View sample comparative psychology of motor systems research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Locomotor Networks: Introduction and History
In the animal kingdom, various kinds of locomotion—such as swimming, walking, flight, and crawling—have evolved. Understanding locomotor function is of vital scientific interest, because locomotion serves as multipurpose behavior in various more complex behavioral programs and issues. Understanding the neuronal mechanisms underlying locomotion has long attracted scientists as a case study well suited to understanding nervous system function in general and for medical use and robotics.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
To understand locomotor systems requires a multilevel approach ranging from the cellular level (i.e., identifying the neurons involved, their intrinsic properties, the properties of their synaptic connections, and the role of particular transmitters and neuromodulators) to the system level (i.e., determining the functional integration of these networks in complete motor programs). Our current understanding of locomotor networks is the outcome of investigating and comparing various invertebrate and vertebrate locomotor systems in which rhythmic behaviors can be studied on multiple levels, ranging from the interactions between identifiable neurons in identified circuits to the analysis of gait. This review will focus on (a) the principles of cellular and synaptic construction of central pattern-generating networks for locomotion, (b) their location and coordination, (c) the role of sensory signals in generating a functional network output, (d) the main mechanisms underlying their ability to adapt through modifications, and (e) basic features in modulating the network function.
Due to the limited space available for this introduction to this lively and fast-developing field in neurosciences, the authors will restrict citations mostly to recent, in-depth reviews on individual aspects mentioned and will refer to original articles only as specifically needed.
Understanding the Act of Progression: Historical Aspects
The problem of how locomotion is generated has been considered for more than 2,400 years, starting in the time of Aristotle (about 400 B.C.). Between the second and third centuries A.D., the Aristotelian concept of a vital pneuma (transformed from the omnipresent ether by the lungs and transported by the bloodstream to the muscles) as the ultimate cause underlying locomotor ability was first modified by Galen, who discovered that nerves originating in the brain and spinal cord innervate the muscles. In the seventeenth century, Descartes and Borelli integrated Galen’s discoveries in a more mechanically based theory, suggesting that muscles contract by a corpuscular animal spirit released from the nervous system. In the mid-nineteenth century (and based on the work in the eighteenth century by Schwammerdam, Galvani, and others), Matteucci, Helmholtz, and du Bios-Reymond discovered the electrical properties of axons and their implication for neuromuscular transmission (the modern term synapse was adopted much later by Sherrington in 1897). Benefiting from the general progress in detailed anatomical knowledge and with a new basic concept (Cajal’s neuron doctrine), late nineteenth-century physiology initiated a common understanding of the nervous system’s function and its role in the generation and control of behavior (for an in-depth review of the early history, see Bennett, 1999).
The Neural Basis of Locomotor Pattern Generation: A First Concept
At the end of the nineteenth century, the discovery of proprioceptive pathways in the nervous system (e.g., by Bell, Golgi, and Kühne in the mid- nineteenth century; early review: Sherrington, 1900), the description of numerous different reflex responses in the limbs of monkeys, dogs, and cats after skin or nerve stimulation (establishing what are called reflex laws; Pflüger, 1853) and the apparent resemblance of these reflex responses, including scratch reflexes and spinal stepping, to parts of the limb-movement cycle during real locomotion led to the idea that the antagonistic activation of effector organs during locomotion might be triggered by feedback from sense organs in the skin and the moving parts of the body. Coordinated limb movement during locomotion was thought to be the result of a chain arrangement of these reflex arcs. Remarkably, this concept already included the principle of a reciprocal innervation of antagonistic muscles (Sherrington, 1905a, 1905b) and the demonstration of postural reflexes (Sherrington, 1900a).
Toward a Concept of Central Control of Locomotion
In the early twentieth century, the putative role of the spinal cord in the basic generation of locomotion was established by experiments in mammals, mainly in the dog and the cat, that could still produce alternating leg movements after the brain was disconnected (Brown, 1911, 1912; Sherrington, 1905a, 1905b). In cats, Brown (1911) demonstrated that the spinal cord was capable of producing locomotor patterns after complete deafferentation of the moving limb. He concluded that alternating rhythmic movements derive from a central spinal process and proposed a simple half-center model as the basis of the alternating activity of flexors and extensors during walking: Each half-center is responsible for activating either flexors or extensors, and both half-centers are connected by reciprocal inhibition in order to silence one center while the other is active. Muchlater, when reciprocal inhibition was first shown on the interneuron level in the spinal cord (Jankowska, Jukes, Lund, & Lundberg, 1967a, 1967b), the suggested spinal network organization incorporated the basic features of Brown’s half-center model.
The Concept of a Central Pattern Generator (CPG)
The combined evidence from the first half of the twentieth century suggested that the central nervous system does not necessarily require sensory feedback to generate rhythmic movement resembling repetitive behaviors such as locomotion. This conclusion emerged from experiments in a variety of invertebrate and vertebrate species, in which the ability to generate a patterned rhythmic activity was not abolished by (a) paralysis using neuromuscular blockers to prevent proprioceptive input evoked by movements, (b) deafferentation, or (c) the complete physical isolation of the nervous system from all sources of possible feedback (for a review, see Grillner, 1985; see also Delcomyn, 1980). The ensemble of neuronal elements necessary and sufficient for the production of locomotor patterns was defined as a central pattern generator (CPG; Grillner & Zangger, 1975; Wilson, 1961). However, since the motor patterns observed after deafferentation are often imprecise and sometimes even lack important elements of motor output as compared to intact conditions (e.g., Grillner & Zangger, 1979; Pearson, 1985; Sillar, Clarac, & Bush, 1987), the validity of such a concept for completely central locomotor pattern generation was questioned (e.g., Bässler, 1987, 1988; Pearson, 1985). At present it is clear that, in the majority of locomotor systems, sensory feedback and CPG networks interact to generate the functional locomotor program, whereby sense organs form integral elements of the pattern-generating mechanisms (e.g., see the review in Büschges & El Manira, 1998; Pearson, 1995; Prochazka & Mushahwar, 2001) with only few exceptions (e.g., Arshavsky, Orlovsky, Panchin, Roberts, & Soffe, 1993).
Organization of Neural Networks for Locomotion
Most locomotor patterns have in common that they are based on rhythmic movements (i.e., cyclic motor patterns). Each cycle can be generally divided into two phases, a power stroke and a return stroke. During the power stroke, locomotor organs exert force against the surrounding medium and move the organism relative to its environment; during the return stroke, the locomotor organs are moved back to their starting position for the next power stroke. In walking, for example, the power stroke of the locomotor cycle is the stance phase, when the limb is on the ground and generates force to propel the body relative to the ground, either forward or backward. The return stroke of the leg is the swing phase, during which the leg is moved back to its starting position. In general, antagonistic muscles of leg joints exhibit phases of alternating activity during the generation of stepping movements. In vertebrates like fish or agnaths, swimming is generated by a rostral-caudally or caudal-rostrally directed undulating contraction wave, depending on the direction of swimming. This contraction wave wanders along the trunk musculature. In every cycle, the myotomal musculature of both sides of each segment contracts in an alternating fashion.
In the next section we will review the main features of current knowledge of the construction of neural networks and the mechanisms underlying the generation of locomotor patterns in vertebrates and in invertebrates.
When considering the generation of locomotor programs, several different aspects and levels of nervous control are important (Figure 5.1). For a very detailed review of this, see Orlovsky, Deliagina, and Grillner (1999), where the following summary of present knowledge was presented. The highest level of control is represented in the decision to locomote. Activation of this system is mediated by external or internal cues, such as sensory stimuli or motivation. The decision to locomote activates two different systems of descending control. One system has command-like features and controls the starting and stopping of the locomotor program as well as the intensity (e.g., speed) of locomotion. In vertebrates, the reticulospinal pathways, receiving signals from the mesencephalic locomotor region and the subthalamic locomotor region and sending their axons into the spinal cord, are elements of this system (e.g., Armstrong, 1988; Jordan, Brownstone, & Noga, 1992; Mori, Matsuyama, Kohyama, Kobayashi, & Takakusaki, 1992; Orlovsky et al., 1999).
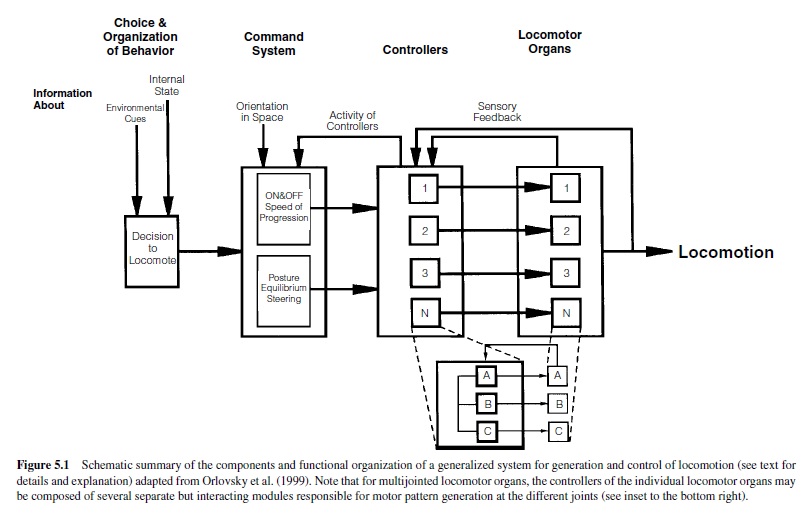
In invertebrates, groups of interneurons or individual descending interneurons have been identified that serve command-like functions in the initiation and maintenance of motor programs (e.g., Bowerman & Larimer, 1974a, 1974b; Brodfuehrer & Friesen, 1986a, 1986b, 1986c; Gamkrelidze, Laurienti, & Blankenship, 1995; Kupfermann & Weiss, 1978). The second system is in charge of generating and controlling the animal’s posture and equilibrium during locomotion, as well as its direction of locomotion. In vertebrates, the cerebellum, brainstem, and spinal cord serve this system; in invertebrates, this system is distributed among various ganglia. The information from these two systems is fed into the neuronal networks of the locomotor system itself, the controllers, located downstream close to the locomotor organs in spinal segments (vertebrates) or ganglia (invertebrates). The construction and action of these controllers will be our main focus. The controllers (Figure 5.1) encompass the neuronal networks, including the CPGs that generate activity of the locomotor organs by driving specific sets of motoneurons. These motoneurons form the neuronal output stage and innervate the muscles moving the locomotor organs. Rhythmic motoneuron activity is generated by alternating excitatory and inhibitory synaptic impulses from the premotor neural networks, the controllers, to the motoneurons. The generation of functional locomotor programs often relies on feedback about the executed action from each level to the next higher level. Information about the activity of the controllers is fed back to the command level. Sensory information reporting the actual movement generated by the locomotor organs is fed back to the controllers. Therefore, in many locomotor systems, sense organs have to be considered important elements enabling the systems to generate functional locomotor programs (see also Orlovsky et al., 1999). Finally, locomotor systems consisting of a multitude of locomotor organs need to generate coordinating mechanisms to adjust and time the sequence of movements among the individual locomotor organs (e.g., Cruse, 1990).
Marked differences appear to exist in the degree of coupling between the actions of individual controllers for locomotor systems, on the one hand, and a multitude of locomotor organs, on the other. For example, evidence suggests that the wing-control system in the locust flight system acts in general as one integrated common pattern generator for driving all four wings (Robertson & Pearson, 1983; Waldron, 1967). However, more recent evidence gathered from various vertebrate and invertebrate organisms suggests that each locomotor organ may indeed have its own controller—that is, each segment of a lamprey for swimming (Grillner et al., 1995), each leg of a vertebrate or invertebrate (Bässler & Büschges, 1998; Orlovsky et al., 1999) and each leg of a human (Gurfinkel, Levik, Kazennikov, & Selionov, 1998) for walking, and each wing of an insect for flying (Ronacher, Wolf, & Reichert, 1988). The construction of such controllers has been well studied for the generation of the swimming motor pattern in mollusks and lower vertebrates (Arshavsky et al., 1993; Grillner et al., 1995) and annelids (Brodfuehrer, Debski, O’Hara, & Friesen, 1995) and for walking pattern generation in crustaceans (Cattaert & LeRay, 2001), insects (Bässler & Büschges, 1998), anurans (Cheng et al., 1998), and mammals (Orlovsky et al., 1999). In humans, less evidence is presently available on the construction principles of the limb controllers themselves, but present data suggest that the main features in the organization of the walking control system of humans has similarities to those of both cats and arthropods (for a recent summary and comparison, see Orlovsky et al., 1999).
The complexity of the controllers’ construction depends on (a) the complexity of the locomotor organs and (b) the requirements of the locomotor behavior to be generated out. Thus, the complexity of the controllers increases with the segmentation of the locomotor organs, from unitary wings to multisegmented legs. For example, in Clione, a mollusk, the swimming motor activity is generated by elevation and depression of wing-like appendages (Arshavsky et al., 1998). In invertebrates and vertebrates, however, walking is generated by the movements of multijointed limbs, which requires the coordination of the activities of several individual leg joints (Bässler, 1983; Grillner, 1979; Orlovsky et al., 1999). Controllers that need minimal sensory feedback, such as the one controlling locomotion in Clione, are constructed more simply than are controllers governing locomotor programs that depend on sensory feedback, such as walking systems. Pattern generation in the latter locomotor systems relies heavily on sensory signals about movements of the joints and the limbs, signals about forces or strain exerted on each segment of the limb, and coordinating signals between adjacent limbs (e.g., Bässler & Büschges, 1998; Pearson, 1995; Prochazka, 1996a). This also applies to the walking system of humans (Dietz, 1992; Gurfinkel et al., 1998; Sinkjaer, Andersen, Ladouceur, Christensen, & Nielsen, 2000).
Construction Principles of Pattern-Generating Networks for Locomotion
Central neuronal networks have been identified that are capable of generating ongoing rhythmic activity in motoneurons that contribute to the cyclic locomotor output generated for swimming, walking, and flying in vertebrates and invertebrates (described previously). These networks can also be activated in very reduced preparations either by the application of drugs, by sensory stimulation, or by stimulation of higher order centers in the central nervous system (for a comparative review, see Orlovsky et al., 1999). Using these approaches, more or less complete patterns of a locomotor program can be generated, allowing their detailed investigation. Neuronal networks have been analyzed on several levels: first, the operational level, focusing within the systems level on mechanisms for the generation of functional motor programs (e.g., Bässler & Büschges, 1998; Grillner, 1979); second, the level of the neuronal networks themselves, analyzed by investigating the topologies of the neural network and the synaptic interactions among its elements (e.g., Arshavsky et al., 1993; Bässler & Büschges, 1998; Grillner et al., 1995; Kiehn, Housgaard, & Sillar, 1997; Roberts, 2000); and third, a subject that has drawn a lot of attention lately: the cellular and subcellular levels and the cellular properties of individual neurons or neuron classes within the networks and their role in generating rhythmic locomotor activity (e.g., Dale, 1997; Grillner, Wallen, Hill, Cangiano, & El Manira, 2001). Finally, in the past two decades, simulation studies using artificial neural networks or computer models have increasingly helped to investigate the necessity and sufficiency of neuronal mechanisms and the construction of the neuronal networks underlying the generation of locomotor patterns as presently understood (e.g., reviews in Cruse et al., 1995; Grillner et al., 1995).
Despite differences among phyla, species, and locomotor tasks, it has become clear by now that there are some specific common outlines of networks generating rhythmic locomotor activity. Two prominent basic neural network topologies have been identified: mutual inhibition and forward excitation–reciprocal inhibition.
