Sample Genes And Behavior Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Explaining behavior in genetic terms is no small task. Behavior draws on the activities of a wide range of genes acting at many different times in an individual’s life. Model organisms have provided insight into the role of genes in behavior that have major implications for humans. Many of the mutations and genetic variants (alleles) that affect behavior in Drosophila and mice have proved to be subtle changes in genes that, when more severely mutated, produce wider ranging effects. Studies of mutants induced in the laboratory have identified a variety of genes that affect behavior: genes that regulate other genes (transcription factors), regulatory enzymes (protein kinases), and signaling molecules in the brain (neuropeptides and receptors). Their study has made major contributions to our understanding of biological rhythms, learning, memory, and social behavior, and has helped explain newer findings on naturally occurring variants in behavior.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Introduction
Unitary explanations for human behavior have a dismal history in the modern world. Whether economic, cultural, psychological, or genetic, attempts to reduce human behavior to a single influence inevitably fail to give a full account and have often produced distortions with unfortunate political consequences. Charting the relationship between genes and behavior can therefore be a perilous activity. The challenges are many: the difficulty of defining genetic and nongenetic factors, the difficulty of understanding the interactions among these many factors, the many steps that intervene between the expression of a gene and the manifestation of a behavior, and the inescapable fact that each individual is the unique product of a series of historical accidents. Historical uniqueness also applies to nonhuman biological individuals. It is a hallmark of the biological world due to the fact that no particular combination of genes and experiences is ever replicated exactly in nature.
Studies of genes and behavior in model organisms offer one way out of this conundrum. To begin with, it is far easier to control environmental conditions and genetic heterogeneity in the laboratory, and such studies are generally undertaken keeping environmental conditions as uniform as possible and genetic makeup as well defined as possible. But this would be no help if the phenomena and principles are fundamentally different from those of humans. Fortunately, they appear to be similar by an ever-increasing number of criteria. One of the most striking outcomes of modern biology and the sequencing of genomes is the similarity of genes between distantly related creatures. The fruit fly Drosophila melanogaster and humans share a great many of the same genes in common. Similarly, they share considerable conservation of metabolic and signaling pathways at the cellular level. Most strikingly, and most relevant for this discussion, there is growing evidence for conservation at the level of behavior and its molecular mechanisms. This has been shown most convincingly, thus far, for several behaviors in Drosophila: circadian rhythms (Young 1998), learning and memory (Dubnau and Tully 1998), and most recently sleep (Greenspan and Ferveur 2000). Thus, there is good reason to believe that the characteristics, interactions, and contributions of genes in the behavior of model organisms will provide important guidelines for how to think about the role of genes in human behavior.
Traditionally, there have been two separate, and often hostile, approaches to behavior genetics in model organisms: the measurement and manipulation of naturally occurring variation in laboratory or wild caught strains, and the isolation and manipulation of newly induced mutations. The former assayed the extent of relatively mild mutational variation that survives in the world and exerts effects on behavior, without being able to know the identity of any of the genes or their mechanism of action. The latter concentrated exclusively on identifying genes and mechanisms subserving particular behaviors, but did so by producing relatively drastic genetic lesions that did not reflect the permissable variation that would have been worked on by natural selection. Both have been relevant to humans, but a synthesis between the two, made possible by recent advances in molecular biology, holds the most promise for useful insights. In this research paper, a few salient examples of gene effects on behavior are described as illustrations of general principles that are emerging.
2. Severe Single-Gene Mutants
2.1 Circadian Rhythms
The clearest contribution single-gene mutants have made is in the realm of identifying individual genes that are central to behavioral mechanisms. The unraveling of the cellular mechanism of the circadian clock began with the discovery of the period gene through the isolation of long-day, short-day, and arrhythmic mutants in Drosophila (Fig. 1; Konopka and Benzer 1971) and proceeded with the isolation and cloning of additional mutants in flies, fungi, and mice (Young 1998). What has emerged is a picture of the circadian clock as a feedback loop in which each cell counts out its own 24 hour period by means of an oscillating cycle of gene readout in which some of the products accumulate up to a point, then begin to dampen their own synthesis through a series of time delays between the various stages of gene expression: transcription, translation, and import back into the nucleus (Fig. 1). This serves as the central regulator for specific implementation of rhythmic activities. The cellular mechanism of the circadian clock, as well as the clock’s phenomenology, appears to be universal in the biological world and many of the actual genes involved, such as period, are well conserved between flies and mammals. The human relevance is thus transparent.
