View sample cancer research paper on colorectal cancer. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Epidemiology
Incidence
In terms of incidence, colorectal cancer ranks fourth in frequency among males, with approximately 550 000 new cases a year worldwide, and third among females, with an estimated 473 000 new cases. The age-standardized incidence is 20.1 per 100 000 a year for males and 14.6 for females. Mortality is about one-half that of incidence with approximately 530 000 deaths in 2002 (males, 280 000; females, 250 000). With an estimated 2.8 million persons alive within 5 years of diagnosis, colorectal cancer represents 11.5% of all prevalent cancer cases, the second most prevalent cancer in males after prostate cancer (1 515 000 cases) and the third most prevalent cancer in females after breast and cervix uteri (1 315 000 cases).
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
In general, the incidence of colorectal cancer is high in developed countries (North America, Western Europe, Australia, New Zealand, and Japan) and low in Africa and Asia, with incidence rates varying by as much as 25-fold worldwide. In high-risk populations, the ratio of incidence between colon and rectal cancer incidence is 2:1 or more, whereas colon and rectal cancer rates are generally similar in low-risk countries. The geographical differences in incidence rates, and the disparity in colon and rectal cancer, are likely to be due to environmental exposures and dietary factors.
Colorectal cancer is rare before the age of 40. Incidence increases significantly in each successive decade of age. The lifetime incidence of sporadic colorectal cancer is approximately 5%, with nearly 50% of the cases occurring after the age of 70.
Incidence rates have been declining in the United States since 1985. This decline has been attributed to increased screening and therapeutic interventions, following the then U.S. President Ronald Reagan’s diagnosis of colorectal cancer in 1985. Incidence rates have tended to stabilize in recent years in Western Europe, suggesting that similar trends toward a decline may be seen in the near future. On the other hand, incidence is increasing rather rapidly in countries where overall risk was formerly low, especially in Japan. A proportional shift in tumors from the rectum and sigmoid to the colon has been reported.
Survival
Improvement in survival has been reported recently in some European countries and in the United States. In Europe, there was an overall 10% increase in relative survival between 1978–80 and 1987–89, more marked in Western than Northern Europe. Improved diagnostic and treatment procedures, allowing earlier diagnosis and a more effective treatment, with reduction of perioperative mortality and better adjuvant chemotherapy are all potential explanations for the improvement in colorectal cancer survival.
Population-based data from cancer registries suggest that colorectal cancer survival is higher in the United States than in Europe. The overall relative survival was 65.2% in the United States for the 1991–2000 period, whereas the European average 5-year relative survival was only 51% for colon cancer and 48% for rectal cancer. One study (Gatta, 2003) reported that the survival advantage for colorectal cancer patients in the United States can only be partly explained by differences in the distribution of subsite and morphology and that the main explanatory difference was the proportion of adenocarcinomas arising in a bowel polyp (13% in the United States vs. 2% in Europe). In this study (Gatta, 2003), the proportion of cases with unspecified subsite was higher in Europe than the United States (10% vs. 2%) and the 5-year survival 13–22% higher in the United States than in Europe for each anatomical subsite. Five-year survival was also higher in the United States than Europe for each morphological group, with the exception of non-microscopically verified cases, which were more frequent in the United States than in Europe (16% vs. 3%). Survival data for the United States, obtained from nine cancer registries in the U.S. Surveillance, Epidemiology and End-Results (SEER) study, may be subject to methodological flaws and are not fully representative of the United States as a whole either. To date, there is no definitive explanation to this survival difference. The first population-based survival comparison between the United States and Europe since the 1960s, the CONCORD study, is currently in progress and will provide new data.
There are important regional differences in survival across Europe reflecting differences in the effectiveness of health-care systems, availability of diagnostic and therapeutic facilities, and quality of treatment. Whereas the 5-year relative survival for men with colon cancer is higher than 55% in Spain, France or Austria, it is below 45% in Denmark, Wales or countries in Eastern Europe (26.3% for Poland). Relative survival rates for men diagnosed with colon cancer during 1982–92 in five developing countries – China, Cuba, India, the Philippines, and Thailand – ranged from 28% to 42%.
