View sample stress and immune function research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The term stress has been criticized for being too general or nonspecific. For some it is a stimulus, whereas for others it is a response or a combination of stimulus and response. For some it is an inherently psychological process mediated solely by the nervous system and affecting mental health, whereas for others it is a physical syndrome due primarily to exercise or physical insult and mediated by damage or injury to bodily tissue. Over the past 10–20 years, however, depictions of stress and discussions of how it operates and affects us have become more integrated and have been increasingly used as an exemplar or illustration of important mind-body connections or pathways linking environments, behaviors, and biological changes to health and well-being. These characterizations describe stress as a relatively nonspecific series of biological and psychological changes that support coping and adaptation to threat, harm, danger, or demand posed by the environment. The evolution of conceptions of stress and the increasing focus on stress-related changes in the immune system are the principal topics of this research paper.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Stress is derived from an inherently adaptive process. People live in a constantly changing world in which physical and social conditions require continuous adjustment and adaptation. Many of these changes are small and some are imperceptible, involving little effort in adjusting to them. When people drive their cars, they must make more-or-less continuous, small adjustments with the steering wheel; they are often unaware of these adjustments. However, the sudden appearances of major barriers or obstacles (turns, other vehicles, potholes) ordinarily require bigger adjustments that demand more effort and awareness. These are similar to more substantial environmental changes that call for more effort for successful adaptation. Mental and physical resources must be marshaled to support this effort, and this mobilization includes many cognitive and bodily changes associated with stress. Some changes are disruptive and are perceived as aversive either because they produce harm or loss or because they pose a threat for future harm or loss (e.g., Lazarus & Folkman, 1984). In response to such appraisals, psychological, behavioral, and physiological responses are activated that enable people to engage in coping strategies that promote adaptation to or accommodation to the situation.
The general readying responses and nonspecific activation associated with coping constitute what most theorists call stress; it helps people achieve adaptation. Stress becomes detrimental when coping strategies are ineffective, demands exceed ability to cope, or activation of psychological, behavioral, and physiological response systems is sustained or unusually intense. Among the systems affected is the immune system, a key component of overall survival because of its critical role in protection from disease. Although all stress response systems work in concert, the immune system has received considerable attention because of its role in infection and disease. The relationships among stress, coping, and immunity are explored after separate discussions of major conceptual models of stress, the strategies people use to cope with stress, and the growing literature on stress-related immune changes.
The Stress Construct
Although the concept of stress is widely acknowledged, there is little scientific consensus about its definition. Most theorists agree that stress can be adaptive, that it is precipitated by demand or by threatening or harmful events, and that it is often associated with negative moods. Because both biological and psychological response systems are activated, some theorists argue that stress is an emotion. Others maintain that stress is best described as a general state of arousal that prompts and supports action directed toward dealing with the stimulus (e.g., Baum, 1990; Mason, 1971). Operational definitions of stress also vary and can include measures of precipitating events (such as the number of major life changes a person experiences in a given time frame); psychological, physiological, or behavioral responses common in stress; or both the event and the responses. Regardless of how it has been measured, stress appears to be a fundamental component of adjustment and adaptation to environmental change. Theories of stress have evolved over the past 100 years to describe the variety of response patterns to threat, harm, or loss that occur, and work by Cannon, Selye, and Mason in particular has set the stage for modern depictions of stress.
Cannon (1914) was one of the first scientists to study stress. He agreed with major theorists of his time that the body needed to maintain a state of equilibrium. Anything that disturbed or threatened this equilibrium prompted the organism to respond in ways that would restore homeostasis. Some stressful events elicited negative emotions such as anger and fear, and these emotions were associated with activation of the sympathetic nervous system (SNS) and the release of hormones that stimulated other areas in the body. These neurohormonal changes prepared the organism to engage in one of two adaptive behavioral responses: fight or flight. The organism was readied either to stand its ground and fight off the stressor or to flee the situation. Much of this work was based on crude bioassay techniques and an incomplete knowledge of sympathetic physiology, but much of what Cannon proposed has been verified by more recent research. For example, we now know that catecholamines (i.e., epinephrine and norepinephrine) are released during stress or arousal and accomplish many of the actions proposed by Cannon.
Selye (1956/1984) developed a different model of stress, the general adaptation syndrome (GAS), based on activity in the hypothalamic-pituitary-adrenocortical (HPA) axis. Beginning his work in the 1930s, Selye characterized stress as a triad of physiological responses to noxious events that included ulceration of the digestive system, enlargement of the adrenal gland, and involution of lymphoid tissues (Selye, 1936). He argued that these responses were driven by activation of the HPA axis and were seen regardless of the type of stressor presented. During initial stages of alarm, large increases in corticosteroids were seen immediately following presentation of the stressor, signifying resource mobilization. After mobilization was accomplished, responding shifted to the second stage, during which the organism resisted or acted to overcome the stressor. Corticosteroids in the adrenal cortex were replenished and adaptation was usually achieved. If adaptation was precluded—as in cases of extreme or persistent stress exposure—exhaustion was reached.This final stage was characterized by a depletion of resources and could result in chronic debilitating disease or death.
Mason (1971) expanded on these models by examining stress responding in a variety of physiological systems. Building on Cannon’s work and investigating other bodily systems not directly related to arousal of the SNS or HPA axis, he showed that predictable changes occurred in many systems. Different kinds of stressors elicited different kinds of changes, but emotional responses to stressors such as anger and fear were not specific to the stressor. This meant that the emotional arousal that characterized different stressors made them feel the same across different situations. He concluded that stress was a unified catabolic response serving to maintain the high levels of circulating glucose needed by the body in order to sustain prolonged resistance.
Cannon (1914) and Selye (1956/1984) accurately identified the SNS and the HPA axis as principal drivers of stress responding. Activation of these systems continues to be the focus of studies examining physiological pathways by which stress is induced. Mason’s (1971) work integrated these two systems and expanded prevailing views of stress to include general activation across a multitude of biological systems. Other response pathways such as psychological and behavioral responses received less attention in these earlier models. Cannon and Mason incorporated emotional responses such as fear and anger into their theories, considering negative emotions as mechanisms through which threatening stimuli evoked physiological responses. Mason went further in integrating these levels of response, but the conceptions of stress that grew in the fields of biology and medicine were fundamentally different from those developing simultaneously in psychology and psychiatry.
Psychological theories of stress developed independently of these biological models and focused on individual variability in stress responding. Lazarus (1966) argued that stress was the product of the interaction between the person and his or her environment. It was not enough for a stimulus to occur; people had to appraise the event as stressful and—through processes of secondary appraisals—decide on what available coping strategies could be used to deal with the situation and whether the problem should be attacked or accommodated. These appraisal processes then elicited appropriate psychological, physiological, and behavioral responses.
Central to this model were the processes of cognitive appraisals and coping, both of which were important psychological variables that moderated the relationship between stressful events and bodily reactions. Lazarus (1966) regarded stress as a transactional process in which an individual was constantly acquiring new information and reappraising the situation. Lazarus and Folkman (1984) later expanded on this model and defined stress as the “particular relationship between the person and the environment that is appraised by the person as taxing or exceeding his or her resources and endangering his or her well-being” (p. 19).
This model of stress (Lazarus, 1966; Lazarus & Folkman, 1984) has been the foundation for much of the stress research conducted in the past 40 years. However, it has not been without critics; moreover, as new information has been discovered about the myriad of cognitive processes involved and of bodily responses and health-related outcomes associated with stress, researchers have modified and expanded on this model. Hobfoll (1989) and Hobfoll and Lily (1993) have defined stress in the context of resource loss and conservation. Individuals actively sought to gain and maintain resources; stress was the result of the loss, the threat of loss, or the lack of gain of these resources. In response to stress, individuals acted to minimize the amount of loss experienced. Rahe and Arthur (1978) based their model of stress on the occurrence of major life changes as precipitating events and defined coping as the conscious efforts people used to try to reduce these responses. In contrast, psychological defenses such as denial and repression were unconscious efforts that helped to deflect the initial perception of the significance of the events. Although longterm use of these psychological defenses could result in pathology, short-term use was beneficial in reducing initial psychophysiological responses. Sustained psychophysiological responding not alleviated by coping or defensive efforts lead to illness symptoms, behavior, and disease.
More recently, McEwen (1998; McEwen & Stellar, 1993) proposed a model of allostasis and allostatic load to explain the relationship between stress and disease. Like the earlier biological models of stress proposed by Selye (1936) and Cannon (1914), allostatic load theory focused on physiological response pathways—in particular, those regulatory systems that were highly reactive to external stimuli (e.g., the SNS and the HPA axis). Adaptation to challenges was achieved through changes in these allostatic systems, and this process was termed allostasis. Allostasis corresponded to Cannon’s notion that organisms respond in ways to restore homeostasis when equilibrium was threatened or disturbed; it also corresponds to Selye’s conceptualization of the phases of alarm and resistance. Also similar to these earlier theories, allostasis models assumed that prolonged activation of a physiological system could promote disease. Prolonged activation may have been caused by episodic or repeated acute activation, lack of adaptation, or the failure of the system to shut off or turn on properly. It caused wear and tear on the body, termed allostatic load. Allostatic load represented the cumulative effects of stressful life challenges as well as biological, lifestyle, and environmental risk factors for disease such as genetics, health behaviors (smoking, diet, drinking), and disruptions in the sleep-wake cycle.Although the general concept of allostatic load was appealing because it offered a broad framework for studying risk factors for disease, it was difficult to operationalize and study.
