View sample memory systems research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Early Views on Multiple Memory Systems
The notion that there is more than one kind of memory is an old one, richly woven into the history of theorizing and research in philosophy, psychology, and neuroscience. In 1804 the French philosopher Maine de Biran proposed what may be the first formal theory of multiple memory systems. He viewed all cognition and memory as based on a fundamental mechanism of habit, a concept similar to the current term association. In his proposal, habits were simple and automatic mechanisms, but they had a broad applicability. Habits were viewed as mediating acquired behaviors that operate independently of conscious control and conscious recollection. In addition, the habit mechanism was also viewed as the basis for more complex, consciously mediated aspects of memory. Main de Biran elaborated his scheme into three distinct forms of memory, each based on the fundamental habit mechanism but also distinct in its contents and properties. One form was called representative memory, characterized as expressed in the conscious recollection of a “well-circumscribed idea.” The second, designated mechanical memory, refers to situations in which the habit mechanism doesnotgeneratearecalledidea,butinsteadonlyafacilitation of the repetition of a movement. Finally, sensitive memory refers to when the habit mechanism generates a feeling—or fantastic, albeit vague or obscure image—without recalling the ideas behind it. Thus, mechanical memory was seen as expressing habits in the form of coordinated actions, and sensitive memory as a habit expressed in the form of an affective component. These two kinds of memory had in common that they could operate without conscious recall and could be the source of the most inflexible and obstinate behaviors.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Maine de Biran developed his formulation without experiments or consideration of the anatomy or functions of brain systems. And there is no record that Maine de Biran’s theory had significant influence over successive developments in memory research. Yet, as it turns out, he was prescient in describing a division of memory systems that is—as you will see—strongly supported by modern cognitive neuroscience.
There has been much progress—and many detours—in both psychological and biological studies on the brain and memory systems before Maine De Biran’s scheme was rediscovered. The history of this area has largely preserved the notion of an elemental habit mechanism bolstered by the early discoveries about the existence of reflexes and their conditionability, and many theories have preserved a distinction between simple habits and conscious memory, albeit sometimes in the form of debates in which habits and recollection were polarized as alternatives.
A century after Maine de Biran, the notion of habit as a fundamental mechanism and memory as a more complex phenomenon associated with consciousness was widely held. William James (1890/1918) wrote of them in separate chapters in his treatise Principles of Psychology. James considered habit a very primitive mechanism that is common among biological systems and due to plasticity of the organic materials. Within the nervous system, habits were viewed as nothing more than the ready discharge of a wellworn reflex path. But James also attributed to habit great importance in the development of more complicated behavioral repertoires. He suggested that well-practiced behaviors and skills—including walking, writing, fencing, and singing—are mediated by concatenated reflex paths, organized to generate the serial production of movements and unconscious sensations leading to other movements and sensations. He thought of habits as eliminating the need for conscious supervision after a behavior becomes routine; moreover, he recommended early and frequent reinforcement of good habits as a key exercise in ethical and cognitive development.
James distinguished memory as something altogether different from habit, albeit based on that mechanism, a very complicated phenomenon with many facets. James is perhaps best known for having originated the distinction between primary memory and secondary memory. Primary memory is what we today call short-term or working memory. It is a short-lived state in which new information has achieved consciousness and belongs to our stream of thought. James viewed primary memory as the gateway by which material would enter secondary memory or what we now call longterm memory. James defined secondary memory as “the knowledge of an event, or fact, of which meantime we have not been thinking, with the additional consciousness that we have thought or experienced it before” (p. 648). In addition to its personal and temporal aspects, the full characterization of memory was framed in terms of two other properties—its structure as an elaborate network of associations and its basis in habit mechanisms. Thus, James theorized a mechanistic basis for how habits could be elaborated for the formation of multiple and linked associations to support the richness of memory. Thus, the underlying foundation of recall was a complex yet systematic set of associations between any particular item and anything co-occurring in one’s previous experiences with the item. He argued that when we search for a memory, we navigate through the elaborate network of the associations—and if successful, locate the sought memory among them. The goodness of memory, he believed, was as much dependent on the number of associations in the network as on the strength of those associations.
The Experimental Era: Debates on the Fundamental Basis of Memory
At the outset of experimental approaches to memory, reductionism reigned.The goal was to identifying basic mechanism of habit as an explanation of memory, eliminating the need for allusions to consciousness. This approach was known as behaviorism, and its origins began separately in the United States and Russia (see Eichenbaum & Cohen, 2001, for review). At the turn of the twentieth century, Thorndike had invented his puzzle box, with which he observed cats learning to manipulate a door latch to allow escape from a holding chamber. Around the same time, Small introduced the maze to studies of animal learning, inspired by the famous garden maze at Hampton Court in London. By 1907 Watson had published his accounts on maze learning by rats, and by 1913 he had written his behaviorist manifesto, formalizing it the next year in his systematic exposition—claiming we need never return to terms such as consciousness.
Independently in the early 1900s, Pavlov and Bechterev (physiologists in Russia) had been experimenting on autonomic nervous system reflexes in dogs. Pavlov was studying the physiology of digestion and observed that dogs would secrete saliva not only when given food, but also when presented with an arbitrary stimulus following repeated pairings of the arbitrary stimulus and food delivery. He called this phenomenon the conditioned reflex. Bechterev studied the respiratory motor reflex by which cold applied to the skin produces a reflexive catching of the breath, and he discovered that an arbitrary stimulus applied repeatedly at the same time as the cold would eventually set off the same reflex by itself. The neurology of the conditioned reflex—especially as elaborated by Sherrington—gave biological validity to what behaviorists saw as the elemental mechanism of learned behavior.
There were debates about the distinctions between the fundamental association in Pavlovian conditioning versus that in Thorndike’s instrumental learning—specifically, whether the critical association was between the stimulus and the response or the stimulus and the reinforcer. Despite this difference, the two viewpoints came to be referred to collectively as stimulus-response or S-R learning, and we should consider them as offering a physiological instantiation of the habit mechanism.To the theorists of this time, having a full accounting of S-R learning would solve the problem of memory.
Yet there were detractors from this prominent theme. Early challenges to behaviorism came from the psychologists such as Yerkes and Kohler, whose observations on great apes led them to conclude that animals did not learn complex problems by a combination of random trial and error and eventual reinforcement of a correct solution; rather, at least the higher animals had insights into relationships between means and ends. Tolman (1932) was perhaps the most successful in challenging behaviorism because he developed operational definitions for mentalistic processes including purposive behavior and expectancy. Tolman’s goal was to get behind the behavior—not by specifying particular elements of habits or their linkage, but by identifying the complex cognitive mechanisms, purposes, expectations, and insights that guided behavior. Tolman’s basic premises were that learning generally involved the acquisition of knowledge about the world—in particular, about relationships between and among stimuli and their consequences—and that this knowledge led to expectancies when the animal was put in testing situations. He argued that learning involved the creation of what he called a cognitive map that organized the relations among stimuli and consequences based on interconnections between groups of stimuli. Moreover, he rigorously tested these ideas using the same species (rats) and mazelearning paradigms that were a major focus of the prominent S-R theorists. In a series of studies he showed that rats were capable of solving maze problems by taking novel detours or shortcuts, and they exhibited a capacity for latent learning, in which they acquired problem solutions in the absence of reinforcement. Collectively, in each of these studies rats showed they were capable of learned behaviors that were not previously reinforced and therefore could not be mediated by S-R representations.
A parallel debate emerged from studies on human verbal memory. On the side of reductionism was Herman Ebbinghaus (1885), who had admired the mathematical analyses that had been brought to the psychophysics of perception and sought to develop similarly precise and quantitative methods for the study of memory. Ebbinghaus had rejected the use of introspection as capable of providing evidence on memory. He developed objective assessments of memory in savings scores that measured retention in terms of the reduction in trials required to relearn material, and he used statistical analyses to test the reliability of his findings. Furthermore, to create learning materials that were both simple and homogeneous in content, Ebbinghaus invented the nonsense syllable, a meaningless letter string composed of two consonants with a vowel between. With this invention he avoided the confounding influences of interest, beauty, and other features that he felt might affect the memorability of real words, and he simultaneously equalized the length and meaningfulness of the items—that is, by minimizing the former and eliminating the latter. Ebbinghaus was and is still hailed as a pioneer of systematic scientific methodology in the study of human verbal memory. His studies and those that followed provided a detailed characterization of the acquisition and retention of arbitrary associations, as well as examined many phenomena of verbal memory.
