View sample sex behavior research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
In this research paper we summarize the progress that has been made in understanding sexual differentiation, as well as the hormonal and neural mechanisms that drive and direct male and female sexual behavior. We begin with the question of why sexual reproduction is by far the most common means of propagating multicellular species even though asexual reproduction is theoretically much faster and easier. We then describe copulatory patterns that are common across mammalian species and summarize various laboratory tests of sexual behavior. Because hormones are important for sex differentiation in all mammalian and avian species, and because hormones also activate sexual behavior in adulthood, we discuss the means by which hormones exert their influence. We next describe the hormonal and neural control of female sexual behavior, followed by a similar treatment of the control of male sexual behavior. In each case, we first summarize the effects of systemically administered drugs and hormones or other treatments that affect more than one brain area.We then review the information concerning the specific brain areas that are implicated in the control of the behavior, including effects of lesions and stimulation, local application of drugs and hormones, and measures of neural activity. Finally, we observe that the hormonal and neural mechanisms that control sexual behavior are similar to the mechanisms that regulate other social behaviors. We close with a series of questions that remain unanswered and that may form a basis for future research. We hope that the reader will gain an understanding of the theoretical context in which sexual reproduction is embedded as well as an appreciation for both the similarities and the variations in the means by which sexual motivation is translated into successful reproduction.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Why Sex?
Why is sexual reproduction so prevalent? A sexual reproduction potentially results in twice as many offspring per generation and costs much less time and energy. Most multicellular animals spend large amounts of time and energy sizing up the field of potentia lmates and then preparing to copulate with the most desirable of the lot.The fact that sexual reproduction persists in the face of obvious disadvantages suggests that very important benefits accrue to sexually produced offspring. One advantage of sexual reproduction is that the recombination of genes promotes survival in times of environmental change. It also promotes differential adaptation to various niches in a time of relative constancy and decreases the ability of pathogens to exploit a single genotype. Another advantage is that some offspring will inherit fewer harmful mutations than either parent and will therefore be advantaged, whereas other offspring inherit more than the average number of harmful mutations and are more likely to die before reproducing, thereby carrying a large load of mutations to the grave (Kondrashov, 1988). In addition, meiotic recombination allows for repair of DNA. Lack of such recombination of the Y chromosome has resulted in evolutionary degeneration so that it is now only a shadow of its partner, the X chromosome, with which it now aligns only in the uppermost region.
Sex Differentiation
Fish and Reptiles
Sexual reproduction implies differentiation into different sexes. One might think that because of the importance of sex differentiation, evolutionarily early mechanisms for differentiation would have been conserved. However, a brief perusal of such mechanisms provides a richly varied list. Among fish there are both simultaneous and sequential hermaphrodites. Simultaneous hermaphrodites have ovotestes that produce both eggs and sperm; these fish alternate between masculine and feminine patterns of behavior. Sequential hermaphrodites begin life as one sex and then change to the other in response to environmental or genotypic influences. Among other species of fish, males are polymorphic; that is, there are two male phenotypes. For example, among plainfin midshipmen, Type I males are larger and are territorial, whereas Type II males resemble females and sneak into the nest sites of Type I males and release their sperm (reviewed in Nelson, 2000).
Among some species of reptiles, sex differentiation is determined genetically, as in birds and mammals. However, in many species of lizards, turtles, and crocodiles, the ambient temperature during incubation of the eggs determines whether offspring will be male or female. In some species, warmer temperatures produce females; in others, warm temperatures produce males. In snapping turtles and crocodiles, females are produced if temperatures are either very high or very low. Apparently, the temperature influences the differentiation of the embryonic gonad into either a testis or an ovary; however, the mechanism for this influence is not known (Crews, Bull, & Billy, 1988).
As in fish, the males of some species of lizards exhibit multiple phenotypes. High levels of both testosterone and progesterone during early posthatching development result in territorial males with orange and blue dewlaps (skin flaps) attached to their throats (Moore, Hews, & Knapp, 1998). Low levels of testosterone and progesterone produce nonterritorial males with plain orange dewlaps. The presence of high corticosterone as a result of stress in adulthood determines whether nonterritorial males are sedentary or nomadic. Thus, interactions between early and late hormonal influences and environmental factors determine the coloration and behavior of these lizards.
Birds
In birds a ZZ chromosomal configuration confers maleness, whereas females have a ZW chromosomal pattern. The homogametic sex (the sex with two of the same type of sex chromosomes)isthedefaultsexforreproductivebehaviorpatterns (Balthazart & Ball, 1995). Thus, birds with two Z chromosomes will develop male-typical reproductive behaviors in the absence of gonadal secretions. Secretion of estrogen by ovaries of ZW individuals organizes female-typical reproductive behavior patterns. However, for singing behavior, female-typical lack of singing is the default condition, unless testosterone masculinizes the song-producing neural circuits. For other types of behavior, neither the male- nor femaletypical pattern is the default condition. The W chromosome directs the differentiation of the left primitive gonad to become an ovary, which secretes estrogen, causing the right Müllerian duct to degenerate. The lack of a W chromosome, and of the resultant production of estrogen, results in retention of both Müllerian ducts.
Mammals
In mammals the homogametic sex is the female, with an XX chromosomal pattern. In the absence of hormones, a phenotypic female develops. A gene on the Y chromosome, the sex-determining region of the Y chromosome (SRY), produces a locally acting protein that causes the primitive gonads to differentiate into testes. The testes then secrete androgens and peptide hormones that produce masculine differentiation. Differentiation of the external genitalia is mediated primarily by a metabolite of testosterone, 5-alpha-dihydrotestosterone (DHT), which binds with higher affinity than testosterone to the intracellular androgen receptor. Testosterone is converted to DHTby the enzyme 5-alpha-reductase, which is present in the genital skin of embry onic males and females. Under androgenic influence the genital folds that would become labia in females fuse into a scrotum; the genital tubercle enlarges into a penis, rather than a smaller clitoris; and the genital groove fuses to become a duct for both urine and semen.
Because females possess 5-alpha-reductase and androgen receptors, their genitalia may be partially masculinized if they are exposed to high concentrations of androgens during development, as in congenital adrenal hyperplasia (CAH). Individuals with CAH lack or have insufficient amounts of the enzyme that produces cortisol, the major glucocorticoid in humans. As a result of the lack of negative feedback from cortisol, blood concentrations of adrenocorticotropic hormone (ACTH) are high; they stimulate the adrenal cortex to produce excess adrenal androgens that partially masculinize a female fetus’s genitalia. Conversely, if males lack 5-alphareductase, their genitalia will be incompletely masculinized, and they may be thought to be female at birth (ImperatoMcGinley, Guerrero, Gautier, & Peterson, 1974). However, the pubertal surge of testosterone is sufficient to masculinize the genitals, and the individuals become phenotypically, as well as genotypically, male.
Another disorder of differentiation is androgen insensitivity syndrome, in which a genetic male (XY chromosome pattern) lacks androgen receptors. As a result, the testosterone produced by internal testes cannot masculinize the body, and the individual is phenotypically female. However, she lacks female internal genitalia and is sterile.
Individuals whose genitals are ambiguous at birth are called intersexes. Controversy has arisen over the medical and psychological treatment of intersexes. Often, babies born with small penises or large clitorises have been subjected to surgical “correction,” usually reducing the size of the penis or clitoris and forming a vagina. It was thought that gender identity is very malleable and that an individual could easily adopt the gender role that was assigned. However, this surgical reconstruction usually left the individual with greatly diminished, or absent, genital sensations, and frequently with little information, counseling, or medical follow-up (FaustoSterling, 2000). Because of these problems, new guidelines for the management of intersexuality have been proposed (Diamond & Sigmundson, 1997).
In most male rodents, differentiation of brain mechanisms controlling sexual behavior and endocrine function is mediated by estradiol, which is produced from testosterone by the enzyme aromatase. Females usually are not masculinized by their own and their mother’s estradiol because estradiol is bound to alpha-fetoprotein, which keeps estradiol circulating in the blood, rather than entering cells to bind to estrogen receptors. Exposure to excess estradiol during early development can masculinize female rodents so that they display masculine sexual preferences and behavior patterns if they are given estradiol or testosterone in adulthood (McCarthy, Schlenker, & Pfaff, 1993). Among primates and guinea pigs, androgens, rather than estradiol, are the primary masculinizing and defeminizing hormones (Goy & McEwen, 1980).
Although the neural bases of reproductive behavior are permanently differentiated early in life, hormones are required during adulthood in order to activate the patterns that were previously organized. This finding has been referred to as the organizational-activational distinction.
Patterns of Sexual Behavior in Mammals
Female Reproductive Cycles
Female reproductive cycles consist of a preovulatory follicular phase, during which the follicle surrounding the oocyte (immature egg) secretes increasing amounts of estrogen and promotes the development of the oocyte. Following ovulation, the remnant of the follicle, the corpus luteum, secretes progesterone, which prepares the uterus for implantation of a fertilized egg.
There are three types of reproductive cycles in female mammals. Type 1 cycles, characterized by spontaneous ovulation and a spontaneous luteal phase, are exhibited by primates (including women), dogs, and guinea pigs. As the follicle grows, it secretes more and more estrogen in response to follicle-stimulating hormone (FSH) from the anterior pituitary. When the level of estrogen rises high enough, it triggers a positive feedback response, in which a surge of luteinizing hormone (LH), accompanied by a smaller surge of FSH, is released from the anterior pituitary. Luteinizing hormone causes the oocyte to undergo its first meiotic division and to break free of the surrounding follicle. The follicle then becomes the corpus luteum (yellow body) and secretes progesterone and smaller amounts of estrogen, which increase the vascularization of the uterus. If the oocyte is fertilized by a sperm, it will undergo its second meiotic division, develop into a blastocyst in the fallopian tube or uterine horn, and implant in the highly vascularized uterus. If fertilization does not occur, the lining of the uterus is either sloughed off, as in humans and other great apes, or resorbed.
Type 2 cycles require the stimuli derived from copulation in order to induce ovulation, but when ovulation does occur, the luteal phase is spontaneous. Type 2 cycles are characteristic of animals that live solitary lives, including cats, rabbits, voles, and ferrets. Thus, ovulation, and in some cases behavioral estrus as well, occurs only when a male is present.
Animals with Type 3 cycles ovulate spontaneously but require copulatory stimuli to induce a luteal phase. Rats, mice, and hamsters exhibit Type 3 cycles. Both Type 2 and Type 3 cycles minimize the amount of time spent in a nonpregnant state and are seen in animals that tend to be short-lived and to produce a large number of offspring.
Because females are fertile for only a relatively brief period, it is usually important for them to advertise their sexual interest. Attractive chemosensory pheromones are released, and in some species physical changes in the genital region occur. In addition, the female may engage in a series of proceptive behaviors, which are defined as those that indicate the female’s motivation to engage in sexual activity.These behaviors may include approaches to a male, alternating approaches and withdrawals, prolonged eye contact, vocalizations, and presentation of the genital region. The third component of female sexuality, in addition to attractivity and proceptivity, is receptivity. Behaviors associated with receptivity include postures that permit the male to copulate successfully. All three components of female sexuality (attractivity, proceptivity, and receptivity) are enhanced by estrogen, which also leads to ovulation and therefore fertility. It is not surprising that evolutionary processes ensure that the most attractive females, from a male’s perspective, are those that are the most fertile and also those that display the greatest sexual motivation and responsiveness.
Copulatory Patterns Common Across Mammalian Species
Some patterns of copulation are common across species. In many mammals copulation is preceded by the male’s investigation of the female’s genitals, which allows him to determine whether she is receptive and provides him with sexually arousing stimuli. If the female is receptive, the male will mount from herrear,claspherflankswithhisforepaws,andbeginaseriesof rapid, shallow thrusts with his pelvis. Usually, the male’s penis is at least partially erect during this thrusting. In response to flank contact or the actual mount, the female will typically display lordosis, a rather rigid posture in which her back is flat or concave and her tail is deflected. By exposing the vagina, lordosis makes it possible for the male to achieve intromission (insertion of his penis into the female’s vagina).
If the male does not detect the vagina with his penis soon after he begins thrusting, he usually dismounts and either reapproaches the female or engages in other activities. If a male rodent does detect the female’s vagina, he typically performs a deeper, intravaginal thrust, followed immediately by a springing dismount. This springing dismount is usually used as the measure of intromission in rats and many other rodents because it is reliably associated with penile insertion (Sachs, 1983). After an intromission male rodents typically groom their genitals and wait for a minute or two before mounting again. Male rats ejaculate after about 10 such intromissions. Ejaculation is characterized by a deeper intravaginal thrust, a much slower dismount, prolonged genital grooming, and ultrasonic vocalizations during the postejaculatory interval of quiescence.
In other species, such as mice, the male maintains the intromission and shows repeated intravaginal thrusting before ejaculating (Mosig & Dewsbury, 1976). Male ungulates may ejaculate immediately after intromission (Lott, 1981). Dogs and other canids begin to ejaculate soon after penile insertion, but their penis swells to such an extent that it remains locked in the vagina for up to 30 min, thereby promoting sperm transport (Beach & LeBoeuf, 1967). Ejaculation is usually accompanied by rhythmic contractions of skeletal muscles and the striated muscles of the perineal area.
Ejaculation is typically followed by genital grooming and a period of sexual quiescence. The postejaculatory interval of quiescence may last for less than 30 s in Syrian hamsters (Bunnell, Boland, & Dewsbury, 1976), for 5 to 10 min in rats, or hours or days in other species (Dewsbury, 1972). During this time male rats make ultrasonic calls, and male gerbils stomp their feet. Toward the end of the period, introduction of a novel female may elicit renewed copulation. The lack of copulation during the postejaculatory interval does not result from erectile failure, at least in some species. In rats, for example, ex copula touch-based erections are actually enhanced following an ejaculation (O’Hanlon & Sachs, 1980). At the end of the postejaculatory interval, copulation is likely to occur again.
A male rat may achieve up to seven or eightejaculations before reaching sexual satiety, which lasts for several days. After reaching satiety with one female, some males can be induced to begin copulating with a new female. This phenomenon is sometimes referred to as the Coolidge effect, a reference to an anecdote involving President and Mrs. Calvin Coolidge. When visiting afarm, Mrs. Coolidge observed that one rooster mated repeatedly during her visit to the chicken pen and asked the farmer to call Mr. Coolidge’s attention to the rooster’s activities when the President visited the facility. When the farmer relayed the message later that day, Mr. Coolidge asked the farmer to point out to Mrs. Coolidge that the repeated activity was directed toward many different hens.
