View sample cancer research paper on brain tumors. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Malignant brain and nervous system tumors account for 18 820 new cancer diagnoses each year in the United States, or 1.3% of all primary incident cancers, and 12 820 or 2.3% of annual cancer deaths (American Cancer Society, 2006). Each year more than 41 130 new benign or malignant primary brain tumors are diagnosed among residents of the United States (CBTRUS, 2005). Worldwide the incidence rate of primary malignant brain and nervous system tumors ranges from 5.8 per 100 000 person-years for males in developed countries (4.1 per 100 000 females) to 3.0 per 100 000 person-years for males in less developed countries (2.1 per 100 000 person-years females) (Ferlay et al., 2004). Brain tumors are the second leading cause of death from neurological disease, second only to stroke, and the eleventh most common cause of death from cancer in the United States. Five-year survival rates published by the American Cancer Society show that survival from 1974 to 2001 for all types of brain tumors combined significantly improved in the United States; however, the average survival rate from 1995 to 2001 was still only 33% (American Cancer Society, 2006). The average 5-year brain tumor survival rate for 22 European countries when including follow-up data through 1998 was 18% (Sant et al., 2003). However, differences in survival by country are likely to be heavily influenced by variation in histology types diagnosed in each country.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Benign tumors can result in similar symptoms and prognoses as malignant tumors. For this reason, many cancer registries have routinely included both benign and malignant intracranial tumors. The U.S. Surveillance, Epidemiology, and End Results (SEER) network of population-based cancer registries now reports both benign and malignant CNS tumors. Brain tumor patients may present with general symptoms, such as headaches and seizures, which result from an increase in intracranial pressure. One-third of patients present with headaches and one-fifth with seizures. Other symptoms may include specific motor, speech, or sensory deficits resulting from compression of the corresponding region of the brain; slow-growing tumors may present with change in character only.
The discussion in this research paper will include benign and malignant tumors of the brain, cranial nerves, and cranial meninges, which account for 95% of all CNS tumors and 93% of all nervous system tumors. For simplicity, this group of tumors will be called brain tumors or, when benign tumors are excluded, brain cancer. The term central nervous system tumors (or cancer) indicates that tumors of the spinal cord and spinal meninges are included along with brain tumors, and nervous system (NS) tumors indicates that tumors of the peripheral nerves are included as well. Three major histologic groups are used in the descriptive tables of this research paper, corresponding to International Classification of Diseases for Oncology (ICD-O-3) morphology codes 9530–9534 and 9537–9539 for gliomas, 9530–9534 and 9537–9539 for meningiomas, and 9540–9571 for nerve sheath tumors. The statistics reported on incidence will focus on data from Surveillance, Epidemiology, and End Results (SEER).
Classification
Anatomic Classification
Tumors of the central nervous system include tumors of the brain, cranial nerves, cerebral meninges, spinal cord, and spinal meninges. These subsites are represented by the International Classification of Diseases for Oncology (ICD-O) codes C70.0–C72.9. We will not include tumors of sites such as the eye and the pituitary gland, which appear to be etiologically distinct.
Histopathology
The WHO classification of tumors system has allowed for international standardization of CNS malignancies based on cell of origin and histopathologic features, including degree of anaplasia. Tumors are classified into one of four grades: grade 1 is benign, grade 2 is considered low grade, while grades 3 and 4 are considered high grade or malignant. The most common are tumors of neuroepithelial origin, including astrocytomas, oligodendrogliomas, mixed gliomas, ependymal tumors, choroid plexus tumors, glial tumors of uncertain origin, neuronal and mixed neuronalglial tumors, neuroblastic tumors, pineal parenchymal tumors, and embryonal tumors. The majority of CNS primary tumors are of astroglial origin, called gliomas. In the United States, according to recent data from the Los Angeles County Cancer Surveillance Program, gliomas account for 55% and 40% of primary CNS tumors among men and women, respectively (Table 1). The astrocytic tumors account for 80% of gliomas and include astrocytomas (grade II), anaplastic astrocytomas (grade III), and glioblastoma multiforme (grade IV). These represent increasing grades of anaplasia and clinical virulence of tumors of astrocytic cells. Two clinical variants of glioblastoma multiforme (GBM), primary and secondary, have been described, with primary GBM representing the more common (75%) of the two forms. Primary GBM is believed to develop de novo and grows very aggressively without evidence of a malignant precursor lesion. Secondary GBM is believed to arise from a low-grade lesion that has undergone genetic alterations to transition to a high-grade tumor. Oligodendrogliomas are classified as tumors with a cellular morphology most closely resembling that of the normal oligodendrocyte. There are two grades of oligodendrogliomas in the WHO classification system, where grade II is the lowest grade of oligodendroglioma and grade III (anaplastic) is the highest.
Tumors in the cranium can also originate from structures surrounding the brain, including the meninges, cranial nerves exiting the brain, blood vessels, or primitive remnants left from early development. Tumors of the peripheral nerves represent a subheading of the WHO classification scheme that includes schwannomas, neurofibromas, and malignant peripheral nerve sheath tumors. Acoustic schwannomas, which originate from the eighth cranial nerve, account for 90% of CNS schwannomas and 8% of all CNS tumors. Using recent data from the Los Angeles Cancer Surveillance Program in the United States, tumors of the meninges account for approximately 16% and 35% of central nervous system tumors in men and women respectively (Table 1). These originate in the cells of the dura mater, which is the covering for the brain and spinal cord. Tumors of the last three subheadings include lymphomas and hemopoietic tumors, germ cell tumors, and tumors of the sellar region. Tumors of the sellar region include craniopharyngiomas, tumor-like processes growing from epithelial rests remaining from Rathke’s pouch, the progenitor for the pituitary gland.
