Sample Genetics Of Complex Traits Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The purpose of this research paper is to present a schema for conceptualizing the sources of individual differences in lifespan developmental processes. The two broad domains of genetic and environmental factors are presented as co-acting in their influence on complex traits, with the relative influence differing from trait to trait. Furthermore, intricate interactions are possible among genes and between genes and environments. The system is dynamic, with the possibility that the relative contributions of genetic and environmental sources can change over time. Newly available methods assure finer-grained descriptions of the interrelationships of genes and environment in the ‘architecture’ of complex systems in the near future.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Mendelian Genetics
Prior to the research of Mendel, the general conceptualization of the hereditary material was of an amorphous, miscible substance, in which the contributions of the parents were blended and influences from the environment altered the mix. Mendel’s ingenious contribution was to postulate the existence of discrete elements (later termed ‘genes’). Further postulations, in abridged form, were that an organism possesses many such genes, and each occurs as a pair, with an organism receiving one element from each parent. There are different forms of the genes (later to be named ‘alleles’). Just as an individual received one allele for each gene from each parent, it transmits only one of each pair to its offspring. The alleles do not blend and are not modified during the association with their partner alleles or with the alleles of other genes, but are transmitted from the organism in the same functional condition in which they were contributed to the organism. Likewise, environmental factors do not alter the nature of the elements.
A critical distinction in Mendelian genetics is made between the phenotype, the measured attribute, and the relevant genes, or genotype, and the crux of the theory was the set of rules determining the relationships that could obtain between genotype and phenotype.
Mendelian genetics deals with categories. In the ideal case, all individuals are assignable unequivocally to one of two or three categories. A particular reason that the genotype phenotype distinction is important is that the relationship between the two is not always additive. Knowing the phenotype is not always unambiguously informative about genotypes. For example, in one classical Mendelian situation, those individuals possessing two copies of a particular allele of the gene belong to category A, those possessing two copies of another allele of the same gene belong to category B, and those possessing one copy of each also belong to category B. That is to say, one copy of the allele ‘for’ category B is as effective as two. This relationship is described as dominance; the allele for which one copy is as effective as two is the dominant allele, and the other is termed the recessive allele. The two genotypes with identical alleles are called homozygotes, and the genotype with one each is called a heterozygote. Other relationships are possible: In some situations, the relationship between allelic ‘dose’ and phenotype is strictly additive, and intermediate degrees of dominance are found for other phenotypes.
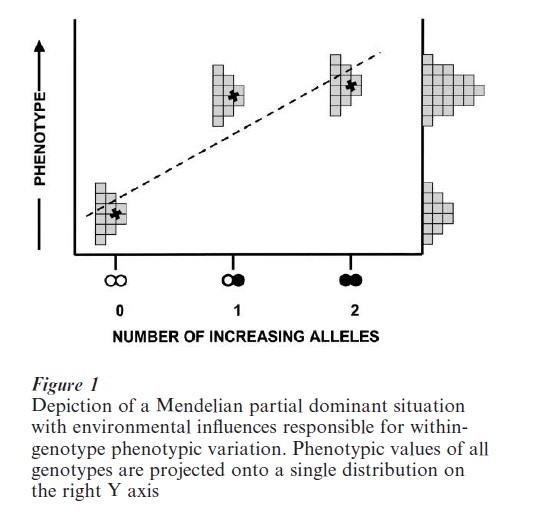
Even when dominance is present, there is, of course, an additive component to the relationship. Thus, the phenotypic variability can be regarded as due to an additive component and to dominance deviations. Another major reason that the distinction between genotype and phenotype is critical is that genes may not be the sole determinants of the phenotypic outcome. The basic principle of multiple influences underlying quantitative genetics is illustrated in Fig. 1. Phenotypic values are shown for a number of individuals of each genotype for a single gene with a partially dominant functional relationship to the phenotype. The within-genotype variability is assumed to be due to environmental circumstances that have uniquely affected each individual. A projection of these phenotypic values onto the right Y-axis reveals a distribution, the variability of which is attributable both to genetic differences (with some of this genetic influence due to the additive effect of allelic ‘substitution’ (represented by the dashed regression line), and some due to dominance (the deviations of the X’s marking the means of each group from the regression line) and also with a portion of the variance due to environmental differences among the individuals. The consequence when more than one gene can affect the phenotype will be discussed below.
