View sample stress and emotion in early childhood research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Stress is a fact of life. Even before birth, successful adaptation requires responding to stressors and regulating stress reactions. What causes us to react and how we regulate stress change during development and differ among individuals. These differences affect our physical and emotional health and determine whether we experience events as threats or challenges. In this research paper we adopt a developmental psychobiological approach to the study of stress in early development. Further, we explore the intimate, but not isomorphic, relations among emotions, temperament, and stress.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Developmental psychobiologists approach the study of stress from a systems perspective (e.g., Gottlieb, Whalsten, & Lickliter, 1998). (Note that to reduce the overall length of the paper, the number of citations had be limited. Whenever possible we have cited review papers rather than original studies. We hope that the interested reader is able to use these reviews to find the original studies supporting the points made in this research paper.) This perspective, with its roots in epigenetic approaches to comparative psychology (e.g., Kuo, 1976), is shared by many developmental frameworks (e.g., Lerner, 1986; Sameroff, 1983). Accordingly, the stress system is viewed as hierarchically organized into reciprocally influencing systems and subsystems. Whereas understanding organization on one level requires understanding the roles played by systems at lower levels of organization, reductionistic explanations are viewed as misleading. Plasticity is seen as an inherent characteristic of living systems; nonetheless, with development, plasticity is expected to narrow. Understanding the boundaries of plasticity and recognizing the processes involved in narrowing the range of likely adaptations as development proceeds are central to research on the developmental psychobiology of stress.
The developmental systems perspective is overwhelmingly complex. Coherence of a sort is achieved by reference to several critical propositions. First, development proceeds through activity-dependent processes. At all levels of the organism, the critical question is how that activity shapes future responses to, creation of, and selection of experiences. Second, activity involves not only responses, but also regulation of responses; thus, no reaction of the organism can be understood without an equal focus on how the reaction is regulated. Finally, the systems that regulate development do not stop at the skin, but extend into the social contexts that are essential for the survival of the developing young.
The developmental psychobiology of stress is eclectic. Because the neural systems underlying emotions and emotionality influence the activation and regulation of behavioral and physiological responses to stressors, developmental psychobiological research on stress is intimately related to neuroscience research on emotions and temperament. Theory and research in these domains, however, are not always consistent with a developmental systems approach. Temperament theorists, for example, often adopt main effect rather than transactional models in studies of the development of temperament (Kagan, 1994), whereas neuroscience research is often overly reductionist (see discussion by West & King, 2001). Nevertheless, the emphasis on neural plasticity in neuroscience (e.g., Hann, Huffman, Lederhendler, & Meinecke, 1998) and psychobiological models of temperament (Rothbart, Derryberry, & Posner, 1994) provides bridges from these research domains to developmental psychobiological research on stress.
In this research paper we review what is known about the development of activity and regulation of the two arms of the stress system, the limbic-hypothalamic-pituitaryadrenocortical (L-HPA) and brain-stem norepinephrine/ sympathetic-adrenomedullary (NE-SAM) systems. We begin with an overview of the neurobiology of the L-HPA system and the autonomic nervous system, emphasizing the SAM system. Next we describe limbic and cortical circuits involved in the ability to anticipate threat and engage in preparatory responses and the way these circuits modulate and may be modulated by the L-HPAand SAM systems. This is followed by a discussion of what is known about the ontogeny of these systems and of the way individual differences in the development of reactivity and regulation of these systems may be related to temperament and caregiving. We conclude with some thoughts about the need for basic research examining the development of stress systems in order to better our understanding of the origins of individual differences in stress reactivity and regulation. We begin, however, with a general discussion of the concept of stress as it is used in the psychobiological literature.
The Psychobiology of Stress
Stress is difficult to define. Like the terms motivation and emotion, periodically there are calls to strike stress from the scientific lexicon (e.g., Engle, 1985). Stress variously refers to objective events (stressors), subjective psychological states (being stressed), and physiological responses (e.g., increases in cortisol). Following Selye (1975), in this research paper we refer to the events that precipitate stress reactions as stressors and the responses to those events as stress reactions. Events that have the potential to stimulate stress responses are not stressors for all individuals or at all ages. Intraindividual processes mediate the effect of the event on the response (e.g., Frankenhaeuser, 1979). Stress results when the demands of internal or external events exceed immediately available resources. These demands may be physiological, including being overheated, chilled, and so on. They may also be psychological, including perceived threat, failure of expectation, and social rejection. Such conditions threaten well-being and require a shifting of metabolic resources to fuel the processes needed for self-protection. This shift in metabolic resources favors systems involved in immediate survival and threat-related learning processes. When intense or prolonged, this metabolic shift limits activity in systems performing functions that are future oriented, including functions directed at growth and repair. Shifting resources to maintain organism viability is termed allostasis or stability through change (McEwen, 1998). The capacity to respond to stress through allostatic adjustments is necessary for survival. Increasing evidence suggests that when stress responses are limited or acute, they tend to enhance functioning. However, these adjustments have costs that, if frequent or prolonged, may undermine health and development. Thus, as important as activation is in understanding the psychobiology of stress, an understanding of the processes that regulate stress reactions is critical.
Two systems orchestrate stress responses in mammals: the L-HPA and the NE-SAM systems (Johnson, Kamilaris, Chrousos, & Gold, 1992). These systems interact in complex ways at all levels of their organization. In the early 1900s Cannon (1936) argued that the SAM system was responsible for coordinating the physiological and behavioral responses necessary to meet external challenges to the constancy of the internal milieu. Building on Bernard’s theory that organisms have evolved complex adaptive mechanisms to stabilize their internal states, Cannon proposed the concept of fight/flight to describe the behavioral functions of the SAM system. Later, when Selye (e.g., 1975) presented his theory of the general adaptation syndrome, attention shifted from the SAM to the L-HPAsystem. Both Cannon and Selye recognized that thoughts and emotions could produce increases in sympathetic and adrenocortical activity even when there were no physical threats to homeostasis. However, it was not until researchers understood that activity of the pituitary gland was under the regulation of hypothalamic releasing and inhibiting factors that the outlines of our current understanding of stress and its relations to the neurobiology of emotion and cognition began to be discerned. It is now well recognized that the SAM and L-HPA system are regulated in part by forebrain structures and pathways, including regions in the prefrontal cortex (Johnson et al., 1992). As in all areas of neuroscience, most of what we know is based on animal research and, when conducted in humans, generally involves adults. Thus, caution is necessary in extrapolating the information presented here to human infants and children.
Contemporary formulations of stress describe a loosely integrated system consisting of neuroanatomical and functional subsystems. Below the neck, stress biology centers on the regulation of glucocorticoids or CORT (cortisol in primates, corticosterone in rodents) and catecholamines, primarily norepinephrine and epinephrine (NE and EPI) (e.g., Johnson et al., 1992). In the periphery, CORT and catecholamines operate to increase the energy available for action through inhibiting glucose uptake into storage sites and liberating energy from fat and protein stores. Concurrently, they stimulate increases in cardiovascular and pulmonary function to support the increased motor activity needed in times of challenge. Finally, in concert with central components of the stress system, they function to modulate the biology of growth and repair, including digestion, physical growth, immune function, and reproduction. In the brain, the stress system is orchestrated through reciprocal interactions among NE and hypothalamic and extra-hypothalamic corticotropinreleasing hormone (CRH).
Levels of the stress system mature and become organized overthecourseofdevelopment.Inhumans,thehypothalamicbrain-stem level develops largely during the prenatal period. Development and integration of limbic and hypothalamicbrain-stem circuits likely occur over the course of infancy (Vazquez,1998).Thefrontalcortexisalsoinvolvedintheregulation of limbic and hypothalamic nuclei. The long period of development of the frontal cortex that extends into adolescence (Huttenlocher, 1994) likely means that a protracted period of development of stress reactivity and regulation in humans exists. A prolonged period of postnatal development of the stress system also suggests that postnatal experience may play critical and multiple roles in emerging individual differences in stress reactivity and regulation (e.g., Heim, Owen, Plotsky, & Nemeroff, 1997). Next we describe each level of the stress system in more detail.
The Limbic-Hypothalamic-Pituitary-Adrenocortical System
The L-HPA system orchestrates mammalian stress biology through the activity of CRH (e.g., Nemeroff, 1996). CRH is a neuroactive peptide produced in the hypothalamus and in extra-hypothalamic sites. In the hypothalamus its production begins the cascade of events that culminates in increased production of CORT by the adrenal glands. Along with several other secretagogues, CRH regulates the production of adrenocorticotropic hormone (ACTH) by the anterior pituitary (for review, see Palkovits, 1987). Released into general circulation, ACTH binds to receptors on adrenocortical cells in the cortex of the adrenal glands and stimulates the biosynthesis and release of CORT into general circulation. Negative feedback regulates L-HPA activation and CORT production. Current evidence suggests that negative feedback is a widely distributed system involving CORT receptors in, but not limited to, the prefrontal cortex, hypothalamus, hippocampus, and the anterior pituitary gland (e.g., de Kloet, Vreugdenhil, Oitzl, & Joels, 1998; Sanchez, Young, Plotsky, & Insel, 2000).
CRH-producing cells in the hypothalamus receive input from other limbic, hypothalamic, and brain-stem nuclei. As discussed later, NE is a major stimulus of CRH activity in response to psychological stressors. However, multiple neurotransmitter and neuropeptide systems, beyond the NE system, are involved in regulating CRH (Palkovits, 1987). Furthermore, hypothalamic CRH-producing cells also receive input from other nuclei in the hypothalamus, particularly those involved in daily energy flow (Dallman et al., 1993). The net result is that the production of cortisol is not a direct reflection of the individual’s emotional state. Rather, it reflects the extent to which signals impinging on the hypothalamus from all sources indicate that extraordinary resources can and must be expended in order to meet the demands of the moment.
Balancing internal and external demands is reflected not only in CRH activity at the level of the hypothalamus, but also in CRH activity at extra-hypothalamic sites (Nemeroff, 1996). CRH is produced in many brain structures that are involved in associating fear and anxiety with activation of the stress system, including the amygdala and prefrontal cortex. In addition, one subtype of the CRH receptor, CRH1, appears specifically to mediate fear-related functions, whereas increasing evidence suggests that CRH2 receptors are more involved in anxiety states (Steckler & Holsboer, 1999). The neuroanatomy of the CRH system has lead to the (likely overly simplistic) view of CRH as the central orchestrator of the stress system, both in terms of endocrine and behavioral responses.
CORT has figured prominently in research on the health consequences of chronic stress. One common fallacy about the L-HPA system is that CORT is necessarily bad for one’s health and development. In fact, the relationship between CORT and healthy adaptation is an inverted-U function. Although it appears that chronic or frequent high CORT can be detrimental, it is equally apparent that insufficient CORT has negative consequences (McEwen, 1998). One hypothesis is that the basis for this inverted-U function lies in the two receptors for CORT, termed mineralocorticoid receptors (MR) and glucocorticoid receptors (GR), and the different functions they mediate (de Kloet et al., 1998). According to this hypothesis, MRs primarily mediate processes that sustain and promote mental and physical health, whereas GRs mediate effects that shunt metabolic resources from growth and repair to catabolic activities needed to manage immediate threats. MRs tend to be occupied when CORT levels are in the basal range. GRs become occupied as CORT levels rise in response to stressors. As GRs become occupied, CRH activity in the hypothalamus is restrained and the stress response is terminated. Activation of this system and activation of GRs is normal and probably has beneficial effects. However, when GRs are occupied chronically, GR-mediated biochemical events can threaten neuronal viability and downregulate or reduce the GRs available to terminate the stress response, leading to an increase in CORT production. Thus, frequent or prolonged elevations in CORT have been postulated to be one cause of subsequent heightened and prolonged CORT elevations following trauma or chronic adversity. Importantly, early experiences in rodents shape the MR and GR receptor systems (e.g., Caldji et al., 1998; Levine, 1994). Conditions associated with adequate maternal care result in increased MR/GR ratios that allow better containment of the stress response and promotive effects associated with MR occupation to be produced across a wider range of CORT production. Histories of inadequate nurturance result in the opposite pattern of decreased MR/GR ratios.
