is 8 (√̅4̅х̅1̅6̅). In this example, 50% responding would occur closer to 8 s than to 10 s.
Other experiments indicate that animals time linearly and that they can start and stop their interval clocks. How animals time has produced much theorizing. It has been variously suggested that rats time by employing a pacemaker (e.g., Church, Meck, & Gibbon, 1994), by using oscillators (e.g., Gallistel, 1994), or by using behaviors that predictably fill given intervals (e.g., Killeen & Fetterman, 1988).
Gallistel and Gibbon (2000) have presented a timing theory that is highly ambitious in that it attempts to explain a wide rangeofphenomena.The theory has been used to explain phenomena as different as delayed classical conditioning to extinction following different reward schedules in instrumental conditioning. Whatever the fate of this theory, it is clear that animals such as rats and pigeons have well-developed capacities for timing events. How extensively this timing capacity enters into learning and cognition appears to be a major issue that will occupy investigators over the near future.
Memory
Memory is among the most intensively investigated topics in animal cognition. Animal memory may be studied either in its own right or as a mechanism controlling learning and performance. Determining under what conditions forgetting occurs is an example of the former; examining the capacity of the memory of nonreward stored on one trial to be retrieved on the next trial so as to correctly anticipate the reward outcome on that trial is an example of the latter.
It is now recognized that animals can retain information over long temporal periods. This was not always known. In the early days of the investigation of animal learning, laboratory data suggested that animals possessed only fleeting memory. A popular procedure devised by W. S. Hunter (1913) in the early 1900s is a case in point. In Hunter’s procedure, called delayed reaction, animals that were retained in a delay chamber could determine which of three doors lead to food because a light was flashed in front of the correct door. After the light went off animals ran from the delay chamber to the doors after varying retention intervals. Rats failed at this task with delays of as little as 10 s. Raccoons were able to respond correctly following a delay of up to 25 s.
Contrast such poor performance with some subsequent findings obtained under both field conditions and in the laboratory. A certain bird, Clark’s nutcracker, stores the seeds of pine cones in individual caches in the late summer and early spring when food is plentiful, recovering the seeds months later when food is scarce. It is estimated that the bird stores many thousands of seeds in caches of a few seeds each. A high percentage of seeds is recovered by the bird. Skinner (1950) trained pigeons to peck for food at a spot on an illuminated key. Following a 4 year retention interval the pigeons were tested and immediately pecked the correct key. Wendt (1937) trained a dog to withdraw its foot at the sound of a tone paired with shock. After a 30 month retention period, foot withdrawal to the tone occurred on 80% of the test trials, only a slight drop from the prior training session.
A currently debated topic concerns whether memory is a unitary system or is composed of two or more subsystems. Some examples of currently postulated subsystems are procedural versus declarative memory, semantic versus episodic memory, and long-term versus short-term memory (see, e.g., Spear & Riccio, 1994). In the animal area one of the most popular distinctions is that between working memory and reference memory. Working memory is concerned with keeping track of information that may change from one trial to the next. Reference memory is concerned with isolating important relationships in the situation that are stable over trials. As an example, consider rats rewarded for a running response on every other trial, under a single alternation schedule of rewarded and nonrewarded trials. Rats so trained may eventually come to run faster on rewarded than on nonrewarded trials. In this situation working memory would be used to determine whether reward or nonreward occurred on the prior trial and thus whether reward or nonreward is to occur on the current trial. Reference memory would be employed to learn that rewards and nonrewards occur according to a particular rule or schedule—a single alternating one.
Working memory, unlike short-term memory, may be effective following long retention intervals. In the singlealternation situation, as indicated earlier, rats have employed the memory of the reward outcome on the prior trial to anticipate the reward outcome correctly on the current trials when trials were separated by 24 hr (see, e.g., Capaldi, 1994). The single-alternation situation is useful for understanding a second popular distinction between memories in the animal area as well as in human memory: that between retrospective and prospective memory. In the case of the single-alternation schedule, retrospective memory would consist of retaining the memory of poor reward or nonreward over the retention interval, utilizing that memory to determine whether responding on the current trial should be fast or slow. Employing prospective memory, the animal would determine at the time of reward or nonreward whether it should run fast or slow on the subsequent trial, thus making it unnecessary to retain the memory of reward or nonreward over the retention interval.
Memory may be analyzed in terms of three stages. The first, encoding, refers to the stage in which the memory is formed. The second, retention, refers to the persistence of the memory over time. The third, retrieval, refers to recall of the stored memory. We may fail to remember because of poor retrieval cues. This occurs when the cues at retrieval differ from the cues that accompany storage. Interference may also be responsible for forgetting. In proactive interference, memory for material learned earlier may interfere with material learned later. In retroactive interference, material learned later may interfere with material learned earlier.
Apopular procedure employed to study animal memory (it is sometimes used with people as well) is delayed matching to sample (DMTS). In this procedure a subject (say, a pigeon) is initially trained to peck each of three keys arranged in a horizontal row on the wall of an apparatus called an operant chamber. A typical trial begins by exposing a stimulus— the sample stimulus, say, a horizontal line on a white background—on the center key, with the side keys being blank. After the pigeon has observed the horizontal line, or sample stimulus, for some period (or has pecked it), the center key goes blank. There then ensues the retention interval in which all three keys remain blank. When the appropriate retention interval has elapsed the side keys are illuminated, one with the horizontal line and the other with a vertical line. These are called the comparison stimuli. Acorrect response, which may produce food reward, consists of pecking the side key that contains the comparison stimulus matching the sample— in the present example, the horizontal line. The horizontal and vertical lines may be presented equally often as samples in an irregular fashion over trials. The positions of the comparison stimuli are varied irregularly over trials such that each may appear equally often on the right key and on the left key.
Both retroactive and proactive interferences have been demonstrated in the DMTS situation. Retroactive interference has been investigated as follows. Typically the chamber is dark during the retention interval of a DMTS task. If, after the sample stimulus is removed, the chamber is illuminated, correct responding may decrease substantially. Proactive interference is a major factor in DMTS (see, e.g., Wright, Urcuioli, & Sands, 1986). For example, memory is much better when many rather than few sample stimuli are used. This is because presenting only a few sample stimuli increases proactive interference. In an interesting experiment employing monkeys, when trial unique stimuli were employed, retention was good even at a 24 hr interval.
Several models of Pavlovian conditioning emphasized memory. In an early model suggested by Wagner (1976), rehearsal of the conditioned stimulus was stressed. According to Wagner, surprising events are better rehearsed and thus better remembered than expected events. For example, on the first trial, a surprising tone may be strongly rehearsed together with the subsequent shock because of surprise. This would lead to a strong increment in the capacity of the tone to signal shock. On subsequent trials in which shock is expected following tone, and thus surprise is reduced, little or no increase in learning may occur.
A person may be asked to remember, say, 12 items consisting of 3 items in each of four different categories: flowers, foods, furniture, and animals. On outputting the items the person may do so by category: 3 flowers, followed by 3 animals, and so on. Organization processes of this sort are of concern in animal memory. For example, in a study by Roberts (1998), a 12-arm radial maze, which consists of a central platform with a number of arms branching out at equal angles, was baited with four each of three different types of food, always in the same arms over successive trials. For example, cheese might be placed in arms 1, 3, 5, and 8 on successive trials. The rats learned to take the food items in a particular order, each of the four preferred foods first, the four least preferred foods last. The ability of rats to employ one entire series of items to predict another series of items correctly, as considered earlier, is another example of complex organization processes in rats.