Mutual Inhibition
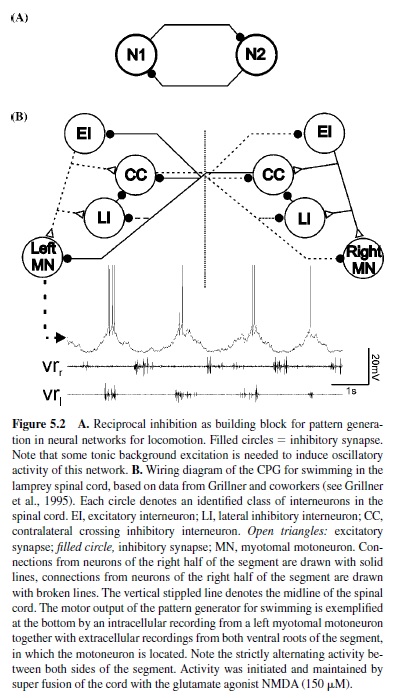
This construction principle found in various locomotor systems is based on mutual inhibition between neurons or groups of neurons within the neuronal networks (Figure5.2, panel A). Each group of neurons is in charge of generating one phase of the locomotor activity. Through this mechanism, only one group of neurons is active at any given time once the activity of the network has been started. Transition between the activity of the two neurons or groups of neurons emerges through mechanisms that either generate fatigue in the activity of the currently active group of neurons or enable the silent, inactive group of neurons to escape inhibition. Such topology is called half-center construction, and, long before experimental verification was possible, Brown (1911) conceived it for the generation of alternating activity during stepping in the cat. Mutual inhibition has been identified as a building block underlying the generation of alternating motor activity in the neuronal networks for swimming in vertebrates (lampreys [Buchanan, 1982; Grillner, 1985] and tadpoles [Roberts, Dale, Evoy, & Soffe, 1985; Soffe, Clarke, & Roberts, 1984]) and for swimming and other locomotor behaviors, such as crawling, in invertebrates (mollusks [Arshavsky, Beloozerova, Orlovsky, Panchin, & Pavlova, 1985a, 1985b, 1985c, 1985d; Getting, 1981; Getting, Lennard, & Hume, 1980; Katz, Getting, & Forst, 1994] and annelids [Friesen & Hocker, 2001; Friesen, Poon, & Stent, 1978; Stent et al., 1978]). For example, in the lamprey, swimming-network alternating activity of both sides of each segment is based on mutual inhibition between groups of crossed inhibitory neurons on each side of the spinal cord (Figure 5.2, panel B; Buchanan, 1982; Grillner, 1985).
Forward Excitation and Reciprocal Inhibition
Another identified network interaction is forward excitation from one neuron to another neuron via a delay and reciprocal inhibition from the second neuron to the first neuron (Figure 5.3, panel A). In the CPG network for locust flight, this element has been found to underlie alternating activation of wing elevator and depressor motoneurons, representing some kind of switch-off mechanism (Figure 5.3, panel B; Robertson & Pearson, 1983, 1985). For example, activity of one neuron (type 301) increases and excites another neuron (type 501) through the action of an excitatory influence with a certain delay. At some point, neuron 501 is pushed past its spike threshold and activated. Its activity then in turn terminates the activity of 301 through the inhibitory synapse (Robertson & Pearson, 1985). Ongoing rhythmic activity in such a circuit relies on some mechanism that enables neuron 301 to have pacemaker or burst-producing properties (described presently).
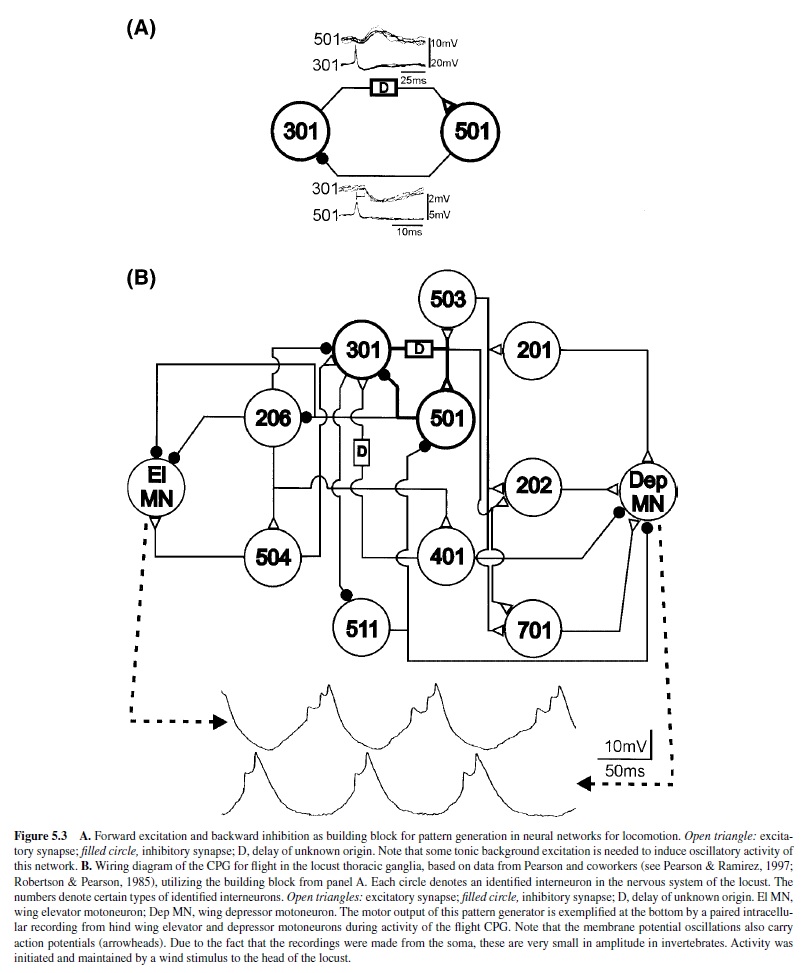
Finally, it is now known that other locomotor systems also combine different types of building blocks for the generation of rhythmic motor patterns, as with the locomotor network of the nudibranch Tritonia, which includes both elements previously described (Getting, 1981; Getting et al., 1980; Katz et al., 1994).
Although no definite information on network topology is available at this time, the finding that spinalized primates can produce locomotor patterns provides evidence that CPGs for locomotor activity also exist in the spinal cords of higher mammals (Fedirchuck, Nielsen, Petersen, & Hultborn, 1998). Evidence is also growing for the existence of spinal CPGs’ controlling locomotion in humans (Calancie et al., 1994; Dimitrijevic, Gerasimenko, & Pinter, 1998).
In general, locomotor networks are constructed redundantly—that is, they contain multitudes of these small neuronal circuits (e.g., five in case of the locust flight CPG; Grimm & Sauer, 1995). They thereby gain substantial robustness against synaptic noise or functional failure in individual neuronal elements.
The pattern of activity generated by locomotor networks need not be two-phased, as the motor output often suggests. Locomotor networks can be constructed and operate in a way that leads them to generate a rhythm with more than the two phases that are obvious from the locomotor program. For example, the locust flight system generating a two-phase motor output for wing elevation and depression is driven by the output of a three-phase neuronal network (Robertson & Pearson, 1985).
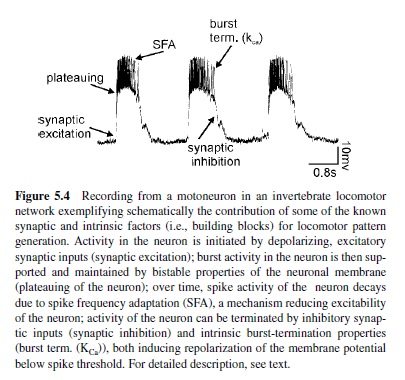
As the previous description makes clear, synaptic interactions within neuronal networks are important prerequisites for generating the rhythmic motor activity underlying locomotion. In addition, intrinsic properties of neurons contribute to and cooperate with the network topology in the generation of rhythmic motor activity. Intrinsic properties of neurons are generated within neurons themselves and have been studied in great detail. Some of the most prominent ones are summarized in Figure 5.4 and will be briefly introduced here:
- Plateau potentials. Besides the generation of action potentials, neurons can be capable of generating plateau potentials, which are spike-like, quasi-stable operating characteristics. A plateau potential is basically a prolonged, rather slow regenerative depolarization (Hille, 1991). It usually results from a voltage-dependent inward current mechanism: Sufficient depolarization initiates an inward current flow, which causes further depolarization, leading to a self-sustained depolarized state of the neuron. The membrane potential remains in this depolarized state for some time. Since the membrane potential of the plateau is usually above spike threshold, the plateau phase is characterized by burst activity of the neuron. Asufficient hyperpolarizing synaptic input or a mechanism for burst termination (discussed next) can terminate the plateau by turning off the voltagedependent inward current. In-depth reviews on the role of this property for pattern generation are found in Kiehn et al. (1997), Marder and Calabrese (1996), and Pearson and Ramirez (1992).
- Burst termination properties. In addition to inhibitory synaptic inputs, intrinsic properties of neurons can contribute to terminating bursts of activity. One example is the calcium-dependent potassium (KCa) channel (Hille, 1991). During strong bursts of action potentials, calcium ions enter a neuron through cation channels underlying the depolarization and the burst of activity. Over time, this leads to an accumulation of Ca2 ions in the neuron, which in turn activate KCa The potassium outward current initiates a hyperpolarization of the neuron below its spike threshold and thereby terminates its depolarization and activity (e.g., Grillner & Wallen, 1985; in-depth review by Grillner et al., 1995).
- Spike frequency adaptation (SFA). There are presently many examples of the activity of neurons adapting over time to a given depolarization in membrane potential. The mechanism behind this phenomenon is often the slow after-hyperpolarization (sAHP) that follows each action potential (Hille, 1991; Schwindt & Crill, 1984). The sAHP is generated by KCa With the generation of action potentials, not only Na but also Ca2 ions enter a neuron. These Ca2 ions activate a KCa channel, which initiates an sAHP of the neuron following spike activity. sAHPs accumulate over time and can thereby reduce the excitability of a neuron and thus its activity (in-depth review in Grillner et al., 2000).
- Intrinsic oscillations or endogenous bursting. Neurons can be capable of steadily producing phases of alternating activity consisting of bursts and silence. This property is called endogenous bursting or intrinsic oscillation (Hille, 1991). The underlying ionic mechanisms are diverse here, as well. For example, the active phase of a neuron can display similarities to plateau potentials. There are also automatic ionic mechanisms, that is, conductances in the neuron that terminate activity after some time by opening ion channels (e.g., K channels). These allow an outward current to hyperpolarize the membrane potential below spike threshold. The next cycle of activity is then started either by rebound properties of the neuron or by a tonic background excitation (Grillner & Wallen, 1985; Hochman, Jordan, & Schmidt, 1994; Sigvardt, Grillner, Wallen, & Van, 1985; Sillar & Simmers, 1994b).
Through the action of premotor neuronal networks, a basic rhythmic activity is generated that must be modified for a functional locomotor pattern, depending on the complexity of the locomotor organs and the locomotor task executed. Getting (1989) coined the term building block for identified types of network connections, synaptic properties, and intrinsic neuronal properties in charge of generating rhythmic motor activity.
The controllers of the locomotor organs can contain a multitude of such pattern-generating networks. Where the locomotor organ is segmented (e.g., for walking), the number of pattern-generating networks can be increased as well (Büschges, Schmitz, & Bässler, 1995; Cheng et al., 1998; Edgerton, Grillner, Sjöström, & Zangger, 1976). For example, in the stick insect walking system, each of the three main joints of each leg is driven by an individual neural network capable of generating rhythmic motor activity (Büschges et al.). The activity of the individual pattern generators can be coupled by sensory signals (e.g., Hess & Büschges, 1999; summary in Bässler & Büschges, 1998; and discussed later in this paper). Similar results have recently been presented for the cervical spinal cord controlling the forelimb of a vertebrate, the mudpuppy (Cheng et al.). In this investigation, evidence was presented that the motoneuron pools innervating the elbow joint, that is, the flexor and extensor, are driven by one central pattern-generating network for each of the two antagonistic muscle groups moving the tibia—the flexor and the extensor. These findings verified an old hypothesis, the unit-burst generator concept initially proposed by Edgerton et al., who suggested that there are unitary central patterngenerating networks present in the vertebrate spinal cord for each muscle group of the limb.
The basic rhythmic activity of the pattern-generating networks is shaped for a functional locomotor output by sensory signals from the locomotor organs and synaptically transmitted to the output elements of the locomotor system, the motoneurons. There are only a few examples of locomotor systems in which motoneurons themselves are elements of the pattern-generating networks, for example, in crustaceans (Chrachri & Clarac, 1989), annelids (Poon, Friesen, & Stent, 1978), and a lower vertebrate, the tadpole (Perrins & Roberts, 1995a, 1995b, 1995c).
Location of Pattern-Generating Networks for Locomotion
As stated previously, rhythmic locomotor activity is generated within the controllers of the locomotor organs. These controllers are the neuronal networks located in the central nervous system (CNS), mostly in close apposition to locomotor organs (i.e., in the segments from which the locomotor organs arise and from where they are innervated). Segmental organization of the organism or segmental structure of the locomotor organs has no prejudicative meaning for the localization of the pattern-generating networks in the nervous system. Let us consider the generation of locomotor patterns on the level of rhythmic activity that drives one locomotor organ, for example, the chain of myotomal segments in swimming in the lamprey and the tadpole, each wing in flying (or swimming), or each limb in terrestrial locomotion.
With few exceptions, the controllers for locomotion are distributed across several segments of the CNS. The patterngenerating network for locust flight is a distributed neuronal network encompassing the three thoracic ganglia and some condensed abdominal neuromeres attached to the metathoracic ganglion (Robertson & Pearson, 1985). Distribution is also present in the walking-pattern-generating networks of vertebrates, for example, for the forelimb in the cervical spinal cord (Cheng et al., 1998) and for the hindlimb in the lumbar spinal cord (e.g., Cazalets, Borde, & Clarac, 1995; Kjaerulff & Kiehn, 1996). In the lamprey and the tadpole, the CPG for swimming that drives the chains of myotomal segments is distributed along the segments of the spinal cord (as discussed previously). Similarly, the pattern-generating networks for crawling and swimming in the leech are distributed along the chain of segmental ganglia (comparative summary in Orlovsky et al., 1999). However, these locomotor systems are special in the sense that, for swimming, the motor pattern results from the coordinated action of the subsequent segments of the organism; that is, the locomotor organ is the organism itself. For all controllers generating swimming movements, it is known that the nervous system of each individual segment contains neural networks capable of generating a rhythmic motor output for the segment. This is most obvious in the CPG for swimming in Clione, which is generated by a network of interneurons, most of which are located in the pedal ganglia of the nonsegmented organism (Arshavsky et al., 1985a, 1985b, 1985c, 1985d, 1985e). Only in arthropod walking systems has a clear segmental organization been found, with the controller of each leg restricted mainly to the segmental ganglion of the locomotor organ, which has been studied in great detail for the stick insect (summary in Bässler & Büschges, 1998).
Regarding mammalian locomotion, important new findings were recently presented on the organization and location of the pattern-generating networks for the individual limbs. Individual neuronal networks for the generation of rhythmic motor activity for both elbow flexor and elbow extensor motoneuron pools were identified in the mudpuppy forelimb (Cheng et al., 1998). The data presented support the unitburst generator concept of locomotion described earlier. Similarly, lesion experiments in the neonatal rat lumbar spinal cord have revealed that the CPGs controlling hindlimb movements are distributed throughout the hindlimb enlargement and most likely also in the lower thoracic cord (Cazalets et al., 1995; Kjaerulff & Kiehn, 1996). Together with additional evidence, this suggests that in mammals, too, the CPG for the hindlimb is not a unitary entity, but is again composed of several unit-burst generators controlling single muscles or joints. Between the lumbar segments, the capability to generate rhythmic activity declines from rostral to caudal (summary in Kiehn & Kjaerulff, 1998). In patients with spinal cord injuries, it was reported that the higher the level of the injury was, the more normal the locomotor pattern appeared (Dietz, Nakazawa, Wirz, & Erni, 1999). This indicated that, also in humans, the CPGs for locomotion are not restricted to specific levels of the spinal cord.
Sensory Signals Controlling Locomotor Activity
In the majority of locomotor systems, sensory signals are utilized, first, to generate a functional locomotor pattern, and second, to stabilize the locomotor pattern, by adapting to biomechanical changes and responding to unexpected events. Third, sensory information plays a crucial role in controlling the posture and equilibrium of the locomotor system during the behavioral task (Macpherson, Fung, & Jacobs, 1997; Orlovski et al., 1999). Such information is gathered from multiple sensory systems and integrated in the networks controlling locomotion and related to the current position and condition of the body and the limbs (e.g., the phase of a movement). The dependence of motor control systems on proprioceptive signals has been well characterized in a statement by Prochazka (1996b): “You can only control what you sense.” Proprioceptors located in muscles and joints characterize the positions of the limbs, and together with exteroreceptors they sense contact with the ground or obstacles and the load carried by the limb. Their general role is to establish the temporal order of the locomotor pattern and to reinforce ongoing activity (Bässler & Büschges, 1998; Duysens, Clarac, & Cruse, 2000; Grillner, 1979; Pearson, 1995; Pearson & Ramirez, 1997; Prochazka).