Autonomic Regulation
Although the L-HPA system now figures prominently in research on stress, the older focus on the SAM system has not been lost (see review by Johnson et al., 1992). Consider the catecholamines EPI and NE. EPI is produced by the adrenal medulla and then released into general circulation. EPI acts as a stress hormone, whereas NE produced at synapses is a neurotransmitter. Both EPI and NE act to energize and mobilize the organism for action. Neurons of the hypothalamus and other cell groups within the brain stem are the central coordinators of the sympathetic nervous system (SNS). In the brain, NE-producing neurons originating in the locus coeruleus (LC) project widely throughout the cortex. Although the LC has often been considered a component of central autonomic control, there is little evidence to support this view. LC projections seem to be involved in arousal (Saper, 1995). In addition, LC neurons project to the CRHproducing cells in the hypothalamus, serving as a primary stimulus of increased CRH production and sensitization in response to emotional stressors. In a parallel but independent system, CRH-producing neurons in the amygdala project to the LC, bringing activity of the LC under the regulation of extra-hypothalamic CRH. The central nucleus of the amygdala also stimulates activity of the SAM system via projections to the lateral hypothalamus and brain-stem autonomic nuclei. Although the SAM system has long been associated with stress, its activity is not specific to threatening or aversive events. Instead, because of the role of the sympathetic system in supporting rapid energy mobilization, its activity tends to track conditions requiring effort and information processing more generally, rather than those involving distress and uncertainty about outcomes more specifically (e.g., Frankenhaeuser, 1979). Despite this, frequent mobilization of the sympathetic system, particularly in the presence of elevated CORT, can threaten physical health.
The SAM system forms one arm of the autonomic nervous system (ANS). The other arm of this system is the parasympathetic nervous system (PNS). Unlike the SAM system, which is sometimes referred to as a diffuse or mass-discharge system, the PNS tends to be more fine-tuned, having discrete effects on the organ systems that it innervates (Hugdahl, 1995). Similar to the health-promotive effects of MRs for the L-HPA system, the PNS primarily promotes anabolic activities concerned with the conservation and restoration of energy (Porges, 1995a, 1995b). The presence of PNS terminals on most organs and tissues innervated by the SAM system allows the PNS to serve as a major regulator of sympathetic effects. Furthermore, although both the PNS and SAM systems have been viewed as efferent systems that carry out work dictated by the brain, both systems also have afferent projections to the brain. These afferent projections not only inform the brain about the status of organs and tissues in the periphery but also allow autonomic regulation of the central nervous system.
Parasympathetic neuronal projections leave the brain through several cranial nerves including the 10th cranial, or vagus nerve, which has been the focus of most of the psychophysiological research relating activity of the PNS to stress and emotion (Porges, 1995a, 1995b). In the following description we draw heavily from Porges’s work, which has stimulated much of the developmental work on emotion and stress (see also the review by Beauchaine, in press). The primary fibers of the vagus nerve originate in two nuclei in the medulla: the dorsal motor nucleus of the vagus (DMNX), which regulates visceral functions, and the nucleus ambiguus (NA), which regulates functions associated with communication and emotion. In addition, a third medullary nucleus, the nucleus tractus solitarius (NTS) receives many of the afferent projections traveling through the vagus from peripheral organs. In his polyvagal theory, Porges (1995a) argued that this trinity of nuclei forms the central regulatory component of the vagal system. Efferent projections from the NA, the smart vagus (Vna), are the principal vagal component in vagal cardiac and bronchomotor regulation. The intimate associations between Vna and facial and vocal expressions of emotion, in combination with afferent projections through the NTS, provide pathways through which emotion regulation may contribute to stress regulation, and vice versa. Though still speculative, this polyvagal theory offers a number of insights into the potential role of the PNS in regulating stress biology (Porges, 1995b). Specifically, high-baseline Vna should increase the individual’s ability to cope effectively with stress by permitting the lifting of what Porges termed the vagal break, allowing rapid increases in sympathetic activity to shift metabolic resources quickly in response to challenge. In addition, feedback to the NTS via afferent projections of the vagal system should stimulate CNS containment of both the L-HPA and SAM system reactivity.
Limbic Regulation
The physiology of stress can be activated and regulated with little or no input from limbic or cortical centers. Limbiccortical involvement provides the opportunity to anticipate threats to homeostasis before they are actualized, allowing for preparatory, defensive responses. Integration of corticolimbic with hypothalamic-brain-stem stress systems also means that feedback and afferent projections of the L-HPA, NE-SAM, and vagal systems influence cognitive-emotional behavior. All attempts to describe the neurobiology of emotion and stress trace their history to work by Papez as elaborated by MacLean (1952). Accordingly, emotions involve the integration of neural structures that include hypothalamic and brain-stem nuclei, along with structures such as the amygdala, hippocampus, cingulate gyrus, and orbitofrontal cortex (see Figure 5.1).
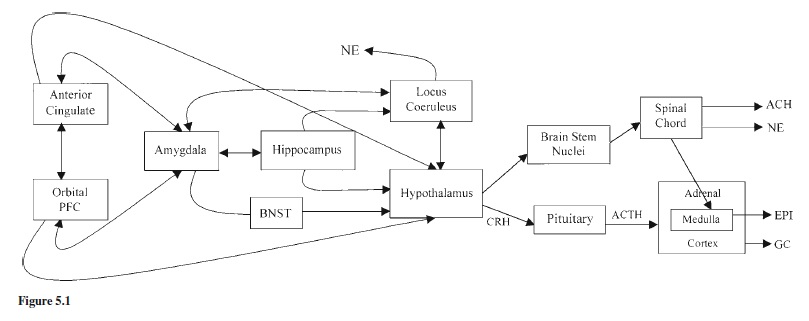
The amygdala has long been known to mediate adrenocortical responses to psychosocial stressors (Palkovits, 1987). Its role in negative emotion and conditioned fear is also now well established (for review, see Rosen & Schulkin, 1998). The amygdala and the bed nucleus of the stria terminalis (BNST) form the core structures in current views of the neurobiology of fear, anxiety, and emotional activation of the stress system. The amygdala is comprised of multiple nuclei that are richly interconnected with other parts of the brain. The central nucleus of the amygdala (CEA) has widespread influence over the L-HPA, NE-SAM, and vagal systems via amygdalofugal and stria terminalis pathways. Lesions of the amygdala and surrounding cortex in adult animals prevent elevations in stress hormones to psychological stressors such as physical restraint but do not prevent elevations to physical stressors such as illness or surgery. Such lesions also affect negative emotionality and impair fear conditioning. Although some have speculated that the CEA is involved in anxiety (e.g., with regard to behavioral inhibition, see Kagan, 1994), the role of the CEA in anxiety has recently been questioned. Indeed, Davis has argued that the BNST is more centrally involved in regulating anxious affectivity (for discussion, see Rosen & Schulkin, 1998). Nonetheless, although controversy exists regarding the roles of the CEA and BNST in the regulation of fear versus anxiety, both structures and their circuits are involved in the regulation of L-HPA and SAM system responses to events that elicit negative emotionality.
Current views hold that the threshold for activating the CEA and BNST is regulated by extra-hypothalamic CRH. Similar to stimulation of the CEA, microinfusions of CRH into the CEA produce fear behaviors in primates (reviewed by Rosen & Schulkin, 1998). The fear-inducing effects of CRH are mediated by CRH1 receptors, and experiences that increase fearful reactions to events also tend to increase CRH1 receptors in these regions (for review, see Steckler & Holsboer, 1999). There is also increasing evidence that CRF2 receptors may be involved in regulating anxiety and related states. These facts would seem to argue for a close coupling between fear/anxiety and elevations in CORT. As reflected in syndromes such as posttraumatic stress disorder (PTSD), however, this is not always the case. Whereas elevated NE and EPI have been described in PTSD, remarkably, basal cortisol levels are normal or even suppressed and the L-HPA response to stressors is often dampened although levels of CRH are increased (see review by Yehuda, 1998). Nevertheless, emotion-modulated startle responses, which are believed to reflect responsivity of the CEA and BNST, are increased in animal models of PTSD and are further enhanced by infusions of CRH especially in the presence of high CORT (see review by Rosen & Schulkin, 1998). Odd as it may seem, the limbic CRH and hypothalamic CRH systems appear only loosely coupled. It is not uncommon to find dissociations between these levels of the CRH system and, consequently, between activity of the L-HPA and NE-SAM systems. There is some suggestion that these dissociations may be the result of prolonged elevations in CORT (e.g., Rosen & Schulkin, 1998). In animal models, prolonged CORT elevations produce increased activity of CRHproducing cells in the CEA but decreased activity of similar cells in the hypothalamus. Adrenalectomy (i.e., eliminating CORT) has the opposite effect. Dissociations of this sort may contribute to the development of anxiety disorders (see also Cameron & Nesse, 1988).
Frontal Regulation
Frontal regulation of the limbic, hypothalamic, and brain-stem circuits involved in stress and emotion is a comparatively new frontier in stress research. Although it has long been recognized that the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) play critical roles in regulating emotional behavior (e.g., MacLean, 1952), their roles in regulating activity of the L-HPA and autonomic systems are increasingly appreciated. Indeed, the degree and breadth of interconnectivity between the amygdala and frontal cortex in primates have been one of the surprising findings of the last two decades (Emery & Amaral, 2000). Perhaps especially in primates, the frontal cortex appears to play a central role in stress reactivity and regulation. In this section we briefly describe OFC and ACC regulation of the stress system. Then we broaden the discussion to current views of the roles played by analytic reasoning and positive affectivity.
The OFC and medial cortex have numerous reciprocal connections to the amygdala and other limbic regions (Price, 1999). These connections support the integration of sensory and affective signals, allowing the organization of behavior in relation to reward and punishment. They are also critically important in organizing and modulating behavior so that it is appropriate to the social context. It has been hypothesized that the OFC and its connections to the amygdala and other limbic regions help to mediate attachment effects on stress reactivity and regulation (Schore, 1996). This argument is supported by evidence that the OFC and medial prefrontal regions have connections with hypothalamic and brain-stem regions that regulate behavioral, neuroendocrine, and autonomic stress responses. Thus, activity in this region may be important in modulating autonomic and neuroendocrine stress responses.
Technically, the ACC is part of the limbic system. However, it has both cortical and limbic functions and serves, in many ways, to balance activity in the prefrontal regions of the brain with activity in the limbic-hypothalamic areas. The ACC long has been associated with emotion. Most critical to this review, dysregulation of autonomic and neuroendocrine stress reactions are produced by lesions of the ACC (e.g., Diorio, Viau, & Meaney, 1993). The ACC also subserves cognition. It has been hypothesized that the cognitive and emotion functions of the ACC involve two subdivisions, a dorsal cognitive and rostral-ventral affective division (Bush, Luu, & Posner, 2000). According to this perspective, the cognitive division is considered part of the anterior attention network, a distributed attentional network that contributes to executive functioning. The emotional division, on the other hand, is connected to the OFC and medial prefrontal cortex, to the amygdala, and to hypothalamic and brain-stem regions involved in the regulation of stress physiology (e.g., Price, 1999).
Posner and Rothbart (2000) argued that the anterior attention network forms the basis of the effortful control dimension of temperament. Effortful control is believed to contribute importantly to the regulation of social and emotional behavior, particularly when effortful inhibition of actions and emotion are required. Recent evidence that the cognitive and emotional subdivisions of the ACC reciprocally regulate each other may provide one mechanism whereby effortful control exerts inhibitory effects on negative affect and stress physiology (Drevets & Raichle, 1998).