Animals such as birds, rats, and monkeys have been shown to possess highly impressive memories—impressive from the standpoint of retaining a considerable amount of information over long intervals (consider Clark’s nutcracker), and from that of being able to organize discrete events into useful wholes (identified as chunks in the “Serial Learning” section). Memory investigations are perhaps as illuminating as any other in suggesting that animals are not merely passive learners but actively process information.
Spatial Learning
Animals may have to move around in space for a variety of reasons: to find mates, to forage for food, to escape predators. Thus, spatial learning is of vital importance to a wide variety of animals. Spatial learning is an area of intense investigation under both laboratory and field conditions and includes a variety of topics, ranging from the navigational abilities of birds to maze learning in rats. Accordingly, spatial learning occupies the attention of an equally diverse array of investigators, ranging from evolutionary biologists to experimental psychologists.
Consider an application of evolutionary thinking to spatial learning. Gaulin and Fitzgerald (1989) reasoned that spatial abilities would be genetically selected-for more often in males than in females of polygymous species of meadow voles, because the male maintains a larger home range in which to seek potential mates or resources to attract mates. They compared the polygamous meadow voles to the monogamous pine voles. They found, first of all, that sex difference in favor of a larger home range occurred in male meadow voles but not male pine voles.They compared the two species on a variety of mazes of increasing difficulty, finding that males outperformed females in meadow voles but not in pine voles. Moreover, the hippocampus, which is considered to be of importance in spatial learning, was found to be larger in meadow voles than in pine voles (Jacobs, Gaulin, Sherry, & Hoffman, 1990).
Sex differences in spatial learning occur in a variety of species, including humans. Human males outperform human females in a variety of spatial learning tasks ranging from mental rotation of objects to map reading. Human females outperform human males in recalling the locations of objects interspersed in a room, and the difference is larger for incidental learning than for direct learning. Silverman and Eals (1992) interpret these sex differences in humans in evolutionary terms. It is believed that in the Pleistocene (the period in which much human evolution is considered to have occurred), males tended to hunt, and so traveled greater distances than females (favoring male spatial learning), whereas females gathered items such as vegetables (which favored learning the location of items by females).
Spatial learning thus provides a good illustration of the evolutionary approach to animal and human cognition. The evolutionary approach assumes that the cognitive ability possessed by a species was designed by evolution to meet the demands of its particular environment. Thus the evolutionary approach is fully prepared to discover that a given species may possess a unique adaptation. Unique adaptations are hardly rare, and several have already been mentioned: echolocation in bats, dancing in bees, language in humans. That each of these has been considered to be instinctive by some does not necessarily lessen their importance to cognition. For example, although our species’ability to master language may be instinctive to a great degree, there is reason to suppose that language development was a major factor in human problem solving, tool using, and related cognitive activities (see, e.g., Bickerton, 1998).
The radial maze mentioned earlier is an important tool used to investigate spatial learning and other problems in the laboratory. Apellet of food is placed at the end of each arm of the maze. The rat is placed on the central platform and is free to enter the arms. Rats easily master radial mazes, as indicated by their entering each arm only once. Efficient performance of this sort may itself have an instinctive or unlearned basis. It has long been known that in, say, a T-maze, the rats, having obtained food in one arm, typically avoid that arm in favor of responding to the other arm, a behavior sometimes called win-shift. In foraging in the wild it may be of benefit to animals such as rats to avoid going back to a location in which food was obtained because the availability of food at that source may be less likely than at some new source.
Not only are eight-arm radial mazes solved efficiently by rats, but so, too, are mazes containing a greater number of arms. The most impressive of these was a hierarchical maze employed by Roberts (1998). That maze consisted of eight primary arms. At the end of each primary arm were three branching secondary arms. The rats performed very well in this maze under a variety of conditions. For example, under one condition the rats were allowed to enter the primary arms with some of the secondary arms blocked off. In a subsequent test phase the rats entered the previously blocked secondary arms with a high degree of accuracy. This finding indeed suggests that the rat’s memory for spatial location is well developed. Another indication of the rat’s impressive memory in this situation is the difficulty of producing retroactive inhibition. In retroactive inhibition, as indicated earlier, memory of an initial task is reduced by provision of a subsequent task prior to testing of the initial task. Various means of producing retroactive inhibition in the radial maze have been used, including learning another maze in a different room. In an impressive experiment by Beatty and Shavalia (1980), rats exhibited little to no retroactive interference when they were required to enter four arms of a different radial maze within a 4 hr retention interval.
Rats can learn radial mazes by employing either distal cues, such as the shape of the room, landmark cues, such as objects in the environment, or intramaze cues, such as light differences in the appearance of the maze in different areas. In a very interesting experiment Williams and Meck (1991) reported that in the radial maze distal cues were used more often by males than by females, with landmarks cues being used more often by females than by males.
How animals represent spatial conditions is a topic of great interest. According to one view, animals such as rats may possess a cognitive map of the environment that consists of both a general framework, within which objects are located relative to each other, and general features of the environment, such as its shape (O’Keefe & Nadel, 1978). Gallistel (1990) provides a somewhat different definition of a cognitive map, in that it is evidenced by any orientation based upon computing distance. Others suggest that the concept of a cognitive map is not necessary to explain spatial learning.According to this view, animals acquire a set of memories of local views of the environment associated with the particular movements that take them from one place to another (e.g., Leonard & McNaughton, 1990).
As much as any topic, the ability of animals to go from one place to another brings together the topics of instinct and cognition. Consider the indigo bunting, a bird studied by Emlen (1970). The bird migrates over great distances. Yet, although migration is a species-typical behavior, specific migratory routes are learned by the bird by its exposure to the star pattern in the sky. In what follows it is possible to describe only a few examples of the many procedures that have been used to study map like learning in various species of animals.
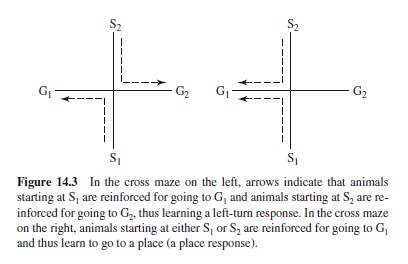
In going from one place to another, do animals learn to make specific responses or do they acquire more general, cognitively informed spatial information? An early and hotly contested attempt to resolve this issue involved what came to be known as the place versus response controversy. This may be illustrated by considering the two cross mazes shown in Figure 14.3.Two groups of rats might be used, both being trained to traverse the maze from each of two different start locations, S1 and S2. The difference is this: The group on the left is rewarded for making a specific response (turning left), going from S1 to G1 and from S2 to G2; the group on the right is rewarded for going to a specific place, from S1 to G1 and from S2 to G1. Thus the response group is rewarded for going to two different places whereas the place group is rewarded for making two different responses, left (S1–G1) and right (S2–G1). As a review (Restle, 1957) of the extensive literature in this area indicated, rats learn to do both. If trained in a visually rich, well-illuminated environment, the place group is superior to the response group. In an impoverished, dimly illuminated environment the response group is superior to the place group.