Other sensory information involved in the generation and control of locomotor behavior itself is provided by visual cues. Visual cues play a decisive role in controlling goal direction in locomotion, allowing the preadjustment of the locomotor activity and the interpretation of visual flow that yields information on walking speed and direction (review in Rossignol, 1996b). Furthermore, together with the vestibular apparatus or comparable gravity-sensor systems in invertebrates, visual input is involved in controlling the body’s orientation in space. This is especially important for animals locomoting in a three-dimensional environment (i.e., flying or swimming; Orlovsky et al., 1992; Reichardt, 1969; Ullen, Deliagina, Orlovsky, & Grillner, 1995).
The following sections briefly review the major sensory systems and their roles in the control of locomotion.
Visual Regulation of Locomotion
Visual control of locomotion is very powerful. Apparently, visual input is used to direct locomotion, to avoid obstacles on the way to reach a target, and for orientation. Due to its complex nature, very little was known until recently about the visual control of locomotion; however, advances in computer technology allowing artificial simulation of the optical system (as with virtual reality; Warren, Kay, Zosh, Duchon, & Sahuc, 2001) provided deeper insight into the mechanisms of visuomotor control.
As introduced by Gibson (1958), movements of the body in space generate a continuously changing pattern of motion on the retina, a pattern referred to as optic flow. This selfinduced optic flow must be distinguished in speed and direction from the optic flow induced by moving objects. Confusion about this distinction is typically experienced by a person who is sitting in one train and observing another train, and is unable to identify which train is moving. Generally, optical flow is used to assess the velocity of the locomotion and the direction of self-movement. Consequently, information gained from visual flow is used to control multiple aspects of locomotion, including goal-directed spatial behavior, locomotor speed, and gross adaptation to environmental changes. The association of changes in optic flow with changes in movement is so strong that artificially induced or perturbed optic flow can modulate the velocity and direction of locomotion or even initiate locomotor behavior. This is an observation commonly found throughout the animal kingdom, for example in lobsters and crayfish. A front-to-rear optokinetic stimulation provided by horizontal stripes on an underlying treadmill can trigger forward locomotion with the velocity depending on the velocity of the stripes (Davis & Ayers, 1972). Insects flying tethered inside a striped drum will tend to turn in the direction in which the drum is rotated (Reichardt, 1969), and expanding the size of a target during the time a gerbil is walking (giving the impression that the target is getting closer) causes the animal to decrease its velocity (Sun, Carey, & Goodale, 1992).
Powerful effects of changes in visual flow have also been reported in humans. For example, during forward walking in a room in which the walls can be displaced, moving them forward (instead of backward as it would appear during forward locomotion) will create the impression of walking backward, despite contradictory proprioceptive signals from the limbs (Lee & Thompson, 1982). Toddlers who have just learned to walk will tip over if the walls are set in motion (Stoffregen, Schmuckler, & Gibson, 1987). Comparable experimental approaches showed that, as in animals, walking velocity in humans is adjusted to visually perceived walking speed (Konczak, 1994; Prokop, Schubert, & Berger, 1997). Thus, visual input during locomotion in vertebrates and invertebrates is used not only to avoid obstacles, but also to guide locomotor direction and velocity.
In the field of visual locomotor control, there is an ongoing discussion whether visual flow is the dominant optical influence on target-directed locomotion or whether the walking direction is simply determined by the current body orientation and the perceived direction of the target. In light of this dispute, it has recently been demonstrated that humans do not guide locomotion by relying either on visual flow alone or on egocentric direction alone. Both components are probably used in a complementary way—for example, when the optical flow is reduced or distorted: On a grass lawn or at night, behavior tends to be governed by egocentric direction (Harris & Carre, 2001; Rushton, Harris, Lloyd, & Wann, 1998; Warren et al., 2001; Wood, Harvey, Young, Beedie, & Wilson, 2000).
Visual information is also essential to perform anticipatory foot placement in order to avoid obstacles. To avoid bumping into an object, it is crucial to calculate how much time is left for corrective action. The distance to the obstacle is relevant only in relation to the speed of self-motion. Thus, visual information is used in a feed-forward rather than an on-line control mode to regulate locomotion. Humans do not fixate on obstacles as they step over them, but perform a planning in the steps before (Hollands & Marple Horvat, 1996, 2001; Patla, Adkin, Martin, Holden, & Prentice, 1996; Patla & Vicker, 1996). This feed-forward information is very important in the control of walking, and it is not possible to walk more than 8 s or 5 m without visual feedback (Lee & Thompson, 1982).
Compared to our knowledge of invertebrate systems, knowledge about the mechanisms of visual locomotor control in vertebrates is rather incomplete. The approach of recording cortical cells during visually guided locomotion in cats demonstrated increased firing in pyramidal cells when modification of the step cycle was required to clear an obstacle (Drew, Jiang, Kably, & Lavoie, 1996). It has been suggested that the increased discharge is used to modify the step cycle, since it has been shown that inactivation of these cortical areas results in the inability to clear visible obstacles.Acomparable feed-forward mechanism to anticipate collision was recently described in locusts (Hatsopoulos, Gabbiani, & Laurent, 1995). The lobula giant motion detectors (LGMD) in the locust optic lobe (Strausfeld & Nässel, 1981) are neurons that receive inputs from afferents that are sensitive to local motion over the entire visual hemifield (Rowell, O’Shea, &Williams, 1977) and that respond most strongly to objects approaching the eye (Rind & Simmons, 1992). LGMD synapse directly to a large neuron (descending contralateral motion detector, or DCMD), which is involved in the generation and control of flight and jump maneuvers (Pearson, Heitler, & Steeves, 1980; Robertson & Pearson, 1983). In the visual system of the fly, a number of processing stages have been identified and several interneurons with sensitivity to various directions of motion have been found (Borst & Egelhaaf, 1989), making the visual system of flies the best-described model in visual processing (reviewed in Krapp, 2000).
Proprioceptive Regulation of Locomotion
The question of how proprioceptive signals regulate locomotor activity has been an intensive field of research, including studies in humans, cats, and various arthropods (reviewed in Büschges & El Manira, 1998; Duysens et al., 2000; Pearson, 1995; Pearson & Ramirez, 1997; Prochazka, 1996b). These studies made it clear that there are common principles in the proprioceptive control of locomotion throughout the entire animal kingdom, indicating their general importance in the generation of functionally relevant locomotor movements (Pearson, 1993). A prominent example providing evidence that local proprioceptive reflex pathways are strongly involved in the control of stepping is the finding that spinalized cats walking with their hindlimbs on a treadmill are able to adapt the speed of stepping to the speed of the treadmill belt (Brown, 1911). The only explanation for this ability is that sensory feedback from proprioceptors in the limbs is involved in controlling the step cycle. Generally, proprioceptive feedback serves two separate functions in the control of locomotion: (a) the control of phase transition and (b) the regulation of the magnitude of muscle activity.
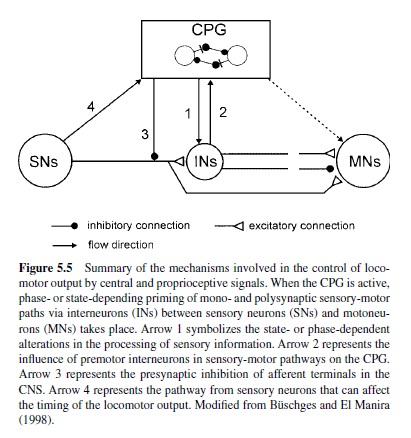
A common principle in sensory control of locomotion is the task- or phase-dependency of sensory feedback. Transmission in proprioceptive reflex pathways, for example, strongly depends on the motor task and the phase of the movement (reviewed in Büschges & El Manira, 1998; Pearson, 1995). For example, reflex pathways can generate opposite motor outputs of varying strength or gain, depending on the actual behavior or the phase of the movement (e.g., standing compared with walking; Figure 5.5). This phenomenon has been termed reflex reversal and is found in vertebrates (Pearson, 1993, 1995; Prochazka, 1996b) as well as in invertebrates (Bässler & Büschges, 1998; Cattaert & LeRay, 2001; Clarac, Cattaert, & LeRay, 2000). This flexibility of reflex pathways ensures that the motor output is adjusted properly to the actual behavioral task, depending on the behavioral and the biomechanical states of the locomotor apparatus.
Proprioceptive Control of Phase Transition
Sherrington (1910) already introduced the concept that somatosensory afferents from the limbs are involved in the regulation of the step cycle during walking in vertebrates. One mechanism regulating the duration of the stance phase in vertebrates is hip extension, since preventing hip extension in a limb prevents the onset of the swing phase and thus of stepping in cats and rats (e.g., Fouad & Pearson, 1997a; Grillner & Rossignol, 1978). The afferents responsible for signaling the hip angle and subsequently for the initiation of the swing phase are probably muscle spindles in hip flexor muscles (Hiebert, Whelan, Prochazka, & Pearson, 1996). Another signal in the control of the step cycle in vertebrates arises from Golgi tendon organs and muscle spindles from extensor muscles (Conway, Hultborn, & Kiehn, 1987; Whelan, Hiebert, & Pearson, 1995b). Both sensors are active during stance, with the Golgi tendon organs providing a gauge of the load carried by the leg (reviewed in Dietz & Duysens, 2000). The excitatory activity during walking is opposite to its inhibiting action during standing (reflex reversal). The functional consequence is that the swing phase is not initiated until the load is taken off the limb (otherwise, balance would be lost), as occurs at the end of the stance phase when the weight of the animal is borne by the other limbs.
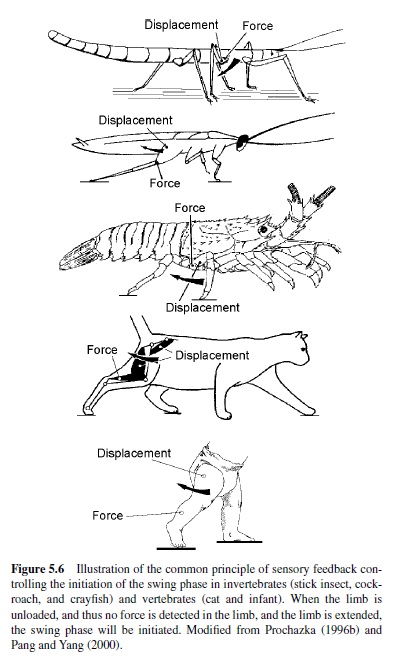
The fact that, at the end of the stance phase, signals both about joint and limb displacement and about load on the limb are involved in the initiation of the swing phase is a general rule in vertebrate and invertebrate walking systems. It has been commonly found in stick insects, cockroaches, lobsters, cats, and humans (Figure 5.6; Anderson & Grillner, 1983; Bässler & Büschges, 1998; Clarac, 1982; Pang &Yang, 2000; Pearson, 1993; Wendler, 1974). In the stick insect, for example, two types of proprioceptors have been found to influence the timing of the onset of the swing phase. These sensors are (a) the campaniform sensillae, which measure load on a limb orstrainonthecuticle,inamanneranalogoustotheGolgitendon organs in vertebrates, and (b) the femoral chordotonal organ, which, by being stretch-sensitive in a manner analogous to muscle spindles in vertebrates, signals the movement and position of the femur-tibia joint (Bässler & Büschges).
Prochazka (1996b) has formulated a general rule for the transition from the stance phase to the swing phase during stepping in vertebrates:
IF extensor force low
AND hip extended
THEN initiate swing.
However, phase transitions controlled by proprioceptive signals are not only found in walking systems (reviewed in Pearson,1993;Pearson&Ramirez,1997).Intheflightsystem of insects, especially well studied in locusts, sensory information about movements of the wings is also utilized for phase transition in motor activity. Two wing-sensory systems—that is, the wing tegulae—a hinge mechanoreceptor, and the stretch receptors control the initiation and duration of elevator activity during flight motor activity (Wolf & Pearson, 1988). Similarly, in vertebrate swimming (e.g., in the lamprey spinal locomotor network), sensory signals that report bending of the spinal cord contribute to the alternation of motor activity between the myotomal motoneuron pools of both sides of the spinal cord (Grillner et al., 1995).
The functional significance of regulating phase transition by means of afferent pathways might be to limit a movement, such as the amount of leg extension (Whelan et al., 1995b) or the amplitude of wing depression during flight (Wolf & Pearson, 1988), to a range compatible with effective function. A second advantage of afferent phase control is to ensure that a certain phase of the movement is not initiated until a defined biomechanical state has been reached. This allows the transition without destabilization of the system.
Regulation of the Magnitude of Muscle Activity
The second principle of locomotor control found in vertebrates and invertebrates is the control of motor activity via afferent feedback (Duysens et al., 2000; Pearson, 1993). A generalization emerging from studies in various walking systems is that afferent feedback from leg proprioceptors contributes to the generation of stance-phase activity (see Figure 5.6). For example, in invertebrates such as the stick insect, the chordotonal organ in the femur of the front leg signals flexion of the femur-tibia joint during the stance phase.
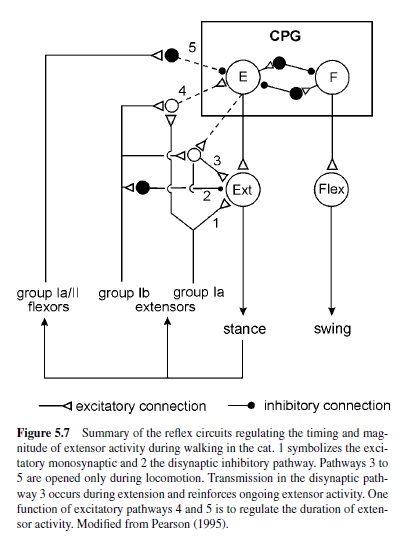
In walking animals, these sensory signals reinforce the activity in flexor motoneurons (Bässler, 1986) as a result of the action of a parallel and distributed neuronal network driving the leg motoneurons (Büschges, Sauer, & Bässler, 2000). Also, in the walking system of the crayfish, sensory signals are utilized to reinforce motor activity during stance (El Manira, DiCaprio, Cattaert, & Clarac, 1991; Sillar, Skorupski, Elson, & Bush, 1986). In vertebrates, sensory signals underlying the reinforcement of motor activity arise both from Golgi tendon organs and primary muscle spindle afferents (Guertin, Angel, Perreault, & McCrea, 1995; McCrea, Shefchyk, Stephens, & Pearson, 1995; Whelan et al., 1995b). At least three excitatory reflex pathways transmit proprioceptive information from extensor muscles to motoneurons or the CPG (Figure 5.7): (a) the well-known monosynaptic pathway from muscle spindles to motoneurons; (b) a disynaptic pathway from spindles and Golgi tendon organs that is opened during the stance phase; and (c) a polysynaptic pathway. The latter pathway includes the extensor half-center in a way that also controls the timing of the stepping pattern (Pearson & Ramirez, 1997). The neural mechanisms that contribute to the modulation of sensory pathways in the control of locomotion have been investigated in great detail in the past decade. Two key factors are currently known: (a) In many locomotor systems the actual motor output is the result of the action of a distributed neural network that can modulate the magnitude of motor activity generated by differentially weighting (or opening and closing) individual parallel (sometimes opposing) interneuronal pathways between sense organs and motoneurons (Bässler & Büschges, 1998; Büschges et al., 2000; Cattaert & LeRay, 2001; Pearson, 1995; also refer to Figure 5.5). Phase dependency of this mechanism in the locomotor cycle is generated by the action of the central pattern generators. (b) Phasic modulation of efficacy of the individual pathways from sense organs onto motoneurons are the target of pre- and postsynaptic modulatory mechanisms at the intercalated synapses, for example, presynaptic inhibition (Clarac, El Manira, & Cattaert, 1992; Nusbaum, El Manira, Gossard, & Rossignol, 1997; Rossignol, 1996a; and Figure 5.5). In humans, as well, the load carried by the extensor muscles increases the magnitude of their activity (Dietz & Duysens, 2000; Stephens & Yang, 1999). In cats, removing feedback from these afferents reduces extensor activity by more than 50% and, in humans, the contribution to extensor muscle activity has been estimated to be about 30% (Yang, Stein, & James, 1991).
The general integration of proprioceptive feedback in locomotor systems throughout the animal kingdom strongly indicates the functional necessity of such a feature. The benefits are the appropriate and effective control of motor rhythm, integrating biomechanical changes and external perturbations in the system.