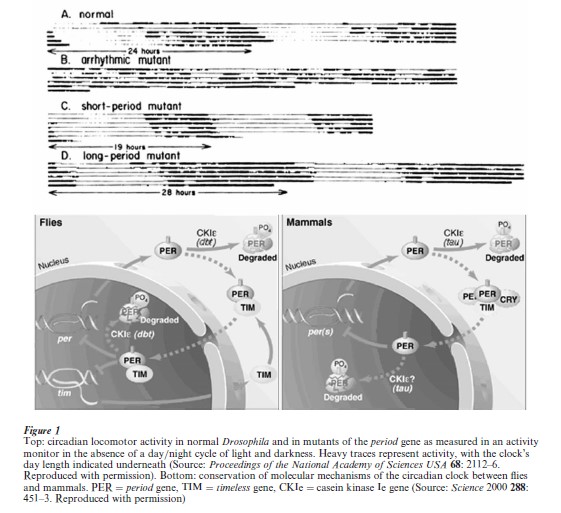
2.2 Learning And Memory
Learning and memory have many features common to flies, rodents, and humans. Behaviorally, all are capable of associative conditioning and the memory that is induced shows multiple phases that can be distinguished by pharmacology and by training regimen (Dubnau and Tully 1998). In the fly, learning takes the form of distinguishing between two odors and avoiding the one that has previously been paired with electric shock. Long-term memory is produced by repeated training trials separated by suitable time intervals. The original Drosophila learning mutant, dunce (Dudai et al. 1976), is defective in initial acquisition as well as in subsequent retention of the learned behavior (Fig. 2). Care was taken to ascertain that these mutant flies were normal in all other relevant faculties that are required for the learning task: olfaction, locomotor activity, and shock reactivity.
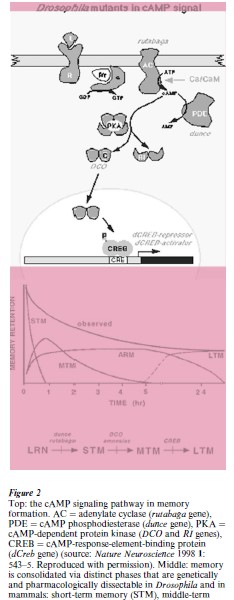
The product of the dunce gene was subsequently identified as an enzyme that is part of the signaling cascade in nerve cells that utilizes the intracellular messenger molecule cyclic AMP (Fig. 2). The gene encodes a cAMP-phosphodiesterase, the enzyme that degrades cyclic AMP. Mutations in other components of this signaling system, rutabaga in the synthetic enzyme (adenylate cyclase), and Dco and RI in the key enzyme regulated by cyclic AMP (cAMP-dependent protein kinase), were subsequently shown to also affect learning and memory in the fly (Dubnau and Tully 1998). The cyclic AMP signaling system had already been implicated in neuronal plasticity as central to the mechanism that modifies efficacy at synapses in the circuitry underlying learning. The isolation of mutants affecting the system from unbiased, open-ended mutant screens and the demonstration that they affected classical conditioning in the intact animal gave universality to the finding. Mouse mutants in the synthetic enzyme (adenylate cyclase) show analogous deficits in learning (Silva et al. 1998). The relevance to humans has been borne out by the fact that there are currently several memory enhancement drugs in clinical trial that target cAMP phosphodiesterase.