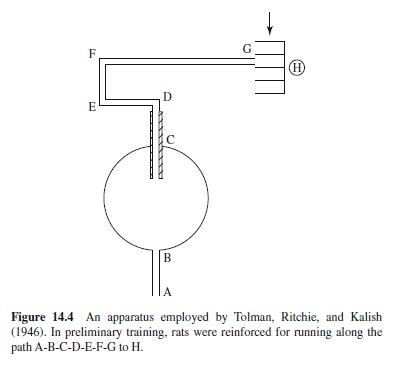
Another of the various procedures employed to determine whether rats learn specific responses or more general spatial information involved the maze shown in Figure 14.4. Path AB was the starting path. The rats ran along the paths B-C-DE-F-G. H was a dim light. Figure 14.5 shows how the rats were tested. Path AD was blocked. Path 5 led to the original goal and the dim light, H. If the animals were learning a specific response, then presumably they would begin by going left, selecting paths 10 to 18. If they were learning to go to a specific place (H) then they would select a novel initial response (go to the right) along paths 9 to 1. Of 56 rats that were tested, 3 failed to respond on the test trials. Of the remaining 53 rats, 36% selected Path 5; the distribution of choices for the remaining 34 rats is shown in Figure 14.6. If choices from paths 9 to 1 are regarded as novel (right turn instead of a left turn), then more than 87% of the rats selected a novel route different from that learned in initial training.
This ability to use a novel route to a goal has been examined in bees (J. L. Gould, 1986). Bees are first trained to go from the hive to Place A located to the west of the hive, as shown in Figure 14.7. Following this training, bees are caught and removed in an opaque box to a new spatial location to the south, Place B. Place A is not visible from Place B and the bees have never flown from B to A. What will the bees do? Will they return to the hive and then go from the hive to A? Or will they fly directly from B to A? Most bees fly directly from Place B to Place A. This behavior, Gould suggests, indicates that the bee is using a map-like representation of its environment.
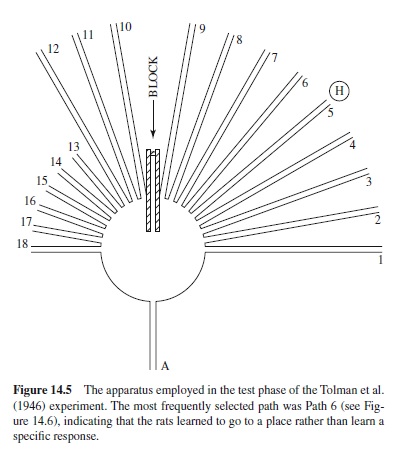
Since bees and rats can employ novel routes to reach a goal, it is perhaps not surprising that the chimpanzee can do the same. In an interesting experiment by Menzel (1973), a chimpanzee was carried on the shoulders of one experimenter around a large area (4,000 m2) while a second experimenter hid food in each of 18 randomly selected locations. The chimpanzees, when released, were highly successful in locating the hidden food, retrieving an average 13 of the 18 items. In so retrieving the hidden items, the animals neither followed the routes along which they had been taken, nor responded haphazardly and at random. Rather, they followed efficient routes that minimized the distance to the items.
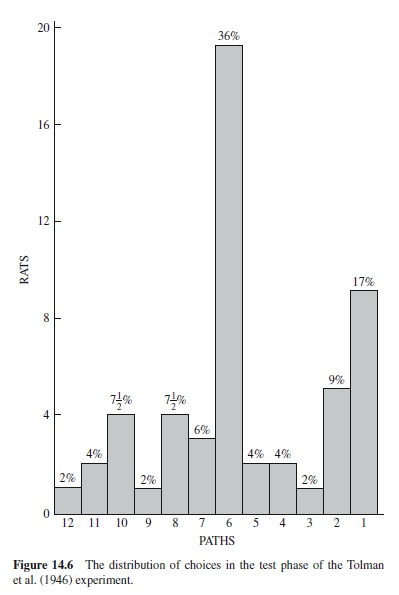
Another indication that chimpanzees can form a map-like representation of the environment comes from a study by Menzel, Premack, and Woodruff (1978). In that study, chimpanzees were shown an impoverished TV picture of where food was hidden in a rather complicated area containing trees, hills, and large objects. They were more successful in reaching the goal than were control animals given no information. Thus chimpanzees can match the information provided on a flat TV screen to spatial locations in the three-dimensional environment.

Language Learning in Animals
It is quite clear that animals communicate in the sense that certain behaviors in one animal are capable of producing specific and predictable behaviors in another animal. Thus, as indicated, a foraging bee’s dance when it returns to the hive indicates to the bee’s conspecifics the location of food (VonFrisch, 1950, 1967). As another example, vervet monkeys can communicate to their conspecifics whether an observed predator is a leopard, a snake, oraneagle (Seyfarth & Cheney, 1990). In the case of the vervet, it is quite clear that some degree of learning is involved. For example, young, inexperienced vervets may mistakenly give the eagle call on spying a nondangerous bird.
Hockett (1960) identified 13 characteristics of human language that can be presented in any system of communication. These are shown in Table 14.1.

Hockett (1960) suggests that characteristics 1 through 5 (vocal auditory channel, broadcast transmission and directional reception, rapid fading [transitoriness], interchangeability, and total feedback) are common to a variety of animal species, including some birds and many mammals. Characteristics 6 through 8 (specializing, semanticity, arbitrariness) are to be found in primates. Displacement, the ability to consider things that are remote in either space or time, can be found only in humans. Other particularly human characteristics are productivity (the ability to say novel things), duality of patterning (which refers to making words from phonemes), and arbitrariness (the fact that words do not resemble the objects they signify in any physical sense).
Although animals are capable of communication, that they are capable of acquiring language is another matter, and one fraught with continuing controversy. On the one hand, there can be little doubt that many of the investigators of ape language, such as the Rumbaughs (e.g., Rumbaugh, 1977) and the Gardners (e.g., 1969), are quite convinced that chimpanzees possess the capacity to acquire language or some aspects of language. On the other hand, other investigators of ape language such as Terrace (1979) are equally convinced that no such thing has been demonstrated. To complete the picture, importantly, many linguists and other language experts are strongly of the opinion that no animal has come even close to demonstrating the sort of language ability displayed by humans (see, e.g., Healy, 1980; Pinker, 1994). They point out, among other things, that although children acquire complex language skills practically effortlessly, heroics and extensive training efforts are required to get chimpanzees to master relatively simple skills that may, at best, approximate language.
Some early unsuccessful attempts to teach language to animals involved raising chimpanzees or other primates at home and tutoring them in spoken English (C. Hayes, 1951; K. J. Hayes & Hayes, 1952). It came to be realized that the chimpanzees’ vocal apparatus is not designed for speaking. Beatrice andAllen Gardner (1969) overcame the difficulty by teaching sign language to an infant, female chimpanzee named Washoe. After 51 months of training, Washoe had acquired 122 signals. It was asserted by the Gardners that Washoe could combine signals into phrases of some two to four items. An often-cited example is that Washoe gave two signs on seeing a swan: the sign for water followed by the sign for bird.
Another approach was to get Sarah, a now famous chimpanzee, to place symbols for objects on a board (Premack & Premack, 1983). Sarah was then thought to write sentences on the board. For example, Sara was given an apple if she placed on the board the symbols for give and apple.
