View sample Synapse Ultrastructure Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The chemical synapse, which is the primary location for communication between neurons, is characterized by the apposition of two neuronal membranes with unequal membrane densities and a cluster of synaptic vesicles close to the synaptic site. Synaptic ultrastructure refers to the study of the physical components that make up the chemical synapse. This research paper examines some of the important findings in this area and considers the potential functional relevance of changes in synaptic ultrastructure that have been observed following neuronal activation, neural lesions, learning, and memory.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Why Is Synaptic Ultrastructure Important?
Ramon y Cajal (1893; cited in Bliss and Collingridge 1993) theorized that learning and memory formation resulted from the formation of new synapses. Others speculated that learning might result from changes in the relative strength or efficacy of existing synapses. Donald Hebb (1949) postulated that one neuron’s involvement in firing a second neuron would strengthen the connection between the two neurons. The most likely location for this type of change in connective strength is the synapse. At the time of Hebb’s proposal there was little evidence to suggest that synapses were capable of change, either anatomically or physiologically. Based on advances in research methodology, however, it is clear that synapses are indeed capable of these types of changes.
As a result of this early research (Cajal 1893, Hebb 1949), the structure and functioning of the synapse has become an area of intense study. While neuronal, axonal, and dendritic changes are also undoubtedly important, the synapse probably represents the primary location for activity dependent neural plasticity. Research on synapses includes examining synaptic chemical transmission, how synapses form, and the differences in synaptic connections throughout the brain. While describing specific synaptic components has also been an important pursuit, quantifying the number and dimensions of synapses is the primary goal of research on synapse ultrastructure.
Quantifying synapses may be especially important
for functional reasons when synapses change in number or dimension following neural activation, neural lesions, learning, and memory formation. While a comprehensive review of the literature in this area is beyond the scope of this research paper, the basic components of the synapse are described, some of the research examining the structural plasticity of the synapse is discussed, and a series of ultrastructural changes that may support the anatomical storage of information is proposed.
2. Basic Ultrastructural Components Of The Synapse
The following is a brief description of the basic physical components of the synapse. The synapse can be divided into presynaptic components, the synaptic cleft, and postsynaptic components. The presynaptic component usually arises from the end of an axonal branch and the postsynaptic component is usually a dendrite or a dendritic spine. Although many other synaptic contact types exist (e.g., axo-axonic (axon to axon), axosomatic (axon to neuron cell body), etc.), the majority of synapses in the more plastic commonly studied regions of the brain (e.g., the hippocampus and cerebral cortex) consist of axon terminals contacting dendritic spines.
The synaptic junction contains a specialized area referred to as the active zone where synaptic transmission is known to take place. This area is composed of two plasma membranes separated by a cleft, with dense structural specializations (referred to as densities, dense projections, or thickenings; see Fig. 1). The presynaptic axon terminal contains synaptic vesicles situated within the presynaptic dense projections. These dense projections are tufts of electron dense material assumed to be essential for vesicular release. The postsynaptic element contains the postsynaptic density (PSD), which is comprised of various proteins (Bloomberg et al. 1977) and is associated with actin, a calcium calmodulin dependent protein kinase (Cohen et al. 1985). While PSDs are also associated with other kinases etc., actin may play a particularly important role in synaptic ultrastructural change as it is involved in maintaining the structure of the postsynaptic element (see Sect. 3.2). The PSD is also the site of postsynaptic neurotransmitter receptors and is considered integral to modulation of synaptic events (Seikevitz 1985).
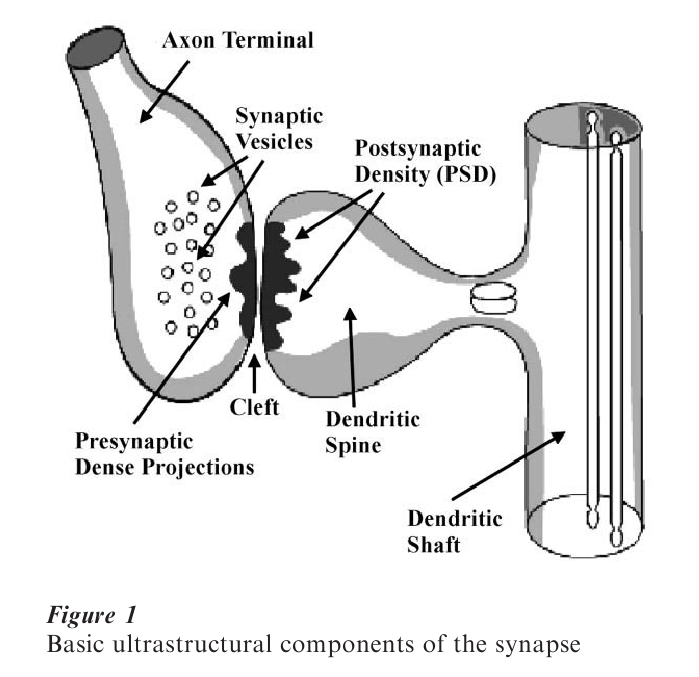
Gray (1959) proposed that chemical synapses come in two basic morphological types (Type I and Type II). Type I synapses are thought to be excitatory, house round vesicles, and have a wider PSD region (asymmetric synapses). Type II synapses are thought to be inhibitory, house oblong or oval vesicles and have a smaller PSD (symmetric synapses).