As additional mutants accumulated, comparison of the time course of memory decay revealed some interesting differences between them (Fig. 2), suggesting that they differentially affected memory retention. This suggestion received major support from studies of another component of the cAMP signaling system: the Creb (cAMP-response-element-binding) protein that regulates genes induced by cAMP. Creb exists in both repressor and activator forms. A repressor form of the gene, when engineered to be expressed in the fly just prior to training, completely blocks the formation of long-term memory while permitting normal shortand medium-term memory (Dubnau and Tully 1998). In contrast, an activator form of the gene, when engineered to be expressed just prior to training, produces long-term memory after a single trial, in contrast to the usual 10 trials with intervals that are required to induce long-term memory (Fig. 2). These findings not only placed the differential genetic effects on memory phases on firmer ground, they were also subsequently extrapolated to mice and rats (Silva et al. 1998). A Creb mutant in the mouse that reduces, but does not eliminate, repressor activity fails to form long-term memory in a fear-avoidance paradigm. Analogous results have been obtained in the rat after injection of a Creb gene blocker (anti-sense RNA) to specifically reduce levels of the protein.
A variety of other mutants disrupting learning that also affect synaptic efficacy have also been obtained in the fly and mouse, including those affecting other regulatory enzymes (calcium/calmodulin-dependent protein kinase, fyn kinase) and receptors (NMDA and acetylcholine). The studies of the cAMP cascade stand out, however, for the depth to which they have taken the analysis through their insight into the mechanism of differential memory phases.
memory (MTM), anesthesia-resistant memory (ARM), and long-term memory (LTM) appear sequentially, and each has a progressively slower rate of decay (source: Trends Neuroscience 1995 18: 212–7). Bottom: a genetic model of information flow showing where in the pathway single gene mutant and pharmacological disruptions have their primary effects (source: Cell 1994 79: 35–47. Reproduced with permission)
2.3 Social Behavior
Genetic studies of social behavior have great relevance for humans and for considerations of human evolution. A productive avenue into this question has been taken in rodents, based on neuroendocrinological studies of the behavioral effects of oxytocin and vasopressin. Administration of these hormones produce complementary effects on a variety of behaviors including affiliative, sexual, maternal, territorial, and aggressive behavior as well as on social memory (Young et al. 1998). The detailed neural mechanisms for the hormones’ actions have yet to be elaborated. A mouse mutant that lacks oxytocin altogether (Oxt−/−) shows a distinct deficit in social memory as reflected by male olfactory investigation of a female in repeated exposures. The effect is more selective than predicted from the range of effects when oxytocin is administered. Moreover, Oxt−/− mutant mice are normal for olfactory responses to other, nonsocial odors, and normal for learning tasks unrelated to this social behavior.
An increase in aggression between males was also observed in the oxytocin-deficient mice. This observation falls into line with a growing number of other mouse mutant studies reporting increases in aggression. These range from mutants in receptors for serotonin or adenosine, to synthetic enzymes for the neurotransmitters GABA or nitric oxide, to regulatory enzymes (calcium/calmodulin-dependent protein kinase II) to cell adhesion molecules. In contrast to mutants of circadian rhythms or of learning and memory, there seem to be a great many genetic lesions that produce aggression in mice. When one encounters such promiscuity in genetics, it is usually diagnostic of a system that is subject to many nonspecific kinds of disruption, with all of the caveats to interpretation that accompany effects on such a system, as opposed to a sensitive indicator of key mechanistic components.
2.4 Range Of Action Of Behavioral Genes
The foregoing examples highlight the mechanistic insights that have been gained from behavioral studies of laboratory-induced mutants. An important criterion for these studies was the relative refractoriness of the behavior to mutational disruption: mutations in most genes do not affect these behaviors. The question then arises of the relative specificity of these genes to the behaviors they disrupt. Are these ‘genes for’ behaviors such as rhythms, memory, or social life, as have often been alluded to in evolutionary discussions of behavior (e.g., Wilson 1978)? For the mutants described above, as well as for the vast majority of behavioral mutants studied in both flies and mice, the answer appears to be negative. By and large, the genes that produce behavioral mutants are not limited to affecting only one behavior or only one aspect of the animal. They exert influences over many facets of the organism’s biology (the genetic term for this is ‘pleiotropy’).
