View sample cancer research paper on diet and cancer. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Historical Background
The idea that food might be a cancer risk factor, or might be used for cancer prevention (or even treatment), started in ancient times. Hieroglyphic inscriptions and papyri manuscripts suggest that physicians in Ancient Egypt were able to distinguish between benign and malignant tumors. They used various food compounds to treat some cancers, e.g., stomach and uterine cancers were treated with barley and pig ears. In 168 BC Galen, a Roman physician, wrote that unhealthy diet and bad climate were directly connected to cancer. In the Middle Ages (1676), Wiseman suggested that cancer might arise from ‘‘an error in diet,’’ and recommended avoiding ‘‘salt, sharp and gross meats’’.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
In 1811, Howard proposed that constipation might be an important cancer risk factor. Lambe, a Fellow of the Royal College of Physicians of London, suggested in 1815 that excess of food consumption, and especially excess of meat consumption, might lead to cancer development.
At the beginning of the twentieth century, Shaw proposed eating more vegetables and less foods of animal origin, less alcohol, tea, and tobacco to reduce cancer risk.
The real beginning of the scientific studies of diet in relation to cancer risk started from the observations of Peyton Rous, when in 1914 he observed that restriction of food consumption delayed the development of cancer metastases in mice. This study was followed by the extensive animal research that demonstrated an association between obesity and mortality from cancer beginning in the 1920s. Other early epidemiological studies of diet and cancer were a case-control study conducted by Stocks and Karn in England and Wales in 1933 and an ecological study of oral cancer in India conducted by Orr. These suggested that low consumption of vegetables and fruit might increase cancer risk. In 1937, Hoffman (a founder of the American Cancer Society) concluded that ‘‘excessive nutrition,’’ especially fatty, sugary foods, white bread, and meat are important cancer risk factors.
Despite a profusion of experimental studies in animals beginning in the 1950s and epidemiologic studies in humans in the late 1960s, the role of diet in cancer tended to be discounted, in favor of alternative theories of genetic errors, viruses, or exposure to specific chemical carcinogens. Nevertheless, experimental studies by Tannenbaum and Silverstone indicated that diet was an important environmental factor that influenced cancer incidence. In 1969, the Irish surgeon Denis Burkitt (who identified Burkitt’s lymphoma) hypothesized that low consumption of dietary fiber in the Western diet accounted for the rise of various chronic diseases, including colon cancer.
Methodological Issues
In their classic analysis of the Causes of Cancer (1981) Doll and Peto characterized diet as a ‘‘chronic source of both frustration and excitement to epidemiologists.’’ Based on ‘‘strong but indirect evidence’’ they estimated that the possible reduction of the cancer deaths in the United States that could be achieved, at least in principle, by dietary modification was around 35%, with the range of acceptable estimates from 10 to 70%. Their estimate varied for different cancer sites (almost 90% for stomach and colorectal cancers, 50% for cancers of endometrium, gallbladder, pancreas, and breast, and approximately 20% for lung, larynx, bladder, cervix, uteri, pharynx, and esophageal cancers). They concluded that most of the cancers that are currently common could be made less so by suitable modification of national dietary practices.
In 1992, Doll narrowed the range of the likely percentages of cancer deaths attributable to diet to 20–60% (Doll, 1992). However, there is still not enough precise and reliable evidence of what exact dietary changes, other than maintenance of a healthy body weight and limiting or avoiding consumption of certain types of food would be of major importance in reducing various cancer risks in the human population.
Many hypothetical and actual ways of how dietary factors may increase cancer risk have been described, and most are still relevant:
- Ingestion of powerful, directly acting carcinogens or their precursors, including carcinogens in natural foods (e.g., pyrrolizidine alkaloids in the Senecio plant, safrole in sassafras, bracken fern), carcinogens produced on cooking (e.g., benzo[a]pyrene and other polycyclic hydrocarbons while cooking meat or fish by broiling, smoking, or frying reusing fat), carcinogens produced in stored food by microorganisms (e.g., aflatoxin produced by Aspergillus flavus in peanuts);
- Carcinogen formation in the body from substrates such as nitrites, nitrates, secondary amines), altering intake or excretion of cholesterol and bile acids, and altering the bacterial flora of the bowel;
- Affected transport, activation, and/or deactivation of carcinogens, including altering concentration in feces or duration of contact with feces, induction or inhibition of enzymes that affect carcinogen metabolism, and deactivation or prevention of formation of short-lived intracellular species;
- Effects on promotion of cells, including vitamin A deficiency, retinol-binding protein, and other factors affecting stem cell differentiation;
- Overnutrition, including effects on age at menarche, adipose-tissue-derived estrogens, and others (Doll and Peto, 1981).
Many fundamental questions are difficult to study due to the high correlation among the multiple specific components of diet, the challenge of measuring nutritional intake at critical periods of development (in utero, during rapid periods of growth, before first pregnancy, etc.), variations in the food supply over time or the form in which nutrients are delivered, and the more limited range of intake in cohort studies conducted in developed countries than exists internationally. Few studies have directly measured the degree to which cancer risk can be modified by changes in nutrition in adulthood. Even more challenging are the complex interactions likely to occur among nutritional factors, and/or between diet and individual susceptibility factors.
Despite these challenges, hypotheses and supporting evidence regarding the relationship of diet to cancer can be obtained from a variety of sources. In vitro studies and animal experiments are helpful in examining mechanisms of action, although they alone do not establish direct relevance to humans (Ames et al., 1987). Metabolic or biochemical studies in humans, such as studying the association of diet with estrogen profiles or markers of DNA damage do not examine cancer endpoints directly, but can be invaluable in the interpretation of other forms of evidence (Willett, 2006).