Increases in the size and functional connectivity of the ACC with development may also help explain children’s increasing ability to use cognitive coping strategies to regulate emotion, behavior, and stress (e.g., Rothbart, Derryberry, et al., 1994; Wilson & Gottman, 1996).
In addition, affect influences activity of the cognitive and affective subdivisions of the ACC. Positive emotion has been shown to support the cognitive ACC and enhance executive functioning (Ashby, Isen, & Turken, 1999), whereas negative emotion has been shown to decrease activity in the cognitive division (Bush et al., 2000). Thus, conditions that produce anger, fear, and other strong negative affects, if intense, may disrupt children’s effortful regulation of their behavior and make it difficult for them to engage in tasks requiring executive function. This ability to dampen negative affects and/or reassert more positive affective states may be critical in regulating stress. Some individuals seem to be able to do this better than others. As discussed in the next section, individual differences may partly reflect asymmetry in neural activity in the prefrontal cortex.
Emotional activity in the prefrontal cortex appears to be lateralized, with activity (for review, see Davidson, 1994; Davidson & Slagter, 2000) in the right prefrontal cortex supporting negative affectivity, while activity in the left supports positive affectivity. It is interesting to note that baseline asymmetry predicts susceptibility to negative and positive emotion-eliciting stimuli and may index the extent of prefrontal-cortex inhibition of limbic-hypothalamic stress circuits. Specifically, greater activity in the right prefrontal cortex may result in disinhibition of the stress system, whereas greater activity in the left prefrontal cortex may help contain and terminate stress reactions. It is not yet clear how this laterality is related to the functioning of specific frontal structures involved in the regulation of the stress response. Nonetheless, the focus on right-frontal asymmetry is consistent with evidence that there is a right bias in the reactive components of the stress system. In rodents there is evidence that the right, not the left, medial frontal cortex mediates neuroendocrine and autonomic responsivity to stressors (Sullivan & Grafton, 1999). Similarly, both sympathetic (Kagan, 1994) and parasympathetic regulation of the heart show a right bias (Porges, 1995a). Hyperactivity in the right frontal regions, then, may reflect a bias not only to negative emotions but also to hyperactivation of the stress system.
Although most of the attention has been on negative emotionality, recently there has been increased attention on positive emotions in stress regulation. Positive affectivity has been associated with problem-focused coping (Folkman & Moskowitz, 2000), perhaps because it supports the engagementofthecognitiveACCandexecutivefunctions.Similarly, positive affectivity as reflected in greater left than right frontal activity has been associated with self-reported pBibliography: for approach-oriented coping strategies (Davidson & Irwin, 1999). This is consistent with Davidson’s argument that lateralization of emotion in the frontal lobes reflects differential motor biases, with negative emotions organized to support withdrawal and freezing and positive emotions organized to support approach. Greater left than right frontal activity has also been associated with more rapid termination of CEAgenerated fear reactions. Davidson and colleagues have suggested that a left-sided bias in the emotion system may allow individuals to experience negative emotions and produce stress reactions to threat, but then to dampen these responses rapidly once the threat has been removed.
Summary
The physiology of stress and emotions is complex. While we are beginning to develop a much richer understanding of the neurobiological bases of both emotions and stress, most of the work has yet to be conducted with humans. Furthermore, we know the least about infants and young children. Information about neurobiology, however, can serve as a guide in our attempts to construct a psychobiological account of the development of stress and emotion in early childhood. In addition, the information we are accumulating on young children—when inconsistent with models based on adults or animals—can challenge researchers in neuroscience to provide explanations that are more congruent with the human developmental data. We turn now to what we know about stress and emotions in early human development.
Psychobiological Studies of Stress and Emotion in Children
Psychobiological studies of stress in human infants and children are relatively new. Until the early 1980s, researchers in human development were largely limited to examining heart rate–behavior associations. Only a handful of child studies assessing CORT and catecholamines existed (see review by Gunnar, 1986). After 1980, research on the psychobiology of stress in children burgeoned as the result of the availability of salivary assays for cortisol and theoretical advances in psychophysiology (e.g., Berntson, Cacioppo, & Quigley, 1993; Davidson, 1994; Kirschbaum & Hellhammer, 1989; Porges, 1995a). These technical and theoretical advances corresponded to a heightened interest in the physiological basis of temperament (e.g., Kagan, 1994, 2001). We cover the temperament research later when we discuss individual differences. First we describe what is known at this point about the ontogeny of stress reactivity and regulation in infancy and early childhood.
Developmental Periods of Stress Reactivity and Regulation
Prenatal Origins
The ontogeny of human stress reactivity and regulation begins well before birth. By 18 to 20 weeks gestation, increases in NE and CORT are observed to invasive surgical procedures (e.g., Giannakoulpoulous, Teixeira, Fisk, & Glover, 1999; Giannakoulpoulous, Sepulveda, Kourtis, Glover, & Fisk, 1994). With increased gestational age, basal levels of cortisol and ACTH rise (Economides, Nicolaides, Linton, Perry, & Chard, 1988; Murphy, 1982), and heart rate decreases but becomes more variable and coupled with fetal movement (e.g., DiPietro, Costigan, Shupe, Pressman, & Johnson, 1999). By the latter part of gestation individual differences in fetal movement and heart rate show modest stability and predict maternal reports of infant temperament during the early postnatal months (e.g., DiPietro, Hodgson, Costigan, Hilton, & Johnson, 1996). In general, active fetuses and those with higher heart rates associated with fetal motor movement are described as more difficult, less predictable, and more physically active in early infancy.
Experience begins to shape the infant’s stress system before birth. In animal models where maternal stress can be manipulated experimentally, a wide range of environmental (e.g., loud noises) and psychosocial (e.g., entry into new social groups) stressors during pregnancy result in offspring that are more behaviorally and physiologically stress reactive (e.g., Weinstock, 1997). Activity of the maternal L-HPA axis appears to be a mediating factor because controlling maternal CORT levels during these stressors reduces the influence of maternal stress during pregnancy on the offspring’s development (e.g., Barbazanges, Piazza, Moal, & Maccari, 1996). One pathway through which maternal CORT may influence the fetus’s developing stress system is via effects on placental CRH production (Wadhwa, Garite, & Sandman, 2001). During gestation, the placenta produces a large number of hormones and peptides, including CRH, that maintain the integrity of the fetal-maternal placental unit. As the placenta enlarges during pregnancy, CRH levels increase. Placental CRH binding protein, a molecule that traps CRH, and the anticortisol effects of rising estrogen levels protect both the mother and fetus from activation by stress hormones. CRH binding protein during early pregnancy and the latter part of the third trimester stimulates fetal L-HPA maturation and contributes to the initiation of labor and delivery. Nonetheless, whereas the CRH molecule is necessary for healthy development of the fetus, it also may provide a mechanism through which maternal stress can influence the development of the infant’s stress system.
Proving that maternal stress influences fetal development in humans is hampered by our inability to perform controlled experiments. Nevertheless, evidence now exists that maternal perceptions of high stress and low social support during pregnancy are correlated with higher maternal ACTH and CORT levels, higher maternal CRH levels (which are of placental origin), fetuses with higher and less variable heart rates, and newborns delivered earlier with lower birth weights (e.g., Huizink, de Medina, Mulder, Visser, & Buitelaar, 2000; DiPietro et al., 1996; Wadhwa et al., 2001). Lower versus higher socioeconomic status is also associated with many of these same effects (e.g., DiPietro et al., 1999).
As yet, there are very few prospective studies in humans of the relations between maternal stress during pregnancy and postnatal measures of infant behavior and stress system activity. However, in contrast to one early study that failed to find any association between maternal L-HPA activity and infant temperament (Vaughn, Bradley, Joffe, Seifer, & Barglow, 1987), several recent studies have yielded positive findings (Huizink et al., 2000; Wadhwa et al., 2001). In these latter studies, controlling for a variety of obstetric and psychosocial risk factors, higher maternal CORT and ACTH levels during pregnancy were associated with maternal reports and observational measures of infant negative emotional reactivity and nonadaptability.
Although still preliminary, these studies suggest a transactional view of the fetal origins of infant stress reactivity and regulation. The placenta, which is of fetal origin, expresses genes that both influence and are influenced by maternal hormone levels. Maternal stress hormone levels, in turn, are influenced by obstetric factors and by the mother’s reactions to the challenges of her daily life. Impinging on the fetus, these influences may affect the activity of the developing stress system and contribute to the organization of postnatal temperament. Undoubtedly, this is a vast oversimplification of the complex interweaving of organismic and environmental processes that shape the developing stress system prior to birth. Furthermore, birth is not the endpoint of these shaping processes.
Early Postnatal Development
Although it was once thought that the neonatal L-HPA axis was hyporesponsive at birth, this is not the case (for review, see Gunnar, 1992). The newborn displays graded behavioral, endocrine, and autonomic responses to aversive medical procedures. Furthermore, the healthy newborn is remarkably capable of regulating stress. Stressors such as heel-stick blood draws, circumcision, and physical exams produce increases in heart rate, decreases in vagal tone, and elevations in CORT; however, following such stressors the parameters of these systems return rapidly to baseline (e.g., Gunnar, Porter, Wolf, & Rigatuso, 1995).
The healthy neonate has powerful biobehavioral regulatory mechanisms at its disposal. Sleep is one of these mechanisms. Sleep is critical to stress regulation throughout life (Dahl, 1996). Newborns spend the majority of their time asleep, and in the young infant sleep periods are dominated by active or REM sleep as compared to slow-wave or quiet sleep (Anders, 1975). Quiet sleep appears to serve restorative functions in the newborn similar to the restorative functions it serves at later stages of the life cycle. This has been equated with the concept of a stimulus barrier in early infancy that protects the newborn from overwhelming stimulation (e.g., Tennes, Emde, Kisley, & Metcalf, 1972). Indeed, stressors alter sleep in the newborn, increasing the ratio of quiet to active sleep (for discussion, see Gunnar, 1992). In animal models the shift into sleep following stress has been shown to be facilitated by the rise in CORT and other stress biochemicals that increase in response to noxious stimulation (e.g., Born, de Kloet, Wenz, Kern, & Fehm, 1991). Thus it may be that stressors stimulate elevations in stress biochemicals that, in turn, facilitate the shift to quiet sleep supporting a return to homeostasis.
In addition to sleep, feeding and tactile stimulation appear to serve stress regulatory functions for the newborn. Blass (e.g., 1996) has recently shown that several components of nursing operate to calm the neonate through opioid- and nonopioid-mediated pathways. Sucking produces calming through nonopioid pathways in both human infants and rat pups. Sucking and swallowing are complex motor acts that engage and are regulated by the vagal system (e.g., Porges, 1995a). Thus, the vagal system may be partially responsible for the behavioral calming produced by nonnutritive sucking. In contrast, the calming and analgesic effects of sweet tastes appear to be opioid mediated. Thus, rat pups given a sucroseflavored liquid are slower to remove their paws from a hot plate, and this effect is blocked if the pups are first pretreated with an opioid antagonist. Similar calming effects of sucrose have been demonstrated in human newborns. In addition to activating opioid-mediated analgesic pathways, sweet tastes also produce facial expressions of positive affect and increase left-sided anterior EEG activity (Fox & Davidson, 1986). Although it is unlikely that this EEG activity reflects frontal lobe generators in the neonate, it may reflect activity of deeper structures such as the amygdala that also show asymmetric organization and are rich in opioid receptors (Pitkanen, Savander, & LeDoux, 1997).
Attention and alerting also may be components of the calming effects of sucrose. For example, Barr, Young, Wright, and Hendricks (1997) noted that quinine, an aversive taste, calms crying newborns. They have shown that in response to either sucrose or quinine newborns do not quiet and fall asleep; rather, they enter a sustained calm, alert state. Soothing practices that engage the vestibular and proprioceptive systems (i.e., picking the infant up, rocking) also appear to be most effective when they produce a calm, alert state (e.g., Brackbill, 1975). One interpretation is that these practices disrupt crying by engaging the infant’s orienting and attentional mechanisms (Rothbart, Posner, & Rosicky, 1994). As discussed later, attentional mechanisms play a central role in stress regulation.