Role of Exteroceptive Input
Compared to proprioceptive control, much less knowledge has been gathered on the role of exteroceptive inputs (e.g., cutaneous afferents in vertebrates) in the control of locomotion. Exteroceptors in the skin have a strong influence on the central pattern generator for walking (Forssberg, 1979) and on brainstem areas controlling locomotion (Drew, Cabana, & Rossignol, 1996). One important function is to respond to unpredicted perturbations from obstacles on the ground. To be functionally meaningful, these reflexes are strongly modulated during the gait cycle, as has been demonstrated in cats (Abraham, Marks, & Loeb, 1985; Andersson, Forssberg, Grillner, & Lindquist, 1978; Duysens, Loeb, & Weston, 1980; Forssberg, 1979) and humans (Duysens, Trippel, Horstmann, & Dietz, 1990; Yang & Stein, 1990). A mechanical stimulus applied to the dorsal part of a paw in cats or to a cutaneous nerve in humans during the onset and middle of the swing phase produces a strong, short latency excitement of flexor motoneurons and inhibition of extensor motoneurons to increase elevation of the limb and clear the obstacle. Forssberg introduced this reflex pattern as the stumbling corrective response. In contrast, the same stimulus applied during the end of the swing phase and during the stance phase produces the opposite response; the limb cannot be lifted at this phase in the step cycle because otherwise the animal or person would fall. However, the stimulus evokes increased flexor activity in the subsequent step. This state-dependent modulation has been found to be mediated by the convergence of primary afferents and the output from the CPG to premotor interneurons (Degtyarenko, Simon, & Burke, 1996).
Exteroceptive signals, such as cutaneous stimuli, are also known to trigger swimming or turning in animals. A welldefined system has been described for swimming behavior in tadpoles: A brief stimulus to the skin, (e.g., of the head) can trigger sustained swimming or struggling sequences, depending on stimulus intensity and duration (Soffe, 1991). This response is mediated via a single skin-sensory pathway directly accessing the CPGs in the spinal cord: the Rohon-Beard sensory neurons.
In conclusion, in many locomotor systems, sensory input plays a prominent role in shaping the motor output of the controllers toward functional locomotor behavior. The major tasks of sensory signals are (a) to control the direction of locomotion, (b) to control posture and equilibrium, (c) to control phase transitions in the step cycle, (d) to control the magnitude of muscle activity, (e) to avoid obstacles, and finally, (f) to respond to perturbations.
Plasticity in Motor Systems
For many years, the central nervous system in adult mammals has been seen as a hard wired and rigid structure.The same was believed about the nervous systems of invertebrates, whose relatively short life spans were thought to make adaptive processes in the nervous system unnecessary. This view has changed, and today it is accepted that the CNS in vertebrates and invertebrates is capable of major reorganizations in response to injury or loss of parts of the nervous system under experimental or pathological conditions (Meinertzhagen, 2001; Raineteau & Schwab, 2001). Theoretically, reorganization can occur on multiple levels in preexisting neural circuits: by changing synaptic strength (referred to as synaptic plasticity), by anatomical reorganization through the sprouting of uninjured axonal branches and dendrites (referred to as anatomical plasticity), or by changes in neuronal properties. Because axonal plasticity after injuries to the CNS is frequently associatedwithfunctionalrecovery,ongoingresearch in adult vertebrates focuses on understanding the mechanism of plasticity, which could lead to new treatments for patients suffering from stroke or traumatic injuries of the brain or spinal cord.
This research paper will introduce examples of plastic rearrangements in locomotor systems of the nervous system and discuss possible mechanisms of spontaneous recovery after injuries to the nervous system. Lately, this topic has been extensively reviewed by Bizzi, Tresch, Saltiel, and d’Avella (2000), Pearson (2000), and Raineteau and Schwab.
Injury-Induced Adaptations of Central Pattern-Generating Networks
Aprominentexampleofplasticityinalocomotorsystemisthe finding that cats with complete thoracic spinal cord transection can be trained to walk on a treadmill with their hind limbs (Barbeau & Rossignol, 1994; De Leon, Hodgson, Roy, & Edgerton, 1998; Lovely, Gregor, Roy, & Edgerton, 1990; and reviewed in Rossignol, 2000). Directly after spinalization, cats that had their forelimbs placed on a platform could not self-support their hindquarters during stepping movements on a treadmill. Within 2 weeks, these cats gained partial weight control and were sometimes even able to place the plantar surface of the paws on the treadmill belt.After about four weeks, themovementofthetreadmillbeltwasabletotriggerlocomotion, and the cats could transiently support their own weight (Barbeau & Rossignol; Lovely et al.; De Leon et al.). Consistent with the idea of the activity-dependent acquisition of a motor task, the training effects persist. With longer delays in training, however, the effects of the treadmill training cease (De Leon et al., 1999). In humans, too, treadmill training has proven its validity in the rehabilitation phase of patients with spinal cord injuries (Behrman & Harkema, 2000; Dietz, Colombo, & Jensen, 1994; Wernig & Muller, 1992). Regular training with partial weight support by suspending patients in a parachute belt over a treadmill increased the return of rhythmic muscle activation, improved weight support capability, and decreased spasticity in patients with anatomically incomplete spinal cord injury. A mechanism probably involved in training-induced functional recovery is the enhanced excitability of spinal pattern-generating networks after spinal cord injury (De Leon et al., 1999; Tillakaratne et al., 2000).
Plasticity in Afferent Pathways Controlling Locomotion
The finding that treadmill training in spinalized cats enhances locomotor recovery indicated that adaptive changes in the pattern-generating networks are driven by sensory signals from the stepping limbs. A good example that afferent pathways are modifiable is the conditioning of the wellknown H-reflex in a learning task in rats, monkeys, and humans (Segal & Wolf, 1994; Wolpaw, 1997). It is also known that, after injuries to the CNS (especially the spinal cord), reflexes are exaggerated (Burke, Gillies, & Lance, 1970; Hochman & McCrea, 1994; Nelson & Mendell, 1979). One factor in the increased reflex gain is the sprouting of sensory afferents and a simultaneous increase in their effectiveness.
Another example of injury-induced reflex plasticity is the finding that afferent pathways that are involved in the initiation of the swing phase and in reinforcing extensor activity are enhanced by partial denervation of extensor muscles (Gritsenko, Mushahwar, & Prochazka, 2001; Pearson, Fouad, & Misiaszek, 1999; Whelan et al., 1995a). Increases in proprioceptive reflex strength occur within a week and are paralleled by the recovery of stepping. The location of the adaptive changes is probably in the lumbar spinal cord, since the amplitudes of group I (rising from Golgi tendon organs and muscle spindles) field potentials from a spared synergistic muscle are increased in the intermediate nucleus of lumbar segments (Fouad & Pearson, 1997b). The finding that a comparable increase in the effectiveness of group I input from this muscle can also be found in chronically spinalized cats also indicates that reflex adaptations are occurring at the level of the spinal cord (Bouyer, Whelan, Pearson, & Rossignol, 2001). In the light of the adaptive capabilities of the spinal cord, Pearson (2001) recently reviewed the role of plasticity in reflex function and in the recovery of locomotion after injuries to the CNS.
The fact that plasticity can occur on several levels (e.g., that spinal reflex pathways are able to learn) has been also demonstrated by Lou and Bloedel (1988), who showed that decerebrated walking ferrets were able to change the trajectory of the swing phase when an obstacle was interjected into the step cycle during the swing phase.
In insects as well, the removal of sensory organs involved in the regulation of locomotion or even amputation of a limb can be compensated. For example, in the locust flight system, complete or partial removal of the tegulae (mechanoreceptors at the wing base) leads to compensatory anatomical and synaptic rearrangements resulting in functional recovery of flight motor behavior within 2 weeks following the lesion (Büschges, Ramirez, Driesang, & Pearson, 1992a, 1992b; Fischer & Ebert, 1999; Gee & Robertson, 1996; Wolf & Büschges, 1997). Interestingly, it is reported that this recovery occurs spontaneously and does not depend on training or activity. The plasticity of locomotor systems can also be expressed on an immediate short-term scale. An example of such injury-induced plasticity is the recovery of walking after leg amputation in cockroaches. The walking pattern adapts to the loss of the limb by switching interleg locomotor coordination from hexapods to that of tetrapods (Hughes, 1957; Wilson, 1966).
Injury-Induced Plasticity in the Corticospinal Tract
Due to limited self-repair after traumatic injuries to the CNS in higher vertebrates, it is believed that functional recovery, too, is rather limited. However, significant functional recovery occurs after stroke (Ferrucci et al., 1993; Speach & Dombovy, 1995) traumatic head injury (Sbordone et al., 1995), or spinal cord injury (Bracken et al., 2000). The underlying mechanisms of this spontaneous recovery are rather unclear. A phenomenon often observed in patients and animal models after such injuries and intensive rehabilitative training conducted parallel to functional recovery or in training paradigms is found at the cortical level in rearrangements of the sensory and motor representation maps (Bruehlmeier et al., 1998; Levy, Amassian, Traad, & Caldwell, 1990; Nudo, Milliken, Jenkins, & Merzenich, 1996;Wu & Kaas, 1999; and see Klintsova & Greenough, 1999, for a review). The underlying mechanisms of these rearrangements are probably multiple and rather unclear. It has been suggested that unmasking horizontal connections between the cortical subregions may play a crucial role (Chen, Corwell, Yaseen, Hallett, & Cohen, 1998; Huntley, 1997; Jacobs & Donoghue, 1991). A possible contribution of structural plasticity in descending corticospinal tract (CST) axons has been discussed since the recent finding that the CST in adult rats has a greater potential for anatomical rearrangements than was previously believed. Injury-induced sprouting and increased collateralization was found in severed CST fibers rostral to the lesion, in parallel with shifts in hindlimb motor cortex representations (Fouad, Pedersen, Schwab, & Brösamle, 2001). Structural adaptations have also been reported in spared CST fibers. Significant anatomical plasticity was correlated with spontaneous functional recovery in a grasping task in adult rats (Weidner, Ner, Salimi, & Tuszynski, 2001). It is thus currently speculated that such spontaneous growth of lesioned and unlesioned axons contributes to changes in cortical motor representations and to functional recovery after incomplete spinal cord lesions.
Functional and anatomical studies clearly show that the potential for adaptive reorganization in the adult nervous system in vertebrates and invertebrates has been underestimated. Adaptive changes can occur on several levels of the nervous system and are often activity dependent. Current approaches to experimentally increase the plastic capabilities of the nervous system, together with the progress in understanding rehabilitative strategies, might open new avenues in the treatment of injuries to the nervous system.
Modulation of Locomotor Activity
In order to initiate, maintain, adapt, or terminate locomotor activity to meet the requirements of the current environmental conditions, neuronal networks for motor control must be flexible.Whereasthefast,task-dependent,cycle-by-cycleadaptation of locomotor activity is achieved by sensory feedback, which, in a broad sense, can be viewed as being an integral part of the pattern-generating networks, neuromodulatory inputs can reshape the motor output by affecting intrinsic network properties of motor circuits and thus provide the general flexibility observed in motor behavior. The term neuromodulation has been in common usage for more than two decades and was originally defined as the alteration of the cellular or synaptic properties of a neuron mediated by a substance released from another neuron (Kaczmarek & Levitan, 1987; Kupfermann, 1979). However, this definition no longer strictly conforms to many other definitions of neuromodulation used in scientific literature, primarily because in many systems neurotransmission and neuromodulation apparently involve common mechanisms and address common targets (for an overview of the large variety of phenomena nowadays referred to as neuromodulation, see Katz, 1999). This section introduces the main mechanism of generating locomotor flexibility, the modulation of locomotor network function by neural active substances (neuromodulators).
Sources of Neuromodulators
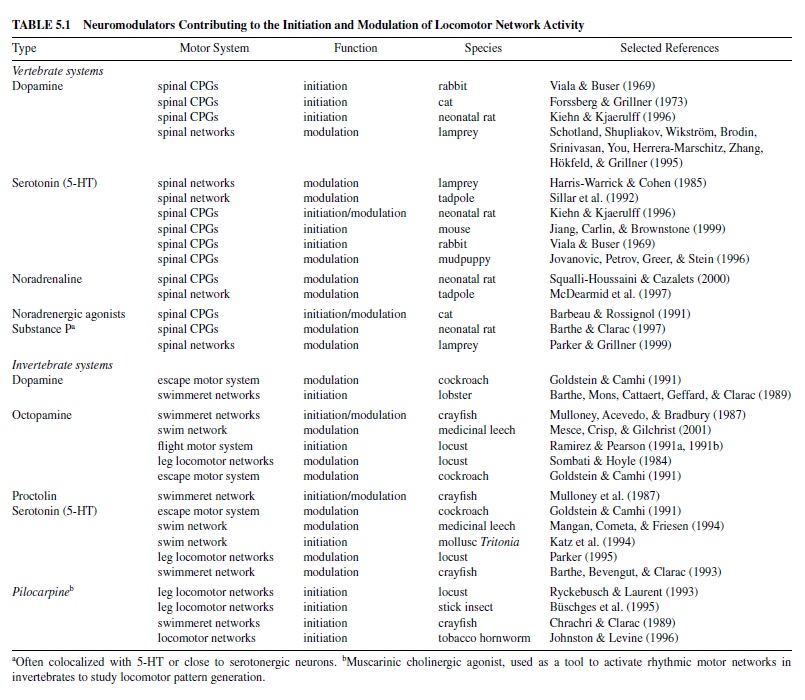
In vertebrates and invertebrates, neuromodulators are mostly released from cell groups consisting of relatively few neurons located in the CNS, but outside the specific locomotor circuits and not participating in the basic locomotor rhythm generation (referred to as extrinsic neuromodulation; Katz, 1999). Most of the modulators of vertebrate locomotor networks are synthesized in distinct cell clusters in the brainstem that produce, among other things, the biogenic amines serotonin (5-HT, nucleus raphe), noradrenaline (norepinephrine, locus coeruleus), or dopamine. In many vertebrate systems, the axonal projections of these relatively few cells however, can supply large areas of the brain and spinal cord (Kuypers & Huisman, 1982; van Mier, Joosten, van Rheden, & ten Donkelaar, 1986). Invertebrate neuromodulators, such as the neuropeptide proctolin, are normally released from neurosecretory cells either clustered in cerebral ganglia regions or from single, paired, or small groups of cells spread over the whole ventral nerve cord (Beltz & Kravitz, 1987; Keshishian & O’Shea, 1985; Stevenson & Sporhase-Eichmann, 1995). This not only enables the coordinated release of neuromodulators to defined targets within a particular CPG, but also underlies the simultaneous control of locomotor networks consisting of multiple CPGs (e.g., networks driving leg joints or spinal networks for swimming). For vertebrates and invertebrates, classic neuromodulators associated with locomotor systems and their controllers—that is, the spinal cord in vertebrates and the segmental ganglia in invertebrates—are summarized in Table 5.1.
Effects of Neuromodulators on the Output of Locomotor Networks
In principle, neuromodulators alter the expression of motor patterns by affecting the controllers of a locomotor system that drives the effector organs, such as the muscles in the body wall or within limbs or wings. The best-recognized function of neuromodulators in vertebrate and invertebrate motor control is the alteration of ongoing motor activity. Such alterations in the intensity of locomotor activity are important, for instance, in adjusting the instantaneous speed of locomotion, which involves acceleration as well as deceleration and termination, or in changing locomotor intensity. Furthermore, in a variety of vertebrate and invertebrate species, neuromodulators are able to initiate locomotor activity (Table 5.1).
Initiation of Locomotor Activity
In the simplest examples, initiation of long-lasting periods of locomotor activity often requires no more than a short external stimulus that triggers long-lasting, self-sustained network activity (e.g., escape swimming in tadpoles; Roberts, 1990). However, locomotor activity can also be initiated by administration of neuromodulators. Intravenous injection of dopamine (L-DOPA) elicits locomotor activity in spinalized cats on a treadmill (Forssberg & Grillner, 1973; Jankovska et al., 1967a, 1967b). As well as in rabbits (Viala & Buser, 1969) and decerebrated adult rats (Iles & NicolopoulosStournaras, 1996). Intrathecal application of noradrenaline (Kiehn, Hultborn, & Conway, 1992) or intravenous injection of adrenoreceptor agonists to acutely (Forssberg & Grillner) or chronically spinalized cats (e.g., Barbeau & Rossignol, 1991) also evokes locomotor activity (reviewed in Rossignol et al., 1998). Sublesional transplantation of noradrenergic embryonic neurons from the locus coeruleus into the spinal cord (i.e., close to the locomotor networks) is also able to trigger automatic locomotion in spinalized cats (Yakovleff et al., 1989). In addition, noradrenaline or dopamine, when applied to in vitro spinal cords of vertebrates, activate the locomotor networks (Kiehn & Kjaerulff, 1996; Kiehn, Sillar, Kjaerulff, & McDearmid, 1999; Squalli-Houssaini & Cazalets, 2000).
