View sample cancer research paper on gastric cancer. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Introduction
Gastric cancer remains one of the most common cancers in the world, although it has declined substantially in many countries. It remains the fourth most common cancer in the number of new cases, and the second most common cause of death from cancer. According to global estimates (Parkin et al., 2005), 934 000 new cases of gastric cancer occurred in 2002, accounting for 8.6% of all new cases of cancer. The number of deaths from gastric cancer was estimated to be 700 000 (10.4% of all cancer deaths). It can be roughly inferred from the mortality-to incidence ratio (0.75) that the prognosis of gastric cancer is generally poor. Five-year survival is little more than 30% in developed areas and 20% in developing areas.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Most gastric cancers are adenocarcinomas arising from the gastric epithelium. Adenocarcinomas can be classified into two histological types: The intestinal type is more frequent in men and in older persons, while the diffuse type is more frequent at younger ages and shows little difference in frequency by sex. It has been hypothesized that variation in the intestinal type of adenocarcinoma is the main component of international and temporal variation in the incidence of gastric cancer, but the hypothesis is controversial. It is of interest to know whether etiological factors are different for the intestinal and diffuse types, but epidemiological studies, though limited, have not supported a differential etiology for the two histological types of gastric cancer. The stomach is classified anatomically into cardia (near the esophagus), fundus, corpus, and antrum (near to the duodenum). Cancers of the cardia seem to differ from cancer in other parts of the stomach with respect to etiological factors. There is evidence that the incidence of adenocarcinoma of the gastric cardia has increased in recent decades in Europe and North America.
Chronic atrophic gastritis is a well-established precursor lesion. The prevalence of chronic atrophic gastritis is higher in populations with higher rates of gastric cancer, and the risk of gastric cancer is substantially increased in individuals with chronic atrophic gastritis. It is hypothesized that atrophic gastritis advances to intestinal metaplasia, dysplasia, and finally the intestinal type of gastric cancer.
Descriptive Features
Geographic Variation
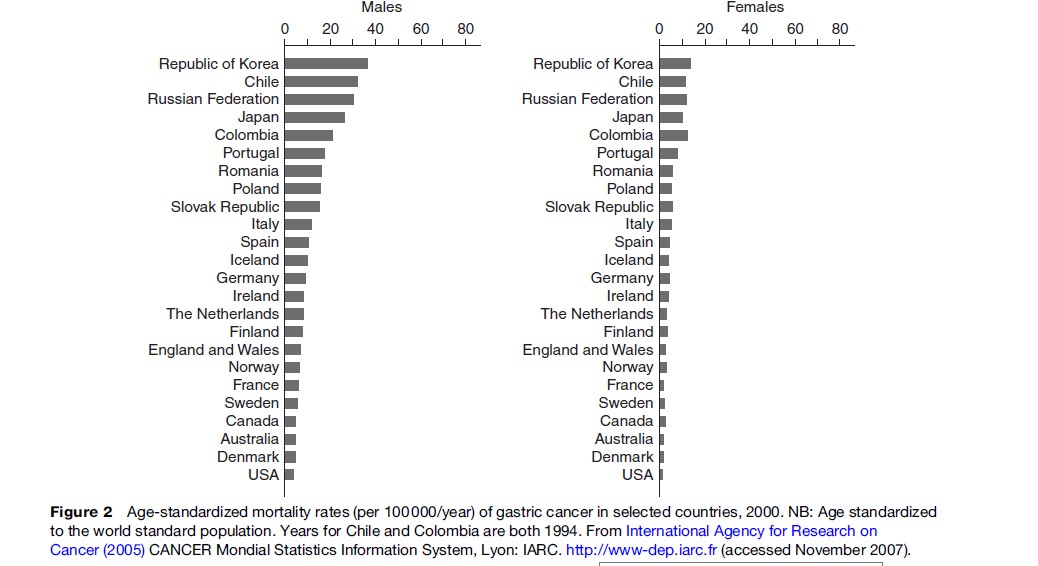
