Sample Genetic Aspects of Race Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Natural species are genetically polymorphic, in two related, but distinct ways. Different individuals may carry different alleles of the same gene (polymorphism within populations), and different populations may be characterized by different alleles, or by different frequencies of the same alleles (polymorphism among populations). Differences among populations depend on various evolutionary factors, but populations that exchange migrants tend to resemble each other, whereas mutually isolated groups and communities tend to diverge genetically.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
The relative importance of polymorphism within and among populations varies across species. In many plant and animal species, the largest share of genetic diversity occurs among populations or groups thereof. When that is the case, most individuals of unknown origin can be assigned to one group on the basis of their genetic characteristics, with a high degree of statistical confidence. If distinct groups occupy different geographical areas, it is customary to define them as subspecies or races. The question is how faithfully this concept also describes the levels and patterns of genetic diversity observed among humans. One of the latest definitions of race is as follows: ‘A large population of individuals who have a significant fraction of their genes in common, and can be distinguished from another race by their common gene pool’ (Vogel and Motulsky 1986). But the concept of race is older than genetics. Long before Mendel the term referred to people sharing the same ‘blood,’ that is, to groups of genealogically-related individuals, especially those of noble families (Furetiere 1690, cited in Cohen 1991). In the eighteenth and nineteenth centuries, the term race came to designate constant human types, and came to be used not only to describe, but also to explain, human diversity. People who look different belong to different races, and they are different because they belong to different races. That view is still widespread; it seems based on an empirical observation, and on an assumption. The observation is that biologically related people look alike, and the assumption is that our species is naturally subdivided in clusters of individuals who look alike because they are genealogically related, or races.
Is that assumption based on solid scientific grounds? Or are human races arbitrary groups of populations, which do not share a common evolutionary history more than other populations classified into different races? Only in the second half of the twentieth century has it become possible to address this question using genetic data. For the purposes of this research paper, the hypothesis being tested is whether the observed patterns of genetic diversity suggest that humankind is subdivided in nearly discrete, and internally homogeneous, groups. The results of those tests, and their evolutionary implications, will be summarized in this research paper.
1. Listing Human Races
Prior to measuring genetic differences within and among human groups, one has to define these groups. The many lists of races which have appeared in the course of history may be regarded as successive attempts to identify the set of physical traits which leads to the best discrimination of groups, that is, traits for which the among-group component of variation is maximum. Ideally, once such ‘best’ physical traits are identified, most individuals and populations should be classified unambiguously into the appropriate group, except perhaps for a minority of them, traditionally regarded as derived from interracial crosses.
Table 1 summarizes some lists of human races proposed in the last three centuries. Although this selection is necessarily incomplete (the interested reader is referred to Cohen 1991 and Molnar 1998) a problem, and a trend, are evident. The problem is, any group defined on the basis of morphological traits harbors some degree of internal variation, and different criteria, say skin color and skull shape, lead to different classifications. This is clearly shown by the fact that, although the existence of human races has been challenged only recently (Livingstone 1964), little agreemement has evidently been reached on how many races exist, and which. The trend is a consequence of the problem. As more and more groups came in contact with the European scientists who were developing these classifications, the number of proposed races increased steadily.