This approach also had its detractors. Most prominent among these, perhaps, was the British psychologist Fredric Bartlett (1932), whose work stands in stark contrast to the rigorous methods introduced by Ebbinghaus. Bartlett differed in two major ways. First, his interest was in the mental processes used to recover memories—that is, in remembering more so than in learning. He was not so interested in the probability of recall, as dominated Ebbinghaus’s approach, but in what he called “effort after meaning”—the mental processing taken to search out and ultimately reconstruct memories. Second, Bartlett shuddered at the notion of using nonsense syllables as learning materials. By avoiding meaningful items, he argued, the resulting memories would necessarily lack the rich background of knowledge into which new information is stored. Indeed, the subtitle of Bartlett’s book Remembering is indeed A Study in Experimental and Social Psychology, thus highlighting his view that real memory is embedded in the full fabric of a lifetime of experience, prominently including one’s culture.
Barlett’s main strategy was called the method of repeated reproduction. His most famous material was a short folk tale titled “The War of the Ghosts,” which was adapted from the original translation by the explorer Franz Boaz. He selected this story for several reasons: The syntax and prose were derived from a culture quite different from that of his British experimental subjects, the story contents lacked explicit connections between some of the events described, and the tale contained dramatic and supernatural events that would evoke vivid visual imagery on the part of his participants. These qualities were, of course, exactly the sort of thing Ebbinghaus worked so hard to avoid with his nonsense syllables. But Bartlett focused on these features because he was primarily interested in the content and structure of the memory obtained and less interested in the probability of recall of specific items.
Barlett made three general observations on this and other reproductions of the story: First, the story was considerably shortened, mainly by omissions. Second, the syntax became more modern and taken from the participant’s culture. Third, the story became more coherent and consequential. From these observations Bartlett concluded that remembering was not simply a process of recovery or forgetting of items,but that memory seemed to evolve over time. Items were not lost or recovered at random; rather, material that was more foreign to the subject, lacked sequence, or was stated in unfamiliar terms was more likely to be lost or changed substantially in both syntax and meaning, becoming more consistent with the subject’s common experiences.
To account for these observations, Bartlett developed an account of remembering known as schema theory. In his view, the simplest schemas were habit-like traces of items in sequential order of experience. But he elaborated this lowlevel mechanism, arguing that our experience of particular sequences builds up en masse; particular past events are more or less dated or placed in relation to other associated particular events in a dynamic organization from which one can construct or infer both specific contents of memories and their logical order. Bartlett proposed that remembering is therefore a reconstructive process and not one of mere reproduction, as Ebbinghaus preferred and as would guide lowlevel rote memory.
Reconciliation: Multiple Memory Systems
The evidence provided by Tolman, Barlett, and others did not resolve the debate, but in general led to more complex constructs of S-R models. The issue has now, however, been largely resolved by the introduction of cognitive neuroscience, and evidence that both habit-like and recollective memory exist and are mediated by distinct neural systems. I describe here two particularly compelling lines of evidence that support this reconciliation, one from the literature on maze learning in rats and the other a classic study in the field of human neuropsychology.
The debate on learning in animals became focused on the central issue of whether rats acquire maze problems by learning specific turning responses or by developing an expectancy of the place of reward.The issue was addressed using a simple T-maze apparatus wherein response versus place strategies could be directly compared by operational definitions (Figure 19.1). The basic task involves the rat beginning each trial at the base of the T and being rewarded at the end of only one arm (e.g., the one reached by a right turn). The accountings of what was learned in this situation differ strongly by the two theoretical approaches. In this situation, then—according to S-R theory—learning involves acquisition of the reinforced left-turn response. By contrast, according to Tolman’s account, learning involves the acquisition of a cognitive map of the environment and the expectancy that food was to be found at a particular location in the test room. The critical test involved effectively rotating the T by exactly 180° so that the choice arms still ended at the same two loci (albeit which arms reach those loci are now exchanged), and the start point would now be at the opposite end of the room. The S-R theorist would predict that a rat would continue to make the previously reinforced right-turn response at the choice point, leading it to a goal location different from that where the food was provided during training. By contrast, the prediction of Tolman’s account was that the rat would switch to a left-turn response in order to arrive at the expected location of food in the same place in the room where it was originally rewarded.
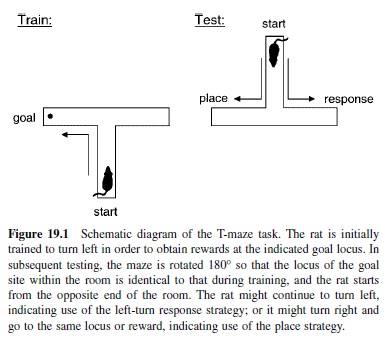
Tolman provided initial evidence in favor of his prediction, but subsequent efforts to replicate this result were mixed. A decade of these experiments indicated that place learning was more often favored, but that there were conditions under which response learning was preferred. His analysis indicated that the nature of the available cues was the primary determining factor for the differences in the results. In general, whenever there were salient extramaze visual cues that differentiated one goal location from the other, a place representation predominated. Conversely, when differential extramaze cues were not prominent, the response strategy would predominate. Such a pattern of results did not, of course, declare a winner in the place versus response debate. Instead these results suggested that both types of representation are available to the rat and that the rat might use either one under conditions of different salient cues or response demands.
This story does not end there. A most elegant explanation of how rats could use both strategies was recently provided by Packard and McGaugh (1996). In this experiment, rats were trained for a week on the T-maze task, then given the rotated-maze probe trial. Then they were trained for another week with the maze in its original orientation and finally presented with an additional probe trial. Packard and McGaugh found that normal rats initially adopted a place representation as reflected in their strong preference for the place of the previous goal during the first probe trial. However, after the additional week of overtraining, normal rats switched, now adopting a response strategy on the final probe test. Therefore, under these training circumstances, rats developed both strategies successively. Their initial acquisition was guided by the development of a cognitive map, but subsequent overtraining led to development of the response habit.
But Packard and McGaugh’s experiment went beyond merely confirming that the same rats can use both learning strategies. In addition to the pure behavioral testing, Packard and McGaugh also examined whether different brain systems supported these different types of representation. Prior to training, all animals had been implanted with indwelling needles that allowed injection on the probe tests of a local anesthetic or saline placebo directly and locally into one of two brain structures—the hippocampus or the striatum. The results on normal animals previously described were from those subjects that were injected with placebo on both probe tests. However, the effects of the anesthetic were striking. On the first probe trial, animals that were injected with anesthetic into the striatum behaved just as control subjects had— they were predominantly place learners, indicating that the place representation did not depend on the striatum. But the animals that had been injected with anesthetic into the hippocampus showed no preference at all, indicating that they relied on their hippocampus for the place representation and that this was the only representation normally available at that stage of learning. On the second probe test, a different pattern emerged. Whereas control subjects had by that time acquired the response strategy, animals given an anesthetic in the striatum lost the turning response and instead showed a striking opposite preference for the place strategy. Animals given an injection of anesthetic into the hippocampus maintained their response strategy.
Combining these data, a clear picture of the evolution of multiple memory representations emerges. Animals normally develop an initial place representation that is mediated by the hippocampus, and no turning-response representation develops in this initial period. With overtraining, a response representation that is mediated by the striatum is acquired and indeed, it predominates over the hippocampal place representation. The latter is not, however, lost—it can be uncovered, so to speak, by inactivating the striatum and suppressing the turning response strategy. These findings offer compelling evidence that elements of both the S-R and the cognitive map views were right: There are distinct types of memory for place and response, which are distinguished by their performance characteristics as well is by the brain pathways that support such characteristics. It is notable that the Packard and McGaugh experiment was preceded by many other studies demonstrating a specific role for the hippocampus in memory, as well as by a few studies showing specificity in the involvement of the striatum in the acquisition of habits. But this particular study is most striking both in the elegance of the dissociation and in its contact with the history of views on habit and cognitive memory.
In the field of human memory research, the discovery of multiple memory systems came from two major breakthroughs in the study of patients with pervasive, global amnesia.The first of these breakthroughs came with the report by Scoville and Milner (1957) of what has become probably the most famous neurological patient in the literature—the man known by his initials H. M. This patient had the medial temporal lobe area removed to alleviate his severe epileptic attacks. H. M. consequently suffered what appeared to be a nearly complete loss of the ability to form new long-term memories: His impairment—tested over the last 40 or so years—has been shown to extend to verbal and nonverbal memory, spatial and nonspatial memory; indeed, it seems to cut across all categories of learning materials. Yet a second line of discovery about global amnesia revealed a spared domain of learning capacity. Even from the outset, a few exceptions to the otherwise pervasive deficit were apparent. H. M. was able to learn new motor skills, and he showed a facilitation of perceptual identification resulting from prior exposure to objects or words (an effect that later came to be understood as reflective of a preserved priming).