Wide international variation in both incidence and mortality of gastric cancer has long been recognized. The incidence rates are highest in registries in Japan, Korea, and northeast China, with annual age-standardized rates of 70–80 per 100 000 in men and 35 in women (Figure 1). Relatively high rates of incidence are recorded in South America and Eastern Europe. The rates are lower in populations in Western Europe and Scandinavian countries, and lowest in North America, Oceania, and South Asia. It is well known that Portugal has the highest rate in Western Europe. Mortality statistics indicate an international variation similar to that noted from the incidence data (Figure 2). Cancer mortality statistics in Russia and Korea have become available very recently, while high rates of mortality from gastric cancer in Chile and Portugal have been well recognized. Both incidence and mortality data demonstrate that rates in males are 1.5–2.5 times higher than those of females in all populations and countries. Apart from China, regional variation within countries is much less than international variation between countries. For example, in Japan, the difference between the highest mortality rates in the northern part along the Sea of Japan and the lowest rates in southern Kyushu is approximately threefold. In England and Wales, there is a twofold difference between North and South. In China, by contrast, there is an eightfold difference between provinces with the highest and lowest mortality rates.
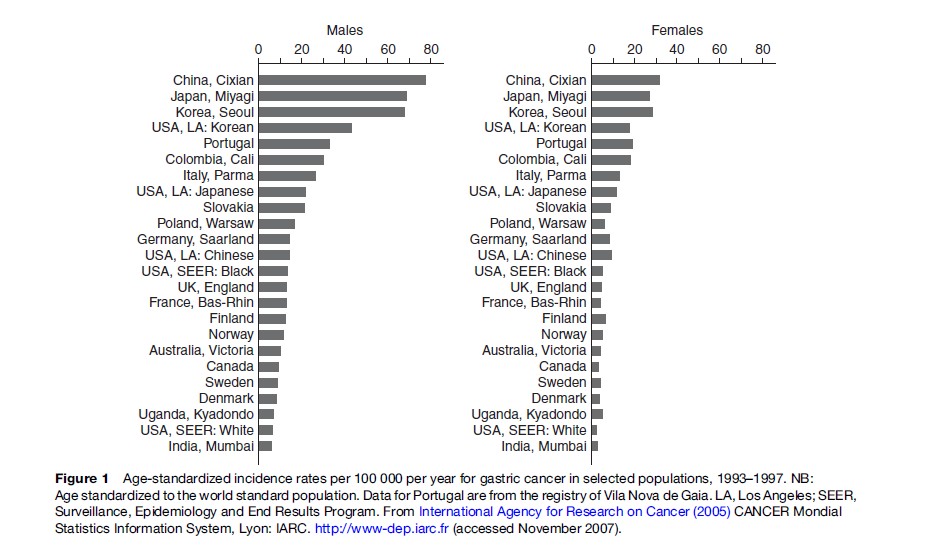
Multiethnic registries in the United States provide incidence rates by ethnicity within the same geographic region. Koreans and Japanese living in Los Angeles have much lower incidence than Koreans and Japanese living in their home countries, but still have higher incidence than African and Caucasian Americans in Los Angeles. This type of information alone strongly indicates that environmental factors are important in the etiology of gastric cancer. Migrant studies provide more definite information regarding the role of genetic and environmental factors and also the time of exposure. As early as 1969, Haenszel and Kurihara reported a historical finding that the first-generation migrants (born in Japan) tended to maintain the high risk of gastric cancer as experienced by Japanese in Japan, but the second generation (born in the United States) had a risk of gastric cancer closer to that of Caucasians in the United States. Similar findings have been observed in other migrant studies in Colombia, Australia, and Brazil. Exposure to environmental factors in early life is considered to be crucial in the development of gastric cancer.