Serotonin (5-HT) is a neuromodulator that can initiate locomotion in some vertebrates, but not in others. In the rabbit (Viala & Buser, 1969) and the neonatal rat (Cazalets, SqualliHoussaini, & Clarac, 1992; Kiehn & Kjaerulff, 1996), 5-HT induces alternating rhythmic activity in muscle antagonists. Furthermore, sublesional transplantation of serotonergic brain stem cells into the spinal cord of chronically spinalized rats was shown to activate spinal locomotor networks and to improve locomotion (Feraboli-Lohnherr, Orsal, Yakovleff, Gimenez y Ribotta, & Privat, 1997). However, 5-HT cannot initiate locomotion in the cat (Barbeau & Rossignol, 1991), the lamprey (Harris-Warrick & Cohen, 1985), or the tadpole (Sillar, Wedderburn, & Simmers, 1992). Finally, in some vertebrates, acetylcholine can cause a strong activation of spinal pattern generators (Cowley & Schmidt, 1994; Panchin, Perrins, & Roberts, 1991). In invertebrates, injection or bath application of neuromodulators to isolated nervous systems elicits locomotion or locomotor-like pattern (summary in Table 5.1). However, we do not at present fully understand what mechanisms underlie such an initiation or whether neuromodulators function to activate locomotor networks in vivo (e.g., Pearson, 1993). Evidence from invertebrate systems suggests that neuromodulators might either directly alter cellular properties of specific network neurons (Kleinhaus & Angstadt, 1995; Ramirez & Pearson, 1991a, 1991b), resulting in locomotor activity onset, or activate modulatory pathways external to the locomotor network, successively initiating pattern generation (Katz & Frost, 1995).
Modulation of Ongoing Locomotor Activity
In general, a neuromodulator can alter ongoing locomotor activity by affecting three major parameters of the locomotor pattern: (a) It can change the cycle period of the locomotor pattern; (b) it can change muscle force within each activity cycle by altering the duration and intensity of motoneuronal activity bursts; and (c) it can change the coordination of the activity cycles, not only between different neuron pools driving particular muscles (e.g., within one limb during walking) but also the longitudinal coordination of body segments (e.g., during swimming in aquatic animals).
In vertebrates, noradrenergic pathways particularly affect the duration of the movement cycle. For example, administration of noradrenergic agonists increases spinalized cats’ step-cycle length during walking (reviewed in Ribotta et al., 1998). Noradrenaline and its agonists consistently lengthen cycle periods during swimming in amphibian tadpoles (Fischer, Merrywest, & Sillar, 2001; McDearmid, Scrymgeour-Wedderburn, & Sillar, 1997). However, an increased duration of the movement cycles is also mediated by 5-HT and dopamine (Grillner et al., 1995; Kiehn & Kjaerulff, 1996). In a wide range of vertebrates, 5-HT increases the duration and intensity of the activity bursts within each movement cycle—not only in swimming animals, such as the lamprey (Harris-Warrick & Cohen, 1985) and amphibian tadpoles (Sillar et al., 1992), but also in the locomotor systems of walking animals, such as rabbits (Viala & Buser, 1969), rats (Kiehn & Kjaerulff, 1996; Squalli-Houssaini et al., 1993), and cats (Barbeau & Rossignol, 1991). Finally, modulators such as noradrenaline, 5-HT, and dopamine not only influence the longitudinal coordination of the locomotor pattern between successive body segments in swimming animals (Fischer et al., 2001; Grillner et al., 1995), but also shift the activation of particular muscles within a step cycle and thus may alter the complete movement pattern of the limb (Kiehn & Kjaerulff).
In many systems, the effects of neuromodulators are mediated via numerous pharmacologically distinct receptor subclasses (for 5-HT see, e.g., Wedderburn & Sillar, 1994; Wikstrom, Hill, Hellgren, & Grillner, 1995), enabling a multimodal control of the motor pattern. In vertebrates, direct pharmacological activation of, for example, the adrenoreceptors, which are defined as putative target receptors for catecholamines such as noradrenaline (e.g., Hirst & Nield, 1980), can modulate motor output (Barbeau & Rossignol, 1991; Forssberg & Grillner, 1973; Kiehn et al., 1992), with each subclass affecting particular facets of the motor pattern (Fischer et al.; Squalli-Houssaini & Cazalets, 2000).
Neuromodulators Affect Cellular Properties and the Synaptic Efficacy of Network Neurons
Most of the classic neuromodulators exert their effects by changing intrinsic properties of one or a few network neurons or of one or more particular synaptic connections, which affects the overall network output, resulting in a more or less extensive alteration of the motor pattern (discussed previously). For many motor systems, more than one neuromodulatory substance is known (e.g., Grillner, Parker, & El Manira, 1998; Schotland et al., 1995), each of which has distinct effects on the network output (Kiehn & Kjaerulff, 1996; Sillar, Keith, & McDearmid, 1998) and must be coordinated for proper pattern modulation. However, in the majority of motor systems, we are just beginning to understand how such multiple and sometimes even contradictory modulatory inputs are processed and integrated and thus enable a functional pattern modulation. The following sections summarize the most common elementary effects of neuromodulators acting extrinsically on particular cellular and synaptic properties of neurons within locomotor networks (for an in-depth review, see Kiehn & Katz, 1999).
Alteration of Intrinsic Cellular Properties of Network Neurons
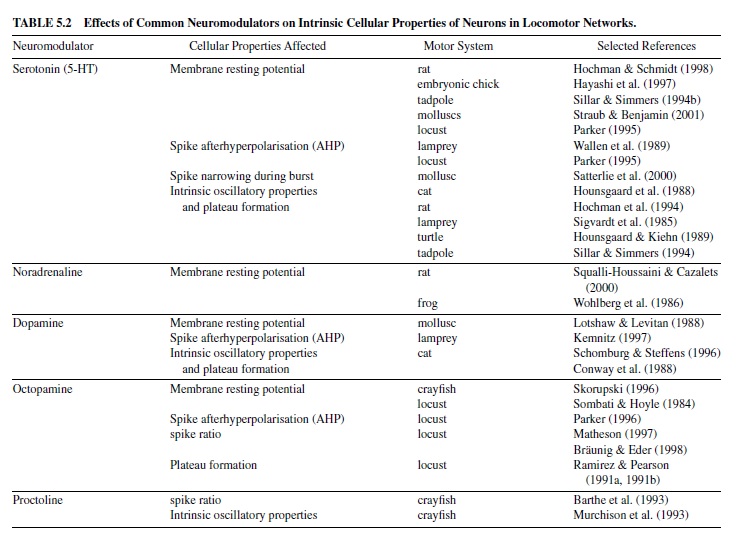
Features of neuronal activity such as action potential formation, spike rate, and activity threshold of neurons may vary widely among the different classes of neurons within a network. The shape of a particular type of neuron’s characteristic activity pattern in response to synaptic drive depends on intrinsic biophysical membrane parameters, that is, on its set of steady and transient voltage-dependent ionic conductances within the membrane. These intrinsic cellular properties determine (a) membrane resting potential (i.e., the state of excitability of a neuron); (b) burst activity, including SFA (i.e., codetermining the activity period of a neuron); (c) mechanisms underlying membrane bistability (i.e., the ability of plateau potential generation to maintain a prolonged period of activity); and (d) the mechanisms enabling a postinhibitory rebound (PIR) of a neuron (helping to escape a phase of strong inhibition)—all of which may contribute to the basic shaping of the network output. Most of the classic neuromodulators alter such intrinsic cellular properties by affecting one or more transient ionic conductances. An overview is given in Table 5.2.
Alteration of Synaptic Transmission Between Network Neurons
Besides their effects on intrinsic cellular properties, neuromodulators can affect synaptic transmission either by targeting presynaptic neurons (i.e., resulting in an altered amount of transmitter release; e.g., Shupliakov, Pieribone, Gad, & Brodin, 1995) or by changing the responses of the postsynaptic cell to a transmitter (e.g., by alterating particular membrane properties; Parker, 1995). In spinal locomotor networks, biogenic amines such as 5-HT and noradrenaline can control locomotor intensity by increasing or decreasing the amount of the inhibitory transmitter released from neurons responsible for the reciprocal coupling between antagonistic motoneuron pools. Strengthening (or weakening) an inhibitory phase between two consecutive movement cycles causes a delayed (or an earlier) onset of activity in the succeeding cycle and thus modulates the cycle duration during ongoing locomotor activity (e.g., Sillar et al., 1998). During locomotor activity, the properties of the synaptic transmission between neurons in a locomotor network can also depend on the connection’s activityhistory(i.e.,onthepreviouscyclesofmovementinthe same episode of locomotion, so-called activity-dependent synaptic plasticity; e.g., Parker & Grillner, 2000). Neuromodulators can affect these activity-dependent properties of a synaptic connection, enabling synaptic metaplasticity (reviewed in, e.g., Parker, 2001), which adds a further degree of functional flexibility to the network output. In some cases, neuromodulators can even reverse the sign of a particularsynaptic connection (Johnston, Peck, & Harris-Warrick, 1993).
Conclusions
Research in the field of locomotor control has greatly benefited from studies in lower vertebrates and invertebrates, such as the lamprey and tadpole for swimming pattern generation and the crayfish and stick insect for walking pattern generation. These animal models, in being experimentally well accessible, helped to unravel basic principles for neuronal networks controlling locomotion. However, a major outcome of the past research in various lower and higher animal species is that many of the mechanisms described also contribute to locomotor pattern generation in higher vertebrates (including humans) that are often less accessible experimentally, indicating that common principles appear to underlie the design of locomotor networks in the entire animal kingdom. However, it must be acknowledged that we are still far from completely understanding the mechanisms underlying locomotor network function, even in the few networks that have been characterized in detail on the cellular level. Important issues that must be addressed in the future are (a) the mechanisms underlying descending control and action selection (e.g., changes in gait or locomotor speed and selection of particular locomotor responses); (b) the structure and functioning of pattern-generating networks for terrestrial locomotion, such as walking, including the level of cell-to-cell interaction and mechanisms of coupling limbs, joints, and segments; and (c) interactions among neuromodulatory influences on locomotor patterns and neuronal activity.
Another major outcome of recent locomotor research is that networks are by no means hardwired but are flexible regarding their topology and the properties of the connections between their components. This not only enables a functional flexibility during ongoing behavior but also underlies the ability of these networks to establish locomotor ability during the development of an individual, to maintain locomotion throughout its life span as well as to enable a certain degree of functional recovery (following, e.g., the damage of peripheral nerves and muscles or the CNS). Discovering the mechanisms underlying such adaptive rearrangements in the adult mammalian nervous system is of immediate clinical interest because they are frequently related to functional recovery, and might offer a key to the treatment of patients with injuries of the CNS.
New techniques that were recently established offer experimental access to locomotor systems on levels required to address all these questions. For example, the development of in vitro preparations of the spinal cord for mammals opened new perspectives in exploring their complex neuronal networks. Recentadvancesinneurogenetics,inparticulartheavailability of so-called knockout mutants, offer new approaches to studying a wide range of functional levels within motor systems ranging from the role of single molecules (e.g., receptors or transmitters)tomuchhigherlevels(e.g.,supraspinalprocesses involved in the generation and control of locomotion).
Bibliography:
- Abraham, L. D., Marks, W. B., & Loeb, G. E. (1985). The distal hindlimb musculature of the cat: Cutaneous reflexes during locomotion. Experimental Brain Research, 58, 594–603.
- Andersson, O., Forssberg, H., Grillner, S., & Lindquist, M. (1978). Phasic gain control of the transmission in cutaneous reflex pathways to motoneurons during “fictive” locomotion. Brain Research, 149(2), 503–507.
- Andersson, O., & Grillner, S. (1983). Peripheral control of the cat’s step cycle. II: Entrainement of the central pattern generators for locomotion by sinusiodal hip movements during fictive locomotion. Acta Pysiologica Scandinavica, 118, 229–239.
- Armstrong, D. M. (1988). The supraspinal control of mammalian locomotion. Journal of Physiology, 405, 1–37.
- Arshavsky, Y., Beloozerova, I. N., Orlovsky, G. N., Panchin, Y., & Pavlova, G.A. (1985a). Control of locomotion in marine mollusc Clione limacina. I: Efferent activity during actual and fictitious swimming. Experimental Brain Research, 58(2), 255–262.
- Arshavsky, Y., Beloozerova, I. N., Orlovsky, G. N., Panchin, Y., & Pavlova, G. A. (1985b). Control of locomotion in marine mollusc Clione limacina. II: Rhythmic neurons of pedal ganglia. Experimental Brain Research, 58(2), 263–272.
- Arshavsky, Y., Beloozerova, I. N., Orlovsky, G. N., Panchin, Y., & Pavlova, G. A. (1985c). Control of locomotion in marine mollusc Clione limacina. III: On the origin of locomotor rhythm. Experimental Brain Research, 58(2), 273–284.
- Arshavsky, Y., Beloozerova, I. N., Orlovsky, G. N., Panchin, Y., & Pavlova, G. A. (1985d). Control of locomotion in marine mollusc Clione limacina. IV: Role of type 12 interneurons. Experimental Brain Research, 58(2), 285–293.
- Arshavsky, Y., Beloozerova, I. N., Orlovsky, G. N., Panchin, Y., & Pavlova, G. A. (1985e). Control of locomotion in marine mollusc Clione limacina. V: Photoinactivation of efferent neurons. Experimental Brain Research, 59(1), 203–205.
- Arshavsky, Y., Deliagina, T. G., Orlovsky, G. N., Panchin, Y., Popova, L. B., & Sadreyev, R. I. (1998). Analysis of the central pattern generator for swimming in the mollusk Annals of the New York Academy of Sciences, 860, 51–69.
- Arshavsky, Y., Orlovsky, G. N., Panchin, Y., Roberts, A., & Soffe, S. R. (1993). Neuronal control of swimming locomotion: Analysis of the pteropod mollusc Clione and embryos of the amphibian Trends in Neuroscience, 16(6), 227–233.
- Barbeau, H., & Rossignol, S. (1991). Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Research, 546, 250–260.
- Barbeau, H., & Rossignol, S. (1994). Enhancement of locomotor recovery following spinal cord injury. Current Opinion in Neurology, 7(6), 517–524.
- Barthe, J. Y., Bevengut, M., & Clarac, F. (1993). In vitro, proctolin and serotonin induced modulations of the abdominal motor system activities in crayfish. Brain Research, 623, 101–109.
- Barthe, J. Y., & Clarac, F. (1997). Modulation of the spinal network for locomotion by substance P in the neonatal rat. Experimental Brain Research, 115, 485–492.
- Barthe, J. Y., Mons, N., Cattaert, D., Geffard, M., & Clarac, F. (1989). Dopamine and motor activity in the lobster Homarus gammarus. Brain Research, 497, 368–373.
- Bässler, U. (1983). Neural basis of elementary behavior in stick insects. Berlin: Springer-Verlag.
- Bässler, U. (1986). On the definition of central pattern generator and its sensory control. Biological Cybernetics, 24, 47–49.
- Bässler, U. (1987). Timing and shaping influences on the motor output for walking in the stick insect. Biological Cybernetics, 55, 397–401.
- Bässler, U. (1988). Functional principles of pattern generation for walking movements of stick insect forelegs: The role of femoral chordotonal organ afferences. The Journal of Experimental Biology, 136, 125–147.
- Bässler, U. (1993). The femur-tibia control system of stick insects: A model system for the study of the neural basis of joint control. Brain Research Reviews, 18, 207–226.
- Bässler, U., & Büschges, A. (1998). Pattern generation for stick insect walking movements: Multisensory control of a locomotor program. Brain Research Reviews, 27(1), 65–88.
- Behrman, A. L., & Harkema, S. J. (2000). Locomotor training after human spinal cord injury: A series of case studies. Physical Therapy, 80(7), 688–700.
- Beltz, B. S., & Kravitz, E. A. (1987). Physiological identification, morphological analysis, and development of identified serotonin-proctolin containing neurons in the lobster ventral nerve cord. Journal of Neuroscience, 7, 533–546.
- Bennett, M. R. (1999). The early history of the synapse: From Plato to Sherrington. Brain Research Bulletin, 50(2), 95–118.
- Bizzi, E., Tresch, M. C., Saltiel, P., & d’Avella, A. (2000). New perspectives on spinal motor systems. Nature Reviews Neuroscience, 1, 101–108.
- Borst, A., & Egelhaaf, M. (1989). Principles of visual motion detection. Trends in Neuroscience, 12, 297–306.
- Bouyer, L. J., Whelan, P. J., Pearson, K. G., & Rossignol, S. (2001). Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. Journal of Neuroscience, 21(10), 3531– 3541.
- Bowerman, R. F., & Larimer, J. L. (1974a). Command fibres in the circumoesophageal connectives of crayfish. I: Tonic fibres. The Journal of Experimental Biology, 60, 95–117.