The archetypal circadian rhythms gene, period, regulates the song produced by a male fly during courtship as well as its 24-hour rhythms (Greenspan and Ferveur 2000). The male’s song, produced by vibrating the wing, consists of a series of ‘pulses’ which sound like bumps occurring at the rate of about 30 per second. The rhythm lies in the slight but regular variation in the interval between pulses (Fig. 3) as the male gradually increases then decreases this interval to produce a smoothly wavering song over a period of approximately 60 seconds. Female flies respond to the male’s song by becoming more receptive to their courtship advances. Mutants in the period gene that affect the circadian rhythm have corresponding effects on song rhythm such that long-day mutants have an 80-second rhythm, short-day mutants have a 40-second rhythm, and arrhythmic mutants have no regular rhythm (Fig. 3).
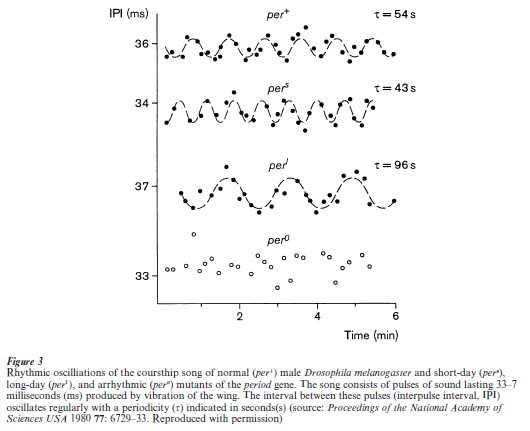
The period mutant also affects timing on scale greater than the 24-hour period: short-day and long- day mutants have a shorter and longer developmental time course, respectively, from egg to adult compared to normal controls. These effects of period occur on timescales an order of magnitude larger (10 days) or several orders of magnitude smaller (60 seconds) than the 24-hour clock. Distant as they are from the circadian timescale, they are timing mechanisms nonetheless and therefore not outside of the realm already affected by the mutants. It remains a mystery, however, as to how the relatively slow mechanism of feedback onto gene expression (Fig. 1) can also regulate a 60-second rhythm. One clue comes from a more divergent consequence of per and several other rhythm mutants that was revealed in pharmacological studies of cocaine responsiveness, in which they fail to become sensitized. There is no circadian or timing- dependent aspect to this phenomenon. Instead, it appears to involve a different use of their function as gene regulators: induction of the enzyme responsible for sensitization fails to occur in the mutants.
Mutants affecting learning and memory are no less wide-ranging. The dnc mutation produces sterility in female flies, defects in embyronic development, and shorter lifespan. The most severe Creb mutants are lethal in both flies and mice. Finally, Oxt−/− female mice are defective in lactation, as well as in infant vocalization.
The conclusion that genes producing behavioral mutants are wide-ranging in their effects (pleiotropic) is based on a large enough sampling of mutants from two different organisms to consider it likely to be the rule rather than the exception (Hall 1994, Pflugfelder 1998, Mouse Knockout & Mutation Database, http://research.bmn.com/mkmd/). It would be a mistake, therefore, to ignore it in analyses of how genes affect behavior, or in considerations of what kinds of behavioral variation are evolutionarily permissable. A sobering conclusion that emerges from this recognition is that the path from gene to behavior is extraordinarily complex, winding its way through all possible levels of biological organization, from gene regulation through cellular biochemistry, through communication between cells, through large-scale patterns of activity in the nervous system, finally emerging as the manifest behavioral output itself.
3. Mild Natural Variants
Natural variation in behavior exists between and within species, and provides a major substrate for natural selection. Divergence in behavior between species is thought to be a critical component of the speciation process itself, whereas behavioral variants within a species provide it with the flexibility needed to weather the unpredictability of external conditions. Until recently, the identity and mechanistic nature of these behavioral variants has been a black box. Analyses were confined to statistical treatments of overall behavioral variation (e.g., Ehrman and Parsons 1981). A growing list of these variants has now been characterized molecularly and functionally. As foreshadowed by the experience with laboratory mutants, many have proved to be subtle variations of genes that are capable of more severe and wider ranging effects.