Stage at diagnosis is probably the major prognostic factor. It has recently improved in many countries because of better information with earlier visits to the doctor for digestive tract symptoms and more widespread use of endoscopy. The proportion of tumors diagnosed at an advanced stage remains too high, however, with 20–30% of patients having clinically detectable metastases at the time of the initial diagnosis. Stage at diagnosis is the major determinant of survival differences between European populations.
Age is also a major prognostic factor. In Europe, 5-year relative survival for colon cancer declines slowly with increasing age up to 74 years (from 56% to 51% between 45–54 years to 65–74 years), but fell sharply in patients over 74 years (46%). The prognosis of elderly patients is bad and, contrary to what was observed for younger patients, there was no significant change for survival in elderly patients. Those patients are more likely to be diagnosed with advanced stage and less likely to receive optimal treatment. This is a major public health problem since, in the near future, the majority of colorectal cancers will occur in patients aged 75 and above.
It is well established that socioeconomic status is a major prognostic factor for many cancers, including colorectal cancer. In England and Wales, improvements in survival were usually greater for those living in affluent areas than those in deprived areas, even after correction for the widening differences in overall mortality between rich and poor. The overall increase in survival during the 1990s was significantly associated with a widening deprivation gap in survival, suggesting the influence of delay or less effective access to diagnosis and treatment for patients living in more deprived areas.
In the United States, racial disparities are particularly pronounced. Whereas colorectal cancer mortality rate decreased approximately 1.9% a year for white patients, it has fallen by 0.8% a year for African-American patients. The 5-year relative survival was 65% for Whites and 55% for African-Americans. Much of this difference in survival is explained by differences in stage at diagnosis. This apparent race effect is in fact a socioeconomic effect: African-Americans have lower median incomes, are more likely to be poor, less likely to have health insurance, and more likely to have less than a high school education than white patients.
Risk Factors And Causes
The risk factors for colorectal cancer are both environmental and genetic. There is no family history in approximately 70% of colorectal cancer, and these cases are considered to be sporadic. Roughly 10–30% of colorectal cancers occur among patients with a familial risk. Fewer than 5–10% of colorectal cancer cases develop in the setting of defined hereditary cancer syndrome with an inherited predisposition related to specific germline mutation. Familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC, or Lynch syndrome) are the most common of these familial colon cancer syndromes (Figure 1).
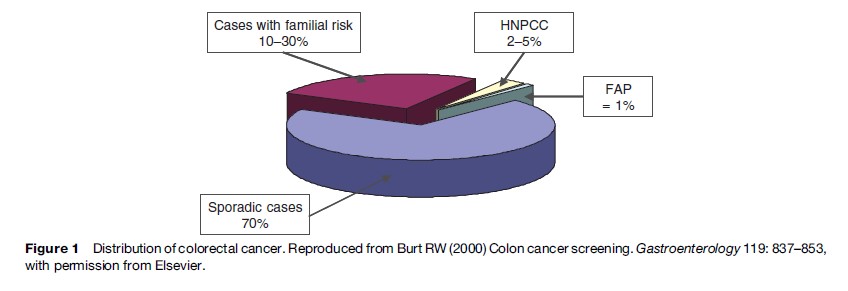
Familial Cancer Syndromes
Familial Adenomatous Polyposis
FAP is caused by a germline mutation in the adenomatous polyposis coli (APC) gene and inherited in an autosomal dominant pattern. FAP and its variants account for less than 1% of colorectal cancers. Patients with the classical form of FAP develop hundreds to thousands of colon polyps, usually starting in their teenage years. All patients will develop colorectal cancer from the colon polyps by age 40 and must be offered a prophylactic colectomy to prevent cancer. A milder type of familial adenomatous polyposis, called autosomal recessive familial adenomatous polyposis, has been identified and is related to mutations of the MYH gene. People with the autosomal recessive type have fewer polyps than those with the classic type.
