View sample Synapse Formation Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Formation of specialized contacts, the so-called synapses, between presynaptic axonal terminals of neurons and their postsynaptic targets, such as neurons and muscle cells, is a decisive step in building the nervous system. It enables rapid formation transfer between contacting cells via spatially and temporally restricted activation of postsynaptic receptors by neurotransmitters secreted from the nerve terminals. Considerable progress in the understanding of this complex phenomenon came from studies of synaptogenesis of the vertebrate neuromuscular junction (Fig. 1A). During the last decade investigations of glutamatergic neuron-neuron contacts in mammalian brains (Fig. 1B) and the neuromuscular junction in Drosophila highlighted the diversity of molecular interactions and universality of certain principles underlying formation of different types of synapses. These two issues are addressed in the present paper.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Approaching The Target
Growth cones are guided to their targets by attractive and repulsive cues that can be diffusible or integrated into the cell surface or the extracellular matrix (Mueller 1999). Semaphorins, ephrins and their receptors constitute large groups of guidance molecules that evoke repulsive and adhesive responses, often bidirectionally in the interacting cells. Cell adhesion molecules of the immunoglobulin superfamily promote neurite outgrowth via homophilic and heterophilic interactions with a variety of extracellular matrix and membrane-bound molecules. Recently, the L1 immunoglobulin superfamily molecule has also been found to be involved in ephrin receptor mediated repellent signaling. Netrins compose a family of proteins that can attract or repel axons, acting via distinct netrin receptors. Binding of recognition molecules to receptors activate signal transduction mechanisms, mostly involving receptor and non-receptor tyrosine kinases. These signals have been shown to converge in the regulation of activities of several small guanosine triphosphate (GTP) binding proteins of the Rho subfamily. Two of these proteins, Cdc42 and Rac1, are under the control of attractive guidance cues, whereas Rho is under control of repulsive guidance cues (Hall 1998). Recent studies show that one and same molecule can evoke attractive or repulsive responses depending on the internal state of the cell. For instance, three different extracellular cue molecules—recognition molecule netrin-1 brainderived neurotrophic factor (BDNF) and neurotransmitter acetylcholine—induce attractive or repellent turning of the growth cones depending on the intracellular level of cyclic adenosine monophosphate (AMP) (Ming et al. 1997).
2. Selecting The Target
Selection of the appropriate postsynaptic target and search strategies of axonal growth cones are interrelated processes. Removal of the repulsive cues or their axonal receptors often results in a decrease in axon bundling, so-called defasciculation, indicating that axon–axon interactions are no longer favored over axon–substrate interactions. This enables the fibers to grow into the regions that normally are not invaded and there form ectopic synapses. At the Drosophila neuromuscular junction, the immunoglobulin superfamily molecule fasciclin II promotes and semaphorin II inhibits promiscuous synaptogenesis. These molecules are expressed by all muscles in wild-type flies. A more restricted expression is seen for netrin B which is synthesized by a subset of muscles where it attracts some axons and repels others. Underexpression of repellent molecules or overexpression of adhesion-promoting fasciclin II upregulate formation of promiscuous and ectopic synapses. These experiments argue against the view that preand postsynaptic partners have unique complementary labels, and show that the relative balance of adhesive and repellent molecules controls the number of the synapses formed (Winberg et al. 1998). This is also demonstrated by genetic deletion of semaphorin IIIa and its receptor, neuropilin, which resulted in defasciculation and abnormal spreading of cranial nerves in mice. Also, more synapses were formed on wild-type hippocampal neurons expressing the neural cell adhesion molecule (NCAM), the closest mammalian homolog of fasciclin II, when compared to neighboring neurons from NCAM-deficient mice maintained in co-cultures. However, NCAM-deficient neurons maintained in a no-choice situation— separately from NCAM-expressing cells—receive normal numbers of contacts. This implies that NCAM mediates one of several signals which are integrated by the developing axon to confer preference for contact formation at sites where the integrated signal is optimal (Dityatev et al. 2000).