Molecular Genetic Characteristics
Advances in molecular genetics are of growing importance for the correct classification of brain tumors, as well as the prediction of patient prognosis (i.e., survival) and treatment response. Details have been described in recent reviews (Ohgaki, 2005). As with other human malignancies, the pathogenesis of brain tumors is known to be associated with inactivation of tumor suppressor genes (TSG) and/or the activation of oncogenes, which may occur through several mechanisms including gene mutation, chromosomal loss, chromosomal gain or amplification, and methylation. In the sections below, we focus on several of the more common molecular genetic characteristics of brain tumors.
Chromosomal Loss
Molecular studies indicate that gliomas frequently have loss of several chromosomal regions, referred to as loss of heterozygosity (LOH), at chromosomal regions 1p, 9p, 10q, 17p, 19q, and 22q. Frequent LOH suggests that inactivation of tumor suppressor genes in these regions may contribute to the development of gliomas. LOH 10q is one of the most frequent genetic alterations in gliomas, occurring in 40–50% of astrocytomas (grades II–III) and 60–80% of glioblastomas. Loss of one specific tumor suppressor gene on chromosome 10, named phosphatase and tensin homolog (PTEN), is associated with high-grade tumors and has been observed in 15–40% of glioblastomas. Mutations in PTEN can cause disruptions in cellular growth, migration, apoptosis or cellular death, and interaction with the extracellular matrix. Chromosomal loss is also a common distinguishing feature of oligodendrogliomas. Loss of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q) is present in 50–90% of oligodendrogliomas and is considered a marker of better patient outcome. Oligodendrogliomas from patients without loss of 1p/19q may instead have loss of chromosomal regions 9p and 10q; these losses frequently occur in the presence of amplification (i.e., multiple copies) of the oncogene known as epidermal growth factor receptor (EGFR).
Loss of chromosomal region 17p or mutations in the tumor suppressor gene p53 located in the 17p region is believed to play a major role in both the formation of low-grade glioma and in the transition of low-grade gliomas to malignant glioblastoma. This tumor suppressor gene is known to be involved in many types of cancer, potentially through a regulatory role in apoptosis and cell cycle control. TP53 mutations are the earliest detectable change in gliomas and have been observed in as many as 53% of low-grade and anaplastic astrocytomas (WHO grades II and III, respectively) and 65% of glioblastomas. TP53 mutations are more common among the secondary glioblastomas (65%) than primary glioblastomas (28%) (Ohgaki, 2005). Some studies suggest that there is no difference in survival between patients with or without a p53 mutation, while others suggest there may be a survival benefit.
Mutations or loss of part of the promoter of the tumor suppressor gene retinoblastoma (RB1) can decrease gene activity and therefore diminish its ability to regulate cell cycle control. Mutations in RB1 have been detected primarily in high-grade gliomas, suggesting RB1 mutations represent a late event in astrocytoma development. Cyclin-dependent kinase inhibitor-2A (CDKN2A, also known as P16) is a second tumor suppressor gene in the RB1 pathway that contributes to cell cycle regulation. Loss of chromosomal regions of this tumor suppressor gene are found in high-grade gliomas and some types of oligodendrogliomas.
Chromosomal Gain
The epidermal growth factor receptor gene (EGFR), located on chromosome arm 7p, is a pro-oncogene involved in cellular proliferation. Amplification or chromosomal gain through accumulation of multiple gene copies are rarely found in low-grade gliomas and anaplastic oligodendrogliomas (<10%) but frequently found (40%) in primary glioblastomas (Ohgaki, 2005). Overexpression of the protein coded by EGFR is less common (<10%) among secondary glioblastomas, but common in primary glioblastomas (>60%). The frequency of EGFR amplification in brain tumors in the United States is more common in white non-Hispanic cases than other racial/ ethnic groups. The prognostic value of EGFR is controversial. Some studies suggest EGFR amplification or overexpression may contribute to radiotherapy resistance and to overall poor survival; however, these differences may be due to confounding by age, because EGFR is more common in older patients and survival significantly decreases with age. EGFR amplification was not associated with survival in a recent meta-analysis (Huncharek and Kupelnick, 2000); however, some studies suggest EGFR is predictive of poor prognosis when present in younger patients.
Demographic Patterns
Incidence And Mortality
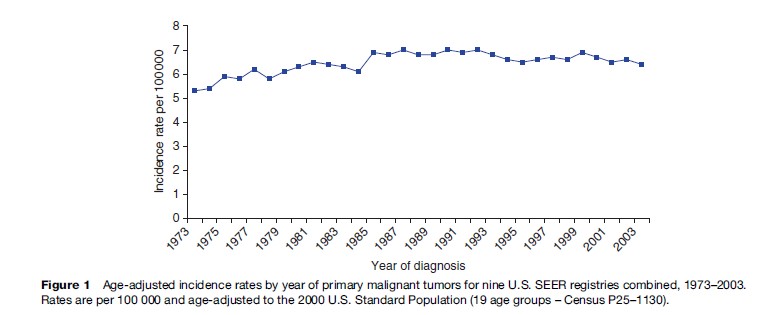
From the 1970s through the mid-1990s, incidence rates of primary brain tumors steadily increased in the United States (Figure 1), leading to speculation that the number of people being diagnosed with brain tumors was rising due to changes in the environment or lifestyle exposures. Age-adjusted incidence rates of primary malignant brain tumors increased by 22% from 1973 to 2003, using data from nine U.S. SEER registries (SEER Program, 2006). However, two reports using SEER incidence data and the U.S. National Center for Health Statistics mortality data found that the changing patterns of incidence and mortality were best explained by increasing use of better diagnostic equipment, in particular computed tomography (CT) and magnetic resonance imaging (MRI) (Smith et al., 1998; Legler et al., 1999). Legler et al. found that from 1975 to 1995, incidence rates were significantly increasing only among children under 15 years of age and adults 65 years of age and older. During this same time period, mortality declined for the youngest age group and increased among the oldest age group. The difference in mortality by age may be explained in part by the fact that low-grade tumors that have better prognosis are more common in children and high-grade tumors that have poorer prognosis are more common in adults. The authors also showed that use of CT and MRI procedures by doctors for their patients 65 years and older increased during the mid-1980s and early 1990s, suggesting greater use of available technology to diagnose patients.