The source of genotypic diversity is, of course, the existence of different alleles. These alternative forms of the gene arise through mutational events, and their ultimate fate in a population depends upon their influence on the reproductive ‘fitness’ of their possessors, among other matters (see Falconer and Mackay 1996, Hartl and Clark 1997). In any population, the relative frequencies of the alternative alleles (there may be many more than two, but this discussion will be restricted to the simple case of two) will ordinarily be far from equal. Thus, the different genotypes will also not be equally frequent. If p represents the frequency of the most abundant allele (say A1) and q represents the least frequent one (A2), and if mating is at random, then the relative frequencie s of the geno types A1A1, A1A2, and A2A2 will be p2, 2pq, and q2, respectively. Different values of p and q will thus obviously lead to different genotype frequencies, and thus to different means and to different variances.
2. Polygenic Inheritance
For many phenotypes, it is not possible to assign individuals to categories; instead, they are arrayed along a continuous distribution. Although for a time there was a conjecture that different genetic systems were engaged in the categorical and continuous, a model (Fisher 1918) that postulated the existence of many loci, each performing according to Mendelian rules, but each having only a small influence on the same phenotype, resolved the issue. The collective effect of these multiple genes (polygenes) yields statistical expectations of resemblance of relatives of different degrees of biological relationship. For example, our understanding of the mechanism of gene transmission informs us that a parent shares half of his or her alleles with a child; grandparents share one quarter of their alleles with their grandchildren; siblings share half of their alleles on average; fraternal twins are just like siblings in this regard, and identical twins share all of their alleles. Comparison of the degree of similarity of phenotypes to the fraction of shared alleles permits the estimation of the proportions of phenotypic variance that can be attributed to genetic sources and to environmental sources. Each of these broad categories can be further divided. The overall genetic component can be described in terms of an additive component and a non-additive one. The non-additive component itself can be subdivided into effects arising from dominance, which can be regarded as the interaction of alleles at a locus, and from epistasis, which is the interaction of genotypes at different loci. This latter effect will be discussed in more detail later.
In the quantitative genetic model, environment is usually defined by exclusion—it is all of the influences that are non-genetic; i.e., not arising in a causal sense from the coded material of the DNA. Thus the scope and range of environment in this conceptualization is enormous, including in its compass factors from cellular biochemistry to peer group dynamics. This environmental contribution to phenotypic variance can also be partitioned in a number of ways, depending on the purpose of the research and on the data available. Long-term environmental effects may be distinguished from short term ones, for example, for developmental studies. One common distinction is that between environmental effects that are shared by family members and non-shared environmental influences that are experienced uniquely by individuals.
A simple, broad-stroke conceptual model represents the phenotype as the additive outcome of genetic and of environmental influences. Algebraically, this can be stated as
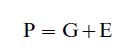
In a system such as this, the variances can be described as
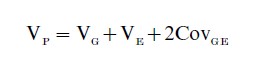
As noted previously, these terms are further divisible. Thus, as noted, VG includes both an additive component (VA) and a nonadditive (VNA) component, and VE is similarly subdivisible. These components yield an estimate of the relative influence of the two general domains. (There are also formulations that include a component of stochastic or of nonlinear dynamic influences [see Molenaar et al. 1993, Finch and Kirkwood 2000].) Measurement error is usually found in the environmental term, and as will be discussed below, there are also interaction terms that can be of great importance. The most commonly encountered index is that of heritability, which is the proportion of total phenotypic variance that is due to genetic differences among individuals. In the narrow sense, heritability is VA/VP, and in the broad sense, it is VG /VP. These two values are differentially useful for different descriptive and predictive purposes (see, for example, Falconer and Mackay 1996). The same information can be captured by an index of environmentality (1-h2), of course, but this term is infrequently encountered. It is very important to recognize that heritability and environmentality are descriptive statistics describing the state of affairs of a specific population under specific environmental circumstances. Different gene pools, or different environmental forces, might yield quite different estimates. Note also that high heritability does not mean that there is no conceivable environmental influence that can affect the phenotype. It means that in the population that was studied, there was either no sufficiently powerful environmental influence, or that if one existed, it impinged on so few of the population that it had little effect on the overall variability. Thus high heritability for a condition such as Attention Deficit Hyperactivity Disorder should not inhibit the search for environmental interventions. The classical single locus exemplar of this point is that of phenylketonuria, in which the homozygous recessive condition for a particular allele at the phenylalanine hydroxylase locus results in devastating mental retardation in ordinary environmental circumstances. However, the understanding of the metabolic process in which phenylalanine hydroxylase is involved led to the development of a rational intervention—a diet without phenylalanine, which cannot be metabolized normally by the homozygous recessive individuals.