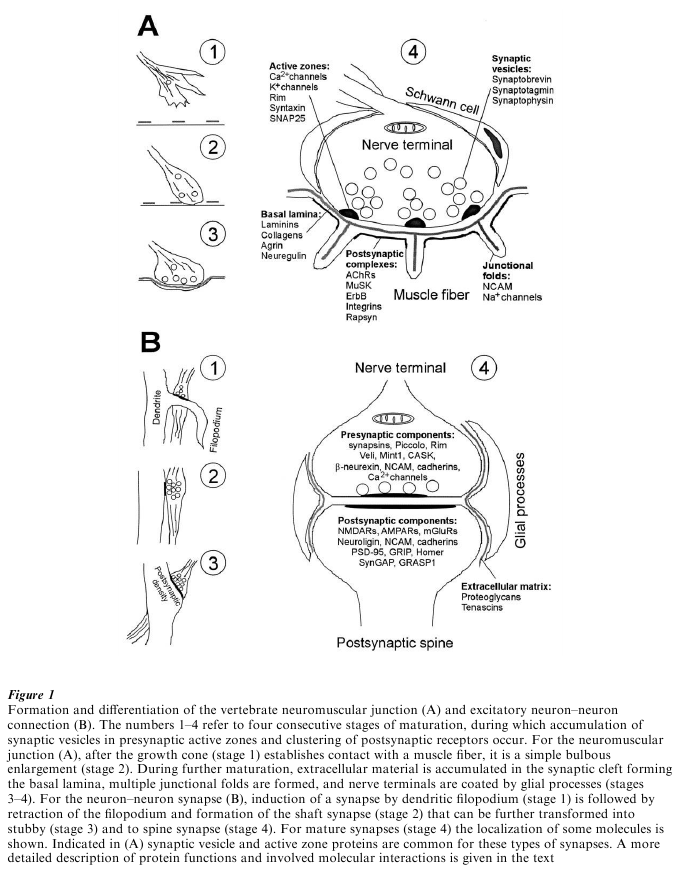
Figure 1 Formation and differentiation of the vertebrate neuromuscular junction (A) and excitatory neuron–neuron connection (B). The numbers 1–4 refer to four consecutive stages of maturation, during which accumulation of synaptic vesicles in presynaptic active zones and clustering of postsynaptic receptors occur. For the neuromuscular junction (A), after the growth cone (stage 1) establishes contact with a muscle fiber, it is a simple bulbous enlargement (stage 2). During further maturation, extracellular material is accumulated in the synaptic cleft forming the basal lamina, multiple junctional folds are formed, and nerve terminals are coated by glial processes (stages 3–4). For the neuron–neuron synapse (B), induction of a synapse by dendritic filopodium (stage 1) is followed by retraction of the filopodium and formation of the shaft synapse (stage 2) that can be further transformed into stubby (stage 3) and to spine synapse (stage 4). For mature synapses (stage 4) the localization of some molecules is shown. Indicated in (A) synaptic vesicle and active zone proteins are common for these types of synapses. A more detailed description of protein functions and involved molecular interactions is given in the text
Cooperative actions of some N-acetyl-galactosamine-terminated glycoconjugates, N-cadherin and BDNF are known to regulate formation of synapses of retinal axons in the chick optic tectum. Laminaspecific connections in the hippocampus illustrate that synaptogenic cues can be expressed by ‘guidepost’ cells that are not definitive synaptic targets but transient targets for growing axons. Such ‘guidepost’ cells, for instance, are Cajal–Retzius cells which occupy the stratum lacunosum-moleculare and calbindin-positive interneurons occupying the stratum radiatum of the hippocampus. During early development, entorhinal axons form synapses onto Cajal–Retzius cells before the dendrites of pyramidal cells reach the molecular layer. Calbindin-positive interneurons are preferred targets for commissural axons. Later, most of guidepost cells die and synapses are transferred to the pyramidal dendrites (Sanes and Yamagata 1999).
3. Exchanging Signals
When an axon approaches its postsynaptic target, it releases anterograde signals and receives retrograde influences from the postsynaptic side. As discussed below, anterograde signals can induce clustering of postsynaptic receptors at synaptic sites and down regulation of receptor expression at nonsynaptic sites. Also, when neonatal muscles, destined to become fastor slow-twitch, are denervated and cross-reinnervated with each other’s nerves, they acquire contractile characteristics appropriate for their new innervation. Such ‘conversion’ of postsynaptic cells is mediated by acetylcholine release and electrical activity inducing changes in expression of proteins encoding fiber typespecific isoforms of contractile proteins.