Survival
Age-adjusted 5-year survival rates for all malignant primary brain tumors in the United States, as catalogued by the National Cancer Institute from 1989 to 1996 were 31% (Gurney and Kadan-Lottick, 2001). While this represents an improvement from rates observed in 1974–76 (23%), the survival rates remain extremely low. Five-year survival rates recently published by the American Cancer Society, show that the average survival rate from 1995 to 2001 is still only 33% (American Cancer Society, 2006). Similarly, the weighted 5-year brain cancer survival rate calculated for 22 European countries through 1998 was also low, reaching only 18%.
Survival rates are dependent on a number of patient and tumor characteristics, including age and histologic type of tumor. For example, the SEER 5-year survival rate for patients aged 0–19 years of age at diagnosis is 65%, while the rate for those aged 65–74 years or 75 years and older are 6.5% and 3.6%, respectively (Ries et al., 2000).
A study using SEER data that examined malignant brain tumor survival by histology (Davis et al., 1998) also found that survival declined with increasing age at diagnosis. For the most part, the survival rates between the sexes did not differ. The survival rate for patients with glioblastoma whose brain tumor was diagnosed between the years 1986 and 1991 was only 1% for all ages combined. The rate of survival was 34% for astrocytomas and 84% for pilocytic astrocytomas, which occur predominantly in children. Survival from medulloblastoma for all age groups combined improved from 40% in the 1970s to 60%. The survival rates for oligodendroglioma also substantially improved to 65% between 1986 and 1991. Five-year survival rates remained similar over the 20 years for ependymomas (60%), mixed gliomas (51%), other gliomas (23%), and gliomas not otherwise specified (NOS) (17%).
It is thought that improved diagnostic abilities, treatment modalities, and earlier age at diagnosis may account for the improvements in survival of astrocytomas, medulloblastomas, and oligodendrogliomas over the last 20 years.
Incidence patterns of primary brain tumors by age, gender, and race are described using data from SEER.
Age
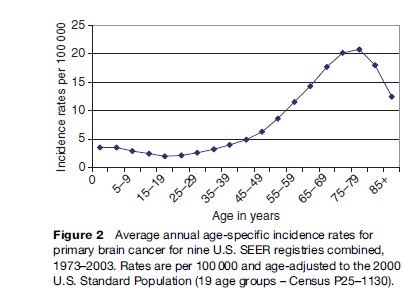
Incidence rates of primary brain tumors show a bimodal peak by age. Incidence rates of tumors vary by histologic type; however, for all tumor types combined, incidence rates peak in early childhood (generally by age 5 years), decline and increase again from approximately ages 25 through 75 years of age. The average annual age-specific incidence of brain tumors in nine SEER registries is shown in Figure 2.
Gender
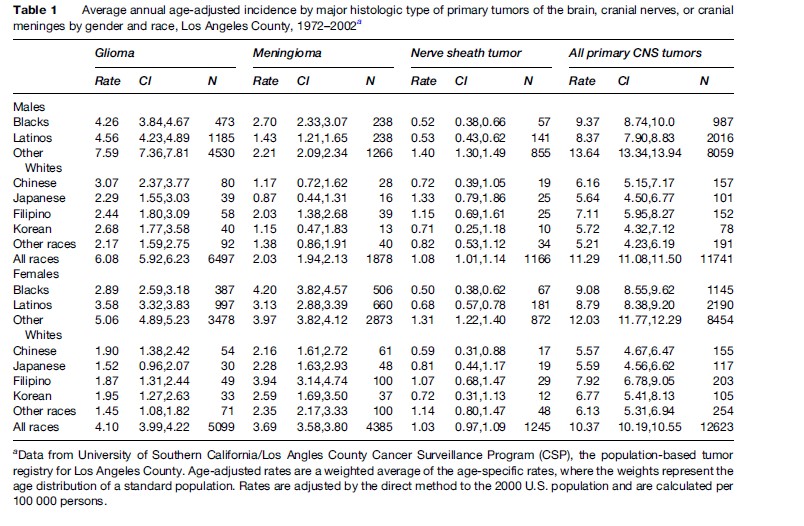
Table 1 shows the age-adjusted annual incidence for the major histologic groups of primary brain tumors by sex and racial/ethnic group in Los Angeles County, CA for 1972–2002. For all histologic types and races combined, the rate is higher in men than in women. However, incidence rates vary considerably by histologic subtype. Glioma rates are higher in males than in females in each racial/ethnic group and meningiomas are higher in women.
Race And Ethnicity
The incidence of gliomas in the United States is highest in non-Spanish-surnamed Whites for males and females, while the incidence of meningiomas is highest in Blacks for males and females (see Table 1).
Environmental Factors
This section reviews recent findings from epidemiologic studies, which have investigated the association between human brain tumors and various potential risk factors.