Epidemiological studies of diet and cancer are a relatively new area of research. During the last 30 years, approaches for assessing dietary intake (e.g., standardized questionnaires to assess intakes of foods from which nutrient intakes can be calculated, biochemical determinations of body tissues, anthropometric measurements) have been developed and have been shown to be informative. Early epidemiological studies of diet and cancer were mostly correlational: They compared the disease rates in populations with the population per capita consumption of specific dietary factors. These studies, especially international correlational studies, have several strengths (such as large contrasts in dietary intake, large populations, more stable dietary patterns). However, the primary problem is that many potential determinants of cancer other than the dietary factor under consideration may vary between areas with a high and low cancer incidence (e.g., genetic predisposition, other dietary factors, including energy intake, and other environmental or lifestyle factors). Traditionally, such studies have been considered the weakest form of evidence (Willett, 2006).
Other types of epidemiological studies are based on comparing cancer occurrence in subgroups within a population that consumes unusual diets (often defined by religious or ethnic characteristics) with the general population. Generally providing nearly the same inferential strength as ecological studies, such studies may be particularly useful when a hypothesized association is not observed (Willett, 2006). In addressing the possibility that the correlations observed in the ecological studies are due to genetic factors, migrant studies can be particularly useful, as well as for examining the latency or relevant times of exposure.
Many of the weaknesses of correlational studies are potentially avoidable in case-control studies (when information about previous diet is obtained from the patient with studied disease and compared to that without the disease) or cohort investigations (when information on diet is obtained from disease-free subjects who are then followed to determine disease rates according to levels of dietary factors). However, in case-control studies, biases due to selection or recall could often occur. Another problem is the selection of an appropriate control group: Diet may influence the incidence of many diseases, and it is often difficult to identify disease groups that are unrelated to the aspect of diet under investigation. Methodological sources of inconsistency may be particularly problematic in nutritional epidemiology because of the inherent biological complexity resulting from nutrient–nutrient interactions, and that may produce inconsistent findings (Willett, 2006). Prospective cohort studies avoid most of the potential sources of methodological bias associated with case-control studies, providing the opportunity to obtain repeated assessments of diet over time and to examine the effects of diet on a wide variety of diseases. However, to conduct such a study, it is necessary to enroll tens of thousands of subjects even for common cancers. The use of detailed questionnaires makes this kind of study expensive. Consequently, for diseases with low frequencies, case control studies will continue to play a role in nutritional epidemiology. Due to current uncertainty about measuring diets in early life, whether either study design will be able to address the influence of childhood diet on disease occurring decades later remains unclear (Willett, 2006).
The randomized trial (optimally, double-blind) is in principle the most rigorous evaluation of dietary hypotheses. However, such experiments in humans are only justified when considerable nonexperimental data establish a rationale for undertaking a trial; they provide preliminary data on potential risks and benefits. Experimental studies are particularly useful for evaluating hypotheses on minor dietary components (e.g., whether a specific micronutrient can reduce cancer risk). However, controlled trials of dietary factors also have several limitations, including uncertainty of the time between changes in dietary factor levels and expected changes in cancer incidence, decreased compliance in following the diet during an extended trial, the tendency of enrolled participants to be highly selected on basis of health consciousness and motivation, and other factors (Willett, 2006).
Cancer risk reduction includes two strategies: (1) Cancer prevention (reduction of carcinogen exposure) and (2) protection (intervention to stimulate mostly endogenous mechanisms of the organism to reduce the risk from exposure to carcinogens). Table 1 presents some carcinogenic and protective effects of dietary compounds in animal models and in humans. The majority of these results suggesting that dietary compounds have carcinogenic or protective roles need further analysis in larger studies to confirm the observed effects. Table 1 shows that diet is both an important cancer risk factor and a cancer protective factor for a variety of cancers, including nongastrointestinal locations, confirming the generalized effects (both damaging and protective) of foods consumed for the entire human body.
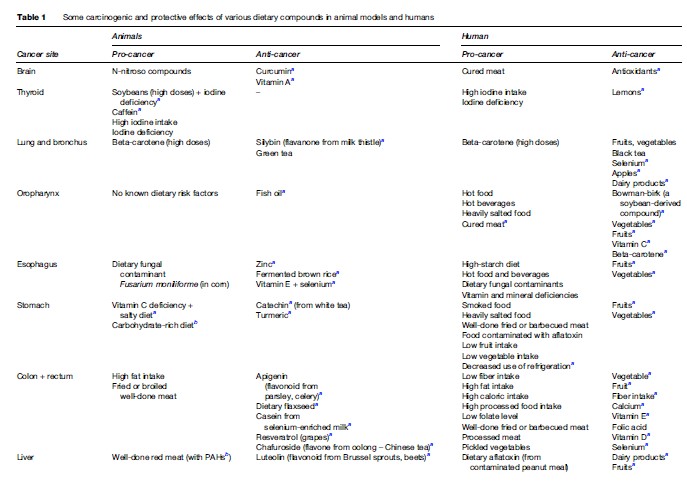
Dietary Factors That Increase Cancer Risk
Dietary factors can modify cancer risk in several different ways at multiple stages of the carcinogenic process. Direct carcinogens, or initiators, cause structural damage or malfunction of the genes that regulate cell proliferation, DNA repair, the survival of damaged cells, and the ability of cells to invade and migrate. These may occur naturally or be produced during cooking, digestion, or the metabolism of certain foods. For example, aflatoxin is a carcinogenic fungus that occurs naturally in moldy grains, heterocyclic amines are produced by frying meat and fat at high temperatures, acrylamide is generated from carbohydrates when cooking foods such as French fries and pancakes, polycyclic aromatic hydrocarbons are produced by grilling and charring foods, nitrosamines are generated in the stomach during digestion, and large numbers of free radicals are formed during the metabolism of food to energy.