Still another approach provided chimpanzees with keys on a computer exhibiting lexigrams. The lexigrams were different geometric patterns, each of which represented something such as a request (please) or an item (banana). Lana, one of the chimpanzees employed in this research, could request items by pressing the lexigrams in a particular order: please machine give banana.
Terrace (1979) trained a young chimpanzee named Nim Chimpsky (a play on the name of the famous linguist Noam Chomsky). Nim was taught sign language. As a result of his research, Terrace concluded that there was no evidence suggesting that Nim had learned language. Nim, it was concluded, was either imitating his trainer or was merely exhibiting a rather straightforward form of serial learning. Terrace’s criticisms brought into question any attempt to teach language to an animal that evolved stringing together items, such as gestures or lexigrams, because these could easily be interpreted as forms of serial learning based on learning simple associations.
Following Terrace’s telling criticisms, a new tactic was adopted by the Rumbaughs (e.g., Savage-Rumbaugh et al., 1993). Instead of attempting only to get animals to reproduce items such as signals or gestures, they also required the animals to comprehend items. For example, Kanzi, a pigmy chimpanzee (or bonobo), might be told “Kanzi go out to the hall and get the ball.” By teaching Kanzi many words, presenting him with many sentences, and issuing commands to him using different word orders (e.g., Savage-Rumbaugh et al., 1993), the researchers concluded that Kanzi had acquired language in a meaningful sense. Indeed, in terms of sentence compression Kanzi was said to rival a child, Alia, up until the time she became 2 years old, after which age Alia increasingly surpassed Kanzi. The approach involving the ability to comprehend language has also been employed with the dolphin (e.g., Herman, 1986).
If children are more or less totally deprived of language experience when quite young, they exhibit serious deficiencies in language use later in life (Bickerton, 1998; Candland, 1993). On the other hand, if children are provided with minimum language experienced they use language normally later in life. Indeed, as Bickerton (1998) has described, children deprived of normal language manage on their own to create a fully functional language in a single generation. This has occurred, for example, when people who speak many different languages are thrown together (as on plantations) and communicate by means of what is called a pidgin. Pidgins are very simple ways of communicating that consist of two to three words. Children exposed to a pidgin, in the absence of explicit instruction, create a much more sophisticated language called a Creole. A similar thing happens when deaf children are exposed to parents or others who may use sign language badly. These facts and others (see Pinker, 1994) have caused language experts to suggest that the language gulf between humans and other animals is a matter of kind and not of degree.
From an evolutionary standpoint, there is no particular reason that our closest relative, the chimpanzee, should possess even a rudimentary language ability. Humans and chimpanzees separated from each other—that is, no longer shared a common ancestor—about 5 to 7 million years ago. Language ability may have evolved in the human line after we separated from the chimpanzee. Perhaps species more closely related to ours, such as the extinct Homo erectus, possessed language, or at least something approximating human language. Whatever the truth may be, the language gulf between humans and extant animals such as the chimpanzee is very large and may be one of kind, not degree.
Pidgins, as indicated, are very simple ways of communicating and consist of a few words being strung together like beads on a string. Bickerton (1998) has suggested that animals such as chimpanzees and bonobos are capable, at most, of acquiring a pidgin. According to Bickerton, children under 2 years of age use a pidgin, which is acquired on the basis of general intellectual capacity. At above 2 years of age, children begin to acquire language on the basis of a mechanism specifically shaped by evolution for this purpose, and often called a language acquisition device. In Bickerton’s view, the language capacities of both mature apes and children under 2 years of age are on a par and do not represent true language. Interestingly, as indicated before, Kanzi, a bonobo, was able to follow verbal commands as well as Alia, a child, up until Alia was 2 years of age, whereupon Alia increasingly surpassed Kanzi in comprehension.
Experts are not agreed on many important aspects of language that may bear upon animal abilities along this line. To consider merely one line of disagreement, Pinker (1994) is of the opinion that language evolved slowly and in stages over the 350,000 or so generations separating our species from the chimpanzee. Bickerton (1998) believes that language evolved in two stages, a pidgin stage followed by a Creole-type stage. Pinker’s view allows that we might find in animals some intermediate language stage(s) between that of a pidgin and a Creole. Bickerton’s view does not allow this and suggests we will never observe in animals a form of language more complicated than a pidgin. Both Pinker and Bickerton think that language is a hardwired capacity of our species, independent of general intelligence. If, as some (e.g., S. J. Gould, 1987) think, language is a result of an increase of intelligence in humans, then it should be possible to find in animals increasingly better forms of language correlated with better intelligence. Although these are interesting speculations, definite and secure knowledge concerning animal language ability lies in future research.
Still another view suggesting that language in humans is fundamentally different from that in animals was suggested by Dunbar (1993). Dunbar suggests that language evolved in three principle stages. Australopithecus cines, an early ancestor, had a primate system of vocalization similar to that seen in present-day apes such as chimpanzees. This could not be called language. With Homo erectus, a close relative of our species, a simple kind of language evolved that prompted bonding between individuals. About 50,000 or so years ago, our own species, Homo sapiens, developed fully modern language that was employed for more than social boding. Essential language developed into a useful means for communicating abstract ideas. Thus Dunbar, like Bickerton and Pinker, is of the opinion that as far as language is concerned, the gulf between man and other animas is one of kind and not degree.
Evolution and Cognition
Several characteristics of the approach to animal and human cognition currently employed within experimental psychology are noteworthy. Workers in human cognition all but ignore animal learning and cognition. Workers in animal cognition, for the most part, employ a limited number of species, most notably rats and pigeons. The cognitive capacities isolated for examination in animal cognition are often those similar to those possessed in abundance by humans. Do animals possess numerical abilities? Can animals make inferences of the sort If A = B and B = C, then A = C? To what extent do human theories of serial learning apply to animals? Are animals, like humans, capable of self-recognition? By virtue of examining these and other questions much useful information has been provided for better understanding both animal and human cognition. Furthermore, there is ample reason for believing that at least in some cases similar processes are in operation over a broad range of animal species. The prime example of this is the sort of associative learning examined in Pavlovian and instrumental conditioning.
It is probably the case that most (if not all) experimental psychology and perhaps most social scientists accept evolution. This being so, Symons (1987) wrote a paper entitled “If we’re Darwinians, what’s the fuss about?” The fuss, according to Symons, is about this: “Perhaps the central issue of psychology is whether the mechanisms of the mind are few, general and simple, on the one hand, or numerous, specific and complex on the other” (p. 126). As indicated previously, and speaking generally, many experimental psychologists tend toward the former assumptions whereas many evolutionary biologists and psychologist tend toward the latter, and some incline toward the view that the modules are in communication with each other (Mithen, 1996). To employ metaphors, experimental psychologists might compare the mind to a general-purpose computer, whereas evolutionary biologists and psychologists often compare the mind to a Swiss army knife, a general-purpose tool having a diversity of different functions.