N-Nitroso Compounds (Nocs)
N-nitroso compounds (NOCs) and their precursors are experimental carcinogens (Lijinsky, 1992) that have been investigated as potential causes of brain tumors in humans. NOCs are divided into two major categories based on their chemical structure – nitrosamines and nitrosamides. Nitrosamines are carcinogenic in animal studies, but have never been shown to cause tumors in the brain or spinal cord. Nitrosamides are direct-acting agents that can cause DNA adducts and have been shown to be potent nervous system carcinogens in a variety of species. Ethylnitrosourea (ENU), a type of nitrosamide, has been shown to be a neurocarcinogen in rats, mice, rabbits, opossums, and monkeys through various routes of administration and when given in a single dose or through chronic low-dose exposure. Transplacental exposure is the most potent route of exposure, as only one-fiftieth (1/50) of the dose of ENU required to induce tumors in adult animals is necessary to cause 100% tumor induction in fetal animals (Ivankovic, 1979).
Population Exposure To Nocs
The major sources of population exposure to NOCs in the United States are tobacco smoke, cosmetics, automobile interiors, cured meats, and some types of pesticides (National Research Council Committee on Diet Nutrition and Cancer, 1982). Other sources of NOC exposure include soaps, shampoos, hand lotions, rubber baby bottle nipples, and pacifiers. Endogenous formation of NOCs in humans occurs in the stomach or bladder when both an amino compound and a nitrosating agent are present simultaneously. Food is a primary source of both highly concentrated nitrite solutions (e.g., from cured meats) and amino compounds (e.g., in fish and other foods, and in many drugs). Drinking water also contains nitrate (in the absence of vitamins), but this is a minor source unless levels are extraordinarily high.
Diet And Vitamin Supplementation
The majority of hypotheses regarding dietary factors and brain tumors have focused on cured meats (sources of N-nitroso compounds), antioxidants, and inhibitors of nitrosation, including vitamins C and E. Eight of ten epidemiologic studies that investigated the role of cured meats during pregnancy found a significant positive association between the frequency of maternal cured meat intake by the mother (individual or combined cured meats) and the risk of childhood brain tumors (CBT) (Baldwin and Preston-Martin, 2004). The individual foods most consistently found to be associated with increased risk for CBT were hot dogs and bacon. In a study of adults, serum levels of ascorbic acid (vitamin C) and alpha and gamma-tocopherol (vitamin E) were both inversely related to glioblastoma risk (Schwartzbaum and Cornwell, 2000). Furthermore, high intake of fruits and vegetables has been associated with lower risk of childhood brain tumors in several studies of CBT (maternal intake of fruits and vegetables during pregnancy) and has also been reported in a number of studies of adult brain tumors.
The apparent reduction in risk of brain tumor development associated with fruits, vegetables, and vitamin intake may be interpreted as supportive of the N-nitroso hypothesis due to the inhibition of the endogenous formation of NOCs in the presence of vitamins C or E, or the apparent protective effect may be due to another mechanism such as the inhibition of free radical formation in the brain.
Radiation
High-dose exposure to ionizing radiation is the only established environmental cause of brain tumors. Several studies have shown an excess risk of brain tumors following exposure to high-dose therapeutic radiation (2500 cGy) for primary malignancies or benign conditions (Wrensch et al., 2002). Meningioma appears to be the most common brain tumor resulting from radiotherapy for other medical conditions; however, tumors of other histological types have occurred, including glioblastoma.
One of the strongest pieces of evidence supporting a role of therapeutic radiation and brain tumor risk comes from an Israeli cohort study of adults irradiated as children for tinea capitis (i.e. ringworm of the scalp); adults with a history of irradiation were at high risk of developing nerve sheath tumors of the head and neck (RR = 33.1 or exposed children were 33 times more likely to develop a nerve sheath tumor), intermediate risk of meningioma (RR = 9.5), and lowest risk of glioma (RR = 2.6). Recent updated results from this cohort show that risk remains elevated after 40 years of follow-up for both benign meningiomas and malignant brain gliomas (Sadetzki et al., 2005).
The association between brain tumors and prior exposure to low-dose, diagnostic X-rays remains less certain. A Swedish case-control study found that X-rays of the head and neck were associated with increased risk of adult brain tumors (OR 1.64; 95% CI 1.04–2.58) and that this risk was strongest among individuals irradiated 5 or more years before tumor diagnosis (OR 2.10; 95% CI 1.25–3.53) (Hardell et al., 2001). Epidemiologic studies have found that prior dental radiography, particularly in earlier decades when doses were high, was associated with subtentorial, intracranial meningiomas (Preston-Martin and White, 1990). Other studies, however, did not confirm these findings.
Electric And Magnetic Fields
In the 1990s, numerous studies of electric and magnetic fields were completed to investigate the potential association with child and adult brain tumor risk. Electric and magnetic fields induce weak electric currents in the body; however, they can neither break bonds nor heat tissue. Several studies that have explored the association between exposure to electromagnetic fields (EMFs) and brain tumor risk have been recently reviewed (Kheifets, 2001). Although early studies of childhood brain tumors and residential exposure (both from ambient fields and use of electrical appliances) were suggestive of an increased risk, findings from the most recent studies have produced inconsistent results. Methodologic limitations of studies have included small sample sizes, in particular small numbers of subjects with high levels of exposure; measurement errors; lack of a standardized reference interval for the evaluation of magnetic fields; and incomplete evaluation of potential confounding variables. Exposure to radiofrequency sources is difficult to quantify in the laboratory setting; their measurement in large epidemiologic populations is even more challenging.
Studies of residential exposure to EMF and adult brain tumors have provided little evidence that EMF is a risk factor for brain tumors. A meta-analysis of 29 studies of occupational sources of EMF and brain cancer found a small increase in risk among electrical workers (Kheifets et al., 1995), but there was substantial heterogeneity in findings across studies due to differences in study design, occupations included, and exposure assessment methods.