Time Trend
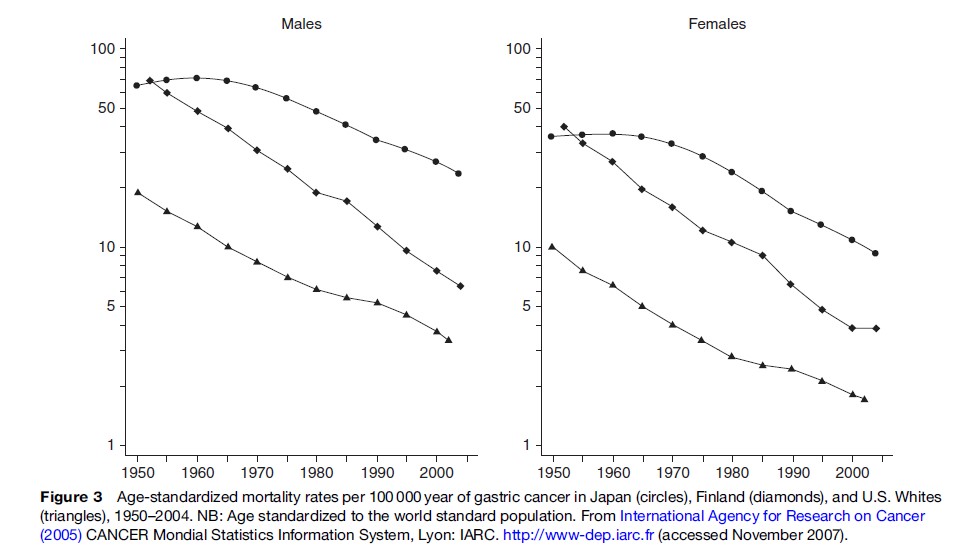
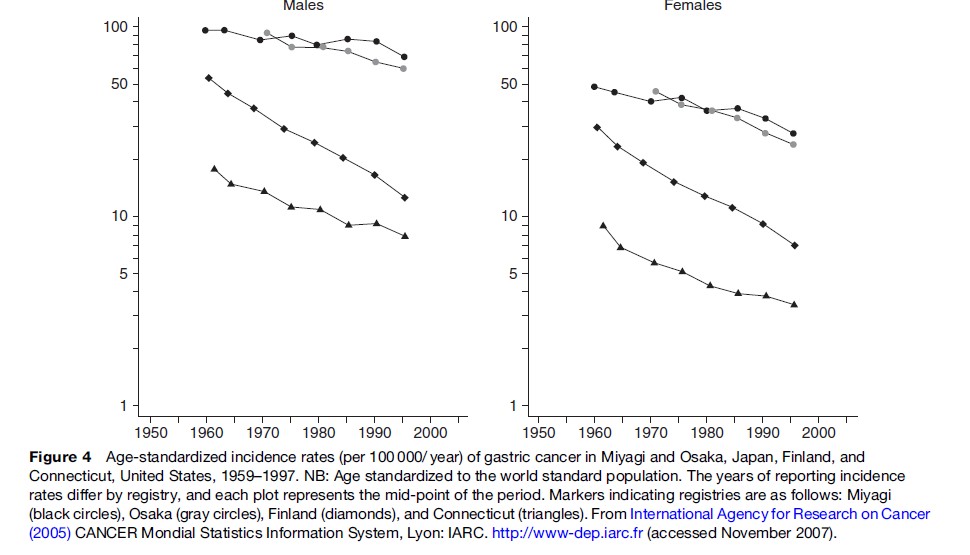
The decline in gastric cancer incidence and mortality is a global phenomenon, although the starting point and the rate of decline differ somewhat in different countries and populations. This phenomenon was aptly described in 1986 as an unplanned triumph by Howson et al. (1986) who suggested that the decline was due to widespread use of refrigerators; the advent of refrigerators resulted in decreased consumption of salted, preserved foods and increased consumption of fresh vegetables and fruits (see the section titled ‘Diet’). The most extensive analysis of time trends in gastric cancer as well as other sites of cancer was conducted by Coleman and colleagues (1993), based on incidence and mortality data up to the late 1980s. The decline in mortality from gastric cancer started much later in Japan than in countries in Europe and North America (Figure 3). It is notable that mortality rates in Japan and Finland were at the same level in the 1950s. In Japan, the incidence of gastric cancer has not declined as rapidly as the trend in mortality (Figure 4). The increasing divergence between incidence and mortality in Japan can probably be ascribed to nationwide screening for gastric cancer. The greater decline in mortality than in incidence may be attributable to earlier detection and more effective treatment. Alternatively, gastric cancers that are pathologically malignant but clinically more benign may have been detected more frequently because of screening. The incidence has declined more in females than in males (Figure 4) and it is less likely that the so-called overdiagnosis is more frequent in men than in females.