- Bowerman, R. F., & Larimer, J. L. (1974b). Command fibres in the circumoesophageal connectives of crayfish. II: Phasic fibres. The Journal of Experimental Biology, 60, 119–134.
- Bracken, M. B., Aldrich, E. F., Herr, D. L., Hitchon, P. W., Holford, T. R., Marshall, L. F., Nockels, R. P., Pascale, V., Shepard, M. J., Sonntag, V. K., Winn, H. R., & Young, W. (2000). Clinical measurement, statistical analysis, and riskbenefit: Controversies from trials of spinal injury. Journal of Trauma 48(3), 558–561.
- Bräunig, P., & Eder, M. (1998). Locust dorsal unpaired median (DUM) neurons directly innervate and modulate hindleg proprioceptors. The Journal of Experimental Biology, 201, 3333– 3338.
- Brodfuehrer, P. D., Debski, E. A., O’Gara, B. A., & Friesen, W. O. (1995). Neuronal control of leech swimming. Journal of Neurobiology, 27(3), 403–418.
- Brodfuehrer, P. D., & Friesen, W. O. (1986a). Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. I: Output of Tr1 and Tr2. Journal of Comparative Physiology A, 159(4), 489–502.
- Brodfuehrer, P. D., & Friesen, W. O. (1986b). Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. II: Role of segmental swim-initiating interneurons. Journal of Comparative Physiology A, 159(4), 503–510.
- Brodfuehrer, P. D., & Friesen, W. O. (1986c). Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. III: Sensory inputs to Tr1 and Tr2. Journal of Comparative Physiology A, 159(4), 511–519.
- Brown, G. T. (1911). The intrinsic factors in the act of progression in mammals. Proceedings of the Royal Society of London, B, 84, 308–319.
- Brown, G. T. (1912). The factors in rhythmic activity of the nervous system. Proceedings of the Royal Society of London, Series B, 85, 278–289.
- Bruehlmeier, M., Dietz, V., Leenders, K. L., Roelcke, U., Missimer, J., & Curt, A. (1998). How does the human brain deal with a spinal cord injury? European Journal of Neuroscience, 10(12), 3918–3922.
- Buchanan, J. T. (1982). Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: Synaptic interactions and morphology. Journal of Neurophysiology, 47(5), 961–975.
- Burke, D., Gillies, J. D., & Lance, J. W. (1970). The quadriceps stretch reflex in human spasticity. Journal of Neurology, Neurosurgery and Psychiatry, 33(2), 216–223.
- Büschges, A., & El Manira, E. (1998). Sensory pathways and their modulation in the control of locomotion. Current Opinion in Neurobiology, 8(6), 733–739.
- Büschges, A., Ramirez, J.-M., & Pearson, K. G. (1992). Reorganization of sensory regulation of locust flight after partial deafferentation. Journal of Neurobiology, 23, 31–43.
- Büschges, A., Ramirez, J.-M., Driesang, R., & Pearson, K. G. (1992). Connections of the forewing tegulae in the locust flight system and their modification following partial deafferentation. Journal of Neurobiology, 23, 44–60.
- Büschges, A., Sauer, A. E., & Bässler, U. (2000). Adaptive behavior and intelligent systems without symbols and logic. In H. Cruse, J. Dean, & H. Ritter (Eds.), Prerational intelligence (Vol. 1, pp. 267–296). Boston: Kluwer.
- Büschges, A., Schmitz, J., & Bässler, U. (1995). Rhythmic patterns in the thoracic nerve cord of the stick insect induced by pilocarpine. The Journal of Experimental Biology, 198, 435–456.
- Büschges, A., & Wolf, H. (1999). Phase-dependent presynaptic modulation of mechanosensory signals in the locust flight system. Journal of Neurophysiology, 81, 959–962.
- Calancie, B., Needham-Shropshire, B., Jacobs, P., Willer, K., Zych, G., & Green, B. A. (1994). Involuntary stepping after chronic spinal cord injury. Brain, 117, 1143–1159.
- Cattaert, D., & Le Ray, D. (2001). Adaptive motor control in crayfish. Progress in Neurobiology, 63(2), 199–240.
- Cazalets, J. R., Borde, M., & Clarac, F. (1995). Localisation and organisation of the central pattern generator for hindlimb locomotion in newborn rats. Journal of Neurophysiology, 15, 4943– 4951.
- Cazalets, J.-R., Squalli-Houssaini, Y., & Clarac, F. (1992). Activation of the central pattern generators for locomotion by serotonin and excitatory aminoacids in neonatal rat. Journal of Physiology, 455, 187–204.
- Chen, R., Corwell, B., Yaseen, Z., Hallett, M., & Cohen, L. G. (1998). Mechanisms of cortical reorganization in lower-limb amputees. Journal of Neuroscience, 18(9), 3443–3450.
- Cheng, J., Stein, R. B., Jovanovic, K., Yoshida, K., Bennett, D. J., & Han, Y. (1998). Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus maculatus)spinalcord. JournalofNeuroscience,18(11),4295–4304.
- Chrachri, A., & Clarac, F. (1989). Induction of rhythmic activity in motoneurons of crayfish thoracic ganglia by cholinergic agonists. Neuroscience Letters, 77(1), 49–54.
- Chrachri, A., & Clarac, F. (1989). Synaptic connections between motor neurons and interneurons in the fourth thoracic ganglion of the crayfish, Procambarus clarkii. Journal of Neurophysiology, 62(6), 1237–1250.
- Clarac, F. (1982). Proprioceptive functions of invertebrates. Journal of Physiology (Paris), 78(7), 665–680.
- Clarac, F., Cattaert, D., & Le Ray, D. (2000). Central control components of a “simple” stretch reflex. Trends in Neuroscience, 23(5), 199–208.
- Clarac, F., El Manira, A., & Cattaert, D. (1992). Presynaptic control as a mechanism of sensory-motor integration. Current Opinion in Neurobiology, 2(6), 764–769.
- Conway, B. A., Hultborn, H., & Kiehn, O. (1987). Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research, 68(3), 643–656.
- Conway, B. A., Hultborn, H., Kiehn, O., & Mintz, I. (1988). Plateau potentials in alpha-motoneurons induced by intravenous injection of L-dopa andclonidine in the spinal cat. Journal of Physiology, 405, 369–384.
- Cowley, K. C., & Schmidt, B. J. (1994). Acomparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neuroscience Letters, 171, 147–150.
- Cruse, H. (1990). What mechanisms coordinate leg movement in walking arthropods? Trends in Neurosciences, 13(1), 15–21.
- Cruse, H., Bartlin, C., Breifert, M., Schmitz, J., Brunn, D. E., Dean, J., & Kindermann, T. (1995). Walking: A complex behaviour controlled by simple networks. Adaptive Behavior, 3, 385– 418.
- Dale, N. (1997). Role of ionic currents in the operation of motor circuits in the Xenopus. In P. S. G. Stein, S. Grillner, A. I. Selverston, & D. G. Stuart (Eds.), Neurons, Networks and Motor Behavior (pp. 92–96). Cambridge: MIT Press.
- Davis, W. J., & Ayers, J. L., Jr. (1972). Locomotion: Control by positive feedback optokinetic responses. Science, 177, 183–185.
- Degtyarenko, A. M., Simon, E. S., & Burke, R. E. (1996). Differential modulation of disynaptic cutaneous inhibition and excitation in ankle flexor motoneurons during fictive locomotion. Journal of Neurophysiology, 76(5), 2972–2985.
- De Leon, R. D., Hodgson, J. A., Roy, R. R., & Edgerton, V. R. (1998). Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. Journal of Neurophysiology, 79(3), 1329–1340.
- De Leon, R. D., Hodgson, J. A., Roy, R. R., & Edgerton, V. R. (1999). Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. Journal of Neurophysiology, 81(1), 85–94.
- Delcomyn, F. (1980). Neural basis of rhythmic behaviour in animals. Science, 210, 492–498.
- Dietz, V. (1992). Human neuronal control of automatic functional movements: Interaction between central programs and afferent input. Physiological Reviews, 72(1), 33–69.
- Dietz, V., Colombo, G., & Jensen, L. (1994). Locomotor activity in spinal man. Lancet, 344(8932), 1260–1263.
- Dietz, V., & Duysens, J. (2000). Significance of load receptor input during locomotion: A review. Gait Posture, 11(2), 102–110.
- Dietz, V., Nakazawa, K., Wirz, M., & Erni, T. (1999). Level of spinal cord lesion determines locomotor activity in spinal man. Experimental Brain Research, 128(3), 405–409.
- Dimitrijevic, M. R., Gerasimenko, Y., & Pinter, M. M. (1998). Evidence for a spinal central pattern generator in humans. Annals of the New York Academy of Science, 860, 360–376.
- Drew, T., Cabana, T., & Rossignol, S. (1996). Responses of medullary reticulospinal neurons to stimulation of cutaneous limb nerves during locomotion in intact cats. Experimental Brain Research, 111(2), 153–168.
- Drew, T., Jiang, W., Kably, B., & Lavoie, S. (1996). Role of the motor cortex in the control of visually triggered gait modifications. Canadian Journal of Physiology and Pharmacology, 74(4), 426–442.
- Duysens, J., Clarac, F., & Cruse, H. (2000). Load-regulating mechanisms in gait and posture: Comparative aspects. Physiological Reviews, 80(1), 83–133.
- Duysens, J., Loeb, G. E., & Weston, B. J. (1980). Crossed flexor reflex responses and their reversal in freely walking cats. Brain Research, 197(2), 538–542.
- Duysens, J., Trippel, M., Horstmann, G. A., & Dietz, V. (1990). Gating and reversal of reflexes in ankle muscles during human walking. Experimental Brain Research, 82(2), 351–358.
- Edgerton, V. R., Grillner, S., Sjöström, A., & Zangger, P. (1976). Central generation of locomotion in vertebrates. In R. M. Herman, S. Grillner, & D. G. Stuart (Eds.), Neural control of locomotion (pp. 439–464). New York: Plenum Press.
- El Manira, A., DiCaprio, R. A., Cattaert, D., & Clarac, F. (1991). Monosynaptic interjoint reflexes and their central modulation during fictive locomotion in crayfish. European Journal of Neuroscience, 3, 1219–1232.
- Fedirchuck, J. R., Nielsen, J., Petersen, N., & Hultborn, H. (1998). Pharmacologically evoked fictive motor patterns in the acutely spinalized marmoset monkey (Callithrix jacchus). Experimental Brain Research, 122, 351–361.
- Feraboli-Lohnherr, D., Orsal, D., Yakovleff, A., Gimenez y Ribotta, M., & Privat, A. (1997). Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: Specific role of serotonergic neurons. Experimental Brain Research, 113, 443–454.
- Ferrucci, L., Bandinelli, S., Guralnik, J. M., Lamponi, M., Bertini, C., Falchini, M., & Baroni, A. (1993). Recovery of functional status after stroke: A postrehabilitation follow-up study. Stroke, 24(2), 200–205.
- Fischer, H., & Ebert, E. (1999). Tegula function during free locust flight in relation to motor pattern, flight speed and aerodynamic output. The Journal of Experimental Biology, 202, 711–721.
- Fischer,H.,Merrywest,S.D.,&Sillar,K.T.(2001).Adrenoreceptormediated modulation of the spinal locomotor pattern during swimming in Xenopus laevis European Journal of Neuroscience, 13, 977–986.
- Forssberg, H. (1979). Stumbling corrective reaction: A phasedependent compensatory reaction during locomotion. Journal of Neurophysiology, 42, 936–953.
- Forssberg, H., & Grillner, S. (1973). The locomotion of the acute spinal cat injected with clonidine i.v. Brain Research, 50, 184–186.
- Fouad, K., & Pearson, K. G. (1997a). Effects of extensor muscle afferents on the timing of locomotor activity during walking in adult rats. Brain Research, 749(2), 320–328.
- Fouad, K., & Pearson, K. G. (1997b). Modification of group I field potentials in the intermediate nucleus of the cat spinal cord after chronic axotomy of an extensor nerve. Neuroscience Letters, 236(1), 9–12.
- Fouad, K., Pedersen, V., Schwab, M. E., & Brösamle, C. (2001). Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Current Biology, 11, 1766–1770.
- Friesen, W. O., & Hocker, C. G. (2001). Functional analyses of the leech swim oscillator. Journal of Neurophysiology, 86(2), 824–835.
- Friesen, W. O., Poon, M., & Stent, G. S. (1978). Neuronal control of swimming in the medicinal leech. IV: Identification of a network of oscillatory interneurons. The Journal of Experimental Biology, 75, 25–43.
- Gamkrelidze, G. N., Laurienti, P. J., & Blankenship, J. E. (1995). Identification and characterization of cerebral ganglion neurons that induce swimming and modulate swim-related pedal ganglion neurons in Aplysia brasiliana. Journal of Neurophysiology, 74(4), 1444–1462.
- Gee, C. E., & Robertson, R. M. (1996). Recovery of the flight system following ablation of the tegulae in immature adult locusts. The Journal of Experimental Biology, 199, 1395–1403.
- Getting, P. A. (1981). Mechanisms of pattern generation underlying swimming in I: Neuronal network formed by monosynaptic connections. Journal of Neurophysiology, 46(1), 65–79.
- Getting, P. A. (1989). Emerging principles governing the operation of neural networks. Annual Reviews in Neuroscience, 12, 185–204.
- Getting, P. A., Lennard, P. R., & Hume, R. I. (1980). Central pattern generator mediating swimming in I: Identification and synaptic interactions. Journal of Neurophysiology, 44(1), 151–164.
- Gibson, J. J. (1958). Visually controlled locomotion and visual orientation in animals. British Journal of Psycology, 49, 182–189.
- Goldstein, R. S., & Camhi, J. M. (1991). Different effects of the biogenic amines dopamine, serotonin and octopamine on the thoracic and abdominal portions of the escape circuit in the cockroach. Journal of Comparative Physiology A, 168, 103–112.
- Grillner, S. (1979). Interaction between central and peripheral mechanisms in the control of locomotion. Progress in Brain Research, 50, 227–235.
- Grillner, S. (1985). Neurobiological bases of rhythmic motor acts in vertebrates. Science, 228, 143–149.
- Grillner, S., Cangiano, L., Hu, G., Thompson, R., Hill, R., & Wallen, P. (2000). The intrinsic function of a motor system: From ion channels to networks and behavior. Brain Research, 886(1-2), 224–236.
- Grillner, S., Deliagina, T., Ekeberg, O., El Manira, A., Hill, R. H., Lansner, A., Orlovsky, G. N., & Wallen, P. (1995). Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends in Neurosciences, 18(6), 270–279.
- Grillner, S., Parker, D., & El Manira, A. (1998). Vertebrate locomotion: A lamprey perspective. Annals of the New York Academy of Sciences, 860, 1–18.
- Grillner, S., & Rossignol, S. (1978). On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Research, 146, 269–277.
- Grillner, S., & Wallen, P. (1985). The ionic mechanisms underlying N-methyl-D-aspartate receptor-induced, tetrodotoxin-resistant membrane potential oscillations in lamprey neurons active during locomotion. Neuroscience Letters, 60(3), 289–294.
- Grillner, S., Wallen, P., Hill, R., Cangiano, L., & El Manira, A. (2001). Ion channels of importance for the locomotor pattern generation in the lamprey brainstem-spinal cord. Journal of Physiology, 533(1), 23–30.
- Grillner, S., & Zangger, P. (1975). How detailed is the central pattern generation for locomotion? Brain Research, 88(2), 367–371.
- Grillner, S., & Zangger, P. (1979). On the central generation of locomotion in the low spinal cat. Experimental Brain Research, 34(2), 241–261.
- Grimm, K., & Sauer, A. E. (1995). The high number of neurons contributes to the robustness of the locust flight: CPG against parameter variation. Biological Cybernetics, 72, 329–335.
- Gritsenko, V., Mushahwar, V., & Prochazka, A. (2001). Adaptive changes in locomotor control after partial denervation of triceps surae muscles in the cat. Journal of Physiology, 533(1), 299–311.
- Guertin, P., Angel, M. J., Perreault, M. C., & McCrea, D. A. (1995). Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. Journal of Physiology, 487(1), 197–209.
- Gurfinkel, V. S., Levik, Y. S., Kazennikov, O. V., & Selionov, V. A. (1998). Locomotor-like movements evoked by leg muscle vibration in humans. European Journal of Neuroscience, 10(5), 1608–1612.
- Harris, M. G., & Carre, G. (2001). Is optic flow used to guide walking while wearing a displacing prism? Perception, 30, 811–818.