Females with Type 2 or Type 3 reproductive cycles require the stimulation of copulation to trigger ovulation or a luteal phase, respectively. For example, spines on the male cat’s penis scratch the female’s vagina, a rather painful way to induce ovulation (Type 2 cycle). Female rats typically require five or six intromissions, separated by approximately 2-min intervals, to elicit a luteal phase (Type 3 cycle). In the wild, or in seminatural environments, female rats pace their interactions with males in order to achieve the correct timing and number of intromissions before ejaculation (McClintock, 1987). Indeed, there was a higher rate of pregnancy in females receiving 5 paced intromissions than in those receiving 10 nonpaced intromissions (Erskine, 1985). Females tested in a place-conditioning apparatus spent more time in the paced mating compartment, but not in the nonpaced mating compartment, compared to a neutral compartment (Paredes & Alonso, 1997). Females developed place preferences even if they were not actively pacing, if males were placed into their compartments at their preferred intervals (Jenkins & Becker, 2001b). Thus, copulation was rewarding only if it occurred at the female’s preferred intercopulatory interval.
In addition to triggering a luteal phase, multiple intromissions or intravaginal thrusting may increase the number of sperm in the male’s ejaculate and facilitate sperm transport in the female’s reproductive tract (Adler & Toner, 1986), or promote male-female bonding (reviewed in Carter, DeVries, & Getz, 1995). On the other hand, copulation is energetically expensive, and lengthy copulation may expose the animals to predation. Therefore, copulatory behavior reflects a balance of selection pressures imposed by the physical, biological, and social environments.
Testing Paradigms
Use of a Limited Number of Species
As in other areas of biology, most research on sexual behavior has been done on a limited number of species, most of them rodents. The rat is a common model because it is relatively inexpensive and there is currently much information on its neural and endocrine systems. However, focusing on a limited number of species limits the opportunities to identify interesting variations and to correlate neural and behavioral variations.
Tests of Sexual Motivation
It is useful to distinguish between sexual motivation and copulatory performance. However, these concepts may be difficult to measure. Lesions or drugs may alter the ability to detect or interpret stimuli, perform copulatory movements, or remember stimuli associated with previous sexual encounters. Drugs or lesions may cause general malaise, and altered stimuli from one partner may inhibit the behavior of the other, thereby compounding the copulatory deficits.
Mount and intromission latencies are common measures of sexual motivation. However, intromission latency depends on the ability to achieve an erection, as well as on motivation. There are several tests of sexual motivation that are not based directly on copulatory behavior. In place-preference tests one partner is initially allowed to copulate in one of two interconnected areas and to be alone in the other area. The subject later spends time in the side previously associated with copulation or in the side it inhabited alone. In a second technique a subject must cross an obstruction in order to reach a sexual partner. Another test of sexual motivation is the X-maze or cross-maze, in which a sexual partner is placed into one of four interconnected goal boxes; the other three goal boxes contain different objects or remain empty. The number of choices of each goal box, the latencies to reach each goal box, and the number of no-choice trials are measured. A fourth technique uses a bilevel apparatus in which a male and female are initially allowed to copulate throughout the apparatus. The subject is later placed alone into the apparatus, and the number of times he or she changes levels, presumably in search of the partner, is tabulated. A final measure is lever pressing for a secondary reinforcer that has been paired with copulation. In several of these tasks motivational factors are confounded with motor ability or with the ability to learn the secondary reinforcement task. Therefore, care must be given to the choice of test to be used and to the interpretation of results.
Tests of Female Attractivity
Female attractivity is measured primarily by allowing a male to spend time with one female or another. In some tests the bedding from a female’s home cage, which presumably contains the pheromones excreted either directly from the anogenital region or in the urine, is presented to a male, who spends time in contact with the bedding of the estrous female or that of a nonestrous female, a male, or clean bedding.
Tests of Female Proceptivity
Female proceptivity is measured by direct observation of behaviors that increase the likelihood of sexual contact. These include approach to the male, display of the genital region, and species-typical behaviors, such as hopping and darting by female rats and increased eye contact and tongue flicking by primates.
Tests of Female Receptivity
Female receptivity is usually measured as the number of receptive postures displayed divided by the number of mounts by a male (lordosis quotient). Some tests of receptivity include a quantification of lordosis quality, ranging from 0 (no lordosis behavior) to 1 (brief stationary posture with flat back), 2 (slightly concave back), 3 (markedly concave back), and 4 (markedly concave back, a posture that is held for several seconds).
Tests of Male Copulatory Behavior
Male copulatory behavior is usually quantified in tests that use both temporal and behavioral criteria to determine test length. In this way the initiation of sexual behavior can be distinguished from the ability to copulate to ejaculation after copulation has begun. Other test paradigms allow the male to mate until he achieves sexual satiety, defined as failure to resume copulation within a specified time after the last ejaculation.
Male rat copulatory behavior has been analyzed into four weakly correlated factors (Sachs, 1978). First, a copulatory rate factor includes the interintromission interval, the ejaculation latency, the time from an ejaculation to the termination of ultrasonic vocalization, and the postejaculatory interval before the next intromission. These four measures are highly correlated in tests of normal males, but they can be dissociated by experimental treatments; therefore, they may be controlled by separate physiological mechanisms. Three other factors included an initiation factor based primarily on mount and intromission latencies, an intromission ratio factor based on the number of intromissions divided by the number of mounts plus intromissions, and an intromission count factor based on the number of intromissions preceding ejaculation. A later factor analysis, based on copulation tests in bilevel chambers, identified an anticipatory factor, reflecting the number of times the male changed levels, in addition to the four factors just noted (Pfaus, Damsma, et al., 1990).
Tests of Penile Function
Erection, intromission, and ejaculation can be easily observed in studies of monkeys, dogs, cats, and many other species, including humans. However, in rodents these penile components of copulation are more often inferred than observed. In some experiments an angled mirror was placed under a clear floor of a test cage to facilitate observation of penile actions; in other experiments the female’s vagina was inspected after copulation for evidence of sperm. Because genital reflexes are difficult to measure while the male is copulating, paradigms have been developed for monitoring them ex copula. However, different physiological mechanisms may control erection in different contexts (reviewed in Sachs, 2000).
Spontaneous erections can occasionally be observed when a male is alone in his home cage or in a neutral arena. Such erections can be increased by various drugs, in which case they are called drug-induced erections. The number of spontaneous erections is increased in the presence of an inaccessible estrous female (Sachs, Akasofu, Citron, Daniels, & Natoli, 1994) or the volatile odors of an estrous female (Kondo, Tomihara, & Sakuma, 1999). These noncontact erections are a model for psychogenic erections in humans and appear to have physiological bases similar to those of spontaneous and drug-induced erections.
Touch-based erections have been elicited by manually stimulating the penes of dogs or other species. However, tactile stimulation of the penis in rats and other rodents inhibits erection. Therefore, Hart developed a technique that exerts pressure at the base of the penis of rats (1968) or mice (Sachs, 1980). The male is restrained in a supine position, and the penile sheath is retracted, exposing the glans penis. The continuing pressure of the retracted sheath around the base of the penis elicits a series of erections. Penile anteroflexions (flips) may also occur. Occasionally, semen is emitted, usually as a result of drug administration. These ex copula reflexes are similar in form and mechanical basis to those used in copula (Holmes, Chapple, Leipheimer, & Sachs, 1991); however, the temporal relations are different, as are the hormonal mechanisms of control (Sachs, 1983).
The Urethrogenital Reflex
Another ex copula genital response is the urethrogenital reflex, which has been proposed as a model for the human orgasmic reflex. This reflex has been elicited in both male and female rats that had been anesthetized and spinally transected (McKenna, Chung, & McVary, 1991). Typically, the urethra is filled with saline under pressure, and then the pressure is rapidly released. The reflex consists of rhythmic contractions of the pelvic muscles, with similar timing as in human orgasm.
Principles of Hormonal Action
Genomic Effects
Hormones are blood-borne chemical messengers that are produced and released by endocrine glands and that act on tissues located at some distance from the secreting gland. Because they circulate throughout the body, the specificity of their action depends on the presence of specialized receptors in the target tissues or organs. Most of the cellular receptors that are important for sexual behavior act by initiating or repressing transcription of certain genes. According to the most widely accepted model of hormonal action, a steroid hormone molecule binds to its cognate receptor in the cytoplasm of the cell. The hormone-receptor complex then enters the cell nucleus, where it dimerizes (links to another hormonereceptor complex); the dimer then binds to a hormone response element upstream of a structural gene and initiates transcription of the appropriate mRNA, which is in turn translated into a protein. The resultant protein may be a regulator of transcription of additional genes, or it may be an enzyme, a receptor, or a structural protein. Some hormonal effects may be exerted indirectly by increased activity impinging on downstream neurons.
The importance of steroid receptors has been demonstrated by profound deficits in masculine and feminine sexual behavior observed in male and female mice that lack the classic estrogen receptor (ER). These knockout (ERKO) animals usually exhibit little or no copulatory behavior (Ogawa et al., 1998; Wersinger et al., 1997). Administration of estradiol, with or without progesterone, to ovariectomized female ERKO mice did not result in receptivity (Rissman, Early, Taylor, Korach, & Lubahn, 1997). Furthermore, male mice frequently behaved aggressively toward ERKO female intruders but never to wild-type females, suggesting that attractivity was also impaired by the lack of estrogen receptors (Ogawa et al., 1996).
Animals lacking progesterone receptors (progesterone receptor knockout mice, or PRKOs) have also been produced. PRKO females do not ovulate, and after ovariectomy they do not respond behaviorally to estradiol or progesterone injections (Mani, Blaustein, & O’Malley, 1997). Similar results were obtained when estrogen-primed female rats were injected with antisense to the progesterone receptor into the ventromedial nucleus of the hypothalamus (VMH), an important area for the control of receptivity (Ogawa, Olazabal, Parhar, & Pfaff, 1994). (Antisense oligonucleotides bind to mRNA for the designated protein, thereby preventing translation of the protein.) Male PRKO mice, however, showed a copulatory deficit only on their first copulatory tests (Phelps, Lydon, O’Malley, & Crews, 1998).
Rapid, Nongenomic Effects
Besides their slow, genomically mediated effects, steroid hormones may have rapid effects. For example, estrogen had very rapid effects on neuron membranes (Xiao & Becker, 1998). In addition, progesterone and its metabolites acted in an agonist-like manner to increase functioning of gammaaminobutyric acid (GABAA) receptors (Majewska, Harrison, Schwartz, Barker, & Paul, 1986), thereby increasing chloride influx and hyperpolarizing neurons. Testosterone has affected cell firing in the medial preoptic area (MPOA) of castrated male rats within minutes (Pfaff & Pfaffman, 1969) or seconds (Yamada, 1979). Furthermore, neurons in slices from the MPOA showed changes in firing rates within minutes of estrogen or testosterone administration via the perfusion medium (Silva & Boulant, 1986). On the other hand, hours or days of steroid hormone replacement are required to restore copulation in gonadectomized animals. Although rapid membrane effects of estrogen are not sufficient for induction of estrus in female rats and rapid effects of testosterone are not sufficient to restore sexual behavior of castrated male rats, they may contribute to such facilitation. For example, rapid effects of progesterone in the ventral tegmental area (VTA) of the midbrain prolonged lordosis in female rats and hamsters (Frye & Vongher, 1999).
There is evidence for a rapid effect of testosterone on erectile function (Sachs & Leipheimer, 1988). Electromyograph (EMG) recordings during tests of touch-based erections revealed penile muscle activity in some testosterone-treated castrated rats within 5 min after injection. However, testosterone did not restore erection at that time. Inhibition of protein synthesis by the antibiotic anisomycin did not affect the short-latency (within 24 hr) activation of touch-based erections by testosterone (Meisel, Leipheimer, & Sachs, 1986). Therefore, protein synthesis was not a necessary component of the hormonal activation of touch-based erections.
Activation of Female Sexual Behavior By Gonadal Hormones
Dependence of Most Nonprimate Species on Steroid Hormones
Females of virtually all nonmammalian species that have been tested mate only during a period of elevated blood estrogens (Crews & Silver, 1985). Most nonprimate female mammals are also completely dependent on hormones to elicit proceptive and receptive sexual behaviors. The estrous cycles of rats are typically four (occasionally five) days long. During two days of diestrus, plasma concentrations of estrogen and progesterone are low, although estrogen begins to rise during the second day of diestrus. On the day of proestrus, estrogen peaks in the afternoon, followed several hours later by progesterone. Hormone levels then fall precipitously, so that estrogen is at basal levels by the beginning of the day of estrus; progesterone declines to its nadir by the middle of the day of estrus. Female rats are receptive only during the evening of proestrus. Some ovariectomized female rats can be induced to become receptive following injections of low doses of estrogen alone, but most require a subsequent surge of progesterone. One function of the initial surge of estrogen is to up-regulate the production of progesterone receptors, stimulation of which then elicits the proceptive and receptive behaviors.
On the other hand, female sheep require progesterone before estrogen (Robinson, 1954). Other rodents, such as prairie voles (Carter, Witt, Auksi, & Casten, 1987) and hamsters (Wynne-Edwards, Terranova, & Lisk, 1987), require only estrogen for receptivity. Female musk shrews aromatize circulating testosterone to estradiol in the preoptic area and hypothalamus (Rissman, 1991). Thus, there is much variability in the pattern of hormone secretion, but females of most nonprimate species require hormones associated with ovulation in order to become sexually receptive.
Increased Likelihood of Copulation by Periovulatory Female Primates
Sexual behavior in female primates is less dependent on hormones. Female monkeys readily display proceptive and receptive behaviors throughout their 28-day cycle in laboratory tests with a single male partner. However, sexual interest is heightened during the periovulatory period. This increase is especially noticeable in female monkeys in the wild or in seminatural environments, where females are subjected to aggression and harassment by other females if they show proceptive behaviors toward a male (Wallen, 1990). As a result, females are willing to risk this aggression only around the time of ovulation, when high levels of estrogen increase sexual motivation.
In humans, too, there may be increased sexual interest around the time of ovulation. Among women in stable sexual relationships, there is relatively little variation in the frequency of copulation throughout the menstrual cycle (Adams, Gold, & Burt, 1978). However, there is a peak in erotic thoughts and in autoerotic activity around the time of ovulation and a smaller increase shortly before menstruation (Adams et al., 1978; Slob, Bax, Hop, Rowland, & Van der Werff ten Bosch, 1996). This pattern of increased periovulatory sexual interest and of a secondary peak shortly before the onset of menstruation was also observed among lesbians (Matteo & Rissman, 1984).
Hormonal Control of Sensory Processes
One way in which hormones facilitate sexual behavior is by enhancing the processing of sensory information. Females have generally greater sensitivity for chemosensory stimuli, including species-specific pheromones, than do males (Doty, Applebaum, Zusho, & Settle, 1985).This sensitivity is further enhanced by increased periovulatory estrogen concentrations. Both female mice and women are able to use chemosensory stimuli to express preferences for males with certain immune system markers that are different from their own (reviewed in Wedekind & Penn, 2000). The resultant increase in ability of the offspring’s immune system to recognize a greater variety of invaders contributes to their survival. Males are unable to detect these differences.