3. Ultrastructural Plasticity Of The Synapse
It has become clear that neuronal growth, changes in connectivity, and changes in activity are associated with modulation of the structure of synapses (Bailey and Kandel 1993). Changes have been observed in synaptic number, synaptic curvature, synaptic size, and synaptic configuration (e.g., perforated synapses, see Sect. 3.4). Please note that this research paper does not include a consideration of the ultrastructure of dendritic spines (see Harris and Kater 1994 for review).
3.1 Quantifying Synaptic Number
When examining the ultrastructure of synapses in a given brain region the initial analysis usually involves some estimate of the total number of synapses in that region. In early research synapses were often counted on a single plane within the region of interest. That is, researchers used an electron microscope to photograph one large two-dimensional area and simply counted how many synapses were present. Gundersen (1977) and others began to realize that this form of counting involved a serious bias. Coggeshall and Lekan (1996) point out that considering only one plane biases the synaptic counts because the number of two-dimensional synaptic profiles observed depends not only on the actual number of whole synapses but also on the size, shape, orientation, etc. of the synapses. As a result, the method of using stereological dissectors to estimate synaptic number was developed. Put simply this involves examining a series of micrographs (photographs) that move through the tissue in three dimensions. Synapses are only counted once when they first appear which rules out the bias identified above and includes all synapses present (see Geinisman et al. 1996b for more details). Synaptic number in a brain region is assumed to important functionally as more synapses would yield stronger connections between neurons (Petit 1995).
3.2 Changes In Synaptic Curvature
Changes in synaptic curvature are also known to occur following various forms of synaptic activation (see Petit 1995 for review). Markus and Petit (1989) evaluated synaptic curvature using four possible states: concave (presynaptic element protrudes into the postsynaptic element), flat (no curvature), convex (postsynaptic element protrudes into the presynaptic element), and irregular (w-shaped or more than one curvature). One possible explanation for curvature changes is that plastic internal actin and myosin cytoskeletal networks may create changes in response to activity dependent calcium influx. Fifkova (1985) found that cytoskeletal networks that contain only highly plastic actin structures are found exclusively in developing neurons and mature dendritic spines (see Deller et al. 2000 for an update on this research).
Functionally, curvature is thought to effect synaptic efficacy by increasing contact area and the probability of the transmitters reaching their target (see Markus and Petit 1989 for review). Computer modeling experiments have also suggested that concave shaped synapses, seem to confine the diffusion of presynaptic intracellular calcium (Ghaffari et al. 1997). This would lead to higher calcium concentrations in the presynaptic terminal and an increased probability of vesicular (transmitter) release.
3.3 Changes In Synaptic Size
Most researchers have theorized that larger synapses are stronger synapses. Conceivably, more neurotransmitter could be released (presynaptically), more postsynaptic receptors could be present, and more conductance through to the postsynaptic cell could occur (Petit 1995). A recent study used cultured cortical neurons to study the effect of synaptic size on the quantal capacity o f the synapse (Mackenzie et al. 1999). By imaging Ca2+ and then conducting morphological analyses on the same synapse, Mackenzie et al. concluded that synaptic size correlates positively with the amplitude of the postsynaptic response. This suggests that larger synapses create larger synaptic signals.