Tumor promoters are substances that accelerate cell turnover so that genetically damaged cells multiply more rapidly and have greater likelihood of acquiring additional mutations needed for malignant transformation. Substances such as capsaicin in chili pepper or salt in foods preserved by salting irritate the lining of the digestive tract, as do bile acids released into the colon during the digestion of fat. The resulting inflammation accelerates cell turnover and creates an environment favorable to tumor development.
A third category of dietary factors that affect cancer risk interfere with cellular defense mechanisms that normally detoxify or remove carcinogens, repair DNA, and/or trigger death in damaged cells. For an abnormal cell to become cancerous, it must evade numerous host defense mechanisms.
Macronutrients And Increased Cancer Risk
Among the dietary macronutrients hypothesized to increase cancer risk, those that have been most extensively studied are fat and meat intake. Per capita fat consumption is highly correlated with cancers of the breast, colon, and prostate in international correlation studies, but these do not identify which of the many factors associated with economic development are causally related to the risk of these cancers. Prospective epidemiological studies have not found consistent associations between total fat and either breast or colon cancer, nor did the Women’s Health Initiative find a statistically significant reduction in invasive breast cancer risk over an 8.1-year average follow-up period. These studies have not been able to evaluate reductions in fat intake during critical periods of breast development, nor have extreme reductions in fat intake been achieved and maintained over many years. While it is still possible that greater reductions in fat intake do reduce breast cancer risk, this does not appear to be a feasible large-scale intervention for women in Western countries. Higher levels of animal, but not vegetable, fat have been associated with increased prostate cancer risk in some studies. More consistent associations have been observed between the consumption of red and processed meat and colon cancer risk. Overall, the more powerful and less biased studies have not supported an association between fat intake during midlife and cancer incidence. The effects of diet during earlier periods of life remain to be explored (Willett, 2006).
The issues of energy (calorie) intake and energy balance are currently of great interest to cancer researchers, because of multiple lines of evidence linking obesity and weight gain to many different cancers. In animal studies, restriction of energy intake reduced the occurrence of many tumors (with reduced growth) (Weindruch and Walford, 1982). In humans, an excessive energy intake as assessed by obesity indices is strongly related to risks of cancers of endometrium, colon, breast (postmenopausal), kidney, esophagus (adenocarcinoma), and biliary tract (International Agency for Research on Cancer, 2002), and recent evidence suggests that greater adiposity is also associated with death due to leukemia, lymphoma, myeloma, and cancer of the pancreas (Calle et al., 2003). However, energy intake is difficult to measure directly in epidemiological studies because it is influenced by body size as well as by variations in self-reported intake of various foods. What is still unknown is whether, associated with a specific type of fat (saturated, unsaturated, or polyunsaturated) or with overall calorie intake, fat can promote carcinogenesis directly and at the same time be the major source of calories (Strickland and Kensler, 2004).
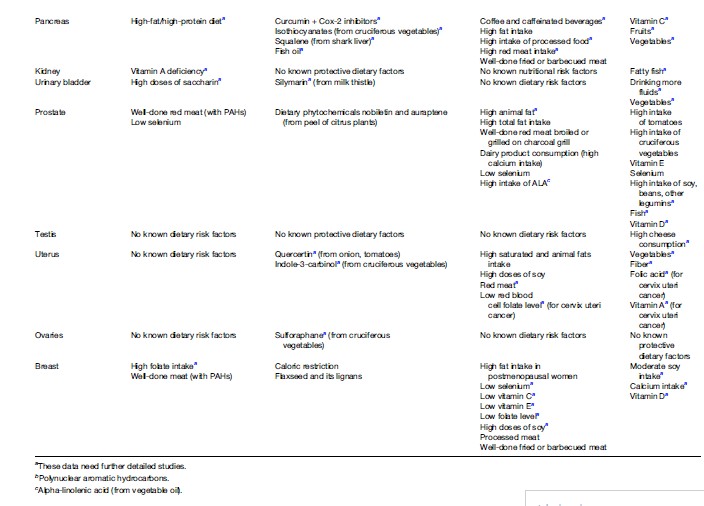
Several recent meta-analyses studied an association of certain diets and cancer risk in humans. For example, they showed an increased risk of colorectal cancer from high intake of fat, pickled vegetables, and red/processed meat (see Table 2). High consumption of dietary alphalinolenic acid, dairy products, and associated high calcium intake were suggested as risk factors for prostate cancer.
Direct impact of foods on the gastrointestinal mucosa causes its chronic irritation or inflammation, resulting in increased cellular proliferation, local production of growth factors, and oxidative stress, thus enhancing carcinogenesis. As a result, higher risks of oral and esophageal cancers are associated with increased intake of alcohol, salt-preserved meat and fish, smoked food, charcoal grilled meat, and hot beverages.
Some foods and nutrients have been recently discussed as cancer risk factors (according to American Cancer Society publications). There is currently no evidence that bioengineered food, cholesterol, and coffee increase cancer risks.
Food Preservation Methods
Curing food (predominantly by adding nitrates, nitrites, and salt) is one of the most common methods of preservation, especially for meat. Several studies showed that consumption of considerable amounts of cured meat is associated with an increased risk for colorectal and stomach cancers. Some studies showed an increased risk of upper aerodigestive tract and brain cancers. An increased risk for childhood brain tumors has been shown to be associated with cured meat consumption by the mother during pregnancy. Adult glioma risk was higher in persons who consumed high levels of cured bacon and ham.