The second breakthrough came in 1980 when Cohen and Squire proposed that these exceptions to amnesia were indicative of a large domain of preserved learning capacities in amnesia. Their conclusion was based on the observation of complete preservation of the acquisition and retention of a perceptual skill (reading mirror-reversed words) in patients with amnesia. These patients showed fully intact skilled performance, yet were markedly impaired both in recognizing the particular words on which they trained and in recollecting their training experiences. These investigators were struck by the dissociation between the ability to benefit or otherwise have performance shaped by a series of training experiences—an ability that appeared fully normal in the amnesic patients—and the capacity to explicitly remember or consciously recollect those training experiences or their contents, which was markedly impaired in the patients. Cohen and Squire attributed the observed dissociation—together with the earlier findings of spared memory in amnesia—to the operation of distinct forms of memory, which they called procedural memory and declarative memory, respectively. These forms of memory were seen as functionally distinct memory systems—one dedicated to the tuning and modification of networks that support skilled performance, and the other to the encoding, storage, and retrieval on demand of memories for specific facts and events. These functionally distinct memory systems were tied to separate brain systems, with declarative memory seen as critically dependent on the medial temporal-lobe and midline diencephalic structures damaged in various amnesias. Procedural memory was seen as mediated by various brain systems specialized for particular types of skilled performance.
Three Major Memory Systems in The Brain
A general, anatomically based framework for some of the major memory systems has emerged from many experiments (like those just described) that provide dissociations among the role of specific brain structures in different forms of memory, combined with the known anatomical pathways of the key structures. In this section I provide an anatomical framework and a preliminary overview of the functional distinctions among these pathways. Subsequent sections elaborate on the functional distinctions in greater detail.
Asketch of some of the most prominent memory pathways currently under investigation is provided in Figure 19.2 (for a similar outline, see Suzuki, 1996). In this scheme, the origin of each of the memory systems is the vast expanse of the cerebral cortex, focusing in particular on the highest stages of the several distinct sensory and motor processing hierarchies— the cortical association areas. The cerebral cortex thus provides major inputs to each of three main pathways of processing related to distinct memory functions. One pathway is to the hippocampus via the parahippocampal region. As introduced previously, this pathway supports the cognitive form of memory, Tolman’s cognitive maps, and declarative memory in humans. The main output of hippocampal and parahippocampal processing is back to the same cortical areas that provided inputs to the hippocampus and are viewed as the long-term repository of declarative memories.
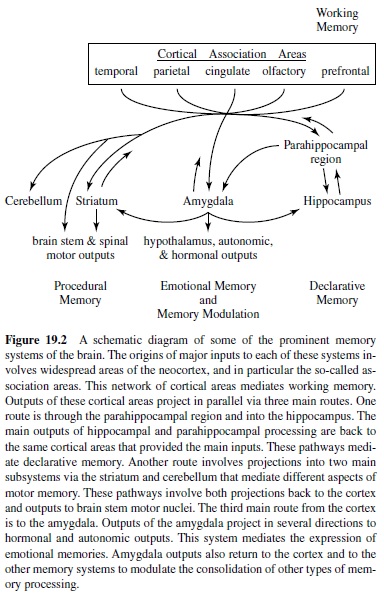
The other two main pathways highlighted here involve cortical inputs to specific subcortical targets as critical nodal points in processing leading to direct output effectors. One of these systems involves the amygdala as a nodal stage in the association of exteroceptive sensory inputs to emotional outputs effected via the hypothalamic-pituitary axis and autonomic nervous system, as well as emotional influences over widespread brain areas. The putative involvement of this pathway in such processing functions has led many to consider this system as specialized for emotional memory.
The other system involves the striatum as a nodal stage in the association of sensory and motor cortical information with voluntary responses via the brain stem motor system. The putative involvement of this pathway in associating cortical representations to specific behavioral responses has led many to consider this system as specialized for habit or skill learning, two forms of procedural memory. An additional, parallel pathway that mediates different aspects of sensorimotor adaptations involves sensory and motor systems pathways through the cerebellum.
The distinct roles of these systems have been compellingly demonstrated in many multiple-dissociation experiments, three of which are summarized here. The first study involves a triple dissociation of memory functions in rats that showed three different patterns of sparing and impairment of memory following damage to the hippocampus, amygdala, and striatum. The other two studies involve double dissociations of memory functions in humans with specific types of brain damage. Taken together, the findings suggest a similar set of memory functions supported by homologous brain areas in animals and humans.
One of the most striking dissociations among memory functions supported by separate brain structures comes from a study by McDonald and White (1993). This study involved multiple experiments in which separate groups of rats were trained on three different versions of the spatial radial maze task (Figure 19.3). Each version of the task used the same maze, the same general spatial cues and approach responses, and the same food rewards. But the stimulus and reward contingencies of each task differed, each focusing on a different kind of memory processing demand. For each task, performance was compared across three separate groups of rats operated to disrupt hippocampal pathways, or the amygdala, or the striatum. In addition, different methods of brain damage were compared. Hippocampal system disruption was accomplished by a fornix transection or by a neurotoxic lesion of the hippocampus. Damage to the amygdala and the striatum was accomplished by electrolytic or neurotoxic lesions of the lateral nucleus of the amygdala or dorsal part of the neostriatum, where cortical sensory input arrive in these structures.
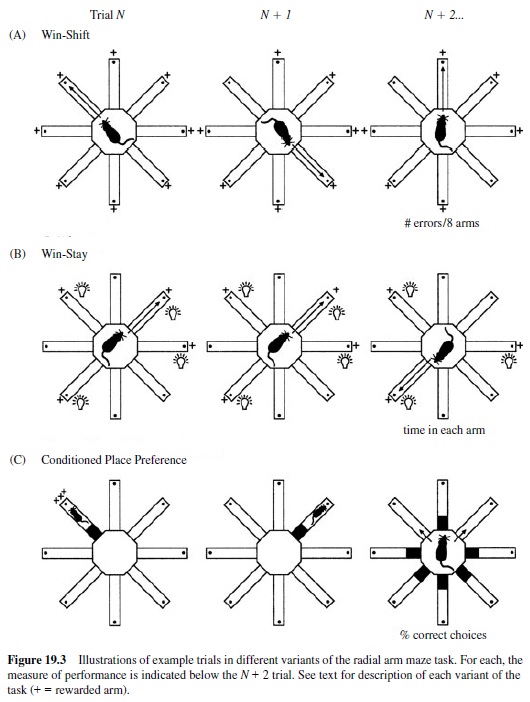
One test was the conventional spatial working memory version of the radial maze task (Figure 19.3). In this version of the task, an eight-arm maze was placed in the midst of a variety of extramaze stimuli in the testing room, providing animals with the opportunity to encode the spatial relations among these stimuli as spatial cues. On every daily trial, a food reward was placed at the end of each of the eight maze arms, and the animal was released from the center and was allowed to retrieve the rewards. Optimal performance would entail entering each arm only once and subsequently avoiding already visited arms in favor of the remaining unvisited arms. The central memory demand of this task was characterized as a win-shift rule; such a rule emphasizes memory for each particular daily episode with specific maze arms. Also, the task requires flexible use of memory by using the approach into previously rewarded locations to guide the selection of other new arms to visit. Based on these characteristics of the memory demands, it was expected that performance on this task would require the hippocampal system.
They found that normal animals learned the task readily, improving from nearly chance performance (four errors out of their first eight arm choices) on the initial training trial to an average of fewer than half an error by the end of training. Consistent with expectations, damage to the hippocampal system resulted in an impairment on this version of the radial maze task. Compared to normal animals, rats with fornix transections made more errors by entering previously visited maze arms. By contrast, amygdala and striatum lesions had no effect on task performance.