- Harris-Warrick, R. M., & Cohen, A. H. (1985). Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. The Journal of Experimental Biology, 116, 27–46.
- Hatsopoulos, N., Gabbiani, F., & Laurent, G. (1995). Elementary computation of object approach by a wide-field visual neuron. Science, 270, 1000–1003.
- Hayashi, T., Mendelson, B., Phelan, K. D., Skinner, R. D., & Garcia-Rill, E. (1997). Developmental changes in serotonergic receptor-mediated modulation of embryonic chick motoneurons in vitro. Brain Research and Developmental Brain Research, 10, 21–33.
- Hess, D., & Büschges, A. (1999). Role of proprioceptive signals from an insect femur-tibia joint in patterning motoneuronal activity of an adjacent leg joint. Journal of Neurophysiology, 81(4), 1856–1865.
- Hiebert, G. W., Whelan, P. J., Prochazka, A., & Pearson, K. G. (1996). Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of Neurophysiology, 75(3), 1126–1137.
- Hille, B. (1991). Ionic channels of excitable membranes (3rd ed.). New York: Sinauer Associates.
- Hirst, G. D., & Neild, T. O. (1980). Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature, 283, 767–768.
- Hochman, S., Jordan, L. M., & Schmidt, B. J. (1994). TTX-resistant NMDA-receptor mediated voltage oscillations in mammalian lumbar motoneurons. Journal of Neurophysiology, 72, 2559– 2562.
- Hochman, S., & McCrea, D. A. (1994). Effects of chronic spinalization on ankle extensor motoneurons. II: Motoneuron electrical properties. Journal of Neurophysiology, 71(4), 1468–1479.
- Hochman, S., & Schmidt, B. J. (1998). Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord. Journal of Neurophysiology, 79, 743–752.
- Hollands, M. A., & Marple-Horvat, D. E. (1996). Visually guided stepping under conditions of step cycle–related denial of visual information. Experimental Brain Research, 109, 343–356.
- Hollands, M. A., & Marple-Hovat, D. E. (2001). Coordination of eye and leg movements during visually guided stepping. Journal of Motor Behavior, 33, 205–216.
- Hounsgaard, J., Hultborn, H., Jespersen, B., & Kiehn, O. (1988). Bistability of alpha-motoneurons in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. Journal of Physiology, 405, 345–367.
- Hounsgaard, J., & Kiehn, O. (1989). Serotonin-induced bistability of turtle motoneurons caused by a nifedipine-sensitive calcium plateau potential. Journal of Physiology, 414, 265–282.
- Hughes, G. M. (1957). The co-ordination of insect movements. II: The effect of limb amputation and the cutting of commissures in the cockroach (Blatta orientalis). The Journal of Experimental Biology, 34, 306–333.
- Huntley, G. W. (1997). Correlation between patterns of horizontal connectivity and the extend of short-term representational plasticity in rat motor cortex. Cerebral Cortex, 7, 143–156.
- Iles, J. N., & Nicolopoulos-Stournaras, S. (1996). Fictive locomotion in the decerebrated rat. Experimental Brain Research, 109, 393–398.
- Jacobs, K. M., & Donoghue, J. P. (1991). Reshaping the cortical motor map by unmasking latent intracortical connections. Science, 251, 944–957.
- Jankowska, E., Jukes, M. G., Lund, S., & Lundberg, A. (1967a). The effect of DOPA on the spinal cord. 5: Reciprocal organization of pathways transmitting excitatory action to alpha motoneurons of flexors and extensors. Acta Physiologica Scandinavica, 70(3), 369–388.
- Jankowska, E., Jukes, M. G., Lund, S., & Lundberg, A. (1967b). The effect of DOPA on the spinal cord. 6: Half-centre organization of interneurons transmitting effects from the flexor reflex afferents. Acta Physiologica Scandinavica, 70(3), 389– 402.
- Jiang, Z., Carlin, K. P., & Brownstone, R. M. (1999). An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Research, 816, 493–499.
- Johnston, B. R., Peck, J. H., & Harris-Warrick, R. M. (1993). Dopamine induces sign reversal at mixed chemical-electrical synapses, Brain Research, 625, 159–164.
- Johnston, R. M., & Levine, R. B. (1996). Crawling motor patterns induced by pilocarpine in isolated larval nerve cords of Manduca sexta. Journal of Neurophysiology, 76(5), 3178–3195.
- Jordan, L. M., Brownstone, R. M., & Noga, B. R. (1992). Control of functional systems in the brainstem and spinal cord. Current Opinion in Neurobiology, 2, 794–801.
- Jovanovic, K., Petrov, T., Greer, J. J., & Stein, R. B. (1996). Serotonergic modulation of the mudpuppy (Necturus maculatus) locomotor pattern in vitro. Experimental Brain Research, 111, 57–67.
- Kaczmarek, L. K., & Levitan, I. B. (1987). Neuromodulation: The biochemical control of neuronal excitability. New York: Oxford University Press.
- Katz, P. S. (1999). What are we talking about? Modes of neuronal communication. In P. S. Katz (Ed.), Beyond Neurotransmission: Neuromodulation and its importance for information processing (pp. 1–28). Oxford: Oxford University Press.
- Katz, P. S., & Frost, W. N. (1995). Intrinsic neuromodulation in the Tritonia swim CPG: Serotonin mediates both neuromodulation and neurotransmission. Journal of Neurophysiology, 74, 2281–2294.
- Katz, P. S., Getting, P. A., & Frost, W. N. (1994). Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature, 367, 729–731.
- Kemnitz, C. P. (1997). Dopaminergic modulation of spinal neurons and synaptic potentials in the lamprey spinal cord. Journal of Neurophysiology, 77, 289–298.
- Keshishian, H., & O’Shea, M. (1985). The distribution of a peptide neurotransmitter in the postembryonic grasshopper central nervous system. Journal of Neuroscience, 5, 992–1004.
- Kiehn, O., Housgaard, J., & Sillar, K. T. (1997). Basic building blocks of vertebrate spinal central pattern generators. In P. S. G. Stein, S. Grillner, A. I. Selverston, & D. G. Stuart (Eds.), Neurons, networks and motor behaviour (pp. 47–59). Cambridge: MIT Press.
- Kiehn, O., Hultborn, H., & Conway, B. A. (1992). Spinal locomotor activity in acutely spinalized cats induced by intrathecal application of noradrenaline. Neuroscience Letters, 143, 243–246.
- Kiehn, O., & Katz, P. S. (1999). Making circuits dance: Neuromodulation of motor systems. In P. S. Katz (Ed.), Beyond neurotransmission (pp. 275–317). New York: Oxford University Press.
- Kiehn, O., & Kjaerulff, O. (1996). Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. Journal of Neurophysiology, 75, 1472–1482.
- Kiehn, O., & Kjaerulff, O. (1998). Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Annals of the New York Academy of Sciences, 860, 110–129.
- Kiehn, O., Sillar, K. T., Kjaerulff, O., & McDearmid, J. R. (1999). Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. Journal of Neurophysiology, 82, 741–746.
- Kjaerulff, O., & Kiehn, O. (1996). Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord. Journal of Neurophysiology, 775, 1472–1482.
- Kleinhaus, A. L., & Angstadt, J. D. (1995). Diversity and modulation of ionic conductances in leech neurons. Journal of Neurobiology, 27, 419–433.
- Klintsova, A. Y., & Greenough, W. T. (1999). Synaptic plasticity in cortical systems. Current Opinion in Neurobiology, 9(2), 203–208.
- Konczak, J. (1994). Effects of optic flow on the kinematics of human gait: A comparison of young and older adults. Journal of Motor Behavior, 26, 225–236.
- Krapp, H. G. (2000). Neuronal matched filters for optic flow processing in flying insects. In M. Lappe (Ed.), International review of Neurobiology, 44: Neuronal processing of optic flow. New York: Academic Press.
- Kupfermann, I. (1979). Modulatory actions of neurotransmitters. Annual Review of Neuroscience, 2, 447–465.
- Kupfermann, I., & Weiss, K. R. (1978). The command neuron concept. The Behavioral and Brain Sciences, 1, 3–39.
- Kuypers, H. G. J. M., & Huisman, A. M. (1982). The new anatomy of the descending brain pathways. In B. Sjölund & A. Björklund (Eds.), Brain stem control of spinal mechanisms (pp. 29–54). Amsterdam: Elsevier Biomedical Press.
- Lee, D. N., & Thomson, J. A. (1982). Vision in action: The control of locomotion. In D. J. Ingle, M. A. Goodale, & R. J. W. Mansfield (Eds.), Analysis of visual behavior (pp. 411–433). Cambridge: MIT Press.
- Levy, W. J., Amassian, V. E., Traad, M., & Cadwell, J. (1990). Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Research, 510(1), 130–134.
- Lou, J. S., & Bloedel, J. R. (1988). A new conditioning paradigm: Conditioned limb movements in locomoting decerebrate ferrets. Neurosci Lett, 84,185–190.
- Lotshaw, D. P., & Levitan, I. B. (1988). Reciprocal modulation of calcium current by serotonin and dopamine in the identified Aplysia neuron R15. Brain Research, 439, 64–76.
- Lovely, R. G., Gregor, R. J., Roy, R. R., & Edgerton, V. R. (1990). Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Research, 514(2), 206–218.
- Macpherson, J. M., Fung, J., & Jacobs, R. (1997). Postural orientation, equilibrium, and the spinal cord. Advances in Neurology, 72, 227–232.
- Mangan, P. S., Cometa, A. K., & Friesen, W. O. (1994). Modulation of swimming behavior in the medicinal leech. IV: Serotonininduced alteration of synaptic interactions between neurons of the swim circuit. Journal of Comparative Physiology A, 175, 723–736.
- Marder, E., & Calabrese, R. L. (1996). Principles of rhythmic motor pattern generation. Physiological Reviews, 76(3), 687–717.
- Matheson, T. (1997). Octopamine modulates the responses and presynaptic inhibition of proprioceptive sensory neurons in the locust Schistocerca gregaria. The Journal of Experimental Biology, 200, 1317–1325.
- McCrea, D. A., Shefchyk, S. J., Stephens, M. J., & Pearson, K. G. (1995). Disynaptic group I excitation of synergists ankle extensor motoneurons during fictive locomotion in the cat. Journal of Physiology, 487(2), 527–539.
- McDearmid, J. R., Scrymgeour-Wedderburn, J. F., & Sillar, K. T. (1997). Aminergic modulation of glycine release in a spinal network controlling swimming in Xenopus laevis. Journal of Physiology, 503, 111–117.
- Meinertzhagen, I. A. (2001). Plasticity in the insect nervous system. Advances in Insect Physiology, 28, 84–167.
- Mesce, K. A., Crisp, K. M., & Gilchrist, L. S. (2001). Mixtures of octopamine and serotonin have nonadditive effects on the CNS of the medicinal leech. Journal of Neurophysiology, 85, 2039– 2046.
- Mori, S., Matsuyama, K., Kohyama, J., Kobayashi, Y., & Takakusaki, K. (1992). Neuronal constituents of postural and locomotor control systems and their interactions in cats. Brain Development, 14(20), 109–120.
- Mulloney, B., Acevedo, L. D., & Bradbury, A. G. (1987). Modulation of the crayfish swimmeret rhythm by octopamine and the neuropeptide proctolin. Journal of Neurophysiology, 58, 584– 597.
- Murchison, D., Chrachri, A., & Mulloney, B. (1993). A separate local pattern-generating circuit controls the movements of each swimmeret in crayfish. Journal of Neurophysiology, 70, 2620–2631.
- Nelson, S. G., & Mendell, L. M. (1979). Enhancement in Iamotoneuron synaptic transmission caudal to chronic spinal cord transection. Journal of Neurophysiology, 42, 642–654.
- Nudo, R. J., Milliken, G. W., Jenkins, W. M., & Merzenich, M. M. (1996). Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience, 16, 785–807.
- Nusbaum, M. P., El Manira, A., Gossard, J.-P., & Rossignol, S. (1997). Presynaptic mechanisms during rhythmic activity in vertebrates and invertebrates. In P. S. G. Stein, S. Grillner, A. I. Serlverston, & D. G. Stuart (Eds.), Neurons, networks, and behavior (pp. 225–235). Cambridge: MIT Press.
- Orlovsky, G. N., Deliagina, T. G., & Grillner, S. (1999). Neuronal control of locomotion. Oxford: Oxford University Press.
- Orlovsky, G. N., Deliagina, T. G., & Wallen, P. (1992). Vestibular control of swimming in lamprey. I: Responses of reticulospinal neurons to roll and pitch. Experimental Brain Research, 90(3), 479–488.
- Panchin, Y., Perrins, R. J., & Roberts, A. (1991). The action of acetylcholine on the locomotor central pattern generator for swimming in Xenopus The Journal of Experimental Biology, 161, 527–531.
- Pang, M. Y. C., & Yang, J. F. (2000). The initiation of the swing phase in human infant stepping: Importance of hip position and leg loading. Journal of Physiology, 528.2, 389–404.
- Parker, D. (1995). Serotonergic modulation of locust motor neurons. Journal of Neurophysiology, 73, 923–932.
- Parker, D. (2001). Spinal cord plasticity: Independent and interactive effects of neuromodulator and activity-dependent plasticity. Molecular Neurobiology, 22, 55–80.
- Parker, D., & Grillner, S. (1999). Long-lasting substanceP-mediated modulation of NMDA-induced rhythmic activity in the lamprey locomotor network involves separate RNA- and protein-synthesis-dependent stages. European Journal of Neuroscience, 11, 1515–1522.
- Parker, D., & Grillner, S. (2000). The activity-dependent plasticity of segmental and intersegmental synaptic connections in the lamprey spinal cord. European Journal of Neuroscience, 12, 2135–2146.
- Patla, A. E., Adkin, A., Martin, C., Holden, R., & Prentice, S. (1996). Characteristics of voluntary visual sampling of the environment for safe locomotion over different terrains. Experimental Brain Research, 112, 513–522.
- Patla, A. E., & Vickers, J. N. (1997). Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport, 8, 3661–3665.
- Pearson, K. G. (1985). Are there central pattern generators for walking and flight in insects? In W. J. P. Barnes & M. Gladden (Eds.), Feedback and motor control in invertebrates and vertebrates (pp. 307–316). London: Croom-Helm.
- Pearson, K. G. (1993). Common principles of motor control in vertebrates and invertebrates. Annual Reviews in Neuroscience, 16, 265–297.
- Pearson, K. G. (1995). Proprioceptive regulation of locomotion. Current Opinion in Neurobiology, 5(6), 786–791.
- Pearson, K. G. (2000). Neural adaptation in the generation of rhythmic behavior. Annual Reviews of Physiology, 62(1), 723–753.
- Pearson, K. G. (2001). Could enhanced reflex function contribute to improving locomotion after spinal cord repair? Journal of Physiology, 533(1), 75–81.
- Pearson, K. G., & Duysens, J. E. J. (1976). Functions of segmental reflexes in the control of stepping in cockroaches and cats. In R. E. Herman, S. Grillner, D. Stuart, & P. Stein (Eds.), Neural control in locomotion (pp. 519–538). New York: Plenum Press.
- Pearson, K. G., Fouad, K., & Misiaszek, J. E. (1999). Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. Journal of Neurophysiology, 82(1), 370–381.
- Pearson, K. G., Heitler, W. J., & Steeves, J. D. (1980). Triggering of locust jump by multimodal inhibitory interneurons. Journal of Neurophysiology, 43, 257–278.
- Pearson, K. G., & Ramirez, J.-M. (1992). Parallels with other invertebrateandvertebratemotorsystems.InR.M.Harris-Warrick, E. Marder,A. I. Selvertson, & M. Moulins (Eds.), Dynamic biological networks (pp. 263–281). Cambridge: MIT Press.
- Pearson, K. G., & Ramirez, J.-M. (1997). Sensory modulation of pattern-generating circuits. In P. S. G. Stein, S. Grillner, A. I. Serlverston, & D. G. Stuart (Eds.), Neurons, networks, and behavior (pp. 225–235). Cambridge: MIT Press.
- Perrins, R., & Roberts, A. (1995a). Cholinergic contribution to excitation in a spinal locomotor central pattern generator in Xenopus Journal of Neurophysiology, 73, 1005–1012.
- Perrins, R., & Roberts, A. (1995b). Cholinergic and electrical motoneuron-to-motoneuron synapses contribute to on-cycles excitation during swimming in Xenopus Journal of Neurophysiology, 73, 1013–1019.
- Perrins, R., & Roberts, A. (1995c). Cholinergic and electrical synapses between synergistic spinal motoneurons in the Xenopus laevis Journal of Physiology, 485, 135–144.
