View sample cancer research paper on pancreatic cancer. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
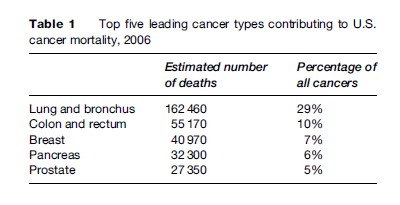
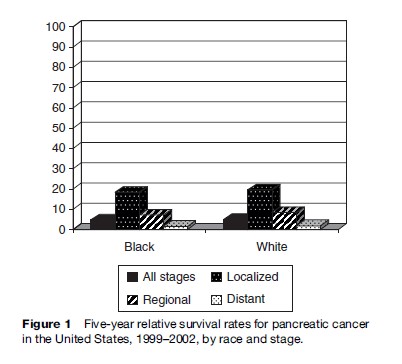
Cancer of the pancreas is relatively uncommon but, because it is rapidly fatal, it is a major source of cancer mortality. In 2002, it was estimated that over 227 000 individuals would have died of pancreatic cancer worldwide (Parkin et al., 2005). Pancreatic cancer is the eighth leading cause of cancer death in the world and the fourth leading cause of cancer deaths in the United States (Table 1). In the United States and in Europe, the overall 5-year survival rate for this cancer is less than 5%. Survival is higher among patients diagnosed with localized tumors (5-year survival rate is under 20%) (Figure 1), but, unfortunately, very few cancers are still localized at diagnosis. Symptoms for pancreatic cancer, such as abdominal pain, are nonspecific, and when they develop the cancer is usually advanced. The location of the pancreas, lack of specific symptoms, and lack of biomarkers for early detection all contribute to the high mortality rates. Survival rates have only improved slightly over the past decade because of the lack of significant medical advancement in the treatment for pancreatic cancer.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Pancreatic Cancer Pathology
Classification
The large majority of pancreatic cancers are adenocarcinoma that develop in the exocrine pancreas and are thought to originate in ductal cells, although the exact cell of origin is still unknown (Bardeesy and DePinho, 2002). Islet cell tumors of the endocrine pancreas and other pancreatic tumors account for less than 15% of pancreatic cancers. Other rare types of pancreatic cancers include sarcomas and lymphomas. Pancreatic tumors can arise anywhere in the pancreas but are more often found in the head of the pancreas (70–80% of tumors) than in the body or tail.
In most epidemiologic studies, pancreatic cancer is used synonymously with exocrine pancreatic cancer, as endocrine cancers are not usually included in the analyses or represent only a small fraction of the total cases.
Diagnosis
Pancreatic cancer is particularly challenging to diagnose for several reasons: there is no screening test for early detection, symptoms are nonspecific or absent until late in the progression of the disease, and the pancreas is difficult to access. Weight loss, abdominal pain, and jaundice, common first symptoms of this cancer, are often a consequence of the tumor interfering with nearby organs, especially the bile duct, liver, and stomach. Other signs of pancreatic cancer include late-onset diabetes, changes in bowl habits, vomiting, and acute pancreatitis (Germanos et al., 2006).
Technological advances have greatly improved the diagnosis and staging of pancreatic cancer; these include contrast-enhanced computed tomography (CT), endoscopic ultrasonography (EUS), magnetic resonance imaging (MRI), and endoscopic retrograde cholangiopancreatography (ERCP). These diagnostic modalities can identify smaller tumors and provide staging data necessary to determine whether tumors can be surgically removed. However, despite improvements, challenges remain; accuracy of tumor and nodal staging varies by imaging modality, and failure to detect small liver metastases and peritoneal dissemination remains problematic across all imaging modalities (Germanos et al., 2006). The use of EUS-guided fine needle aspiration (FNA) allows for histologic diagnosis of primary tumors, lymph node, and distant metastases.
In many countries in which these expensive instruments are not widely available, histopathological confirmation of pancreatic cancer remains low and varies dramatically by country, even within developed countries (mean of 34% in the UK compared to mean of 78% in the United States) (Parkin et al., 2002). Consequently, the criteria for confirmation of pancreatic cancer cases in epidemiologic studies often do not include pathology reports and can contribute to misclassification of disease outcome.
Treatment
Surgical removal of pancreatic tumors is the only treatment option that can improve survival rate. Unfortunately, only a small number of patients diagnosed with localized disease are eligible for surgery (less than 10% of all cases in the United States (Ries et al., 2006)). The 5-year survival rate is approximately 20% among those who undergo this procedure but, despite the slightly better prognosis, most of these patients die within 7 years of surgery (Germanos et al., 2006). Adjuvant therapy in combination with surgery is considered standard care; chemoradiotherapy after surgical resection has been shown to prolong survival by a few months (Germanos et al., 2006), but it is mostly used for palliative care. Chemoradiotherapy has no survival benefits among cancer patients who do not undergo surgery, but it is often used for palliative care.
Descriptive Epidemiology of Pancreatic Cancer
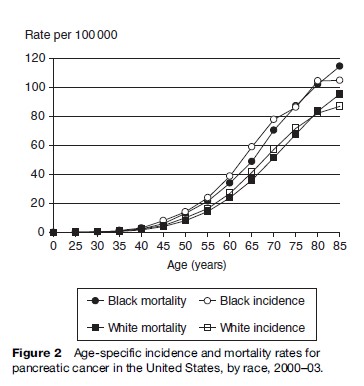
Pancreatic cancer almost never occurs prior to the third decade of life, but after the age of 35 years, incidence rates increase exponentially and peak in the seventh and eighth decades (Figure 2). Throughout the world, men have higher incidence and mortality rates than women (8.5 per 100 000 in men vs. 5.0 in women in developed countries; 2.4 in men vs. 1.6 in women in developing countries in 1990). In the United States, Blacks have higher incidence and mortality rates than Whites (10.2 per 100 000 in Blacks vs. 6.6 in Whites, for age-adjusted incidence rates, 1996–2000; Figure 2) (Ries et al., 2003).