Note that a gene is an unobserved hypothetical element both in a Mendelian system and in a polygenic system. The basic sequence involved in identifying a Mendelian inheritance pattern is to observe a categorical distribution of some phenotype, then to determine if the transmission pattern within families accords to Mendelian rules. In a polygenic, multifactor system, the issue is whether the pattern of resemblance among relatives conforms to expectations of the quantitative model. It is not required to know anything concrete about the gene or genes involved in either of these situations. But in fact, historically, while the range of application of Mendelian rules was being explored and the elaboration of the quantitative genetic model was proceeding, much was also being learned about the physical reality of genes. The key observation was that small bodies in the nucleus of cells behave according to rules similar to those of Mendel’s elements. The hypothesis that these small bodies, the chromosomes, were the vehicles of the genes was quickly confirmed, and the field of cytogenetics emerged. Examination of the fine structure of chromosomes revealed distinctive banding patterns that permitted specification of chromosomal location, and subsequent research showed that the Mendelian elements resided in fixed positions on the chromosomes. The position of a gene came to be called its locus, for obvious reasons. The term, locus, was a terminological advance, making it possible to distinguish easily between the concept of gene, which everyone has, and the alleles, which different individuals can have in different combinations.
Throughout the first half of the twentieth century, a major biological mystery concerned the mechanism through which genes exert their influence. In midcentury occurred the revolution of molecular biology that discovered the chemical nature of genes and revealed the processes through which the genetic information in DNA is transcribed into RNA and translated into protein. This work connected the genetics of hypothetical genes to anatomy, physiology, immunology, endocrinology, biochemistry, neurobiology—to all of the disciplines that have explored the biological functioning of the organism. The question of the mechanism of gene action is thus answered in broad principle (although the details of specific cases may be unknown). Genes have no special route of influence to the phenotype; they operate through the complex systems comprising the familiar structures and functions of the body.
Complex systems such as this have some properties of great importance for the developmental sciences. A fundamental feature is the existence of feedback relationships of the sort involved in regulated processes, such as the familiar negative feedback in homeostatic systems. Another related feature is the typical presence of redundant pathways leading to the phenotype. With such properties, deficiencies in a particular element of a pathway might be compensated by up-regulation of other elements in that pathway, or by enhanced activity in the parallel pathway. Kacser and Burns (1979) have emphasized that in biochemical pathways with feedback loops, it becomes impossible to specify the impact of any element without specifying the other elements in the system. In general, the simple notions of linear, unidirectional causation must yield to circular or network causal concepts (Sattler 1986). Specific to interpretation of genetic influences, we are cautioned that the effect of genotypic differences at a particular locus may be greatly influenced by other loci in the system, or by the environment in which the organism exists. The magnitude and complexity of these interactions will be illustrated later.
3. New Tools
Recent developments in molecular genetics have provided some remarkable new methods with which to approach the issues identified in this review. Some of the most relevant are the following.
3.1 Quantitative Trait Loci
Although the original quantitative genetic model postulated an indefinitely large number of loci with equal small effects, it has long been suspected that within a polygenic set there may exist a distribution of effect sizes. Prior to the genome projects that have provided chromosomal markers throughout the genomes of human beings and several model species, attempts to locate such loci have been fraught with difficulties. With the availability of the new markers, it has become possible to identify segments of the chromosomes in which are located genes that have a detectable influence on the variability of a phenotype. This is obviously a critical step on the way to further characterization of the locus at the molecular level. These individuated polygenes are termed quantitative trait loci (QTLs).