Nitrates are added to meat or fish while processing for antibacterial purposes (against Clostridium botulinum) and to improve the product’s appearance (reacting with myoglobin it produces an attractive pink-red color). Nitrates and nitrites are precursors of N-nitroso compounds (NOCs), some of the strongest carcinogens. In fish processing, the nitrate salt is often used, a source of both salt and NOCs (from the reaction between nitrates and secondary amines in fish). People may be exposed to NOCs from exogenous (mainly from tobacco and eating preserved or heat-treating food) and endogenous (formation by the human body) sources. Vegetables are also important sources of dietary nitrates, but they are very rich sources of vitamin C – a known N-nitrosation inhibitor. Therefore, modern meat-processing technologies add ascorbic acid to cured foods to reduce N-nitrosation reaction and NOC concentration.
Smoked-cured food may be preserved by directly burning wood, or by adding so-called liquid smoke (made by condensing smoke from burning wood). Both of these methods expose foods to potentially carcinogenic substances, including polycyclic aromatic hydrocarbons (PAHs).
Fermentation methods (when cultures of naturally occurring organisms may be encouraged to flourish to promote mold growth with distinctive tastes and textures) are used in many foods, such as bread, matured cheeses, yogurt, soy sauce, and wines. In animal models, it was shown that fermented food has chemopreventive effects. However, if contaminated with mycotoxin, fermented food caused an increase in stomach and liver cancer incidence in Japan (Kinosita et al., 1968).
Food preservation by irradiation is used to kill harmful organisms in food to extend its shelf life (e.g., shellfish, strawberries, and other soft fruits), to prevent potatoes from sprouting, and to destroy dangerous microorganisms on poultry. There is currently no evidence that this might be carcinogenic for humans. However, it has been proposed that irradiated food should be marked to inform consumers about the mode of preservation. Further studies of possible association between consuming irradiated food and colon cancer risk are needed (especially in susceptible populations).
The Cooking Process
Recent studies have evaluated the association of specific cancers with methods of cooking meat. Frying or broiling produces potential carcinogens, including PAHs and heterocyclic amines (HCAs), which are associated with increased risk of colon, breast, liver, and prostate cancers and lymphomas in animals (Strickland and Kensler, 2004). HCAs are formed from the cooking of muscle meats, such as beef, pork, fowl, and fish, by amino acids and creatine reacting at high cooking temperatures. At least 17 different HCAs may be associated with human cancer risk. Individuals who eat their beef medium-well or well-done had more than three times the risk of stomach cancer than those who ate their beef rare or medium-rare. Non-muscle meat sources of protein (e.g., milk, eggs, tofu, liver) have very few, if any HCAs (naturally or when cooked). Lower levels of HCAs are likely to form during oven roasting and baking. Meat that was microwaved for 2 min prior to cooking had a 90% decrease in HCA content (U.S. National Cancer Institute, 2004). At this moment, no U.S. Federal Agency monitors HCA content in cooked meats, and there is no precise measure of the quantity of HCAs necessary to increase cancer risk.
Heating some foods to a temperature of 120 C (248 F) can produce acrylamide, a chemical compound whose primary use is to make polyacrylamide and acrylamide copolymers. Potato chips and French fries have been found to contain relatively high levels of acrylamide (National Cancer Institute, 2002). Acrylamide has been classified by the International Agency for Research on Cancer (IARC) as ‘‘probably carcinogenic to humans (Group 2A)’’ (from evidence of its carcinogenicity in experimental animals, and because it is metabolized to a genotoxic compound, glycidamide, in both rodents and humans).
Sweeteners And Additives
Artificial sweeteners are often used instead of sucrose (table sugar) to sweeten foods and beverages. One of the most popular of them, saccharin, in 2000 was removed from the U.S. National Toxicology Program’s Report on Carcinogens, where it had been listed since 1981 (suspected to cause urinary bladder cancer) as an anticipated human carcinogen. However, high doses of saccharin cause bladder stones comprised of sodium saccharin and bladder cancer in male rats. The International Agency for Research on Cancer (IARC) and U.S. National Toxicology Program (NTP) removed saccharin from the list of human carcinogens because saccharin did not cause bladder stones in humans, and the increased frequency of bladder cancer in the male rats was considered to be secondary to chronic bladder inflammation resulting from the stones.
Studies of another sweetener, aspartame, which was suspected of increasing the risk for human brain tumors, did not confirm the association. Currently three artificial sweeteners – acesulfame potassium, Sucralose, and Neotame – are authorized for use in food in the United States: More than 100 safety studies showed no evidence that they cause cancer in humans. Recent studies concluded that cyclamate, banned in 1969 by the U.S. Food and Drug Administration (FDA) as possibly associated with human urinary bladder cancer, was not a carcinogen (U.S. National Cancer Institute, 2006).
Nutritional Factors That May Decrease Cancer Risk
Macronutrients And Foods
The question of the specific role of dietary components in cancer prevention remains largely unanswered. The overall recommendation to eat an abundant amount of fruits and vegetables has not changed for 25 years. Recent evidence that is less susceptible to methodological bias does not support a major benefit from increasing fruit and vegetable consumption for reducing cancer risk. The possibility remains that higher intakes of specific fruits and vegetables, or their specific substances, may reduce the risk of specific cancers (e.g., folic acid for reducing colon cancer risk and lycopene for reducing prostate cancer risk) (Willett, 2006).