Sex, Age, And Social Class
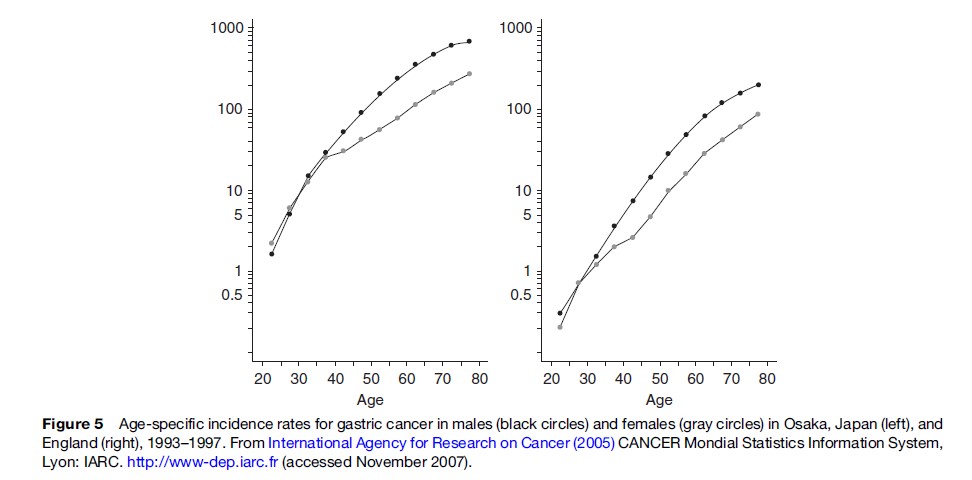
Men have gastric cancer incidence and mortality rates approximately twice as high as women in most countries and populations, mainly at ages 40 and older: There is no difference at younger ages (Figure 5). With few exceptions, this phenomenon is seen worldwide, although divergence in the rates between men and women begins at slightly different ages in different populations. The lack of sex difference at younger ages is compatible with the observation that the diffuse type of gastric cancer is more common at younger ages, with no clear sex difference. Lower socioeconomic status is consistently associated with an increased risk of gastric cancer in different countries. The most likely explanation is that factors etiologically linked to gastric cancer are more prevalent in individuals ranked at lower socioeconomic status.