- Pflüger, A. (1853). Die sensorischen Funktionen des Rückenmarks der Wirbelthiere nebst einer neuen Lehre über die Leitungsgesetze der Reflexionen. Berlin.
- Poon, M., Friesen, W. O., & Stent, G. S. (1978). Neuronal control of swimming in the medicinal leech. V: Connexions between the oscillatory interneurons and the motor neurons. The Journal of Experimental Biology, 75, 45–63.
- Prochazka, A. (1996a). The fuzzy logic of visuomotor control. Canadian Journal of Physiology and Pharmacology, 74(4), 456–462.
- Prochazka, A. (1996b). Proprioceptive feedback and movement regulation. In L. Rowell & J. T. Shepherd (Eds.), Handbook of physiology (pp. 89–127). New York: Oxford University Press.
- Prochazka, A., & Mushahwar, V. K. (2001). Spinal cord function and rehabilitation: An overview. Journal of Physiology, 533(1), 3–4.
- Prokop, T., Schubert, M., & Berger, W. (1997). Visual influence on human locomotion: Modulation of changes in optic flow. Experimental Brain Research, 114, 63–70.
- Raineteau, O., & Schwab, M. E. (2001). Plasticity of motor systems after incomplete spinal cord injury. Nature Reviews Neuroscience, 2(4), 263–273.
- Ramirez, J.-M., & Pearson, K. G. (1991a). Octopamine induces bursting and plateau potentials in insect neurons. Brain Research, 549, 332–337.
- Ramirez, J.-M., & Pearson, K. G. (1991b). Octopaminergic modulation of interneurons in the flight system of the locust. Journal of Neurophysiology, 66, 1522–1537.
- Reichardt, W. (1969). Movement perception in insects. In W. Reichardt (Ed.), Processing of optical data by organisms and by machines (pp. 465–493). New York: Academic Press.
- Ribotta, M. G., Orsal, D., Ferboli-Lohnherr, D., & Privat, A. (1998). Recovery of locomotion following transplantation of monoaminergic neurons in the spinal cord of paraplegic rats. Annals of the New York Academy of Sciences, 860, 393–411.
- Rind, F. C., & Simmons, P. J. (1992). Orthopteran DCMD neurons: A reevaluation of responses to moving objects. I. Selective responses to approaching objects. Journal of Neurophysiology, 68, 1654–1682.
- Roberts, A. (1990). How does a nervous system produce behaviour? A case study in neurobiology. Scientific Progress (Oxford), 74, 31–51.
- Roberts, A. (2000). Early functional organization of spinal neurons in developing lower vertebrates. Brain Research Bulletin, 53(5), 585–593.
- Roberts,A.,Dale,N.,Evoy,W.H.,&Soffe,S.R.(1985).Synapticpotentials in motoneurons during fictive swimming in spinal Xenopus Journal of Neurophysiology, 54(1), 1–10.
- Robertson, R. M., & Pearson, K. G. (1983). Interneurons in the flight system of the locust: Distribution, connections, and resetting properties. Journal of Comparative Neurology, 215(1), 33–50.
- Robertson, R. M., & Pearson, K. G. (1985). Neural circuits in the flight system of the locust. Journal of Neurophysiology, 53(1), 110–128.
- Ronacher, B., Wolf, H., & Reichert, H. (1988). Locust flight behaviour after hemisection of individual thoracic ganglia: Evidence for hemiganglionic premotor centers. Journal of Comparative Physiology A, 163, 749–759.
- Rossignol, S. (1996a). Neural control of stereotypic limb movements. In L. B. Rowell & J. T. Sheperd (Eds.), Handbook of physiology (pp. 173–216). New York: Oxford University Press.
- Rossignol, S. (1996b). Visuomotor regulation of locomotion. Journal of Physiology and Pharmacology, 74, 418–425.
- Rossignol, S. (2000). Locomotion and its recovery after spinal injury. Current Opinion in Neurobiology, 10(6), 708–716.
- Rossignol,S.,Chau,C.,Brunstein,E.,Giroux,N.,Boyer,L.,Barbeau, H., & Reader,T.A. (1998). Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Annals of the New York Academy of Sciences, 860, 346–359.
- Rowell, C. H. F., O’Shea, M., & Williams, J. L. D. (1977). The neuronal basis of a sensory analyser: The acridid movement detector system. The Journal of Experimental Biology, 68, 157–185.
- Rushton, S. K., Harris, J. M., Lloyd, M. R., & Wann, J. P. (1998). Guidance of locomotion of foot uses perceived target location rather than optic flow. Current Biology, 8, 1191–1194.
- Ryckebusch, S., & Laurent, G. (1993). Rhythmic patterns evoked in locust leg motor neurons by the muscarinic agonist pilocarpine. Journal of Neurophysiology, 69, 1583–1595.
- Satterlie, R. A., Norekian, T. P., & Pirtle, T. J. (2000). Serotonininduced spike narrowing in a locomotor pattern generator permits increases in cycle frequency during accelerations. Journal of Neurophysiology, 83, 2163–2170.
- Satterlie, R. A., & Spencer, A. N. (1985). Swimming in the Pteropod Mollusk, Clione-Limacina. 2. Physiology. The Journal of Experimental Biology, 116, 205–232.
- Sbordone, R. J., Liter, J. C., & Pettler-Jennings, P. (1995). Recovery of function following severe traumatic brain injury: A retrospective 10-year follow-up. Brain Injury, 9(3), 285–299.
- Schomburg, E. D., & Steffens, H. (1996). Bistable characteristics of motoneuron activity during DOPA induced fictive locomotion in spinal cats. Neuroscience Research, 26, 47–56.
- Schotland, J., Shupliakov, O., Wikstrom, M., Brodin, L., Srinivasan, M., You, Z. B., Herrera-Marschitz, M., Zhang, W., Hokfelt, T., & Grillner, S. (1995). Control of lamprey locomotor neurons by colocalized monoamine transmitters. Nature, 374, 266–268.
- Schwindt, P. C., & Crill, W. E. (1984). Membrane properties of the cat spinal motoneurons. In R. A. Davidoff (Ed.), Handbook of the spinal cord (pp. 199–242). New York: Marcel Dekker.
- Segal, R. L., & Wolf, S. L. (1994). Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Experimental Neurology, 130(2), 202–213.
- Sherrington, C. S. (1900a). The muscular sense. In E. A. Schäfer (Ed.), Textbook of physiology (Vol. 2, pp. 1002–1025). Edinburgh:
- Sherrington, C. S. (1900b). On the innervation of antagonistic muscles (sixth note). Proceedings of the Royal Society, 66, 60–67.
- Sherrington, C. S. (1905a). On reciprocal innervation of antagonistic muscles (seventh note). Proceedings of the Royal Society, 76B, 160–163.
- Sherrington, C. S. (1905b). On the reciprocal innervation of antagonistic muscles (eighth note). Proceedings of the Royal Society, 76B, 269–279.
- Sherrington, C. S. (1910). Flexor reflex of the limb, crossed extension reflex, and reflex stepping and standing. Journal of Physiology (London), 40, 28–121.
- Shupliakov, O., Pieribone, V. A., Gad, H., & Brodin, L. (1995). Synaptic vesicle depletion in reticulospinal axons is reduced by 5-hydroxytryptamine: Direct evidence for presynaptic modulation of glutamatergic transmission. European Journal of Neuroscience, 7, 1111–1116.
- Sigvardt, K. A., Grillner, S., Wallen, P., & Van, D. P. (1985). Activation of NMDA receptors elicits fictive locomotion and bistable membrane properties in the lamprey spinal cord. Brain Research, 336, 390–395.
- Sillar, K. T., Clarac, F., & Bush, B. M. H. (1987). Intersegmental coordination of central neural oscillators for rhythmic movements of the walking legs of crayfish, lenisculus. The Journal of Experimental Biology, 131, 245–264.
- Sillar, K. T., Reith, C. A., & McDearmid, J. R. (1998). Development and aminergic neuromodulation of a spinal locomotor network controlling swimming in Xenopus Annals of the New York Academy of Sciences, 860, 318–332.
- Sillar, K. T., & Simmers, A. J. (1994a). 5HT induces NMDA receptor-mediated intrinsic oscillations in embryonic amphibian spinal neurons. Proceedings of the Royal Society of London B, 255, 139–145.
- Sillar, K. T., & Simmers, A. J. (1994b). Oscillatory membrane properties of spinal cord neurons that are active during fictive swimming in Rana temporaria European Journal of Morphology, 32, 185–192.
- Sillar, K. T., Skorupski, P., Elson, R. C., & Bush, B. M. H. (1986). Two identified afferent neurons entrain a central locomotor rhythm generator. Nature, 323, 440–443.
- Sillar, K. T., Wedderburn, J. F., & Simmers, A. J. (1992). Modulation of swimming rhythmicity by 5-hydroxytryptamine during post-embryonic development in Xenopus laevis. Proceedings of the Royal Society London B, 250, 107–114.
- Sinkjaer, T., Andersen, J. B., Ladouceur, M., Christensen, L. O., & Nielsen, J. B. (2000). Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. Journal of Physiology, 523(3), 817–827.
- Skorupski, P. (1996). Octopamine induces steady-state reflex reversal in crayfish thoracic ganglia. Journal of Neurophysiology, 76, 93–108.
- Soffe, S. R. (1991). Triggering and gating of motor responses by sensory stimulation: Behavioral selection in Xenopus Proceedings of the Royal Society of London B, Biological Sciences, 246, 197–203.
- Soffe, S. R., Clarke, J. D., & Roberts, A. (1984). Activity of commissural interneurons in spinal cord of Xenopus Journal of Neurophysiology, 51(6), 1257–1267.
- Sombati, S., & Hoyle, G. (1984). Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. Journal of Neurobiology, 15, 481–506.
- Speach, D. P., & Dombovy, M. L. (1995). Recovery from stroke: Rehabilitation. Baillieres Clinical Neurology, 4(2), 317– 338.
- Squalli-Houssaini, Y., & Cazalets, J.-R. (2000). Noradrenergic control of locomotor networks in the vitro spinal cord of the neonatal rat. Brain Research, 852, 100–109.
- Squalli-Houssaini, Y., Cazalets, J. R., & Clarac, F. (1993). Oscillatory properties of the central pattern generator for locomotion in neonatal rats. Journal of Neurophysiology, 70, 803–813.
- Stent, G. S., Kristan, W. B., Jr., Friesen, W. O., Ort, C. A., Poon, M., & Calabrese, R. L. (1978). Neuronal generation of the leech swimming movement. Science, 200, 1348–1357.
- Stephens, M. J., & Yang, J. F. (1999). Loading during the stance phase of walking in humans increases the extensor EMG amplitude but does not change the duration of the step cycle. Experimental Brain Research, 124(3), 363–370.
- Stevenson, P. A., & Sporhase-Eichmann, U. (1995). Localization of octopaminergic neurons in insects. Comparative Biochemistry and Physiology A, Physiology, 110(3), 203–215.
- Stoffregen, T. A., Schmuckler, M. A., & Gibson, E. J. (1987). Use of central and peripheral optical flow in stance and locomotion in young walkers. Perception, 16(1), 113–119.
- Straub, V. A., & Benjamin, P. R. (2001). Extrinsic modulation and motor pattern generation in a feeding network: A cellular study. Journal of Neuroscience, 21, 1767–1778.
- Strausfeld, N. J., & Nässel, D. R. (1981). Neuroarchitecture of brain regions that subserve the compound eyes of crustacea and insects. In H. Autrum (Ed.), Comparative physiology and evolution of vision in invertebrates (Vol. 2, part 6B, pp. 1–132). Berlin: Springer.
- Sun, H.-J., Carey, D. P., & Goodale, M. A. (1992). A mammalian model of optic-flow utilization in the control of locomotion. Experimental Brain Research, 91, 171–175.
- Tillakaratne, N. J., Mouria, M., Ziv, N. B., Roy, R. R., Edgerton, R., & Tobin, A. J. (2000). Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res, 60, 219–230.
- Ullen, F., Deliagina, T. G., Orlovsky, G. N., & Grillner, S. (1996). Visual potentiation of vestibular responses in lamprey reticulospinal neurons. European Journal of Neuroscience, 8(11), 2298–2307. van Mier, P., Joosten, H. W., van Rheden, R., & ten Donkelaar, H. J. (1986). The development of serotonergic raphespinal projections in Xenopus laevis. International Journal of Developmental Neurosciences, 4, 465–475.
- Viala, D., & Buser, P. (1969). The effects of DOPA and 5-HTP on rhythmic efferent discharges in hindlimb nerves in the rabbit. Brain Research, 12, 437–443.
- Waldron, I. (1967). Mechanisms for the production of the motor output pattern in flying locusts. The Journal of Experimental Biology, 47, 201–212.
- Wallen, P., Buchanan, J. T., Grillner, S., Hill, R. H., Christenson, J., & Hokfelt, T. (1989). Effects of 5-hydroxytryptamine on the afterhyperpolarization, spike frequency regulation, and oscillatory membrane properties in lamprey spinal cord neurons. Journal of Neurophysiology, 61, 759–768.
- Warren, H. W., Kay, B. A., Zosh, W. D., Duchon, A. P., & Sahuc, S. (2001). Optic flow is used to control human walking. Nature Neuroscience, 4(2), 213–216.
- Wedderburn, J. F., & Sillar, K. T. (1994). Modulation of rhythmic swimming activity in post-embryonic Xenopus laevis tadpoles by 5-hydroxytryptamine acting at 5HT1a receptors. Proceedings of the Royal Society of London B, 257, 59–66.
- Weidner, N., Ner, A., Salimi, N., & Tuszynski, M. H. (2001). Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proceedings of the National Academy of Science, 98(6), 3513–3518.
- Wendler, G. (1964). Laufen und Stehen der Stabheuschrecke Carausius morosus: Sinnesborstenfelder in den Beingelenken als Glieder von Regelkreisen. vergl. Physiol., 48, 198–250.
- Wernig, A., & Muller, S. (1992). Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia, 30(4), 229–238.
- Whelan, P. J., Hiebert, G. W., & Pearson, K. G. (1995a). Plasticity of the extensor group I pathway controlling the stance to swing transition in the cat. Journal of Neurophysiology, 74(6), 2782– 2787.
- Whelan, P. J., Hiebert, G. W., & Pearson, K. G. (1995b). Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Experimental Brain Research, 103, 20–30.
- Wikstrom, M., Hill, R., Hellgren, J., & Grillner, S. (1995). The action of 5-HT on calcium-dependent potassium channels and on the spinal locomotor network in lamprey is mediated by 5-HT1A-like receptors. Brain Research, 678, 191–199.
- Wilson, D. M. (1961). The central nervous control of flight in a locust. The Journal of Experimental Biology, 38, 471–490.
- Wilson, D. M. (1966). Insect walking. Annual Review of Entomology, 11, 103–122.
- Wohlberg, C. J., Hackman, J. C., & Davidoff, R. A. (1987). Epinephrine and norepinephrine modulate neuronal responses to excitatory amino acids antagonists in frog spinal cord. Synapse, 1, 202–207.
- Wolf, H., & Büschges, A. (1997). Plasticity of synaptic connections in sensory-motor pathways of the adult locust flight system. Journal of Neurophysiology, 78, 1276–1284.
- Wolf, H., & Pearson, K. G. (1988). Proprioceptive input patterns elevator activity in the locust flight system. Journal of Neurophysiology, 59, 1831–1853.
- Wolpaw, J. R. (1997). The complex structure of a simple memory. Trends in Neuroscience, 20(12), 588–594.
- Wood, R. M., Harvey, M. A., Young, C. E., Beedie, A., & Wilson, T. (2000). Weighting to go with the flow? Current Biology, 10, 545–546.
- Wu, C. W., & Kaas, J. H. (1999). Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. Journal of Neuroscience, 19(17), 7679–7697.
- Yakovleff, A., Roby-Brami, A., Guezard, B., Mansour, H., Bussel, B., & Privat, A. (1989). Locomotion in rats transplanted with noradrenergic neurons. Brain Research Bulletin, 22, 115–121.
- Yang, J. F., & Stein, R. B. (1990). Phase-dependent reflex reversal in human leg muscles during walking. Journal of Neurophysiology, 63(5), 1109–1117.
- Yang, J. F., Stein, R. B., & James, K. B. (1991). Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Experimental Brain Research, 87(3), 679–687.
- Zhao, F.-Y., & Roberts, A. (1998). Assessing the roles of glutamatergic and cholinergic synaptic drive in the control of fictive swimming frequency in young Xenopus Journal of Comparative Physiology A, 183, 753–758.