The regulatory roles for feeding and nonnutritive sucking led Blass (1996) to argue that the mother serves as a shield to buffer the infant from pain and facilitate the restoration of growth processes following periods of stress system activation. Although the concept of mother as shield is attractive, she may not shield all stress-sensitive systems equally (see also Hofer, 1987). Being held, fed, and allowed to suckle appear to have their largest effects on behavioral distress, are less clearly capable of buffering heart rate responses to painful stimulation, and have no apparent impact on CORT responses to either painful or nonpainful stressors (e.g., Gunnar, 1992). Thus, the layers of stress regulation appear to be loosely coupled in the newborn. This is to be expected given the wide range of cultural variation in patterns of holding, carrying, and feeding, and given beliefs about whether and how quickly to respond to infant crying (e.g., Barr, 1990). If soothing practices were tightly coupled to stress regulation, it would seem unlikely that such variations would exist.
Variations in how much the infant is held when not distressed, however, do appear to affect the duration of crying bouts (e.g., Barr, 1990). In addition, breast feeding versus bottle feeding also appears to affect infant irritability and behavioral responsivity to stressors (e.g., Hughes, Townsend, & Branum, 1988). We do not know whether caregiving variations shape differentially responsive stress systems in humans, although in rodent models variations surrounding feeding and contact (licking and grooming) have such effects (e.g., Caldji et al., 1998). Also, there is evidence that activity of the L-HPAsystem is affected by experience in early life. In newborns, repeated exposure to the same handling stressor results in habituation of the CORT response, although with two exposures at a 24-hour interval, behavioral responses do not habituate (e.g., Gunnar, 1992). Pain, in contrast, may sensitize behavioral and physiological components of the stress system (e.g., Taddio, Katz, Ilarslch, & Koren, 1997).
The First Two Years
It has been suggested that there are two periods of marked change in biobehavioral organization during the first year of life (Emde, Gaensbauer, & Harmon, 1976). The first, between two and four months of age, has been described as the three-month revolution when almost every facet of infant functioning exhibits reorganization. The second is during the later half of the first year, when the emergence of independent locomotion appears to produce dramatic neurobehavioral reorganization (e.g., Campos, Kermoian, & Witherington, 1996; Fox & Bell, 1993). This latter period is also associated with the emergence and organization of secure base behavior (e.g., Bowlby, 1969) and inhibition of approach to novel or strange events and people (e.g., Bronson, 1978). Both of these periods are associated with marked changes in stress reactivity and regulation.
Two to Four Months. Several research groups have used well-baby examinations and childhood immunizations as stressors in developmental studies of stress in infancy (e.g., Gunnar, Brodersen, Krueger, & Rigatuso, 1996; Lewis & Ramsay, 1995). As in the newborn period, CORT increases markedly to exam inoculations at 2 months of age. Heart-rate and vagal-tone changes to inoculations have not been studied, but physical exams elicit significant increases in heart rate and decreases in vagal tone in the 2-month-old infant (White, Gunnar, Larson, Donzella, & Barr, 2000). Both physical exams and inoculations elicit fussing and crying at this age (Gunnar et al., 1996; Lewis & Ramsay, 1995). When facial expressions are coded based on discrete muscle groups, expressions during inoculations at this age reflect generalized distress, rather than more specific negative emotions such as fear or anger, as will be the case by the second year of life (Izard, Hembree, Dougherty, & Spizzirri, 1983). Probing the reasons for the change in CORT response to a physical exam, results showed that it was not because the exam produced less behavioral distress in 12-week-old and older infants (as reviewed in Gunnar, 2000). Nor did the change appear to be due to the greater organization of the circadian rhythm in cortisol that emerges around three months. The decreased CORT response to handling around three months could reflect as-yetunexamined maturation of negative feedback controls of the L-HPA axis. Indeed, feedback regulation of the L-HPA axis changes during early postnatal development in the rodent (Vazquez, 1998).
The L-HPA system is not the only stress-sensitive system to exhibit changes in regulation between 2 and 4 months. Developmental changes in fussing and crying have been well documented (as reviewed in Barr, 1990). The amount of time spent fussing and crying increases from birth to around 6 to 8 weeks and then declines. This developmental pattern in fussing and crying has been described in several cultures with markedly different early child-care practices, suggesting that it may be a universal phenomenon (see Barr, 1990). The basis for this developmental increase and subsequent decline is unknown; however, it raises the question of whether at around 2 months of age the infant might be particularly vulnerable to stress. Certainly, this is the period when some infants develop colic (e.g., Gormally & Barr, 1997), which by definition reflects dysregulation of the behavioral component of the stress system.
If this period of heightened irritability constitutes a stressvulnerable period, we might expect that infants with colic would be especially vulnerable to hyperresponsivity of the L-HPA and SAM systems. This possibility was recently examined by subjecting 2-month-olds with and without colic to the physical exam stressor paradigm (White, et al., 2000). Remarkably, although the physical exam produced inconsolable crying in many of the infants with colic, changes in CORT, heart rate, and vagal tone were significant but did not differ between groups. These data add to the body of literature indicating that fussing and crying, the primary behavioral measures used to index stress in early infancy, are not always indicative of individual differences in the activity of stress-sensitive physiological systems. Again, the layers of the stress system appear to be only loosely coupled.
Several other systems that are relevant for stress research also undergo developmental shifts during these early months of life. These include sleep, attention, and the parasympathetic nervous system. Changes in sleep emerge gradually, but for most a more mature day-night sleep organization is characteristic of the infant by 3 to 4 months of age (e.g., Coons, 1987). Unfortunately, although sleep and the regulation of the L-HPA and autonomic nervous systems are interrelated (Follenius, Brandenberger, Bandesapt, Libert, & Ehrhart, 1992; Porges, Doussard-Roosevelt, Stifter, McClenny, & Riniolo, 1999), little is known about the relations between stress regulation and the ontogeny of sleep in human infants and children.
Recently, there has been increasing interest in attention and emotion regulation. Early in the first year, between roughly 3 and 4 months, the development of the posterior attention system, which is thought to be involved in the ability to orient attention, may allow increased regulation of infant distress (e.g., Rothbart, Posner, et al., 1994). Development of the posterior attention system also may play a role in the regulation of stress physiology. With the development of this system, gaze aversion and distraction appear to become coping strategies for the infant that are used in increasingly coordinated and sophisticated ways to regulate behavioral arousal and distress over the course of the first year (e.g., Field, 1981).
Attention regulation has been related to ascending influences of the vagal system, particularly the component regulated by the nucleus ambiguus (Vna). Porges (1995a) argued that basal Vna tone may index the capacity to modulate cardiac-CNS activity to sustain attention to the environment. Maturational increases in basal Vna tone can be seen (Porges & Fox, 1986), presumably reflecting myelination of the neural systems underlying vagal regulation. With maturation of the Vna system, the infant’s capacity to regulate arousal through regulating attention and vice versa is expected to increase. Recently, research on vagal tone has shifted from an exclusive focus on basal tone to an interest in the dynamics of the vagal responses to stimulation. According to Porges’s (1995a) polyvagal theory, suppression of Vna activity allows increases in sympathetic activity, whereas increases allow the infant to engage in social approach and remain calm. Modulating Vna activity thus is viewed as a necessary support for social and attentional regulatory strategies. Huffman et al. (1998) recently argued that not until close to 3 months of age would infants evince the capacity to regulate the Vna system to support orienting and soothing. They demonstrated that among 12-week-olds, high basal Vna was associated with less irritability, whereas delta Vna during testing was related to duration of attention. Both measures were related to measures of soothability.
In sum, the systems influencing stress reactivity and regulation undergo rapid maturation during the early months of life. Three months of age has been described as a qualitative turning point in early infancy from which the infant emerges prepared to engage and sustain a broader range of interactions with the environment. By 3 months the elevations in CORT that have characterized neonatal responses are no longer observed, on average, to handling stressors. Fussing and crying become increasingly dissociated from activity of the HPAsystem. Vagal tone increases, and some infants show increased competence in using vagal regulation to sustain attention and engagement during challenging stimulation. In addition, more clearly established day-night rhythms may facilitate the regulation of behavioral and physiological responses to potentially stressful stimulation. Unfortunately, we need to know much more about the integration of these various components of the stress system through this developmental period.
Later Infancy. Responsivity of the L-HPA system to stressors appears to undergo another change in the latter part of the first year of life (points below are reviewed in Gunnar, 2000). Elevations in CORT to inoculation procedures are roughly comparable at 4 and 6 months of age; however, by the second year of life (i.e., 12, 15, or 18 months), on average, infants do not exhibit elevations in CORT to these procedures. Similarly, maternal separation, stranger approach, unfamiliar and arousing events, and frustrating tasks do not readily provoke increases in cortisol in children older than 12 months. Whether this decrease in CORT responsivity emerges gradually or abruptly has not been determined, nor have the processes accounting for this change been identified. What has been shown is that there are individual differences in whether the infant exhibits an inhibition of the CORT response to stressors by the end of the first year. Examination of CORT increases at 6 and 15 months using the inoculation paradigm revealed that while most infants failed to elevate CORT at 15 months, some showed increases that were as large or larger than those typically observed at 6 months. These high CORT reactive infants tended to be the ones with an insecure attachment relationship to the parent who accompanied them during the exam-inoculation procedure. The role of relationships in the development of individual differences in stress reactivity and regulation will be discussed more fully below. Here we only note that these data suggest that the organization of secure-base behavior in the latter part of the first year may play a role in the developmental changes in CORT responsivity observed during this age period.
The latter part of the first year is a period of emotional reorganization. In addition to changes in secure-base behavior and distress responses to separation from attachment figures, other developmental changes in negative emotionality are also observed. Given the emphasis on fear-stress relations in neuroscience, the fact that this period is associated with increased behavioral inhibition is of particular interest. In rodents, developmental changes in behavioral inhibition are related to increased CORT responses near the end of the period of relative CORT hyporesponsivity in early development (Takahashi & Rubin, 1993). Administering CORT to the young rat pup speeds up the emergence of behavioral inhibition. This has been taken as evidence that CORT facilitates maturation of fear circuits in the rat brain. There have been too few studies of adrenocortical activity and the development of behavioral inhibition in humans to conclude that a similar pattern does not exist in late infancy. However, the correspondence in humans of increased fearfulness and decreased CORT responsivity over the last part of the first year suggests that the developmental psychobiology of fear and stress may be very different in human infancy.
One reason for the apparent difference may be that fear is rarely the emotion expressed in infant research. More typical is wariness or inhibition of approach combined with increased proximity to caregivers, followed by interest and affiliation/exploration (Sroufe, Waters, & Matas, 1974). It is not clear whether wariness is less intense fear or a response that reflects conflict between approach and avoidance (Bronson, 1978). However, wariness in the face of unknown people, objects, and events emerges gradually over the latter part of the first year and is tempered by experience, context, and the controllability of stimulation (e.g., Bronson, 1978; Gunnar, 1980; Sroufe et al., 1974). Many of these same factors are well known to temper physiological responses to threatening stimuli in studies of adults and animals (e.g., Lefcourt, 1973; Maier, Ryan, Barksdale, & Kalin, 1986).
It is important to note that wariness or behavioral inhibition emerges around the same period when infants are increasingly able to control proximity to both safe havens and exciting, new stimulation. Functionalist approaches to emotion argue that in most instances emotions serve to organize, not disorganize, behavior (Campos et al., 1996; Panksepp, 1996). Accordingly, wary responses to the unknown may serve to check the infant’s tendency to approach things that are new, foster increased proximity to attachment figures in new situations, and thus provide a window of opportunity for caregivers to warn infants away from situations that are dangerous (Waters, Matas, & Sroufe, 1975). Social referencing, or the infant’s tendency near the end of the first year to look to caregivers for their appraisals of unfamiliar or strange events, provides another avenue through which caregivers can curb infant curiosity at a time when infant mobility is increasing (Campos & Stenberg, 1981). Campos et al. (1996) argued that an epigenetic-constructionist perspective on emotional development is helpful in understanding the reorganization of emotions near the end of the first year. This perspective, which is consistent with the developmental psychobiological approach, may also help us understand the organization of fear/wariness and stress in infants near the end of the first year of life.