Etiology
Helicobacter Pylori
H. pylori infection has emerged as an important etiological factor in the past 20 years. There is no doubt that H. pylori infection is a cause of gastric cancer, as concluded by the expert panel of the International Agency for Research on Cancer in 1994. Evidence for the causal link with H. pylori infection is primarily based on findings from prospective studies. In the year 1991, three prospective studies all reported an increased risk of gastric cancer in individuals who were seropositive for H. pylori infection. The subjects in these pioneering studies were subscribers to the Kaiser-Permanente Medical Care Program in the United States, Japanese participants in the Honolulu Heart Program, and participants in the British United Provident Association (BUPA) and the Caerphilly collaborative heart disease (CCHD) studies. Serum IgG antibody to H. pylori was measured using frozen stored blood samples in cases of gastric cancer and matched controls within each cohort. These initial findings have been corroborated by subsequent prospective studies. A pooled odds ratio of 2.36 (95% confidence interval, 1.98–2.81) was reported for the association between H. pylori infection and gastric cancer in a combined analysis of 12 case–control studies nested in prospective cohorts (Helicobacter and Cancer Collaborative Group, 2001). Importantly, H. pylori infection was exclusively associated with non-cardia cancer, with an odds ratio of 3.0 (95% CI, 2.3–3.8), while the odds ratio for cancer of the gastric cardia was 1.0 (95% CI, 0.7–1.4). It was also observed that the increased risk of non-cardia cancer associated with H. pylori infection was attenuated when H. pylori infection was assessed closer to the time of cancer diagnosis. H. pylori infection is probably lost in the development of gastric atrophy, which precedes the occurrence of carcinoma in many patients. It is thus not surprising that the results from case–control studies are rather inconsistent. There is no difference between the two histological types (intestinal and diffuse) in the magnitude of the increased risk associated with H. pylori infection.
Despite established causality, H. pylori infection alone cannot sufficiently explain the descriptive features of gastric cancer. As illustrated in the EUROGAST study, which showed a positive geographical correlation at international level between H. pylori infection and gastric cancer risk, there is no difference in the prevalence of H. pylori infection between men and women in many populations, whereas incidence and mortality rates of gastric cancer are 1.5–2.5 times higher in males than in females across populations. There is also a notable variation in gastric cancer rates among populations with similar rates of H. pylori infection. This phenomenon is referred to as the African or Asian enigma: H. pylori infection is fairly high, but gastric cancer rates are generally low in African and southeast Asian countries. Furthermore, only a very small proportion of individuals infected with H. pylori develop gastric cancer. Host and environmental factors, as well as the virulence of the bacterium, have recently become of interest as determinants of the outcome of H. pylori infection. The virulence of H. pylori differs with the presence of cytotoxin-associated gene A (CagA). Strains that are positive for CagA induce more severe inflammation and are associated with a higher risk of gastric cancer. Interleukin (IL)-1b, a pro-inflammatory cytokine and a potent inhibitor of gastric acid secretion, is upregulated during H. pylori infection. Specific genotypes thought to enhance IL-1b production are associated with an increased risk of hypochlorhydria and of gastric cancer in response to H. pylori infection. Among the dietary factors, it is suggested from a mechanistic viewpoint that vitamin C may be protective and that salt intake may synergistically enhance the risk of gastric cancer.
Diet
In 1997, the World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) issued an expert report on diet and cancer based on a comprehensive literature review. The summary for diet and gastric cancer is presented in Table 1. The main conclusion is that high consumption of vegetables and fruit convincingly confers a lower risk of gastric cancer and that high intake of salt or salted foods probably increases the risk. Refrigeration of food is indirectly protective by reducing salt intake. The importance of these two dietary factors is also acknowledged in the joint report of the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) issued in 2003, although the evidence for a protective effect of vegetables and fruit is regarded as less convincing.
The reduced risk of gastric cancer associated with high consumption of vegetables and fruit has been observed in a large number of case–control studies in different populations, but supportive findings from prospective studies are limited. Decreased risk has been more consistently observed with the consumption of fresh vegetables and citrus fruits. Garlic and other allium vegetables are of particular interest, because of the abundant experimental evidence on the anticarcinogenic effects of allium compounds (e.g., allyl sulfides). High consumption of garlic and other allium vegetables was shown to be related to a decreased risk of gastric cancer in China and Italy, where allium vegetables are commonly consumed.