Global variations in incidence and mortality rates for pancreatic cancer are large, but perhaps not as striking as those observed for other cancers. Reported pancreatic cancer rates are lower in developing countries, with the exception of central and temperate South America, where high mortality rates are also noted. In Europe, the highest mortality rates are observed in Hungary, the Czech Republic, and Austria, and the lowest rates are found in Latvia and Albania (Bray et al., 2002). In the United States, mortality rates are highest in the Northeast and around the Mississippi River. Part of the global and regional variation in mortality rates is likely to be due to detection capabilities and completeness of case ascertainment (e.g., percentage of autopsied deaths), but differences in smoking prevalence are also responsible. In addition to smoking, other modifiable risk factors are likely to account for some of the unexplained differences observed within developed countries.
In most developed countries, pancreatic cancer mortality rates in men decreased slightly between 1980 and 2000, but remained fairly stable in women. The decreased rates have been predominantly driven by the decline in cigarette smoking prevalence; for example, in the United States, the drop in pancreatic cancer rates in men mirrored the significant decline in smoking prevalence (50% to 25% male current smokers between 1965 and 2000). Mortality rates in both sexes have increased over the past four decades in a number of countries in which rates had been low in the mid-1950s, such as Japan, Spain, Italy, Bulgaria, Poland, and Yugoslavia. In Japanese men ( Japan), age-standardized mortality rates increased from 1.4 per 100 000 to 12.5 per 100 000 between 1950 and 1995 (Lin et al., 1998).
Pancreatic Cancer Risk Factors
Tobacco
Cigarette smoking is the most consistent risk factor for pancreatic cancer and is estimated to account for approximately 25% of all pancreatic cancer cases. Over 40 studies have reported positive associations for cigarette smoking and pancreatic cancer. Current smokers have a two-to threefold higher risk of pancreatic cancer than nonsmokers. In contrast, former smokers have little excess pancreatic cancer risk. Dose–response relationships have been observed for dose and duration of smoking, but the increase is not as dramatic as for lung cancer. Two studies observed stronger dose–response relationships when smoking was calculated over the last 15 years (compared to lifetime smoking) (Howe et al., 1991; Fuchs et al., 1996). Excess risk from smoking cigarettes disappears within a decade of smoking cessation.
Few studies have examined noncigarette tobacco smoke in relation to pancreatic cancer. Current smokers only of cigars may have a modest increase in pancreatic cancer risk compared to never-smokers (relative risk 1.2–1.6) and inhalation may be critical (RR 2.7, 95% confidence interval (CI) 1.5–4.8 for cigar smokers who inhale vs. never-smokers) (Shapiro et al., 2000). In contrast to cigar smoking, few studies have observed a higher risk of pancreatic cancer for smokers who only smoke pipes. In a recent case-control study of noncigarette smokers, smokeless tobacco use was associated with a substantial increase in risk of pancreatic cancer (RR 3.5, 95% CI 1.1–10.6 for those using >2.5 ounces/week vs. never-users) (Alguacil and Silverman, 2004).
The exact mechanisms underlying the causal association between tobacco smoke and pancreatic cancer are unclear. Tobacco-specific nitrosamines, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (commonly referred to as NNK), are the most likely tobacco constituents involved in pancreatic carcinogenesis in humans (Hecht, 2003). Nitrosamines are potent pancreatic carcinogens in animal models, and levels of NNK in samples of human pancreatic juice are significantly higher in smokers than nonsmokers (Prokopczyk et al., 2002). Furthermore, as NNK levels are approximately 15 times higher in smokeless tobacco than in mainstream smoke, the strong positive association for smokeless tobacco is consistent with a role for NNK in pancreatic carcinogenesis. However, given that smoking cessation results in a relatively rapid return to the incidence rates experienced by never-smokers, it is likely that smoking acts at later stages in carcinogenesis (e.g., promotion of initiated cells). Therefore, tobacco smoke may be involved in carcinogenesis through its pro-inflammatory response (e.g., oxidative damage from free radicals), or through other mechanisms that promote cell proliferation and angiogenesis.
Hereditary Pancreatic Cancer
It has been estimated that approximately 5% of pancreatic cases are due to hereditary factors (Lynch et al., 1996). An inherited component to pancreatic cancer has been suggested by a number of observational studies; individuals with a close relative with pancreatic cancer have a three to fivefold higher risk of pancreatic cancer. In a large prospective study of family pancreatic cancer (FPC) kindred, defined as at least two family members with pancreatic cancer, members of those families with at least one first-degree relative with pancreatic cancer had a ninefold greater risk of developing pancreatic cancer (standardized incidence ratio, SIR 9.0, 95% CI 4.5–16.1) than the SEER population (Klein et al., 2004). In contrast, family members genetically unrelated to a patient with pancreatic cancer had a SIR of 2.4 (95% CI 0.06–13.5) compared to the general population (Klein et al., 2004), suggesting that genetic factors are much stronger than environmental factors shared by these families.
Major genes responsible for FPC have yet to be identified. In the United States, the Pancreatic Cancer Genetic Epidemiology (PACGENE) Consortium has been formed to investigate susceptibility genes in these families, and a similar consortium exists in Europe (EUROPAC). A recent segregation analysis among 287 pedigrees provided the first formal evidence for a rare major gene in the etiology of pancreatic cancer (Klein et al., 2002), although the ‘gene’ could be a collection of susceptibility genes, which may include known cancer genes. Excess incidence of pancreatic cancer has been observed in a number of rare inherited cancer syndromes, including Peutz-Jeghers, hereditary breast-ovarian cancer (HBOC), familial atypical multiple mole melanoma syndrome (FAMMM), hereditary pancreatitis, hereditary non-polyposis colorectal carcinoma (HNPCC), Fanconi anemia, and cystic fibrosis. Germ-line mutations in the tumor suppressor genes involved in these hereditary cancer syndromes may also play a role in FPC, but the extent of this is unclear. BRCA2 mutations appear to contribute to FPC; different mutations in BRCA2 have been reported in 12–19% of patients with FPC (Murphy et al., 2002; Hahn et al., 2003).