According to an epigenetic-constructionist perspective, developmental changes in one system can generate experiences that set the stage for widespread biobehavioral changes. In addition, changes in the person bring about bidirectional changes in person-environment relations that set the stage for further development. During the latter part of the first year, learning to crawl and then to walk dramatically alters the infant’s relations with the environment. Independent locomotion changes the events and obstacles that the infant encounters daily and requires the development of strategies for managing the environment that are markedly different from those that serve the prelocomotor infant (e.g., Campos et al., 1996). Self-produced locomotion appears to be critical in organizing fear reactions to one particular situation: heights. Infants placed on the deep side of a visual cliff where depth cues indicate they should fall fail to show increases in heart rate prior to the onset of crawling, but they do show such increases after a few weeks of crawling experience. From this epigenetic-constructionist perspective, the critical emotion-organizing feature of motor acquisitions is the increase in agency and intentionality that they allow the infant. Increased experiences of agency and intentionality, in turn, may affect the extent to which the infant appraises events based on his or her certainty of being able to control them (Gunnar, 1980).
Infants are responsive to the contingency of stimulation early in infancy (Watson & Ramey, 1972). Within a few months of birth, infants exhibit positive affect to events that are contingent on their actions, as well as anger or sadness when a previously contingent event begins to occur noncontingently. However, it is not until close to a year of age that the infant’s control over producing stimulation determines whether a potentially distressing event produces crying and avoidance or positive affect and approach (for a review, see Gunnar, 1980). Over the course of the second year of life, increases are observed in children’s attempts to control directly or alter situations that produce inhibition of approach (e.g., Parritz, 1996). Furthermore, by 12 months of age, approach versus avoidance of strangers reflects the responsiveness of the stranger to the infant’s actions, and thus the stranger’s controllability (e.g., Mangelsdorf, 1992). Thus, by the first birthday, and increasingly over the second year of life, stress reactivity and regulation may be influenced by the infant’s sense of agency or perceived control.
Another approach to understanding changes in emotionality during the latter part of infancy has focused on the development of the frontal lobes (e.g., Bell & Fox, 1992). Many of the social and cognitive accomplishments emerging during the latter part of the first year and throughout the second year depend on the development of the prefrontal cortex and its connections with brain systems involved in motor development and emotion (e.g., Dawson, Panagiotides, Klinger, & Hill, 1992; Diamond, 2000). Maturation of frontal functioning, like other aspects of brain development, is expected to reflect genetically-programmed, activity-dependent neural processes that are supported by the child’s interactions with the environment. It is important to note that using 8-monthold infants, Bell and Fox (1997) showed that one to four weeks of independent locomotion was associated with the degree of mass neuronal excitability in the frontal cortex, greater activity over left than right frontal leads, and the ability to tolerate longer delays on a classic frontal lobe task (i.e., the A not B task). These data suggest that as the infant approaches the second year, motor acquisitions that dramatically alter the infant’s control over approaching and avoiding stimulation co-occur with maturational changes in anterior regions of the brain. These changes likely underlie the developmental changes in the organization of emotional and physiological responses to stressors that are observed around the first birthday and increasingly over the second year of life (e.g., Campos et al., 1996). Unfortunately, there are no studies as of yet examining the relations between the developmental changes just described and the responsivity of the autonomic or neuroendocrine system to stressors.
The Toddler and Preschool Period
Development of frontal regions of the brain should allow increasing control over emotional behavior and physiological stress responses (Dawson et al., 1992). Indeed, marked increases in self-control of negative emotionality develop between 1 and 3 years (e.g., Kopp, 1989). Studies focusing on individual differences have shown correlations between expressive language development and regulation of negative emotions and social engagement and between both of these domains and cardiac vagal tone (e.g., Bornstein & Suess, 2000). The study of emotion regulation has dominated research on emotional development in the last decade, despite problems in definition and operationalization (Thompson, 1994). The research and theorizing of Posner and Rothbart (e.g., 2000) provided much needed focus in this area.They argued that maturation of the anterior attentional network permits effortful regulation of behavior, including emotional behavior. In line with these predictions, Kochanska, Murray, and Harlan (2000) have shown that children who perform better on tasks designed to assess effortful control also are better at suppressing both positive and negative emotional expressions. Stroop tasks that require inhibition of response to a prepotent stimulus activate the frontal attentional network in imaging studies of adults (Posner & Petersen, 1990). A version of the Stroop that was designed for 2- and 3-year-olds has revealed increases in accuracy over this age period (GerardiCaulton, in press). In addition, at 30 months, when some but not all children were able to perform the task, more competent performance was negatively correlated with parent reports of child negative emotionality. Effortful regulation of behavior, nonetheless, undoubtedly involves multiple neural systems; thus, these studies provide only the first insights into the neural bases of self-regulation and its development.
Presumably, as the child develops increasing ability to regulate emotions, she should also become increasingly capable of regulating physiological stress reactions (Stansbury & Gunnar,1994).Thisassumptionisspeculativelybasedonseveral arguments. First, with the development of the anterior attentional network, the child should be able to engage the cognitive component of the anterior cingulate cortex, thus suppressing activity of the emotional component (Bush et al., 2000). This should help inhibit and constrain the reactivity of limbic components of the stress system. Second, to the extent that emotion regulation also involves increased activity in the left prefrontal cortical regions, the child should become increasingly capable of using positive affect and approachoriented behavioral strategies for managing potentially stressful situations (Davidson & Irwin, 1999; Dawson et al., 1992). Third, the ability to regulate negative emotions should foster social competence and better social relationships with peers and adults (e.g., Eisenberg et al., 1993). This ability, in turn, should enhance the child’s opportunities to use positive and supportive social relationships to cope with stressful situations. Social competence should also reduce the likelihood that the child’s behavior will create stressful interactions with others (for review, Gunnar, 2000). Thus far, no studies examiningdevelopmentalchangesinthepresumedneuralsubstrate of emotion regulation and changes in stress reactivity or regulation have been reported. There have been studies of individual differences in effortful control, emotion regulation, and physiology, as reviewed in the next section.
Individual Differences
Questions about the origins of individual differences form the core of developmental research on stress. Most of this research deals with temperament and the argument that some children are biologically predisposed to be more stress reactive than are others. Some research, however, focuses on the importance of early experiences in the shaping of stress reactivity. These research foci come together in arguments about the relations between temperament and attachment and in studies of experience and the continuity of behavioral dispositions.As in other areas of developmental research, main effect arguments based on either nature or nurture explanations are giving way to transactional models that are more consistent with the developmental psychobiological perspective.
Stress and Temperament
Most studies of stress and temperament deal with behavioral inhibition. In this section we draw heavily on several excellent recent summaries of this research (see Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Kagan, in press; Stevenson-Hinde & Shouldice, 1996). As conceptualized by Kagan (2001), about 10% of children are extremely anxious and inhibited in their reactions to unfamiliar events. Consistent with the neurobiology linking fear and stress, a lower threshold for activation of fearanxiety circuits in the CEA (or perhaps the BNST) is believed to form the basis of extreme inhibition to the unknown. Kagan argued that extremely negative reactions to stimulation at 4 months of age reflect activity of the CEAand thus predict fearful, anxious reactions to the unknown in later infancy and childhood. With development, behavioral inhibition may be more readily seen to social than to nonsocial stimuli, leading some to talk of social reticence rather than behavioral inhibition when discussing this temperamental disposition in preschoolers and older children. Fox and colleagues (e.g., Schmidt & Fox, 1999) argued that at least two forms of social reticence may have different neurobiological substrates. These forms differ in the extent to which a reticent or shy child is also motivated to be social. Although both patterns reflect a diathesis to be stressed in social situations, children who are both sociable and shy may experience the most conflict between response tendencies and thus may find new social situations to be the most stressful or challenging.
Continuity in extreme inhibition has been examined in several longitudinal studies. Generally speaking, shyness shows modest continuity across childhood, although children selected to be extremely shy often become less so with age (e.g., Kagan, in press; Stevenson-Hinde & Shouldice, 1996). Less continuity has been noted for children selected for high reactivity early in infancy (Fox et al., 2001). Most infants who show extreme negative reactivity at 4 months do not remain behaviorally inhibited into childhood; nevertheless, a small subset does remain so. As discussed later, there is some evidence that consistently inhibited children may be more extreme in their physiological markers of inhibition.
Studies of heart rate and heart rate variability or vagal tone constitute the largest body of literature on physiological differences between extremely inhibited and uninhibited children. In several cohorts, children identified as extremely inhibited during infancy have been shown to have higher and more stable baseline heart rates and lower vagal tone (for a review, see Kagan, in press). Higher and more stable baseline heart rates continue to distinguish behaviorally inhibited children throughout the preschool years. However, with continued development these baseline differences in heart rate become more difficult to find. Thus, several studies of children 7 years and older have failed to obtain differences between shy, inhibited children and uninhibited children using baseline cardiac measures, although heart rate changes in response to social stressors differentiate these groups (e.g., Marshall & Stevenson-Hinde, 1998; Schmidt, Fox, Schulkin, & Gold, 1999). In studies of CORT activity there is also evidence that between 3 and 7 years of age children become increasingly capable of maintaining the normal diurnal decrease in CORT under normative conditions of social challenge (i.e., a day at daycare; Dettling, Gunnar, & Donzella, 1999). Furthermore, positive correlations between vagal tone and age have been reported over this age period (e.g., Donzella, Gunnar, Krueger, & Alwin, 2000). It may be that by about 7 years of age maturation of these systems allows children, including those who are more fearful or inhibited, to maintain basal functioning even in less protected contexts.
Recently, in attempts to understand the underlying neurobiological differences between extremely inhibited and uninhibited children, researchers have examined more direct indexes of the forebrain systems presumably involved in fearfulness and negative emotionality. To this end, startle amplitude, a measure presumably mediated by the CEA, has been employed in several studies. At 9 months, infants selected at four months for extreme negative reactivity have been shown to exhibit larger startle reactions during stranger approach. Tested again at 4 years, however, larger startle amplitudes were not found for these children, although at this older age only baseline startle was examined, and this might not reflect the same underlying neural circuits (see Schmidt et al., 1997).
Right frontal EEG asymmetry has also been examined in relation to behavioral inhibition. Schmidt and Fox (1999) recently reviewed their studies of EEG asymmetry on 81 children selected at 4 months because they were either high negative, high positive, or low reactive in their behavioral responses to stimulation. At 9 and 24 months, but not at 14 months, high negative infants exhibited greater right frontal activity. At 48 months, the 4-month groupings no longer predicted differences in frontal EEG asymmetry; however, asymmetry scores at 48 months were significantly correlated with concurrent measures of social reticence. When children who were continuously extreme in inhibition were examined separately from those who became less inhibited with age, the continuously inhibited children exhibited greater right frontal asymmetry at 9, 14, and 48 months (Fox et al., 2001).
Consistent with the relative lack of baseline physiological difference between inhibited and uninhibited school-aged children, Schmidt et al. (1999) recently failed to find baseline asymmetry differences in 7-year-olds selected to be extremely shy. However, they did find that these children showed a greater increase in right frontal EEG asymmetry as the social stressor became more intense. Thus, right frontal EEG asymmetry does seem to be associated with behavioral inhibition, although as with other physiological measures, differences are not always obtained, even when extreme groups are chosen. As with other stress-sensitive physiological systems, the capacity to detect baseline differences related to behavioral inhibition may decrease with age.