Hereditary Nonpolyposis Colorectal Cancer
HNPCC, Lynch syndrome, is an autosomal dominant syndrome that accounts for 2–3% of colorectal cancers. It is caused by germline mutations of mismatch repair genes (see the section titled ‘Pathogenesis’). Colon cancers in HNPCC are characterized by early age of onset and predominant involvement in the right (or ascending) side (or part) of the colon. Cancer risk is also increased for endometrial, ovary, stomach, small bowel, the hepatobiliary system, and the urinary system. Clinical criteria defining HNPCC have been developed (Amsterdam criteria, Bethesda guidelines).
Genetic counseling is mandatory when a familial cancer syndrome is suspected.
Familial Or Personal History Of Colorectal Cancers Or Adenomatous Polyps
Patients with a personal or a familial history of colorectal cancer or adenomatous polyps have a greater risk of colorectal cancer than the general population. After resection of a single colorectal cancer, the risk of metachronous (subsequent) primary colorectal cancer in the first 5 years is between 1.5 and 3%. First-degree relatives of persons with colon cancer have a two to threefold increased risk of colorectal cancer.
Inflammatory Bowel Disease
Studies have reported a five to 15-fold increased risk of colorectal cancer in patients with ulcerative colitis (UC), a chronic and inflammatory disease of the large bowel. The risk factors for colorectal cancer in UC include longer duration of disease, greater extent of disease (pancolitis), associated primary sclerosing cholangitis, and the finding of precancerous dysplasia of the bowel. Patients with Crohn’s disease (CD), another inflammatory bowel disease, are also at increased risk for colorectal cancer, but the risk is not as great as that in UC. Recent studies suggest that colorectal cancer in patients with preexisting inflammatory bowel disease may be less frequent than previously believed, possibly because of effective therapies and prevention strategies (periodic colonoscopic examinations with biopsies to identify dysplasia and cancer).
Environmental Risk Factors In Sporadic Cases
Studies of migrants clearly show that the risk of colon cancer is related to environmental factors: When populations moved from low-risk to high-risk areas, the incidence of colorectal cancer increased rapidly within the first generation, implying that dietary and other environmental factors constitute a major component of risk.
Recognized risk factors are related to lifestyle and environment. There are strong correlations between risk of colorectal cancer and the intake of a diet high in red and processed meat or in animal fat. Physical inactivity and excess body weight are consistent risk factors. Insulin resistance and subsequent hyperinsulinemia is induced by excess energy intake and some aspects of the Western diet (e.g., saturated fats and refined carbohydrates). High alcohol consumption, probably in combination with a diet low in some micronutrients such as folate and methionine, and smoking early in life are all likely to increase risk of colon cancer. Whereas previous studies have identified a role for dietary fibers in the pathogenesis of colorectal cancer, recent epidemiologic studies have tended not to support a strong influence of fiber. Fiber supplementation had no significant protective effect for the development of recurrent colorectal adenomas in randomized controlled studies.
Some protective factors have been identified such as a diet high in fruit and vegetables, regular physical activity, and the regular use of aspirin or nonsteroidal antiinflammatory drugs. A meta-analysis of three controlled trials concluded that calcium supplementation significantly decreased the risk of recurrence of colorectal adenomas.
Pathogenesis
Colorectal carcinogenesis is a long-term multistep process, the adenoma-to-carcinoma sequence, with each step corresponding to key generic events (Figure 2). Specific germline mutations are responsible for the inherited colorectal cancer, while the accumulation of somatic mutations over time, linked to environmental exposures and affected by individual genetic susceptibility, results in a pathologic evolution from premalignant adenoma to invasive cancer in sporadic cases. It is estimated that the complete adenoma-to-carcinoma sequence can take from 7 to 15 years.