The general recommendation to reduce total calories and fat consumption and to increase fiber is a good one with regard to cardiovascular disease prophylaxis, but whether it is effective in cancer prevention remains unproven (Meyskens, 2004). Large prospective cohort studies and several large randomized trials recently indicated that dietary fiber has not been associated with the risk of colon cancer (Schatzkin et al., 2000; Terry et al., 2001). A modest inverse association was observed in a multicenter cohort study in Europe (Bingham et al., 2003), but the confounding by intake of folic acid (see the section titled ‘Micronutrients’ below) was not considered, which is plausible because whole grains, fruits, and vegetables are the primary dietary sources of both nutrients (Willett, 2006).
Salt is hypothesized to act as a local irritant, with positive associations of salt intake with the risk of gastric cancer shown in several case-control studies. Moreover, this hypothesis is compatible with the striking decline in gastric cancer in most industrialized countries because refrigeration reduced the need for salt for preservation. However, the supply of fresh fruits and vegetables and the reduced level of Helicobacter pylori infection are important factors in the decrease in gastric cancer incidence (Willett, 2006).
Some foods are currently under study for their ability to reduce cancer risk. To date, there is no clear evidence to support the role of garlic, olive oil, organic foods, and tea in human cancer prevention. Well-controlled studies are needed to confirm whether these and other foods or additives, such as selenium or soy supplements, might be helpful in cancer prevention.
Micronutrients
Approximately 20–30% of Americans consume multivitamin supplements daily, indicating a strong public interest in the prevention of cancer and other chronic diseases through a nutrition-based approach. However, there are still many uncertainties concerning the potentially cancer-preventive effects of supplements in humans. Vitamins A, D, E, C, folic acid, and calcium are among the most widely discussed micronutrients.
There are two forms of vitamin A – preformed vitamin A and carotenoids – and the distinction between these two forms has practical implications, because preformed vitamin A is obtained only from foods derived from animal sources or vitamin supplements, whereas carotenoid precursors of vitamin A are obtained almost entirely from plant sources (Willett, 2006). In several large trials (Group TA-TBCCPS, 1994; Omenn et al., 1996), increases in lung cancer incidence of 18% and 28% were registered in persons at high risk for lung cancer (they were taking beta-carotene and combined beta-carotene/preformed vitamin A supplements, respectively).
Several recent studies showed that increased vitamin D levels could decrease the risk of colon, prostate, breast, and ovarian cancers. This points to a need to rethink current recommendations on sun exposure and vitamin D intake, which might be too restrictive to provide much, if any, of the cancer-prevention benefits of vitamin D. However, it is currently unclear what to do with sun exposure recommendations. Both the increased risk of skin cancer from sun exposure and its potential to lower the risk of cancer (and osteoporosis) should be taken into account. The National Academy of Science lists adequate intake of vitamin D at 600 IU per day for adults over 70 years old, and 200 IU per day in those who are 50 and younger, well under the level where real cancer-prevention benefits seem to start. To reach these effective levels, most people would need to take a vitamin D supplement regularly, since most foods are relatively poor in vitamin D. Fatty fish is one of the best sources.
Overall data about the role of vitamin E in cancer prevention remains unclear. That may be the result of only modest numbers of specific cancers in the studies that have been reported, but a large beneficial effect of vitamin E against cancer seems unlikely (Willett, 2006).
In case-control studies, the evidence for a protective effect of vitamin C has been seen for laryngeal, oral, esophageal, and stomach cancers, and cervical dysplasia. However, taking into account the experience with betacarotene and lung cancer, supportive data from large cohort studies or randomized trials is important for confirming these relationships (Willett, 2006).
Several studies (both case-control and cohort) have demonstrated evidence of an inverse association between folate intake and colon cancer risk (Giovannucci, 2002). Some studies reported an inverse association between folate intake and breast cancer incidence among regular alcohol consumers (Zhang et al., 2003).
At present the results of several studies support a modest benefit of higher calcium intake in colon cancer prevention. However, the practical implication of that finding is currently unclear, because high calcium intake has been associated with higher risk of prostate cancer.
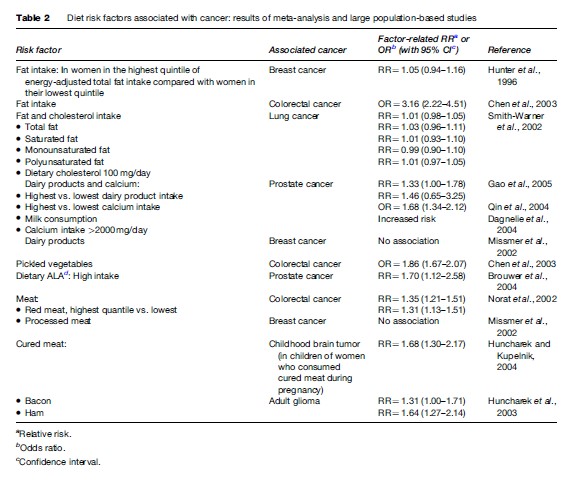
Chemoprevention is the use of either natural or synthetic substances, or their combination, to block, reverse, or retard carcinogenesis. More than 5000 phytochemicals were identified in fruits, vegetables, and grains, with many still unknown. The interactions between the various components within a food, or in food combinations, may explain why isolated dietary components may not be as efficient for cancer prevention as a whole food (see Table 3). What foods should be combined for maximum cancer prevention remains to be determined. Whole green tea is more effective than epigallocatechin gallate in inhibiting TNF-a release and increasing the percentage of human lung cancer cells undergoing apoptosis. Combining soy phytochemicals and green tea extracts appeared to be more effective in inhibiting tumor angiogenesis, reducing estrogen receptor (ER)-alpha, and lowering serum insulin-like growth factor (IGF)-1 in estrogen-dependent human breast tumors implanted into severe combined immunodeficient mice than when either is provided alone. However, food may contain antagonistic components, for example, soy has a reduced ability to inhibit aberrant crypt foci compared to isolated genistein in colon cancer in rats.