The second test involved a variant of the same radial maze task (Figure 19.3). In this version, the maze was again surrounded by a curtain, and lamps were used to cue particular mazearms.Onthefirsttrialofeachdailytrainingsession,four arbitrarily selected arms were illuminated and baited with food, whereas the other four arms were dark and had no food. After the first occasion a lit arm was entered, that arm was rebaited so that the animal could return to the arm for a second reward. Subsequently, that lamp in that particular arm was turned off and no more food was provided at that arm. Thus, here the task was characterized by awin-stay rulein which animals could approach any lit arm at any time and could even reexecute the approach to a particular arm for reward one time in each daily trial. This version of the task minimized the availability of spatial cues; indeed, it associated rewards with different sets of locations across days. Also, it did not require memory for recent episodes or flexible expression of memory. Thus, performance was not expected to rely upon the hippocampal system. Instead, this task would seem to require memory processes associated with learning consistent with stimulus-response contingencies or simple response habits and so was expected to rely on the striatal system.
Results showed that normal control subjects learned the appropriate behavioral responses to the lit arms gradually over several training sessions. In the first few sessions, they selected lit arms on only 50% of the trials, but by the end of training, they performed at about 80% correct. Consistent with expectations, animals with striatal damage were impaired, barely exceeding chance performance even with extended training. By contrast, animals with fornix transections succeeded in learning and even outperformed the control subjects in learning rate. Animals with amygdala lesions were unimpaired, learning the task at a normal rate.
The third test involved yet another variant of the radial maze task in which animals were separately conditioned to be attracted to one maze arm and habituated to another arm, without performing specific approach movements to either of the arms (Figure 19.3). In this version, the maze was surrounded by a curtain to diminish the salience of spatial cues. Six of the maze arms were blocked off to make them inaccessible, and one of the remaining two arms was illuminated by proximal lamps, whereas the other was only dimly illuminated. After a preliminary session in which rats could explore both available arms, conditioning proceeded with daily exposures to one of the two arms. For each rat, either the lit or the dark arm was associated with food by confining the animal in that arm for 30 min with a large amount of food on four separate trials. On another four trials, the same animal was confined for the same period of time to the other arm, but with no food. Thus, in half of the rats, the lit arm was associated with food availability and the dark arm was not; for the other half of the rats, the opposite association was conditioned. In a final test session, no food was placed on the maze and the access to both the lit and dark arms was allowed. The amount of time spent in each arm for a 20-min session was recorded to measure the preference for each of the two arms. This version of the radial maze task emphasized the strong and separate associations between food reward or absence of reward with a particular maze arm defined by a salient nonspatial cue. This task minimized the availability of spatial relations among stimuli. Also, because the same lit and dark arms used during training were re-presented in testing, the task did not require memory for specific episodes and flexible expression of memory, nor did it require reproduction of specific habitual approach responses. Thus, it was not expected that either the hippocampal system or the striatum would be critical to learning. Instead, learning would seem to depend on memory processes associated with emotional conditioning, and it so was expected to depend upon the amygdala.
They found that normal animals showed a strong preference for the arm associated with food, typically spending 50–100% more time in the maze arm in which they had been fed compared to the arm where no food was previously provided. Consistent with expectations, rats with amygdala damage showed no conditioned preference for the cue arm associated with food. By contrast, rats with fornix transections or striatal lesions showed robust conditioned cue preferences.
A very similar pattern of observations has emerged from analyses of human amnesia. In both studies, the learning and memory capacities of amnesic patients with damage to the medial temporal lobe was compared with that of nonamnesic patients—that is, humans with brain pathologies not producing the classic amnesic syndrome. The two studies differ in their focus on comparing classic amnesia with more specific disorders of learning and memory resulting from damage to the amygdala or striatum, respectively.
In one study, Bechara et al. (1995) examined three patients with selective damage to the hippocampus or amygdala. One patient suffered from Urbach-Wiethe disease, a disorder resulting in selective bilateral calcification of the tissue of the amygdala and sparing the adjacent hippocampus.Another patient experienced multiple cardiac arrests and associated transient hypoxia and ischemia that resulted in selective bilateral hippocampal atrophy, sparing the neighboring amygdala. The third patient suffered herpes simplex encephalitis, resulting in bilateral damage to both the amygdala and hippocampus.
This study focused on a form of autonomic conditioning involving an association between a neutral stimulus and a loud sound. The conditioning stimulus (CS+) was either a monochrome color slide or a pure tone. Participants were initially habituated to the CS+ as well as to several like stimuli (different colors or tones) that would be presented as CSstimuli. Subsequently, during conditioning the CSs were presented in random order for 2 s each. Each presentation of the CS+ was terminated with the unconditioned stimulus (US), a loud boat horn that was sounded briefly. Autonomic responses to these stimuli were measured as skin conductance changes through electrodermal recordings.
Normal controls showed skin conductance changes to the US and robust conditioning to the CS+, with smaller responses to the CS- stimuli. The patient with selective amygdala damage showed normal unconditioned responses to the US, but failed to develop conditioned responses to the CS+ stimuli. By contrast, the patient with selective hippocampal damage showed robust skin conductance changes to the US and normal conditioning to the CS+ stimuli. This patient also showed responsiveness to the CS- stimuli, but clearly differentiated these from the CS+ s. The patient with combined amygdala and hippocampal damage failed to condition, even though he responded to the US.
After the conditioning sessions, the subjects were debriefed with several questions about the stimuli and their relationships. Control subjects and the patient with selective amygdala damage answered most of these questions correctly, but both patients with hippocampal damage were severely impaired in recollecting the task events. These findings demonstrate a clear double dissociation, with a form of emotional conditioning disrupted by amygdala damage and declarative memory for the learning situation impaired by hippocampal damage. The finding that these different forms of memory for the identical stimuli and associations are differentially affected by localized brain damage further supports the notion of multiple memory systems.
In another study, Knowlton, Mangels, and Squire (1996) examined patients in the early stages of Parkinson’s disease— associated with degeneration of neurons in the substantia nigra resulting in a major loss of input to the neostriatum— and amnesic patients with damage to the medial temporal lobe or to associated regions of the diencephalon.
Subjects were trained in a probabilistic classification learning task formatted as a weather prediction game. The task involved predicting the one of two outcomes (rain or shine) based on cues from a set of cards. On each trial, one to three cards from a deck of four was presented. Each card was associated with the sunshine outcome only probabilistically— either 75%, 57%, 43%, or 25% of the time—and the outcome with multiple cards was associated with the conjoint probabilities of the cards presented in any of 14 configurations. After presentation of the cards for each trial, the subject was forced to choose between rain and shine, and was then given feedback as to the outcome. The probabilistic nature of the task made it somewhat counterproductive for participants to attempt to recall specific previous trials because repetition of any particular configuration of the cues could lead to different outcomes. Instead the most useful information to be learned concerned the probability associated with particular cues and combinations of cues—acquired gradually across trials, much as habits or skills are acquired.
Over a block of 50 trials, normal subjects gradually improved from pure guessing (50% correct) to about 70% correct, a level consistent with the optimal probability of accuracy in this task. However, the patients with Parkinson’s disease failed to show significant learning, and the failure wasparticularly evident in those patients with more severe Parkinsonian symptoms. By contrast, amnesic patients were successful in learning the task, achieving levels of accuracy not different from that of controls by the end of the 50-trial block.
Subsequent to training on the weather prediction task, these subjects were debriefed with a set of multiple-choice questions about the types of stimulus materials and nature of the task. Normal subjects and those with Parkinson’s disease performed very well in recalling the task events. But the amnesic subjects were severely impaired, performing near the chance level of 25% correct. These findings demonstrate a clear double dissociation, with habit or skill learning disrupted by neostriatal damage and declarative memory for the learning events impaired by hippocampal or diencephalic damage, providing further evidence for the view that different forms of memory are represented for the identical learning materials within parallel brain systems.
Elaborating the Role of the Three Major Memory Systems
Thisanalysissofarhasofferedonlyapreliminaryviewintothe distinct functions of the hippocampal, striatum, and amygdala ascomponentsofseparatememorysystems.Theremainderof this research paper extends these characterizations, offering greater detail on the full anatomy of the pathways involved in these systems and on their different functional roles. The evidence for these characterizations comes mainly from anatomical neuropsychological studies of the effects of selective damage within these systems. I limit the discussion of a few particularly strong examples of experiments that reveal the scope and nature of their role in memory. For a more comprehensive treatment of each of these and other memory systems, the reader is referred to Eichenbaum and Cohen (2001).
The Declarative Memory System
Ideally, to the extent that the early analyses of cognitive memory are correct, this system should have all the properties of recollective memory outlined by Maine de Biran, James, Tolman, and Bartlett. Indeed, it appears that their characterizations fit the modern description of hippocampaldependent memory functions quite well. Recall that the common theme in all those theoretical frameworks is that cognitive memory is a network of associations built up from linking the records of many experiences and the ability to search the network via the recollective process for memories; this network employs those memories to solve a myriad of problems.