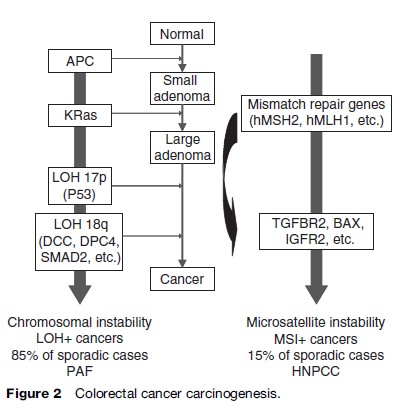
Recent progress in molecular biology has identified at least two types of colorectal tumors that are distinct by their carcinogenic processes (Figure 2). The first group, LOH-positive (loss of heterozygosity) colorectal cancers, is characterized by a chromosomal instability with hyperploidy and allelic losses involving preferentially chromosome 18q and chromosome 17p. Mutations of tumor-suppressor genes (e.g., APC, DCC, SMAD4, SMAD2, p53) and oncogenes (e.g., K-ras, c-myc, c-erb-2) are characteristic. Mutations in the APC gene occur early in the process, while others generally occur later. This mechanism is responsible for about 85% of sporadic colorectal cancers and involved in patients with PAF.
The second group, MSI-positive (microsatellite instability) colorectal cancers, is characterized by genetic instability. Microsatellites are genomic regions in which short DNA sequences (or a single nucleotide) are repeated. During DNA replication, mutations occur in some microsatellites, resulting in instability. These abnormalities are usually repaired by the mismatch-repair proteins, coded by mismatch-repair genes (e.g., hMSH2, hMLH1, or hMSH6). The repair is inefficient in tumors with germ-line or acquired mutations of these genes. HNPCC is the hereditary form of this pathway, which is responsible for approximately 15% of sporadic colorectal cancers. Besides oncogenes and tumor-suppressor genes, which are also involved in the chromosomal instability mechanism, further tumor suppressor genes (e.g., BAX, IGF2R, and TGFBR2) are also involved in this pathway.
Screening
Colorectal cancer is a common disease, fatal if left untreated, and usually preceded for many years by a benign lesion that can be detected before clinical symptoms. It therefore meets the main requirements to justify mass screening, providing the screening test is effective and acceptable, and treatment for screening-detected disease is more effective than treatment for disease diagnosed later, when clinical symptoms arise. The objectives of screening are to prevent death from colorectal cancer by detecting and treating curable cancers and to prevent colorectal cancer from arising in the first place by removing adenomas.
The fecal occult blood test (FOBT), sigmoidoscopy, and colonoscopy (direct visual inspection of the lower bowel or the entire bowel using a flexible telescope) have all been considered screening tests for colorectal cancer, but only FOBT has been extensively evaluated as a screening tool at the population level.
Mass screening using FOBT, followed by a colonoscopy in the event of a positive test result to detect the source of bleeding, is effective in reducing mortality from colorectal cancer. A study conducted in Minnesota among 46 551 volunteers aged between 50 and 80 years showed the efficacy of 2-yearly rehydrated FOBT in reducing colorectal cancer mortality by 21%. This result may not be directly transposable to the general population because the compliance of the general population with FOBT is much lower than that of volunteers, and the rehydration technique used to examine the stool sample for occult blood in that study is known to produce unduly high rates of positivity (incorrectly suggesting blood in the feces), and thus of subsequent colonoscopies; this is not compatible with mass screening. Four population-based studies have been set up to evaluate the effectiveness of biennial FOBT screening in more typical conditions of medical practice. The interest of the study conducted in Sweden (Kewenter, 1988) is limited because the screening test was only performed twice. Acceptability of the test ranged between 53 and 67% at the first screening; positivity rates were 2.1% initially and 1.3% on average in the successive rounds. Colorectal cancer mortality was significantly lower in the screening population compared with the control population (mortality ratio, 0.82 to 0.86). The reduction in mortality was more pronounced in those who participated at least once (mortality ratio, 0.61 to 0.67). A meta-analysis including all controlled trials of screening for colorectal cancer using the Hemoccult® FOBT has reported a reduction in colorectal cancer mortality of 16% (RR, 0.84; CI, 0.77–0.93) among persons allocated to screening. When adjusted for screening attendance, the mortality reduction was 23% (RR 0.77, CI: 0.57–0.89). Overall, it may be concluded from these studies that if 10 000 people were offered a biennial Hemoccult screening program and two-thirds attended for at least one Hemoccult test, 8.5 deaths (CI, 3.6–13.5) from colorectal cancer would be prevented over 10 years.