Evolution and Cognition: Implications
At one time, unlike now, animal and human learning and cognition were seen as highly related (see, e.g., McGeoch & Irion, 1952). Workers in the two areas shared a variety of important assumptions and considered, very probably, that choosing animals or humans for study involved little more than a strategic decision. If an attitude of common purpose was once the rule of the day, then it appears to be no more. On the one hand, many workers in human learning and cognition do not appear to regard animal learning and cognition as especially relevant to their concerns. It may be that many workers in human learning and cognition see the intellectual gap between our species and others to be very wide, so wide as to be one of kind and not of degree. According to this view, animal cognition has little to offer human cognition.
Interestingly, and perhaps ironically, many workers in animal learning and cognition may hold a contrary opinion, that is, that humans and animals differ cognitively, but only in degree. This inference seems to make sense when one examines some of the concerns popular in the study of animal cognition. As we have seen, workers in animal cognition are interested in determining the extent to which animals possess language, can use numerical information, imitate others, and so on. What these concerns have in common, of course, is that they are things that are done by humans and done very well. Needless to say, perhaps, these are legitimate problems, ones well worth studying.
The attitudes of workers in animal and human cognition may stem from the same source: an incorrect understanding of how evolution shapes cognitive adaptations. Both camps appear to accept some version of a continuity view, that as we go from so-called “lower” to “higher” animals, intellectual capacity increases. Workers in human learning and cognition, as indicated, appear to believe for whatever reason (e.g., that intermediate species became extinct) that human intellectual capacity, at least in some significant respects, differs from that of animals in kind and therefore that animal cognition may be ignored. Workers in animal learning and cognition also accept some version of the continuity view but appear to believe, generally speaking, that intellectual development of various sorts has progressed far enough in animals to make them useful objects of study. If the foregoing analysis is correct, then new more useful approaches to learning and cognition are being ignored by both animal and human workers.
There are certain implications that follow from an evolutionary approach embracing both discontinuity and continuity of intellectual development over species, which may well result in a closer, if not close, reuniting of animal and human learning and cognition. On the one hand, although there may well be some capacities unique to humans (e.g., the capacity for language), there are undoubtedly others that are widely shared over species and that, accordingly, should benefit from a comparative approach. A prime example here is spatial learning. As we saw, spatial learning is well developed in many species, and in some species (including our own), males seem better at spatial learning than females. This difference seems related to the different demands placed on males and females in the environment in which they evolved.
Thus, animals that had to move about a good deal, that had to go from place to place to find food or mates or to escape danger, would be expected to be better at spatial learning than animals that had fewer such demands. Note the difference between the emphasis here and that previously described as shared by many workers in human and animal cognition: The emphasis is not on the degree of correspondence between the cognitive capacity of humans versus other animals, but on the relation between particular animals and the specific demands of the environment in which those animals evolved. That is, to better understand the degree or kind of cognitive capacity an animal might possess, a first step might well involve a better acquaintance with the problems that animals faced in the environment in which it evolved.
In Pavlovian conditioning, as indicated, a tone may be presented a few seconds prior to food, and the relation between these events may be learned as indicated by the animal’s coming, with training, to salivate to the tone. Pavlovian learning of this sort appears to be common in a variety of species (see, e.g., Wasserman & Berglan, 1998). Presumably this is because learning such Pavlovian relationships is important to survival in many species. That is, it is hardly uncommon for events of biological significance to be reliably signaled by other events—a rustle of leaves indicating an approaching predator, circling vultures indicating a dead animal and a potential meal, and so on. If this is so, then as expected, learning Pavlovian relations is common to a wide variety of species.
If, as indicated, regularities in the environment play an important role in shaping an animal’s cognitive capacities, then it becomes incumbent upon investigations to obtain knowledge about regularities and behavior in that environment. We have already seen how our understanding of spatial learning in animals and humans has benefited from such an approach. To suggest another familiar example, we have seen how our understanding of language has benefited from examining real-life situations in which children exposed to a pidgin develop on their own, within a single generation, a more complex form of language called a Creole. As another example, observations of chimpanzees in the wild suggest that they possess a high level of cognitive capacity that might otherwise go unnoticed. Field observation of 14 different groups of widely separated chimpanzees suggests that they may be characterized as possessing a culture (Whiten & Boesch, 1999). By culture it is meant that the various groups of chimpanzees engage in complex behaviors that are obviously learned and, in addition, are passed on from one generation to the next.
An outstanding example of such complex behavior is termite fishing, in which chimpanzees insert thin, flexible strips of bark into termite mounds to extract the insects, which they eat. This behavior is practiced by 8 of the 14 groups of chimpanzees observed. However, it is practiced differently by different groups. For example, once the insects have swarmed up the stick, 3 of the 8 groups pull the stick through their fists and eat the gathered prey. This technique is used by some other groups of chimpanzees who also pull the stick through their mouths. To cite another complex behavior, 3 of the 14 groups of chimpanzees use small sticks to remove bone marrow inside the bones of monkeys they have killed. The point here, at least as suggested by Whiten and Boesch (1999), is not merely that termite fishing and removing bone marrow with sticks are complex learned behaviors, but that they are passed on from one generation to the next, and so, in the opinion of some, indicate that chimpanzees possess a culture. Whiten and Boesch (1999) indicate that still other animals may possess a culture, as (for example) populations of whales that sing in different dialects and hunt in different ways. Are the sorts of behaviors engaged by chimpanzees and possibly other animals, such as whales, really manifestations of culture, on par in some respects with human culture as suggested by Whiten and Boesch (1999)—or is it something simpler? It may be, for example, that termite fishing is composed of many component behaviors, gradually and accidentally learned over time by one or two members of a chimpanzee group, which are then acquired by other members according to the same principles that explain other forms of instrumental learning. Laboratory studies that, for example, compare learning by imitation in chimpanzees with such learning in a variety of other animals, including humans and birds, can help determine whether ascribing culture to chimpanzees is valid and useful. The plain implication of the previous analysis is that experimental psychologists, if they are to better understand learning and cognition, must supplement laboratory studies of learning and cognition with real-life field studies of animals dealing with problems of evolutionary adaptation in their environments.
Although the strategy just outlined is already being followed in some cases, unfortunately, the practice is less widespread than is desirable. However, it does seem to be growing. For example, the behavior system approach assumes that in Pavlovian conditioning, an unconditioned stimulus (UCS) such as food or shock activates an underlying organization of behavior appropriate to that UCS (for the food UCS, the feeding system, for shock, the defensive system). According to this view, a conditioned stimulus (CS) will come to elicit responses that are components of the behavior system activated by the UCS. Thus, not only must preorganized response systems be considered but also a particular species’sensitivity to different stimuli and its underlying motivational system. The usefulness of this approach has been demonstrated in a variety of laboratory experiments that were cognizant of the animals’ species-typical behaviors. As merely one example, Timberlake and Washburne (1989) examined predation in seven different species of rodents. Characteristic differences in prey-capture of crickets by the rodents were related to species-typical behavior (e.g., more carnivorous species captured prey more quickly and reliably), and the response of each species to a CS, a rolling ball bearing, resembled that particular species’typical response to actual prey.
Felial imprinting (e.g., baby ducklings’ following a parent) is another example of how an understanding of species-typical behavior may be profitably used to conduct and illuminate laboratory studies. Some of the so-called “attributes” of imprinting based on field studies have been shown by laboratory studies not to be entirely correct. For example, the supposed irreversibility of imprinting was questioned by Salzen and Meyer (1968), who showed that chicks imprinted on one ball could be shifted to another ball and reversed. Other important characteristics ascribed to imprinting (e.g., that imprinting is a specialized form of learning, differing in several important respects from other forms of learning) seem more feasible (see, e.g., Bateson, 1990). For example, imprinting occurs more readily to species-typical stimuli than to artificial stimuli.