Somatosensory input from the flank area is also enhanced by estrogen in female rats. Pressure on the flank before and during a mount increases the likelihood or intensity of lordosis. The size of the receptive fields (the areas on the skin that elicit an electrophysiological response) of sensory nerves increases following estrogen administration to ovariectomized females (Kow, Montgomery & Pfaff, 1979). As a result, the flanks become more sensitive to the stimuli that elicit lordosis.
Systemicallyadministered Drugs Affect Female Sexual Behavior
The slow, genomically mediated effects of steroid hormones on copulatory behavior are mediated primarily by up- or down-regulation of some aspect of neurotransmitter function. Because neurotransmitters often act synergistically in more than one site and because the site of action often is not known a priori, systemic administration of drugs can be advantageous. However, drugs can have interfering actions at different sites. Therefore, to gain a full understanding of neurotransmitter influences, some experiments should administer drugs widely throughout the system, whereas in other experiments drugs should be targeted to specific sites. Table 12.1 summarizes the effects on female sexual behavior of drugs and treatments that affect neurotransmitter function in more than one brain area.
Brain Areas Implicated in the Activation of Female Sexual Behavior
Neural Control of Proceptivity
Medial Preoptic Area and Ventromedial Hypothalamus
Pfaff (1999) suggested that the MPOAis important for active proceptive behaviors; it then must decrease its activity during the stationary lordosis posture, which is promoted by the ventromedial hypothalamus (VMH). Estrogen increased the facilitative effect of the preoptic area on the midbrain locomotor region, thereby enhancing proceptive behaviors (Takeo & Sakuma, 1995).
Dopamine release in the MPOAof ovariectomized female rats increased with the onset of sexual receptivity following estrogen and progesterone injections (Matuszewich, Lorrain, & Hull, 2000). Dopamine concentrations increased further when a male was introduced and the animals copulated; however, a significant increase occurred only in a nonpacing environment, although dopamine metabolites increased in both pacing and nonpacing conditions. Perhaps the lack of an increase in dopamine was related to the smaller number of copulatory behaviors in the pacing environment.
Nucleus Accumbens
Dopamine release in the mesolimbic dopamine tract, which ends in the nucleus accumbens (NAcc) and several other limbic sites, may be critical for the rewarding aspects of paced mating. Dopamine is released in the NAcc and dorsal striatum of female rats (Mermelstein & Becker, 1995; Pfaus, Damsma, Wenkstern, & Fibiger, 1995) and hamsters (Meisel, Camp, & Robinson, 1993) during paced, but not during nonpaced, copulation. Furthermore, paced copulation is rewarding for female rats, but nonpaced copulation is not (Oldenburger, Everitt, & de Jonge, 1992; Paredes & Alonso, 1997). Even when the female is not actively in control of copulatory intervals and the male is removed and replaced at her preferred interval, dopamine is released (Becker, Rudick, & Jenkins, 2001), and the female develops a conditioned place preference for the copulatory arena (Jenkins & Becker, 2001b).
Neural Control of Receptivity
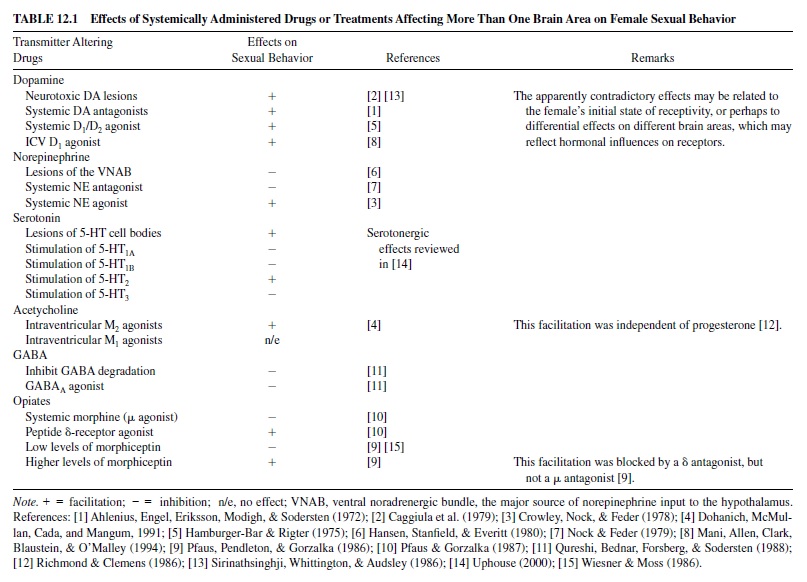
Pfaff and Schwartz-Giblin (1988) have provided a thorough and elegant model of the neural mechanisms that control lordosis (see Figure 12.1). The functions of the five modules that they identified range from slow hormone-mediated disinhibition of behavior to moment-to-moment postural adjustments.
The Forebrain Module
The forebrain exerts primarily inhibitory effects, but there are selective facilitative effects. Electrolytic lesions of the lateral septum increased receptivity in female rats (Nance, Shryne, Gordon, & Gorski, 1977), whereas electrical stimulation inhibited lordosis in female hamsters (Zasorin, Malsbury, & Pfaff, 1975). Thus, the septal area is generally inhibitory to receptivity in female rodents. Olfactory bulbectomy increased lordosis responses in hormone-primed female rats (Lumia, Meisel, & Sachs, 1981) and in gonadally intact, cycling female rats (Al Satli & Aron, 1977). However, removal of only vomeronasal input inhibited responding in female hamsters (Mackay-Sim & Rose, 1986).
The Hypothalamic Module
The hypothalamic module is the primary site for slow hormone-mediated effects. Estradiol and progesterone bind to their respective receptors in the VMH and the preoptic area and initiate RNA transcription and protein synthesis. These hormones also alter the electrophysiological responsiveness of neurons in the hypothalamus. There are apparently contradictory reports concerning the role of the preoptic area in controlling lordosis behavior of female rats. On the one hand, lesions of the MPOA facilitated lordosis responding in hormonally primed females, and electrical stimulation inhibited lordosis (Takeo, Chiba, & Sakuma, 1993). However, in another study MPOA lesions inhibited lordosis (Bast, Hunts, Renner, Morris, & Quadagno, 1987). Even in animals whose receptive behavior was enhanced by MPOA lesions, proceptive behavior was suppressed (Hoshina, Takeo, Nakano, Sato, & Sakuma, 1994), suggesting that the intact MPOA inhibits receptivity and facilitates proceptivity.Asingle-neuron recording experiment suggests a resolution of the apparent contradiction: Different groups of neurons, located in slightly different subregions of the preoptic area, may promote proceptive behavior and lordosis (Kato & Sakuma, 2000).
Lesions of the VMH have consistently impaired lordosis responses and also increased male-typical behavior (Dörner, Döcke, & Götz, 1975). Conversely, electrical stimulation facilitated lordosis (Pfaff & Sakuma, 1979). Sequential implantation of estradiol and progesterone into the VMH was sufficient to restore receptivity in ovariectomized rats (Rubin & Barfield, 1983). Furthermore, estrogen injections in ovariectomized female rats induced transcription of RNA for the progesterone receptor selectively in the ventromedial hypothalamus of females, but not in males (Lauber, Romano, & Pfaff, 1991). Progesterone’s ability to facilitate behavior was blocked by administration of antisense to the progesterone receptor into the VMH (Ogawa et al., 1994). Estradiol also increased mRNA for preproenkephalin (the precursor of enkephalin) in the VMH (Romano, Harlan, Shivers, Howells, & Pfaff, 1988). Enkephalin mRNA synthesis in the VMH was highly correlated with the female rat estrous cycle (Funabashi, Brooks, Kleopoulos, Grandison, Mobbs, & Pfaff, 1995).
Estradiol priming is essential for the release of norepinephrine in the VMH of female rats during copulation (Vathy & Etgen, 1988). Norepinephrine stimulates (α1 receptors, which are associated with increased neural activity of the VMH and with the activation of lordosis (Kow, Weesner, & Pfaff, 1992). Microinjection of an (α1 antagonist (prazosin) into the VMH inhibited receptivity (Etgen, 1990).
The effects of several other neurotransmitters on lordosis are mediated by the VMH.Acetylcholine, possibly acting via muscarinic M3 receptors, and serotonin, acting via 5-HT2C receptors, increased neural activity in the VMH and increased lordosis; conversely, stimulation of 5-HT1A receptors inhibited VMH neural activity and lordosis (reviewed in Pfaff, Schwartz-Giblin, McCarthy, & Kow, 1994; Uphouse, 2000). Oxytocin also increased both VMH neural activity (Kow, Johnson, Ogawa, & Pfaff, 1991) and lordosis (Witt & Insel, 1991) and may override the inhibitory effects of stress (McCarthy, McDonald, Brooks, & Goldman, 1996). The oxytocinergic neurons that innervate the VMH are located in the paraventricular nucleus of the hypothalamus (PVN). Oxytocin neurons in the PVN also project to the spinal cord, where they promote lordosis responding.
The Midbrain Module
The primary role of the midbrain module is to transform the slow effects of hormonal stimulation of the hypothalamus into the rapid, behaviorally relevant activity that mediates receptive behavior. Major output neurons from the preoptic area and VMH to the midbrain central gray contain substance P, prolactin, and gonadotropin releasing hormone (GnRH), all of which are important facilitators of lordotic responding (reviewed in Pfaff et al., 1994). Microinjection of GnRH into the central gray facilitated lordosis, whereas microinjection of an antiserum to GnRH blocked lordosis (Sakuma & Pfaff, 1980). Similar results were observed with substance P (Dornan, Malsbury, & Penney, 1987) and prolactin (Harlan, Shivers, & Pfaff, 1983) and antibodies to these peptides. Within the central gray acetylcholine (Richmond & Clemens, 1986) and GABA(McCarthy, Pfaff, & Schwartz-Giblin, 1991) also contribute to the facilitation of lordosis. The central gray in turn sends output to the reticulospinal neurons of the lower brain stem module. Lesions of the central gray impair lordotic responding (Hennessey, Camak, Gordon, & Edwards, 1990) and abolish the facilitative effects of VMH stimulation (Sakuma & Pfaff, 1979).
The Lower Brain Stem Module
The lower brain stem module integrates sensory input from the spinal cord in order to perform moment-to-moment corrections of posture. The vestibular organs and proprioceptors throughout the body also provide input that is essential for maintaining the rigid lordotic posture and accommodating the weight of the male.
The Spinal Cord Module
Thespinalcordreceivesandprocessestherelevantsomatosensory input; it also receives descending facilitative input and generates the motor output. The characteristic dorsiflexion of lordosis requires intersegmental coordination. The important sensory stimuli are pressure applied to the flanks, posterior rump, and perineal area. Estrogen increases the size of the receptive fields of neurons in these areas (Kow et al., 1979). Because the combined sensory input from several nearby regions summates to elicit the lordosis response, this increase in receptivefieldsizegreatlyincreasestheprobabilitythatmountingby the male will elicit lordosis.
The Urethrogenital Reflex
As noted earlier, the urethrogenital reflex has been proposed as a model for the human orgasmic reflex (McKenna et al., 1991). It can be elicited in both male and female rats and appears to have similar mechanisms of control in both sexes (Vathy & Marson, 1998).
Summary of Circuitry Controlling Female Proceptive and Receptive Behavior
In summary, most of the forebrain inhibits receptive behavior. The hypothalamus, especially the VMH, is the primary site at which estrogen and progesterone have their slow, genomically mediated facilitative effects on lordosis. Some neurons in the MPOA may facilitate proceptive behavior and inhibit lordosis, and others may do the opposite. The VMH communicates with the midbrain module, particularly the central gray, via axons carrying neuropeptides including GnRH, substance P, and prolactin. These neuropeptides alter the responsiveness of neurons to the rapid, behaviorally relevant stimuli that control the behavior. The central gray, in turn, interacts with the lower brain stem, which produces the postural changes of lordosis. The spinal cord both receives the somatosensory input that initiates the lordosis response and also transmits the motor signals to the deep back muscles that produce the response.
Activation of Male Sexual Behavior By Gonadal Hormones
Dependence of Copulation on Recent Exposure to Testosterone
Male sexual behavior is heavily dependent on hormones. Increasing production of testosterone at puberty increases sexual activity; after castration, sexual activity declines. There is usually more testosterone than is necessary to facilitate sexual behavior; the excess is necessary for sperm production in the testes. Thus, small reductions of testosterone typically do not affect behavior.
Time Course of Changes in Copulation Following Castration
Although androgens are almost completely eliminated from the body within 24 hr after castration (Krey & McGinnis, 1990), male rats often continue to copulate for days or weeks. The threshold for ejaculation (number of intromissions required to trigger ejaculation) actually decreases for some days aftercastration,whereasintromissionlatenciesandpostejaculatory intervals increase (Davidson, 1966).
The behavioral changes following castration occur in a characteristic sequence. Ejaculation is lost first, then intromission, and mounting last. This sequential loss occurs in part because the different behavioral elements depend on different peripheral target mechanisms. For example, unlike intromission, mounting is not dependent on tactile sensitivity of the penis or erectile function, and ejaculation requires even more sensory and motor competence than does intromission. The various elements may also depend on different central circuits, which have different hormonal requirements.
The effects of castration in men are more variable than in animals. Kinsey concluded, on the basis of anecdotal accounts, that castration had relatively little effect on sexual function in most men (Kinsey, Pomeroy, & Martin, 1948). However, a review of prospective studies of men castrated as “treatment” for sexual offenses revealed that half to two thirds of the men rapidly lost sexual interest, whereas the rest reported a gradual waning of sexual activity, with 10% continuing to have intercourse for up to 20 years (Heim & Hursch, 1979).
Time Course of Changes in Copulation Following Testosterone Restoration
After copulation has been lost, exogenous testosterone restores copulatory elements in the reverse order in which they were lost. Restoration occurs over 5 to 10 days (Putnam, Du, Sato, & Hull, 2001), which suggests that long-term genomic effects mediate the restoration of copulation. In support of this conclusion, inhibition of protein synthesis with anisomycin blocked the effects of testosterone on copulatory behavior (McGinnis & Kahn, 1997). However, anisomycin did not disrupt, and in some cases even facilitated, testerone’s restoration of touch-based erections (Meisel et al., 1986).
The Role of Testosterone Metabolites in Maintaining and Restoring Copulation
Testosterone works primarily via metabolism to either estradiol or DHT. Unlike testosterone, DHT cannot be aromatized to estradiol; therefore, it can be used to differentiate androgenic versus estrogenic effects of testosterone. Some target cells produce both estradiol and DHT and have both estrogen and androgen receptors.