Medical Conditions
Chronic Pancreatitis
The most common cause of chronic pancreatitis is alcoholism, but other causes include biliary tract disease, and hereditary and tropical pancreatitis. Establishing the role of chronic pancreatitis in pancreatic cancer has been difficult because chronic pancreatitis is a rare condition in the general population, and large cohorts of pancreatitis patients are necessary to obtain sufficient pancreatic cancer cases to examine the relation between the two diseases. In addition, these studies have been plagued by misclassification of disease, as pancreatic cancer can sometimes be initially mistaken for pancreatitis, and the distinction between acute and chronic pancreatitis can be difficult. Cohort studies of chronic pancreatitis patients have found extremely high rates of pancreatic cancer: patients with this condition are 16 times more likely to develop pancreatic cancer than those without it. The excess risk remained high after removing patients who developed cancer within the first 5 years after diagnosis of chronic pancreatitis (SIR ¼ 14, 95% CI 8–23) (Lowenfels et al., 1933).
In contrast, other studies using national records of patients with pancreatitis and linking them to incident pancreatic cancer cases (using cancer registries) reported high relative risks in the first 4 years of follow-up but weaker associations with longer follow-up periods, thus suggesting that the association may not be causal. In one study, however, the risk of pancreatic cancer in patients with chronic pancreatitis was still high (RR 4.6, 95% CI 2.6–7.5) compared with the general population, for years 5 through 9 following the diagnosis of pancreatitis (Karlson et al., 1997). This observation is consistent with chronic pancreatitis playing a role in the progression of pancreatic cancer. It has been proposed that the prolonged inflammation observed in chronic pancreatitis patients is what initiates or aids the progression of a pancreatic tumor (Lowenfels et al., 1999). Smoking may also exacerbate the association between pancreatitis and pancreatic cancer (Talamini et al., 1999).
The hereditary form of pancreatitis develops at a very early age (usually under 21 years of age). It is found in multiple family members, and is present in the absence of alcohol use. A multicenter study of hereditary pancreatitis reported eight cases of pancreatic cancer with an estimated risk ratio of 53 (95% CI 23–105) (Lowenfels et al., 1997). Very high rates of pancreatic cancer have also been reported among patients with tropical pancreatitis, another rare form of pancreatitis that is not associated with alcohol use (SIR 100, 95% CI 37–218, compared to the general population) (Chari et al., 1994). The association between hereditary and tropical pancreatitis and pancreatic cancer may be stronger than among typical cases of pancreatitis, because these conditions develop at younger ages.
Because of various limitations in studies that have examined pancreatitis and pancreatic cancer, including small numbers of pancreatic cancer cases and inability to control for smoking, it is difficult to establish the exact strength of the underlying association. However, based on the existing data, including the studies on hereditary and tropical pancreatitis, there is sufficient evidence to suggest that chronic pancreatitis is causally related to pancreatic cancer. Given the rarity of this condition in the general population ( 0.04%), less than 1% of pancreatic cancers are likely to be attributable to chronic pancreatitis.
Cholecystectomy And Cholelithiasis
A large number of studies have examined the association between gallbladder disease and gallbladder removal and pancreatic cancer, but findings have been inconsistent. As nonspecific gastrointestinal symptoms resulting from an underlying pancreatic cancer can lead to the diagnosis of cholelithiasis and even to cholecystectomy prior to a pancreatic cancer diagnosis, studies that do not remove individuals with a recent diagnosis of gallbladder disease (<1 year) may be biased. Overall, a number of studies have reported an elevated risk of pancreatic cancer among individuals with a history of gallbladder disease (cholelithiasis) or with a history of gallbladder removal (cholecystectomy). In one study, a 70% increase in risk remained after allowing 20 or more years between cholecystectomy and tumor diagnosis (Silverman et al., 1999). In contrast, a number of other studies did not observe an association between cholelithiasis or cholecystectomy and pancreatic cancer risk, or in some studies the association was no longer positive after removing those who had cholelithiasis or cholecystectomy less than a year before interview or diagnosis of pancreatic cancer.
Most studies examining cholecystectomy or cholelithiasis did not control for obesity, which is a known risk factor for gallbladder disease; consequently, it is possible that previous positive associations were confounded by obesity. Given the lack in consistency across studies, it is unlikely that gallbladder disease or cholecystectomy play an important role in pancreatic carcinogenesis. However, a modest increase in risk ( 20%) for cholecystectomy cannot be completely ruled out.
Diabetes Mellitus
Adult-onset diabetes, or type 2 diabetes, has been consistently associated with an elevated risk of pancreatic cancer in observational studies. Nonetheless, there has been substantial controversy over the role of type 2 diabetes and in determining whether it is a cause or simply a consequence of pancreatic cancer. Reverse causation could not be ruled out as an explanation for positive findings from earlier case-control studies, because diabetes is often a manifestation of pancreatic cancer. To understand better the role of diabetes, a number of observational studies collected information on duration of diabetes. In 1995, a metaanalysis estimated that individuals with at least 5 years’ duration of diabetes had a twofold higher risk of pancreatic cancer than those without diabetes (95% CI 1.2 to 3.2) (Everhart and Wright, 1995). In an updated meta-analysis based on a larger number of studies, the association between chronic diabetes (5 or more years) and pancreatic cancer was slightly weaker than the earlier meta-analysis, but still statistically significant (RR 1.5, 95% CI 1.3–1.8, compared to nondiabetics) (Huxley et al., 2005). In this meta-analysis, results from 28 cohort studies were slightly weaker than those obtained from 22 case-control studies (Huxley et al., 2005).