Dissociations between behavioral and physiological indexes of fear and stress are often noted (e.g., Quas, Hong, Alkon, & Boyce, 2000). Some of these dissociations may reflect the lack of specificity of the physiological measures. Thus, for example, low vagal tone may reflect low emotional expressivity and not just high fearful inhibition (e.g., Cole, Zahn-Waxler, Fox, Usher, & Welsh, 1996; Porges, 1995a). More specific measures of sympathetic activity may help clarify cardiac associations with extremely inhibited behavior (e.g., pre-ejection period; Berntson et al., 1993). Selecting children based on physiological extremes, and not merely behavioral extremes, may also be useful (see Fox, 1989). However, even when all of these analytic choices are made, it is likely that the associations between physiology and behavior will remain elusive. We suggest that this is because context and the resources children need to cope with challenge moderate relations between temperament and the activity of these stress-sensitive physiological systems.
Studies of CORT and temperament make this last point most clearly. While higher CORT levels for shy, inhibited children have been noted in several studies (for review, see Gunnar, 2000), particularly in new social situations, it is often the extroverted children who exhibit greater CORT responsivity (e.g., Davis, Donzella, Krueger, & Gunnar, 1999). At first glance this seems incongruous. Why would extroverted children be stressed by meeting other children, an activity that they seem to enjoy? However, activation of the stress system should help children mobilize the resources they need to facilitate adaptation to new situations. Perhaps extroverted children are better at mobilizing to meet social challenge. If so, the critical question may be not whether children react initially, but how rapidly they dampen their reactivity. Indeed, there is evidence that as social situations become familiar, socially competent, outgoing children show reduced CORT activity and associations between high CORT activity and negative, emotional temperament become more likely (see Gunnar, 1994). However, even when young children are familiar with the social situation, higher stress system activity is less often associated with shyness and more often associated with behaviors such as low frustration tolerance and aggression—behaviors that cause preschoolers to be disliked by their peers. In fact, peer rejection appears to be an important predictor of high CORT levels in preschool classrooms (Gunnar, Tout, de Hann, Pierce, & Stansbury, 1997). Combined, these findings strongly suggest that behavioral inhibition is not the only temperamental disposition associated with greater stress reactivity in young children.
Furthermore, they point to the importance of context and relationships in determining how temperamental differences among children impact the activity of stress-sensitive physiological systems. In the following section we deal with caregiver-child interaction. However, it is important to note that the psychobiology of stress in early childhood includes peer- as well as adult-child relations.
Stress and Caregiving Relationships
In work with animals it is well documented that maternal behavior shapes the reactivity of stress-sensitive systems (e.g. Caldji et al., 1998; Levine, 1994). In rats, dams that spend the most time licking and grooming their pups and exhibit well-organized nursing behavior have pups that grow up to be less fearful of novelty, compared to the offspring of mothers low in these behaviors. Anumber of neurobiological changes accompany these differences in fear reactions, including more rapid containment of the HPA stress response, less evidence of CRH activity in the CEA, BNST, and LC, and decreased NE in response to psychosocial stressors (Caldji et al., 1998). In primates, there is strong evidence that contact with the mother buffers the stress response (e.g., Levine & Weiner, 1988). As long as the infant monkey can gain access to the mother, elevations in CORT to a variety of stressors are reduced, even though the infant may still show agitated, distressed behavior.
In human adults, supportive social relationships moderate the impact of stressful life circumstances on emotional and physical health (Berkman, 1995). In young children, studies of the quality of mother-infant attachment have yielded evidence that secure attachment relationships function to regulate the activity of stress-sensitive systems (see review by Gunnar, 2000). This has been demonstrated during the strange situation task used to assess attachment security, as well as when attachment security is examined as a moderator of temperament-physiology associations in response to other stressful stimuli. Under both kinds of testing situations, both insecure avoidant (A) and insecure resistant (C) infants exhibit larger and more prolonged CORT and heart rate increases (see also Spangler & Grossmann, 1993; Sroufe & Waters, 1979). Furthermore, behavioral indexes of distress or inhibition appear to be associated with heightened CORT responses only when infants and toddlers are tested in the presence of a parent with whom they have an insecure attachment relationship (e.g., Spangler & Schieche, 1998). Similar results have been obtained for preschool-aged children. Thus, among 4.5-year-olds, highly inhibited children who were insecurely attached had the highest heart rates during mild social challenges, whereas highly inhibited children who were securely attached had the lowest heart rates (Stevenson-Hinde & Marshall, 1999). Thus, although there is an ongoing debate between attachment and temperament theorists over whether these attachment classifications reflect behavioral inhibition rather than relationship quality (e.g., Belsky & Rovine, 1987), the preponderance of the evidence indicates that differences in stress reactivity between children in secure and insecure relationships are not a reflection of temperament. Rather, they reflect the role of attachment security in moderating relations between temperamental fearfulness and stress system activity.
Attachment theorists argue that infants form secure relationships with caregivers who are sensitive and responsive to their signals. A number of researchers have questioned the strength of these associations (e.g., Goldsmith & Alansky, 1987); however, evidence suggests that these qualities in a caregiver are associated with reduced stress system activity. Infants interacting with an insensitive, unresponsive mother or those randomly assigned to an unresponsive babysitter have been shown to produce increasing levels of CORT during play bouts (e.g., Gunnar, 2000; Spangler, Schieche, Ilg, Maier, & Ackermann, 1994). Furthermore, mothers suffering from clinical depression, who often have trouble being sensitive and responsive, have infants who tend to show right frontal EEG asymmetry and higher CORT levels (e.g., Dawson & Ashman, 2000). Controlling for numerous other factors, it appears that the depressed mothers’ unresponsive, intrusive behavior shapes higher CORT and greater right frontal EEG activity (see Dawson & Ashman, 2000). In addition, the timing of maternal periods of clinical depression appears to matter. Periods of depression during the infant’s first year have been shown to predict higher CORT levels at age 3, whereas periods of depression during the second and third year predict greater right frontal EEG patterns at three years. Dawson and colleagues speculate that their findings may reflect sensitive periods for the organization of different aspects of the stress system; however, they also note that these data are preliminary and need replication.
Although the results just described strongly suggest that sensitive, responsive parenting regulates the young child’s stress system, there is some evidence that overly responsive, overly solicitous parenting may do the opposite. Thomasgard and Metz (1993) argued that from the best of intentions parents may feel that they need to step in to protect their anxious child from upsetting experiences and may intrude into their ongoing activities in ways that are overly protective and overly solicitous. Overly solicitous, intrusive caregiving during threat is associated with larger CORT increases among toddlers and predicts insecure attachment classification (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996).
Similarly, parents who are extremely affectionate with and solicitous of infants selected for high negative reactivity at 4 months are more likely to have infants who become extremely inhibited in their second year than are less affectionate and solicitous parents (Arcus, Gardner, & Anderson, 1992). Such parenting might increase fearfulness and stress reactivity because it is insensitive, is organized more around the parent’s anxiety about the child than the child’s actual needs, and reduces the child’s experiences of self-regulation. Regardless, these data suggest that we need more studies of caregiving in stressful circumstances to understand how what the caregiver does during periods of high challenge influences both the parent-child relationship and the child’s developing stress system.
Animal studies document that not only normal variations in caregiving but also early maltreatment influence the developing stress system. There are too few studies of early maltreating rearing environments in humans to know whether early experiences shape the stress system in our species (for review, see Gunnar, 2000). In several studies of children who were severely maltreated during their first years of life, elevations in baseline CORT and catecholamine production have been noted several years after removal from their maltreating contexts. Similarly, in a recent study of women who were sexually abused during childhood, heightened ACTH responses to a social stressor were found both for those who were and those who were not clinically depressed. However, the situations that result in maltreatment in humans are complex. Any of a number of factors beyond caregiver-infant interaction, including lack of adequate stimulation, malnutrition, and inadequate medical care might be involved in producing alterations in the development of the stress system. As in other domains of early human experience, proving that maltreatment at the hands of caregivers early in development has permanent effects on the developing stress system will be difficult.
Summary and Conclusions
The last several decades have seen tremendous advances in our understanding of the neurobiology of the human stress system. Research on the development of stress reactivity and regulation in infants and children has burgeoned in recent years due largely to the development of noninvasive measurement techniques. However, we are still far from understanding the processes through which individual differences in stress become organized. Repeatedly throughout this review we have noted where basic information is lacking. Much of this information involves normative data on the organization of stress reactivity and regulation at different points during early development. As in the study of emotional development more generally, we have much more information about individual differences in stress reactivity than we do about normative patterns of development and change. However, unless we develop this latter body of knowledge, it will be difficult to explicate the origins of individual differences in stress reactivity and regulation. Research on temperament, especially extremely inhibited temperament, has motivated much of the research on early stress reactivity and regulation. Although this work has been aimed at documenting the stability of temperamental differences among children, the evidence strongly suggests that most children do not remain extremely fearful or inhibited throughout infancy and early childhood. Change is as likely, or even more likely, than stability. Studies of caregiver-child interactions indicate that qualities of care, including sensitivity and responsiveness, are related to reactivity and regulation of the stress system in infants and young children. It seems likely that transactional processes shape the development of the stress system in humans as they appear to in other mammals. These processes, including the child’s role in influencing the nature of his or her experiences, remain largely unexplored. We argue that an adequate understanding of the development of stress and emotion in early childhood will require attention both to the transactional nature of the influences that shape differences in stress responsivity among individuals and to the ways these transactions emerge and change during the early years of life.
Bibliography:
- Anders, T. (Ed.). (1975). Maturation of sleep patterns in the newborn infant. New York: Spectrum.
- Arcus, D., Gardner, S., & Anderson, C. (1992). Infant reactivity, maternal style, and the development of inhibited and uninhibited behavioral profiles. Paper presented at the International Society for Infant Studies, Miami, FL.
- Ashby, F. G., Isen, A. M., & Turken, A. U. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychological Review, 106(3), 529–550.
- Barbazanges, A., Piazza, P. V., Moal, M. L., & Maccari, S. (1996). Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. Journal of Neuroscience, 16(12), 3943–3949.
- Barr, R. G. (1990). The early crying paradox: A modest proposal. Human Nature, 1(4), 355–389.
- Barr, R. G., Young, S. N., Wright, J. H., & Hendricks, L. A. (1997). Differential response to intraoral sucrose, quinine, and corn oil in crying human newborns. Physiology and Behavior, 62(2), 317–325.
- Beauchaine, T. (in press). Vagal tone, development, and Gray’s motivational theory: Toward an integrative model of autonomic nervous system functioning in psychopathology. Development and Psychopathology.
- Bell, M. A., & Fox, N. A. (1992). The relations between frontal brain electrical activity and cognitive development during infancy. Child Development, 63, 1142–1163.
- Bell, M. A., & Fox, N. A. (1997). Individual differences in object permanence performance at 8 months: Locomotor experience and brain electrical activity. Developmental Psychobiology, 31(4), 287–297.
- Belsky, J., & Rovine, M. (1987). Temperament and attachment security in the strange situation: An empirical rapproachement. Child Development, 58(3), 787–795.
- Berkman, L. F. (1995). The role of social relations in health promotion. Psychosomatic Medicine, 57, 245–254.
- Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114(2), 296–322.
- Blass, E. M. (1996). Mothers and their infants: Peptide-mediated physiological, behavioral and affective changes during suckling. Regulatory Peptides, 66(1–2), 109–112.
- Born, J., de Kloet, E. R., Wenz, H., Kern, W., & Fehm, H. L. (1991). Gluco- and antimineralocorticoid effects on human sleep: A role of central corticosteroid receptors. American Physiological Society, E183–E188.
- Bornstein, M. H., & Suess, P. E. (2000). Physiological selfregulation and information processing in infancy: Cardiac vagal tone and habituation. Child Development, 71(2), 273–287.
- Bowlby, J. (1969). Attachment and Loss: Attachment (Vol. 1). New York: Basic Books.