Estrogen and DHT differentially affect copulation and ex copula reflexes. In male rats estrogen maintained or restored most copulatory elements (Davidson, 1969). Furthermore, systemicinhibitionofaromataseoradministrationofestrogen receptor antagonists inhibited restoration of copulation by testosterone (Vagell & McGinnis, 1997). Similar inhibitory effects of aromatase inhibition were found in castrated, testosterone-treated monkeys (Zumpe, Clancy, Bonsall, & Michael, 1996). Neither DHT (Beyer, Larsson, Perez-Palacios, & Morali, 1973) nor another nonaromatizable androgen (methlytrienolone, R1881; Baum, Kingsbury, & Erskine, 1987) restored or maintained copulation in castrated malerats. DHTand R1881 were also ineffective in male gerbils (Yahr & Stephens, 1987), hamsters (Christensen, Coniglio, Paup, & Clemens, 1973), pigs (Levis & Ford, 1989), and rams (Parrott, 1986). Finally, several synthetic androgens (5-alphaandrostanediols) that can be aromatized to estradiol, but not 5-alpha-reduced to DHT, were even more effective than testosterone in restoring sexual behavior in castrated rats (Morali et al., 1993) or mice (Ogawa et al., 1996). The effectiveness of estradiol, and the ineffectiveness of DHT, gave rise to the aromatization hypothesis. Thatis, the aromatization of testosterone to estradiol is the critical step in the maintenance or restoration of copulation in males of numerous species.
However, estrogen cannot maintain full copulatory behavior. Estrogen-treated castrates displayed fewer behavioral ejaculation patterns than did males treated with testosterone or a combination of estrogen and DHT (Putnam, Panos, & Hull, 1998; Vagell & McGinnis, 1997). Furthermore, estradiol alone was unable to restore partner preference, and the nonsteroidal aromatase inhibitor fadrozole failed to block the effects of testosterone on partner preference (Vagell & McGinnis, 1997). The estrogen receptor antagonist RU-58668 also did not block testosterone’s restoration of copulation or partner preference in male rats, but it did inhibit restoration of scent marking (Vagell & McGinnis, 1998). Similar results were observed in male hamsters; fadrozole failed to inhibit testosterone’s restoration of copulation or anogenital investigation of an estrous female hamster (Cooper, Clancy, Karom, Moore, & Albers, 2000). Therefore, the activation of estrogen receptors is not always sufficient to stimulate copulation or partner preference in male rats or hamsters, and estrogen receptor antagonists do not always render testosterone ineffective.
Furthermore, stimulation of androgen receptors does sometimes contribute to testosterone’s effects. For example, the nonsteroidal antiandrogen flutamide reduced testosterone’s ability to restore copulation in castrated male rats (Vagell & McGinnis, 1998). It also inhibited the restoration of partner preference, scent marking, and 50-kHz (“attraction”) vocalizations. An antiandrogen with greater affinity for the androgen receptor (-trifluoro-2-methyl-4-nitro-mlactoluidide, SCH-16423) eliminated all copulatory behavior in most male rats treated with a dose of testosterone that restored ejaculation in all control males (McGinnis & Mirth, 1986). Therefore, stimulation of estrogen receptors is not sufficient for full restoration of copulation in male rats or hamsters.
In a number of other species, the aromatization hypothesis has little or no support. DHT is sufficient for maintenance or restoration of copulation in mice (Luttge & Hall, 1973), deer mice (Clemens & Pomerantz, 1982), rabbits (Ågmo & Södersten, 1975), guinea pigs (Butera & Czaja, 1989), and monkeys (Phoenix, 1974).
Effects of Hormone Deprivation and Replacement on Ex Copula Penile Responses
Animal Studies
Compared to copulation, touch-based reflexes could be reinstated much more rapidly. In castrated male rats, touch-based erections were increased 24 hr after testosterone replacement, with normal levels of erections reached by 48 hr (Gray, Smith, & Davidson, 1980). The same males were tested for copulation 52 hr after testosterone replacement, and only 1 of 10 males mounted. Noncontact erections were also lost more rapidly after castration, and were restored sooner by testosterone, compared to copulation (Manzo, Cruz, Hernandez, Pacheco, & Sachs, 1999). Therefore, the hormonal stimulation of noncontact and touch-based erections may be similar but may differ from hormonal control of copulation, at least with regard to temporal factors.
Another difference in hormonal control of penile reflexes, compared to copulation, is the ineffectiveness of estrogen and the effectiveness of DHT in restoring or maintaining touchbased (Gray et al., 1980; Meisel, O’Hanlon, & Sachs, 1984) or noncontact (Manzo et al., 1999) erections in rats. DHT was the active androgen that also maintained nitric oxide– mediated erections in rats (Lugg, Rajfer, & Gonzalez-Cadavid, 1995), as discussed later. The DHT regimens that maintained or restored reflexes were ineffective in copulation tests (Gray et al., 1980; Meisel, O’Hanlon, et al., 1984). Furthermore, treatment of testosterone-replaced castrated rats with the androgen receptor antagonist flutamide (Gray et al., 1980) or with a 5-alpha-reductase inhibitor (Bradshaw, Baum, &Awh, 1981) blocked the restorative effects of testosterone. Therefore, as with temporal factors, the hormonal mechanisms that control ex copula erections are different from those that regulate copulation.
Although estrogen is ineffective in ex copula reflex tests, it can maintain erections during copulation. The fact that an erection actually occurred during copulation, and not just the behavioral pattern associated with intromission, was verified by placing nontoxic tempera paint into the female’s vagina (O’Hanlon, Meisel, & Sachs, 1981). Estrogen-treated males had as high a percentage of intromission patterns in which they actually achieved insertion as did control males. Furthermore, EMG recordings from the bulbospongiosus muscle revealed that the duration, frequency, and average amplitude were at least as great in estrogen-treated castrates as in testosterone-treated castrates (Holmes & Sachs, 1992). Sachs (1983) suggested that a copulatory behavioral cascade was organized in the brain and included activation of reflexes that were not observable in noncopulatory contexts. Elicitation of reflexive erections may depend primarily on disinhibition of the lumbosacral spinal circuits. Whereas estrogen cannot disinhibit reflexes in ex copula tests, it can apparently activate those same reflexes in the context of copulation.
Studies on Human Males
There is usually little (Raboch, Mellan, & Starka, 1975) or no (Pirke, Kockott, Aldenhoff, Besinger, & Feil, 1979) difference in testosterone concentrations in men with erectile dysfunction compared with normally functioning men. However, testosterone replacement in hypogonadal men increased erectile function (Davidson, Camargo, & Smith, 1979; O’Carroll, Shapiro, & Bancroft, 1985). Furthermore, the loss of erection in hypogonadal men is restricted to nocturnal and spontaneous erections and not erections stimulated by fantasizing or viewing erotic films (Bancroft & Wu, 1983; LaFerla, Anderson & Schalch, 1978). Thus, the effect of androgen on erection in men is context-sensitive, as it is in rats.
Exogenous testosterone treatments in eugonadal men (which produced supraphysiological concentrations), as well as in hypogonadal men (which produced normal concentrations), increased subjective sexual arousal ratings in response to sexual audiotapes (Alexander et al., 1997). These treatments also increased the bias to attend to sexual auditory stimuli in a dichotic listening task. However, there did not appear to be a strong correlation between endogenous testosterone levels in eugonadal men and sexual behavior (Brown, Monti, & Corriveau, 1978).
Testosterone and DHT were equally effective in stimulating sexual activity in agonadal men, and in normal men treatment with an estrogen receptor blocker or an aromatase inhibitor had no effect on sexual function (Gooren, 1985). An exception to this finding comes from an unusual case of a man receiving combined estrogen and progesterone to alleviate menopausal-like symptoms after undergoing castration (Davidson et al., 1979). In this man a normal level of sexual activity was maintained without androgen treatment. However, because progesterone is a precursor of testosterone, a slight increase in testosterone may have been sufficient to stimulate sexual function.
Effects of Systemically Administered Drugs on Male Sexual Behavior
Table 12.2 summarizes the effects on male sexual behavior of systemically administered drugs and of treatments that affect neurotransmitter function in more than one brain area.
Brain Areas Implicated in Control of Male Sexual Behavior
Sensory Systems
Chemosensory Systems
The main and accessory olfactory bulbs receive chemosensory information from receptors in the nasal epithelium and vomeronasal organ, respectively. Generally, damage to the olfactory system impairs male sexual behavior. In male hamsters, bilateral bulbectomy abolished copulation (Murphy, 1980; Winans & Powers, 1974). When destruction of receptors in the nasal epithelium was combined with vomeronasal nerve cuts or deafferentation of the vomeronasal pump, copulation was also severely disrupted (Meredith, Marques, O’Connell, & Stem, 1980). In rats the primary effects of olfactory bulbectomies were a reduction in the percentage of rats that copulated to ejaculation and a slowing of copulation (reviewed in Meisel, Sachs, & Lumia, 1984). Early reports of sexual impairment by bulbectomy attributed the effects to anosmia alone. However, the olfactory bulbs also have nonsensory influences because peripheral deafferentation was often less debilitating than was olfactory bulbectomy (reviewed in Cain, 1974). Olfaction plays a less critical role in the control of male sexual behavior in nonrodent species (reviewed in Hull, Meisel, & Sachs, 2002).
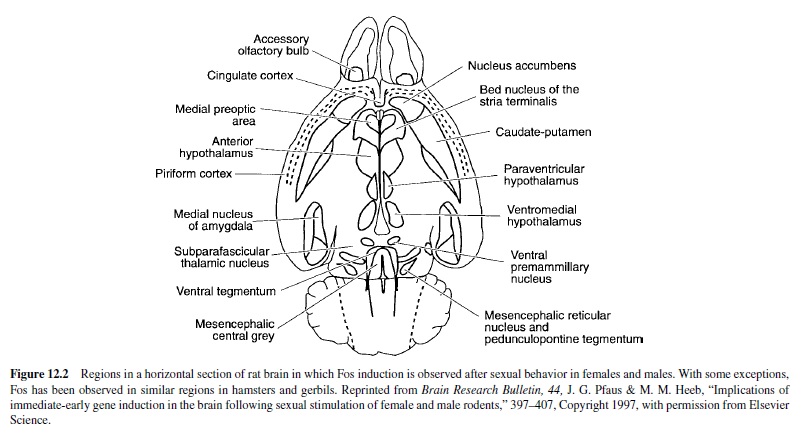
Cells in the olfactory bulbs are activated after copulation and after exposure to a sexually relevant olfactory stimulus. Fos immunoreactivity (ir) is used as a measure of regionspecific cellular activity following a stimulus, in this case sexual behavior. (See Figure 12.2 for a diagram of the brain areas activated by sexual behavior in male or female rodents.) In the accessory olfactory bulb of male hamsters, Fos-ir increased after copulation; this increase was also observed in males whose main olfactory system was ablated with zinc sulfate (Fernandez-Fewell & Meredith, 1994). Thus, pheromonal stimulation of the vomeronasal organ and the accessory olfactory bulb is sufficient for cellular activation in the central vomeronasal pathway of male hamsters. In male rats, Fos-ir was increased in the accessory olfactory bulbs after exposure to bedding from an estrous female; an even higher amount of Fos-ir was observed after copulation (Kelliher, Liu, Baum, & Sachs, 1999).
Somatosensory Systems
Mechanoreceptors in the penis supply somatosensory information to the brain and contribute to sexual arousal. These receptors are more responsive when the penis is erect (Johnson, 1988) or near core body temperature, as it is during erection (Johnson & Kitchell, 1987). Major somatosensory input from the penile skin, prepuce, and glans penis enter the central nervous system in the spinal cord mainly via the dorsal nerve of the penis (reviewed in Steers, 2000).
Auditory System
Male and female rats produce ultrasonic vocalizations during copulation; these vocalizations are believed to facilitate copulation (reviewed in Barfield & Thomas, 1986). During mating, the male produces a 50-kHz vocalization, which is associated with arousal, and a 22-kHz vocalization following ejaculation (Barfield & Geyer, 1972). There is also increased vocalization by the female in response to the male’s vocalizations (White, Gonzales, & Barfield, 1993).
Amygdala
Basolateral Nuclei
Lesions of the basolateral amygdala did not impair copulation in male rats (Kondo, 1992) or hamsters (Lehman & Winans, 1982), but did inhibit bar pressing for a secondary reinforcer that had been paired with access to a female (Everitt, 1990). Therefore, the basolateral amygdala may be important for the motivational aspects of male sexual behavior or for learning the appropriate associations, but not for copulatory performance.
Corticomedial Nuclei
Unlike lesions of the basolateral amygdala, lesions of the corticomedial nuclei clearly impaired male sexual behavior in rats (Dominguez, Riolo, Xu, & Hull, 2001; Kondo, 1992), hamsters (Lehman, Winans, & Powers, 1980), and gerbils (Heeb & Yahr, 2000). These animals required more time to reach an ejaculation and displayed fewer ejaculations than did control animals. Furthermore, medial amygdala lesions blocked the facilitative effects on copulation of preexposure to an estrous female (de Jonge, Oldenburger, Louwerse, & Van de Poll, 1992) and reduced the number of noncontact erections (Kondo, Sachs, & Sakuma, 1997). Thus, the medial amygdala facilitates the response to and assimilation of sexually exciting stimuli.
The medial and anterior cortical nuclei of the amygdala receive projections from the olfactory system (reviewed in McDonald, 1998). Both exposure to chemosensory stimuli from estrous female rats and increasing amounts of copulation elicited increasing amounts of Fos-ir in the medial amygdala and in several downstream sites that are important for male sexual behavior (Baum & Everitt, 1992; Robertson et al., 1991; Veening & Coolen, 1998). Chemosensory stimuli and copulation also induced increasing Fos-ir patterns in hamsters (Kollack-Walker & Newman, 1997), gerbils (Heeb & Yahr, 1996), prairie voles (Wang, Hulihan, & Insel, 1997), and musk shrews (Gill, Wersinger, Veney, & Rissman, 1998).
Theposterodorsalregionofthemedialamygdala(MeApd) contains a high concentration of androgen receptors. Androgen-sensitive neurons in this region that project to the MPOA are activated selectively by ejaculation (Gréco, Edwards, Zumpe, & Clancy, 1998). The MeApd is part of an interconnected ejaculation-specific circuit that may promote sexual satiety in rats (Coolen, Olivier, Peters, & Veening, 1997), hamsters (Parfitt, Coolen, Newman, & Wood, 1996; Parfitt & Newman, 1998), and gerbils (Heeb & Yahr, 1996).
Bed Nucleus of the Stria Terminalis
The bed nucleus of the stria terminalis (BNST) has reciprocal connections with the medial amygdala and the MPOA. Lesions of the BNST increased the number of intromissions required to elicit an ejaculation, increased postejaculatory intervals, and decreased the number of ejaculations (Emery & Sachs, 1976); these effects are similar to those observed following lesions of the medial amygdala. In addition, exposure to a sexually relevant olfactory stimulus, noncontact erections, or mating increased Fos-ir in the BNST of male rats (Kelliher et al., 1999) and hamsters (Fernandez-Fewell & Meredith, 1994).