Retrograde signaling plays an important role in elimination of incorrect or excessive synaptic inputs. Most vertebrate muscle fibers are initially innervated by more than one motor axon, retaining only a single input during early postnatal life. Similarly, projections from both eyes initially overlap in the lateral geniculate nucleus, with eye-specific laminae forming from the initially diffuse projections late in embryogenesis. These processes appear to depend on postsynaptic electrical activity. First, competition and selection of afferents could then arise due to limited amounts of maintenance factors released from postsynaptic cells and taken up by axons in an activity-dependent manner. Nitric oxide and neurotrophic factors, like BDNF, nerve growth factor (NGF) and fibroblast growth factor 2 (FGF2), are among the favorite candidate molecules mediating retrograde strengthening of synapses. Second, electrical activity can evoke release of proteases such as thrombin or plasminogen activator, destabilizing cell interactions at the nerve terminal unless they are protected by endogenous inhibitors. Interestingly, the heparan sulfate proteoglycan agrin, implicated in clustering of nictotinic acetylcholine receptors (AChRs) at the neuromuscular junction, may function also as a protease inhibitor. Third, postsynaptic activity can redistribute the localization of adhesion molecules leading to retraction of axons and loss of synapses via downregulation of adhesive interactions in inappropriate sites, whereas synaptic strengthening should involve accumulation of adhesion molecules, such as NCAM, or changes in their molecular configuration as has been shown for cadherins, which dimerize upon depolarization. Such dimers are protected from proteolytic degradation and can form transsynaptic associations (Tanaka et al. 2000).
4. Postsynaptic Differentiation
Maturation of synaptic contacts leads to the accumulation of transmitter receptors at postsynaptic sites. If AChRs are uniformly distributed in immature muscles at a density of 1000 µm-2, their expression in the mature muscles exceeds 10000 µm−2 at the synapse, whereas the density drops to 10 µm−2 extrasynaptically. Many molecules have been reported to promote clustering of AChRs in vitro. Of these, only one has so far been implicated in synaptogenesis in vivo. This is the nerve-derived alternatively spliced isoform of agrin that interacts with the transmembrane protein tyrosine kinase MuSK. A crucial effector of receptor clustering downstream of MuSK is the cytoplasmic protein rapsyn that bears distinct domains responsible for association with the membrane, multimerization, and interaction with receptors. Functionally similar synapse-associated proteins were discovered in neuronal synapses (Garner et al. 2000). They bear protein motifs called PDZ domains that can bind ligandor voltage-gated ion channels and adhesion molecules. In Drosophila, discs-large protein interacts with and determines synaptic localization of fasciclin II and Shaker type of voltage-gated K+ channels. In vertebrates, the PDZ domaincontaining postsynaptic density protein (PSD-95), glutamate receptor interacting protein (GRIP), and Homer interact with N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-isoxazole-4propionic acid (AMPA) and metabotropic glutamate receptors, respectively. PSD-95 can also interact with voltage-gated K+ channels, the synaptic cell adhesion molecule neuroligin, and kainate receptors, whereas GRIP interacts with ephrin B ligands. These complexes are linked to the postsynaptic cytoskeletal matrix and signaling cascades. For example, PSD-95 and GRIP are likely to couple glutamate receptors to the Ras GTPase-activating protein SynGAP and GDP-GTP exchange factor GRASP1, respectively.
Differential expression of transmitter receptors in postsynaptic sites at the vertebrate neuromuscular junction is achieved not only by clustering of diffusely distributed receptors, but also by transcriptional activation of AChR genes in the muscle in subsynaptic nuclei, and transcriptional repression of AChR expression in nonsynaptic nuclei. Repression of synthesis is r egulated by postsynaptic activity, inducing entry of Ca2+ and activation of protein kinase C. Critical targets of this kinase are transcriptional factors (myoD, myf5, MRF4, and myogenin) that downregulate transcription of AChRs by binding to the socalled E-box containing sequences in several AChR subunit genes. These repressive signals can be counteracted at synaptic sites by the nerve-derived neuregulin that is incorporated into synaptic basal lamina and activates synthesis of AChRs in myocytes. Neuregulin acts via the epidermal growth-factor-related receptor tyrosine kinase and downstream cascades of effector molecules, including Ras, Raf, and Erk kinases. These effectors regulate the activity of transcription factors of the ets family, which can bind to N-box elements in some AChR subunit genes. Neuregulins also trigger synthesis of some receptors in the central nervous system and some proteins are likely to be synthesized locally at synaptic sites in dendrites.