3.4 Changes In Synaptic Configuration
One of the main changes in synaptic ultrastructure is the transition of some synapses from a continuous synapse to a perforated synapse. A perforated synapse can be defined as any synapse with a discontinuous or segmented PSD (Geinisman et al. 1991). Carlin and Siekevitz (1983) proposed that at some optimal size the postsynaptic material would perforate, eventually segment, and potentially new simple synapses would form. There is, however, evidence against the notion that synapses split to form new simple synapses. Jones et al. (1991) found few double-headed spines, axospinous non-perforated synapses lying adjacent to one another, and spinules completely traversing the presynaptic terminals of perforated synapses. Functionally, perforations may indicate synapses ready to divide and this may lead to more synapses and a larger neural signal (Toni et al. 1998). Alternatively, perforations may allow calcium channels to be located closer to the vesicular release apparatus, which could result in greater neurotransmitter release (Jones et al. 1991).
4. Changes In Synaptic Ultrastructure Following Neural Events
As mentioned in Sect. 1, a comprehensive review of the literature on changes in synaptic ultrastructure is beyond the scope of this research paper. As a result synaptic changes following the onset of disease and other models are not discussed. The following is a selection of findings associated with models that involve activation of synapses primarily in the hippocampal formation due to its involvement in learning and memory.
4.1 Long-Term Potentiation
Bliss and Lomo (1973) are credited with the discovery of long-term potentiation (LTP), which has proven to be a popular model system for learning and memory. Put simply LTP is a sustained increase (hours, days, or weeks) in the amplitude of the response evoked in a cell, or population of cells, following tetanic (repeated) stimulation of those cells. The ultrastructural characteristics of synapses have been shown to change following the induction of LTP (Geinisman et al. 1991, Desmond and Levy 1983, Weeks et al. 1999). It is not clear, however, what role, if any, these changes play in the enhanced neural signal observed during the maintenance of LTP (see Sect. 3.2–3.4 for some possibilities).
While LTP can be induced in several brain regions, the majority of the research on changes in synaptic ultrastructure following LTP has been conducted in the hippocampus. Geinisman et al. (1996a) found more numerous axo-dendritic synapses (synapses directly on the dendritic shaft) 13 days following the induction of LTP in the rat dentate gyrus (a sub-region of the hippocampus). Functionally, these axo-dendritic synapses may have a more direct effect on the soma and could therefore combine to create a potentiated signal (see Harris and Kater 1994 for discussion). Desmond and Levy (1983) analyzed synaptic curvature in the rat dentate gyrus and found that the number of concave shaped synapses increased as early as 2 minutes after the induction of LTP. General increases in synaptic size following LTP have also been reported (Chang and Greenough 1984, Desmond and Levy 1988). These studies found an enlargement of the presynaptic and postsynaptic elements, and an increase in length of the presynaptic dense projections and postsynaptic density (PSD).
Geinisman et al. (1991) found an increase in the number of perforated synapses per neuron following the induction of LTP in the rat dentate gyrus. Importantly, the significant increase in perforated synapses observed at 1 hour post-induction was no longer evident at 13 days post-induction (Geinisman et al. 1996a). Buchs and Muller (1996) utilized calcium accumulation markers to identify activated synapses and found that these activated synapses were more perforated following LTP induction. Toni et al. (1999) reported an increase in synaptic perforations in activated synapses during the first 30 minutes following in vitro LTP induction in the rat CA1 area (another subregion of the hippocampus). At 60 minutes postinduction, they found an increase in the proportion of double synapses (two spines connected to the same presynaptic terminal) suggesting that perforated synapses may eventually split to form new simple synapses. Contradictory evidence has come from Sorra and Harris (1998) who did not find any ultrastructural changes 2 hours post-LTP induction in area CA1 of the rat.
In a series of experiments, Weeks et al. (1999, 2000, 2001) considered the pattern of synaptic ultra- structure at 1 hour, 24 hours, and 5 days post-LTP induction. LTP was associated with an increase in concave shaped synapses at 24 hours. Synapses were larger overall at 1 hour and 5 days but not different at 24 hours. These differences in length were particularly evident in concave-shaped synapses, which were longer at 1 hour, shorter at 24 hours, and longer at 5 days. The proportion of perforated synapses was increased at 1 hour post-LTP induction but did not differ from controls at later time periods. Taken together and added to the other research in this area these results form a model of ultrastructural remodeling that occurs in activated synapses following LTP. Synapses appear to initially grow in size, become concave in shape and perforate by 1 hour post-LTP induction, either split or form new smaller concave synapses at 24 hours and then grow again in size by 5 days.