All of the aforementioned theories characterize the processes through which stress unfolds differently, but they all assume that stress has an adaptive function involving arousal of bodily response systems that prompt changes that reestablish homeostasis. When stress is unusually intense or sustained, it cancausepathology. Integrative depictions of stress define it as “a negative emotional experience accompanied by predictable biochemical, physiological, and behavioral changes that are directed toward adaptation either by manipulating the situation to alter the stressor or by accommodating its effects” (Baum, 1990, p. 653).After an appraisal of a situation as threatening or harmful, activation of both specific and nonspecific responses continues until the source of stress is eliminated or its effects have been accommodated. This catabolic fight-or-flight reaction is beneficial in the short term but can result in negative physical and mental health effects if these emergency responses are extreme or prolonged. Variability in the stress process occurs through environmental and personal factors that alter stress appraisals and choice of coping efforts.
Coping
Coping is central to most modern conceptions of stress. Stress motivates people to adapt and reduce the aversive effects of a stressor or one’s reactions to it; the biological changes that occur support these efforts to manipulate or accommodate these aversive conditions. There are many ways in which people cope, and these methods have been divided into two broad categories based on whether the action is directed at manipulating-altering the situation or at palliating negative emotions (Lazarus & Folkman, 1984; Steptoe, 1989). The first of these actions is problem-focused coping, which includes using overt or covert behavioral or cognitive strategies designed to alter the situation or people’s relationship to the situation. Behavioral strategies are usually purposeful efforts to eliminate or change the stressor or its proximity; such strategies include running away, avoiding the location of the stressor, and using aggressive behaviors such as fighting an attacker. Manipulations of the event or attempts to terminate the stressor are also used. Cognitive strategies often focus on plans of action or on redefining the situation in a less threatening manner. Specific strategies include seeking information about the problem, selectively attending to certain aspects of the situation, or using cognitive reappraisal and reframing. Problem-focused strategies are more likely to be chosen than are more emotion-focused options and appear to be the most effective when people have some control over the situation (DeGroot, Boeke, Bonke, & Passchier, 1997; Folkman & Lazarus, 1980). For example, breast cancer patients who viewed their disease as controllable and used problemfocused coping reported fewer symptoms of depression and anxiety soon after their diagnosis (Osowiecki & Compas, 1999). In another study, breast cancer patients reported better adjustment to their disease if they engaged in planning or positive reinterpretation (Ben-Zur, Gilbar, & Lev, 2001).
Attempts to decrease emotional responses to an aversive event without altering the situation are termed emotionfocused coping. These are generally accommodative in nature and are designed to alter reactions to a stressor rather than the stressor itself. If problem-focused coping can be thought of as danger control or attempts to change the source of a threat, emotion-focused coping would be distress control or action taken to minimize upset or unhappiness (Leventhal, 1980). People may increase their alcohol consumption, cigarette smoking, eating, and use of other substances during stress (e.g., Alexander & Walker, 1994; Greeno & Wing, 1994). Engaging in these appetitive behaviors allows for a temporary respite from dealing with the stressful situation; these behaviors do nothing to change or improve the situation, however, and some are independently associated with health risks. People may also use cognitive emotion-focused strategies such as denial, wishful thinking, and rationalization.
If effective, emotion-focused coping should decrease psychophysiological responding. The term repressive-defensive coping has been used to describe people who report low levels of anxiety (i.e., negative emotion) but who exhibit high physiological arousal at the same time. Repressors are also identified by their reports of low anxiety but high defensiveness or social desirability (e.g., King, Taylor, Albright, & Haskell, 1990). This emotion-focused coping style is associated with greater resting cardiovascular arousal as well as with greater increasesincardiovascularreactivityandgreaterimmunosuppression in response to a stressful task (e.g., Jammer & Leigh, 1999; King et al., 1990).
In hindsight, some emotion-focused strategies appear to be maladaptive. While people are engaging in these strategies, little or nothing is done to improve the situation, which has remained the same or has worsened because no counteractions have been taken (Brenner, Melamed, & Panush, 1994; Redeker, 1992). Consistent with this idea is that emotionfocused coping is often associated with worsening of stress symptoms and disease outcomes. For example, use of avoidance and self-blame has been positively associated with blood pressure reactivity to acute tasks, use of denial has been positively associated with progression to AIDS in patients with HIV infection, and use of wishful thinking coping prospectively predicted symptoms of posttraumatic stress disorder in victims of motor vehicle accidents (Dolan, Sherwood, & Light, 1992; Dougall, Ursano, Posluszny, Fullerton, & Baum, 2001; Kohlman, Weidner, & Messina, 1996; Leserman et al., 2000). Prolonged use of these strategies may preclude the use of more effective strategies, especially if something could have been done to control the situation (Lazarus, 1993). However, in situations in which stressors are short-lived, dissipate on their own (without any problem-focused coping), or both, emotionally focused strategies that insulate people from threat and make distress less likely may be very adaptive (DeGroot et al., 1997). Similarly, in situations characterized by little or no control, these strategies are often preferred and can be adaptive (DeGroot et al., 1997; Folkman & Lazarus, 1980). For example, use of avoidance was associated with less perceived stress in people donating blood, and patients with terminal illnesses who perceived that they had little control over their illness used more emotion-focused coping and less problem-focused coping than did patients who had nonterminal diseases (Kaloupek & Stoupakis, 1985; Kausar &Akram, 1998). When stressors or responses are extreme or prolonged, emotion-focused coping may help to diffuse psychophysiological arousal enough so that the person can conserve energy, reappraise the situation, and decide on more appropriate strategies (cf. Pilette, 1983; Rahe &Arthur, 1978).
Problem-focused and emotion-focused coping are not mutually exclusive and are typically used together or sequentially to alleviate stress. Although many specific types of these strategies have been identified, no single strategy has been found to be universally effective or ineffective. Coping is a dynamic process that constantly changes as the person discovers new information and tries new techniques for dealing with the situation(Aldwin&Brustrom,1997).Ratherthanconsistency in use of coping, flexibility in the types of strategies used and the relative use of problem-focused and emotion-focused coping appears to be more adaptive (Cantor & Norem, 1989; Lester, Smart, & Baum, 1994; Vitaliano, Maiuro, Russo, & Becker, 1987). Choice of coping can be influenced by the person’s perceived control over the situation (as mentioned previously) and by a myriad of other event and person characteristics such as perceived self-efficacy, social support, and personality (e.g., Fleishman, 1984; Gerin, Litt, Deich, & Pickering, 1995; Thoits, 1986). This variability in coping helps to explain the variability seen in psychological, behavioral, and physiological responses to stress.
Responses to Stress
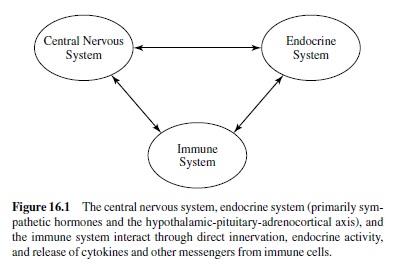
As suggested earlier, stress is driven by biological changes that affect a number of bodily systems and prepare organisms to act quickly and decisively. Recently, there has been a burgeoning interest in the possible consequences of stress on immune outcomes. The relatively new interdisciplinary field of psychoneuroimmunology examines the bidirectional interactions among the immune system and other psychological and physiologicalresponsepatterns.Theimmunesystemworksin concert with other stress systems as a sensory and regulatory system (see Figure 16.1) and is the body’s primary defense against infection and disease. Before immune changes in response to stress are described, changes in other psychological, behavioral, and physiological systems are reviewed.
Psychological and Behavioral Responses and Stress
Stressors or appraisals of them as posing threat or excess demand elicit a broad array of responses, including disturbances in mood and behavior. Increases in negative emotions such as anxiety, depression, fear, or anger are often reported during stressor exposure and can persist in some cases long after exposure has ceased (e.g., Delahanty, Dougall, et al., 2000; Dew & Bromet, 1993; Ursano, Fullerton, Kao, & Bhartiya, 1995). During periods of stress, people also experience increases in negative cognitions such as unwanted or uncontrollable thoughts and memories of the stressor (e.g., Dougall, Craig, & Baum, 1999). These cognitions can help people process and cope with the event (Creamer, Burgess, & Pattison, 1992; Greenberg, 1995; Horowitz, 1986). However, some of these thoughts are unwanted, unbidden, and uncontrollable; they may become acute stressors in their own rights, helping to perpetuate chronic stress (Baum, Cohen, & Hall, 1993; Craig, Heisler, & Baum, 1996). For example, the occurrence of event-related intrusive thoughts predicted long-term distress and physiological responding in victims who were injured in motor vehicle accidents, who were recovery workers at an airplane crash site, and who experienced personal loss as a result of a hurricane (Dougall et al., 1999). Stress associated withtheseintrusivethoughtsmayalsointerferewithdecisionmaking processes, problem solving, or judgments, contributing to new sources of stress and impairing overall efficiency.