Of course, it is not only the study of animal cognition that can benefit from a better understanding of species-typical behavior. We have seen, for example, that children exposed to a pidgin convert it to a more complex Creole in a single generation, supplying a good reason for believing, as Pinker (1994) asserts, that language is an instinct. Perhaps we will come to better understand this language instinct as we learn more about other complex (or even simple) instincts in humans and other animals. That is, similar processes may underlie various complex instincts, such as the language instinct in humans and echolocation in bats.
What the future may hold is difficult to say. However, there are signs that the current divide between animal and human learning and cognition may diminish, returning us to a view more commonly held in the 1930s, 40s, and 50s. This time around it may be a much better informed view. It should benefit from three areas that are much more sophisticated today than in the past: cognitive neuroscience (e.g., Rapp, 2001), behavior genetics (e.g., Bailey, 1998), and evolutionary psychology (e.g., Crawford & Krebs, 1998). Some would argue that opening laboratory psychology up to these more biologically informed influences should help to place the field in a more proper, and thus more useful, perspective. That is, it should moderate what some see as the extreme environmentalism that has dominated psychology during the past 75 years or so (see, e.g., Pinker, 1994; Tooby & Cosmides, 1992), replacing it with a more balanced view. This more balanced view would hold that environmental influences are indeed important, but that these work through evolved mechanisms to determine behavior. Two prime examples of such evolved mechanisms cited in this research paper are the language acquisition device of humans, and mechanisms of echolocation in bats.
Bibliography:
- Asendorpf, S. B., Warkentin, V., & Baudonniere, P. M. (1996). Social awareness and other awareness: Vol. 2. Mirror selfrecognition, social contingency awareness and synchronic imitation. Developmental Psychology, 32, 313–321.
- Bailey, J. M. (1998). Can behavior genetics contribute to evolutionary behavioral science? In C. Crawford & D. L. Krebs (Eds.), Handbook of evolutionary psychology: Ideas, issues, and applications (pp. 211–233). Mahwah, NJ: Erlbaum.
- Baron-Cohen, (1995). Mindblindness. Cambridge, MA: MIT Press.
- Bateson, P. P. G. (1990). Is imprinting such a special case? Philosophical Transaction of the Royal Society of London, 329B, 125–131.
- Beatty, W. W., & Shavalia, D. A. (1980). Spatial memory in rats: Time course of working memory and effects of anesthetics. Behavioral and Neural Biology, 28, 454–462.
- Bickerton, D. (1998). The creation and re-creation of language. In C. Crawford & D. L. Krebs (Eds.), Handbook of evolutionary psychology: Ideas, issues, and applications (pp. 613–634). Mahwah, NJ: Erlbaum.
- Boysen, S. T., & Berntson, G. G. (1989). Numerical competence in a chimpanzee (Pan Troglodytes). Journal of Comparative Psychology, 103, 23–31.
- Boysen, S. T., & Capaldi, E. J. (Eds.). (1993). The development of numerical competence: Animal and human models. Hillsdale, NJ: Erlbaum.
- Brannon, E. M., & Terrace, H. S. (2000). Representation of numerosities 1–9 by Rhesus Macaques (Macaca mulatta). Journal of Experimental Psychology: Animal Behavior Processes, 26, 31–49.
- Burns, R. A., Dunkman, J. A., & Detloff, S. L. (1999). Ordinal position in the serial learning of rats. Animal Learning & Behavior, 27, 272–279.
- Byrne, R. W. (1998). So much easier to attract straw men. Behavior and Brain Sciences, 21, 116–117.
- Candland, D. K. (1993). Feral children and clever animals. New York: Oxford University Press.
- Capaldi, E. J. (1992). The organization of behavior. Journal of Applied Behavior Analysis, 25, 575–577.
- Capaldi, E. J. (1993). Animal number abilities: Implications for a hierarchical approach to instrumental learning. In S. T. Boysen & E. J. Capaldi (Eds.), The development of numerical competence: Animal and human models (pp. 191–209). Hillsdale, NJ: Erlbaum.
- Capaldi, E. J. (1994). The sequential view: From rapidly fading stimulus traces to the organization of memory and the abstract concept of number. Psychonomic Bulletin & Review, 1, 156–181.
- Capaldi, E. J. (1998). Counting behavior. In G. Greenberg & M. M. Haraway (Eds.), Comparative psychology: A handbook (pp. 817–822). New York: Garland Publishing.
- Capaldi, E. J., Alptekin, S., & Birmingham, K. M. (1996). Instrumental performance and time between reinforcements: Intimate relation to learning or memory retrieval? Animal Learning & Behavior, 24, 211–220.
- Capaldi, E. J., Birmingham, K. M., & Miller, R. M. (1999). Forming chunks in instrumental learning: The role of overshadowing. Animal Learning & Behavior, 27, 221–228.
- Capaldi, E. J., & Lynch, D. (1966). Patterning at 24-hour ITI: Resolution of a discrepancy more apparent than real. Psychonomic Science, 6, 229–230.
- Capaldi,E.J.,&Minkoff,R.(1966).Rewardscheduleeffectsatarelatively long intertrial interval. Psychonomic Science, 6, 321–322.
- Capaldi, E. J., & Miller, D. J. (1988a). Counting in rats: Its functional significance and the independent cognitive processes that constitute it. Journal of Experimental Psychology: Animal Behavior Processes, 14, 3–17.
- Capaldi, E. J., & Miller, D. J. (1988b). Number tags applied by rats to reinforcers are general and exert powerful control over responding. Quarterly Journal of Experimental Psychology, 40B, 279–297.
- Capaldi, E. J., & Miller, R. M. (2001a). Molar vs. molecular approaches to reward schedule and serial learning phenomena. Learning and Motivation, 32, 22–35.
- Capaldi, E. J., & Miller, R. M. (2001b). Stimulus control of anticipatory responding in instrumental learning. Animal Learning & Behavior, 29, 165–175.
- Church, R. M., & Deluty, M. Z. (1977). Bisection of temporal intervals. Journal of Experimental Psychology: Animal Behavior Processes, 3, 216–228.
- Church, R. M., & Gibbon, J. (1982). Temporal generalization. Journal of Experimental Psychology: Animal Behavior Processes, 8, 165–186.
- Church, R. M., Meck, W. H., & Gibbon, J. (1994). Application of scalar timing theory to individual trials. Journal of Experimental Psychology: Animal Behavior Processes, 20, 135–155.
- Crawford, C., & Krebs, D. L. (Eds.). (1998). Handbook of evolutionary psychology: Ideas, issues, and applications. Mahwah, NJ: Erlbaum.
- D’Amato, M. R., & Colombo, M. (1988). Representation of serial order in monkeys (Cebus apella). Journal of Experimental Psychology: Animal Behavior Processes, 14, 131–139.
- Darwin, C. (1871). The descent of man and selection in relation to sex. London: John Murray.