- Brackbill, Y. (1975). Continuous stimulation and arousal level in infancy: Effects of stimulus intensity and stress. Child Development, 46, 364–369.
- Bronson, G. (1978). Aversive reactions to strangers: A dual process interpretation. Child Development, 49, 495–499.
- Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Neuroscience, 4, 215–222.
- Caldji, C., Tannenbaum, B., Sharma, S., Francis, D., Plotsky, P. M., & Meaney, M. J. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences, USA, 95(9), 5335–5340.
- Cameron, O. G., & Nesse, R. M. (1988). Systematic hormonal and physiological abnormalities in anxiety disorders. Psychoneuroendocrinology, 13(2), 287–307.
- Campos, J., Kermoian, R., &Witherington, D. (1996).An epigenetic perspective on emotional development. In R. D. Kavanaugh, B. Zimmerberg, & S. Feinman (Eds.), Emotion: Interdisciplinary perspectives (pp. 119–138). Mahwah, NJ: Erlbaum.
- Campos, J., & Stenberg, C. (1981). Perception, appraisal and emotion: The onset of social referencing. In M. Lamb & L. Sherrod (Eds.), Infant social cognition: Empirical and theoretical considerations (pp. 273–314). Hillsdale, NJ: Erlbaum.
- Cannon, W. B. (1936). Bodily changes in pain, hunger, fear and rage. New York: Appleton-Century-Crofts.
- Cole, P., Zahn-Waxler, C., Fox, N. A., Usher, B. A., & Welsh, J. D. (1996). Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology, 105, 518–529.
- Coons, S. (1987). Development of sleep and wakefulness during the first six months of life. In C. Guilleminault (Ed.), Sleep and its disorders in children (pp. 17–27). New York: Raven Press.
- Dahl, R. E. (1996). The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology, 8, 3–27.
- Dallman, M. F., Strack, A. M., Akana, S. F., Bradbury, M. J., Hanson, E. S., Scribner, K. A., & Smith, M. (1993). Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Frontiers in Neuroendocrinology, 14, 303–347.
- Davidson, R. J. (1994). Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology, 6, 741–758.
- Davidson, R. J., & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Neuroscience, 3, 11–21.
- Davidson, R. J., & Slagter, H. A. (2000). Probing emotion in the developing brain: Functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Mental Retardation and Developmental Disabilities Research Reviews, 6(3), 166–170.
- Davis, E. P., Donzella, B., Krueger, W. K., & Gunnar, M. R. (1999). The start of a new school year: Individual differences in salivary CORT response in relation to child temperament. Developmental Psychobiology, 35(3), 188–196.
- Dawson, G., & Ashman, S. (2000). On the origins of a vulnerability to depression: The influence of early social environment on the development of psychobiological systems related to risk for affective disorder. In C. A. Nelson (Ed.), Minnesota Symposia on Child Psychology: Vol. 31. The effects of adversity on neurobehavioral development (pp. 245–280). New York: Erlbaum.
- Dawson, G., Panagiotides, H., Klinger, L. G., & Hill, D. (1992). The role of frontal lobe functioning in the development of infant selfregulatory behavior. Brain and Cognition, 20, 152–175.
- de Kloet, R., Vreugdenhil, E., Oitzl, M. S., & Joels, A. (1998). Brain corticosteroid receptor balance in health and disease. Endocrine Reviews, 19(3), 269–301.
- Dettling, A., Gunnar, M. R., & Donzella, B. (1999). CORT levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology, 24(5), 505–518.
- Diamond, A. (2000). Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development, 71(1), 44–56.
- Diorio, D., Viau, V., & Meaney, M. J. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience, 13, 3839–3847.
- DiPietro, J. A., Costigan, K. A., Shupe, A. K., Pressman, E. K., & Johnson, T. R. (1999). Fetal neurobehavioral development associated with social class and fetal sex. Developmental Psychobiology, 33, 79–81.
- DiPietro, J. A., Hodgson, D. M., Costigan, K. A., Hilton, S. C., & Johnson, T. R. (1996). Fetal neurobehavioral development. Child Development, 67, 2553–2567.
- Donzella, B., Gunnar, M. R., Krueger, W. K., & Alwin, A. (2000). CORT and vagal tone responses to competitive challenge in preschoolers: Associations with temperament. Developmental Psychobiology, 37(4), 209–220.
- Drevets, W. C., & Raichle, M. E. (1998). Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion, 12, 353–385.
- Economides, D. L., Nicolaides, K. H., Linton, E. A., Perry, L. A., & Chard, T. (1988). Plasma CORT and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Therapy, 3, 158–164.
- Eisenberg, N., Fabes, R. A., Bernzweig, H., Karbon, M., Poulin, R., & Hanish, L. (1993). The relations of emotionality and regulation to preschoolers’ social skills and sociometric status. Child Development, 64, 1418–1438.
- Emde, R. M., Gaensbauer, T. J., & Harmon, R. J. (1976). Emotional expressions in infancy: A biobehavioral study. New York: International University Press.
- Emery, N. J., & Amaral, D. G. (2000). The role of the amygdala in primate social cognition. In R. D. Lane & L. Nadel (Eds.), Cognitive neuroscience of emotion (pp. 156–191). New York: Oxford University Press.
- Engle, B. T. (1985). Stress is a noun! No, a verb! No, an adjective. In T. Field, P. McCabe, & N. Sneiderman (Eds.), Stress and coping (Vol. 1, pp. 3–12). Hillsdale, NJ: Erlbaum.
- Field, T. (1981). Infant gaze aversion and heart rate during face-toface interactions. Infant Behavior and Development, 4, 307–316.
- Folkman, S., & Moskowitz, J. T. (2000). Stress, positive emotion, and coping. Current Directions in Psychological Science, 9, 115–118.
- Follenius, M., Brandenberger, G., Bandesapt, J. J., Libert, J. P., & Ehrhart, J. (1992). Nocturnal CORT release in relation to sleep structure. Sleep, 15(1), 21–27.
- Fox, N. A. (1989). Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology, 25(3), 364–372.
- Fox, N. A., & Bell, M. A. (1993). Frontal function in cognitive and emotional behaviors during infancy: Effects of maturation and experience. In B. de Boysson-Bardies & S. de Schonen (Eds.), NATO ASI Series D: Behavioural and Social Sciences: Vol. 69. Developmental Neurocognition: Speech and Face Processing in the First Year of Life (pp. 199–210). Dordrecht, Netherlands: Kluwer.
- Fox, N.A., & Davidson, R. J. (1986). Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia, 24, 417–422.
- Fox, N. A., Henderson, H. A., Rubin, K. H., Calkins, S., & Schmidt, L. A. (2001). Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development, 72, 1–21.
- Frankenhaeuser, M. (1979). Psychobiological aspects of life stress. In S. Levine & H. Ursin (Eds.), Coping and health (pp. 203– 224). New York: Plenum Press.
- Gerardi-Caulton, G. (in press). Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Developmental Science.
- Giannakoulpoulous, X., Sepulveda, W., Kourtis, P., Glover, V., & Fisk, N. M. (1994). Fetal plasma and beta-endorphin response to intrauterine needling. The Lancet, 344, 77–81.
- Giannakoulpoulous, X., Teixeira, J., Fisk, N. M., & Glover, V. (1999). Human fetal and maternal noradrenaline responses to invasive procedures. Pediatric Research, 45, 494–499.
- Goldsmith, H. H., & Alansky, J. A. (1987). Maternal and infant temperamental predictors of attachment: A meta-analytic review. Journal of Consulting and Clinical Psychology, 55(6), 805–816.
- Gormally, S. M., & Barr, R. G. (1997). Of clinical pies and clinical clues: Proposal for a clinical approach to complaints of early crying and colic. Good Practice Guide, 3, 137–153.
- Gottlieb, G., Wahlsten, D., & Lickliter, R. (1998). A developmental psychobiological systems view. In R. Lerner (Ed.), Handbook of child psychology: Vol. 1. Theoretical model of human development (5th ed., pp. 233–274). New York: Wiley.
- Gunnar, M. R. (1980). Contingent stimulation: A review of its role in early development. In S. Levine & H. Ursin (Eds.), Coping and health (pp. 101–120). New York: Plenum Press.
- Gunnar, M. R. (1986). Human developmental psychoendocrinology: A review of research on neuroendocrine responses to challenge and threat in infancy and childhood. In M. Lamb, A. Brown, & B. Rogoff (Eds.), Advances in developmental psychology (Vol. 4, pp. 51–103). Hillsdale, NJ: Erlbaum.
- Gunnar, M. R. (1992). Reactivity of the hypothalamic-pituitaryadrenocortical system to stressors in normal infants and children. Pediatrics, 80(3), 491–497.
- Gunnar, M. R. (1994). Psychoendocrine studies of temperament and stress in early childhood: Expanding current models. In J. Bates & T. Wachs (Eds.), Temperament: Individual differences at the interface of biology and behavior (pp. 175–198). New York: American Psychological Association Press.
- Gunnar, M. R. (2000). Early adversity and the development of stress reactivity and regulation. In C. A. Nelson (Ed.), The Minnesota Symposia on Child Psychology: Vol. 31. The effects of adversity on neurobehavioral development (pp. 163–200). Mahwah, NJ: Erlbaum.
- Gunnar, M. R., Brodersen, L., Krueger, K., & Rigatuso, J. (1996). Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development, 67(3), 877–889.
- Gunnar, M. R., Porter, F., Wolf, C., & Rigatuso, J. (1995). Neonatal stress reactivity: Predictions to later emotional temperament. Child Development, 66, 1–14.
- Gunnar, M. R., Tout, K., de Haan, M., Pierce, S., & Stansbury, K. (1997). Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology, 31(1), 65–85.
- Hann, D. M., Huffman, L. C., Lederhendler, I. I., & Meinecke, D. (Eds.). (1998). Advancing research on developmental plasticity: Integrating the behavioral science and neuroscience of mental health (Vol. 98). Washington, DC: National Institute of Health.
- Heim, C., Owen, M. J., Plotsky, P. M., & Nemeroff, C. B. (1997). The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: Focus on corticotropinreleasing factor. Annals of the New York Academy of Science, 821, 194–207.
- Hofer, M. (1987). Shaping forces within early social relationships. In N. A. Krasnegar (Ed.), Perinatal development: A psychobiological perspective (pp. 251–274). Orlando, FL: Academic Press.
- Huffman, L., Bryan, Y. E., del Carmen, R., Pedersen, F. A., Doussard-Roosevelt, J., & Porges, S. W. (1998). Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development, 69, 624–635.
- Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Cambridge, MA: Harvard University Press.
- Hughes, R. B., Townsend, P. A., & Branum, Q. K. (1988). Relationship between neonatal behavioral responses and lactation outcomes. Issues in Comprehensive Pediatric Nursing, 11(5–6), 271–281.
- Huizink, A. C., de Medina, P. G. R., Mulder, E. J. H., Visser, G. H. A., & Buitelaar, J. K. (2000). Prenatal psychosocial and endocrinologic predictors of infant temperament. In A. C. Huizink (Ed.), Prenatal stress and its effects on infant development (pp. 171–200). Hoorn, Netherlands: Drukkerij van Vliet.
- Huttenlocher, P. R. (1994). Synaptogenesis, synapse elimination, and neural plasticity in human cerebral cortex. In C. A. Nelson (Ed.), Threats to optimal development: Integrating biological, psychological, and social risk factors (Vol. 27, pp. 35–54). Mahwah, NJ: Erlbaum.
- Izard, C. E., Hembree, E. A., Dougherty, L. M., & Spizzirri, C. C. (1983). Changes in facial expressions of 2- to 19-month-old infants following acute pain. Developmental Psychology, 19(3), 418–426.
- Johnson, E. O., Kamilaris, T. C., Chrousos, G. P., & Gold, P. W. (1992). Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews, 16, 115–130.