Medial Preoptic Area
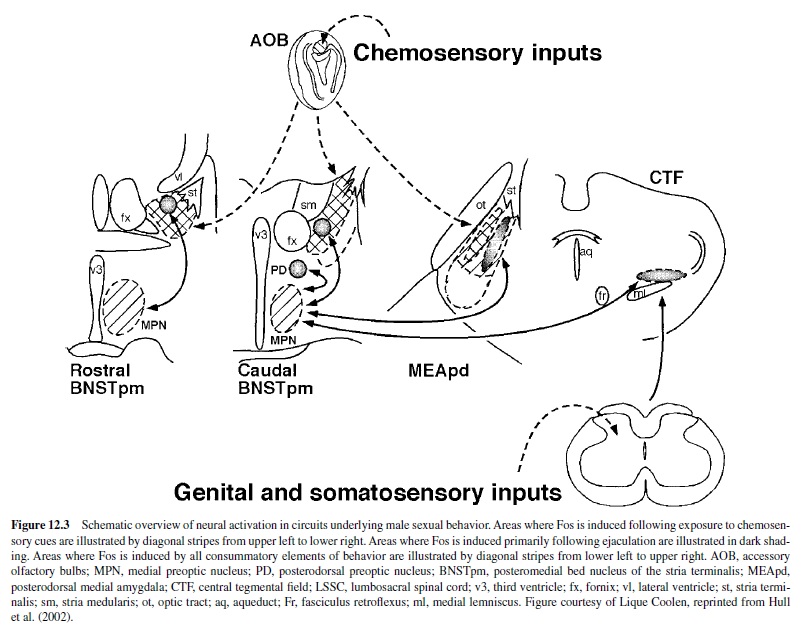
The MPOA is perhaps the most important integrative site for the regulation of male sexual behavior in all vertebrate species that have been tested. It receives indirect input from every sensory modality (Simerly & Swanson, 1986) and sends projections to structures that are critical for the initiation and patterning of copulation (Simerly & Swanson, 1988). See Figure 12.3 for interconnections of the MPOAand other areas important for the control of male sexual behavior.
Effects of Lesions
Damage to the MPOA has consistently impaired male sexual behaviorinrats,monkeys,goats,dogs,cats,mice,guineapigs, hamsters, ferrets, gerbils, snakes, birds, lizards, and fish (reviewedinHulletal.,2002).Becausethenatureofthesexually relevant stimuli and the motor patterns that express copulation vary greatly among species, the fact that MPOA damage impairs sexual behavior in all these different species confirmsthe MPOA’s role as a central integrative node for the regulation of male sexual behavior. The severity of sexual impairment by MPOA lesions is dependent on the lesion’s size and location. Smaller MPOA lesions have variable and less severe effects than do larger lesions (Arendash & Gorski, 1983; Heimer & Larsson, 1966/1967). Lesions of the caudal MPOA, including the rostral anterior hypothalamus, impaired copulation more severely than did those of the rostral MPOA (Van de Poll & van Dis, 1979).
There has been disagreement as to whether the MPOA is important for the appetitive as well as the consummatory aspects of male sexual behavior. Everitt (1990) suggested that the MPOA controls only copulatory performance and is not important for motivation. This was supported by reports that male rats with MPOAlesions pursued estrous females and investigated their anogenital regions (Hansen & Hagelsrum, 1984; Heimer & Larsson, 1966/1967). Similar patterns of behavior were observed in cats (Hart, Haugen, & Peterson,1973) and dogs (Hart, 1974) with MPOA lesions. Furthermore, MPOA lesions did not affect the frequency of masturbation in monkeys (Slimp, Hart, & Goy, 1978) or noncontact erections in rats (Liu, Salamone, & Sachs, 1997b). On the other hand, MPOA lesions diminished preference for a female partner in rats (Edwards & Einhorn, 1986; Edwards, Walter, & Liang, 1996; Paredes, Tzschentke, & Nakach, 1998) and ferrets (Kindon, Baum, & Paredes, 1996; Paredes & Baum, 1995), decreased pursuit of a female by male rats (Paredes, Highland, & Karam, 1993), and precopulatory behavior in marmosets (Lloyd & Dixson, 1988), suggesting that the MPOA is indeed important for sexual motivation.
Effects of Stimulation
Stimulation of the MPOA enhances sexual activity. In rats, electrical stimulation of the MPOAreduced the number of intromissions preceding ejaculation, the ejaculation latency, and the postejaculatory interval (Malsbury, 1971; RodriguezManzo, Pellicer, Larsson, & Fernandez-Guasti, 2000). However, MPOA stimulation did not restore copulation in males that had reached sexual satiety (Rodriguez-Manzo et al., 2000), suggesting that sexual inhibition due to satiety is not mediated by the MPOA. Stimulation of the MPOA has also elicited erection (Giuliano et al., 1997) and the urethrogenital reflex (Marson & McKenna, 1994b).
Fos Studies
Exposure to the odor of an estrous female and increasing amounts of copulation induced increasing amounts of Fos-ir in the MPOAof male rats (Baum & Everitt, 1992; Bressler & Baum, 1996; Robertson et al., 1991; Veening & Coolen, 1998), hamsters (Kollack-Walker & Newman, 1997), and gerbils (Heeb & Yahr, 1996). Noncontact erections and exposure to the bedding of an estrous female also induced Fos-ir in the MPOA of male rats, but the effects were less dramatic than those observed following copulation (Kelliher et al., 1999). Ejaculation-induced Fos-ir in the MPOA was decreased by a D1 antagonist and by lack of previous sexual experience (Lumley & Hull, 1999). In one subregion, the posterodorsal preoptic nucleus, Fos-ir was significantly increased only following ejaculation in male rats (Coolen, Peters, & Veening, 1996), hamsters (Kollack-Walker & Newman, 1997), and gerbils (Heeb & Yahr, 1996).
Microinjection Studies
Microinjection of the classic dopamine agonist apomorphine into the MPOA of male rats facilitated copulation (Hull, Bitran, Pehek, Warner, & Band, 1986) and touch-based genital reflexes (Pehek, Thompson, & Hull, 1989a), whereas a dopamine antagonist impaired appetitive and consummatory measures of sexual behavior, as well as genital reflexes (Warner et al., 1991). Stimulation of D1 receptors in the MPOA promoted parasympathetically mediated erections and speeded copulation, whereas stimulation of D2 receptors shifted the autonomic balance to favor sympathetically mediated seminal emission and ejaculation (Hull, Eaton, Markowski, Moses, Lumley, & Loucks, 1992; Hull et al., 1989; Markowski, Eaton, Lumley, Moses, & Hull, 1994).
Microdialysis Studies
Extracellular dopamine increased in the MPOA of male rats during exposure to an estrous female and during copulation (Hull, Du, Lorrain, & Matuszewich, 1995). Both basal (Lorrain & Hull, 1993) and mating-induced (Lorrain, Matuszewich, Howard, Du, & Hull, 1996) dopamine levels were decreased by reverse dialysis of a nitric oxide synthase (NOS) inhibitor and by castration (Du, Lorrain, & Hull, 1998; Hull et al., 1995). Lesions of the medial amygdala blocked the mating-induced increase but did not affect basal dopamine levels in the MPOA; therefore, the mating-induced dopamine increase was mediated by input from the medial amygdala (Dominguez et al., 2001). These lesions also impaired copulation; microinjection of the dopamine agonist apomorphine into the MPOA restored copulatory ability (Dominguez et al., 2001).
Paraventricular Nucleus of the Hypothalamus
Effects of Lesions
Lesions of the parvocellular PVN inhibited noncontact erections (Liu, Salamone, & Sachs, 1997a) and decreased the quantity of seminal emission during ejaculation (Ackerman, Lange, & Clemens, 1997) but did not impair copulation (Ackerman et al., 1997; Liu et al., 1997a).
Microinjection and Microdialysis Studies
Microinjections of either oxytocin, the classic D1/D2 dopamine agonist apomorphine, or the D2 agonist quinpirole into the PVN facilitated drug-induced and noncontact erections (Argiolas, Melis, Mauri, & Gessa, 1987; Melis, Argiolas, & Gessa, 1987). PVN microinjections of apomorphine (Pehek et al., 1989a) or the D3/D2 agonist quinelorane (Eaton et al., 1991) also increased touch-based erections and seminal emissions. These effects were blocked by intraventricular, but not PVN, administration of an oxytocin antagonist (Melis, Succu, Spano, & Argiolas, 1999b), suggesting that oxytocinergic axon terminals ending outside the PVN, perhaps in the hippocampus (Melis, Stancampiano, & Argiolas, 1992), promote noncontact erections.
Nitric oxide (NO) in the PVN also promotes erections. Microinjection of an NOS inhibitor (L-NAME) into the PVN decreased noncontact erections and impaired copulation (Melis, Succu, Mauri, & Argiolas, 1998). Both noncontact erections and copulation increased NO production in the PVN (Melis et al., 1998; Melis, Succu, Spano, & Argiolas, 1999a). However, reverse dialysis of a different NOS inhibitor (L-NMMA) into the PVN failed to impair copulation, although it did inhibit noncontact erections (Sato et al., 1999), and similar treatment in the MPOA did inhibit copulation (Sato, Horita, Kurohata, Adachi, & Tsukamoto, 1998). Systemic injections of either apomorphine or the D2 agonist quinpirole increased NO production in the PVN and elicited drug-induced erections (Melis, Succu, & Argiolas 1996). Similarly, microinjections of the glutamate agonist N-methyl-D-aspartate (NMDA) into the PVN increased erections and increased NO production in the PVN (Melis, Succu, Iannuci, & Argiolas, 1997). Finally, oxytocin and NOS are colocalized in the PVN (Yamada, Emson, & Hokfelt, 1996).
These studies provide a consistent model of PVN function, in which dopamine (via D2 receptors) or glutamate (via NMDA receptors) activates NO production in oxytocinergic neurons. This intracellular NO increases the release of oxytocin from axon terminals ending elsewhwere, perhaps the hippocampus, where they promote noncontact and druginduced erections and may produce some facilitation of copulation. In addition, oxytocinergic neurons descending from the PVN to the spinal cord may promote seminal emission and ejaculation.
Anterior Lateral Hypothalamus
Important reciprocal connections between the MPOA and several more caudal sites pass through the lateral hypothalamus (Simerly & Swanson, 1988). Lesions that sever these connections are as destructive to copulation as are MPOA lesions (Scouten, Burrell, Palmer, & Cegavske, 1980). However, cell bodies in the anterior lateral hypothalamus (LHA) also influence sexual behavior. Serotonin is released in the LHA at the time of ejaculation, and a selective serotonin reuptake inhibitor (SSRI) microinjected into the LHA delayed the onset of copulation and delayed ejaculation after the male did begin to copulate (Lorrain, Matuszewich, Friedman, & Hull, 1997). Thus, the LHA appears to be one site at which SSRI antidepressants inhibit sexual motivation and ejaculation. One means by which SSRIs in the LHA inhibit sexual motivation may be by decreasing dopamine release in the mesolimbic tract, which is important for many motivated behaviors. Reverse dialysis of serotonin into the LHA resulted in a decrease in dopamine release in the NAcc, a major terminus of the mesolimbic dopamine tract (Lorrain, Riolo, Matuszewich, & Hull, 1999). Thus, serotonin release in the LHA may inhibit sexual motivation by inhibiting dopamine release in the NAcc.
Nucleus Accumbens and Ventral Tegmental Area
Effects of Lesions and Electrical Stimulation
The mesolimbic dopamine tract arises from cell bodies in the VTAand ascends to several forebrain structures, including the NAcc. It is important for behavioral activation, reward, and incentive learning for a variety of motivated behaviors. NAcc lesions decreased noncontact and apomorphine-stimulated erections (Liu, Sachs, & Salamone, 1998). Copulation was unaffected, except for increased intromission latency; amphetamine-stimulated locomotion was also decreased, suggesting a general deficit in behavioral activation. Lesions of the VTA increased the postejaculatory interval but did not affect other measures of copulation (Brackett, Iuvone, & Edwards, 1986). Conversely, electrical stimulation of the NAcc decreased the latencies to mount, intromit, and ejaculate and to resume copulation after ejaculating (Eibergen & Caggiula, 1973; Markowski & Hull, 1995).
Microinjection Studies
Stimulating inhibitory autoreceptors on mesolimbic cell bodies in the VTA delayed the start of copulation and slowed its rate (Hull, Bazzett, Warner, Eaton, & Thompson, 1990); however, it did not affect genital reflexes or the percentage of X-maze trials on which the male chose the goal box containing the female (Hull et al., 1991). Therefore, behavioral activation, rather than sexual motivation or reflexes, was affected by inhibition of mesolimbic activity. Similarly, microinjection of the opioid antagonist naloxone into the VTA decreased level changing in search of a female but did not affect copulation (van Furth & van Ree, 1996).
Manipulations of the NAcc resulted in similar conclusions. Amphetamine shortened the latency to begin copulating, and a dopamine antagonist (cis-flupenthixol) or selective neurotoxic lesions delayed it (reviewed in Everitt, 1990). Microinjection of a D3/D2 agonist (quinelorane) into the NAcc increased the number of trials on which the male did not leave the start area of the X maze but did not affect his choice of the female or copulation after the male reached the female (Moses, Loucks, Watson, Matuszewich, & Hull, 1995). It is not clear whether quinelorane’s effects resulted from stimulation of inhibitory presynaptic autoreceptors or postsynaptic receptors in the NAcc. However, the pattern is consistent with a role for the mesolimbic dopamine system in activation of motivated behaviors, rather than specifically sexual motivation or the pattern of copulatory behavior.
Microdialysis Studies
Dopamine is released in the NAcc of male rats when they are exposed to the odor of a receptive female, but not that of a nonreceptive female or a male, and during copulation (Fumero, Fernandez-Vera, Gonzalez-Mora, & Mas, 1994; Mas, Gonzalez-Mora, Louilot, Sole, & Guadalupe, 1990; Mitchell & Gratton, 1991; Pfaus, Damsma, et al., 1990; Wenkstern, Pfaus, & Fibiger, 1993). Finer temporal analysis, achieved with in vivo voltammetry (Blackburn, Pfaus, & Phillips, 1992; Mas et al., 1990) or microdialysis with capillary chromatography (Lorrain et al., 1999), revealed that dopamine rose with the initial presentation of a female, increased further during copulation, decreased during each postejaculatory interval, and increased again as copulation resumed. Reverse dialysis of serotonin into the LHA decreased extracellular dopamine in the NAcc and prevented the increase during copulation (Lorrain et al., 1999). Therefore, one factor promoting sexual quiescence after ejaculation may be the decreased dopamine release in the NAcc caused by serotonin in the LHA.
Sexually satiated males showed a slight increase in NAcc dopamine when they were presented with a novel female; dopamine rose further during copulation with the new female (Fiorino, Coury, & Phillips, 1997). In addition, repeated injections of amphetamine increased (“sensitized”) the locomotor response to amphetamine and also increased the dopamine response to a female (Fiorino & Phillips, 1999). Sensitized males also had shorter intromission latencies and more copulatory behaviors. Therefore, sensitization to a stimulant drug cross-sensitized to a natural behavior.