Consistent with this prediction, some research has found that stress can be manifested as disruption of behavior and performance. Because attention is typically focused on dealing with stressors when they are present, people who are experiencing stress often report trouble concentrating and poor performance on mundane tasks such as balancing a checking account, monitoring computer screens, or assembling a product (for reviews, see Baba, Jamal, & Tourigny, 1998; Cooper, 1988; Kompier & DiMartino, 1995; Krueger, 1989; McNally, 1997).These effects are likely to be minor but can be detrimental if they are work- or safety-related (e.g., writing a report or driving an automobile). Even transient performance decrements induced by laboratory challenges can persist well after physiological and emotional responding has habituated (Glass & Singer, 1972). Additionally, some people cope with stress by increasing their use of drugs such as alcohol and nicotine, eating diets with less nutritional value, and decreasing physical exercise (Alexander & Walker, 1994; Rosenbloom & Whittington, 1993; Willis, Thomas, Garry, & Goodwin, 1987). Other behavioral changes include increases in aggressive behaviors and deterioration of sleep quality and quantity (e.g., Conway, Vickers, Weid, & Rahe, 1981; Ganley, 1989; Grunberg & Baum, 1985; Mellman, 1997; Sadeh, 1996; Spaccarelli, Bowden, Coatsworth, & Kim, 1997). Prolonged changes in these behaviors may have negative health effects and may potentiate other stress-related responses such as alterations in immunity and other neuroendocrine systems.
Neuroendocrine Responses and Stress
The seminal work of Selye (1956/1984) and Cannon (1914) introduced the now accepted effects of stress on the SNS and the HPAaxis. Both of these systems are mobilized when an individual encounters a stressor. Increases in heart rate and blood pressure as well as increases in the release of catecholamines— particularly norepinephrine and epinephrine—are hallmark indicators of SNS arousal. Activation of the HPA axis results in the release of glucocorticoids (e.g., cortisolinhumans) from the adrenal cortex. More recent research has demonstrated that these two systems do not function independently; they communicate with one other. For example, catecholamines affect the release of ACTH (adrenocorticotropic hormone) from the pituitary, and corticosteroids enhance the cardiovascular effects of epinephrine and norepinephrine (e.g., Axelrod & Reisine, 1984; Davies & Lefkowitz, 1984; Szafarczyck, Malaval, Laurent, Gibaud, & Assenmacher, 1987). Likewise, the SNS and the HPA axis connect with other bodily systems, including (but not limited to) the gastrointestinal system, the hypothalamic-pituitary-gonadal axis and the immune system (de la Torre, 1994; Weiner, 1992). The generalized activation that results from stimulation of one or the other system is considered to be adaptive in that it serves to mobilize physiological resources enabling the person to cope more effectively. Other neurohormones and peptides that are altered during stress include growth hormone, prolactin, vasopressin, endogenous opioids, thyrotropin-releasing hormone, thyroid-stimulating hormone, somatostatin, insulin, glucagon, glucose, testosterone, follicle stimulating hormone, and luteinizing hormone (for reviews, see de la Torre, 1994; Lundberg, 1984; Weiner, 1992). These psychological, behavioral, and physiological response systems work in concert to help the individual adapt to stress, and many are directly tied to changes in immune status.
Immune Responses and Stress
The immune system is constantly surveying the body— fending off foreign pathogens such as viruses and bacteria and clearing away bodily cells that have died or have mutated or been altered, as in the case of viral host cells or cancer cells. The continuum of health and disease is maintained in part by the immune system’s ability to detect and eliminate these infectious and noninfectious agents and to provide mechanisms through which healing can occur. These immune activities have been broken down into two types of immunity—innate and acquired—based on how the cells, structures, and factors in each of these processes counter incursions of pathogens. Innate or natural immunity represents the body’s first line of defense against a pathogen. As the name implies, innate immunity does not require prior exposure to a particular pathogen, nor is it specific to the type of pathogen encountered. A similar cascade of events occurs after each foreign substance is encountered. In contrast, an acquired immune response is specific to the pathogen and usually requires other cells to present the pathogen to the agents of acquired immunity. Acquired immunity also has the ability to remember a pathogen after the immune system has encountered it, resulting in faster and stronger defensive responses upon subsequent exposures. Acquired responses are generally stronger and more effective than innate defenses after exposure has createdmemoryforaparticularpathogen,andtheseaspectsof immunity are the basis for vaccination and inoculation.
A variety of cells and structures comprise the immune system. Most research in psychoneuroimmunology focuses on the number and function of lymphocytes, the specialized white blood cells that recognize antigens and are key components of acquired immunity. There are three major types of lymphocytes: natural killer cells, B lymphocytes, and T lymphocytes (see Figure 16.2). Natural killer (NK) cells are large, granular lymphocytes that do not require prior exposure to a pathogen or altered cell; they function as agents of innate immunity by recognizing and killing virally infected cells and tumor cells spontaneously. They have become popular targets for research in psychoneuroimmunology because of their great sensitivity to stress or psychological variables and because of their apparent relevance to several important disease processes.
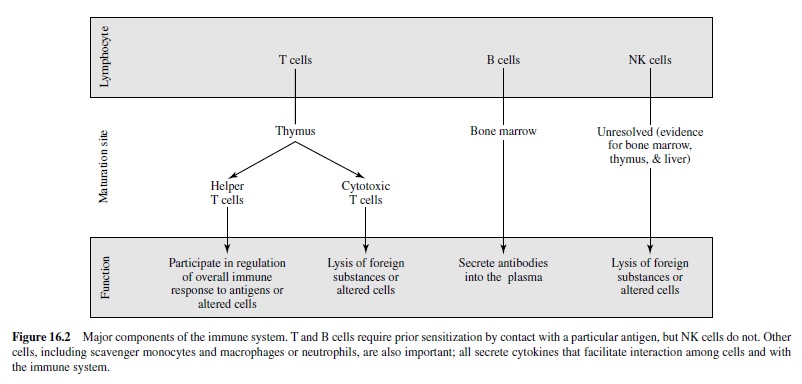
The B and T lymphocytes are agents of acquired immunity. Both originate in the bone marrow, but T cells migrate to the thymus (hence the name T lymphocyte), where they mature into either helper T cells or cytotoxic T cells. Along with different maturational sites, T and B cells have different mechanisms of action. Upon activation and recognition of an antigen, B cells secrete antibodies (or immunoglobulins) that bind to antigens and trigger a cascade of immune events that lead to the elimination of the antigen. This form of immunity is referred to as humoral because the antibodies are free in body fluids (humors). In contrast, T lymphocytes are the major components of cellular immunity and have both regulatory and killer functions. Regulatory T lymphocytes are called helper T cells and recognize nonself pathogens through their presentation by the major histocompatibility complex (MHC). Helper T cells then secrete cytokines that serve to activate B cells and other T cells preferentially. Killer T lymphocytes (cytotoxic T cells) lyse and kill cells that exhibit markers of a specific antigen complex.
Effects of stress on immune system activity vary in a number of different ways depending on the type of immune marker measured, the timing of the assessment, and the type of stressful situation encountered. Considerable research has examined immune reactivity to brief laboratory tasks that are thought to be stressful or challenging. These acute stressors are brief, typically 5–30 min long (e.g., Breznitz et al., 1998; Delahanty, Dougall, Browning, Hyman, & Baum, 1998). Immune changes observed during or immediately after acute stress generally include increases in the numbers of some circulating lymphocytes in the peripheral blood, especially NK cells and cytotoxic T cells (e.g., Breznitz et al., 1998; Herbert et al., 1994; Naliboff et al., 1991). Increases in cell numbers are due in part to migration of lymphocytes into the blood stream from lymphoid organs such as the spleen and lymph nodes (Benschop, Rodriguez-Feuuerhahn, & Schedlowski, 1996). One possible reason for these increases is that lymphocytes travel through the blood during periods of acute stress to reach areas of the body such as the gastrointestinal system and the skin, where they act to ward off infection and to promote healing (Dhabhar, 1998).