3.2 Transgenic Procedures
Among other enticing possibilities, there are now procedures that bring about changes in the DNA molecule constituting a gene so as to prevent its expression (knockout preparations), to alter its regulatory system in order to cause overexpression, or to insert a new gene. These preparations, permitting the examination of the downstream results of altering single loci, provide new avenues of approach to the causal nexus between gene and phenotype.
3.3 Arrays
Wedding of the technological advances of microchips with molecular biology has provided chips on which are arrayed hundreds or thousands of gene segments. When exposed to preparations containing DNA from particular organs or tissues, the chips can identify, through the hybridization of the tissue DNA to the segments on the chips, those genes that are being expressed in the tissue being investigated. Both the bright promise and the methodological issues of this new technology have been well presented by Claverie (1999) and by Lockhart and Barlow (2001).
Although these procedures and techniques are new on the scene, they are already providing powerful complements to the accumulated data on interactions and developmental changes in the architecture of complex phenotypes.
4. Epistasis
The phenomenon of interaction among genes has been known since the early days of the last century. An epistatic variance component can be incorporated in the quantitative genetic model, as well, but the detection of interaction effects in polygenic systems is challenging. There are numerous examples of the dependence of single locus effects on a polygenic background, however. A now-classic result can illustrate. Coleman and Hummel (1975) investigated the phenotypic consequences of homozygosity for a particular allele (db) at the diabetes locus in inbred mice. Inbred strains approximate the condition of being homozygous at all loci (see McClearn and Hofer 1999); animals within a strain are identical, and different inbred strains differ from each other at some unknown number of loci. It was possible to arrange the db/db genotype on the background of two different (but related) inbred strains—C57BL/6 and C57BL/Ks. The phenotypic outcome differed dramatically, in respect to body weight, glucose level, pathophysiology (atrophy of the Islet cells in one strain, and hypertrophy in the other), and in effects on lifespan.
The advent of QTL methods has greatly facilitated the study of epistasis. It has become possible to examine the interaction of these as yet unidentified (but approximately located) loci. In mice, to illustrate the point, QTL interactions have been described affecting blood pressure (Rapp et al. 1998), diabetes (Galli et al. 1999), epileptic seizure frequency (Frankel et al. 1995), and lung cancer (Fijneman et al. 1996).
In human research, an epistatic interaction concerning risk for dementia has been reported. The ε4 allele of the ApoE locus has been implicated by many studies as a risk factor for the development of Alzheimer’s disease. Overall, an allelic dose-response relationship has been observed, with a significant risk in heterozygous condition and an even greater risk in homozygous condition. Kamboh et al. (1995) describe an interaction of this locus and the ACT locus, alleles of which are labeled A and T. If an individual is homozygous TT, the risk associated with two ε4 alleles is not greater than with one. The ε4-associated risk is elevated if individuals are also AA, and individuals homozygous both for ε4 and for A are especially vulnerable.
These few examples represent the scope for the influence of genetic context on the effect of allelic differences at specific loci (i.e., the effect of allelic substitution, or the allelic dose–response effect). Characterization of a gene as ‘the’ gene for some phenotype must be understood to involve acknowledging the potential of this contextual influence.
5. Gene–Environment Interaction
Under the rubric of gene–environment interaction are those phenomena in which the relation of genotype to phenotype is altered as a function of environment, or, conversely, in which the impact of an environmental agency or condition is dependent upon the genotype of the individual exposed to the environment.
This theme can be illustrated by the results of Fosmire et al. (1993), who, in the context of risk factors in Alzheimer’s disease, explored the effects of exposure to high levels of dietary aluminum on brain aluminum levels in five strains of mice. Three strains showed no elevation as a consequence of the exposure, one showed a moderate effect, and one showed a threefold increase.