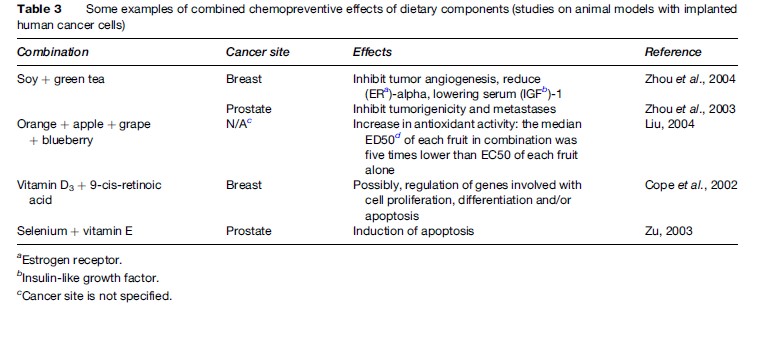
All of the major signaling pathways have been examined as targets for cancer prevention response to one or more dietary components: Carcinogen metabolism, DNA repair, cell proliferation/apoptosis, inflammation, differentiation, oxidant/antioxidant balance, and angiogenesis (see Table 4). The response is complicated, since the effect of dietary components can be cell type and dose-dependent, and any one agent may have multiple mechanisms of action. Bioactive components presenting in food can prevent carcinogenesis in various ways: Blocking metabolic activation, increasing detoxification, or providing alternative targets for electrophilic metabolites. In vitro studies and preclinical models have shown that flavonoids (such as quercetin, rutin, and genistein), phenols (such as curcumin, epigallocatin-3-gallate, and resveratrol), isothiocyanates, allyl sulfur compounds, indoles, and selenium may work as modulators of detoxification enzymes, playing a major role in regulation of the mutagenic and neoplastic effects of chemical carcinogens (phase II enzymes being mediated by the antioxidant response element located in the promoter region of specific genes).
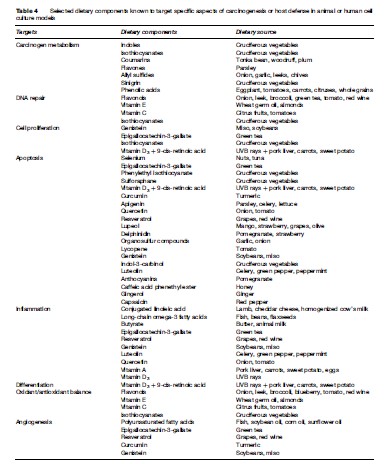
Dietary components that scavenge activated oxygen species, such as flavonoids, vitamin E and C, and isothiocyanates, could stimulate the repair of oxidative DNA damage. Alterations in DNA repair, cell cycle progression, and apoptosis are important molecular targets for dietary components in cancer prevention. Cell proliferation might be modulated by genistein and epigallocathechin-3-gallate, which causes cell-cycle arrest via the induction of CDKs (cyclin-dependent kinase inhibitors) p21 and p27 (they have tumor suppression activity, their expression is controlled by the tumor suppression protein p53) and the inhibition of CDK4, CDK2, cyclin D1, and cyclin E (these kinases have a tumor-stimulating activity). Isothiocyanates can induce p21 expression and inhibit cell proliferation at the G2-M checkpoint. Many dietary cancer-protective compounds, including selenium, epigallocatechin-3-gallate, phenylethyl isothiocyanate, retinoic acid, sulforaphane, curcumin, apigenin, quercetin, and resveratrol, target apoptosis.
Although acute inflammation is usually beneficial, epidemiological data suggest an association between chronic inflammation and subsequent malignant transformation. Conjugated linoleic acid, long-chain omega-3 fatty acids, butyrate, epigallocatechin-3-gallate, curcumin, resveratrol and others (see Table 4) may reduce cancer risk by influencing the inflammatory process.
Polyunsaturated fatty acids, epigallocatechin-3-gallate, resveratrol, curcumin, and genistein may work as inhibitors of tumor angiogenesis.
Some dietary components, such as epigallocatechin-3-gallate (in green tea), genistein (in soybeans), resveratrol (in red wine), and vitamin D3 (UVB rays) have a cancerprotective effect targeting several stages of tumor progression (see Table 4).
Recently, dietary components have been found to exert additive or synergistic effects with pharmaceutical agents by modifying different molecular targets. Diets containing large amounts of olive oil have a significant protective effect against colon cancer that is additive with the inhibitory effects of sulindac, possibly related to regulation of the expression and activity of key proteins involved in prostaglandin-biosynthesis (COX-2) and apoptosis induction (Bcl-2 and caspase-3) pathways. The soy isoflavone daidzein has been reported to improve the capacity of tamoxifen to reduce mammary tumor burden and multiplicity, as well as to increase tumor latency. Recent cell culture and animal studies also suggest that dietary compounds, such as genistein, curcumin, epigallocathechin-3-gallate, resveratrol, indole-3-carbinol, proanthocyanidin, and vitamin D3 enhance the efficacy of cancer chemotherapeutic drugs and radiotherapy by modifying cell proliferation.