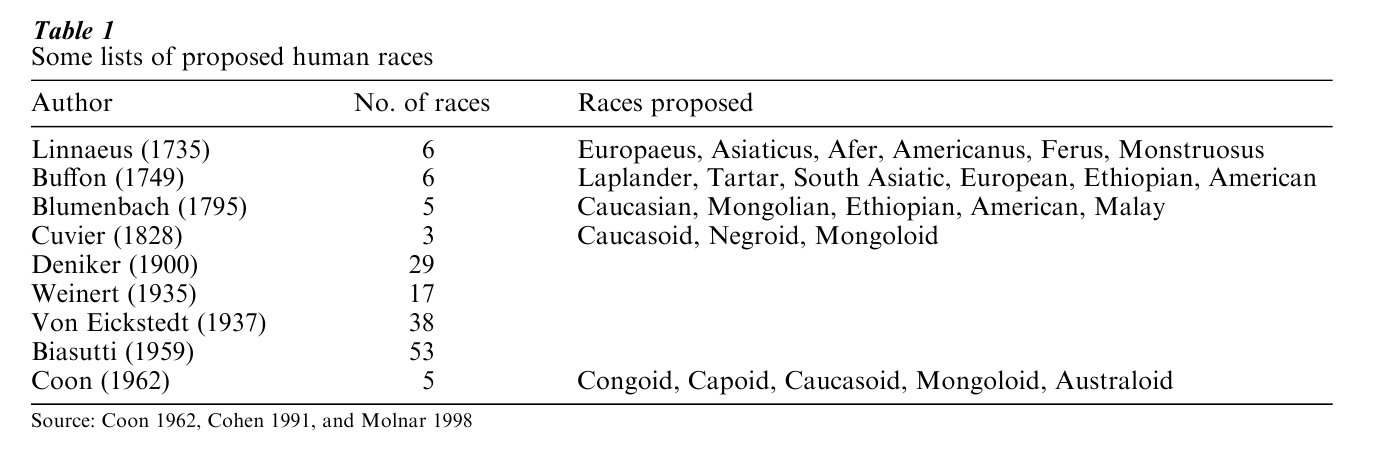
There is an exception to that trend. In 1963, Coon proposed that there are only five subspecies among humans. In that context, subspecies was synonymous to race, and it was defined as a regional population ‘which meets two tests: (1) it occupies a distinct geographical territory; (2) it differs from other subspecies of the same species in measurable characteristics to a considerable degree’ (1962, p. 19). Coon’s definition overlaps with the one given by Vogel and Motulsky (1986), and both can be tested using genetic data.
2. Apportioning Human Diversity
Because there is no agreement on the best system of human racial classification, the groups or putative races being compared in the studies here reviewed were seldom the same. In general, when the groups considered are many, the variance among them is inflated. At the same time, on the other hand, as the number of groups increases, the boundaries between them tend to be ill-defined, which may lead to overestimating variation within groups.
The statistical methods used to apportion human diversity vary too, when one considers different studies. Nevertheless, the conceptual framework is the same, and is as follows. Several groups are defined on the basis of independent evidence. Each individual is compared to all other individuals studied, and a number representing the amount of genetic differentiation between them is calculated. Three components of genetic variance are then estimated from those numbers, namely: (a) between individuals of the same population; (b) among populations of the same group; (c) among major groups (see Lewontin 1972, Latter 1980, Barbujani et al. 1997). In this way, the overall genetic diversity is broken down in three hierarchical components.
2.1 Genetic Diversity At The Protein Level
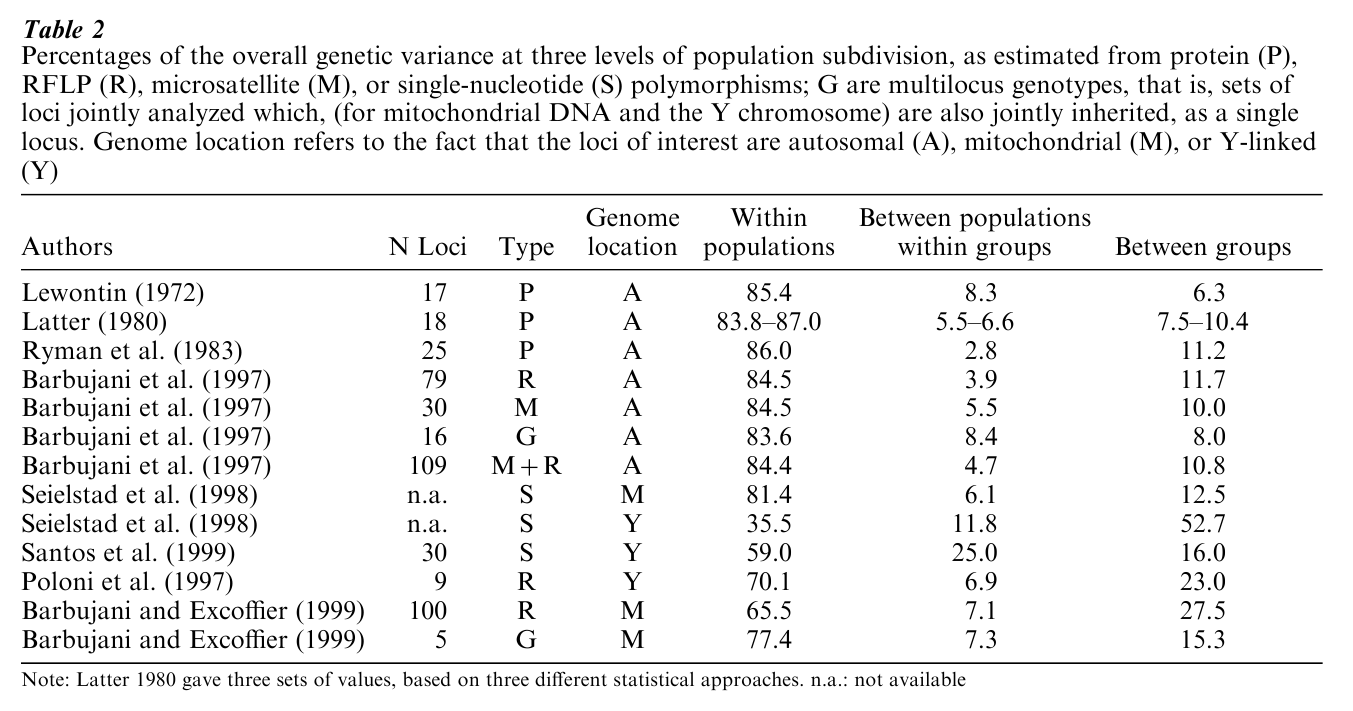
In 1972, Lewontin first quantified the amount of genetic variation within and among major human groups. He chose a seven-race system, comprising Caucasians, Black Africans, Mongoloids, Amerinds, Oceanians (the five groups of Blumenbach’s classification; see Table 1), plus South Asian and Australian aborigines. Analysis of 17 independent genetic loci, for which information on all available populations was collected, gave somewhat different results, but the average proportion of the overall variance that fell within populations was 85.4 percent (with a maximum of 99.7 percent for the Xm locus and a minimum of 63.6 percent for the Duffy locus). Additional differences between populations of the same race represented, on average, 8.3 percent of the total, and differences between races accounted for the remaining 6.3 percent of the total. At the protein level, therefore, variation among seven races seems to represent less than one tenth of the overall genetic diversity of our species.