Cellular Telephones And Other Radiofrequency Exposures
In recent years, public concern has focused on the potential risk of brain tumors due to cell phone usage. However, experimental data indicate that nonthermal radio frequency (RF) waves do not have enough energy to damage DNA, either directly or through epigenetic changes. Additionally, animal studies indicate RF exposure does not increase malignancies in rodents. Several early occupational studies did report associations between RF exposure and hematopoietic or brain cancers (Moulder et al., 2005). Nevertheless, these studies did not provide adequate support for a causal relationship between RF and cancer, due to limitations in design and exposure assessment.
The majority of epidemiologic studies to date do not support an association between cell phone use and brain tumor risk. No excess risk was found in a number of large case-control studies. Two case-control studies did find an elevated risk of brain tumors on the side of the head where the phone was most often held (Muscat et al., 2000; Hardell et al., 2001); however, the conclusions for one of these studies was questioned due to the use of prevalent cases and other design issues. Data from the Interphone Study found no overall increase in risk for temporal glioma or meningioma among cellular phone users (Lonn et al., 2005). Further, a Danish cohort found no increase in brain cancer or any other cancer incidence when including 450 085 mobile phone subscribers during the period of 1982–95 (Johansen et al., 2001). However, a Finnish registry-based study found a significant association (OR 1.5; 95% CI 1.0–2.4) between gliomas and analog cellular phones (Auvinen et al., 2002). No excess of brain cancer was found among employees of a cell phone manufacturing company or in a cohort study designed to examine cancer mortality in Korean War radar and radio technicians (Groves et al., 2002).
Misclassification of exposure is a potential design limitation in all cell phone studies, because RF exposure levels have changed over time with changes in cell phone technology and RF exposure varies with a person’s distance from cellular phone stations at the time they are using a cell phone. Current studies estimate risk for a maximum of 20 years of RF exposure; brain tumor risk for long-term cell phone usage of more than two decades is not known. Despite these limitations, these studies seemingly rule out large and immediate increases in brain cancer rates in cellular phone users.
Trauma
Several case reports and series provide anecdotal evidence that prior head trauma is associated with brain tumor development, but larger studies have not consistently confirmed this relationship. One report describes a case of malignant glioma occurring in the same spot where a man suffered a metal splinter injury 37 years earlier (Sabel et al., 1999); most previous case reports of this sort have involved meningiomas. An international case-control study of head trauma and risk of gliomas and meningiomas found an increased risk of meningioma only among men who had a serious head injury 15–24 years prior to diagnosis (Preston-Martin et al., 1998). Other investigators have found a weak relationship between trauma and meningiomas, suggesting that cellular proliferation due to repair of the dura may explain the increase in risk. However, recall bias is a concern when interpreting these studies because those who developed a brain tumor may be more likely to remember a previous head injury. Large studies from Sweden and Denmark have not confirmed a trauma–brain tumor risk relationship (Inskip et al., 1998; Nygren et al., 2001).
Infectious Agents
Several types of viruses cause brain tumors in experimental animals, including simian immunodeficiency as well as JC and BK viruses. In one of the earliest epidemiologic studies of brain tumors, astrocytomas were associated with positive antibody titers to Toxoplasma gondii (Schuman et al., 1967); however, more recent studies have failed to confirm this association. It is believed that over 62% of the United States population have been exposed to Simian virus 40 (SV 40), a member of the Polyomaviridae family, through administration of polio vaccine contaminated with the virus between 1955 and 1963 (Shah and Nathanson, 1976). Early animal studies revealed the carcinogenic effect of the virus and recent studies detected specific SV40 complexes in brain cancer tissue, suggesting a possible SV 40 role in primary brain tumor pathogenesis (Eddy et al., 1962). The tumorigenicity of other common polyomaviruses, including JC and BK viruses, have been well established through in vitro and animal experimental studies (Inskip et al., 1995). While JC and BK viruses normally do not cause symptoms in healthy individuals, JC and BK viral DNA sequences have been isolated from a number of types of human brain tumors, including medulloblastoma, ependymoma, and gliomas.
Other studies have found associations between risk of maternal infections during pregnancy and childhood brain tumors. Bithell et al. found an increased risk of brain tumors among children of mothers who had chickenpox during the pregnancy (Bithell et al., 1973). History of varicella-zoster infection was related to a reduced risk of adult gliomas if a history of chickenpox or shingles or serum levels of immunoglobulin G antibodies to the virus was used in the analysis (Wrensch et al., 2002). In a recent case-control study, Wrensch et al. reported that glioma cases (particularly those with glioblastoma) had significantly lower levels of anti-varicella zoster virus immunoglobulin G than controls and that cases were less likely than controls to report a history of chickenpox. However, no significant differences were noted between cases and controls for positivity to three other herpesviruses: Epstein-Barr virus, cytomegalovirus, and herpes simplex virus (Wrensch et al., 2002).
Allergies, Epilepsy, And Other Medical Conditions
Several epidemiologic studies have reported a protective association between brain tumor risk and a history of asthma and other allergic conditions. This finding has been consistent across a number of large case-control studies conducted in various countries (Wrensch et al., 2002). One study specifically reported an inverse association of autoimmune disease with both glioma and meningioma, with the greatest reduction in risk found among glioma patients with both asthma and diabetes (Brenner et al., 2002). Three cohort studies using Swedish data provide limited support for this association (relative risk (RR) 0.45; 95% CI 0.19–1.07 and RR 0.45; 95% CI 0.11–1.92 among low-grade and high-grade gliomas, respectively) (Schwartzbaum et al., 2003).