Nigrostriatal Dopamine Tract
The nigrostriatal dopamine tract arises from cell bodies in the substantia nigra, adjacent to the VTA, and projects to the caudate-putamen (dorsal striatum). It contributes to the initiation and control of movement. Degeneration results in Parkinson’s disease, which is characterized by difficulty initiating movements, slowing of movement, and tremor at rest. Bilateral lesions of the substantia nigra slowed copulation and decreased the number of ejaculations (Brackett et al., 1986). In contrast to the immediate release of dopamine in the NAcc when a receptive female was presented, dopamine in the dorsal striatum rose only with the onset of copulation (Damsma, Pfaus, Wenkstern, Phillips, & Fibiger, 1992). This pattern suggests that the nigrostriatal tract is more important for the motor aspects of copulation than for sexual motivation.
Thalamus and Brain Stem
Subparafascicular Nucleus of the Thalamus
The medial parvocellular portion of the subparafascicular nucleus of the thalamus (SPFp) is part of a circuit that responds specifically to ejaculation in rats (Coolen et al., 1996, 1997), hamsters (Kollack-Walker & Newman, 1997), and gerbils (Heeb & Yahr, 1996). Bilateral lesions of the SPFp in gerbils did not affect copulatory performance, suggesting that the SPFp is more important for processing the sensory information resulting from ejaculation than for control of the behavior (Heeb & Yahr, 1996).
Central Tegmental Field and Dorsolateral Tegmentum
The midbrain tegmentum receives sensory input from the genitals, which it relays to the MPOA and anterior hypothalamus; in turn, it receives input from the MPOA, which it sends to the brain stem and spinal cord (Simerly & Swanson, 1986, 1988). Subregions of the tegmentum have been referred to as the central tegmental field (CTF) or the dorsolateral tegmentum (DLT). Combined ipsilateral lesions of the medial amygdala and the CTF abolished copulation-induced Fos-ir in the MPOA, although lesion of either structure alone did not affect Fos-ir (Baum & Everitt, 1992). Unilateral MPOA lesions did not affect Fos-ir in either of those structures; thus, the main flow of information is from CTF and medial amygdala to the MPOA.
Periaqueductal Gray
The periaqueductal gray (PAG) has extensive reciprocal connections with the MPOA (Rizvi, Murphy, Ennis, Behbehani, & Shipley, 1996; Simerly & Swanson, 1986). Bilateral PAG lesions facilitated copulation (Bracket et al., 1986). The caudal two thirds of the PAG contain many androgen and estrogen receptors (Murphy, Shupnick, & Hoffman, 1999). Furthermore, projections from the MPOA terminated in close proximity to steroid-receptor containing PAG neurons, and almost half of the neurons that projected to the nucleus paragigantocellularis (nPGi; discussed next) contained androgen or estrogen receptors (Murphy & Hoffman, 2001).
Nucleus Paragigantocellularis
The nPGi of the medulla contributes to the tonic inhibitory control of spinal mechanisms that control erection and ejaculation. Bilateral lesions of the nPGi increased the number of sexually naive male rats that ejaculated during their first exposure to a receptive female (Yells, Hendricks, & Prendergast, 1992). These lesions also decreased the time and number of intromissions before ejaculation, increased copulatory efficiency, and delayed sexual satiety. Similar lesions decreased the latency to the first touch-based erection and increased the number of anteroflexions (Marson, List, & McKenna, 1992); they also disinhibited the urethrogenital reflex as effectively as did spinal transection (Marson & McKenna, 1990).
Spinal Cord
The spinal cord provides initial processing of somatic and visceral stimuli from the genitals and is the site of somatic and autonomic nuclei that control erection, ejaculation, and detumescence. Both local reflex loops and descending axons from the brain are important for these functions (reviewed in Giuliano & Rampin, 2000).
Effects of Lesions
Transection of the spinal cord dramatically increased touchbased erections (Sachs & Garinello, 1979) and disinhibited the urethrogenital reflex (McKenna et al., 1991). However, acute injections of a local anesthetic decreased the number and magnitude of touch-based erections, in addition to shortening the latency to the first erection (Sachs & Bitran, 1990). The decrease in latency probably resulted from the removal of a supraspinal inhibitory influence; however, the decrease in erections suggests that there is also a descending facilitative influence. The previously observed increase in erections following spinal transection probably resulted from neural reorganization within the spinal cord during the interval between the transection and the test.
Descending serotonergic axons mediate much of the inhibitory influence on touch-based erections and on the urethrogenital reflex. Either intrathecal (around the spinal cord) or intracerebroventricular injections of a serotonin neurotoxin (5,7-dihydroxytryptamine, or 5,7-DHT) disinhibited the urethrogenital reflex in otherwise intact male rats (Marson & McKenna, 1994a), and lesions of the median raphe nuclei (a major source of serotonin neurons) increased the number of touch-based erections (Monaghan, Arjomand, & Breedlove, 1993). In addition to the median raphe nuclei, the nPGi may contribute to the serotonergic inhibition of genital reflexes. A majority of the axons from the nPGi contain serotonin (Marson & McKenna, 1992), and as noted earlier, lesions of the nPGi disinhibited both the urethrogenital reflex and touch-based erections.
Serotonin Receptor Subtypes
Paradoxically, stimulation of 5-HT2C receptors may facilitate erection. Immunoreactivity for 5-HT2C receptors was found on all neurons that projected from the sacral parasympathetic nucleus to the corpus cavernosum and from the motor neurons to the striated penile muscles (Bancilla et al., 1999). Thus, the neurons that are known to control the hemodynamic and striated muscle actions that produce erections possess 5-HT2C receptors, and a systemically administered 5-HT2C agonist (mCPP) facilitated erections in monkeys (Pomerantz, Hepner, & Wurtz, 1993) and rats (Millan, Peglion, Lavielle, & Perrin-Monneyron, 1997).
Intrathecal administration of the 5-HT1A agonist 8-OH-DPAT decreased the number of rats that displayed touch-based erections or seminal emissions; however, similar administration of 8-OH-DPAT resulted in a dramatic facilitation of copulation (Lee, Smith, Mas, & Davidson, 1990). The basis for this discrepancy is not clear; however, a similar discrepancy was observed with intrathecal administration of the dopamine agonist apomorphine, which inhibited touch-based erections but facilitated copulation (Pehek, Thompson, & Hull, 1989b). In addition, intrathecal administration of a GABAB agonist inhibited touch-based erections but had no effect on copulation; a GABAA agonist was almost completely ineffective (Bitran, Miller, McQuade, Leipheimer, & Sachs, 1988).
Oxytocin
Oxytocin, carried by axons from the PVN, provides a major facilitative influence on erection. Administration of oxytocin in the lumbosacral region increased intracavernous pressure (Giuliano, Bernabé, McKenna, Longueville, & Rampin, 2001). Section of the pelvic nerve, the major parasympathetic input to the pelvis, blocked the effect. However, blockade of striated muscle activation did not diminish oxytocin’s effects, providing further evidence that those effects were mediated by the parasympathetic nervous system, rather than by striated penile muscles.
Summary of Circuitry Underlying Mammalian Male Sexual Behavior
The MPOA is the central hub for the control of male copulatory behavior. There are two main sources of sensory input to the MPOA of male rodents. Chemosensory information is processed by the main olfactory system and the vomeronasal system and is relayed via the medial amygdala to the MPOA, BNST, NAcc, and other sites. Somatosensory input from the genitals is processed initially in the dorsal horn of the spinal cord and ascends to the MPOAvia the nPGi, CTF, SPFp, and PVN. One circuit, including the SPFp, the posterodorsal medial amygdala, and the posterodorsal preoptic nucleus, responds specifically to ejaculation and may contribute to sexual satiety. Output from the MPOA is routed via the medial forebrain bundle to the PVN, the midbrain PAG, the midline raphe nuclei, and the nPGi. Many of the neurons responding to sexually relevant sensory input and those providing output to downstream structures contain steroid hormone receptors. Therefore, hormones are able to increase sensitivity to sexually relevant stimuli and to increase responsiveness of motoric output. This circuitry has been highly conserved during evolution, as MPOA lesions in fishes, birds, lizards, and numerous mammals, including humans, impair or abolish male sexual behavior, and lesions of input and output structures also produce similar effects across species.
Sexual Behavior in the Context of Mammalian Social Behavior
Brain areas that are important for male sexual behavior also contribute to female sexual behavior, aggression, and territorial marking (reviewed in Newman, 1999). Furthermore, these areas are richly interconnected, and most contain steroid hormone receptors. Perinatal and adult hormones predispose the network to produce particular patterns of behavior. It is not clear whether each cell contributes to multiple behaviors or whether labeled-line neurons specific for each type of behavior are intermingled within the same structures. Because all of these behaviors contribute to the survival of the species, it may be wise to attend to the common themes underlying these various social behaviors, as well as using analytic techniques for each behavior.
Unanswered Questions and Future Directions of Research
Both in spite of and as a result of the impressive advancements in understanding the physiological bases of sexual behavior, many questions remain partially or completely unanswered. First, there is much to be learned about the anatomical interconnections that control both female and male sexual behavior. Both the neural connections from one place to the next and the neurotransmitter signatures of those neurons are important pieces of the puzzle. There are also questions concerning neurotransmitter interactions. Which neuropeptides are released with classical neurotransmitters and under what circumstances? Which receptor subtypes mediate each effect? There are also questions concerning the intracellular mediators of neurotransmitter and hormonal effects. What changes in gene transcription are induced by specific steroid hormones? How do rapid membrane effects of steroids influence sexual behavior? What changes in gene transcription mediate the effects of previous sexual experience? How does activation of c-fos and other immediateearly genes influence sexual behavior? Finally, broader questions include the interrelationships among sexual and other social behaviors, how species-specific differences in behavior are related to their ecological niches, and how, exactly, motivational fervor elicits particular behavioral responses. These questions should provide the framework for research in the years to come.
Bibliography:
- Ackerman, A. E., Lange, G. M., & Clemens, L. G. (1997). Effects of paraventricular lesions on sex behavior and seminal emissions in male rats. Physiology and Behavior, 63, 49–53.
- Adams, D. B., Gold, A. R., & Burt, A. D. (1978). Rise in femaleinitiated sexual activity at ovulation and its suppression by oral contraceptives. New England Journal of Medicine, 229, 1145–1150.
- Adler, N. T., & Toner, J. P. (1986). The effects of copulatory behavior on sperm transport and fertility in rats. Annals of the New York Academy of Sciences, 474, 21–32.
- Ågmo, A. (1976). Cholinergic mechanisms and sexual behavior in the male rabbit. Psychopharmacology, 51, 43–45.
- Ågmo, A., Paredes, R. G., & Contreras, J. L. (1994). Opioids and sexual behaviors in the male rabbit: The role of central and peripheral opioid receptors. Journal of Neural Transmission, General Section, 97, 211–223.
- Ågmo, A., Paredes, R. G., Sierra, L., & Garces, I. (1997). The inhibitory effects on sexual behavior and ambulatory activity of the mixed GABAA/GABAB agonist progabide are differentially blocked by GABA receptor antagonists. Psychopharmacology, 129, 27–34.
- Ågmo, A., & Picker, Z. (1990). Catecholamines and the initiation of sexual behavior in male rats without sexual experience. Pharmacology, Biochemistry, and Behavior, 35, 327–334.
- Ågmo, A., & Södersten, P. (1975). Sexual behaviour in castrated rabbits treated with testosterone, oestradiol, dihydrotestosterone or oestradiol in combination with dihydrotestosterone. Journal of Endocrinology, 67, 327–332.
- Ahlenius, S., Engel, J., Eriksson, H., Modigh, K., & Sodersten, P. (1972). Importance of central catecholamines in the mediation of lordosis behaviour in ovariectomized rats treated with estrogen and inhibitors of monoamine synthesis. Journal of Neural Transmission, 33, 247–255.
- Ahlenius, S., & Larsson, K. (1990). Effects of selective D1 and D2 antagonists on male rat sexual behavior. Experientia, 46, 1026–1028.
- Albinsson, A., Andersson, G., Andersson, K., Vega-Matuszczyk, J., & Larsson, K. (1996). The effects of lesions in the mesencephalic raphe systems on male rat sexual behavior and locomotor activity. Behavioural Brain Research, 80, 57–63.
- Alexander, G. M., Swerdloff, R. S., Wang, C., Davidson, T., McDonald, V., Steiner, B., & Hines, M. (1997). Androgenbehavior correlations in hypogonadal men and eugonadal men: I. Mood and response to auditory sexual stimuli. Hormones and Behavior, 31, 110–119.
- Al Satli, M., & Aron, C. (1977). Influence of olfactory bulb removal on sexual receptivity in the rat. Psychoneuroendocrinology, 2, 399–407.
- Arendash, G. W., & Gorski, R. A. (1983). Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Research Bulletin, 10, 147–154.
- Argiolas, A., Melis, M. R., Mauri, A., & Gessa, G. L. (1987). Paraventricular nucleus lesion prevents yawning and penile erection induced by apomorphine and oxytocin, but not by ACTH in rats. Brain Research, 421, 349–352.
- Balthazart, J., & Ball, G. F. (1995). Sexual-differentiation of brain and behavior in birds. Trends in Endocrinology and Metabolism, 6, 21–29.
- Bancila, M.,Verge, D., Rampin, O., Backstrom, J. R., Sanders-Bush, E., McKenna, K. E., Marson, L., Calas, A., & Giuliano, F. (1999). 5-Hydroxytryptamine2C receptors on spinal neurons controlling penile erection in the rat. Neuroscience, 92, 1523– 1537.
- Bancroft, J., & Wu, F. C. (1983). Changes in erectile responsiveness during androgen replacement therapy. Archives of Sexual Behavior, 12, 59–66.
- Barbeau, A. (1969). L-DOPA therapy in Parkinson’s disease: A critical review of nine years’experience. Canadian Medical Association Journal, 101, 791–800.
- Barfield, R. J., & Geyer, L. A. (1972) Sexual behavior: Ultrasonic postejaculatory song of the male rat. Science, 176, 1349–1350.
- Barfield, R. J., & Thomas, D. A. (1986). The role of ultrasonic vocalizations in the regulation of reproduction in rats. Annals of the New York Academy of Sciences, 474, 33–43.
- Bast, J. D., Hunts, C., Renner, K. J., Morris, R. K., & Quadagno, D. M. (1987). Lesions in the preoptic area suppressed sexual receptivity in ovariectomized rats with estrogen implants in the ventromedial hypothalamus. Brain Research Bulletin, 18, 153–158.
- Baum, M. J., & Everitt, B. J. (1992). Increased expression of c-fos in the medial preoptic area after mating in male rats: Role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience, 50, 627–646.