- Kagan, J. (1994). Galen’s prophecy. New York: Basic Books.
- Kagan, J. (2001). Temperamental contributions to affective and behavioral profiles in childhood. In S. G. Hofmann & P. Di Bartolo (Eds.), Social phobia and social anxiety: An integration. Boston: Allyn and Bacon.
- Kirschbaum, C., & Hellhammer, D. H. (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22, 150–169.
- Kochanska, G., Murray, K. T., & Harlan, E. T. (2000). Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology, 36(2), 220–232.
- Kopp, C. B. (1989). Regulation of distress and negative emotions: A developmental view. Developmental Psychology, 25(3), 343– 355.
- Kuo, Z. Y. (1976). The dynamics of behavior development. New York: Plenum Press.
- Lefcourt, H. M. (1973). The functions of the illusions of control and freedom. American Psychologist, 28, 417–425.
- Lerner, R. M. (1986). Concepts and theories of human development (2nd ed.). New York: Cambridge University Press.
- Levine, S. (1994). The ontogeny of the hypothalamic-pituitaryadrenal axis: The influence of maternal factors. Annals of the New York Academy of Sciences, 746, 275–288.
- Levine, S., & Wiener, S. G. (1988). Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuoendocrinology, 13, 143–154.
- Lewis, M., & Ramsay, D. S. (1995). Developmental change in infants’responses to stress. Child Development, 66(3), 657–670.
- MacLean, P. D. (1952). Some psychiatric implications of physiological studies on frontotemporal portions of limbic system (visceral brain). Electroencephalography and Clinical Neurophysiology, 4, 407–418.
- Maier, S. F., Ryan, S. M., Barksdale, C. M., & Kalin, N. H. (1986). Stressor controllability and the pituitary-adrenal system. Behavioral Neuroscience, 100(5), 669–674.
- Mangelsdorf, S. C. (1992). Developmental changes in infantstranger interaction. Infant Behavior and Development, 15, 191–208.
- Marshall, P. J., & Stevenson-Hinde, J. (1998). Behavioral inhibition, heart period, and respiratory sinus arrhythmia in young children. Developmental Psychobiology, 33, 283–292.
- McEwen, B. (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Science, 840, 33–44.
- Murphy, B. P. (1982). Human fetal serum cortisol levels related to gestational age: Evidence of a midgestational fall and a steep late gestational rise, independent of sex or mode of delivery. American Journal of Obstetrics and Gynecology, 144, 276–282.
- Nachmias, M., Gunnar, M. R., Mangelsdorf, S., Parritz, R., & Buss, K. (1996). Behavioral inhibition and stress reactivity: Moderating role of attachment security. Child Development, 67(2), 508–522.
- Nemeroff, C. B. (1996). The corticotropin-releasing factor (CRF) hypothesis of depression: New findings and new directions. Molecular Psychiatry, 1(4), 336–342.
- Palkovits, M. (1987). Organization of the stress response at the anatomical level. In E. R. de Kloet, V. M. Wiegant, & D. de Wied (Eds.), Progress in brain research (Vol. 72, pp. 47–55). Amsterdam: Elsevier Science.
- Panksepp, J. (1996). Affective neuroscience: A paradigm to study the animate circuits for human emotions. In R. D. Kavanaugh, B. Zimmerberg, & S. Fein (Eds.), Emotion: Interdisciplinary perspectives (pp. 29–60). Mahwah, NJ: Erlbaum.
- Parritz, R. H. (1996). A descriptive analysis of toddler coping in challenging circumstances. Infant Behavior and Development, 19, 171–180.
- Pitkanen, A., Savander, V., & LeDoux, J. E. (1997). Organization of intraamygdaloid circuitries in the rat: An emerging framework for understanding function of the amygdala. In J. Smith & G. Schoenwolf (Eds.), Neurulation (pp. 517–523). New York: Elsevier Science.
- Porges, S. W. (1995a). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A poly-vagal theory. Psychophysiology, 32(4), 301–318.
- Porges, S. W. (1995b). Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews, 19(2), 225–233.
- Porges, S. W., Doussard-Roosevelt, J. A., Stifter, C. A., McClenny, B. D., & Riniolo, T. C. (1999). Sleep state and vagal regulation of heart period patterns in the human newborn: An extension of the polyvagal theory. Psychophysiology, 36(1), 14–21.
- Porges, S. W., & Fox, N. A. (1986). Developmental psychophysiology. In M. G. H. Coles, E. Donchin, & S. W. Porges (Eds.), Psychophysiology (pp. 611–625). New York: Guilford Press.
- Posner, M. I., & Petersen, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42.
- Posner, M. I., & Rothbart, M. (2000). Developing mechanisms of self-regulation. Development and Psychopathology, 12(3), 427–442.
- Price, J. L. (1999). Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences, 877, 383–396.
- Quas, J. A., Hong, M., Alkon, A., & Boyce, W. T. (2000). Dissociations between psychobiologic reactivity and emotional expression in children. Developmental Psychobiology, 37, 153–175.
- Rosen, J. B., & Schulkin, J. (1998). From normal fear to pathological anxiety. Psychological Review, 105(2), 325–350.
- Rothbart, M. K., Derryberry, D., & Posner, M. I. (1994). A psychobiological approach to the development of temperament. In J. Bates & T. Wachs (Eds.), Temperament: Individual differences at the interface of biology and behavior (pp. 83–116). Washington, DC: American Psychological Association.
- Rothbart, M. K., Posner, M. I., & Rosicky, J. (1994). Orienting in normal and pathological development. Development and Psychopathology, 6, 635–652.
- Sameroff, A. (1983). Developmental systems: Contexts and evolution. In W. Kessen (Ed.), Handbook of child psychology: Vol. 1. History, theory, and methods (4th ed., pp. 237–294). New York: Wiley.
- Sanchez, M. M., Young, L. J., Plotsky, P. M., & Insel, T. R. (2000). Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience, 20(12), 4657–4668.
- Saper, C. B. (1995). Central autonomic system. In G. Paxinos (Ed.), The Rat Nervous System (pp. 107–135). New York: Academic Press.
- Schmidt, L. A., & Fox, N. A. (1999). Conceptual, biological, and behavioral distinctions among different categories of shy children. In L. A. Schmidt & J. Schulkin (Eds.), Extreme fear, shyness, and social phobia: Origins, biological mechanisms, and clinical outcomes (pp. 47–66). New York: Oxford University Press.
- Schmidt, L. A., Fox, N. A., Rubin, K. H., Sternberg, E. M., Gold, P. W., Smith, C. C., & Schulkin, J. (1997). Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology, 30(2), 127–140.
- Schmidt, L. A., Fox, N. A., Schulkin, J., & Gold, P. W. (1999). Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology, 35(2), 119–135.
- Schore, A. N. (1996). The experience-dependent maturation of a regulatory system in the orbital prefrontal cortex and the origin of developmental psychopathology. Development and Psychopathology, 8, 59–87.
- Selye, H. (1975). Confusion and controversy in the stress field. Journal of Human Stress, 1(2), 37–44.
- Spangler, G., & Grossmann, K. E. (1993). Biobehavioral organization in securely and insecurely attached infants. Child Development, 64, 1439–1450.
- Spangler, G., & Schieche, M. (1998). Emotional and adrenocortical responses of infants to the strange situation: The differential function of emotional expression. International Journal of Behavioral Development, 22(4), 681–706.
- Spangler, G., Schieche, M., Ilg, U., Maier, U., & Ackermann, C. (1994). Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology, 27(7), 425–437.
- Sroufe, L. A., & Waters, E. (1979). Heart rate as a convergent measure in clinical and developmental research. Merrill-Palmer Quarterly, 23(1), 3–27.
- Sroufe, L. A., Waters, E., & Matas, L. (1974). Contextual determinants of infant affective response. In M. Lewis & L. Rosenblum (Eds.), The origins of fear (pp. 49–72). New York: Wiley.
- Stansbury, K., & Gunnar, M. R. (1994). Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development, 59(2–3), 250–283.
- Steckler, T., & Holsboer, F. (1999). Corticotropin-releasing hormone receptor subtypes and emotion. Biological Psychiatry, 46, 1480–1508.
- Stevenson-Hinde, J., & Marshall, P. J. (1999). Behavioral inhibition, heart period, and respiratory sinus arrhythmia: An attachment perspective. Child Development, 70, 805–816.
- Stevenson-Hinde, J., & Shouldice, A. (1996). Fearfulness: Developmental consistency. In A. J. Sameroff & M. M. Haith (Eds.), The five to seven year shift: The age of reason and responsibility (pp. 237–252). Chicago: University of Chicago Press.
- Sullivan, R. M., & Grafton, A. (1999). Lateralized effects of medial frontal lesions on neuroendocrine and autonomic stress responses in rats. Journal of Neuroscience, 19, 2834–2840.
- Taddio, A., Katz, J., Ilarslch, A. L., & Koren, G. (1997). Effects of neonatal circumcision on pain response during subsequent routine vaccination. The Lancet, 340, 599–603.
- Takahashi, L. K., & Rubin, W. W. (1993). Corticosteroid induction of threat-induced behavior inhibition in preweanling rats. Behavioral Neuroscience, 107(5), 860–866.
- Tennes, K., Emde, R., Kisley, A., & Metcalf, D. (1972). The stimulus barrier in early infancy: An exploration of some formulations of John Benjamin. In R. R. Holt & E. Peterfreund (Eds.), Psychoanalysis and contemporary science: An annual of integrative and interdisciplinary studies (Vol. 1, pp. 206–234). New York: MacMillan.
- Thomasgard, M., & Metz, W. P. (1993). Parental overprotection revisited. Child Psychiatry and Human Development, 24, 67–80.
- Thompson, R. A. (1994). Emotion regulation: A theme in search of definition. In N. A. Fox (Ed.), The development of emotion regulation: Biological and behavioral considerations (pp. 25–52). Chicago: University of Chicago Press.
- Vaughn, B. E., Bradley, C. F., Joffe, L. S., Seifer, R., & Barglow, P. (1987). Maternal characteristics measured prenatally are predictive ratings of temperamental “difficulty” on the Carey Infant Temperamental Questionnaire. Developmental Psychology, 23(1), 152–161.
- Vazquez, D. M. (1998). Stress and the developing limbichypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology, 23, 663–700.
- Wadhwa, P. D., Sandman, C. A., & Garite, T. J. (2001). The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Progress in Brain Research, 133, 131–142.
- Waters, E., Matas, L., & Sroufe, A. (1975). Infants’ reactions to an approaching stranger: Description, validation, and functional significance of wariness. Child Development, 46, 348–356.
- Watson, J., & Ramey, C. (1972). Reactions to response-contingent stimulation in early infancy. Merrill-Palmer Quarterly, 13, 219–288.
- Weinstock, M. (1997). Does prenatal stress impair coping and regulation of the hypothalamic-pituitary-adrenal axis? Biobehavioral Review, 21, 1–10.
- West, M. J., & King, A. P. (2001). Science lies its way to the truth . . . really. In E. Blass (Ed.), Handbook of behavioral neurobiology, Vol. 13: Developmental psychobiology, developmental neurobiology, and behavioral ecology: Mechanisms and early principles. New York: Plenum.
- White, B. P., Gunnar, M. R., Larson, M. C., Donzella, B., & Barr,
- G. (2000). Behavioral and physiological responsivity, and patterns of sleep and daily salivary cortisol in infants with and without colic. Child Development, 71(4), 862–877.
- Wilson, B. J., & Gottman, J. M. (1996). Attention-The shuttle between emotion and cognition: Risk, resiliency, and physiological bases. In E. M. Hetherington & E. A. Blechman (Eds.), Stress, coping, and resiliency in children and families (pp. 189–238). Mahwah, NJ: Erlbaum.
- Yehuda R. (1998). Psychoneuroendocrinology of post-traumatic stress disorder. Psychiatric Clinics of North America, 21(2), 359–379.