3.1 Differences Between Species: Biological Rhythms
The courtship song produced by Drosophila males is species-specific and plays a role in species recognition for mating (Greenspan and Ferveur 2000). The courtship song of Drosophila melanogaster differs from that of the closely related species Drosophila simulans in several parameters, the most important of which is its rhythmic oscillation (see Sect. 2.4). The D. melanogaster oscillation has a 60-second period, whereas the D. simulans oscillation has a 35-second period. This rhythmic difference is due to a subtle difference in the sequence of the period gene between D. simulans and D. melanogaster: a short segment of the protein coding region that contains repeats of the amino acid pair threonine and glycine (Fig. 3). A D. simulans period gene transferred into D. melanogaster that lack a period gene confers a characteristic D. simulans song rhythm (Wheeler et al. 1991). More specifically, a synthetic gene consisting of the entire D. melanogaster period gene, except for a segment of period from D. simulans consisting of a small region that is highly variable among many Drosophila species, also gave the same characteristic D. simulans song (Fig. 3). The remarkable feature of this finding is that one portion of the sequence was capable of diverging in a species- specific manner, while the rest of the gene stayed constant enough to retain the normal 24 hour circadian rhythmicity.
3.2 Differences Between Species: Social Behavior
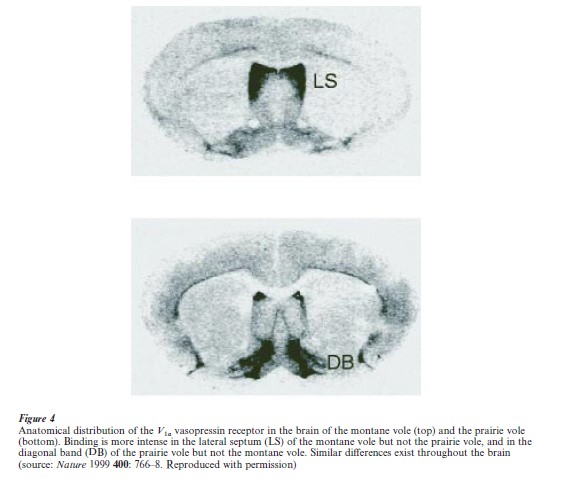
Differences in social behavior are marked in the animal kingdom even between closely related species. Two species of North American vole illustrate this point. The prairie vole (Microtus ochrogaster) is monogamous, biparental, and highly social. They contrast with the montane vole (Microtus montanus), which is promiscuous, maternal, and minimally social (Young et al. 1998). The hormones oxytocin and vasopressin exert complementary effects on these behaviors in the prairie vole, similar to their effects in rats and mice (see Sect. 2.3). Moreover, the anatomical distribution of oxytocin and vasopressin receptors in the brain differs markedly between the two vole species (Young et al. 1999).
Vasopressin administration stimulates affiliative behavior in prairie voles but not in montane voles, nor in mice. When a prairie vole vasopressin receptor gene (V1a) was transferred into mice, thereby creating an anatomical distribution similar to that of the prairie vole, the mice began to exhibit affiliative behavior in response to vasopressin (Fig. 4). This result argues that changing the pattern of V1a distribution is sufficient to alter this behavior. This interpretation is further supported by the fact that the V1a receptor genes are virtually identical between the two vole species that differ in affiliative behavior, except for the presence in prairie voles of a small segment in the region of the gene that is likely to regulate its expression. The presence of this DNA segment correlates well with behavioral and anatomical features of two other species, the pine vole (Microtus pinetorum), which is like the prairie vole behaviorally as well as in its anatomical and molecular characteristics of V1a, and the meadow vole (Mictotus pennsyl anicus), which is like the montane vole in all of these respects. Thus, it appears that subtle evolutionary tinkering with the expression pattern of the vasopressin receptor V1a may account for some of the differences in social behavior between these rodent species.
3.3 Differences Within Species: Circadian Rhythms
If the genetic differences underlying behavioral variation between species are subtle, then those underlying variation within species are downright esoteric. The segment of the period gene that was sufficient to transfer a D. simulans courtship song rhythm into D. melanogaster also varies within the melanogaster species. Two versions (alleles) of the gene predominate: one containing 17 repeats of the pair of amino acids threonine and glycine (Thr-Gly) , and one containing 20 repeats (Thr-Gly) (Costa and Kyriacou 1998). These two alleles are nonrandomly distributed in the wild, following a north–south gradient (known as a ‘latitudinal cline’) from northern Europe down to North Africa with (Thr-Gly) predominating in the north and (Thr-Gly) predominating in the south (Fig. 5). Distributions of this sort are traditionally explained as an adaptive response to climatic variation.