Lewontin’s conclusion that the human racial classification has virtually no genetic or taxonomic significance was variously criticised, not always on scientific grounds. One serious objection was that only a fraction of the total DNA is actually translated into proteins. As a consequence, protein polymorphism may not fully reflect the underlying DNA diversity. Although successive studies of proteins confirmed that more than four fifths of the overall genetic diversity of our species is accounted for by differences among members of the same community (Table 2), skepticism remained widspreaad.
2.2 Genetic Diversity At The DNA Level
The first global study of DNA diversity, based on 109 genetic loci in 16 populations, appeared only 25 years later (Barbujani et al. 1997). The samples were chosen so as to form well-separated groups over the map of the world, whereas geographically intermediate populations were not considered. Therefore, if sampling affected the genetic variances estimated, it did so by increasing the weight of the between-continent components. In addition, this made the samples of each continent fall within one of the racial groups of Blumenbach’s classification, without ambiguity. The loci chosen were 30 short-tandem repeats (STR or microsatellite loci, whose alleles differ in length, that is, in the number of nucleotides they comprise), and 79 biallelic DNA polymorphisms (restriction-fragment length polymorphisms, or RFLP). For 16 RFLPs, compound genotypes referring to subsamples of individuals were available, and variance components were also estimated from them. By jointly analyzing many loci in this way, one may hope to identify significant differences better than by separately considering each gene.
The within-population variance component was significantly greater than 0 at all loci, representing between 54.4 percent and 94.6 percent of the total. Approximately one-third of the loci studied showed significant variance also between populations or between continents. Very similar results were obtained in the analysis of single RFLP polymorphisms, of the multilocus RFLP genotypes, and of the STR loci (Table 2).
Three recent papers provided additional data (Table 2) about genes inherited only from one parent. Every couple possesses four potentially transmissible copies of most chromosomes, two maternal and two paternal. But there is only one (paternal) copy of the Y chromosome; and there are several, presumably identical, copies of the mitochondrial DNA, which are only transmitted by the mother. Population genetics theory predicts that genes on these chromosomes should diverge four times as fast as the biparentally-transmitted (or autosomal) genes considered in the previous studies, all other demographic and evolutionary factors being equal. There is no simple model quantifying the effect of such a fast divergence on components of variance, but the differences among populations and continents ( jointly estimated around 15 percent for autosomic genes) are expected to represent a substantially larger fraction of the genetic variance estimated from Y-chromosome and mitochondrial data.