Individual Susceptibility
Certain genetic polymorphisms influencing food metabolites may be important in diet associations with cancer. Polymorphisms in genes encoding the proteins metabolizing and transporting dietary carcinogens and chemopreventive agents significantly affect cancer risk. Consequently, polymorphisms of gene coding of methylenetetrahydrofolate reductase (this enzyme is critical to the regulation of factors in DNA methylation and synthesis) can influence colorectal cancer risk in affected individuals by altering the cellular response to dietary folate and methionine; this polymorphism is important at the stage of transformation of adenoma to carcinoma. The risk of colon cancer is increased in persons consuming welldone meat who had a rapid-rapid phenotype (NAT2 and CYP1A2): An OR of 6.45 was observed in rapid-rapid type individuals, compared to an OR of 1.87 in rapidslow metabolizers. The OR for rapid-rapid metabolizers consuming rare or medium cooked meat was 3.13, compared to an OR of 0.91 in a rapid-slow phenotype (Lang et al., 1994).
A subject of particular interest is how nutrient intakes modify genetic susceptibility to diseases, especially to cancer. This may provide a scientific basis for cancerpreventive strategies through individual dietary modification. The focus of this scientific pursuit is on the practical activity of entrepreneurs selling dietary recommendations and supplements, which are claimed to be designed specially to individual’s genetic susceptibility to disease (these products often include specifications as nutrigenetic testing, personalized supplements, feed your genes right, and intelligent diet, etc.). Recent testimony by the U.S. Government Accountability Office before the U.S. Senate Special Committee on Aging stated that the nutrigenetic tests purchased from four websites mislead consumers by providing dietary or lifestyle recommendations based on polymorphisms of some genes (Kutz, 2006). In general, research on the interactions between dietary factors and individual genetic susceptibility has not yet developed sufficiently with respect to variations in cancer risk to allow individualized dietary guidelines.
Conclusions And Recommendations
Although many associations of dietary compounds with various cancers have been found, the association does not always mean causation. Causation in cancer risk factors, especially of dietary patterns, is extremely difficult to establish. Some campaigns, such as 5-a-day for better health have been started during the last decade to encourage large-scale dietary changes, but their results are delayed because of a 20to 30-year lag period for changes in cancer morbidity and mortality.
Many studies have been published on specific foods, nutrients, and lifestyle factors and specific cancer risks, but to date no one study provides clear results and recommendations on this subject, and the single new report may sometimes overemphasize contradictory or conflicting results. The generally negative experience with b-carotene as a chemoprevention agent, particularly in lung cancer, mandates caution in extrapolating the potential benefit of a compound from epidemiologic observations without extensive supporting experimental data.
A series of recommendations were generated by numerous governmental and private organizations worldwide and in the United States, including the American Cancer Society (American Cancer Society, 2006), the National Academy of Sciences (Committee on Diet Nutrition and Cancer), World Cancer Research Fund and American Institute for Cancer Research, and others. The following recommendations are typical at present:
- Choose foods and beverages in amounts that help achieve and maintain a healthy weight.
- Eat five or more servings of a variety of vegetables and fruits each day (one serving: fruit: 1 medium apple, banana, orange, ½ cup of chopped, cooked, or canned fruit, 3 4 cup of 100% fruit juice; vegetables: 1 cup of raw, leafy vegetables, ½ cup of other cooked or raw vegetables, chopped, 3 4 cup of 100% vegetable juice; grains: 1 slice of bread, 1 oz of ready-to-eat cereal, ½ cup of cooked cereal, rice, or pasta; meats: 2–3 oz of cooked, lean meat, poultry, or fish).
- Choose whole grains in preference to processed (refined) grains. Choose whole-grain rice, bread, pasta, and cereals. Limit consumption of refined carbohydrates, including pastries, sweetened cereals, and other high-sugar foods.
- Minimize contamination of foods with carcinogens from any source, including those that are natural or occurring during production, processing, and storage.
- Consume alcoholic beverages in moderation.
- Limit consumption of processed and red meats. Choose fish, poultry, or beans as an alternative to beef, pork, and lamb. When you eat meat, select lean cuts and eat smaller portions. Prepare meat by baking, broiling, or poaching, rather than by frying or charbroiling.
Bibliography:
- American Cancer Society (2006) Nutrition and Physical Activity Guidelines for Cancer Survivors. https://www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/nupa-guidelines-for-cancer-survivors.html.
- Ames BN, Magaw R, and Gold LS (1987) Ranking possible carcinogenic hazards. Science 236: 271–280.
- Bingham SA, Day NE, Luben R, et al. (2003) Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 361: 1496–1501.
- Brouwer IA, Katan MB, and Zock PL (2004) Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: A meta-analysis. Journal of Nutrition 134(4): 919–922.
- Cope MB, Steele VE, Eto I, et al. (2002) Prevention of methylnitrosoureainduced mammary cancers by 9-cis-retinoic acid and/or vitamin D3. Oncology Reports 9: 533–537.
- Calle EE, Rodriquez C, Walker-Thurmond K, and Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine 348: 1625–1638.
- Chen J, Giovannucci E, Hankinson SE, et al. (1998) A prospective study of methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms, and risk of colorectal adenoma. Carcinogenesis 19: 2129–2132.
- Chen K, Qiu J-L, Zhang Y, et al. (2003) Meta-analysis of risk factors for colorectal cancer. World Journal of Gastroenterology 9(7): 1598–1600.
- Dagnelie PC, Schuurman AG, Goldbohm RA, et al. (2004) Diet, anthropometric measures and prostate cancer risk: A review of prospective cohort and intervention studies. British Journal of Urology International 93(8): 1139–1150.
- Doll R (1992) The lessons of life: Keynote address to the Nutrition and Cancer Conference. Cancer Research Supplement 52: 2024S–2029S.
- Doll R and Peto R (1981) The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. Journal of the National Cancer Institute 66(6): 1191–1308.
- Gao X, LaValley MP, and Tucker KL (2005) Prospective studies of dairy product and calcium intakes and prostate cancer risk: A meta-analysis. Journal of the National Cancer Institute 97(23): 1768–1777.