Additional evidence for the role of diabetes comes from studies examining the relation between prediagnostic glucose levels and pancreatic cancer. To date, elevated postload or fasting glucose levels have been associated with a higher risk of pancreatic cancer in four cohort studies with 10 to 25 years of follow-up (Gapstur et al., 2000; Batty et al., 2004; Jee et al., 2005; Stolzenberg-Solomon et al., 2005). In these studies, relative risks for biochemically defined diabetes ranged between 1.7 and 4, and dose–response relationships were observed with increasing levels of glucose. In one study, the association between glucose levels (across nondiabetic ranges) and pancreatic cancer was stronger among cases whose blood had been collected 10 or more years prior to cancer diagnosis (Stolzenberg-Solomon, 2005). Taken together, the current data strongly support a causal role for type 2 diabetes in the etiology of pancreatic cancer.
History Of Allergies
A number of observational studies have examined the relation between allergies and pancreatic cancer, usually along with other exposures. In a recent meta-analysis of 14 studies, pancreatic cancer risk was lower among individuals with a history of any allergy than those without allergies (pooled RR 0.82, 95% CI 0.68–0.99) (Gandini et al., 2005). The association was stronger among those with respiratory allergies excluding asthma (RR 0.63, 95% CI 0.52–0.76), or those with dermal allergies (RR 0.66, 95% CI 0.49–0.89), but no association was found for asthma, which is not always related to atopy (RR 1.01, 95% CI 0.77–1.31) (Gandini et al., 2005). A recent prospective study observed similar findings; a history of hayfever alone was associated with a lower risk of pancreatic cancer (RR 0.85, 95% CI 0.77–0.95; 373 pancreatic cancer cases), but no association was observed for a history of asthma alone (RR 1.02, 95% CI 0.85–1.23) (Turner et al., 2005). The biological mechanisms for this association are not known, but it has been proposed that the enhanced immune surveillance (heightened Th2 response) among individuals with allergies can decrease carcinogenesis; for example, a Th2 response stimulates production of anti-inflammatory cytokines, such as interleukin-4, that have been shown to have anticarcinogenic properties (Turner et al., 2006).
Given that having allergies is not a modifiable risk factor, it is not directly obvious how this information might be used to prevent pancreatic cancer. However, understanding the underlying biological mechanisms could provide some important clues into pancreatic carcinogenesis and may present opportunities for the development of new agents for chemoprevention or treatment of this fatal malignancy.
Obesity
A number of early studies did not report any association between body mass index and pancreatic cancer. However, because weight loss is a common symptom of pancreatic cancer, it is possible that earlier studies did not obtain the patient’s weight several years prior to diagnosis. Furthermore, most of the earlier studies had low participation rates and relied heavily on next-of-kin (often called ‘proxies’) to obtain exposure data, such that measurement errors were likely to have occurred. Over the past 5 years, a large number of cohort studies (which are less prone to bias) have reported positive associations between obesity and pancreatic cancer. These data are difficult to refute, but because the evidence is very recent, a number of recent reviews on pancreatic cancer have failed to mention obesity as a potential risk factor. To date, at least 10 prospective cohort studies with a total of over 15 000 cases have reported an elevated risk of pancreatic cancer for obese individuals (BMI 30 kg/m2), compared to individuals of healthy weight (BMI <25), with relative risks typically between 1.5 and 2.0. Although a few prospective studies failed to detect an association with BMI, most of these did not include a category of exclusively obese individuals. In one study in which BMI was not associated with risk, a statistically significant 46% increase in risk was observed among individuals who had gained 12 or more kg as adults, compared to those who gained 2–5 kg. Elevated risks of pancreatic cancer have also been reported for overweight men and women in four recent case-control studies in which only direct interviews were used (i.e., the patient provided the responses, not a relative or friend).
A meta-analysis of 14 studies on obesity and pancreatic cancer risk estimated a 19% increase in risk among obese individuals compared to those with a normal body weight (RR 1.19, 95% CI 1.10–1.29, for BMI 30 kg/m2 vs. 22 kg/m2) (Berrington de Gonzalez et al., 2003). However, the results from this meta-analysis include all studies, regardless of study design, and therefore the overall effect is almost certainly underestimated. The relative risk estimates were higher when the authors excluded case-control studies with proxy data or those studies that had not adjusted for smoking in their analyses.
Obesity results in metabolic abnormalities, including hyperinsulinemia, insulin resistance, and impaired glucose tolerance, which can contribute to the development of diabetes. In vitro studies show that insulin promotes growth of hamster, rat, and numerous human pancreatic cell lines. Studies suggest that islet cell turnover, associated with insulin resistance, may play an important role in pancreatic carcinogenesis. Stimulation of islet cell proliferation enhances pancreatic ductal carcinogenesis in hamsters, and the destruction of islet cells by streptozotocin or alloxan inhibits cancer induction. In a recent study, hamster pancreatic cancer was inhibited by the drug metformin, which normalized insulin levels and the rate of islet cell turnover (Schneider et al., 2001). In a recent prospective study of Finnish male smokers, men with the highest levels of prediagnostic insulin had a twofold increase in risk of pancreatic cancer compared to men with the lowest levels (insulin was measured 5 or more years prior to cancer diagnosis; 95% CI 1.03–3.93) (Stolzenberg-Solomon et al., 2005).
Given the strength of the evidence, obesity is now an accepted risk factor for pancreatic cancer; however, the magnitude of the effect ( 20% increase in risk for every 5 kg/m2 increment in BMI) is not as strong as for other obesity-related cancers.