Logically parallel to evidence such as this on genetic influence on risks of deleterious outcomes engendered by environmental factors is evidence of genetic influence on responsiveness to intended therapeutic interventions. Yaffe et al. (2000), for example, investigated the effects of estrogen used by women 65 years of age and older on risk of cognitive impairment, and found an interaction with ApoE genotype. Risk was not affected in women who possessed at least one ε4 allele, but was reduced in those who were ε4-negative.
A final example illustrating the potential scope and complexities of gene–environment interactions may be taken from the work of Vieira and colleagues (2000). Seventeen QTLs were identified affecting lifespan in Drosophila reared in one of five different environments: three levels of rearing temperature, one involving a brief high temperature exposure, and one involving starvation stress. The results revealed an astounding level of interactions of the QTLs with sex and with environment. Some QTLs were effective only in one or a few environments and not in others; some were effective only in one sex; some were effective in both sexes, but only in one environment; and so on. In a summary statement, the authors reported that all of the genetic variance in this system was involved in some type of interaction!
6. Temporal Considerations
Dynamic change in the network of genetic and environmental factors over time is a key concept for developmental genetics. A fundamentally important principle to emerge from investigations of the mechanisms of gene action is that genes may be turned on and off, or may have their level of expression upregulated or down-regulated. There were temporal aspects to many of the previously cited studies; in a number of researches, the temporal dimension is the major focus. A particularly apposite example is the work of Rogina and Helfand (1995), who measured the expression of a gene in the antennae of Drosophila melanogaster. At hatching, there is little or no activity of this gene, but expression rises systematically to a peak in mid-life, with a subsequent gradual decline to near-zero levels at the end of life. When lifespan was shortened under conditions of an elevated rearing temperature or through the introduction of a different gene that shortens lifespan, the expression of the gene followed the same pattern, but in an accelerated manner, scaling to the proportion of the lifespan rather than to chronological time.
The existence of a program that ‘knows’ what proportion of a lifespan has already passed (and what remains) is an intriguing prospect. But for our present purposes, the important point is that this unambiguous demonstration of lifespan-scaled change in expression of this single gene informs us of the sort of changes that can be taking place in a polygenic system.
An example of dramatic developmental change in a mammalian system is provided by Cheverud et al. (1996) who studied mouse growth. Two sets of QTLs were identified: one affecting early growth but with diminishing influence by 6 weeks of age, and the other coming into play in the interval from 3 to 6 weeks of age.
It remains unclear how many or what proportion of loci can be expected to undergo systematic changes during the lifespan. The new chip technology is poised to generate a flood of data that will illuminate this issue, among many others. As a promissory illustration, Lee and colleagues (1999) have described differences between 5-month and 30-month old mice in respect to gene expression in skeletal muscle. Of 6,347 genes examined, 58 showed a more-than-twofold increase in expression levels and 55 showed a decrease of that magnitude. Although these numbers indicate that only a small percentage of genes are changing expression of this magnitude in this tissue over this time span, the impact of these changes in complex aging systems could be substantial. Information from other tissues is urgently needed, and will undoubtedly be reported soon.
Life stage has also been shown to be critical to the genetics of development in human beings. In a single locus example, Ferrari et al. (1998) showed that the major locus for the vitamin D receptor has an appreciable effect on the bone mass of pre-pubertal girls, but does not have any such influence during post- pubertal adolescence.
Concerning late life, there is a widely shared expectation that with advancing age the cumulative effects of uniquely experienced environmental factors will lead to reduced proportional contribution of genetic factors to that variance. Evidence on shifting variance components in aging has been provided by twin studies on the elderly; the classic expectation is fulfilled in the case of some phenotypes, but not all. Fig. 2 gives results from some biomedical variables included in the Swedish Adoption Twin/Study of Aging (SATSA) (Heller et al. 1993, Hong et al. 1994, McClearn et al. 1994). Estimates were derived from structural equation modeling of genetic, shared environmental, and non-shared environmental components of variance.