- Davis, H., & Pérusse, R. (1988). Numerical competence in animals: Definitional issues, current evidence, and a new research agenda. Behavioral and Brain Sciences, 11, 561–579.
- Dawkins, R. (1976). The selfish gene. London: Oxford University Press.
- Dawkins, R. (1996). The blind watchmaker: Why the evidence of evolution reveals a universe without design. New York: W. W. Norton.
- Dunbar, R. I. M. (1993). Coevolution of neocortical size, group size, and language in humans. Behavioral and Brain Sciences, 16, 681–737.
- Emlen, S. T. (1970). Celestial rotation: Its importance in the development of migratory orientation. Science, 170, 1198–1201.
- Epstein, R., Lanza, R. P., & Skinner, B. F. (1981). “Self-awareness” in the pigeon. Science, 212, 695–696.
- Fodor,J.(1983).Cambridge,MA:MITPress.
- Gallistel, C. R. (1990). The organization of learning. Cambridge, MA: The MIT Press.
- Gallistel, C. R. (1994). Space and time. In N. J. Mackintosh (Ed.), Animal learning and cognition (pp. 221–253). San Diego, CA: Academic Press.
- Gallistel, C. R., & Gibbon, J. (2000). Time, rate and conditioning. Psychological Review, 107, 289–344.
- Gallup, G. G. (1970). Chimpanzees: Self-recognition. Science, 167, 86–87.
- Gallup, G. G. (1977). Self-recognition in primates. American Psychologist, 32, 329–338.
- Gallup, G. G. (1982). Self-awareness and the emergence of mind in primates. American Journal of Primatology, 2, 237–248.
- Gardner, R. A., & Gardner, B. T. (1969). Teaching sign language to a chimpanzee. Science, 165, 664–672.
- Gaulin, S. J. C., & Fitzgerald, R. W. (1989). Sexual selection for spatial-learning ability. Animal Behavior, 37, 322–331.
- Gelman, R., & Gallistel, C. R. (1978). The child’s understanding of number. Cambridge, MA: Harvard University Press.
- Goldstone, R. L., & Kersten, A. (in press). Concepts and categorization. In A. F. Healy & R. W. Proctor (Eds.), Comprehensive handbook of psychology: Vol. 4. Experimental sychology. New York: Wiley.
- Gordon, R. M. (1998). The prior question: Do primates have a theory of mind? Behavior and Brain Sciences, 21, 120–121.
- Gould, J. L. (1986). The locale map of honey bees: Do insects have cognitive maps? Science, 232, 861–863.
- Gould, S. J. (1987). The limits of adaptation: Is language a spandrel of the human brain? Paper presented at the Cognitive Science Seminar for Cognitive Science, Cambridge, MA, MIT.
- Griffin, D. R. (1992). Animal minds. Chicago: University of Chicago Press.
- Haggbloom, S. J., Birmingham, K. M., & Scranton, D. L. (1992). Hierarchical organization of series information by rats: Series chunks and list chunks. Learning and Motivation, 23, 183–199. Hayes, C. (1951). The ape in our house. New York: Harper.
- Hayes, K. J., & Hayes, C. (1952). Imitation in a home-raised chimpanzee. Journal of Comparative and Physiological Psychology, 45, 450–459.
- Healy, A. F. (1980). Can chimpanzees learn a phonemic language? In T. A. Sebeok & J. Umiker-Sebeok (Eds.), Speaking of apes: A critical anthology of two-way communication with man (pp. 141–143). New York: Plenum Press.
- Herman, L. M. (1986). Cognition and language competencies of bottlenosed dolphins. In R. J. Schusterman, J. A. Thomas, & F. G. Wood (Eds.), Dolphin behavior and cognition: Comparative andecologicalaspects(pp.221–252).Hillsdale,NJ:Erlbaum.
- Herrnstein, R. J. (1979). Acquisition, generalization, and discrimination reversal of a natural concept. Journal of Experimental Psychology: Animal Behavior Processes, 5, 116–129.
- Heyes, C. M. (1998). Theory of mind in nonhuman primates. Behavior and Brain Sciences, 21, 101–168.
- Heyes, C. M. (1996). Genuine imitation. In C. M. Heyes & B. Galef (Eds.), Social learning in animals: The roots of culture (pp. 53–71). San Diego, CA: Academic Press.
- Hockett, C. F. (1960). Logical considerations in the study of animal communication. In W. E. Lanyon & W. N. Tavolga (Eds.), Animal sounds and communication (pp. 392–430). Washington, DC: American Institute of Biological Sciences.
- Hoffman, H. S. (1978). Experimental analysis of imprinting and its behavioral effects. The Psychology of Learning and Motivation, 12, 1–37.
- Hull, C. L. (1943). Principles of behavior: An introduction to behavior theory. New York: Appleton-Century.
- Hulse, S. H., & Dorsky, N. P. (1977). Structural complexity as a determinant of serial pattern learning. Learning and Motivation, 8, 488–506.
- Hulse, S. H., Fowler, H., & Honig, W. K. (Eds.). (1978). Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum.
- Hunter, W. S. (1913). The delayed reaction in animals and children. Behavior Monographs, 2, 1–86.
- Jacobs, L. F., Gaulin, S. J. C., Sherry, D., & Hoffman, G. E. (1990). Evolution of spatial cognition: Sex-specific patterns of spatial behavior predict hippocampal size. Proceedings of the National Academy of Sciences, USA, 87, 6349–6352.
- Killeen, P. R., & Fetterman, J. G. (1988). Abehavioral theory of timing. Psychological Review, 95, 274–295.
- Lashley, K. S. (1929). Brain mechanisms and intelligence. Chicago: University of Chicago Press.
- Lashley, K. S. (1951). The problem of serial order in behavior. In L. A. Jeffries (Eds.), Cerebral mechanisms in behavior (pp. 112–136). New York: Wiley.
- Leonard, B., & McNaughton, B. L. (1990). Spatial representation in the rat: Conceptual, behavior, and neurophysiological perspectives. In R. P. Kesner & D. S. Olton (Eds.), Neurobiology of comparative cognition (pp. 363–422). Hillsdale, NJ: Erlbaum.
- Mackintosh, N. J. (1975). Atheory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review, 82, 276–298.
- Mackintosh, N. J. (1995). Categorization by people and by pigeons: The twenty-second Bartlett memorial lecture. Quarterly Journal of Experimental Psychology, 48B, 193–214.
- Marler, P. (1987). Sensitive periods and the roles of specific and generally sensory stimulation in birdsong learning. In J. P. Rauschecker & P. Marler (Eds.), Imprinting and cortical plasticity (pp. 99–135). New York: Wiley.
- Marler, P., & Peters, P. (1989). Species differences in auditory responsiveness in early vocal learning. In R. J. Dooling & S. H. Hulse (Eds.), The comparative psychology of audition: Perceiving complex sounds (pp. 243–273). Hillsdale, NJ: Erlbaum.
- McGeoch, J. A., & Irion, A. L. (1952). The psychology of human learning (2nd ed.). New York: Longmans, Green.
- Menzel, E. W. (1973). Chimpanzee spatial memory. Science, 182, 943–945.
- Menzel, E. W., Premack, D., & Woodruff, G. (1978). Map reading by chimpanzees. Folia Primatologica, 29, 241–249.