More complex fecal occult blood tests, especially immunological tests, have been developed. They are more sensitive, but their specificity at the population level is not well established.
The effectiveness of flexible sigmoidoscopy as a screening tool is currently being tested in randomized trials in England and Italy. Preliminary results of the UK trial suggest that the flexible sigmoidoscopy screening regimen is acceptable, feasible, and safe, with a high prevalence of neoplasia and an acceptable colonoscopy referral rates of 5%.
Colonoscopy remains the gold standard for colorectal exploration. It has the advantage of allowing assessment of the entire colon with the possibility of simultaneous biopsy and removal of any polyps (polypectomy). It cannot, however, be considered as the reference test for mass screening of asymptomatic people in the general population because of its cost, the risks, and the inconvenience to the subject, and it should only be performed when the fecal occult blood test is positive.
Recommendations on colorectal cancer screening in the European Union are as follow:
Faecal occult blood screening should be seriously considered as a preventive measure. The decision on whether or not to embark on these screening programs must depend on the availability of the professional expertise and the priority setting for healthcare resources. If screening programs are implemented they should use the fecal occult blood screening test and colonoscopy should be used for the follow-up of test positive cases. Screening should be offered to men and women aged 50 years to approximately 74 years. The screening interval should be 1–2 years. Other screening methods such as immunological tests, flexible sigmoidoscopy and colonoscopy can at present not be recommended for population screening (Advisory Committee on Cancer Prevention, 2000: 1473–1478).
People with high risk (medical or family history of colorectal cancer or adenomas, inflammatory bowel disease) or very high risk (familial syndromes) should not enter mass screening programs. They deserve individual screening based on colonoscopy at frequent specified intervals.
New screening tests are being developed such as virtual colonoscopy and the stool DNA-based screening test. They are not yet considered to be viable options for mass screening, because their test performance and effectiveness in reducing colorectal cancer mortality have not been characterized in average-risk patients in the community.
Diagnosis And Staging
The most frequent clinical symptoms of patients with colorectal cancer are intestinal bleeding, abdominal pain, and/or a change in bowel habits. Less frequently weakness, anemia without other gastrointestinal symptoms, and weight loss may be the initial symptoms. The most common metastatic sites are the regional lymph nodes, liver, lungs, and peritoneum. Patients may present with signs or symptoms referable to any of these areas.
In addition to a complete physical examination with a digital rectal examination, colonoscopy should be performed when colorectal cancer is suspected. It will allow lesions to be localized anywhere in the large bowel, a biopsy to be taken of any tumor, and removal of any polyps. Biopsies will allow pathological confirmation of the diagnosis. The majority of colorectal cancers are adenocarcinomas, arising from glandular tissue of the lining of the bowel.
Treatment and prognosis will depend upon local and distant disease extension, and complementary tests should be performed once the diagnosis is established. Abdominal and pelvic ultrasound and chest radiography are routinely recommended for the evaluation of regional tumor extension, regional lymphatic spread, and metastases to other organs. The necessity for a preoperative scan with computerized tomography (CT) for all patients with CRC is still being debated because, compared with ultrasound, the information from CT will rarely alter the planned surgical approach and is generally not justified for routine preoperative evaluation of all patients. Local staging of rectal cancer is essential to distinguish early tumors, suitable for treatment by surgery alone, from locally advanced tumors that need additional therapy in order to facilitate surgery and improve outcome. Besides digital rectal examination, endorectal ultrasound and magnetic resonance imaging (MRI) are recommended.