Despite these increases in numbers, the functional ability of T and B lymphocytes in the peripheral blood is impaired as evidenced by the decreased proliferative response of lymphocytes to mitogen presentation in vitro (e.g., Herbert et al., 1994; Manuck, Cohen, Rabin, Muldoon, & Bachen, 1991; Zakowski, Cohen, Hall, Wollman, & Baum, 1994). At any given time an individual typically has only a handful of T and B cells with memory for a specific antigen. However, thousands or millions of cells are often needed to mount an effective defense when the antigen is encountered. To accomplish this, immune cells in circulation proliferate when they encounter specific antigens or very strong mitogens, and the ability to proliferate has been used as a measure of immunocompetence. During or afterstress, these lymphocytes appear to be inhibited in some way, possibly by concomitant stress-related increases in hormones such as epinephrine and cortisol that bind to immune cell membrane receptors and are associated with changes in cell migration and decreased proliferation (e.g., Crary, Borysenko, et al., 1983; Crary, Hauser, et al., 1983; Daynes,Araneo, Hennebold, Enioutina, & Mu, 1995; Kappel, Poulsen, Galbo, & Pedersen, 1998; Sondergaard, Ullum, Skinhoj, & Pedersen, 1999). In contrast, the cytotoxic activity of NK cells appears to incrementally change in proportion to the number of NK cells in the blood. Like stress-induced changes in NK cell numbers, NK cell cytotoxicity increases during exposure to an acute stressor and then rebounds to belowbaselinelevelsafterstressortermination(Breznitzetal., 1998; Delahanty, Wang, Maravich, Forlenza, & Baum, 2000; Schedlowski et al., 1993). This change in overall activity appears to be due to changes in cell number—that is, overall cytotoxicity increases if there are more cells available to kill pathogens and cytotoxicity decreases if there are fewer cells available to kill pathogens (Delahanty, Wang, et al., 2000). In sum, acute stressors elicit mobilization of lymphocytes and transitoryincreasesinsomeagentsofinnateimmunity.Incontrast, episodic or chronic stressors such as bereavement, academic stress, and caregiving for ill family members are associated with general immunosuppression.
Chronic stress is generally associated with lower numbers of peripheral blood lymphocytes—in particular, NK cells and cytotoxic T cells—as well as lower levels of NK cell cytotoxicity and lymphocyte proliferation (e.g., Esterling, KiecoltGlaser, Bodnar, & Glaser, 1994; Herbert & Cohen, 1993; Kiecolt-Glaseretal.,1987).Inaddition,chronicstressappears to affect immune responses during acute stressors—in particular, decreasing rather than increasing numbers of T cells and blunting the increases in NK cell numbers and overall cytotoxicity following acute stressor exposure (e.g., Brosschot et al., 1994; Pike et al., 1997). Evidence for chronic stressrelated immunosuppression has also been found using indirect measures of immune functioning. Chronic stress has been associated with impairments in the ability of wounds to heal, declines in seroconversion as evidenced by antibody titers 6 months after vaccination, and reactivation and recurrence of latent viruses such as herpes simplex and Epstein-Barr virus (e.g., Glaser, Sheridan, Malarkey, MacCallum, & KiecoltGlaser, 2000; Jenkins & Baum, 1995; Kiecolt-Glaser et al., 1987; Kiecolt-Glaser et al., 1988; Kiecolt-Glaser, Marucha, Malarkey, Mercado, & Glaser, 1995; Marucha, KiecoltGlaser, & Favagehi, 1998).
Relationships Between Immune Responses and Other Response Systems
The immune system has bidirectional relationships with other stress response systems; many of these relationships appear to be mechanisms through which stress-related immune changes occur. For example, SNS activation is associated with increases in cell mobilization and decreases in cell proliferation during and shortly after acute stress and with decreases in cell mobilization and decreases in lymphocyte activity as a function of chronic stress (Delahanty, Wang, et al., 2000; Manuck et al., 1991; McKinnon, Weisse, Reynolds, Bowles, & Baum, 1989). Similarly, alterations in other physiological systems such as the HPAaxis and the hypothalamic-gonadal axis have direct effects on immunity and may be important mechanisms in stress-related immune responding (Daynes et al., 1995; Paavonen, 1994; Schuurs & Verheul, 1990). Negative mood and recurrent negative thoughts about the stressor (e.g., Delahanty, Dougall, Craig, Jenkins, & Baum, 1997; Solomon, Segerstrom, Grohr, Kemeny, & Fahey, 1997) and stressrelated changes in behaviors such as decreases in sleep quality and quantity, decreases in exercise, and increases in the intake of recreational drugs may also contribute to immunosuppression (e.g., Eisenstein, & Hilburger, 1998; Galloway & Jokl, 2000; Ironson et al., 1997; Klein, Friedman, & Specter, 1998; McAllister-Sistilli et al., 1998; Watson, Eskelson, & Hartmann, 1984). Not surprisingly, coping styles and resources such as a pessimistic disposition and less available social support have also been associated with immune alterations (e.g., Byrnes et al., 1998; Sieber et al., 1992; Uchino, Cacioppo,&Kiecolt-Glaser,1996).Clearly,therearemultiple pathways through which stress may alter immunity. This cocktail of causal agents contributes to the variability in the relationship between stress and disease processes and suggests several appropriate areas for intervention.
Immune Responses and Health Outcomes
As noted previously, acute exposures to stress appear to mobilize immune cells and to increase some aspects of innate immunity. These changes may be beneficial in warding off opportunistic infections or in guarding against an incursion of pathogens during or shortly after a challenge. In contrast, chronic stress and associated changes in immunity have largely negative effects on resistance to disease processes. If the immune system is not functioning at its optimal level, pathogens and mutated self-cells may escape detection, elimination, or both, and recovery from injuries may be impeded. In fact, people who are experiencing ongoing stress are more susceptible to infectious diseases such as colds, flu, and HIV disease (e.g., Cohen et al., 1998; Stone et al., 1992; for reviews, see Dorian & Garfinkel, 1987; O’Leary, 1990). Additionally, stress has been linked to tumor growth and cancer progression as well as impairments in wound healing (e.g., Ben-Eliyahu, Yirmiya, Liebeskind, Taylor, & Gale, 1991; Bohus, Koolhaas, de Ruiter, & Heijnen, 1992; Kiecolt-Glaser et al., 1995; Marucha et al., 1998; Stefanski & Ben-Eliyahu, 1996). Given these relationships among stress, immunity, and disease, there has been increasing interest in designing and implementing interventions aimed at preventing or minimizing stress-related immune changes.
Stress Management Interventions
Interventions that decrease potentially toxic elements of stress (e.g., anxiety, depression, elevations in blood pressure) should also prevent or reduce stress-related immunosuppression. Probably the most effective way to reduce stress responding is to remove the stimulus. If your car breaks down and you are without transportation, you can either fix it or buy a new one. However, many situations are not easily changed—especially in cases of sudden, acute stressors like assault. A traumatic event like rape can never be undone. Given that direct action is not always feasible or likely to be successful, secondary intervention programs are often used to reduce ongoing stress. Thesestressmanagementtechniquescapitalizeontherelationships among appraisals, coping, and stress responses. By altering characteristics of the situation or of the person such as felt arousal, perceived control, social support, self-efficacy, and cognitive processes, people learn to reappraise the situation and to deal with a stressor more effectively.As a consequence, reductions in psychological, behavioral, and physiological stress responding are seen.
Research examining changes in immunological indexes after stress management interventions have used cognitive and somatic stress reduction techniques. Cognitive techniques are designed to alter appraisals of the situation and the choice of coping strategies in order to reduce or eliminate perceptions of stress (harm, loss, threat, or challenge) or to help people match their coping efforts to the situation at hand (Fava, 2000). These techniques often enhance perceptions of control, predictability, and social support—all important buffers of stress and its associated consequences. Somatic techniques target physiological arousal and use behavioral strategies such as relaxation training, meditation, biofeedback, and exercise to decrease arousal and facilitate appropriate emotional and behavioral responses (e.g., Carrington et al., 1980; Kiecolt-Glaser et al., 1985; LaPerriere et al., 1990; Murphy, 1984).
A number of recent studies have demonstrated that stress management interventions can be effective in buffering immune changes that occur with stress. Fawzy (1994) conducted a group intervention for patients with malignant melanoma and found that 6 months after the intervention, patients displayed increases in numbers of NK cells and large granular lymphocytes, increases in NK cell cytotoxicity, and decreases in numbers of helper T cells. Increases in large granular lymphocytes and NK cell cytotoxicity were associated with decreases in depression and anxiety, and increases in NK cell cytotoxicity predicted lower rates of cancer recurrence. Similarly, Kiecolt-Glaser et al. (1985) found increases in NK cell cytotoxicity and decreases in distress and antibody titers to herpes simplex virus following 1 month of relaxation training. Elderly residents of the same independent-living facilities who were randomly assigned to a social contact group or to a control group exhibited no changes in these stress measures.
Research conducted by Antoni and colleagues (e.g., Antoni et al., 2000; Cruess et al., 2000) has demonstrated that cognitive-behavioral stress management intervention techniques are effective in buffering stress-related immune changes in patients with HIV disease. They found decreases in negative mood, norepinephrine levels, and cortisol to dehydroepiandrosterone sulfate (DHEA-S) ratio levels as well as decreases in antibody titers to herpes simplex virus Type 2 in HIV-positive patients following completion of their multimodal intervention (Antoni et al., 2000; Cruess et al., 2000). In addition to showing these short-term immune changes, patients in the intervention group had greater numbers of cytotoxic T cells than did the waiting-list control patients 6–12 months after the intervention ended (Antoni et al., 2000). Other research groups have found increases in numbers of helper T cells (the cells that are infected by HIV) in HIV-positive patients who participated in stress management interventions (e.g., Eller, 1995; Taylor, 1995). Stress reduction techniques have also been used to prevent stress-related immune changes associated with notification of HIV serostatus. Asymptomatic, healthy gay men who completed an aerobic exercise training program did not experience the increases in negative affect and decreases in NK cell numbers that were observed in the seropositive control group after notification of HIV serological status (LaPerriere et al., 1990).