- Miller, R. R., & Grace, R. C. (in press). Conditioning and learning. In A. F. Healy & R. W. Proctor (Eds.), Comprehensive handbook of psychology: Vol. 4: Experimental psychology. New York: Wiley.
- Mineka, S., & Cook, M. (1988). Social learning and the acquisition of snake fear in monkeys. In T. Zentall & B. G. Galef, Jr. (Eds.), Social learning (pp. 51–73). Hillsdale, NJ: Erlbaum.
- Mithen, S. (1996). The prehistory of the mind. London: Thames and Hudson.
- O’Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford, UK: Clarendon Press.
- Pearce, J. M., & Hall, G. (1980). A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review, 87, 532–552.
- Peterson, I. (2000). Calculating swarms: Ant teamwork suggest models for computing faster and organizing better. Science News, 158, 314–316.
- Pinker, S. (1994). The language instinct: How the mind creates language. New York: William Morrow.
- Premack, D., & Premack, A. J. (1983). The mind of an ape. New York: W. W. Norton.
- Premack, D., & Woodruff, G. (1978). Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences, 4, 515–526.
- Rapp, B. (Ed.). (2001). The handbook of cognitive neuropsychology: What deficits reveal about the human mind. Philadelphia: Psychology Press.
- Restle, F. (1957). Discrimination of cues in mazes: A resolution of the place vs. response question. Psychological Review, 64, 217–228.
- Roberts, W. A. (1981). Retroactive inhibition in rat spatial memory. Animal Learning & Behavior, 9, 566–574.
- Roberts, W. A. (1998). Principles of animal cognition. New York: McGraw-Hill.
- Romanes, G. J. (1882). Animal intelligence. New York: Appleton.
- Rumbaugh, D. M. (Ed.). (1977). Language learning by a chimpanzee. New York: Academic Press.
- Salzen, E. A., & Meyer, C. C. (1968). Reversibility of imprinting. Journal of Comparative and Physiological Psychology, 66, 269–275.
- Savage-Rumbaugh, E. S., Murphy, J., Sevcik, R. A., Brakke, K. E., Williams, S. L., & Rumbaugh, D. M. (1993). Language comprehension in ape and child. Monographs of the Society for Research in Child Development, 58(3–4).
- Seyfarth, R., & Cheney, D. (1990). The assessment by vervet monkeys of their own and another species’ alarm calls. Animal Behaviour, 40, 754–764.
- Silverman, I., & Eals, M. (1992). Sex differences in spatial abilities: Evolutionary theory and data. In J. H. Barkow, L. Cosmides, & J. Tooby (Eds.), The adapted mind: Evolutionary psychology and the generation of culture (pp. 533–549). New York: Oxford University press.
- Skinner, B. F. (1938). The behavior of organisms. New York: Appleton-Century-Crofts.
- Skinner, B. F. (1950). Are theories of learning necessary? Psychological Review, 57, 193–216.
- Spear, N. E., & Riccio, D. C. (1994). Memory: Phenomena and principles. Boston: Allyn & Bacon.
- Spelke, E. S. (2001). Core knowledge. American Psychologist, 55, 1233–1243.
- Spence, K. W. (1936). The nature of discrimination learning in animals. Psychological Review, 43, 427–449.
- Spence, K. W. (1937). The differential response in animals to stimuli varying within a single dimension. Psychological Review, 44, 430–444.
- Sutherland, N. S., & Mackintosh, N. J. (1971). Mechanisms of animal discrimination learning. New York: Academic Press.
- Symons, D. (1987). If we’re all Darwinians, what’s the fuss about? In C. B. Crawford, M. F. Smith, & D. L. Krebs (Eds.), Sociobiology and psychology (pp. 121–146). Hillsdale, NJ: Erlbaum.
- Symons, D. (1992). On the use and misuse of Darwinism in the study of human behavior. In J. H. Barkow, L. Cosmides, & J. Tooby (Eds.), The adapted mind: Evolutionary psychology and the generation of culture (pp. 137–159). New York: Oxford University Press.
- Terrace, H. S. (1979). Nim: A chimpanzee who learned sign language. New York: Knopf.
- Terrace, H.S. (1986). A nonverbal organism’s knowledge of ordinal position in a serial learning task. Journal of Experimental Psychology: Animal Behavior Processes, 12, 203–214.
- Thorndike, E. L. (1911). Animal intelligence: Experimental studies. New York: Macmillan.
- Timberlake, W., & Washburne, D. L. (1989). Feeding ecology and laboratory predatory behavior toward live and artificial moving prey in seven rodent species. Animal Learning & Behavior, 17, 2–11.
- Tolman, E. C. (1932). Purposive behavior in animals and men. New York: Appleton-Century-Crofts.
- Tolman, E. C. (1948). Cognitive maps in rats and men. Psychological Review, 55, 189–208.
- Tolman, E. C., Ritchie, B. F., & Kalish, D. (1946). Studies in spatial learning: Vol. 1. Orientation and the short-cut. Journal of Experimental Psychology, 36, 13–24.
- Tooby, J., & Cosmides, L. (1992). The psychological foundations of culture. In J. H. Barkow, L. Cosmides, & J. Tooby (Eds.), The adapted mind: Evolutionary psychology and the generation of culture (pp. 19–136). New York: Oxford University
- Vaughan, W., Jr., & Greene, S. L. (1984). Pigeon visual memory capacity. Journal of Experimental Psychology: Animal Behaviour Processes, 10, 256–271.
- Von Frisch, K. (1950). Bees, their vision, chemical senses and language. Oxford, UK: Oxford University Press.
- Von Frisch, K. (1967). The dance language and orientation of bees. Cambridge, MA: Belknap Press of Harvard University Press.
- Wagner, A. R. (1976). Priming in STM: An information processing mechanism for self-generated and retrieval-generated depression in performance. In T. J. Tighe & R. N. Leaton (Eds.), Habituation: Perspectives from child development, animal behavior, and neurophysiology (pp. 95–128). Hillsdale, NJ: Erlbaum.
- Wasserman, E. A., & Astley, S. L. (1994). A behavioral analysis of concepts: Its application to pigeons and children. Psychology of Learning and Motivation, 31, 73–132.
- Wasserman, E. A., & Berglan, L. R. (1998). Backward blocking and recovery from overshadowing in human causal judgement: The role of within-compound associations. Quarterly Journal of Experimental Psychology, 51B, 121–138.
- Watanabe, S., Lea, S. E. G., & Dittrich, W. H. (1993). What can we learn from experiments on pigeon concept discrimination? In H. P. Zeigler & H.-J. Bischof (Eds.), Vision, brain, and behavior in birds (pp. 351–376). Cambridge, MA: MIT Press.
- Wendt, G. R. (1937). Two and one-half year retention of a conditioned response. Journal of General Psychology, 17, 178–180.
- Whiten, A. J., & Boesch, C. (1999). Chimps employ culture to branch out. Sciences News, 155, 13–15.
- Williams, C. L., & Meck, W. H. (1991). The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology, 16, 155–176.
- Wright, A. A., Urcuioli, P. J., & Sands, S. F. (1986). Proactive interference in animal memory. In D. F. Kendrick, M. E. Rilling, & M. R. Denny (Eds.), Theories of animal memory (pp. 101–125). Hillsdale, NJ: Erlbaum.