Physical Activity
Data on physical activity and pancreatic cancer are less consistent than for obesity. Hypothetically, because exercise is known to improve glucose tolerance, even in the absence of weight loss, this factor could be an important modifiable risk factor for pancreatic cancer. To date, five studies have reported a lower risk of pancreatic cancer with higher physical activity but an equal number of studies have reported no association. In one study, regular moderate exercise (vs. sedentary) was associated with greater than 50% reduction in risk of pancreatic cancer in both men and women (Michaud et al., 2001). The association was strongest among individuals who were obese (RR 0.59, 95% CI 0.37–0.94, for moderate/heavy exercise vs. sedentary), but was not apparent among those who were not overweight. Because lean individuals are less likely to have metabolic abnormalities, they may not benefit from exercise in the same way as overweight individuals. More studies are needed to elucidate the role of physical activity on pancreatic cancer.
Aspirin And Other Anti-Inflammatory Drugs
Data from experimental studies suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit pancreatic cancer. Furthermore, aspirin and other NSAIDs have been shown to decrease the risk of a number of gastrointestinal cancers, including colorectal, stomach, and esophagus. Inconsistent results have been found in studies examining the use of aspirin and other NSAIDs and pancreatic cancer. The Iowa Women’s Health Study is the only study to date that reported a strong inverse association between aspirin use and the risk of pancreatic cancer (RR 0.40, 95% CI 0.20–0.82, for use six or more times per week compared with no use) (Anderson et al., 2002a), but no information was available on duration of use. Despite a recent study reporting an increase in risk of pancreatic cancer among women taking five or more aspirin per week for more than 10 years (relative risk 1.78, 95% CI 1.18 to 2.60) (Schernhammer et al., 2004), several other studies have not reported any association with aspirin or NSAID use, even for long-term use of aspirin (RR 0.96, 95% CI 0.69–1.33, for 20 or more years of use compared with nonusers ( Jacobs et al., 2004)). Overall, aspirin and other NSAIDs do not appear to play a substantial role in pancreatic cancer.
Dietary Factors
Numerous case-control studies have examined the relation between diet and the risk of pancreatic cancer. For many dietary factors, results have been mixed. Inconsistencies in these findings may be due to a number of inherent problems with these studies, which include use of next-of-kin (proxies) to obtain dietary information, recall bias (when patients remember their past exposures, including diet, differently than healthy controls because of their disease status), low response rates among cases because of high fatality rates, and poor dietary assessment tools. For alcohol and coffee specifically, findings may be biased in population-based case-control studies because heavy alcohol and coffee drinkers may have lower participation rates than nondrinkers. Alternatively, findings may be biased in hospital-based case-control studies because alcohol and coffee may be related to the health conditions afflicting controls that are included for study. Therefore, it is important to look at prospective cohort studies when examining dietary factors in relation to pancreatic cancer risk, as dietary intakes are measured prior to disease diagnosis.
Coffee
At least 13 prospective cohort studies have examined the relation between coffee intake and pancreatic cancer risk; with three exceptions, results from these studies have been null. The International Agency for Research on Cancer (IARC) concluded, based on the existing literature in 1991, that there was little evidence to support a causal relation between coffee and risk of pancreatic cancer (IARC, 1991). The majority of cohort studies conducted since 1991 have been consistent with IARC’s conclusion of no association (World Cancer Research Fund, 1997).
Alcohol
Ten prospective studies have examined the influence of alcohol intake in nonalcoholic populations. Four studies reported an increase in pancreatic cancer with alcohol intake, but the remaining studies observed no associations between alcohol and pancreatic cancer. Given that smoking and alcohol are highly correlated, some of the elevated risks observed may have been due to residual confounding by smoking. However, it is also possible that heavy alcohol drinking does increase the risk of pancreatic cancer (Silverman, 2001); this observation may be mediated through chronic pancreatitis (which is often caused by excessive alcohol consumption). Overall, and given additional data from case-control studies (summarized in the World Cancer Research Fund report (World Cancer Research Fund, 1997)), it appears unlikely that moderate alcohol consumption plays a major role in pancreatic cancer.
Fruit And Vegetables
In 1997, a panel of experts concluded that consumption of fruit and vegetables was ‘probably’ associated with a lower risk of pancreatic cancer; this decision was largely based on case-control data, because few prospective studies had been published at that time (World Cancer Research Fund, 1997). Prospective studies, however, have not confirmed these studies; to date, no association has been observed in five prospective studies. It is likely, therefore, that findings from case-control studies were biased by differential recall of dietary intake or other selection factors, and that overall, consumption of fruit and vegetables is not associated with pancreatic cancer. It remains possible, nevertheless, that certain types of fruit and vegetables, or selected nutrients high in fruit and vegetables, do play a role in pancreatic cancer. For example, cruciferous vegetable consumption (e.g., broccoli, cauliflower, brussels sprouts, cabbage) may decrease the risk of pancreatic cancer (RR 0.70, 95% CI 0.43–1.13, for three or more servings per week compared with less than one serving per week (Larsson et al., 2006b); RR 0.5, 95% CI 0.4–0.8, for the highest compared with lowest quartile of cruciferous vegetable intake (Silverman et al., 1998)). Cruciferous vegetables contain a large variety of compounds with potential anticarcinogenic properties. Another example is folate, which is found in a variety of fruit and vegetables.
Folate
There is substantial evidence to suggest that folate plays a role in colon and breast carcinogenesis; it is involved in DNA repair and synthesis as well as DNA methylation and may thus play a role in a number of cancers. Three prospective cohort studies have reported similar findings for folate. A high dietary intake of folate (i.e., not including supplements) was associated with a 48% lower risk of pancreatic cancer in a Finnish cohort study of smokers (RR 0.52, 95% CI 0.31–0.87) (Stolzenberg-Solomon et al., 2001), and in the same cohort, pre-diagnostic serum folate levels were inversely associated with pancreatic cancer (RR 0.45, 95% CI 0.26–0.88, for high vs. low serum folate levels) (Stolzenberg-Solomon et al., 1999). In a Swedish prospective study, a strong association was reported for dietary folate intake (RR 0.25, 95% CI 0.11–0.59, for 350 vs. <200 mg of folate per day) (Larsson et al., 2006a). Finally, in a combined analysis of two U.S. cohorts, folate intake from diet alone was associated with a lower risk of pancreatic cancer (RR 0.66, 95% CI 0.42–1.03, for the highest vs. lowest quintile of folate intake), but the reduction in risk was not statistically significant (Skinner et al., 2004).