4.2 Learning And Memory
Synaptic ultrastructure has also been shown to change following learning. Reempts et al. (1992) found an increased number of concave perforated synapses in the rat dentate gyrus following the acquisition of a one-way active avoidance task. Concave synapses were also found to be increased in size relative to controls following training. This result is similar to the findings at 1 hour post-LTP induction (Weeks et al. 2000).
Synapse ultrastructure was also analyzed following the hippocampus-dependent trace eye-blink conditioning in the rabbit. Geinisman et al. (2000) found that while the number of synapses did not change following conditioning the PSDs were significantly larger in the trained animals. Geinisman et al. speculated that this increase in size might represent the addition of signal transduction proteins (thus increasing synaptic strength).
Learning that involves the cerebellum has also been associated with changes in synaptic ultrastructure. Kleim et al. (1996) found that motor skill learning caused synaptogenesis (formation of new synapses) in the rat cerebellum. The number of synapses per purkinje cell increased in rats after they learned to navigate an aerial maze compared to motor controls. Further, the number of multiple varicosities (clusters of synapses) increased following learning as did the amount of branching in the purkinje cell processes. Kleim et al. also found that as the number of synapses increased, their average size decreased. This result is similar to the changes observed in the rat dentate gyrus 24 hours post-LTP induction (Weeks et al. 1999).
4.3 Synaptic Ultrastructure Following Neural Lesions
Reactive synaptogenesis (formation of new synapses after a neural lesion) in the hippocampus was examined by Anthes et al. (1993). Following ipsilateral entorhinal cortical lesions, synapses were quantified in the rat dentate gyrus at 3, 6, 10, 15, and 30 days post-lesion. Results showed that the lesions caused an initial 88 percent synaptic loss at day 3, which was followed by rapid synaptogenesis from day 6 through to day 15. Anthes et al. speculated that, following the loss of entorhinal input, previously dormant or suppressed fibers and their synapses became active in the absence of the primary innervation. Interestingly, synaptic size was found to decrease during the phase of rapid synaptogenesis. As synaptogenesis returned to baseline levels (approximately day 15), synaptic size also returned to control or pre-lesion levels. Another important finding was that the number of perforated synapses was greatest at the peak of synaptogenesis (days 10–15) and returned to control levels by day 30 post-lesion. Despite the differences in the length of time post-lesion, the sequence of synaptic structural changes observed by Anthes et al. is very similar to that observed by Weeks et al. (2001) following LTP (see Sect. 4.1).
5. Proposed Sequence Of Synaptic Ultrastructural Change
There are limitations to the scope of the sequence of synaptic ultrastructural changes proposed. This sequence is based primarily on changes observed at excitatory, axo-spinous (axon to dendritic spine) synapses. Therefore, these modifications may not take place in inhibitory, axo-dendritic, or axo-somatic synapses, etc.
The data required to make a detailed comparison between the synaptic structural changes associated with LTP, reactive synaptogenesis, learning, and memory is far from complete but it is tempting to speculate about a common mechanism. One intriguing possibility, which was suggested by the growth of clusters of synapses observed by Kleim et al. (1996), is that a subset of synapses become involved or recruited in the various forms of neural plasticity. This subset of synapses then follows a sequence of structural changes (an initial increase in size, concavity, and perforations, then a decrease in size but an increased number of synapses, followed by a return to baseline levels) to produce more numerous and stronger synapses within the activated groups of afferent fibers. This finding directly supports Hebb’s (1949) postulate (see Sect. 1) and allows for lasting ultrastructural change in synapses that form activated networks within the nervous system. These activated networks may then form a basis for learning, memory, and other psychological processes.