Vegetables and fruit are rich in vitamin C and carotenoids. Vegetables are also a major source of vitamin E and selenium. These micronutrients exert antioxidative effects through different mechanisms and have aroused much interest in the prevention not only of gastric cancer but also of other cancers. Vitamins C and E inhibit the formation of N-nitroso compounds, potential carcinogens for the human stomach (Kono and Hirohata, 1996). Among these micronutrients of anticarcinogenic potential, vitamin C seems to have gained considerable evidence regarding its protective association with gastric cancer. Observational studies have suggested a decreased risk associated with high intake of carotenoids, but supplementation with b-carotene did not decrease the risk of gastric cancer in Finland. On the other hand, combination supplements of b-carotene, vitamin E, and selenium resulted in a 16% reduction in incidence and a 21% reduction in mortality from gastric cancer in Linxian, China, where the inhabitants were marginally deficient in micronutrients.
Folate is abundant in leafy vegetables, and it plays an important role in DNA methylation and DNA synthesis. High folate intake is related to a decreased risk of colorectal cancer, so the relation of folate to gastric cancer risk is naturally of interest. Results from nine case–control studies and two cohort studies are inconsistent, with a pooled relative risk of 0.9 (95% CI, 0.7–1.1) for the highest versus lowest intake (Larsson et al., 2006). However, genetic polymorphism of a key enzyme in folate metabolism, MTHFR C677T, is related to gastric cancer risk, suggesting that folate metabolism is involved in gastric carcinogenesis as well.
Salt itself has no carcinogenic effect, but it is well documented that high salt intake enhances chemical carcinogenesis in the rat stomach. Increased risk associated with high intake of salt and salted foods has mainly been observed in case–control studies, but it has recently been substantiated in prospective studies in Japan. Salt intake in Korea and Japan is the highest in the world, and in parallel with the highest rates of gastric cancer in those countries.
Nitrite reacts with amines, amides, and other proteins to form N-nitrosocompounds. Nitrate is not a nitrosating agent in its own right, but it is reduced to nitrite by bacteria in the mouth and in the achlorhydric stomach. Nitrosation is an acid-catalyzed chemical reaction in the normoacidic condition, and the reaction is also catalyzed by bacteria in the achlorhydric stomach. Vegetables are the major source of nitrate intake, and nitrite intake largely derives from preserved meats. In most circumstances, daily intake of nitrate is 100-fold greater than that of nitrite. Thus epidemiological studies have not adequately addressed the role of nitrate, nitrite, or N-nitrosocompounds in gastric carcinogenesis in humans (Kono and Hirohata, 1996). A possible anticarcinogenic effect of green tea has been a topic in cancer prevention. Green tea extract and epigallocathechin, a major constituent of green tea polyphenol, have been shown to inhibit chemical carcinogenesis in the glandular stomach in rats. High consumption of green tea has been shown to be associated with a reduced risk of gastric cancer in case–control and cohort studies in Japan and China, though such a protective association has not always been replicated in other cohort studies in Japan. Capsaicin, the pungent component of chilli peppers, is known to be both mutagenic and carcinogenic. High consumption of chilli pepper has been reported to be associated with increased risk of gastric cancer in Mexico, India, and Korea.
Alcohol Consumption
A few case–control studies have reported an increased risk of gastric cancer in relation to vodka drinking before breakfast in Poland, and heavy consumption of red wine in Portugal and France. An increased risk of gastric cancer associated with high alcohol consumption has also been reported from small prospective studies in Japan and in the United States. However, a high number of much larger case–control and cohort studies have found no association between alcohol use and gastric cancer risk. The increased risk associated with alcohol use in the more limited studies is probably due to confounding or chance, but the possibility that alcohol intake may increase the risk of cardiac cancer cannot be excluded.
Smoking
Many case–control and cohort studies have fairly consistently showed increased risk of gastric cancer among smokers, but a dose–response relation has not always been observed. The International Agency for Research on Cancer has concluded that smoking is causally related to gastric cancer. Several studies reported that smoking was associated with increased risk of cardia cancer, rather than of non-cardia cancer, but the evidence is not consistent.