Although they are promising, these findings need to be interpreted with caution. Many studies have not found changes in immunity following psychological interventions (e.g., Coates, McKusick, Kuno, & Sites, 1989), and a recent metaanalysis found that stress reduction interventions do not reliably alter immunity (Miller & Cohen, 2001). The effectiveness of stress management interventions in buffering immune responses could be determined by a number of variables. One factor is the extent of stress responding preceding the intervention. In order to reduce stress, the people participating in the intervention need to be experiencing stress. When Miller and Cohen (2001) reexamined the studies they included in their meta-analysis of immune changes following psychological interventions, they found that the few studies that targeted stressed populations demonstrated reliable changes following a stress management intervention. The effectiveness of an intervention also depends on whether immune changes are attributable to stress. Some of the immunosuppression experienced may be a product of chronic disease processes or medical treatment regimens that independently alter immunity, as in the case of cancer or HIV disease. Additionally, stress management interventions are most effective if the participants practice the techniques they learn. In HIV-positive patients, increases in cytotoxic T cells found 6–12 months after a cognitive-behavioral stress management intervention were predicted by greater frequency of use of relaxation techniques at home (Antoni et al., 2000). Likewise, greater practice of relaxation techniques was associated with increases in the percentage of helper T cells during examination stress in medical students(Kiecolt-Glaseretal.,1986).Therefore,dependingon the situation, a number of factors may interact to determine the efficacy of a stress management intervention in preventing or counteracting stress-related immunosuppression.
Conclusions
Stress is a fundamental process characterizing people’s interactions with their environments and responses to challenge. Appraisals of threatening or harmful challenges motivate people to take action to either eliminate these stressors (i.e., problem-focused coping) or protect themselves from the stressor’s negative impact (i.e., emotion-focused coping). These appraisals and coping efforts elicit a series of specific and nonspecific cognitive, behavioral, and physiological responses. Stress responses in turn promote adaptation by mobilizing catabolic energies that support coping efforts. In most cases, people are able to effectively deal with all types of stressors—from minor irritations such as misplacing one’s car keys to extreme stressors such as assault or disaster. People learn from these experiences and move on to encounter new challenges, giving credence to the belief that stress is an inherently adaptive process. Stress becomes detrimental to mental and physical functioning when stressor demands exceed the person’s abilities to cope and responding is prolonged or unusually intense.
One response system that has received considerable attention is the immune system because of its role in the detection and elimination of pathogenic agents. Repeated or protracted exposures to stress appear to suppress immune system functioning and may contribute to increased vulnerability to infection and disease. Alterations in other stress response pathways have important effects on immunity as well and appear to be mechanisms through which many of these stressrelated immune changes occur. By decreasing cognitive, behavioral, and physiological responding, stress reduction interventions are used to facilitate adaptation and have important implications for decreasing vulnerability to disease processes.
Future Directions
Although we have learned a great deal about how the immune system responds during and after stress and how it interacts with other bodily systems, many questions still need further inquiry. Some basic questions about the reciprocal nature of brain-immune system interactions, about mediators between emotional processes or other psychological phenomena and immune system change, and about basic stress-related immunomodulation of disease vulnerability remain to be studied. Gray areas in existing data suggest future directions for both theory and research—not only in psychoneuroimmunology, but also in related disciplines. For example, one question that has plagued chronic stress researchers is how and when acute stress becomes chronic. We know that stress can be an adaptive process, especially in the short-term; more over, some immune changes during acute stress seem consistent with a mobilization of cells and innate immunity and may provide enhanced protection against opportunistic infections. However, chronic stress responding is associated with a broader, longer-term immunosuppression in which some of the conditions for a reduction in host defense are met. When and how does this transition take place? Research has identified event and person variables such as the duration and nature of the stressor and duration of the perceived threat as determinants of chronic stress responding (Baum et al., 1993; Baum, O’Keeffe, & Davidson, 1990). However, these risk factors do not explain all of the variability seen in stress responding. For example, traumatic events that begin and end within minutes, such as powerful storms or tornados, can elicit longterm stress responding in some victims while the majority of victims recover rapidly. These seemingly inconsistent findings are the impetus for research on identifying both situation and person variables that either promote or inhibit chronic stress responding. The coping skills, strategies, and resources people use to deal with a situation have been a prime target of these investigations. Coping has the added advantage of being modifiable, and teaching people appropriate coping strategies is a basic component of many stress management and mental health interventions. Future research designs need to incorporate more frequent longitudinal assessments and examine multimodal assessments of stress responding (i.e., psychological, behavioral, neuroendocrine, and immunological responses) as well as moderating variables such as coping strategies and resources in order to better characterize the time course of stress responding.
Unfortunately, longitudinal psychoneuroimmunological investigations can become very costly and burdensome to study participants. Researchers need to carefully select outcome variables to minimize cost and burden and to maximize relevance, especially with regard to immune indexes. A substantial challenge is that the interaction between the immune system and stress or other psychological and psychobiological processes is still relatively unexplored when compared with stress and other bodily systems such as the cardiovascular system. Investigation of consistent relationships among stress-sensitive systems such as common regulatory features and processes (e.g., sympathetic innervation, endocrine responsivity, distribution of endocrine and neuropeptide receptors, and HPA axis activity) and shared outcomes may yield additional important information about mechanisms underlying immune system changes and about general markers for organismic vulnerability to disease.
Translational research is also integral to psychoneuroimmunology. Investigations of the complex interactions among psychological, neuroendocrine, and immune systems bear directly on basic research on how immune cells react to antigens and interact with each other. However, these basic investigations have undeniable applied or clinical implications and concurrent and derivative translational research that begins to view real-world infectious phenomena in broader terms represents a critical outcome of this research enterprise. For example, research on periodic exacerbations of autoimmune disorders or reactivation of latent viruses provides important basic data about clinical phenomena and their physiological bases; such research suggests important predictive and ameliorative strategies (e.g.,Ackerman, Heyman, Rabin, & Baum, 2000; Jenkins & Baum, 1995; KiecoltGlaser & Glaser, 1987). Additionally, as advancements in immunoassays and assessment techniques occur, psychoneuroimmunology research can better focus on the most appropriate components of immunity for each hypothesis being tested.
Interventions designed to reduce overall stress responding can be effective in minimizing stress-related changes inimmunity. However, as we have discussed, these effects on the immune system are dependent on several factors (i.e., the population has to be experiencing stress, the immune changes have to be associated with stress, and the population has to actively participate and practice the intervention techniques). Future research needs to thoroughly assess whether stress management interventions are first likely to affect immunity and then to identify barriers to participation within the target population. For example, some populations may be more willing to exercise for an hour than to attend a group session and talk about private issues. Barriers related to ethnic, cultural, and socioeconomic factors also need to be considered. Additionally, it may be prudent to reevaluate the outcome measures used and the timing of the assessments. In some instances, such as a preventive intervention in which people are not likely to experience a great deal of stress, immediate measures of stress responding, including immunity, should be unaffected. However, days or weeks later when they next encounter a major stressor, if they use what they learned in the intervention, immune changes should be attenuated. Researchers need to develop longitudinal designs that incorporate these types of issues related to the timing of the intervention in relation to the stressor of interest.
Related to this concern is the fact that the majority of research examining immune changes following psychological interventions have considered populations that are enduring chronic illnesses, such as cancer and HIV disease. Although such research is appropriate and worth while, more of this work needs to be done with other chronically stressed populations— including physically healthy people dealing with a stressor— so that immune processes independent of disease processes can be better understood. Research by Cohen and his colleagues (e.g., Cohen, Doyle, & Skoner, 1999; Cohen et al., 1998) on stress-related vulnerability to experimentally applied viruses has begun to address this concern, as have studies of chronically stressed caregivers or of marital conflict (e.g., Esterling et al., 1994; Kiecolt-Glaser et al., 1987; Kiecolt-Glaseretal., 1988). Studies of psychological trauma and stressrelated immune system change as well as trauma-related health outcomes are also important in this regard (e.g., Inslicht, Hyman, Larkin, Jenkins, & Baum, 2001; Ironson et al., 1997; McKinnon et al., 1989; Segerstrom, Solomon, Kemeny, & Fahey, 1998).
As research continues to explain the complex interactions among stress response pathways and how these responses are altered by appraisal and coping, our understanding of the stress process and its effects on health and disease will increase. Continued characterization of the beneficial as well as harmful effects of stress will improve our ability to identify modifiable risk factors (e.g., lifestyle, appraisal, coping, and social resources) and will advance the design and implementation of interventions to reduce the negative consequences of stress and promote adaptation.
Bibliography:
- Ackerman, K. D., Heyman, R., Rabin, B. S., & Baum, A. (2000). Stress and its relationship to disease activity in multiple sclerosis. International Journal of Multiple Sclerosis, 7, 20–29.