In contrast, folate from supplements (typically from multivitamins) has shown different results. Supplemental folate was associated with an elevated risk of pancreatic cancer in the study of Finnish smokers (RR 1.56, 95% CI 0.90–2.70, for reported users vs. nonusers) (Stolzenberg-Solomon et al., 2001). In the other cohorts, folic acid from supplements was not associated with risk (Skinner et al., 2004; Larsson et al., 2006a).
Findings from these studies suggest that dietary folate may play a role in pancreatic cancer, but it is unclear why folate supplements are not providing similar benefits to those seen with food sources of folate. It has been suggested that folate from supplements may promote the progression of pancreatic cancer in individuals with existing cancer, perhaps from higher exposure levels from supplements (as they are more bioavailable) (Larsson et al., 2006a), or alternatively, that some component of multivitamin supplements (the main source of supplemental folate) is counterbalancing the beneficial effect of folate (Skinner et al., 2004). Therefore, even though data from these large cohort studies suggest that folate intake may be inversely associated with pancreatic cancer risk, folic acid supplement use should not be recommended as a chemopreventive agent for this cancer.
Meat And Fat
Numerous case-control and cohort studies have examined the relation between meat and/or fat intake and pancreatic cancer. Overall, however, findings from these studies have been inconsistent. Studies with positive findings for meat intake are not consistent either, because some report an increase in risk with high beef intake, while others report an increase with high pork intake.
Processed meats are of particular interest, since they contain nitrites, which can be converted to nitrosamines in the stomach, and nitrosamines have been shown to exert carcinogenic properties. In a case-control study conducted in China, consumption of smoked and cured foods increased the risk of pancreatic cancer ( Ji et al., 1995). Recently, a large cohort study reported a positive association between processed meat intake and the risk of pancreatic cancer (RR 1.65, 95% CI 1.35–2.07, for the highest vs. lowest quintile of intake) (Nothlings et al., 2005), but two other cohort studies did not observe an increase in pancreatic cancer risk with higher intakes of processed meats (Stolzenberg-Solomon et al., 2002; Michaud et al., 2003).
Cooking meat at high temperatures can result in the formation of carcinogenic heterocyclic amines and/or polycyclic aromatic hydrocarbons (PAH) (Sinha et al., 1998). Grilling or barbecuing and frying of meat can produce high levels of carcinogens while other methods of preparation, such as broiling or baking, do not form significant levels of these compounds (Sinha et al., 1998). In a case-control study, a high intake of grilled or barbecued red meat was associated with an elevated risk of pancreatic cancer (RR 2.2, 95% CI 1.4–3.4, for the top quintile compared to the bottom two quintiles) (Anderson et al., 2002b). Detailed questions on cooking practices, which are needed to estimate exposure to dietary heterocyclic amines, have not been available in most epidemiologic studies to date. Future studies with more of these data are necessary to elucidate the role of dietary heterocyclic amines in relation to pancreatic cancer.
Carbohydrate And Sugar
A number of studies have examined the relation between carbohydrate intake and the risk of pancreatic cancer. In a large pooled case-control study of 802 cases and 1669 controls from five different countries (SEARCH), a higher risk of pancreatic cancer was observed among individuals consuming a high carbohydrate diet (for top to bottom quintile comparison: RR 2.57, 95% CI 1.64–4.03, after controlling for lifetime cigarette consumption) (Howe et al., 1992). Sugar added to drinks and food, refined sugar, and regular soda consumption have all been associated with the risk of pancreatic cancer.
In a recent cohort study of U.S. women, carbohydrate intake was not associated with pancreatic cancer risk, but an increase in risk was observed with higher intakes of fructose and sucrose and with glycemic load (Michaud et al., 2002). The association with sugar intake was stronger among women who were either overweight or sedentary, two physiological states that are associated with greater insulin resistance. Furthermore, women with both high BMI (≥25 kg/m2) and low physical activity had a statistically significant relative risk of 2.63 for pancreatic cancer when comparing high with low glycemic load intake, but no associations were observed for glycemic load and pancreatic cancer risk among women who had a low BMI (<25 kg/m2) or were physically active.
Although a number of studies suggest carbohydrate intake and/or refined sugars play a role in pancreatic cancer, a number of other studies have found no relation between carbohydrate or sugar and pancreatic cancer risk, thus suggesting that the relationship between diet and pancreatic cancer is complex and likely to depend on a number of factors.
Occupational Exposures
A variety of different occupations have been studied in relation to pancreatic cancer (Anderson et al., 1996), but few findings have been consistent across studies. I discuss the relation between pesticides and pancreatic cancer briefly, as it has received substantial attention and it remains controversial.
Pesticides
A study published in 1992 reported a sevenfold increase in risk of pancreatic cancer among workers who had been exposed to p,p0 -dichlorodiphenyltrichloroethane (DDT) for a mean of 47 months (Garabrant et al., 1992). Although this study raised concerns regarding a possible effect of pesticide exposure, a large number of studies examining the relation between various pesticides and pancreatic cancer among workers with high pesticide exposures have been largely inconsistent. One problem with these studies is that the exact exposure is difficult to quantify and measurement error in establishing exposure level is likely to be large. In a recent case-control study, pancreatic cancer cases had elevated serum levels of DDT compared to controls, but the association was no longer apparent after controlling for serum levels of PCBs (Hoppin et al., 2000). Although it is difficult to rule out an effect of pesticide exposure on pancreatic cancer, pesticides are unlikely to account for a large proportion of pancreatic cancer cases, as elevated exposures are only experienced by a small percentage of the overall population.