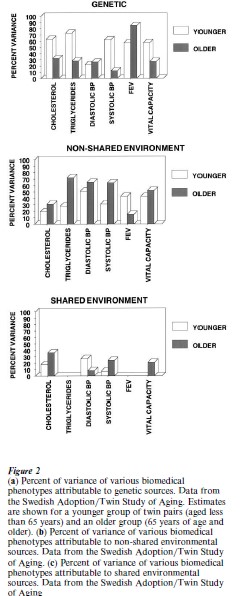
Participants in the study were Swedish identical and fraternal twins, some of whom were reared together and some of whom were separated in early life and reared separately. Estimates were derived separately for a younger group (less than 65 years of age) and an older group (65 years of age and older). Figure 2a gives the estimates of heritability. The difference between the older and younger cohort clearly conforms to the classical expectation of reduced heritability for measures of cholesterol, triglycerides, systolic blood pressure, and vital capacity. Heritability of diastolic blood pressure is, however, modest and essentially the same in both cohorts, and, with respect to forced expiratory volume (FEV), heritability is very substantial in the younger cohort, and even higher in the older cohort.
Fig. 2b gives the estimates for the influence of nonshared environment for these phenotypes. Most of these phenotypes (FEV being the exception) do display the classically expected result of greater non-shared environmental influence in the older group. Shared environmental influences, shown in Fig. 2c, are generally smaller than the nonshared environmental components. An interesting result is the hint that the effects of early shared rearing environment on cholesterol, systolic blood pressure, and vital capacity may be more manifest in the older cohort than in the younger one.
In addition to the fluctuation of genetic influence over the lifespan, there is growing evidence of shorter-term temporal variability. In rats, for example, the liver mRNA concentration of aldolase B, an enzyme of the glycolytic system, declines during fasting, and increases four-to eightfold upon re-feeding of a carbohydrate-rich diet (Munnich et al. 1985). Investigating the mechanisms involved revealed that regulation of the expression of the relevant gene is accomplished very differently in liver, kidney, and small intestine.
Another example of environmentally mediated alteration of gene expression is provided by the work of McMahon and colleagues (1992), who studied the effects of immobilization stress in rats. Among other results, it was found that a single stress resulted in elevation of levels of tyrosine hydroxylase mRNAs in the adrenal glands.
Thus, the responsiveness of the genetic system t2mo the environmental stimulus can occur quickly. From the point of view of behavioral science, an especially pertinent observation is that the expression of a gene in a particular brain region can be classically conditioned (Smith et al. 1992).
7. Summary
The studies reviewed here are but a few, selected, examples from a vast literature pertaining to an enormously broad array of phenotypes observed on species representing the phyletic range from prokaryotes to eukaryotes. Obviously, a brief review such as this can provide only the sketchiest outline. But, hopefully, it provides a hint as to the possibilities of dynamic interaction of genes with genes and of genes with environment and of the changes of this whole complex system over time. It may also serve as a preventive against simplistic interpretations of the old nature vs. nurture variety in issues of lifespan development. The current data are impressive; the anticipated flood of new data from new methodologies will provide us both with sharpened reductionist understanding, and, in the context of the quantitative model, an integrated view appropriate to the complex systems with which the developmental sciences are concerned.