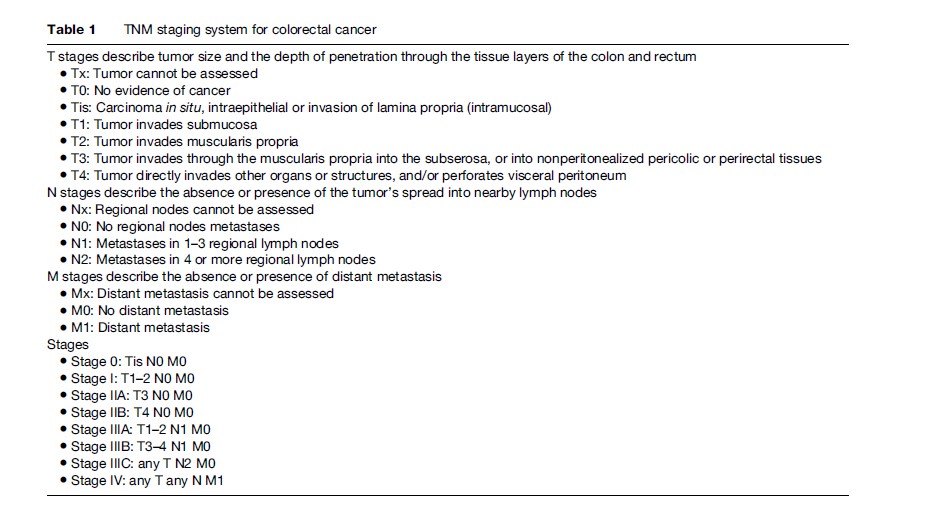
The TNM (tumor, nodes, metastasis) system of the American Joint Committee on Cancer (AJCC) is currently recommended for colorectal cancer staging. It divides colorectal cancers into four stages depending upon depth of the primary ‘tumor,’ presence and number of involved ‘nodes,’ and presence of distant ‘metastases’ (Table 1). TNM stage at diagnosis remains the best indicator of long-term prognosis for both colon and rectal cancer. Five-year survival rates in a series of over 119 000 patients treated between 1991 and 2000 and stratified according to the TNM staging system were as follows: Stage I, 93%; stage IIA, 85%; stage IIB, 72%; stage IIIA, 83%; stage IIIB, 64%; stage IIIC, 44%; and stage IV, 8%.
Treatment
Colorectal cancer management will depend upon disease extension and patient condition. It should be discussed at a multidisciplinary meeting of the specialists treating the patient.
Surgery For Primary Tumor
Radical surgery remains the only potentially curative treatment for colorectal cancer. Major changes in the principles of rectal cancer resection have been recently described, whereas there have been few changes in the principles of colonic cancer resection. Improvements in perioperative care (mechanical bowel preparation, systemic antibiotics, and prevention of blood clots) have reduced the operative morbidity and mortality.
The principles of colonic resection for colon cancer are well accepted. They have not undergone major change over the last 30 years. The principles of radical resection include ligation of the vascular pedicle, draining the tumor, complete resection of the tumor and the adjacent colon with free margins, and resection of any involved contiguous organs. Laparoscopic (key-hole) resection for colon cancer may offer several advantages over conventional laparotomy (open surgery). These include less pain, shorter postoperative ileus (temporary paralysis of bowel muscle movements), shorter hospital stay, faster recovery, and a better cosmetic result. Key-hole surgery can be performed following the same principles as open surgery, with an equivalent quality and the same results in terms of disease control and survival. The laparoscopic approach is considered an acceptable alternative to open surgery for colon cancer.