Contrary to those expectations, mitochondrial variation appeared distributed much like autosomic variation. Such a lower-than-expected population diversity raises evolutionary problems that will not be discussed here, related with possible effects of natural selection on mitochondrial DNA, or with a high prehistoric female mobility. On the other hand, two analyses of the Y chromosome did show an increased differentiation among continents and populations, but confirmed that the largest component of human diversity is within populations (Poloni et al. 1997, Santos et al. 1999). However, in a third study of the Y chromosome, Seielstad et al. (1998) estimated even higher among-continent variances, exceeding 50 per- cent of the total. Note that the values estimated by Santos et al. (1999), which are closer to the other figures listed in Table 2, derive from the analysis of a different set of the same genetic markers, single- nucleotide polymorphisms, that is, variable sites in the Y-chromosome sequence. The fact that such large differences have only been found in the male-transmitted portion of the genome, and only for one set of data, is not easy to interpret.
In synthesis, it seems that the within-population component of the overall genetic diversity of our species is close to 85 percent, regardless of the genetic markers and of the populations studied. Despite the limited polymorphism of the protein markers considered, and despite the choice of different sets of populations, Lewontin’s results essentially are confirmed at the DNA level, for more than 300 genes analyzed so far. Greater among-continent diversities are estimated, as predicted by theory, on the basis of uniparentally-transmitted genes, and the allelic variants of the Y chromosome are more restricted geographically than the alleles of autosomal and mitochondrial loci. Among the plausible explanations of such a high Y-chromosome diversity are, again, selection differences in the males’ and females’ prehistorical migration rates, and a high variability of the males’ reproductive success (discussed in Hammer et al. 1997). The conclusions of the other studies seem rather coherent, and they can be summarized as follows: if we arbitrarily set to 100 the average genetic difference between two very distant members of our species, the expected difference between members of the same community (who are not relatives, of course) seems to be approximately 85, and not 10 or 40, as previously illustrated definitions of race appear to imply.
3. Are We All Equal, Then?
The fact that genetic differences among major human groups are relatively small does not mean that all humans are genetically identical. In fact, the amongpopulation and among-group components of variance are significantly greater than zero, not only for the Y chromosome, but also at several autosomic loci (Barbujani et al. 1997). That fraction of diversity, albeit small in relative terms, contains all the information that population geneticists use to reconstruct human evolutionary past. From its study, we know that both allele frequencies and DNA sequences vary nonrandomly in space, often forming gradients over entire continents (reviewed by Cavalli-Sforza et al. 1994). We also know that zones of sharp genetic change, or genetic boundaries, exist, and often overlap with various kinds of migrational barriers, both physical (mountain ranges, arms of sea) and cultural (especially language boundaries) (Sokal et al. 1990). However, very seldom do genetic boundaries form complete rings around single populations, or groups thereof. Over much of the planet, continuous variation is the rule, with the genetic characteristics of the various regions changing more or less smoothly in the geographical space. Locating on the map the place at which one racial group ends and another one begins proved, so far, an impossible task. But nearly all populations studied are somewhat different from their neighbors.
4. Comparisons With Other Species
The subspecies concept is widely used in zoology and botany, nd the suspicion may be raised that moral and political, rather than scientific, considerations may influence our willingness to use the same concept for humans. Although only a few authors have addressed this problem so far, their results suggest that humans are actually a comparatively uniform species. To cite just one example, the fruit fly Drosophila pseudoobscura shows a 10-fold greater nucleotide diversity than our species (Li and Sadler 1991).
Templeton (1999) compared Barbujani et al.’s (1997) estimates of genetic variances with those referring to 12 large-bodied, highly mobile mammals. Two such species (East African Buffalo and Kenyan Waterbuck) show smaller between-population diversity than humans. However, these are not cosmopolitan species like ours, but dwell in restricted geographical zones. All other species compared, including Elephant, Gazelle, and gray Wolf, show higher, or much higher, differentiation among populations and groups, with values of those variance components reaching 80 percent of the total.
On a smaller taxonomic scale, Ruvolo et al. (1994) compared mitochondrial diversity in the five genera of hominoids, that is, humans and apes. Variation appeared minimal within humans and pygmy Chimpanzees; the latter, once again, occupy a much smaller territory than humans. On the other hand, common Chimpanzee, Orangutan, and especially Gorilla, show much higher genetic differentiation. In particular, the most mitochondrially diverse humans studied appear to be less different than random pairs of lowland gorillas from West Africa.