- Aldwin, C. M., & Brustrom, J. (1997). Theories of coping with chronic stress: Illustrations from the health psychology and aging literatures. In B. H. Gottlieb (Ed.), Coping with chronic stress (pp. 75–103). New York: Plenum Press.
- Alexander, D. A., & Walker, L. G. (1994). A study of methods used by Scottish police officers to cope with work-induced stress. Stress Medicine, 10, 131–138.
- Antoni, M. H., Cruess, D. G., Cruess, S., Lutgendorf, S., Kumar, M., Ironson, G., Klimas, N., Fletcher, M. A., & Schneiderman, N. (2000). Cognitive-behavioral stress management intervention effects on anxiety, 24-hr urinary norepinephrine output, and T-cytotoxic/suppressor cells over time among symptomatic HIVinfected gay men. Journal of Consulting and Clinical Psychology, 68, 31–45.
- Axelrod, J., & Reisine, T. D. (1984). Stress hormones. Science, 224, 452–459.
- Baba, V. V., Jamal, M., & Tourigny, L. (1998). Work and mental health: A decade in Canadian research. Canadian Psychology, 39, 94–107.
- Baum, A. (1990). Stress, intrusive imagery, and chronic distress. Health Psychology, 9(6), 653–675.
- Baum, A., Cohen, L., & Hall, M. (1993). Control and intrusive memories as possible determinants of chronic stress. Psychosomatic Medicine, 55, 274–286.
- Baum, A., O’Keeffe, M. K., & Davidson, L. M. (1990). Acute stressors and chronic response: The case of traumatic stress. Journal of Applied Social Psychology, 20, 1643–1654.
- Ben-Eliyahu, S., Yirmiya, R., Liebeskind, J., Taylor, A., & Gale, R. (1991). Stress increases metastatic spread of a mammary tumor in rats: Evidence for mediation by the immune system. Brain, Behavior, and Immunity, 5, 193–205.
- Ben-Zur, H., Gilbar, O., & Lev, S. (2001). Coping with breast cancer: Patient, spouse, and dyad models. Psychosomatic Medicine, 63, 32–39.
- Benschop, R. J., Rodriguez-Feuuerhahn, M., & Schedlowski, M. (1996). Catecholamine-induced leukocytosis: Early observations, current research, and future directions. Brain, Behavior, and Immunity, 10, 77–91.
- Bohus, B., Koolhaas, J. M., de Ruiter, A. J. H., & Heijnen, C. J. (1992). Psycho-social stress: Differential alterations in immune system functions and tumor growth. In R. Kvetnansky, R. McCarty, & J. Axelrod (Eds.), Stress: Neuroendocrine and molecular approaches (Vols. 1 & 2, pp. 607–621). Philadelphia: Gordon and Breach Science.
- Brenner, G. F., Melamed, B. G., & Panush, R. S. (1994). Optimism and coping as determinants of psychosocial adjustments to rheumatoid arthritis. Journal of Clinical Psychology in Medical Settings, 1, 115–134.
- Breznitz, S., Ben-Zur, H., Berzon, Y., Weiss, D. W., Levitan, G., Tarcic, N., Lischinsky, S., Greenberg, A., Levi, N., & Zinder, O. (1998). Experimental induction and termination of acute psychological stress in human volunteers: Effects on immunological, neuroendocrine, cardiovascular, and psychological parameters. Brain, Behavior, and Immunity, 12, 34–52.
- Brosschot, J. F., Benschop, R. J., Godaert, G. L. R., Olff, M., De Smet, M., Heijnen, C. J., & Ballieux, R. E. (1994). Influence of life stress on immunological reactivity to mild psychological stress. Psychosomatic Medicine, 56, 216–224.
- Byrnes, D. M., Antoni, M. H., Goodkin, K., Efantis-Potter, J., Asthana, D., Simon, T., Munajj, J., Ironson, G., & Fletcher, M.A. (1998). Stressful events, pessimism, natural killer cell cytotoxicity, and cytotoxic/suppressor T cells in HIV Black women at risk for cervical cancer. Psychosomatic Medicine, 60, 714–722.
- Cannon, W. B. (1914). The interrelations of emotions as suggested by recent physiological researches. American Journal of Physiology, 25, 256–282.
- Cantor, N., & Norem, J. K. (1989). Defensive pessimism and stress and coping. Social Cognition, 7, 92–112.
- Carrington, P., Collings, G. H., Jr., Benson, H., Robinson, H., Wood, L. W., Lehrer, P. M., Woolfolk, R. L., & Cole, J. W. (1980). The use of meditation-relaxation techniques for the management of stress in a working population. Journal of Occupational Medicine, 22, 221–231.
- Coates, T. J., McKusick, L., Kuno, R., & Sites, D. P. (1989). Stress reduction training changed number of sexual partners but not immune function in men with HIV. American Journal of Public Health, 79, 885–887.
- Cohen, S., Doyle W. J., & Skoner, D. P. (1999). Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosomatic Medicine, 61, 175–180.
- Cohen, S., Frank, E., Doyle, W. J., Skoner, D. P., Rabin, B. S., & Gawltney, J. M., Jr. (1998). Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychology, 17, 214–223.
- Conway, T. L., Vickers, R. R., Weid, H. W., & Rahe, R. (1981). Occupational stress and variation in cigarette, coffee, and alcohol consumption. Journal of Health and Social Behavior, 22, 155–165.
- Cooper, C. (1988). Predicting susceptibility to short-term stress with the defence mechanism test. Work and Stress, 2, 49–58.
- Craig, K. J., Heisler, J. A., & Baum, A. (1996). Intrusive thoughts and the maintenance of chronic stress. In I. G. Sarason, G. R. Pierce, & B. R. Sarason (Eds.), Cognitive interference: Theories, methods, and findings. The LEA series in personality and clinical psychology (pp. 397–413). Mahwah, NJ: Erlbaum.
- Crary, B., Borysenko, D. C., Sutherland, D. C., Kutz, I., Borysenko, J. Z., & Benson, H. (1983). Decrease in mitogen responsiveness of mononuclear cells from peripheral blood after epinephrine administrationinhumans.JournalofImmunology,130,694–697.
- Crary, B., Hauser, S. L., Borysenko, M., Kutz, I., Hoban, C., Ault, K. A., Weiner, H. L., & Benson, H. (1983). Epinephrine-induced changes in the distribution of lymphocyte subsets in peripheral blood of humans. Journal of Immunology, 131, 1178–1181.
- Creamer, M., Burgess, P., & Pattison, P. (1992). Reaction to trauma: Acognitive processing model. Journal of Abnormal Psychology, 101, 452–459.
- Cruess, S., Antoni, M., Cruess, D., Fletcher, M. A., Ironson, G., Kumar, M., Lutgendorf, S., Hayes, A., Klimas, N., & Schneiderman, N. (2000). Reductions in herpes simplex virus type 2 antibody titers after cognitive behavioral stress management and relationships with neuroendocrine function, relaxation skills, and social support in HIV-positive men. Psychosomatic Medicine, 62, 828–837.
- Davies, A. O., & Lefkowitz, R. J. (1984). Regulation of betaadrenergic receptors by steroid hormones. Annual Review of Physiology, 46, 119–130.
- Daynes, R. A., Araneo, B. A., Hennebold, J., Enioutina, E., & Mu, H. H. (1995). Steroids as regulators of the mammalian immune response. Journal of Investigative Dermatology, 105(1 Suppl.), 14S–19S.
- de la Torre, B. (1994). Psychoendocrinologic mechanisms of life stress. Stress Medicine, 10, 107–114.
- DeGroot, K. I., Boeke, S., Bonke, B., & Passchier, J. (1997). A revaluation of the adaptiveness of avoidant and vigilant coping with surgery. Psychology and Health, 12, 711–717.
- Delahanty, D. L., Dougall, A. L., Browning, L. J., Hyman, K. B., & Baum, A. (1998). Duration of stressor and natural killer cell activity. Psychology and Health, 13, 1121–1134.
- Delahanty, D. L., Dougall, A. L., Craig, K. J., Jenkins, F. J., & Baum, A. (1997). Chronic stress and natural killer cell activity after exposure to traumatic death. Psychosomatic Medicine, 59, 467–476.
- Delahanty, D. L., Dougall, A. L., Hayward, M., Forlenza, M., Hawk, L., & Baum, A. (2000). Gender differences in cardiovascular and natural killer cell reactivity to acute stress following a hassling task. International Journal of Behavioral Medicine, 7, 19–27.
- Delahanty, D. L., Wang, T., Maravich, C., Forlenza, M., & Baum, A. (2000). Time-of-day effects on response of natural killer cells to acute stress in men and women. Health Psychology, 19, 39–45.
- Dew, M. A., & Bromet, E. J. (1993). Predictors of temporal patterns of psychiatric distress during 10 years following the nuclear accident at Three Mile Island. Social Psychiatry and Psychiatric Epidemiology, 28, 49–55.
- Dhabhar, F. S. (1998). Stress-induced enhancement of cell-mediated immunity. Annals of the New York Academy of Sciences, 840, 359–372.