- Baum, M. J., Kingsbury, P. A., & Erskine, M. S. (1987). Failure of the synthetic androgen 17 beta-hydroxyl-17alpha-methyl-estra4,9,11-triene-3-one (methyltrienolone, R1881) to duplicate the activational effect of testosterone on mating in castrated male rats. Journal of Endocrinology, 113, 15–20.
- Beach, F. A., & LeBoeuf, B. J. (1967). Coital behaviour in dogs: I. Preferential mating in the bitch. Animal Behaviour, 15, 546–558.
- Becker, J. B., Rudick, C. N., & Jenkins, W. J. (2001). The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. The Journal of Neuroscience, 21, 3236–3241.
- Benelli, A., Bertolini, A., Poggioli, R., Cavazzuti, E., Calza, L., Giardino, L., & Arletti, R. (1995). Nitric oxide is involved in male sexual behavior of rats. European Journal of Pharmacology, 294, 505–510.
- Beyer, C., Larsson, K., Perez-Palacios, G., & Morali, G. (1973). Androgen structure and male sexual behavior in the castrated rat. Hormones and Behavior, 4, 99–108.
- Bialy, M., Beck, J., Abramczyk, P., Trzebski, A., & Przybylski, J. (1996). Sexual behavior in male rats after nitric oxide synthesis inhibition. Physiology and Behavior, 60, 139–143.
- Bignami, G. (1966). Pharmacologic influences on mating behavior in the male rat: Effects of d-amphetamine, LSD-25, strychnine, nicotine, and various anticholinergic agents. Psychopharmacologia, 10, 44–58.
- Bitran, D., Miller, S. A., McQuade, D. B., Leipheimer, R. E., & Sachs, B. D. (1988). Inhibition of sexual reflexes by lumbosacral injection of a GABAB agonist in the male rat. Pharmacology, Biochemistry, and Behavior, 31, 657–666.
- Blackburn, J. R., Pfaus, J. G., & Phillips, A. G. (1992). Dopamine functions in appetitive and defensive behaviours. Progress in Neurobiology, 39, 247–279.
- Brackett, N. L., Iuvone, P. M., & Edwards, D. A. (1986). Midbrain lesions, dopamine and male sexual behavior. Behavioural Brain Research, 20, 23l–240.
- Bradshaw, W. G., Baum, M. J., & Awh, C. C. (1981). Attenuation by a 5-reductase inhibitor of the activational effect of testosterone propionate on penile erections in castrated male rats. Endocrinology, 109, 1047–1051.
- Bressler, S. C., & Baum, M. J. (1996). Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience, 71, 1063–1072.
- Brown, W. A., Monti, P. M., & Corriveau, D. P. (1978). Serum testosterone and sexual activity and interest in men. Archives of Sexual Behavior, 7, 97–103.
- Bunnell, B. N., Boland, B. D., & Dewsbury, D. A. (1976). Copulatory behavior of the golden hamster (Mesocricetus auratus). Behaviour, 61, 180–206.
- Butera, P. C., & Czaja, J. A. (1989). Effects of intracranial implants of dihydrotestosterone on the reproductive physiology and behavior of male guinea pigs. Hormones and Behavior, 23, 424–431.
- Caggiula, A. R., Herndon, J. G., Jr., Scanlon, R., Greenstone, D., Bradshaw, W., & Sharp, D. (1979). Dissociation of active from immobility components of sexual behavior in female rats by central 6-hydroxydopamine: Implications for CA involvement in sexual behavior and sensorimotor responsiveness. Brain Research, 172, 505–520.
- Cain, D. P. (1974). The role of the olfactory bulb in limbic mechanisms. Psychological Bulletin, 81, 654–671.
- Cantor, J. M., Binik, Y. M., & Pfaus, J. G. (1999). Chronic fluoxetine inhibits sexual behavior in the male rat: Reversal with oxytocin. Psychopharmacology, 144, 355–362.
- Carter, C. S., DeVries, A. C., & Getz, L. L. (1995). Physiological substrates of mammalian monogamy: The prairie vole model. Neuroscience and Biobehavioral Reviews, 19, 303–314.
- Carter, C. S., Witt, D. M., Auksi, T., & Casten, L. (1987). Estrogen and the induction of lordosis in female and male prairie voles (Microtus ochrogaster). Hormones and Behavior, 21, 65–73.
- Christensen, L. W., Coniglio, L. P., Paup, D. C., & Clemens, L. C. (1973). Sexual behavior of male golden hamsters receiving diverse androgen treatments. Hormones and Behavior, 4, 223–229.
- Christiansen, E., Guirguis, W. R., Cox, D., & Osterloh, I. H. (2000). Long-term efficacy and safety of oral Viagra (sildenafil citrate) in men with erectile dysfunction and the effect of randomized treatment withdrawal. International Journal of Impotence Research, 12, 177–182.
- Clark, J. T. (1995). Sexual arousal and performance are modulated by adrenergic-neuropeptide-steroid interactions. In J. Bancroft (Ed.), The pharmacology of sexual function and dysfunction: Proceedings of the Esteve Foundation Symposium VI, Son Vida, Mallorca (pp. 55–68). Amsterdam: Excerpta Medica.
- Clark, J. T., Smith, E. R., & Davidson, J. M. (1984). Enhancement of sexual motivation in male rats by yohimbine. Science, 225, 847–849.
- Clark, J. T., Smith, E. R., & Davidson, J. M. (1985). Evidence for the modulation of sexual behavior by -adrenoceptors in male rats. Neuroendocrinology, 41, 36–43.
- Clemens, L. G., & Pomerantz, S. M. (1982). Testosterone acts as a prohormone to stimulate male copulatory behavior in male deer mice (Peromyscus maniculatus bairdi). Journal of Comparative and Physiological Psychology, 96, 114–122.
- Coolen, L. M., Olivier, B., Peters, H. J., & Veening, J. G. (1997). Demonstration of ejaculation-induced neural activity in the male rat brain using 5-HT1A agonist 8-OH-DPAT. Physiology and Behavior, 62, 881–891.
- Coolen, L. M., Peters, H. J., & Veening, J. G. (1996). Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: A sex comparison. Brain Research, 738, 67–82.
- Cooper, T. T., Clancy, A. N., Karom, M., Moore, T. O., & Albers, H. E. (2000). Conversion of testosterone to estradiol may not be necessary for the expression of mating behavior in male Syrian hamsters (Mesocricetus auratus). Hormones and Behavior, 37, 237–245.
- Crews, D., Bull, J. J., & Billy, A. J. (1988). Sex determination and sexual differentiation in reptiles. In J. M. A. Sitsen (Ed.), Handbook of sexology: Vol. 6. The pharmacology and endocrinology of sexual function (pp. 98–121). New York: Elsevier.
- Crews, D., & Silver, R. (1985). Reproductive physiology and behavior interactions in nonmammalian vertebrates. In N. T. Adler, D. Pfaff, & R. W. Goy (Eds.), Handbook of behavioral neurobiology: Vol. 7. Reproduction (pp. 101–182). New York: Plenum Press.
- Crowley, W. R., Nock, B. L., & Feder, H. H. (1978). Facilitation of lordosis behavior by clonidine in female guinea pigs. Pharmacology, Biochemistry, and Behavior, 8, 207–209.
- Damsma, G., Pfaus, J. G., Wenkstern, D., Philips, A. G., & Fibiger, H. C. (1992). Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: A comparison with novelty and locomotion. Behavioral Neuroscience, 106, 181–191.
- Davidson, J. M. (1966). Characteristics of sex behaviour in male rats following castration. Animal Behavior, 14, 266–272.
- Davidson, J. M. (1969). Effects of estrogen on the sexual behavior of male rats. Endocrinology, 84, 1365–1372.
- Davidson, J. M., Camargo, C. A., & Smith, E. R. (1979). Effects of androgen on sexual behavior in hypogonadal men. Journal of Clinical Endocrinology and Metabolism, 48, 955–958.
- de Jonge, F. H., Oldenburger, W. P., Louwerse, A. L., & Van de Poll, N. E. (1992). Changes in male copulatory behavior after sexual exciting stimuli: Effects of medial amygdala lesions. Physiology and Behavior, 52, 327–332.
- Dewsbury, D. A. (1972). Patterns of copulatory behavior in male mammals. Quarterly Review of Biology, 47, 1–33.
- Diamond, M., & Sigmundson, K. (1997). Management of intersexuality: Guidelines for dealing with persons with ambiguous genitalia. Archives of Pediatric and Adolescent Medicine, 151, 1046–1050.
- Dohanich, G. P., McMullan, D. M., Cada, D. A., & Mangum, K. A. (1991). Muscarinic receptor subtypes and sexual behavior in female rats. Pharmacology, Biochemistry, and Behavior. 38, 115–124
- Dominguez, J., Riolo, J. V., Xu, Z., & Hull, E. M. (2001). Regulation by the medial amygdala of copulation and medial preoptic dopamine release. The Journal of Neuroscience, 21, 349–355.
- Dornan, W. A., Malsbury, C. W., & Penney, R. B. (1987). Facilitation of lordosis by injection of substance Pinto the midbrain central gray. Neuroendocrinology, 45, 498–506.
- Dörner, G., Döcke, F., & Götz, F. (1975). Male-like sexual behaviour of female rats with unilateral lesions in the hypothalamic ventromedial nuclear region. Endokrinologie, 65, 133–137.
- Doty, R. L., Applebaum, S., Zusho, H., & Settle, R. G. (1985). Sex differences in odor identification ability: A cross cultural analysis. Neuropsychologia, 23, 667–672.
- Du, J., Lorrain, D. S., & Hull, E. M. (1998). Castration decreases extracellular, but increases intracellular, dopamine in medial preoptic area of male rats. Brain Research, 782, 11–17.
- Dula, E., Keating, W., Siami, P. F., Edmonds, A., O’Neil, J., & Buttler, S. (2000). Efficacy and safety of fixed-dose and doseoptimizationregimensofsublingualapomorphineversusplacebo in men with erectile dysfunction: The Apomorphine Study Group. Urology, 56, 130–135.
- Eaton, R. C., Markowski, V. P., Lumley, L. A., Thompson, J. T., Moses, J., & Hull, E. M. (1991). D2 receptors in the paraventricular nucleus regulate genital responses and copulation in male rats. Pharmacology, Biochemistry, and Behavior, 39, 177–181.
- Edwards, D. A., & Einhorn, L. C. (1986). Preoptic and midbrain control of sexual motivation. Physiology and Behavior, 37, 329–335.
- Edwards, D. A., Walter, B., & Liang, P. (1996). Hypothalamic and olfactory control of sexual behavior and partner preference in male rats. Physiology and Behavior, 60, 1347–1354.
- Eibergen, R. D., & Caggiula, A. R. (1973). Ventral midbrain involvement in copulatory behavior of the male rat. Physiology and Behavior, 10, 435–441.
- Emery, D. E., & Sachs, B. D. (1976). Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiology and Behavior, 17, 803–806.
- Erskine, M. S. (1985). Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behavioral Neuroscience, 99, 151–161.
- Etgen, A. M. (1990). Intrahypothalamic implants of noradrenergic antagonists disrupt lordosis behavior in female rats. Physiology and Behavior, 48, 31–36.
- Everitt, B. J. (1990). Sexual motivation: A neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neuroscience and Biobehavioral Reviews, 14, 217–232.
- Fausto-Sterling, A. (2000). Sexing the body: Gender politics and the construction of sexuality. New York: Basic Books.
- Fernandez-Fewell, G. D., & Meredith, M. (1994). C-fos Expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: Contribution from vomeronasal sensory input and expression related to mating performance. The Journal of Neuroscience, 14, 3643–3654.
- Fiorino, D. F., Coury, A., & Phillips, A. G. (1997). Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. The Journal of Neuroscience, 17, 4849–4855.
- Fiorino, D. F., & Phillips, A. G. (1999). Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. The Journal of Neuroscience, 19, 456–463.
- Frye, C. A., & Vongher, J. M. (1999). Progestins’ rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABA(A) receptor activity. Journal of Neuroendocrinology, 11, 829–837.
- Fumero, B., Fernandez-Vera, J. R., Gonzalez-Mora, J. L., & Mas, M. (1994). Changes in monoamine turnover in forebrain areas associated with masculine sexual behavior: A microdialysis study. Brain Research, 662, 233–239.
- Funabashi, T., Brooks, P. J., Kleopoulos, S. P., Grandison, L., Mobbs, C. V., & Pfaff, D. W. (1995). Changes in preproenkephalin messenger-RNA level in the rat ventromedial hypothalamus during the estrous cycle. Molecular Brain Research, 28, 129–134.
- Gill, C. J., Wersinger, S. R., Veney, S. L., & Rissman, E. F. (1998). Induction of c-fos-like immunoreactivity in musk shrews after mating. Brain Research, 811, 21–28.
- Giuliano, F., Bernabé, J., Brown, K., Droupy, S., Benoit, G., & Rampin, O. (1997). Erectile response to hypothalamic stimulation in rats: Role of peripheral nerves. American Journal of Physiology, 273, R1990–R1997.
- Giuliano, F., Bernabé, J., McKenna, K., Longueville, F., & Rampin, O. (2001). Spinal proerectile effect of oxytocin in anesthetized rats. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology, 280, R1870–R1877.
- Giuliano, F., Montorsi, F., Mirone, V., Rossi, D., & Sweeney, M. (2000). Switching from intracavernous prostaglandin E1 injections to oral sildenafil citrate in patients with erectile dysfunction: Results of a multicenter European study. Journal of Urology, 164, 708–711.
- Giuliano, F., & Rampin, O. (2000). Central neural regulation of penile erection. Neuroscience and Biobehavioral Reviews, 24, 517–533.
- Goldstein, I., Lue, T. F., Padma-Nathan, H., Rosen, R. C., Steers, W. D., & Wicker, P. A. (1998). Oral sildenafil in the treatment of erectile dysfunction. New England Journal of Medicine, 338, 1397–1404.
- Gooren, L. J. (1985). Human male sexual functions do not require aromatization of testosterone: A study using tamoxifen, testolactone, and dihydrotestosterone. Archives of Sexual Behavior, 14, 539–548.
- Goy, R. W., & McEwen, B. S. (1980). Sexual differentiation of the brain. Cambridge, MA: MIT Press.
- Gray, G. D., Smith, E. R., & Davidson, J. M. (1980). Hormonal regulation of penile erection in castrated male rats. Physiology and Behavior, 24, 463–468.
- Gréco, B., Edwards, D. A., Zumpe, D., & Clancy, A. N. (1998). Androgen receptor and mating-induced Fos immunoreactivity are co-localized in limbic and midbrain neurons that project to the male rat medial preoptic area. Brain Research, 781, 15–24.
- Haensel, S. M., & Slob, A. K. (1997). Flesinoxan: A prosexual drug for male rats. European Journal of Pharmacology, 330, 1–9.
- Hamburger-Bar, R., & Rigter, H. (1975). Apomorphine: Facilitation of sexual behaviour in female rats. European Journal of Pharmacology, 32, 357–360.