Beginning in the 1970s several hypotheses about the function of the hippocampus were proposed; each captured some of these aspects of the earlier views on cognitive memory.The two most prominent early views are summarized here. In 1978, O’Keefe and Nadel assigned Tolman’s cognitive mapping system to the hippocampus. Their account was based on an interpretation of the accumulated voluminous literature on the behavioral effects of hippocampal damage in animals, showing a preponderance of observed impairments in spatial learning versus inconsistent deficits in nonspatial learning following hippocampal damage; it was also based on O’Keefe’s discovery of place cells, hippocampal neurons that fire associated with a rat’s location in its environment. O’Keefe and Nadel’s analysis went well beyond making a simple distinction between spatial and nonspatial learning modalities. Their proposal about spatial learning involved the acquisition of cognitive maps that corresponded roughly, if not topographically, to the salient features of physical environment. They referred to the domain of memory supported by the hippocampus as a locale system that maintains a molar model of spatial relations among objects in the environment—driven by curiosity rather than reinforcement of specific behaviors capable of very rapid learning. By contrast, hippocampalindependent learning was viewed as supported by a taxon system that mediates dispositions of specific stimuli into categories, is driven by reinforcement of approach and avoidance behaviors, and that involves slow and incremental behavioral adaptations.
The other most prominent theory that emerged in this period was Olton, Becker, and Handlemann’s (1979) distinction between working memory and reference memory. Notably, Olton’s use of the term working memory differs in meaning from the same term used in today’s characterizations of a form of short term memory in humans and animals. The memory process Olton conceived would today be viewed as more similar to episodic memory—memory for a particular experience involving one’s own actions—than our current conception of working memory as the contents of current consciousness. To investigate this distinction, Olton invented the radial maze, a maze composed of a central start platform with multiple arms radiating in all directions like the spokes of a wheel. In his classic studies, a bait was placed at the end of each of the arms and then allowed the rat to forage for the food. After several such trials, rats learn to forage efficiently, running down each arm only once without repetition. Good performance requires the animal remember each arm visited in that session and then before the next trial erasing those memories. Olton distinguished working memory from reference memory operationally, using a maze in which many of the arms were never baited. Thus, to be maximally efficient in foraging, animals had to simultaneously demonstrate their capacity for working memory, by visiting each of the baited arms only once, and for reference memory, by consistently avoiding the neverbaited arms.
For a comprehensive review of the experimental tests of these theories, as well as other theories, the reader is referred to Cohen and Eichenbaum (1993). For our purposes here, suffice it to say that the each of these theories was supported by specific experimental findings—thus indicating that each captured a critical aspect of hippocampal system function. However, none of these theories could account for all of the findings, including those that formed the major support for the alternative theories. A formulation that seeks to incorporate the central elements of all of these views within the framework of the earlier conceptualizations of cognitive memory is the account espoused by the present author and his colleagues (see Eichenbaum, 2000; Eichenbaum & Cohen, 2001). According to this view, the hippocampal systems plays a critical role both in episodic memory, as proposed by Olton, and in the development of large-scale organized representations, similar to the proposal of O’Keefe and Nadel (but not limited to physical space).
According to this account, the hippocampal memory system is composed of three major components: cerebral cortical areas, the parahippocampal region, and the hippocampus itself (Burwell, Witter, & Amaral, 1995; Suzuki, 1996), and the major pathways of the system are very similar in rats and monkeys (Figure 19.1). The cerebral cortical areas comprise diverse and widespread association regions that are both the source of information to the hippocampal region and the targets of hippocampal output. They project in different ways to the parahippocampal region, a set of interconnected cortical areas immediately surrounding the hippocampus that in turn project into the hippocampus itself. The main outputs of the hippocampus return to the parahippocampal region, which sends back projections broadly to the same cortical association areas that provided the inputs to the parahippocampal region. This pattern of anatomical organization complements the findings from studies of amnesia, leading to the working hypothesis that the parahippocampal region and hippocampus make their contributions to memory by altering the nature, persistence, and organization of memory representations within the cerebral cortex.
There is emerging evidence that neocortical association areas—the parahippocampal region and the hippocampus— play distinct and complementary roles in this memory system. The roles of these areas may be best contrasted in the results of studies on a simple recognition memory task— called delayed nonmatch to sample (DNMS)—in which subjects must remember single stimulus across a variable memory delay (see Eichenbaum, Alvarez, & Ramus, 2001). For example, in rats performing an odor-guided version of the DNMS task damage to the orbitofrontal cortex resulted in a deficit in the acquisition of the task when the memory delay was minimal, suggesting an important role in perceptual processing or in learning the nonmatching rule (Otto & Eichenbaum, 1992; Ramus & Eichenbaum, 2000). By contrast, rats with damage to the parahippocampal region acquired the DNMS task at the normal rate and performed well at brief memory delays. However, their memories declined abnormally rapidly as the memory delay was extended beyond a few seconds—indicating a selective role in maintaining a persistent memory of the sample stimulus (see also Young, Otto, Fox, & Eichenbaum, 1997). Little if any deficit in nonspatial DNMS is observed following damage to the hippocampus or its connections via the fornix, indicating the parahippocampal region itself mediates the persistence of memories for single items required to perform DNMS.
Parallel results have been obtained in monkeys performing visually guided versions of the DNMS task. Similar to rats, monkeys with damage to the parahippocampal region perform well when the memory delay is brief. When the memory demand is increased by extending the delay period, however, severe deficits in DNMS are observed (Meunier, Bachevalier, Mishkin, & Murray, 1993; Zola-Morgan, Squire, Amaral, & Suzuki, 1989), and these impairments are much more severe than those following damage to the hippocampus (Murray & Mishkin, 1998) or its connections via the fornix (Gaffan, 1994a). Examination of performance on the DNMS task with very brief delays has been difficult because the standard protocol used for monkeys is manual. However, using another recognition task that allowed testing at very brief delays, it has recently been demonstrated that inferotemporal area of the cortex is critical for visual recognition even for a 1-s delay—suggesting a role in perceptual processing as opposed to memory—whereas the parahippocampal region is critical for memory in the same task only when recognition was delayed (Buffalo et al., 1999). The parahippocampal region may also play a role at the intersection of perception and memory, in situations in which perceptual processes depend on learned associations among complex stimulus elements (Eichenbaum & Bunsey, 1995; Murray & Bussey, 1999).
It is notable that memory mediated by the hippocampus itself contributes very little to performance in standard DNMS tasks, in that the deficits observed are modest at most compared to the effects of damage to the cortex or parahippocampal region. However, the hippocampus may play an essential role in other types of simple recognition memory tests (Rampon et al., 2000; Zola et al., 2000; see below) and in recognition memory for configurations of items within scenes or places (Cassaday & Rawlins, 1995; Gaffan, 1994b; Wan, Aggleton, & Malcolm, 1999).
Instead, the findings from studies using animal models point to a critical role for the hippocampus itself in central aspects of declarative memory. To understand this role it is important to consider the fundamental properties of declarative memory, as introduced by Cohen and Squire (1980) and subsequently elaborated by many investigators. We acquire our declarative memories through everyday personal experiences; the ability to retain and recall these episodic memories is highly dependent on the hippocampus in humans (VarghaKhadem et al., 1997). In addition, recent studies have developed animal models of that capture the temporal specificity of events in episodic memory, and have demonstrated critical involvement of the hippocampus itself (Fortin et al., 2002; Kesner et al., 2002; Steele & Morris, 1999). In one study, rats were presented with unique sequences of odors, and probed for their memory of the order in which the items had been presented. Animals with selective hippocampal lesions were severely impaired in remembering the order of events in each episode, even though they could recognize the items as demonstrated in a separate recognition test (Fortin et al., 2002).
The full scope of hippocampal involvement also extends to semantic memory, the body of general knowledge about the world that is accrued from linking multiple experiences that share some of the same information (Squire & Zola, 1998). For example, one can learn about one’s relatives via personal episodes of meeting and talking about family members and then weave together this information into a body of knowledge constituting one’s family tree. Similarly, one can learn about the geographies of one’s neighborhood and hometown by taking trips through various areas and eventually interconnecting them into cognitive maps.