5. Evolution Of Human Genetic Diversity
Understanding human diversity also means to understand how that diversity came about. The low genetic variation among humans, as compared to other species, points to two, not incompatible, evolutionary processes, namely a high dispersal ability leading to limited isolation among populations, and/or a recent origin, too recent indeed for extensive genetic variation to have accumulated ever since.
Various evolutionary scenarios have been proposed to explain the available fossil, archaeological, and genetic evidence on human origins. In Coon’s (1962) view, some 700,000 years ago, the population of our ancestor, Homo erectus, was subdivided geographically in five groups. Coon’s five subspecies of Homo sapiens would differ because they are descended from those groups, with very little interbreeding, if any. This is no longer considered a valid scientific hypothesis, but the idea that the differences among contemporary humans started accumulating before the appearence of anatomically modern humans is still part of the so-called multiregional theory of human evolution (Wolpoff and Caspari 1997). This theory differs from Coon’s in that the major human lineages are here regarded as only partly isolated, with extensive genetic exchange even between distant populations.
The basis of the multiregional theory of human evolution is the observation that certain traits persist in some regions of the earth from H. erectus times through H. sapiens times, and are unique to those regions. However, the statistical significance of that observation has been called into question (Lahr 1996), and it also seems that implausibly high levels of gene flow would be required, for hundreds of millennia, to maintain the unity of our species under a multiregional model (Rouhani 1989). The alternative hypothesis, the so-called out-of-Africa model (Stringer and Andrews 1988), regards the contemporary population as derived from ancestors who lived in Africa until recently. When those people expanded into Eurasia and Australia, perhaps 100,000 years ago, they replaced without admixture previous local populations of Homo erectus and (in Europe) Homo sapiens neandertalensis.
The multiregional and the out-of-Africa models have several implications. If the main human groups separated before the appearance of anatomically modern Homo sapiens, deep differences are to be expected among them, and quite distinct evolutionary lineages should exist, in agreement with Vogel and Motulsky’s (1986) definition of race. On the contrary, if all continental populations derived recently from the same ancestors, even distant human groups should share many alleles, and the differences among groups should be relatively small. The studies reviewed in this research paper seem to clearly support the latter model, although Templeton (1998) argued that high levels of migration could lead to low variances between continents, even under a multiregional model. Be that as it may, most investigators, including Templeton, agree that the available genetic evidence does not suggest the existence of distinct clusters of humans.
Support to this view also comes from studies which, although not explicitly addressing the issue of racial subdivision, are in evident agreement with a recent African origin of humankind. First, genetic diversity is greater in Africa than in any other continent (Armour et al. 1996, Hammer et al. 1997, Jorde et al. 1997), an observation that a multiregional model can hardly explain. Second, genetic diversity within populations decreases as one moves away from Africa, with near populations showing subsets of African genes, and more distant populations showing smaller subsets of those genes (Tishkoff et al. 1996). Third, Neandertal man is considered a direct ancestor of the Europeans, and only of them, under the multiregional model; but the only Neandertalian DNA sequences obtained so far fall out of the range of current human variation, and bear no special resemblance to the European sequences (Krings et al. 1997). All these findings, and other paleontological data, emphasize the crucial role of Africa in human evolution (Foley 1998), and suggest that, during their dispersal in Eurasia, Australia, and the Americas, human groups spent too little time in isolation to significantly diverge genetically and form separate races.
6. Future Prospects
Several problems remain open. The first one has to do with the exact amount of genetic diversity that can be attributed to the various levels of population subdivision. Figures around 10 percent of the total diversity occurring among continents have been estimated by analyzing samples that were well separated in space, so that no ambiguity existed as for the continent each of them belonged to. However, in nature, populations are not so discretely distributed. May that sampling structure have led to overestimating the among-group component of genetic variance? And may the choice of the DNA markers studied (most of them falling in noncoding regions of the DNA) have affected the variance estimates too, perhaps in the opposite direction?
A second question is whether the apparently continuous distribution of genetic variation also implies that recognizable geographical groups do not exist, as has been argued by Templeton (1998). In fact, as previously mentioned, genetic variances among groups, although quantitatively small, are significantly greater than zero at many loci; this may have a meaning.