Recent studies of immunologic biomarkers and gliomas further support the association between allergic conditions and brain tumor risk. In a case-control study of adult glioma, Wiemels et al. found cases had significantly lower serum IgE levels than controls; this was particularly true among subjects reporting food allergies. However, it remains possible that differences in IgE levels were due to changes in IgE after the tumor was diagnosed (Wiemels et al., 2004). Schwartzbaum et al. completed a populationbased case-control study to investigate the association between GBM and common genetic changes in immune pathway genes. The study focused on genes coding for interleukins, which regulate and cause differentiation of cells of the immune system. Investigators found that people with a history of asthma, eczema, and fever were significantly less likely to be diagnosed with a GBM. Two genetic variants in the interleukin-4 receptor were associated with a greater chance of developing a GBM, while a single genetic variant in the interleukin-13 gene was associated with a lower risk of developing GBM (Schwartzbaum et al., 2005). Additional molecular studies are needed to explore the biological basis of this association.
Occupational Exposures
Associations between employment in various occupations and industries and brain tumor risk have been investigated in numerous epidemiological studies, but few associations have been found consistently across studies. Further, results have been limited by difficulties in determining past workplace exposures and by small numbers of people who have been exposed to any specific agent.
Several epidemiological studies have investigated associations between employment in the petrochemical industry and brain tumor risk. One study found an excess of brain cancers among white men with 10 or more years of employment in a particular building complex of interest (Beall et al., 2001). Another case-control study of workers at a petrochemical research facility found positive associations between gliomas and ionizing radiation, n-hexane, organometallics, and amines other than nitrosamines.
Epidemiological studies of brain tumor deaths have reported elevated risk of mortality due to brain tumors for a variety of occupations involving exposure to electromagnetic fields (EMFs). These include electrical or electronic engineers, electronics teachers, and electrical technicians and assemblers, electric utility workers, and workers in the communications industry. A recent review on this subject found that the quality of epidemiological studies of EMF exposure has improved and there is a large body of high-quality data on brain tumor risk and occupational EMF exposure (Ahlbom et al., 2001). No excess brain tumor risk was seen in a cohort of British electrical utility workers (Sorahan et al., 2001). A case-control study conducted in eight Canadian provinces, with emphasis placed on variations in EMF-related risk across different histological types found a pronounced risk for glioblastoma multiforme, but no association for astrocytoma or other brain tumors (Villeneuve et al., 2002).
Smoking And Environmental Tobacco Smoke
Most studies of adult brain tumors have not established any relationship between smoking tobacco and brain cancer. However, a study of adult gliomas in the San Francisco Bay area found that male cases are more likely to report smoking unfiltered cigarettes than controls (Lee et al., 1997). A second study reported that a history of active smoking was associated with a significant dose–response increase in risk of meningioma in men. In the same study, exposure to passive smoke from a spouse on the part of never smokers was associated with increased risk of brain tumors in both sexes (OR = 2.0) and risk increased with increasing duration of exposure ( p for trend = 0.02) (Phillips et al., 2005).
Overall, the epidemiologic data do not support an association between maternal smoking during pregnancy and risk of CBT (Boffetta et al., 2000). However, the metaanalysis of ten studies found a small increase in risk for childhood brain tumors (OR 1.2; CI 1.1–1.4) in relation to father’s smoking during pregnancy. An international collaborative population-based case-control study conducted in seven countries found a small association between paternal smoking before pregnancy and risk of childhood astroglial tumors (OR 1.4) (Cordier et al., 2004). A recent prospective study of 1.4 million Swedish births found an association between maternal smoking during pregnancy and risk of CBT (RR 1.24; 95% CI 1.01–1.53) (Brooks et al., 2004).
Alcohol
Findings relating to parental alcohol intake are mostly unremarkable. Most studies of adult brain tumors find no excess and possibly a reduced risk related to intake of wine and beer.
Use Of Pesticides
Several epidemiologic studies have investigated home and occupational use of pesticides, insecticides, or herbicides as possible etiologic factors for brain tumors. Case-control studies have linked household pest exterminations to the development of childhood brain tumors, while others have found no association with parental pesticide use. A recent review indicated 14 of 17 studies of maternal and childhood pesticide exposure and CBT found positive associations (Zahm, 1999). One large case-control study found an excess risk of CBT related specifically to prenatal exposure to flea and tick products (Pogoda and Preston-Martin, 1997). A cohort study of licensed pesticide applicators (Blair et al., 1983) found an excess risk of brain cancer. More studies are needed to confirm associations seen and to identify which compounds may relate to brain tumor development.
Summary Of Environmental Risk Factors
The cause of most types of brain tumor remain unexplained. High-dose levels of ionizing radiation (IR) are the most clearly established cause of pediatric and adult brain tumors based on both experimental and epidemiologic data. However, few cases are likely to be explained by this factor because precautions are now taken to avoid exposing individuals to unnecessarily high doses of IR. A growing number of epidemiologic studies suggest a strong, protective association between brain tumor risk and a history of asthma and other allergic conditions. Other environmental factors have been less consistently associated with brain tumor risk. Occupational studies suggest that employment in the petrochemical industry or employment involving exposure to electromagnetic fields may increase risk. N-nitroso compounds and pesticide use in the home have been associated with risk of pediatric brain tumors in some studies, but challenges in recreating exposure years in the past limit the strength of conclusions that can be drawn from these data. Current evidence that cell phones, smoking history, or alcohol are risk factors for brain tumors is weak.
Host Factors
Familial Aggregation
The potential heritability and familial clustering of CNS tumors is well documented. The implication of such reports is that shared genes, environment, or gene–environment interaction could be responsible for some neoplasms, but reports estimate that familial gliomas account for 5% or fewer of total cases.
Neurogenetic Syndromes
Various hereditary syndromes have been associated with increased brain tumor risk, including neurofibromatosis-1 and -2, Von Hippel-Lindau disease, Li-Fraumeni syndrome, tuberous sclerosis, and Gorlin’s syndrome. However, these hereditary syndromes are rare and are likely to account for few cases in a population-based series of CNS neoplasia.