In addition, declarative memory for both the episodic and semantic information is special in that the contents of these memories are accessible through various routes. In humans, declarative memory is most commonly expressed through conscious, effortful recollection. This means that one can access and express declarative memories to solve novel problems by making inferences from memory. Thus, even without ever explicitly studying your family tree and its history, you can infer indirect relationships—or the sequence of central events in the family history—from the set of episodic memories about your family. Similarly, without ever studying the map of your neighborhood, you can make navigational inferences from the synthesis of many episodic memories of previous routes taken. Family trees and city layouts are but two examples of the kind of memory space proposed to be mediated by the hippocampal system (Eichenbaum, Dudchenko, Wood, Shapiro, & Tanila, 1999). Within this view, a broad range of such networks can be created, with their central organizing principle the linkage of episodic memories by their common events and places—and a consequent capacity to move among related memories within the network.
These properties of declarative memory depend on the functions of the hippocampus itself. Several experiments have shown that the hippocampus is required in situations in which multiple and distinct but overlapping experiences must be combined into a larger memory representation that mediates flexible, inferential memory expression. For example, in one experiment rats initially learned a series of distinct but overlapping associations between odor stimuli (Bunsey & Eichenbaum, 1996). On each trial, one of two odors was initially presented, followed by a choice between two odors, one of which was baited as the assigned associate for a particular initial odor (Agoes with B, not Y; X goes with Y, not B). Following training on two sets of overlapping odor-odor associations (A-B and X-Y, then B-C and Y-Z), subsequent probe tests were used to characterize the extent to which learned representations could be linked to support inferential memory expression. Control rats learned paired associates rapidly and hippocampal damage did not affect acquisition rate on either of the two training sets. Intact rats also showed that they could link the information from overlapping experiences and employ this information to make inferential judgments in two ways. First, normal rats showed strong transitivity across odor pairings that contained a shared item. For example, having learned that odor Agoes with odor B and that B goes with C, they could infer that A goes with C. Second, control rats could infer symmetry in paired associate learning. For example, having learned that B goes with C, they could infer that C goes with B. By contrast, rats with selective hippocampal lesions were severely impaired, showing no evidence of transitivity or symmetry.
Asimilar characterization accounts for the common observation of deficits in spatial learning and memory following hippocampal damage. For example, in the Morris water maze test, rats or mice learn to escape from submersion in a pool by swimming towards a platform located just underneath the surface. It is important to note that training in the conventional version of the task involves an intermixing of four different kinds of trial episodes that differ in the starting point of the swim.Under this condition, animals with hippocampal damage typically fail to acquire the task (Morris, Garrud, Rawlins, & O’Keefe, 1982). However, if the demand for synthesizing a solution from four different types of episodes is eliminated by allowing the animal to repeatedly start from the same start position, animals with hippocampal damage acquire the task almost as readily as do normal rats and use the same distant spatial cues in identifying the escape site (Eichenbaum, Stewart, & Morris, 1990). Nevertheless, even when rats with hippocampal damage are successful in learning to locate the escape platform from a single start position, they are unable to use this information for flexible, inferential memory expression.Thus, after they were trained to find the platform from a single start position, normal rats readily locate the platform from any of a set of novel start positions. Under these same conditions, however, rats with hippocampal damage fail to readily locate the platform, often swimming endlessly and unsuccessfully in a highly familiar environment.
The view that has emerged from these and many other studies is that the hippocampus plays a central role in the creation of a broad range of memory networks, with their central organizing principle the linkage of episodic memories by their common events and places—and a consequent capacity to move among related memories within the network. The scope of such network reaches to various domains relevant to the lives of animals—from knowledge about spatial relations among stimuli in an environment, to categorizations of foods, to learned organizations of odor or visual stimuli or social relationships. Progress is being made in investigating a variety of these domains.
Procedural Memory Systems
Among the most prevalent kinds of memory we use every day is procedural memory, the habits, skills, and sensorimotor adaptations that go on constantly in the background of all of our intentional and planned behavior. Because this kind of memory generally falls outside of consciousness, we take it for granted. Yet without it we would be forced to think our way through virtually every step we take and every motion we make in our daily tasks. Fortunately there is a motor memory system or systems, a circuitry involving structures of the motor systems of the brain whose plasticity accomplishes the myriad of unconscious learned behaviors we engage almost every waking moment.
Motor memory is generally separated into two general subtypes (Figure 19.2). One type involves the acquisition of habits and skills—the capacity for a very broad variety of stereotyped and unconscious behavioral repertoires. These can involve a simple refinement of particular repeated motor patterns and extend to the learning of long action sequences in response to highly complex stimuli. These abilities reflect both the acquisition of general skills (writing, piano playing, etc.) and the unique elements of personal style and tempo in the expression of these behaviors. A key structure in this subsystem is the striatum. The striatum receives its cortical inputs from the entire cerebral cortex, and these projections are capable of activity-dependent changes in responsiveness. These projections are topographically organized into divergent and convergent projections into modules within the striatum that could sort and associate somatosensory and motor representations. The striatum projects mainly to other components of the basal ganglia and to the thalamus, which project back to both the premotor and motor cortex as well as the prefrontal association cortex (Figure 19.2). It is notable that there are minimal projections of this circuit to the brain stem motor nuclei and none to the spinal motor apparatus.
The other type of procedural memory involves specific sensory-to-motor adaptations—that is, adjustments of reflexes, such as changing the force exerted to compensate for a new load or acquisition of conditioned reflexes that involve novel motor responses to a new sensory contingency, as characterize many instances of Pavlovian conditioning described earlier. A key structure of this subsystem is the cerebellum. The cerebellum receives cortical input from a cortical area much more restricted than the striatum, including only the strictly sensory and motor areas projecting via brain stem nuclei into the lateral part of the cerebellar cortex. Like the striatal subsystem, the cerebellum has a thalamic output route to the cerebral cortex, although the cortical target is also more restricted than that of the striatum—limited to motor and premotor cortex. In addition, the cerebellum receives somatic sensory inputs directly from the spinal cord and has major bidirectional connections with brain stem nuclei associated with spinal cord functions. The functional roles of these two subsystems are discussed in turn.
The Striatal Subsystem
The striatal habit system was introduced via experiments that dissociated this system from the hippocampal and amygdala memory systems. Those experiments provided evidence indicating that a role for the striatum in the acquisition of specific stimulus-response associations, as contrasted with declarative memory and emotional memory functions of the hippocampal and amygdala systems, respectively.
The scope of striatal involvement is not limited to a particular sensory or motivational modality or to a particular type of response output. One study by Viaud, White, and Norman (1989) illustrates some of the range of memory mediated by this system and shows a particularly striking dissociation of regions within the striatum in their effects on inhibition of approach behavior conditioned by different cues. In this study, thirsty rats with lesions of the posteriorventral or ventrolateral regions of the striatum were trained to approach a water spout over several days. Subsequently, they were given foot shocks in the same chamber in the presence of a conditioning cue, which was either a light or an odor. The animals were tested later for their latency to approach the water spout when the conditioning cue was present versus when it was absent. Animals with lesions of the posteriorventral striatum failed to show discriminative avoidance of the light cue but showed good avoidance of the olfactory cue. Conversely, animals with ventrolateral striatal lesions failed to show discriminative avoidance of the olfactory cue but showed good avoidance of the light cue.
Previously the selective role of the striatum in learning specific turning, T-maze, and approach responses in radial maze were shown. Similar dissociations showing striatal function in stimulus-approach learning have extended this role to aversively motivated learning in the water maze (Packard & McGaugh, 1992). In addition, there is further evidence from maze-learning studies that restrict the nature of response learning by this system. In one of these studies, rats were trained on two tasks on different radial mazes (Cook & Kesner, 1988). In a place-learning (allocentric) task, only one arm of an eight-arm maze was consistently baited, and the rat began each trial from any of the remaining arms chosen at random. In a right-left discrimination (egocentric) task, the animal began each trial in the central area of the maze and two randomly chosen adjacent arms were indicated for a choice. The rat had to choose only the left (or for other rats, the right) of the two arms regardless of its absolute location. Here, too, rats with striatal lesions performed well on the place-learning task but did not learn the right-left discrimination task, indicating a selective role in egocentric response learning.
Taken together the literature from studies of damage to the striatum suggests that the deficit following striatal damage is—or includes—an impairment in generating behavioral responses toward important environmental stimuli. The deficit extends to both approach and avoidance responses and to both egocentric spatial and nonspatial stimuli across many modalities. Even this characterization is not sufficiently comprehensive to explain the full range of impairments in animals and humans (see Eichenbaum & Cohen, 2001).
Thus, it is likely that the deficits in egocentric localization and S-R learning in animals with striatal damage may reflect only a subset of the forms of behavioral sequence acquisition mediated by the striatum.