Recent progress in the surgery of rectal cancer allows a better chance of cure, with less mutilation and a better preservation of intestinal and genitourinary function. The most important advance in rectal surgery has been the introduction of the proctectomy with total removal of the mesorectum (total mesorectum excision). The mesorectum is the fatty tissue surrounding the rectum, beneath the peritoneum, which covers the abdominal organs. This procedure allows complete removal of the tumor and the regional lymph nodes that may be affected by the tumor, while sparing the nearby nerves and vessels. The circumferential margin (the minimum distance between tumor and the edge of the tissue removed at surgery) is correlated to recurrence rate, and it should be greater than 1 mm. The local relapse rate after total mesorectum excision is lower than 10%. In specialized centers, more than 80% of surgical resections for rectal cancer are associated with preservation of the anal sphincter.
Neoadjuvant Treatment For Rectal Cancer
Preoperative radiotherapy is recommended for tumors located in the middle or lower rectum that are large, locally invasive, or clinically node-positive (T3/4, N+). In these situations, radiotherapy has been shown to significantly reduce the risk of local recurrence, even after total mesorectum excision, and increase the rate of sphincter preservation. Preliminary results of controlled trials suggest that the addition of concomitant chemotherapy to radiotherapy increases the likelihood of a complete pathologic response and improves local control; neoadjuvant radiochemotherapy is increasingly utilized for locally advanced tumors (fixed tumors, circumferential margin 1 mm on MRI), but its impact on sphincter preservation and survival remains to be proved.
Resection Of Colorectal Cancer Liver Metastases
Approximately 20–30% of patients with metastatic colorectal cancer have liver only metastases, and potentially removable at surgery. Sometimes, initially nonresectable liver metastases may become resectable after chemotherapy. Patients being considered for treatment of hepatic metastases should be discussed by a multidisciplinary team that is experienced in the management of liver metastases. Surgery should be discussed if all macroscopic disease can be resected with clear margins and leave a sufficiently functioning liver. After complete resection, 5-year survival ranged from 25 to 44%, with an operative mortality of 0–6.6%.
Adjuvant Chemotherapy
The use of adjuvant fluorouracil-based chemotherapy is considered standard care in patients with resected stage III colon cancer. Such treatment is associated with an approximately 30% reduction in the risk of disease recurrence, and a 20–30% reduction in mortality. A randomized controlled trial recently demonstrated the superiority of the FOLFOX4 regimen (oxaliplatin plus bolus and infusional 5-fluorouracil) over a regimen based on 5-fluorouracil alone. A 6-month course of FOLFOX4 is presently considered the standard for adjuvant treatment of stage III colon cancer.
Adjuvant chemotherapy is not routinely recommended in resected stage II colon cancer, since its benefit is not clearly demonstrated in this setting. It should be considered for patients with high-risk stage II (e.g., T4 tumors, perforation, bowel obstruction, poor differentiation, inadequate number of examined lymph nodes).
Palliative Chemotherapy
The objectives of palliative surgery are to increase survival and improve quality of life. Major progress has been made over the last 10 years with the use of infusional 5-fluorouracil and the development of regimens using new cytotoxic drugs such as irinotecan (FOLFIRI regimen) or oxaliplatin (FOLFOX regimen). These regimens have been shown to significantly improve the response rate, disease-free survival, and overall survival compared with single-agent 5-fluorouracil, which was the only available drug until the mid-1990s.
More recently, biologic agents cetuximab (an antibody that targets the EGF receptor) and bevacizumab (an antibody that targets the VEGF), in association with chemotherapy, have provided improved results compared to chemotherapy alone.
In more recent clinical trials using new drugs and biological agents, median survival rates were close to 25 months. This is substantial improvement compared to the 10-month median survival observed with 5-fluorouracil-based regimens, but the great majority of patients with metastatic disease remain incurable, with a very poor 5-year survival rate.