Some DNA alleles occur only in one specific population or continent. Individuals of unknown origin who carry those alleles can be assigned with high statistical confidence to the continent they belong to. However, it is not clear yet, and this is a third question, whether also broad human groups can be defined based on the presence of those alleles (rather than on the basis of most loci of the genome, as implied by traditional definitions of race); and it is unclear whether such groups, if any, could be called races. Most studies reviewed in this research paper do not support the idea that the majority of the members of our species can be put into a specific continental or racial group, on the basis of their genotype. This is a statistically tractable problem, and investigation on it is currently in progress. Good candidate genes for discriminating among groups could be the Y-chromosome single-nucleotide polymorphisms which, as mentioned, vary substantially across continents (Seielstad et al. 1998). However, population groups defined on the basis of Y-chromosome diversity only could hardly be called races. A statement such as ‘Human races exist, but only among males’ would sound puzzling.
The genes determining morphological traits such as skull and eye shape, hair texture, or skin color, have not been identified yet, and some have argued that, once they will be known, a clearer identification of human groups will be possible. There are three reasons to doubt it, though. First, as shown in Table 1, centuries of studies of the phenotypes determined by those genes have not led to a consensus on the existing human races; whether and how a better knowledge of the underlying genes may clarify this picture is not obvious. Second, groups sharing traits typically used for racial classification, such as Melanesians and Africans, have been shown to be very distant genetically, both at the protein and at the DNA level (Cavalli-Sforza et al. 1994). Third, morphological traits are determined by the interaction between several genes and environmental factors. Theory shows that this polygenic and multifactorial inheritance results in a continuous distributions of traits, that is, the distributions which are less likely to show the sharp discontinuities necessary to discriminate among groups. Probably, morphological variation reflects differential selection by climate (Owens and King 1999), or the effects of sexual selection, both sometimes leading genetically distant groups to resemble each other.
Grouping individuals in rough categories based on morphology may still be useful for some practical purposes. In countries with extensive recent immigration, different groups may have different disease risks. Despite the low among-continent genetic variances discussed so far, associating a different risk to different, sometimes ill defined, groups (e.g., ‘Hispanics’) may simplify the clinicians’ work. Similar problems also exist in forensic science, for DNA-based personal identification; and similar solutions, based on the definition of somewhat arbitrary racial or ethnic groups, have been put forward to reduce the risk of a wrong decision against a suspect (Nichols and Balding 1991). Despite these examples, genetic studies have shown that humans do not neatly fall within few well-distinct racial groups. Any population differs from its neighbors for several genetic features and, with appropriate sample sizes, these differences may achieve statistical significance. But if the existence of at least one significant genetic difference were the criterion for defining races, every village would be occupied by a different race.
In conclusion, newly discovered polymorphisms, especially those of the Y chromosome, and larger sampling efforts, may modify our ideas on the levels and patterns of human genetic diversity. But at present, clear discontinuities among major groups have not been detected, and there is no clear evidence of largely independent evolution of distinct human lineages. In addition, nobody has yet identified sets of genes whose variation is correlated, so that a classification based on one of them will not be contradicted by the classification based on any other of them. Judging from the available genetic information, therefore, Vogel and Motulsky’s (1986) definition of race does not seem to plausibly describe the structure of the human population. New defintions may be proposed, and, if so, they will have to stand the comparison with the data. At present, the burden of the proof seems on those who maintain that our species is subdivided into races that can be objectively recognized by studying their genes.