The Cerebellar Subsystem
The anatomy and functions of the cerebellum have long been associated with aspects of motor learning, and most studies have focused on its highly organized circuitry and emphasized its mechanisms for reflex adaptations (for review, see Ebner, Flament, & Shanbhag, 1996). Considerable recent attention has focused on Pavlovian eye-blink conditioning as a model learning paradigm in which to study the role of the cerebellum. In this paradigm, rabbits are placed in restraining chambers where they can be presented with a well-controlled tone or light as the conditioning stimulus (the CS), and a photoelectric device records eye-blinks. In classic delay conditioning, this stimulus lasts 250–1,000 ms and coterminates with an air puff or mild electrical shock to the eyelid (the unconditioned stimulus or US) that produces the reflexive, unconditioned eye blink (the UR). After several pairings of the CS and US, the rabbit begins to produced the eye blink after onset of the CS and prior to presentation of the US. With more training, this conditioned response (CR) occurs somewhat earlier, and its timing becomes optimized so as to be maximal at the US onset, showing that not only is a CR acquired, but also a timing of the CR is established.
The role of the cerebellum and associated areas has been studied extensively by Thompson and his colleagues (for a review, see Thompson & Kim, 1996). In their studies they found that permanent lesions or reversible inactivation of one particular cerebellar nucleus—the interpositus nucleus—result in impairments in the acquisition and retention of classically conditioned eye-blink reflexes, without affecting reflexive eye blinks(URs).Additionalcompellingdataindicatingaselective role for the interpositus in this kind of procedural memory come from studies using reversible inactivations of particular areas during training.These studies showed that drug inactivation of the motor nuclei that are essential for production of the CR and UR prevented the elicitation of behavior during training. However, in trials immediately following removal of the inactivation, CRs appeared in full form, showing that the neural circuit that supports UR production is not critical for learning per se. A similar pattern of results was obtained with inactivation of the axons leaving the interpositus or their target in the red nucleus, showing that the final pathway for CR production is also not required to establish the memory trace. By contrast, inactivation of the anterior interpositus nucleus and overlying cortex by drugs (muscimol, lidocaine) or temporary cooling did not affect reflexive blinking, yet resulted in failure of CR development during inactivation and the absence of savings in learning after removal of the inactivation.These results point to a small area of the anterior interpositus nucleus and overlying cerebellar cortex as the essential locus of plasticity in this form of motor learning.
The Emotional Memory System
Perhaps the best studied example of emotional memory involves the brain system that mediates Pavlovian fear conditioning as studied by Joseph LeDoux (1992) and by Michael Davis and their colleagues. This research has focused on the specific elements of the pathways through the amygdala that support the learning of fearful responses to a simple auditory stimulus (Figure 19.1). The critical elements of the relevant amygdala pathways include auditory sensory inputs via the brain stem to circuits through the thalamus. Some of these sensory thalamic areas then project directly to the lateral amygdaloid nucleus. Other thalamic projections follow a route to the primary sensory cortex, then to secondary areas and the perirhinal cortex. Each of these secondary cortical areas are the source of cortical inputs to the amygdala, particularly the lateral and basolateral nuclei of this structure. Those areas of the amygdala project into the central nucleus, which is the source of outputs to subcortical areas controlling a broad range of fear-related behaviors, including autonomic and motor responses.
In this research paper, I provide an overview of the work of LeDoux and his colleagues. LeDoux’s studies have examined the neuropsychology and neurophysiology of these structures in animals during the course of a simple tone-cued fear conditioning task. Rats are initially habituated to an operant chamber, then presented with multiple pairings of a 10-s pure tone terminating with a brief electric shock through the floor of the cage. Subsequently conditioned fear was assessed by measuring the autonomic response as reflected in changes to the tone only in arterial pressure and motor responses as reflected in a stereotypical crouching or freezing behavior when the tone is presented, as well as suppression of drinking sweetened water. Unconditioned responses to the tone were evaluated by presenting other animals with unpaired tones and shocks.
Their initial experiments were aimed at identifying the critical auditory input pathway to the amygdala. Animals with selective lesions in the lateral amygdala show dramatically reduced conditioned responses to the tone in the measures of both autonomic and motor responses. Unconditioned responses (consequent to unpaired presentations) were not affected by this damage. Also, animals with damage to the adjacent striatum performed normally, showing anatomical specificity and that the striatal system is not involved in emotional learning.
Subsequent efforts focused on identifying which of the two prominent auditory input pathways to the lateral amygdala was critical. Broad destruction of all auditory areas of the thalamus eliminated conditioned responses. However, selective ablation of either of the two prominent direct inputs to the lateral amygdala were individually ineffective. Thus, lesions of the medial division of the medial geniculate (including all three nuclei that project directly to the lateral amygdala)—or of the entire auditory cortex that projects to the amygdala—did not reduce either the autonomic or freezing response. However, elimination of both of these inputs produced the full effect seen after lateral amygdala lesions. Thus, for this simple type of conditioning—either the direct thalamic input, which offers a crude identification of a sound, or the thalamocortical input pathway, which provides a sophisticated identification of auditory signal—is sufficient to mediate conditioning.
Additional experiments were aimed at an another component of fear conditioning observed in these studies. After conditioning, when rats are replaced in the conditioning chamber they begin to freeze even before the tone is presented. Thus, rats appear to condition both to the tone and to the environmental context in which tones and shock have previously been paired. This contextual fear conditioning is selective to the environment in which conditioning occurs. Furthermore, contextual fear conditioning can be dissociated from conditioning to the tone by presenting conditioned tones in a different environment. Trained animals do not freeze prior to tone presentation in the different environment, but do freeze when the tone is presented.
Moreover, contextual fear conditioning is mediated by a pathway different from that of tone-cued fear conditioning; to demonstrate this, the animals were trained on the standard version of the task, then assessed freezing both immediately after the rats were placed in the conditioning chamber and then subsequently in response to the tone. Amygdala lesions blocked conditioned freezing to both the context and the tone. By contrast, damage to the hippocampus selectively blocked contextual fear conditioning, sparing the conditioned response to the tone.
The amygdala is also critical to the acquisition of positive affective biases, as demonstrated by the McDonald and White (1993) experiment showing a critical role for the amygdala in conditioned place preferences mediated by food rewards. In addition, many other studies have also indicated a selective role for the amygdala in the acquisition of both positive and negative biases for both primary and secondary reinforcers. Furthermore, these studies indicate that the same brain system that mediates the perception and appreciation of emotional stimuli as well as emotional expression is also the system that is critical to the acquisition, consolidation, and expression of emotional memories.
Conclusion
The preceding overview is not intended to be a comprehensive review of any of the memory systems outlined previously (see Eichenbaum & Cohen, 2001). Nor does it cover all of the brain’s memory systems. In particular, the present review does not consider the prefrontal-posterior cortical network that mediates working memory (see Miller, 2000). In addition, this review did not consider simple forms of sensory adaptation and biasing mediated within the cerebral cortex, such as those that mediate priming (Tulving & Schacter, 1990). And this review did not consider the important role of the amygdala as a key part of a memory modulation system that controls the extent of consolidation of memory in all the systems described previously (see McGaugh, Cahill, & Roozendaal, 1996).
However, the present review does offer an overview of the three major brain systems that mediate the storage and expression of distinct types of long term memory. The hippocampal memory system mediates declarative memory— our capacity to recollect everyday facts and events. The striatal and cerebellar systems mediate forms of procedural memory that allow unconscious acquisition and expression of habits, skills, and sensorimotor adaptations. The amygdala system mediates emotional memory, the unconscious acquisition and expression of biases towards or away from otherwise arbitrary stimuli.
A few closing remarks are in order. Note that the hippocampal system is special in its role in memory per se. The motor and emotional memory systems involve precisely the same brain circuitry as that required for the expression of motor and emotional behavior, respectively. Thus, the forms of unconscious memory these systems mediate can be viewed as tuning and adjustments of the brain’s motor and emotional performance systems. The role of the hippocampus seems special in this regard. It is not clear that the hippocampus has a performance function outside of its role in memory.
Finally, it is noteworthy that in a general sort of way, we have come full circle to the same conclusion about multiple forms of memory reached by Main de Biran 200 years ago. It was he who in 1804 proposed that there were three main kinds of memory, characterized as representative, mechanical, and sensitive memory. Now we call these declarative, motor, and emotional memory, respectively, and rely more or less on the same distinctions in properties of memory to define them. However, we do know much more about the neurobiology of these systems. Modern cognitive neuroscience has shown that the differences between these systems come about because they are mediated by different brain pathways, and their distinctive properties are consequences of the special anatomies and operational characteristics of those systems.