- Dolan, C. A., Sherwood, A., & Light, K. C. (1992). Cognitive coping strategies and blood pressure responses to real-life stress in healthy young men. Health Psychology, 11, 233–240.
- Dorian, B., & Garfinkel, P. E. (1987). Stress, immunity, and illness: A review. Psychological Medicine, 17, 393–407.
- Dougall, A. L., Craig, K. J., & Baum, A. (1999). Assessment of characteristics of intrusive thoughts and their impact on distress among victims of traumatic events. Psychosomatic Medicine, 61, 38–48.
- Dougall, A. L., Ursano, R. J., Posluszny, D. M., Fullerton, C. S., & Baum, A. (2001). Predictors of posttraumatic stress among victims of motor vehicle accidents. Psychosomatic Medicine, 63, 402–411.
- Eisenstein, T. K., & Hilburger, M. E. (1998). Opioid modulation of immune responses: Effects on phagocytes and lymphoid cell populations. Journal of Neuroimmunology, 83, 36–44.
- Eller, L. S. (1995). Effects of two cognitive-behavioral interventions on immunity and symptoms in persons with HIV. Annals of Behavioral Medicine, 17, 339–348.
- Esterling, B. A., Kiecolt-Glaser, J. K., Bodnar, J. C., & Glaser, R. (1994). Chronic stress, social support, and persistent alterations in the natural killer cell response to cytokines in older adults. Health Psychology, 13, 291–298.
- Fava, G. A. (2000). Cognitive behavioral therapy. In G. Fink (Ed.), Encyclopedia of stress (Vol. 1, pp. 484–487). San Diego, CA: Academic Press.
- Fawzy, F. I. (1994). Immune effects of a short-term intervention for cancer patients. Advances, 10, 32–33.
- Fleishman, J. A. (1984). Personality characteristics and coping patterns. Journal of Health and Social Behavior, 25, 229–244.
- Folkman, S., & Lazarus, R. S. (1980). An analysis of coping in a middle-aged community sample. Journal of Health and Social Behavior, 21, 219–239.
- Galloway, M. T., & Jokl, P. (2000). Aging successfully: The importance of physical activity in maintaining health and function. Journal of the American Academy of Orthopaedic Surgeons, 8, 37–44.
- Ganley, R. M. (1989). Emotion and eating in obesity: A review of the literature. International Journal of Eating Disorders, 8, 343–361.
- Gerin, W., Litt, M. D., Deich, J., & Pickering, T. G. (1995). Selfefficacy as a moderator of perceived control effects on cardiovascular reactivity: Is enhanced control always beneficial? Psychosomatic Medicine, 57, 390–397.
- Glaser, R., Sheridan, J., Malarkey, W. B., MacCallum, R. C., & Kiecolt-Glaser, J. K. (2000). Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosomatic Medicine, 62, 804–807.
- Glass, D. C., & Singer, J. E. (1972). Urban stress: Experiments on noise and social stressors. New York: Academic Press.
- Greenberg, M. A. (1995). Cognitive processing of traumas: The role of intrusive thoughts and reappraisals. Journal of Applied Social Psychology, 25, 1262–1296.
- Greeno, C. G., & Wing, R. R. (1994). Stress-induced eating. Psychological Bulletin, 115, 444–464.
- Grunberg, N. E., & Baum, A. (1985). Biological commonalities of stress and substance abuse. In S. Shiffman & T. A. Wills (Eds.), Coping and substance use (pp. 25–62). Orlando, FL: Academic Press.
- Herbert, T. B., & Cohen, S. (1993). Stress and immunity in humans: A meta-analytic review. Psychosomatic Medicine, 55, 364–379.
- Herbert, T. B., Cohen, S., Marsland, A. L., Bachen, E. A., Rabin, B. S., Muldoon, M. F., & Manuck, S. B. (1994). Cardiovascular reactivity and the course of immune response to an acute psychological stressor. Psychosomatic Medicine, 56, 337–344.
- Hobfoll, S. E. (1989). Conservation of resources. American Psychologist, 44, 513–524.
- Hobfoll, S. E., & Lily, R. (1993). Resource as a strategy for community psychology. Journal of Community Psychology, 21, 128–148.
- Horowitz, M. J. (1986). Stress response syndromes (2nd ed.). New York: Jason Aronson.
- Inslicht, S. S., Hyman, K. B., Larkin, G. L., Jenkins, F., & Baum, A. (2001). Domestic violence and health risk: Chronic stress, latent virus antibodies, and health behavior [Abstract]. Psychosomatic Medicine, 63,
- Ironson, G., Wynings, C., Schneiderman, N., Baum, A., Rodriguez, M., Greenwood, D., Benight, C. C., Antoni, M., LaPerriere, A., Huang, H., Klimas, N., & Fletcher, M. A. (1997). Post traumatic stress symptoms, intrusive thoughts, loss and immune function after Hurricane Andrew. Psychosomatic Medicine, 59, 128–141.
- Jammer, L. D., & Leigh, H. (1999). Repressive/defensive coping, endogenous opioids and health: How a life so perfect can make you sick. Psychiatry Research, 85, 17–31.
- Jenkins, F. J., & Baum, A. (1995). Stress and reactivation of latent herpes simplex virus: A fusion of behavioral medicine and molecular biology. Annals of Behavioral Medicine, 17, 116–123.
- Kaloupek, D. G., & Stoupakis, T. (1985). Coping with a stressful medical procedure: Further investigation with volunteer blood donors. Journal of Behavioral Medicine, 8, 131–148.
- Kappel, M., Poulsen, T. D., Galbo, H., & Pedersen, B. K. (1998). Effects of elevated plasma noradrenaline concentration on the immune system in humans. European Journal of Applied Physiology and Occupational Physiology, 79, 93–98.
- Kausar, R., & Akram, M. (1998). Cognitive appraisal and coping of patients with terminal versus nonterminal diseases. Journal of Behavioural Sciences, 9, 13–28.
- Kiecolt-Glaser, J. K., Fisher, L. D., Ogrocki, P., Stout, J. C., Speicher, C. E., & Glaser, R. (1987). Marital quality, marital disruption, and immune function. Psychosomatic Medicine, 49(1), 13–34.
- Kiecolt-Glaser, J. K., & Glaser, R. (1987). Psychosocial influences on herpesvirus latency. In E. Kurstak, Z. J. Lipowski, & P. V. Morozov (Eds.), Viruses, immunity, and mental disorders (pp. 403–411). New York: Plenum Press.
- Kiecolt-Glaser, J. K., Glaser, R., Strain, E. C., Stout, J. C., Tarr, K. L., Holliday, J. E., & Speicher, C. E. (1986). Modulation of cellular immunity in medical students. Journal of Behavioral Medicine, 9, 5–21.
- Kiecolt-Glaser, J. K., Glaser, R., Williger, D., Stout, J., Messick, G., Sheppard, S., Ricker, D., Romisher, S. C., Briner, W., Bonnell, G., & Donnerberg, R. (1985). Psychosocial enhancement of immunocompetence in a geriatric population. Health Psychology, 4, 25–41.
- Kiecolt-Glaser, J. K., Kennedy, S., Malkoff, S., Fisher, L., Speicher, C. E., & Glaser, R. (1988). Marital discord and immunity in males. Psychosomatic Medicine, 50, 213–229.
- Kiecolt-Glaser, J. K., Marucha, P. T., Malarkey, W. B., Mercado, A. M., & Glaser, R. (1995). Slowing of wound healing by psychological stress. Lancet, 346, 1194–1196.
- King, A. C., Taylor, C. B., Albright, C. A., & Haskell, W. L. (1990). The relationship between repressive and defensive coping styles and blood pressure responses in healthy, middleaged men and women. Journal of Psychosomatic Research, 34, 461–471.
- Klein, T. W., Friedman, H., & Specter, S. (1998). Marijuana, immunity and infection. Journal of Neuroimmunology, 83, 102–115.
- Kohlman, C. W., Weidner, G., & Messina, C. R. (1996). Avoidant coping style and verbal-cardiovascular response dissociation. Psychology and Health, 11, 371–384.
- Kompier, M. A., & DiMartino, V. (1995). Review of bus drivers’ occupational stress and stress prevention. Stress Medicine, 11, 253–262.
- Krueger, G. P. (1989). Sustained work, fatigue, sleep loss and performance:Areview of the issues. Work and Stress, 3, 129–141.
- LaPerriere, A., Antoni, M. H., Schneiderman, N., Ironson, G., Klimas, N., Caralis, P., & Fletcher, M. A. (1990). Exercise intervention attenuates emotional distress and natural killer cell decrements following notification of positive serologic status for HIV-1. Biofeedback and Self-regulation, 15, 229–242.
- Lazarus, R. S. (1966). Psychological stress and the coping process. New York: McGraw-Hill.
- Lazarus, R. S. (1993). Coping theory and research: Past, present, and future. Psychosomatic Medicine, 55, 234–247.
- Lazarus, R. S., & Folkman, S. (1984). Stress, appraisal, and coping. New York: Springer.