- Giovannucci E (2002) Epidemiologic studies of folate and colorectal neoplasia: A review. Journal of Nutrition 132: 2350S–2355S.
- Group TA-TBCCPS (1994) The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. New England Journal of Medicine 330: 1029–1035.
- Huncharek M, Kupelnick B, and Klassen H (2001) Paternal smoking during pregnancy and the risk of childhood brain tumors: Results of a meta-analysis. In Vivo 15(6): 535–541.
- Huncharek M, Kupelnick B, and Wheeler L (2003) Dietary cured meat and the risk of adult glioma: A meta-analysis of nine observational studies. Journal of Environmental, Pathology Toxicology and Oncology 22(2): 129–137.
- Hunter DJ, Spiegelman D, Adami HO, et al. (1996) Cohort studies of fat intake and the risk of breast cancer – A pooled analysis. New England Journal of Medicine 334(6): 356–361.
- International Agency for Research on Cancer (2002) Weight Control and Physical Activity. Lyon, France: IARC Press.
- Kinosita R, Ishiko T, Sugiyama S, et al. (1968) Mycotoxins in fermented food. Cancer Research 28: 2296–2311.
- Kutz G (2006) Nutrigenic Testing: Test Purchased from Four Web Sites Misled Consumers. https://www.gao.gov/new.items/d06977t.pdf.
- Lang NP, Butler MA, Massengill J, et al. (1994) Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiology Biomarkers and Prevention 3: 675–682.
- Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of Nutrition 134: 3479S–3485S.
- Meyskens FL (2004) Cancer prevention, screening, and early detection. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB and McKenna WG (eds.) Clinical Oncology, 3rd edn., pp. 425–472. Philadelphia, PA: Elsevier.
- Missmer SA, Smith-Warner SA, Spiegelman D, et al. (2002) Meat and dairy food consumption and breast cancer: A pooled analysis of cohort studies. International Journal of Epidemiology 31(1): 78–85.
- National Cancer Institute (2002) Acrylamide and Cancer Risk. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/acrylamide-fact-sheet.
- National Cancer Institute (2004) Heterocyclic Amines in Cooked Meats. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/cooked-meats-fact-sheet.
- National Cancer Institute (2006) Artificial Sweeteners and Cancer. Questions and Answers. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet.
- Norat T, Lukanova A, Ferrari P, et al. (2002) Meat consumption and colorectal cancer risk: Dose-response meta-analysis of epidemiological studies. International Journal of Cancer 98(2): 241–256.
- Omenn GS, Goodman GE, Thornguist MD, et al. (1996) Effects of a combination of beta-carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine 334: 1150–1155.
- Qin LQ, Xu JY, Wang PY, et al. (2004) Milk consumption is a risk factor for prostate cancer: Meta-analysis of case-control studies. Nutrition and Cancer 48(1): 22–27.
- Schatzkin A, Lanza E, Corle D, et al. (2000) Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. New England Journal of Medicine 342: 1149–1155.
- Smith-Warner SA, Ritz J, Hunter DJ, et al. (2002) Dietary fat and risk of lung cancer in pooled analysis of prospective studies. Cancer Epidemiology Biomarkers and Prevention 11: 987–992.
- Strickland PT and Kensler TW (2004) Environmental factors. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB and McKenna WG (eds.) Clinical Oncology, 3rd edn., pp. 173–189. Philadelphia, PA: Elsevier.
- Terry P, Giovannucci E, Michels KB, et al. (2001) Fruit, vegetables, dietary fiber, and risk of colorectal cancer. Journal of the National Cancer Institute 93: 525–533.
- Weindruch R and Walford RL (1982) Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science 215: 1415–1418.
- Willet WC (2006) Diet and nutrition. In: Schottenfeld D and Fraumeni JF Jr. (eds.) Cancer Epidemiology and Prevention, 3rd edn., pp. 405–421. Oxford, UK: Oxford University Press. World Cancer Research Fund International, American Institute for
- Cancer Research (1997) Food, Nutrition and the Prevention of Cancer: A global perspective. Washington, DC: American Institute for Cancer Research, 1997.
- Zhang SM, Willett WC, Selhub J, et al. (2003) Plasma folate, vitamin B6, vitamin B12, and homocysteine and risk of breast cancer. Journal of the National Cancer Institute 95: 373–380.
- Zhou JR, Yu L, Mai Z, et al. (2004) Combined inhibition of estrogendependent human breast carcinoma by soy and tea bioactive components in mice. International Journal of Cancer 108: 8–14.
- Zhou JR, Yu L, Zhong Y, et al. (2003) Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. The Journal of Nutrition 133: 516–521.
- Zu K and Ip C (2003) Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Research 63: 6988–6995.
- Junien C and Gallou C (2004) Cancer nutrigenomics. In: Simopoulos AP and Ordovas JM (eds.) Nutrigenetics and Nutrigenomics. World Review of Nutrition and Diet 93, pp. 210–269 Basel, Switzerland: Karger.
- Manach C, Scalbert A, Morand C, et al. (2004) Polyphenols: Food sources and bioavailability. American Journal of Clinical Nutrition 79: 727–747.
- https://www.cancer.org/treatment/survivorship-during-and-after-treatment/staying-active/nutrition.html – American Cancer Society. Nutrition for the Person with Cancer.
- https://monographs.iarc.fr/agents-classified-by-the-iarc/ – IARC Monographs of the Evaluation of Carcinogenic Risks to Humans, 2006.
- https://www.cancer.gov/about-cancer/causes-prevention/risk/diet – National Cancer Institute, Cancer Causes and Risk Factors. Diet.