Bibliography:
- Bartlett, F. C. (1932). Cambridge, UK: Cambridge University Press.
- Bechera, A., Tranel, D., Hanna, D., Adolphs, R., Rockland, C., & Damasio, A. R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269, 1115–1118.
- Buffalo, E. A., Ramus, S. J., Clark, R. E., Teng, E., Squire, L. R., & Zola, S. M. (1999). Learning and Memory, 6, 572–599.
- Bunsey, M., & Eichenbaum, H. (1996). Conservation of hippocampal memory function in rats and humans. Nature, 379, 255–257.
- Burwell, R. D., Witter, M. P., & Amaral, D. G. (1995). Perirhinal and postrhinal cortices in the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus, 5, 390–408.
- Cassaday, H. J., & Rawlins, J. N. P. (1995). Fornix-fimbria section and working memory deficits in rats: Stimulus complexity and stimulus size. Behavioral Neuroscience, 109, 594–606.
- Cohen, N. J., & Eichenbaum, H. (1993). Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press.
- Cohen, N. J., & Squire, L. R. (1980). Preserved learning and retention of a pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science, 210, 207–210.
- Cook, D., & Kesner, R. P. (1988). Caudate nucleus and memory for egocentric localization. Behavioral and Neural Biology, 49, 332–343.
- Ebbinghaus, H. (1913). Memory: A contribution to experimental psychology. New York: Dover. (Original work published 1885)
- Ebner, T. J., Flament, D., & Shanbhag, S. (1996). The cerebellum’s role in voluntary motor learning: clinical, electrophysiological, and imaging studies. In J. R. Bloedel, T. J. Ebner, & S. P. Wise (Eds.), The acquisition of motor behavior in vertebrates (pp. 223–234). Cambridge, MA: MIT Press.
- Eichenbaum, H. (2000). Acortical-hippocampal system for declarative memory. Nature Reviews Neuroscience, 1, 41–50.
- Eichenbaum, H., Alvarez, P., & Ramus, S. (2001). Animal models of amnesia. In L. Cermak (Ed.), Handbook of neuropsychology: Vol. 4 (2nd ed., pp. 1–24). Amsterdam: Elsevier Sciences.
- Eichenbaum, H., & Bunsey, M. (1995). On the binding of associations in memory: Clues from studies on the role of the hippocampal region in paired-associate learning. Current Directions in Psychological Science, 4, 19–23.
- Eichenbaum, H., & Cohen, N. J. (2001). From conditioning to conscious recollection: Memory systems of the brain. Oxford, UK: Oxford University Press.
- Eichenbaum, H., Dudchenko, P., Wood, E., Shapiro, M., & Tanila, H. (1999). The hippocampus, memory, and place cells: Is it spatial memory or memory space? Neuron, 23, 1–20.
- Eichenbaum, H., Stewart, C., & Morris, R. G. M. (1990). Hippocampal representation in spatial learning. Journal of Neuroscience, 10, 331–339.
- Fortin, N. J., Agster, K. L., & Eichenbaum, H. (2002). Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience, 5, 458–462.
- Gaffan, D. (1994a). Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: Evidence for multiple memory systems in the primate temporal lobe. Experimental Brain Research, 99, 411–422.
- Gaffan, D. (1994b). Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transection. Journal of Cognitive Neuroscience, 6, 305–320.
- James, W. (1918). The principles of psychology. New York: Holt. (Original work published 1890)
- Kesner, R. P., Gilbert, P. E., & Barua, L. A. (2002). The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience, 116, 286–290.
- Knowlton, B. J., Mangels, J. A., & Squire, L. R. (1996). A neostriatal habit learning system in humans. Science, 273, 1399– 1401.
- LeDoux, J. E. (1992). Brain mechanisms of emotion and emotional learning. Current Opinion in Neurobiology, 2, 191–197.
- Maine de Biran. (1929).The influence of habit on the faculty of thinking. Baltimore: Williams & Wilkins. (Original work published 1804)
- McDonald, R. J., & White, N. M. (1993). A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behavioral Neuroscience, 107, 3–22.
- McGaugh, J. L., Cahill, L., & Roozendaal, B. (1996). Involvement of the amygdala in memory storage: Interactions with other brain systems. Proceedings of the National Academy of Sciences, 93, 13508–13514.
- Meunier, M., Bachevalier, J., Mishkin, M., & Murray, E. A. (1993). Effects on visual recognition of combined and separate ablations oftheentorhinalandperirhinalcortexinrhesusmonkeys.Journal of Neruoscience, 13, 5418–5432.
- Miller, E. K. (2000). The prefrontal cortex and cognitive control. Nature Reviews Neuroscience, 1, 59–65.
- Morris, R. G. M., Garrud, P., Rawlins, J. P., & O’Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683.
- Murray, E. A., & Bussey, T. J. (1999). Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences, 3, 142–151.
- Murray, E .A., & Mishkin, M. (1998). Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. Journal of Neuroscience 18(16), 6568–6582.
- O’Keefe, J. A., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford, UK: Oxford University Press.
- Olton, D. S., Becker, J. T., & Handlemann, G. E. (1979). Hippocampus, space, and memory. Brain and Behavioral Sciences, 2, 313–365.
- Otto, T., & Eichenbaum, H. (1992). Complementary roles of orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed non-matching to sample task. Behavioral Neuroscience, 106, 763–776.
- Packard, M. G., & McGaugh, J. L. (1992). Double dissociation of fornix and caudate nucleus lesions on acquistion of two water maze tasks: Further evidence for multiple memory systems. Behavioral Neuroscience, 106, 439–446.
- Packard, M. G., & McGaugh, J. L. (1996). Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory, 65, 65–72.
- Rampon, C., Tang, Y.-P., Goodhouse, J., Shimizu, E., Kyin, M., & Tsien, J. (2000). Enrichment induces structural changes and recovery from non-spatial memory deficits in CA1 NMDAR1knockout mice. Nature Neuroscience, 3, 238–244.
- Ramus, S. J., & Eichenbaum, H. (2000). Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. Journal of Neuroscience, 20, 8199–8208.
- Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20, 11–21.
- Squire, L. R., & Zola, S. M. (1998). Episodic memory, semantic memory and amnesia Hippocampus, 8, 205–211.
- Steele, R. J., & Morris, R. G. M. (1999). Delay dependent impairment in matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus, 9, 118–136.
- Suzuki, W. A. (1996). Neuroanatomy of the monkey entorhinal, perirhinal, and parahippocampal cortices: Organization of cortical inputs and interconnections with amygdala and striatum. Seminars in the Neurosciences, 8, 3–12.
- Thompson, R. F., & Kim, J. J. (1996). Memory systems in the brain and localization of a memory. Proceedings of the National Academy of Sciences, 93, 13438–13444.
- Tolman, E. C. (1951). Purposive behavior in animals and men. Berkeley: University of California Press. (Original work published 1932)
- Tulving, E., & Schacter, D. L. (1990). Priming and human memory systems. Science, 247, 301–306.
- Vargha-Khadem, F., Gadin, D. G., Watkins, K. E., Connelly, A., Van Paesschen, W., & Mishkin, M. (1997). Differential effects of early hippocampal pathology on episodic and semantic memory. Science, 277, 376–380.
- Viaud, M., White, D., & Norman, M. (1989). Dissociation of visual and olfactory conditioning in the neostriatum of rats. Behavioural Brain Research, 32, 31–42.
- Wan, H., Aggleton, J. P., & Malcolm, W. B. (1999). Different contributions of the hippocampus and perirhinal cortex to recognition memory. Journal of Neuroscience, 19, 1142–1148.
- Young, B. J., Otto, T., Fox, G. D., & Eichenbaum, H. (1997). Memory representation within the parahippocampal region. Journal of Neuroscience, 17, 5183–5195.
- Zola, S. M., Squire, L. R., Teng, E., Stefanacci, L., Buffalo, E. A., & Clark, R. E. (2000). Impaired recognition memory in monkeys after damage limited to the hippocampal region. Journal of Neuroscience, 20, 451–463.
- Zola-Morgan, S., Squire, L. R., Amaral, D. G., & Suzuki, W. (1989). Lesions of perirhinal and parahippocampal cortex that spare the amygdala and the hippocampal formation produce severe memory impairment. Journal of Neuroscience, 9, 4355–4370.