Conclusion
Pancreatic cancer is a devastating disease. No effective treatment exists for the majority of patients who do not have localized disease, and 50% of all patients die within six months of diagnosis. There is currently no population screening for this cancer. Because early onset of the disease is largely asymptomatic, there are few opportunities to diagnose this cancer at early stages. The etiology of pancreatic cancer is poorly understood, and few opportunities for prevention are currently available. Cessation of cigarette smoking is the only widely accepted behavioral change that can reduce the risk of pancreatic cancer. In recent years, accumulating data have suggested that longstanding type 2 diabetes and obesity increase the risk of pancreatic cancer. As a result of the absence of early detection, the difficulty of the diagnostic challenge, the paucity of treatment options, poor survival, and a lack of understanding of its etiology, pancreatic cancer is a major public health challenge. The tobacco epidemic in developing countries and the worldwide obesity epidemic will only increase the public health burden of this disease in the near future.
Bibliography:
- Alguacil J and Silverman DT (2004) Smokeless and other noncigarette tobacco use and pancreatic cancer: A case-control study based on direct interviews. Cancer Epidemiology Biomarkers & Prevention 13: 55–58.
- Anderson KE, Potter JD, and Mack TM (1996) Pancreatic cancer. In: Schottenfeld D and Fraumeni JFJ (eds.) Cancer Epidemiology and Prevention, pp. 725–771. New York: Oxford University Press
- Anderson KE, Johnson TW, Lazovich D, and Folsom AR (2002a) Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. Journal of the National Cancer Institute 94: 1168–1171.
- Anderson KE, Sinha R, Kulldorff M, et al. (2002) Meat intake and cooking techniques: Associations with pancreatic cancer. Mutation Research 506–507: 225–231.
- Bardeesy N and DePinho RA (2002) Pancreatic cancer biology and genetics. Nature Reviews Cancer 2: 897–909.
- Batty GD, Shipley MJ, Marmot M, and Smith GD (2004) Diabetes status and post-load plasma glucose concentration in relation to sitespecific cancer mortality: findings from the original Whitehall study. Cancer: Causes and Control 15: 873–881.
- Berrington de Gonzalez A, Sweetland S, and Spencer E (2003) A metaanalysis of obesity and the risk of pancreatic cancer. British Journal of Cancer 89: 519–523.
- Bray F, Sankila R, Ferlay J, and Parkin DM (2002) Estimates of cancer incidence and mortality in Europe in 1995. European Journal of Cancer 38: 99–166.
- Chari ST, Mohan V, Pitchumoni CS, et al. (1994) Risk of pancreatic carcinoma in tropical calcifying pancreatitis: an epidemiologic study. Pancreas 9: 62–66.
- Everhart J and Wright D (1995) Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. Journal of the American Medical Association 273: 1605–1609.
- Fuchs C, Colditz G, Stampfer M, et al. (1996) A prospective study of cigarette smoking and the risk of pancreatic cancer. Archives of Internal Medicine 156: 2255–2260.
- Gandini S, Lowenfels AB, Jaffee EM, Armstrong TD, and Maisonneuve P (2005) Allergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiology Biomarkers & Prevention 14: 1908–1916.
- Gapstur SM, Gann PH, Lowe W, et al. (2000) Abnormal glucose metabolism and pancreatic cancer mortality. Journal of the American Medical Association 283: 2552–2558.
- Garabrant DH, Held J, Langholz B, Peters JM, and Mack TM (1992) DDT and related compounds and risk of pancreatic cancer. Journal of the National Cancer Institute 84: 764–771.
- Germanos S, Gourgiotis S, Stavrothanasopoulou A, et al. (2006) Diagnostic and therapeutic approach to pancreatic adenocarcinoma. Journal of Gastrointestinal and Liver Disease 15: 257–263.
- Hahn SA, Greenhalf B, Ellis I, et al. (2003) BRCA2 germline mutations in familial pancreatic carcinoma. Journal of the National Cancer Institute 95: 214–221.
- Hecht SS (2003) Tobacco carcinogens, their biomarkers and tobaccoinduced cancer. Nature Reviews Cancer 3: 733–744.
- Hoppin JA, Tolbert PE, Holly EA, et al. (2000) Pancreatic cancer and serum organochlorine levels. Cancer Epidemiology Biomarkers & Prevention 9: 199–205.
- Howe GR, Jain M, Burch JD, and Miller AB (1991) Cigarette smoking and cancer of the pancreas: evidence from a population-based case-control study in Toronto, Canada. International Journal of Cancer 47: 323–328.
- Howe GR, Ghadirian P, Bueno de Mesquita HB, et al. (1992) A collaborative case-control study of nutrient intake and pancreatic cancer within the search programme. International Journal of Cancer 51: 365–372.
- Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, and Woodward M (2005) Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British Journal of Cancer 92: 2076–2083.
- International Agency for Research on Cancer (IARC) (1991) Coffee, tea, mate methylxanthines, and methylglyoxal. IARC Monograph Evaluations of Carcinogenic Risks in Humans 51. Lyon, France: IARC51.
- Jacobs EJ, Connell CJ, Rodriguez C, et al. (2004) Aspirin use and pancreatic cancer mortality in a large United States cohort. Journal of the National Cancer Institute 96: 524–528.
- Jee SH, Ohrr H, Sull JW, et al. (2005) Fasting serum glucose level and cancer risk in Korean men and women. Journal of the American Medical Association 293: 194–202.
- Ji BT, Chow WH, Gridley G, et al. (1995) Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer Epidemiology Biomarkers & Prevention 4: 885–893.