5. Presynaptic Differentiation
Spontaneous and evoked neuromuscular transmission has been seen to begin within minutes after an axon contacts the postsynaptic cell. However, the synaptic activity is initially low, not only because of the small number of postsynaptic receptors, but also because only few vesicles are released. During maturation of a synapse, synaptic vesicles increase in number and become clustered at active zones, whereas some cytoskeletal elements characteristic of the growing axon are lost. These changes are accompanied by an increase in synaptic volume that leads to a prominent increase in synaptic activity. The factors that induce presynaptic differentiation are unknown, but several molecules, which can be retrogradely released or are present in extracellular matrix, have been implicated in stimulating clustering and release of synaptic vesicles. They include neurotrophic factors, BDNF and FGF2, and extracellular matrix molecules such as agrin and synaptic isoforms of laminin. As for the postsynaptic site, presynaptic differentiation involves redistribution of synaptic machinery, and changes in expression of different isoforms of synaptic proteins. Accumulation and mobilization of vesicles is mediated by synapsins and the PDZ domain-containing proteins Piccolo and Rim. Possibly, assembly of pre and postsynaptic components in neuron–neuron connections depends on formation of transsynaptic molecular complexes. Thus, postsynaptically localized neuroligin, associated with PSD-95, can bind to presynaptically localized β-neurexin associated via CASK with two PDZ domain-containing proteins, Veli and Mint1. The latter molecule interacts with N-type Ca2+ channels that are responsible for the entry of Ca2+ and transmitter release at the nerve terminals.
6. Synaptogenesis As A ‘Never Ending Story’
Formation and loss of synapses continue during the whole lifespan of animals. It is likely that high cognitive functions, such as learning and memory, utilize the mechanisms evolved to wire the brain during early development. Indeed, many molecules involved in early synaptogenesis, such as cell adhesion molecules of the immunoglobulin superfamily, integrins, cadherins, and ephrin receptors, have proved to play important roles in long-term potentiation and depression of synaptic connections, underlying learning and memory in adults. However, synaptogenesis in the adult brain occurs in the context of a very different milieu than that of early development. Mature synapses are subject to more severe constraints for further modifications, due to reduced NMDA receptor-mediated currents, and well-developed inhibitory circuitry. Additional restrictions are imposed by the established adhesive interactions within and outside of synapses, and a firm extracellular matrix enriched in repellent signals, thus possibly reducing structural plasticity. It remains to be seen which mechanisms active during development contribute to the formation of synapses in the adult brain and which mechanisms were additionally ‘invented’ to provide stability, but also flexibility in synaptic activity underlying learning and memory.
Bibliography:
- Broadie K 1998 Forward and reverse genetic approaches to synaptogenesis. Current Opinion on Neurobiology 8: 128–8
- Dityatev A, Dityateva G, Schachner M 2000 Synaptic strength as a function of post versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron 26: 207–17
- Fitzsimonds R M, Poo M M 1998 Retrograde signaling in the development and modification of synapses. Physiological Review 78: 143–70
- Flanagan J G, Vanderhaeghen P 1998 The ephrins and Eph receptors in neural development. Annual Review of Neuroscience 21: 309–45
- Hall A 1998 Rho GTPases and the actin cytoskeleton. Science 279: 509–14
- Garner C C, Nash J, Huganir R L 2000 PDZ domains in synapse assembly and signalling. Trends in Cell Biology 10: 274–80
- Lee S H, Sheng M 2000 Development of neuron–neuron synapses. Current Opinion in Neurobiology 10: 25–31
- Ming G L, Song H J, Berninger B, Holt C E, Tessier-Lavigne M, Poo M M 1997 cAMP-dependent growth cone guidance by netrin-1. Neuron 19: 1225–35
- Mueller B K 1999 Growth cone guidance: first steps towards a deeper understanding. Annual Review of Neuroscience 22: 351–88
- Sanes J R, Lichtman J W 1999 Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience 22: 389–442
- Sanes J R, Yamagata M 1999 Formation of lamina-specific synaptic connections. Current Opinion in Neurobiology 9: 79–87
- Schachner M 1997 Neural recognition molecules and synaptic plasticity. Current Opinion in Cellular Biology 9: 627–34
- Tanaka H, Shan W, Phillips G R, Arndt K, Bozdagi O, Shapiro L, Huntley G W, Benson D L, Colman D R 2000 Molecular modification of N-cadherin in response to synaptic activity. Neuron 25: 93–107
- Walsh F S, Doherty P 1997 Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annual Review of Cellular and Developmental Biology 3: 425–56
- Winberg M L, Mitchell K J, Goodman C S 1998 Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell 93: 581–91