Preventive Measures
Few causes of brain tumors in adults or children have been identified. Etiologic associations may be more likely to be detected if analyses are restricted to more homogeneous tumor sets with common morphologic and molecular genetic characteristics. For many subgroups, in order to obtain sufficient numbers of subjects it will become necessary to pool data from studies across geographic areas. We have the most to gain using this approach in studies of the etiology of gliomas, whose causes remain largely unknown. Gliomas appear far more morphologically and genetically diverse than do the other major categories of CNS tumors. Perhaps more is known about the etiology of meningiomas and nerve sheath tumors because each of these major groups represents a more homogeneous entity than do gliomas. Diet, genetics, and immunology will continue to be an important focus of the next generation of epidemiologic studies of brain tumors.
Bibliography:
- Ahlbom IC, Cardis E, Green A, Linet M, Savitz D, and Swerdlow A (2001) Review of the epidemiologic literature on EMF and Health. Environmental Health Perspectives 109 (supplement 6): 911–933.
- American Cancer Society (2006) Cancer Facts & Figures. Atlanta, GA: Surveillance Research.
- Auvinen A, Hietanen M, Luukkonen R, and Koskela RS (2002) Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology 13: 356–359.
- Baldwin RT and Preston-Martin S (2004) Epidemiology of brain tumors in childhood: A review. Toxicology and Applied Pharmacology 199: 118–131.
- Beall C, Delzell E, Rodu B, Sathiakumar N, and Myers S (2001) Cancer and benign tumor incidence among employees in a polymers research complex. Journal of Occupational and Environmental Medicine 43: 914–924.
- Bithell JF, Draper GJ, and Gorbach PD (1973) Association between malignant disease in children and maternal virus infections during pregnancy. British Journal of Preventive Social Medicine 27: 68.
- Blair A, Grauman DJ, Lubin JH, and Fraumeni JF Jr (1983) Lung cancer and other causes of death among licensed pesticide applicators. Journal of the National Cancer Institute 71: 31–37.
- Boffetta P, Tredaniel J, and Greco A (2000) Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: A meta-analysis. Environmental Health Perspectives 108: 73–82.
- Brenner AV, Linet MS, Fine HA, et al. (2002) History of allergies and autoimmune diseases and risk of brain tumors in adults. International Journal of Cancer 99: 252–259.
- Brooks DR, Mucci LA, Hatch EE, and Cnattingius S (2004) Maternal smoking during pregnancy and risk of brain tumors in the offspring. A prospective study of 1.4 million Swedish births. Cancer Causes and Control 15: 997–1005.
- CBTRUS (2005) Statistical Report: Primary Brain Tumors in the United States, 1998–2002. Hinsdale, IL: Central Brain Tumor Registry of the United States.
- Cordier S, Monfort C, Filippini G, et al. (2004) Parental exposure to polycyclic aromatic hydrocarbons and the risk of childhood brain tumors: The SEARCH International Childhood Brain Tumor Study. American Journal of Epidemiology 159: 1109–1116.
- Davis FG, Freels S, Grutsch J, Barlas S, and Brem S (1998) Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on Surveillance Epidemiology, and End Results (SEER) data, 1973–1991. Journal of Neurosurgery 88: 1–10.
- Eddy BE, Borman GS, Grubbs GE, and Young RD (1962) Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology 17: 65–75.
- Ferlay J, Bray F, Pisani P, and Parkin DM (2004) Cancer Incidence Mortality, and Prevalence Worldwide, Verson 2.0. IARC CancerBase No. 5. Lyon, France: IARC Press, 2004.
- Groves FD, Page WF, Gridley G, et al. (2002) Cancer in Korean war navy technicians: Mortality survey after 40 years. American Journal Epidemiology 155: 810–818.
- Gurney JG and Kadan-Lottick N (2001) Brain and other central nervous system tumors: Rates, trends, and epidemiology. Current Opinions in Oncology 13: 160–166.
- Hardell L, Mild KH, Pahlson A, and Hallquist A (2001) Ionizing radiation, cellular telephones and the risk for brain tumours. European Journal of Cancer Prevention 10: 523–529.
- Huncharek M and Kupelnick B (2000) Epidermal growth factor receptor gene amplification as a prognostic marker in glioblastoma multiforme: Results of a meta-analysis. Oncology Research 12: 107–112.
- Inskip PD, Linet MS, and Heineman EF (1995) Etiology of brain tumors in adults. Epidemiology Reviews 17: 382–414.
- Inskip PD, Mellemkjaer L, Gridley G, and Olsen JH (1998) Incidence of intracranial tumors following hospitalization for head injuries (Denmark). Cancer Causes and Control 9: 109–116.
- Ivankovic S (1979) Teratogenic and carcinogenic effects of some chemicals during perinatal life in rats Syrian golden hamsters, and minipigs. National Cancer Institute Monographs 103–115.
- Johansen C, Boice J Jr, McLaughlin J, and Olsen J (2001) Cellular telephones and cancer – A nationwide cohort study in Denmark. Journal of the National Cancer Institute 93: 203–207.
- Kheifets LI (2001) Electric and magnetic field exposure and brain cancer: A review. Bioelectromagnetics Supplement 5: S120–131.
- Kheifets LI, Afifi AA, Buffler PA, and Zhang ZW (1995) Occupational electric and magnetic field exposure and brain cancer: A metaanalysis. Journal of Occupational and Environmental Medicine 37: 1327–1341.
- Lee M, Wrensch M, and Miike R (1997) Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California USA). Cancer Causes and Control 8: 13–24.
- Legler J, Ries L, Smith M, et al. (1999) Cancer surveillance series [corrected]: Brain and other central nervous system cancers: Recent trends in incidence and mortality. Journal of the National Cancer Institute 91: 1382–1390.
- Lijinsky W (1992) Chemistry and Biology of N-nitroso Compounds. Cambridge, UK: Cambridge University Press.