Bibliography:
- Cheverud J M, Routman E J, Duarte F A M, van Swinderen B, Cothran K, Perel C 1996 Quantitative trait loci for murine growth. Genetics 142: 1305–19
- Claverie J-M 1999 Computational methods for the identification of differential and coordinated gene expression. Human Molecular Genetics 8: 1821–32
- Coleman D L, Hummel K P 1975 Influence of genetic background on the expression of mutations at the diabetes locus in the mouse. II. Studies on background modifiers. Israeli Journal of Medical Science 11: 708–13
- Falconer D S, Mackay T F C 1996 Introduction to Quantitative Genetics, 4th edn. Longman, London
- Ferrari S L, Rizzoli R, Slosman D O, Bonjour J P 1998 Do dietary calcium and age explain the controversy surrounding the relationship between bone mineral density and vitamin D receptor gene polymorphisms? Journal of Bone and Mineral Research 13: 3363–70
- Fijneman R J, de Vries S S, Jansen R C, Demant P 1996 Complex interactions of new quantitative trait loci, Sluc1, Sluc2, Sluc3, and Sluc4, that influence the susceptibility to lung cancer in the mouse. Nature Genetics 14: 465–7
- Finch C E, Kirkwood T B 2000 Chance, Development, and Aging. Oxford University Press, New York
- Fisher R A 1918 The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh 52: 399–433
- Fosmire G J, Focht S J, McClearn G E 1993 Genetic influences on tissue deposition of aluminum in mice. Biological Trace Element Research 37: 115–21
- Frankel W N, Valenzuela A, Lutz C M, Johnson E W, Dietrich W F, Coffin J M 1995 New seizure frequency QTL and the complex genetics of epilepsy in EL mice. Mammalian Genome 6: 830–8
- Galli J, Fakhrai-Rad H, Kamel A, Marcus C, Norgren S, Luthman H 1999 Pathophysiological and genetic characterization of the major diabetes locus in GK rats. Diabetes 48: 2463–70
- Hartl D L, Clark A G 1997 Principles of Population Genetics, 3rd edn. Sinauer, Sunderland, MA
- Heller D A, de Faire U, Pedersen N L, Dahlen G, McClearn G E 1993 Reduced importance of genetic influences for serum lipids in the elderly: A study of twins reared apart. New England Journal of Medicine 328: 1150–6
- Hong Y, de Faire U, Heller D A, McClearn G E, Pedersen N 1994 Genetic and environmental influences on blood pressure in elderly twins. Hypertension 24: 663–70
- Kacser H, Burns J A 1979 Molecular democracy: Who shares the controls? Biochemical Society Transactions 7: 1149–60
- Kamboh M I, Sanghera D K, Ferrell R E, DeKosky S T 1995 APOE*4-associated Alzheimer’s disease risk is modified by α1-antichymotrypsin polymorphism. Nature Genetics 10: 486–8
- Lee C-K, Klopp R G, Weindruch R, Prolla T A 1999 Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–3
- Lockhart D J, Barlow C 2001 Expressing what’s on your mind: DNA arrays and the brain. Nature Reviews Neuroscience 2: 63–8
- McClearn G E, Hofer S M 1999 Genes as gerontological variables: Uses of genetically heterogeneous stocks. Neurobiology of Aging 20: 147–56
- McClearn G E, Svartengren M, Pedersen N L, Heller D A, Plomin R 1994 Genetic and environmental influences on pulmonary function in aging Swedish twins. Journal of Gerontology: Medical Sciences 49: M264–8
- McMahon A, Kvetnansky R, Fukuhara K, Weise V K, Kopin I J, Sabban E L 1992 Regulation of tyrosine hydroxylase and dopamine β-hydroxylase mRNA levels in rat adrenals by a single and repeated immobilization stress. Journal of Neurochemistry 58: 2124–30
- Molenaar P C M, Boomsma D I, Dolan C V 1993 A third source of developmental diff Behavior Genetics 23: 519–24
- Munnich A, Besmond C, Darquy S, Reach G, Vaulont S, Dreyfus J O C, Kahn A 1985 Dietary and hormonal regulation of aldolase B gene expression. Journal of Clinical Investigation 74: 1045–52
- Rapp J P, Garrett M R, Deng A Y 1998 Construction of a double congenic strain to prove an epistatic interaction on blood pressure between rat chromosomes 2 and 10. Journal of Clinical Investigation 101: 1591–5
- Rogina B, Helfand S L 1995 Regulation of gene expression is linked to life span in adult Drosophila. Genetics 14: 1043–8
- Sattler R 1986 Biophilosophy: Analytic and Holistic Perspectives. Springer-Verlag, Berlin
- Smith M A, Banerjee S, Gold P W, Glowa J 1992 Induction of c-fos mRNA in rat brain by conditioned and unconditioned stressors. Brain Research 578: 135–41
- Vieira C, Pasyukova E G, Zeng Z-B, Hackett J B, Lyman R F, Mackay T F C 2000 Genotype-environment interaction for quantitative trait loci affecting life span in Drosophila melanogaster. Genetics 154: 213–27
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L 2000 Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology 54: 1949–54