Obesity
A high body mass index is related to an increased risk of adenocarcinoma of the gastric cardia, although the increased risk associated with obesity is much greater for adenocarcinoma of the esophagus. In Sweden, for instance, a high body mass index was associated with a 7.6-fold increased risk of esophageal adenocarcinoma and 2.3-fold increased risk of cardia adenocarcinoma. Gastroesophageal reflux associated with obesity is a possible explanation.
Treatment And Survival
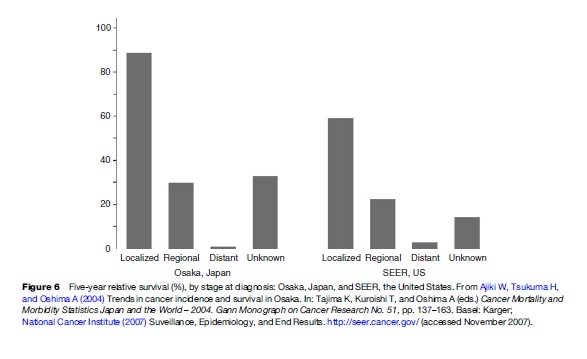
Partial or total gastrectomy (removal of the stomach) is a conventional approach to treatment for surgical cases. Endoscopic mucosal resection has recently been developed for treatment of early cancer (i.e., mucosal or submucosal cancer). Survival depends strongly on the stage of disease at diagnosis and it varies substantially between countries. In Europe, the 5-year age-standardized relative survival for male adults diagnosed in 1990–94 ranged from 9.0% in Poland to 28.9% in Iceland, and the corresponding range for females was from 6.8% in Malta to 37.4% in Austria. The overall 5-year relative survival for men and women combined in 22 European countries was 23% (Sant et al., 2003). In the SEER Program of the United States, the 5-year survival of patients diagnosed during 1988–2003 was 24.1%. In Osaka, Japan, the 5-year relative survival for gastric cancer patients registered during 1993–95 was 48.9%. In the SEER Program and Osaka Cancer Registry, survival rates were reported by stage. The 5-year relative survival for gastric cancer at the localized stage in Osaka is higher than that in the SEER Program areas, but there is little difference in survival between the regional and distant stages (Figure 6). The proportion of the localized stage was far greater in Osaka than in the SEER Program areas (59% vs. 23%), suggesting early stages of gastric cancer are detected because of the mass screening in Japan. The 5-year relative survival of patients with early gastric cancer is greater than 95%.
Cancer survival is generally poorer in patients of low socioeconomic status than for those of higher status, as well as in Blacks as compared with Whites in the United States. No such difference is evident for gastric cancer survival, however (Coleman et al., 2004).
Prevention Strategies
Screening
Gastric cancer screening by means of X-ray photofluorography has been practiced in Japan since the 1960s. The sensitivity and specificity of the double-contrast barium X-ray technique currently used for mass screening in Japan are reportedly 57–90% and 77–91%, respectively. As described above, it is not clear that mass screening is effective in reducing mortality from gastric cancer, although a limited number of case–control and prospective studies have suggested decreased mortality from gastric cancer in individuals with recorded participation in the screening (Watanabe and Fukao, 2001). Nonetheless, 5–6 million persons receive the screening examination annually in Japan. In the year 2003, a total of 5 976 000 persons received X-ray screening in the community or at the workplace, and 5970 cases of gastric cancer were detected among the screenees, resulting in an overall detection rate of 1.0 per 1000 screenees (Okuda et al., 2006).
A new method of screening using serum pepsinogen I and II has recently been introduced in Japan. Pepsinogen I is secreted by the chief cells and mucus neck cells in the fundus of the stomach, and pepsinogen II throughout the stomach and proximal duodenum. A decrease in serum levels of pepsinogen I and in the I/II ratio has been shown to be a good measure of gastric atrophy. The combination of cutoff points of pepsinogen I below 70 mg/L and pepsinogen I/II ratio below 3.0 is currently used as a diagnostic criterion for gastric cancer in screening. The sensitivity and specificity of this criterion are estimated to be 60–96% and 55–84%, respectively. While effectiveness of screening by serum pepsinogen has not been addressed, the use of serum pepsinogen has been incorporated in the screening in Japan as an alternative method or in combination with the X-ray method.