- Karlson BM, Ekbom A, Josefsson S, et al. (1997) The risk of pancreatic cancer following pancreatitis: an association due to confounding? Gastroenterology 113: 587–592.
- Klein AP, Beaty TH, Bailey-Wilson JE, et al. (2002) Evidence for a major gene influencing risk of pancreatic cancer. Genetic Epidemiology 23: 133–149.
- Klein AP, Brune KA, Petersen GM, et al. (2004) Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Research 64: 2634–2638.
- Larsson SC, Hakansson N, Giovannucci E, and Wolk A (2006a) Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. Journal of the National Cancer Institute 98: 407–413.
- Larsson SC, Hakansson N, Naslund I, Bergkvist L, and Wolk A (2006b) Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiology Biomarkers & Prevention 15: 301–305.
- Lin Y, Tamakoshi A, Wakai K, et al. (1998) Descriptive epidemiology of pancreatic cancer in Japan. Journal of Epidemiology 8: 52–59.
- Lowenfels AB, Maisonneuve P, Cavallini G, et al. (1993) Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. New England Journal of Medicine 328: 1433–1437.
- Lowenfels AB, Maisonneuve P, DiMagno EP, et al. (1997) Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. Journal of the National Cancer Institute 89: 442–446.
- Lowenfels AB, Maisonneuve P, and Lankisch PG (1999) Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterology Clinics of North America 28: 673–685.
- Lynch HT, Smyrk T, Kern SE, et al. (1996) Familial pancreatic cancer: a review. Seminars in Oncology 23: 251–275.
- Michaud DS, Giovannucci E, Willett WC, et al. (2001) Physical activity, obesity, height and the risk of pancreatic cancer. Journal of the American Medical Association 286: 921–929.
- Michaud DS, Liu S, Giovannucci E, et al. (2002) Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. Journal of the National Cancer Institute 94: 1293–1300.
- Michaud DS, Giovannucci E, Willett WC, Colditz GA, and Fuchs CS (2003) Dietary meat, dairy products, fat, and cholesterol and pancreatic cancer risk in a prospective study. American Journal of Epidemiology 157: 1115–1125.
- Murphy KM, Brune KA, Griffin C, et al. (2002) Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Research 62: 3789–3793.
- Nothlings U, Wilkens LR, Murphy SP, et al. (2005) Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. Journal of the National Cancer Institute 97: 1458–1465.
- Parkin DM, Muir CS, Whelan SL, et al. (eds.) (2002) Cancer Incidence in Five Continents. Lyon, France: International Agency for Research on Cancer.
- Parkin DM, Bray F, Ferlay J, and Pisani P (2005) Global cancer statistics, 2002. CA Cancer Journal for Clinicians 55: 74–108.
- Pisani P, Parkin DM, Bray F, and Ferlay J (1999) Estimates of the worldwide mortality from 25 cancers in 1990. International Journal of Cancer 83: 18–29.
- Prokopczyk B, Hoffmann D, Bologna M, et al. (2002) Identification of tobacco-derived compounds in human pancreatic juice. Chemical Research in Toxicology 15: 677–685.
- Ries LAG, Eisner MP, Kosary CL, et al. (eds.) (2003) SEER Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute.
- Ries L, Eisner M, Kosary C, et al. (2006) SEER Cancer Statistics Review,1975–2003. Bethesda, MD: National Cancer Institute.
- Schernhammer ES, Kang JH, Chan AT, et al. (2004) A prospective study of aspirin use and the risk of pancreatic cancer in women. Journal of the National Cancer Institute 96: 22–28.
- Schneider MB, Matsuzaki H, Haorah J, et al. (2001) Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 120: 1263–1270.
- Shapiro JA, Jacobs EJ, and Thun MJ (2000) Cigar smoking in men and risk of death from tobacco-related cancers. Journal of the National Cancer Institute 92: 333–337.
- Silverman DT, Swanson CA, Gridley G, et al. (1998) Dietary and nutritional factors and pancreatic cancer: a case-control study based on direct interviews. Journal of the National Cancer Institute 90: 1710–1719.
- Silverman D, Schiffman M, Everhart J, et al. (1999) Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. British Journal of Cancer 80: 1830–1837.
- Silverman DT (2001) Risk factors for pancreatic cancer: a case-control study based on direct interviews. Teratogenesis, Carcinogenesis, and Mutagenesis 21: 7–25.
- Sinha R, Knize MG, Salmon CP, et al. (1998) Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food & Chemical Toxicology 36: 289–297.
- Skinner HG, Michaud DS, Giovannucci EL, et al. (2004) A prospective study of folate intake and the risk of pancreatic cancer in men and women. American Journal of Epidemiology 160: 248–258.
- Stolzenberg-Solomon RZ, Albanes D, Nieto FJ, et al. (1999) Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. Journal of the National Cancer Institute 91: 535–541.
- Stolzenberg-Solomon RZ, Pietinen P, Barrett MJ, et al. (2001) Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. American Journal of Epidemiology 153: 680–687.
- Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, and Albanes D (2002) Prospective Study of Diet and Pancreatic Cancer in Male Smokers. American Journal of Epidemiology 155: 783–792.
- Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. (2005) Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. Journal of the American Medical Association 294: 2872–2878.
- Talamini G, Falconi M, Bassi C, et al. (1999) Incidence of cancer in the course of chronic pancreatitis. American Journal of Gastroenterology 94: 1253–1260.
- Turner MC, Chen Y, Krewski D, et al. (2005) Cancer mortality among US men and women with asthma and hay fever. American Journal of Epidemiology 162: 212–221.
- Turner MC, Chen Y, Krewski D, and Ghadirian P (2006) An overview of the association between allergy and cancer. International Journal of Cancer 118: 3124–3132.
- World Cancer Research Fund, American Institute for Cancer Research (1997) Pancreas Food, Nutrition and the Prevention of Cancer: a Global Perspective. Washington, D.C: American Institute for Cancer Research.