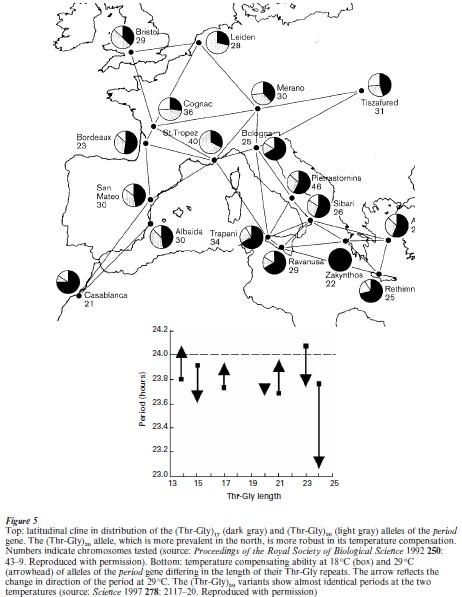
If these variants are undergoing selection, then the functional differences between them become relevant. A clue to such a functional difference was seen in tests of temperature compensation, one of the distinctive features of circadian rhythms which allow coldblooded organisms to maintain their 24 hour cycle at different temperatures. Flies carrying the northerly (Thr-Gly) allele exhibit more robust temperature compensation than those with the more southerly (Thr-Gly) allele (Fig. 5). The temperature range is much greater in the north and presumably imposes greater demands on the temperature-compensating ability of the clock. The size of the effect is small, perhaps reflecting a tradeoff between the temperature compensation and conservation of the overall 24 hour rhythm.
3.4 Differences Within Species: Foraging Behavior
Most natural variation in behavior is not primarily attributable to any single gene. Where multiple genes contribute, the effect often disappears when they are separated from each other in the crosses required for genetic mapping. An exception to this trend is the foraging gene that affects food search behavior by Drosophila larvae.
Natural populations of D. melanogaster harbor two larval types: rovers, who search widely for food, and sitters, who do not (Sokolowski 1998). The difference is not merely locomotor because it is expressed only in the presence of food. Genetic mapping revealed it to be due primarily to a single gene, foraging, and molecular analysis showed it to be an enzyme involved in regulating many physiological processes, cGMP-dependent protein kinase (Fig. 6). Severe mutations of the foraging gene are lethal. The detailed mechanism through which the enzyme alters neuronal and behavioral activity is not known, but it appears to regulate neuronal thresholds.
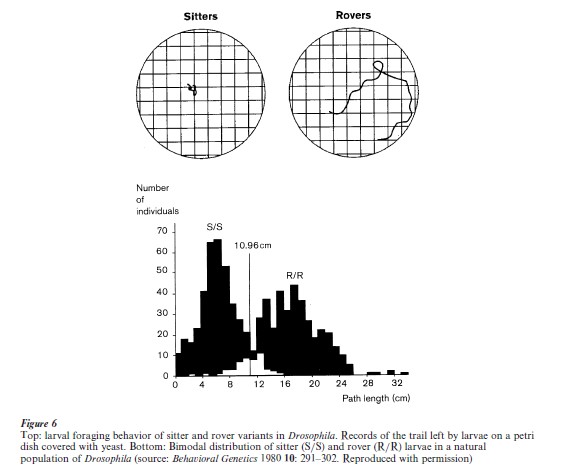
A gene whose product has as wide-ranging functions as the enzyme encoded by foraging must have severe constraints on the kinds of variation that are viable and permissable. The explanation, in this case, appears to be that the magnitude of the natural enzyme variation is relatively small: rovers have 12 percent more activity of the enzyme cGMP-dependent protein kinase than sitters and the levels of its protein and mRNA are correspondingly higher in rovers vs. sitters (Osborne et al. 1997). This small increase thus appears to be sufficient to make a very distinctive difference in behavior, while not seriously impairing viability or obligate functions of the gene.