The public health burden of colorectal cancer can be reduced in all the classical ways: By primary prevention, stopping the disease arising in the first place; by screening to detect and treat disease at an early stage, when the outcome is more favorable; by better clinical investigation to improve the precision of diagnosis; by more effective surgery and chemotherapy; and by palliation, to reduce the pain and suffering of patients who cannot be cured. The balance between these approaches that will be adopted in different countries will depend on the extent of public knowledge and concern, on political priorities, and on the availability of human, physical, and financial resources.
Bibliography:
- Burt RW (2000) Colon cancer screening. Gastroenterology 119: 837–853.
- Gatta G, Ciccolallo L, Capocaccia R, et al. (2003) Differences in colorectal cancer survival between European and US populations: The importance of sub-site and morphology. European Journal of Cancer 39: 2214–2222.
- Kewenter J, Bjork S, Haglind E, Smith L, Svanvik J, and Ahren C (1988) Screening and rescreening for colorectal cancer. A controlled trial of fecal occult blood testing in 27,700 subjects. Cancer 62: 645–651.
- Advisory Committee on Cancer Prevention (2000) Recommendations on cancer screening in the European Union. European Journal of Cancer 36: 1473–1478.
- Calvert PM and Frucht H (2002) The genetics of colorectal cancer. Annals of Internal Medicine 137: 603–612.
- Coleman MP, Rachet B, Woods LM, et al. (2004) Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. British Journal of Cancer 90: 1367–1373.
- Gatta G, Capocaccia R, Sant M, et al. (2000) Understanding variations in survival for colorectal cancer in Europe: A EUROCARE high resolution study. Gut 47: 533–538.
- Greene FL, Page DL Fleming ID, et al. (eds.) (2002) AJCC (American Joint Committee on Cancer) Cancer Staging Manual. New York: Springer-Verlag.
- Lynch HT and de la Chapelle A (2003) Hereditary colorectal cancer. New England Journal of Medicine 348: 919–932.
- Martijn H, Voogd AC, van de Poll-Franse LV, et al. (2003) Improved survival of patients with rectal cancer since 1980: A population-based study. European Journal of Cancer 39: 2073–2079.
- Mitry E, Barthod F, Penna C, and Nordlinger B (2002) Surgery for colon and rectal cancer. Bailliere’s Best Practice and Research in Clinical Gastroenterology 16: 253–265.
- Mitry E, Bouvier AM, Esteve J, and Faivre J (2005) Improvement in colorectal cancer survival: A population-based study. European Journal of Cancer 41: 2297–2303.
- O’Connell JB, Maggard MA, and Ko CY (2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. Journal of the National Cancer Institute 96: 1420–1425.
- Parkin DM, Bray F, Ferlay J, and Pisani P (2005) Global Cancer Statistics, 2002. CA A Cancer Journal for Clinicians 55: 74–108.
- Sant M, Aareleid T, Berrino F, et al. (2003) EUROCARE-3: Survival of cancer patients diagnosed 1990–94: Results and commentary. Annals of Oncology 14(supplement 5): V61–V118.
- Towler BP, Irwig L, Glasziou P, Weller D, and Kewenter J (2003) Screening for colorectal cancer using the faecal occult blood test, Hemoccult (Cochrane Review). The Cochrane Library, Issue 1. Oxford: Update software.
- Vasen HF, Watson P, Mecklin JP, and Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116: 1453–1456.
- Vogelstein B, Fearon ER, Hamilton SR, et al. (1988) Genetic alterations during colorectal-tumor development. New England Journal of Medicine 319: 525–532.
- https://www.asco.org/ – American Society of Clinical Oncology.
- https://www.cancerresearchuk.org/ – Cancer Research UK.
- https://www.iarc.fr/ – International Agency for Research on Cancer.
- http://www.eurocare.it/ – Eurocare – Survival of cancer patients in Europe.
- https://www.cancer.gov/ – National Cancer Institute.
- https://seer.cancer.gov/ – National Cancer Institute, Surveillance Epidemiology and End Results.