Bibliography:
- Armour J A L, Anttinen T, May C A, Vega E E, Sajantila A, Kidd J R, Kidd K K, Bertranpetit J, Paabo S, Jeffreys A 1996 Minisatellite diversity supports a recent African origin for modern humans. Nature Genetics 13: 154–60
- Barbujani G, Excoffier L 1999 The history and geography of human genetic diversity. In: Stearns S (ed.) Evolution in Health and Disease. Oxford University Press, Oxford, UK, pp. 27–40
- Barbujani G, Magagni A, Minch E, Cavalli-Sforza L L 1997 An apportionment of human DNA diversity. Proceedings of the National Academy of Sciences USA 94: 4516–9
- Cavalli-Sforza L L, Menozzi P, Piazza A 1994 The History and Geography of Human Genes. Princeton University Press, Princeton, NJ
- Cohen C 1991 Les races humaines en histoire des sciences. In: Hublin J J, Tillier A M (eds.) Aux Origines d’Homo sapiens. Presses Universitaires de France, Paris, pp. 9–47
- Coon C 1962 The Origin of Races. Knopf, New York
- Foley R 1998 The context of human genetic evolution. Genome Research 8: 339–47
- Hammer M F, Spurdle A B, Karafet T, Bonner M R, Wood E T, Novelletto A, Malaspina P, Mitchell R J, Horai S, Jenkins T, Zegura S L 1997 The geographic distribution of human Y chromosome variation. Genetics 145: 787–805
- Krings M, Stone A, Schmitz R W, Krainitzki H, Stoneking M, Paabo S 1997 Neandertal DNA sequences and the origin of modern humans. Cell 90: 19–30
- Jorde L B, Rogers A R, Bamshad M, Watkins W S, Krakowiak P, Sung S, Kere J, Harpending H 1997 Microsatellite diversity and the demographic history of modern humans. Proceedings of the National Academy of Sciences USA 94: 3100–3
- Lahr M M 1996 The Evolution of Human Diversity. Cambridge University Press, Cambridge, UK
- Latter B D H 1980 Genetic differences within and between populations of the major human subgroups. American Naturalist 116: 220–37
- Lewontin C 1972 The apportionment of human diversity. Evolutionary Biology 6: 381–98
- Li W H, Sadler A 1991 Low nucleotide diversity in man. Genetics 129: 513–23
- Livingstone F B 1964 On the nonexistence of human races. In: Montagu A (ed.) The Concept of Race. Free Press, Glencoe, NY, pp. 46–60
- Molnar S 1998 Human Variation. Races, Types, and Ethnic Groups. Prentice Hall, Upper Saddle River, NJ, p. 19
- Nichols R A, Balding D J 1991 Effects of population structure on DNA fingerprint analysis in forensic science. Heredity 66: 297–302
- Owens K, King M C 1999 Genomic views of human history. Science 286: 451–3
- Poloni E S, Semino O, Passarino G, Santachiara-Benerecetti A S, Dupanloup I, Langaney A, Excoffier L 1997 Human genetic affinities for Y-chromosome P49a,f TaqI haplotypes show strong correspondence with linguistics. American Journal of Human Genetics 61: 1015–35
- Rouhani S 1989 Molecular genetics and the pattern of human evolution: Plausible and implausible models. In: Mellars P, Stringer C B (eds.) The Human Revolution: Behavioural and Biological perspectives on the Origins of Modern Humans. Princeton University Press, Princeton, NJ, pp. 47–61
- Ruvolo M, Pan D, Zehr S, Goldberg T, Disotell T R, von Dornum M 1994 Gene trees and hominoid phylogeny. Proceedings of the National Academy of Sciences USA 91: 8900–4
- Ryman N, Chakraborty R, Nei M 1983 Differences in the relative distribution of human gene diversity between electrophoretic and red and white cell antigen loci. Human Heredity 33: 93–102
- Santos F R, Pandya A, Tyler-Smith C, Pena S D J, Schanfield M, Leonard W R, Osipova L, Crawford M H, Mitchell R J 1999
- The Central Siberian origin for native American Y chromosomes. American Journal of Human Genetics 64: 619–28
- Seielstad M T, Minch E, Cavalli-Sforza L L 1998 Genetic evidence for a higher female migration rate in humans. Nature Genetics 20: 278–80
- Sokal R R, Oden N L, Legendre P, Fortin M J, Kim J Y, Thomson B A, Vaudor A, Harding R M, Barbujani G 1990 Genetics and language in European populations. American Naturalist 135: 157–75
- Stringer C B, Andrews P 1988 Genetic and fossil evidence for the origin of modern humans. Science 239: 1263–8
- Templeton A R 1998 Human races: A genetic and evolutionary perspective. American Anthropologist 100: 632–50
- Tishkoff S A, Dietzsch E, Speed W, Pakstis A J, Kidd J R, Cheung K, Bonne-Tamir B, Santachiara-Benerecetti A S, Moral P, Krings M, Paabo S, Watson E, Risch N, Jenkins T, Kidd K K 1996 Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science 271: 1380–7
- Vogel F, Motulsky A G 1986 Human Genetics: Problems and Approaches, 2nd edn. Springer-Verlag, Berlin, p. 534
- Wolpoff M H, Caspari R 1997 Race and Human Evolution. Simon & Schuster, New York