3.5 Genetic Basis For Selected Behaviors
Artificial selection in the laboratory has been the conventional method for detecting the presence of naturally occurring genetic variation in behavior (Ehrman and Parsons 1981). While such variation has been clearly demonstrable in virtually every behavioral selection attempted, from maze learning in rats to gravity response in Drosophila, there has been no way to identify any of the relevant genes. The behavioral differences are generally due to the contribution of many genes, usually of rather small effect, and thus refractory to conventional mapping techniques. Only their approximate number and putative interactions could be estimated from statistical analyses of selected lines and of F1, F2, and backcross progeny. The advent of QTL mapping (quantitative trait loci) using a widely distributed set of DNA markers has improved the prospects for mapping of such polygenic traits, but significant difficulties still attend the identification of the contributing gene itself.
The identification of natural variants in the foraging locus created the possibility of testing directly its response to selection under conditions likely to be relevant to its role in the wild: differences in population
density. In two different experiments, strains of flies mixed for rovers and sitters were cultured at high or low larval density for over 250 generations. In both cases, the resulting high-density strains had become predominantly rover in behavior and the low-density strains predominantly sitter (Sokowloski 1998). That is, selection had eliminated the vast majority of one foraging allele from each population.
These findings contrast with those on intraspecies variants of the period gene, where the possibility of selection between alleles was inferred from their geographical distribution and made more plausible by the subtle functional differences between alleles. The conditions necessary to impose selection on the foraging locus are easier to imagine and implement, and the outcome leads to the conclusion that being genetically rover is advantageous under crowded conditions, whereas being genetically sitter is advantageous under conditions of low density.
The most common sort of selection experiments have also been the most refractory to analysis. The ability to analyze the genetic basis of the strains whose behavior results from selection for variation in many genes is likely to improve with the advent of genome-wide capabilities to measure differences in gene activity (White et al. 1999). This will likely make it feasible to identify genetic differences between selected strains or natural variants based on detecting asymmetries in the activity patterns of specific genes.
4. Implications For Humans
The foregoing discussion highlights explicit and implicit aspects of the relevance to humans of gene/behavior studies in model organisms. Explicit is the conservation of function (e.g., period for circadian rhythms, Creb for long-term memory), as well as the wide-ranging nature of the genes involved. Implicit is the importance of specific context in any given gene’s effect on behavior and the humility with which the question of genetic influences on behavior must be approached.
4.1 Promiscuity Of Behavioral Genes
The breadth of action of the genes influencing behavior has significant implications for speculation about human behavior and its evolutionary origins. The extent to which a gene can vary is constrained by how widely its effects will radiate through the organism. For genes with wide-ranging (pleiotropic) function, this means that variants with severe effects on the gene’s function are likely to have deleterious consequences. In this light, evolution looks like a balancing act, selecting for genetic variants that do the most to help the organism without doing too much harm. Sometimes this may take the form of a highly restricted alteration in a gene (e.g., anatomical distribution of V a in prairie vole vs. montane vole) and other times as a tradeoff between utility and loss of fitness (e.g., adult sluggishness in sitter). An important implication of this point is that some traits will be carried along for no reason other than their connection to some other effect of the same gene. A further corollary is that not all existing traits have been selected for themselves, and thus cannot be automatically assumed to have adaptive value.
4.2 Context
The nature/nurture dichotomy is the traditional way to describe the putative role of context in affecting gene action, but it only conveys one axis of the problem. Context is as much genetic, consisting of the particular constellation of alleles that an individual possesses, as it is nongenetic, consisting of the myriad environmental factors, both physical and social, that an individual experiences in a lifetime. Interactions between the two further complicate the matter. This means that different alleles of a given gene will not have the same effect in all contexts. A clear example of this was described earlier in the differential ability of two naturally occurring period alleles to compensate for temperature fluctuations (see Sect. 3.3). The (Thr- Gly)17 allele makes the individual more sensitive to its external environment than (Thr-Gly)20. This has important implications for analysis of the genetic contributions to particular behaviors (e.g., heritability).
The effect of an allelic variant on a behavior is also heavily dependent on the genetic context. This is known throughout the genetics literature as the sensitivity of a mutation to ‘genetic background.’ Genetic background is simply another way of describing the particular constellation of alleles at other loci present in the individual. This is exemplified in the interaction between alleles of the foraging locus and those of the Chaser locus, a gene identified based on the ability of certain of its alleles to convert sitters into rovers. In other words, mild alleles of the sort that occur naturally are even more susceptible to variations in genetic context than severe alleles.