Bibliography:
- Anthes D, LeBoutillier J, Petit T 1993 Structure and plasticity of newly formed adult synapses: A morphometric study in rat hippocampus. Brain Research 626: 50–62
- Bailey C, Kandel E 1993 Structural changes accompany memory storage. Annual Review of Physiology 55: 397–426
- Bliss T, Collingridge G 1993 A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–9
- Bliss T, Lomo T 1973 Long lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology 232: 331–56
- Bloomberg F, Cohen R, Siekevitz P 1977 The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. Journal of Cell Biology 78: 204–25
- Buchs P, Muller D 1996 Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proceedings of the National Academy of Science USA 93: 8040–5
- Carlin R, Siekevitz P 1983 Plasticity in the central nervous system; do sysapses divide? Proceedings of the National Academy of Science USA 80: 3517–21
- Chang F, Greenough W 1984 Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Research 309: 35–46
- Coggeshall R, Lekan H 1996 Methods for determining numbers of cells and synapses: A case for more uniform standards of review. Journal of Comparative Neurology 364: 6–15
- Cohen R S, Chung S K, Pfaff D W 1985 Immunocytochemical localization of actin in dendritic spines of the cerebral cortex using colloidal gold as a probe. Cellular and Molecular Neurobiology 5: 271–84
- Deller T, Merten T, Roth S, Mundel P, Frotscher M 2000 Actin-associated protein synaptopodin in the rat hippocampal formation: Localization in the spine neck and close association with the spine apparatus of principal neurons. Journal of Comparative Neurology 418: 164–81
- Desmond N, Levy W 1983 Synaptic correlates of associative potentiation depression: An ultrastructural study in the hippocampus. Brain Research 265: 21–30
- Desmond N, Levy W 1988 Synaptic interface surface area increases with long-term potentiation in the hippocampal dentate gyrus. Brain Research 453: 308–14
- Fifkova E 1985 Actin in the nervous system. Brain Research Review s 9: 187–215
- Geinisman Y, de Toledo-Morrell L, Morrell F 1991 Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Research 566: 77–88
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina I, Beatty M 1996a Synapse restructuring associated with the maintenance phase of hippocampal long-term potentiation. Journal of Comparati e Neurology 367: 413–23
- Geinisman Y, Disterhoft J, Gundersen H, Mcechron M, Persina I, Power J, Van Der Zee E, West M 2000 Remodeling of hippocampal synapses after hippocampus-dependent associative learning. Journal of Comparati e Neurology 417: 49–59
- Geinisman Y, Gundersen H, Van Der Zee E, West M 1996b Unbiased stereological estimation of the total number of synapses in a brain region. Journal of Neurocytology 25: 805–19
- Ghaffari T, Liaw J, Berger T 1997 Impact of synaptic morphology on presynaptic calcium dynamics and synaptic transmission. Society for Neuroscience Abstracts 23: 2105
- Gray E G 1959 Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. Journal of Anatomy 93: 420–33
- Gundersen H J G 1977 Notes on the estimation of the numerical density of arbitrary profiles: The edge eff Journal of Microscopy 111: 219–23
- Harris K, Kater S 1994 Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annual Review of Neuroscience 17: 341–71
- Hebb D 1949 The Organization of Behaviour. Wiley, New York
- Jones D G, Itarat W, Calverly R K S 1991 Perforated synapses and plasticity: A developmental overview. Molecular Neurobiology 5: 217–28
- Kleim J A, Lussing E, Schwarz E, Comery T, Greenough W 1996 Synaptogenesis and FOS expression in the motor cortex of the adult rat after motor skill learning. Journal of Neuroscience 16: 4529–35
- Mackenzie P, Kenner G, Prange O, Shayan H, Umemiya M, Murphy T 1999 Ultrastructure correlates of quantal synaptic function at single CNS synapses. Journal of Neuroscience 19: RC13 (1–7)
- Markus E, Petit T 1989 Synaptic structural plasticity: Role of synaptic shape. Synapse 3: 1–11
- Petit T 1995 Structure and plasticity of the Hebbian synapse: The cascading events for memory storage. In: Spear L P, Spear N E, Woodruff M (eds.) Neurobehavioral Plasticity: Learning, Development and Response to Brain Insults. Erlbaum, Hillsdale, NJ, pp. 185–205
- Reempts J, Dikova M, Werbrouck L, Clincke G, Borgers M 1992 Synaptic plasticity in rat hippocampus associated with learning. Behavioral Brain Research 51: 179–83
- Siekevitz P 1985 The postsynaptic density: A possible role in long-lasting effects in the central nervous system? Proceedings of the National Academy of Science, USA 82: 3494–8
- Sorra K, Harris K 1998 Stability of synapse number and size at 2 hr after long-term potentiation in hippocampal area CA1. Journal of Neuroscience 18: 658–71
- Toni N, Buchs P, Bron C, Muller D 1999 LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402: 421–5
- Weeks A, Ivanco T, LeBoutillier J, Racine R, Petit T 1999 Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: I. The intermediate maintenance phase. Synapse 31: 97–107
- Weeks A, Ivanco T, LeBoutillier J, Racine R, Petit T 2000 Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: II. The induction early maintenance phase. Synapse 36: 286–96
- Weeks A C W, Ivanco T L, LeBoutillier J, Racine R, Petit T 2001 Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: III. Long-term maintenance phase. Synapse 40: 74–84