- Leserman, J., Pettito, J. M., Golden, R. N., Gaynes, B. N., Gu, H., Perkins, D. O., Silva, S. G., Folds, J. D., & Evans, D. L. (2000). Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. American Journal of Psychiatry, 157, 1221–1228.
- Lester, N., Smart, L., & Baum, A. (1994). Measuring coping flexibility. Psychology and Health, 9, 409–424.
- Leventhal, H. (1980). Toward a comprehensive theory of emotion. Advances in Experimental Social Psychology, 13, 139–207.
- Lundberg, U. (1984). Human psychobiology in Scandinavia: II. Psychoneuroendocrinology: Human stress and coping processes. Scandinavian Journal of Psychology, 25(3), 214–226.
- Manuck, S. B., Cohen, S., Rabin, B. S., Muldoon, M. F., & Bachen, E. A. (1991). Individual differences in cellular immune response to stress. Psychological Science, 2(2), 111–115.
- Marucha, P. T., Kiecolt-Glaser, J. K., & Favagehi, M. (1998). Mucosal wound healing is impaired by examination stress. Psychosomatic Medicine, 60, 362–365.
- Mason, J. W. (1971). A re-evaluation of the concept of “nonspecificity” in stress theory. Journal of Psychiatric Research, 8, 323–333.
- McAllister-Sistilli, C. G., Cagiula, A. R., Knopf, S., Rose, C. A., Miller, A. L., & Donny, E. C. (1998). The effects of nicotine on the immune system. Psychoneuroendocrinology, 23, 175– 187.
- McEwen, B. S. (1998). Seminars in medicine of the Beth Israel Deaconess Medical Center: Protective and damaging effects of stress mediators. New England Journal of Medicine, 338, 171–179.
- McEwen, B. S., & Stellar, E. (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101.
- McKinnon, W., Weisse, C. S., Reynolds, C. P., Bowles, C. A., & Baum, A. (1989). Chronic stress, leukocyte subpopulations, and humoral response to latent viruses. Health Psychology, 8(4), 389–402.
- McNally, R. J. (1997). Implicit and explicit memory for traumarelated information in PTSD. In R. Yehuda & A. C. McFarlane (Eds.), Annals of the New York Academy of Science: Psychobiology of posttraumatic stress disorder (Vol. 821, pp. 219–224). New York: New York Academy of Sciences.
- Mellman, T. A. (1997). Psychobiology of sleep disturbances in posttraumatic stress disorder. In R. Yehuda & A. C. McFarlane (Eds.), Annals of the New York Academy of Sciences: Psychobiology of posttraumatic stress disorder (Vol. 821, pp. 142–149). New York: New York Academy of Sciences.
- Miller, G. E., & Cohen, S. (2001). Psychological interventions and the immune system: A meta-analytic review and critique. Health Psychology, 20, 47–63.
- Murphy, L. R. (1984). Stress management in highway maintenance workers. Journal of Occupational Environmental Medicine, 26, 436–442.
- Naliboff, B. D., Benton, D., Solomon, G. F., Morley, J. E., Fahey, J. L., Bloom, E. T., Makinodan, T., & Gilmore, S. L. (1991). Immunological changes in young and old adults during brief laboratory stress. Psychosomatic Medicine, 53, 121–132.
- O’Leary, A. (1990). Stress, emotion, and human immune function. Psychological Bulletin, 108, 363–382.
- Osowiecki, D. M., & Compas, B. E. (1999). A prospective study of coping, perceived control, and psychological adaptation to breast cancer. Cognitive Therapy and Research, 23, 169–180.
- Paavonen, T. (1994). Hormonal regulation of immune responses. Annals of Medicine, 26, 255–258.
- Pike, J. L., Smith, T. L., Hauger, R. L., Nicassio, P. M., Patterson, T. L., McClintick, J., Costlow, C., & Irwin, M. R. (1997). Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosomatic Medicine, 59, 447–457.
- Pilette, W. L. (1983). Magical thinking by inpatient staff members. Psychiatric Quarterly, 55, 272–274.
- Rahe, R. H., & Arthur, R. J. (1978). Life change and illness studies: Past history and future directions. Journal of Human Stress, 4, 3–15.
- Redeker, N. S. (1992). The relationship between uncertainty and coping after coronary bypass surgery. Western Journal of Nursing Research, 14, 48–68.
- Rosenbloom, C. A., & Whittington, F. J. (1993). The effects of bereavement on eating behaviors and nutrient intakes in elderly widowed persons. Journal of Gerontology, 48, S223–S229.
- Sadeh, A. (1996). Stress, trauma, and sleep in children. Child and Adolescent Psychiatric Clinics of North America, 5, 685–700.
- Schedlowski, M., Jacobs, R., Stratmann, G., Richter, S., Hädicke, A., Tewes, U., Wagner, T. O. F., & Schmidt, R. E. (1993). Changes of natural killer cells during acute psychological stress. Journal of Clinical Immunology, 13(2), 119–126.
- Schuurs, A. H. W. M., & Verheul, H. A. M. (1990). Effects of gender and sex steroids on the immune response. Journal of Steroid Biochemistry, 35, 157–172.
- Segerstrom, S. C., Solomon, G. F., Kemeny, M. E., & Fahey, J. L. (1998). Relationship of worry to immune sequelae of the Northridge earthquake. Journal of Behavioral Medicine, 21, 433–450.
- Selye, H. (1936). A syndrome produced by diverse nocuous agents. Nature, 148, 84–85.
- Selye, H. (1984). The stress of life (Rev. ed.). New York: McGrawHill. (Original work published 1956)
- Sieber, W. J., Rodin, J., Larson, L., Ortega, S., Cummings, N., Levy, S., Whiteside, T., & Herberman, R. (1992). Modulation of human natural killer cell activity by exposure to uncontrollable stress. Brain, Behavior, and Immunity, 6, 141–156.
- Solomon, G. F., Segerstrom, S. C., Grohr, P., Kemeny, M., & Fahey, J. (1997). Shaking up immunity: Psychological and immunological changes after a natural disaster. Psychosomatic Medicine, 59, 114–127.
- Sondergaard, S. R., Ullum, H., Skinhoj, P., & Pedersen, B. K. (1999). Epinephrine-induced mobilization of natural killer (NK) cells and NK-like T cells in HIV-infected patients. Cellular Immunology, 197, 91–98.
- Spaccarelli, S., Bowden, B., Coatsworth, J. D., & Kim, S. (1997). Psychosocial correlates of male sexual aggression in a chronic delinquent sample. Criminal Justice and Behavior, 24, 71–95.
- Stefanski, V., & Ben-Eliyahu, S. (1996). Social confrontation and tumor metastasis in rats: Defeat and beta-adrenergic mechanisms. Physiology and Behavior, 60, 277–282.
- Steptoe, A. (1989). Coping and psychophysiological reactions. Advances in Behaviour Research and Therapy, 11, 259–270.
- Stone, A. A., Bovbjerg, D. H., Neale, J. M., Napoli, A., Valdimarsdottir, H., Cox, D., Hayden, F. G., & Gawltney, J. M. (1992). Development of common cold symptoms following experimental rhinovirus infection is related to prior stressful life events. Behavioral Medicine, 18, 115–120.
- Szafarczyck, A., Malaval, F., Laurent, Á., Gibaud, R., & Assenmacher, I. (1987). Further evidence for a central stimulatory action of catecholamines on ACTH release in the rat. Endocrinology, 121, 883–892.
- Taylor, D. N. (1995). Effects of a behavioral stress-management program on anxiety, mood, self-esteem, and T-cell count in HIVpositive men. Psychological Report, 76, 451–457.
- Thoits, P. A. (1986). Social support as coping assistance. Journal of Consulting and Clinical Psychology, 54, 416–423.
- Uchino, B. N., Cacioppo, J. T., & Kiecolt-Glaser, J. K. (1996). The relationship between social support and physiological processes: Areview with emphasis on underlying mechanisms and implications for health. Psychological Bulletin, 119, 488–531.
- Ursano, R. J., Fullerton, C. S., Kao, T., & Bhartiya, V. R. (1995). Longitudinal assessment of posttraumatic stress disorder and depression after exposure to traumatic death. Journal of Nervous and Mental Disease, 183, 36–42.
- Vitaliano, P. P., Maiuro, R. D., Russo, J., & Becker, J. (1987). Raw versus relative scores in the assessment of coping strategies. Journal of Behavioral Medicine, 10, 1–18.
- Watson, R. R., Eskelson, C., & Hartmann, B. R. (1984). Severe alcohol abuse and cellular immune functions. Arizona Medicine, 41, 665–668.
- Weiner, H. (1992). Perturbing the organism: The biology of stressful experience. Chicago: University of Chicago Press.
- Willis, L., Thomas, P., Garry, P. J., & Goodwin, J. S. (1987). A prospective study of response to stressful life events in initially healthy elders. Journal of Gerontology, 42, 627–630.
- Zakowski, S. G., Cohen, L., Hall, M. H., Wollman, K., & Baum, A. (1994). Differential effects of active and passive laboratory stressors on immune function in healthy men. International Journal of Behavioral Medicine, 1(2), 163–184.