4.3 Testability
Human genetics is primarily a correlative science. This applies as much to behavioral genetics as to any other variety. Hypotheses can be tested against observational data, but actual manipulations or ‘test crosses’ cannot, of course, be carried out. One of the principal virtues of model organisms, therefore, is experimental testability of gene function and interaction. Given the increasing evidence for conservation of genetic function between species as distant as Drosophila and mammals, they represent an important contribution to understanding and interpreting possible genetic influences on human behavior. One of these contributions should be a sense of humility before the problem. As the foregoing discussion elaborates, the road from gene to behavior is neither simple nor direct, and is difficult enough where experimental testing is possible. The difficulties are multiplied for natural variants because of the subtlety of their effects and their consequent elusive nature.
The universal importance of context in the action of genes on behavior presents further obstacles to a simple formulation. If combinations of alleles will give different effects due to interactions with each other and with environmental variables, then the problem takes on the aspect of an infinitely sliding scale. Whenever one variable is changed, the relative effect of many of the others may also change. This malleability is no doubt a source of robustness in biological systems, as well as a source for evolutionary change. Understanding it remains one of the central questions in the behavioral sciences.
Bibliography:
- Costa R, Kyriacou C P 1998 Functional and evolutionary implications of natural variation in clock genes. Current Opinions in Neurobiology 8: 659–64
- Dubnau J, Tully T 1998 Gene discovery in Drosophila: new insights for learning and memory. Annual Reviews in Neuroscience 21: 407–44
- Dudai Y, Jan Y N, Byers D, Quinn W G, Benzer S 1976 dunce, a mutant of Drosophila deficient in learning. Proceedings of the National Academy of Sciences USA 73: 1684–8
- Ehrman L, Parsons P A 1981 Behavior Genetics and Evolution. McGraw-Hill Inc., New York
- Greenspan R J, Ferveur J-F 2000 Courtship in Drosophila. Annual Reviews in Genetics 34: 205–32
- Greenspan R J, Tononi G, Cirelli C, Shaw P J 2001 Sleep and the fruit fly. Trends Neuroscience 24: 142–5
- Hall J C 1994 Pleiotropy of behavioral genes. In: Greenspan R J, Kyriacou C P (eds.) Flexibility and Constraint in Behavioral Systems. Wiley, New York
- Konopka R J, Benzer S 1971 Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences USA 68: 2112–6
- Osborne K A, Robichon A, Burgess E, Butland S, Shaw R A, Coulthard A, Pererira S, Greenspan R J, Sokolowski M B 1997 Natural behavior polymorphism due to a cGMPdependent protein kinase of Drosophila. Science 277: 834–36
- Pflugfelder G O 1998 Genetic lesions in Drosophila behavioural mutants. Behavioral Brain Research 95: 3–15
- Silva A J, Kogan J H, Frankland P W, Kida S 1998 CREB and memory. Annual Reviews in Neuroscience 21: 127–48
- Sokolowski M 1998 Genes for normal behavioral variation: recent clues from flies and worms. Neuron 21: 463–6
- Wheeler D A, Kyriacou C P, Greenacre M L, Yu Q, Rutila J E, Rosbash M, Hall J C 1991 Molecular transfer of a speciesspecific behavior from Drosophila simulans to Drosophila melanogaster. Science 251: 1082–5
- White K P, Rifkin S A, Hurban P, Hogness D S 1999 Microarray analysis of Drosophila development during metamorphosis. Science 286: 2179–84
- Wilson E O 1978 On Human Nature. Harvard University Press, Cambridge, MA
- Young L J, Nilsen R, Waymire K G, MacGregor G R, Insel T R 1999 Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400: 766–8
- Young L J, Wang Z, Insel T R 1998 Neuroendocrine bases of monogamy. Trends Neuroscience 21: 71–5
- Young M W 1998 The molecular control of circadian behavioral rhythms and their entrainment in Drosophila. Annual Reviews in Biochemistry 67: 135–52