Eradication Of H. Pylori Infection
Strong evidence that H. pylori infection confers an increased risk of gastric cancer naturally leads to the idea that H. pylori eradication treatment would necessarily prevent the occurrence of gastric cancer. However, there is no evidence in favor of screening for (and eradication of ) H. pylori infection in the prevention of gastric cancer. In a randomized trial in Colombia, the pharmacological eradication of H. pylori resulted in a higher rate of regression of premalignant lesions such as atrophic gastritis, intestinal metaplasia, and dysplasia. On the other hand, there was no clear reduction in the risk of gastric cancer associated with H. pylori eradication treatment in a randomized trial lasting 7.5 years in China. Currently, eradication of H. pylori infection is selectively recommended in high-risk groups, e.g., patients who already have atrophic gastritis, a premalignant condition, and in first-degree relatives of patients with gastric cancer.
Dietary Recommendation
It is estimated in the WCRF/AICR report that gastric cancer risk can be reduced by two-thirds or three-quarters by modification of the diet, i.e., increased consumption of vegetables and fruit and decreased salt intake. More specifically, it is advised to eat 400–800 g (or five or more portions) of a variety of vegetables and fruit daily and to reduce salt intake to less than 6 g per day. The use of refrigeration is also advised to preserve perishable food.
Bibliography:
- Coleman MP, Rachet B, Woods LM, et al. (2004) Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. British Journal of Cancer 90: 1367–1373.
- Helicobacter and Cancer Collaborative Group (2001) Gastric cancer and Helicobacter pylori: A combined analysis of 12 case–control studies nested within prospective cohorts. Gut 49: 347–353.
- Howson CP, Hiyama T, and Wynder EL (1986) The decline in gastric cancer: Epidemiology of an unplanned triumph. Epidemiology Review 8: 1–27.
- Kono S and Hirohata T (1996) Nutrition and stomach cancer. Cancer Causes and Control 7: 41–55.
- Larsson S, Giovannucci E, and Wolk A (2006) Folate intake MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: A meta-analysis. Gastroenterology 131: 1271–1283.
- Okuda J, Ida K, Sakai H, Yokomizo C, Kojima T, and Kato T (2006) Secondary prevention of gastric cancer: Present and future. Clinical Gastroenterology 21: 683–688.
- Parkin DM, Bray F, Ferlay J, and Pisani P (2005) Global cancer statistics, 2002. CA: Cancer Journal for Clinicians 55: 74–108.
- Sant M, Aareleid T, Berrino F, et al. (2003) EUROCARE-3: Survival of cancer patients diagnosed 1990–94 – Results and commentary. Annals of Oncology 14(supplement 5): v61–v118.
- Watanabe Y and Fukao A (2001) Stomach cancer screening. In: Hisamichi S (ed.) Report on the Evaluation of the Efficacy of New Cancer Screening Methods, pp. 81–120. Tokyo: Japanese Public Health Association.
- Shibata A and Parsonnet J (2006) Stomach cancer. In: Schottenfeld D and Fraumeni JF Jr (eds.) Cancer Epidemiology and Prevention, pp. 707–720. Oxford, UK: Oxford University Press.
- World Cancer Research Fund/American Institute for Cancer Research (1997) Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington DC: American Institute for Cancer Research.
- Yamaguchi N and Kakizoe T (2001) Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. The Lancet Onclogy 2: 84–94.
- https://www-dep.iarc.fr/ – CANCER Mondial Statistics Information System, IARC.
- https://seer.cancer.gov/ – Surveillance, Epidemiology, and End Results Program, National Cancer Institute, USA.