- Lonn S, Ahlbom A, Hall P, and Feychting M (2005) Long-term mobile phone use and brain tumor risk. American Journal of Epidemiology 161: 526–535.
- Moulder JE, Foster KR, Erdreich LS, and McNamee JP (2005) Mobile phones, mobile phone base stations and cancer: A review. International Journal of Radiation Biology 81: 189–203.
- Muscat JE, Malkin MG, Thompson S, et al. (2000) Handheld cellular telephone use and risk of brain cancer. Journal of the American Medical Association 284: 3001–3007.
- National Research Council Committee on Diet Nutrition and Cancer (1982) Diet, Nutrition and Cancer. Washington, DC: National Academy Press.
- Nygren C, Adami J, Ye W, et al. (2001) Primary brain tumors following traumatic brain injury – A population-based cohort study in Sweden. Cancer Causes and Control 12: 733–737.
- Ohgaki H (2005) Genetic pathways to glioblastomas. Neuropathology 25: 1–7.
- Phillips LE, Longstreth WT Jr, Koepsell T, Custer BS, Kukull WA, and van Belle G (2005) Active and passive cigarette smoking and risk of intracranial meningioma. Neuroepidemiology 24: 117–122.
- Pogoda JM and Preston-Martin S (1997) Household pesticides and risk of pediatric brain tumors. Environmental Health Perspectives 105: 1214–1220.
- Preston-Martin S and White SC (1990) Brain and salivary gland tumors related to prior dental radiography: Implications for current practice. Journal of the American Dental Association 120: 151–158.
- Preston-Martin S, Pogoda JM, Mueller BA, et al. (1998) Prenatal vitamin supplementation and risk of childhood brain tumors. International Journal of Cancer Supplement 11: 17–22.
- Ries LA, Eisner MP, and Kosary CL (2000) SEER Cancer Statistics Review 1973–1977. http://www-seer.ims.nci.nih.gov/Publications/ (accessed February 2008).
- Sabel M, Felsberg J, Messing-Junger M, Neuen-Jacob E, and Piek J (1999) Glioblastoma multiforme at the site of metal splinter injury: A coincidence? Case report. Journal of Neurosurgury 91: 1041–1044.
- Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, and Novikov I (2005) Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiation Research 163: 424–432.
- Sant M, Aareleid T, Berrino F, et al. (2003) EUROCARE-3: Survival of cancer patients diagnosed 1990–94: Results and commentary. Annals of Oncology 14(supplement 5): 61–118.
- Schuman LM, Choi NW, and Gullen WH (1967) Relationship of central nervous system neoplasms to Toxoplasma gondii infection. American Journal of Public Health and the Nation’s Health 57: 848–856.
- Schwartzbaum J, Ahlbom A, Malmer B, et al. (2005) Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Research 65: 6459–6465.
- Schwartzbaum JA and Cornwell DG (2000) Oxidant stress and glioblastoma multiforme risk: serum antioxidants, gamma-glutamyl transpeptidase, and ferritin. Nutrition and Cancer 38: 40–49.
- Schwartzbaum J, Jonsson F, Ahlbom A, et al. (2003) Cohort studies of association between self-reported allergic conditions, immunerelated diagnoses and glioma and meningioma risk. International Journal of Cancer 106: 423–428.
- Shah K and Nathanson N (1976) Human exposure to SV40: Review and comment. American Journal of Epidemiology 103: 1–12.
- Smith MA, Freidlin B, Ries LA, and Simon R (1998) Trends in reported incidence of primary malignant brain tumors in children in the United States. Journal of the National Cancer Institute 90: 1269–1277.
- Sorahan T, Nichols L, van Tongeren M, and Harrington JM (2001) Occupational exposure to magnetic fields relative to mortality from brain tumours: Updated and revised findings from a study of United Kingdom electricity generation and transmission workers, 1973–97. Occupational and Environmental Medicine 58: 626–630.
- Surveillance Epidemiology and End Results (SEER). Program SEER*Stat Database: Incidence-SEER 9 Regs Public Use Nov 2005 Sub (1973–2003), Linked to County Attributes. Total US, 1969–2003
- https://seer.cancer.gov/.
- Villeneuve PJ, Agnew DA, Johnson KC, and Mao Y (2002) Brain cancer and occupational exposure to magnetic fields among men: Results from a Canadian population-based case-control study. International Journal of Epidemiology 31: 210–217.
- Wiemels JL, Wiencke JK, Patoka J, et al. (2004) Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Research 64: 8468–8473.
- Wrensch M, Bondy ML, Wiencke J, and Yost M (1993) Environmental risk factors for primary malignant brain tumors: A review. Journal of Neuro-oncology 17: 47–64.
- Wrensch M, Fisher JL, Schwartzbaum JA, Bondy M, Berger M, and Aldape KD (2005) The molecular epidemiology of gliomas in adults. Neurosurgery Focus 19: E5.
- Wrensch M, Minn Y, Chew T, Bondy M, and Berger MS (2002) Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro-oncology 4: 278–299.
- Zahm SH (1999) Childhood leukemia and pesticides. Epidemiology 10: 473–475.
- Deitrich M, Block G, Pogoda JM, Buffler P, Hechts S, and PrestonMartin S (2005) A review: Dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors. Cancer Causes and Control 16(6): 619–635.
- Ohgaki H and Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathologica (Berlin) 109: 93–108.
- Wrensch M, Bondy ML, Wiencke J, and Yost M (1993) Environmental risk factors for primary malignant brain tumors: A review. Journal of Neuro-oncology 17: 47–64.
- Wrensch M, Fisher JL, Schwartzbaum JA, Bondy M, Berger M, and Aldape KD (2005) The molecular epidemiology of gliomas in adults. Neurosurgery Focus 19: E5.