- Hansen, S., & Hagelsrum, L. J. (1984). Emergence of displacement activities in the male rat following thwarting of sexual behavior. Behavioral Neuroscience, 98, 868–883.
- Hansen, S., Stanfield, E. J., & Everitt, B. J. (1980). The role of ventral bundle noradrenergic neurones in sensory components of sexual behaviour and coitus-induced pseudopregnancy. Nature, 286, 152–154.
- Harlan, R. E., Shivers, B. D., & Pfaff, D. W. (1983) Midbrain microinfusions of prolactin increase the estrogen-dependent behavior, lordosis. Science, 219, 1451–1453.
- Hart, B. L. (1968). Alteration of quantitative aspects of sexual reflexes in spinal male dogs by testosterone. Journal of Comparative and Physiological Psychology, 66, 726–730.
- Hart, B. L. (1973). Effects of testosterone propionate and dihydrotestosterone on penile morphology and sexual reflexes of spinal male rats. Hormones and Behavior, 4, 239–246.
- Hart, B. L. (1974). The medial preoptic-anterior hypothalamic area and sociosexual behavior of male dogs: A comparative neuropsychological analysis. Journal of Comparative and Physiological Psychology, 86, 328–349.
- Hart, B. L., Haugen, C. M., & Peterson, D. M. (1973). Effects of medial preoptic-anterior hypothalamic lesions on mating behavior of male cats. Brain Research, 54, 177–191.
- Heeb, M. M., & Yahr, P. (1996). C-fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience, 72, 1049–1071.
- Heeb, M. M., & Yahr, P. (2000). Cell-body lesions of the posterodorsal preoptic nucleus or posterodorsal medial amygdala, but not the parvicellular subparafascicular thalamus, disrupt mating in male gerbils. Physiology and Behavior, 68, 317–331.
- Heim, N., & Hursch, C. J. (1979). Castration for sex offenders: Treatment or punishment? A review and critique of recent European literature. Archives of Sexual Behavior, 8, 281–304.
- Heimer, L., & Larsson, K. (1966/1967). Impairment of mating behavior in male rats following lesions in the preoptic-anterior hypothalamic continuum. Brain Research, 3, 248–263.
- Hennessey, A. C., Camak, L., Gordon, F., & Edwards, D. A. (1990). Connections between the pontine central gray and the ventromedial hypothalamus are essential for lordosis in female rats. Behavioral Neuroscience, 104, 477–488.
- Holmes, G. M., Chapple, W. D., Leipheimer, R. E., & Sachs, B. D. (1991). Electromyographic analysis of male rat perineal muscles during copulation and reflexive erections. Physiology and Behavior, 49, 1235–1246.
- Holmes, G. M., & Sachs, B. D. (1992). Erectile function and bulbospongiosus EMG activity in estrogen-maintained castrated rats vary with behavioral context. Hormones and Behavior, 26, 406–419.
- Hoshina, Y., Takeo, T., Nakano, K., Sato, T., & Sakuma, Y. (1994). Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behavioural Brain Research, 61, 197–204.
- Hull, E. M., Bazzett, T. J., Warner, R. K., Eaton, R. C., & Thompson, J. T. (1990). Dopamine receptors in the ventral tegmental area modulate male sexual behavior in rats. Brain Research, 512, 1–6.
- Hull, E. M., Bitran, D., Pehek, E. A., Warner, R. K., & Band, L. C. (1986). Dopaminergic control of male sex behavior in rats: Effects of an intracerebrally infused agonist. Brain Research, 370, 73–81.
- Hull, E. M., Du, J., Lorrain, D. S., & Matuszewich, L. (1995). Extracellular dopamine in the medial preoptic area: Implications for sexual motivation and hormonal control of copulation. The Journal of Neuroscience, 15, 7465–7471.
- Hull, E. M., Eaton, R. C., Markowski, V. P., Moses, J., Lumley, L. A., & Loucks, J. A. (1992). Opposite influence of medial preoptic Dl and D2 receptors on genital reflexes: Implications for copulation. Life Sciences, 51, 1705–1713.
- Hull, E. M., Lumley, L. A., Matuszewich, L., Dominguez, J., Moses, J., & Lorrain, D. S. (1994). The roles of nitric oxide in sexual function of male rats. Neuropharmacology, 33, 1499–l504.
- Hull, E. M., Meisel, R. L., & Sachs, B. D. (2002). Male sexual behavior. In D. W. Pfaff (Ed.), Hormones, brain, and behavior (pp. 3–137). New York: Academic Press.
- Hull, E. M., Warner, R. K., Bazzett, T. J., Eaton, R. C., Thompson, J. T., & Scaletta, L. L. (1989). D2/D1 ratio in the medial preoptic area affects copulation of male rats. Journal of Pharmacology and Experimental Therapeutics, 251, 422–427.
- Hull, E. M., Weber, M. S., Eaton, R. C., Dua, R., Markowski, V. P., Lumley, L., & Moses, J. (1991). Dopamine receptors in the ventral tegmental area affect motor, but not motivational or reflexive, components of copulation in male rats. Brain Research, 554, 72–76.
- Imperato-McGinley, J., Guerrero, L., Gautier, T., & Peterson, R. E. (1974). Steroid 5-alpha-reductase deficiency in man: An inherited form of male pseudohermaphroditism. Science, 186, 1213– 1215.
- Jenkins, W. J., & Becker, J. B. (2001a). Role of the striatum and nucleus accumbens in paced copulatory behavior in the female rat. Behavioural Brain Research, 121, 119–128.
- Jenkins, W. J., & Becker, J. B. (2001b). Sexual behavior that occurs at the female rat’s preferred pacing interval is reinforcing [Abstract]. Hormones and Behavior, 39,
- Johnson, R. D. (1988). Efferent modulation of penile mechanoreceptor activity. In A. Iggo & W. Hamann, (Eds.), Progress in brain research: Vol. 74. Transduction and cellular mechanisms in sensory receptors (pp. 319–324). Amsterdam: Elsevier.
- Johnson, R. D., & Kitchell, R. L. (1987). Mechanoreceptor response to mechanical and thermal stimuli in the glans penis of the dog. Journal of Neurophysiology, 57, 1813–1836.
- Kato, A., & Sakuma, Y. (2000). Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Research, 862, 90–102.
- Kelliher, K. R., Liu, Y. C., Baum, M. J., & Sachs, B. D. (1999). Neuronal Fos activation in olfactory bulb and forebrain of male rats having erections in the presence of inaccessible estrous females. Neuroscience, 92, 1025–1033.
- Kindon, H. A., Baum, M. J., & Paredes, R. J. (1996). Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Hormones and Behavior, 30, 514–527.
- Kinsey, A. C., Pomeroy, W. B., & Martin, C. E. (1948). Sexual behavior in the human male. Philadelphia: W. B. Saunders.
- Kollack-Walker, S., & Newman, S. W. (1997). Mating-induced expression of c-fos in the male Syrian hamster brain: Role of experience, pheromones, and ejaculations. Journal of Neurobiology, 32, 481–501.
- Kondo, Y. (1992). Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiology and Behavior, 51, 939–943.
- Kondo, Y., Sachs, B. D., & Sakuma, Y. (1997). Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behavioural Brain Research, 91, 215–221.
- Kondo, Y., Tomihara, K., & Sakuma, Y. (1999). Sensory requirement for noncontact penile erection in the rat. Behavioral Neuroscience, 113, 1062–1070.
- Kondrashov, A. S. (1988). Deleterious mutations and the evolution of sexual reproduction. Nature, 336, 435–440.
- Kow, L. M., Johnson, A. E., Ogawa, S., & Pfaff, D. W. (1991). Electrophysiological actions of oxytocin on hypothalamic neurons in vitro: Neuropharmacological characterization and effects of ovarian steroids. Neuroendocrinology, 54, 526–535.
- Kow, L. M., Montgomery, M. O., & Pfaff, D. W. (1979). Triggering of lordosis reflex in female rats with somatosensory stimulation: Quantitative determination of stimulus parameters. Journal of Neurophysiology, 42, 195–202.
- Kow, L. M., Weesner, G. D., & Pfaff, D. W. (1992) Alpha 1-adrenergic agonists act on the ventromedial hypothalamus to cause neuronal excitation and lordosis facilitation: Electrophysiological and behavioral evidence. Brain Research, 588, 237–245.
- Krey, L. C., & McGinnis, M. Y. (1990). Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: Relationships with gonadotropin secretion. Journal of Steroid Biochemistry, 35, 403–408.
- LaFerla, J. L., Anderson, D. L., & Schalch, D. S. (1978). Psychoendocrine response to sexual arousal in human males. Psychosomatic Medicine, 40, 166–172.
- Lal, S., Laryea, E., Thavundayil, J. X., Nair, N. P., Negrete, J., Ackman, D., Blundell, P., & Gardiner, R. J. (1987). Apomorphine-induced penile tumescence in impotent patients: Preliminary findings. Progress in Neuropsychopharmacology and Biological Psychiatry, 11, 235–242.
- Lauber, A. H., Romano, G. J., & Pfaff, D. W. (1991). Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology, 53, 608–613.
- Lee, R. L., Smith, E. R., Mas, M., & Davidson, J. M. (1990). Effects of intrathecal administration of 8-OH-DPAT on genital reflexes and mating behavior in male rats. Physiology and Behavior, 47, 665–669.
- Lehman, M. N., & Winans, S. S. (1982). Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: Autoradiographic and behavioral analyses. Brain Research, 240, 27–41.
- Lehman, M. N., Winans, S. S., & Powers, J. B. (1980). Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science, 210, 557–560.
- Levis, D. G., & Ford, J. J. (1989). The influence of androgenic and estrogenic hormones on sexual behavior in castrated adult male pigs. Hormones and Behavior, 23, 393–411.
- Leyton, M., & Stewart, J. (1992). The stimulation of central kappa opioid receptors decreases male sexual behavior and locomotor activity. Brain Research, 594, 56–74.
- Liu, Y. C., Sachs, B. D., & Salamone, J. D. (1998). Sexual behavior in male rats after radiofrequency or dopamine-depleting lesions in nucleus accumbens. Pharmacology, Biochemistry, and Behavior, 60, 585–592.
- Liu, Y. C., Salamone, J. D., & Sachs, B. D. (1997a). Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behavioral Neuroscience, 111, 136l–1367.
- Liu, Y. C., Salamone, J. D., & Sachs, B. D. (1997b). Lesions in medial preoptic area and bed nucleus of stria terminalis: Differential effects on copulatory behavior and noncontact erection in male rats. The Journal of Neuroscience, 17, 5245–5253.
- Lloyd, S. A., & Dixson, A. F. (1988). Effects of hypothalamic lesions upon the sexual and social behaviour of the male common marmoset (Callithrix jacchus). Brain Research, 463, 317–329.
- Lorrain, D. S., & Hull, E. M. (1993). Nitric oxide increases dopamine and serotonin release in the medial preoptic area. NeuroReport, 5, 87–89.
- Lorrain, D. S., Matuszewich, L., Friedman, R. D., & Hull, E. M. (1997). Extracellular serotonin in the lateral hypothalamic area is increased during postejaculatory interval and impairs copulation in male rats. The Journal of Neuroscience, 17, 9361–9366.
- Lorrain, D. S., Matuszewich, L., Howard, R. V., Du, J., & Hull, E. M. (1996). Nitric oxide promotes medial preoptic dopamine release during male rat copulation. NeuroReport, 8, 3l–34.
- Lorrain, D. S., Riolo, J. V., Matuszewich, L., & Hull, E. M. (1999). Lateral hypothalamic serotonin inhibits nucleus accumbens dopamine: Implications for sexual refractoriness. The Journal of Neuroscience, 19, 7648–7652.
- Lott, D. F. (1981). Sexual behavior and intersexual strategies in American bison. Zhurnal für Tierpsychologie, 56, 97–114.
- Lugg, J. A., Rajfer, J., & Gonzalez-Cadavid, N. F. (1995). Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology 136, 1495–1501.
- Lumia, A. R., Meisel, R. L., & Sachs, B. D. (1981). Induction of female and male mating patterns in female rats by gonadal steroids: Effects of neonatal or adult olfactory bulbectomy. Journal of Comparative and Physiological Psychology, 95, 497–509.
- Lumley,L.A.,&Hull,E.M.(1999).EffectsofaD1 antagonistandof sexual experience on copulation-induced Fos-like immunoreactivityinthemedialpreopticnucleus.BrainResearch,829,55–68.
- Luttge, W. G., & Hall, N. R. (1973). Differential effectiveness of testosterone and its metabolites in the induction of male sexual behaviorintwostrainsofalbinomice.HormonesandBehavior,4, 3l–43.
- Mackay-Sim, A., & Rose, J. D. (1986). Removal of the vomeronasal organ impairs lordosis in female hamsters: Effect is reversed by luteinising hormone-releasing hormone. Neuroendocrinology, 42, 489–493.
- Maeda, N., Matsuoka, N., & Yamaguchi, I. (1994). Role of the dopaminergic, serotonergic and cholinergic link in the expression of penile erection in rats. Japanese Journal of Pharmacology, 66, 59–66.
- Majewska, M. D., Harrison, N. L, Schwartz, R. D., Barker, J. L., & Paul, S. M. (1986). Steroid hormone metabolites are barbituratelike modulators of the GABA receptor. Science, 232, 1004– 1007.
- Malmnas, C. O. (1976). The significance of dopamine, versus other catecholamines, for L-dopa induced facilitation of sexual behavior in the castrated male rat. Pharmacology, Biochemistry, and Behavior, 4, 521–526.
- Malsbury, C. W. (1971). Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiology and Behavior, 7, 797–805.
- Mani,S.K.,Allen,J.M.C.,Clark,J.H.,Blaustein,J.D.,&O’Malley, B. W. (1994). Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science, 265, 1246–1249.
- Mani, S. K., Blaustein, J. D., & O’Malley, B. W. (1997). Progesterone receptor function from a behavioral perspective. Hormones and Behavior, 31, 244–255.
- Manzo, J., Cruz, M. R., Hernandez, M. E., Pacheco, P., & Sachs, B. D. (1999). Regulation of noncontact erection in rats by gonadal steroids. Hormones and Behavior, 35, 264–270.
- Markowski, V. P., Eaton, R. C., Lumley, L. A., Moses, J., & Hull, M. (1994). A D1 agonist in the MPOA area facilitates copulation of male rats. Pharmacology Biochemistry and Behavior, 47, 483–486.
- Markowski, V. P., & Hull, E. M. (1995). Cholecystokinin modulates mesolimbic dopaminergic influences on male rat copulatory behavior. Brain Research, 699, 266–274.
- Marson, L., List, M. S., & McKenna, K. E. (1992). Lesions of the nucleus paragigantocellularis alter ex copula penile reflexes. Brain Research, 592, 187–192.
- Marson, L., & McKenna, K. E. (1990). The identification of a brainstem site controlling spinal sexual reflexes in male rats. Brain Research, 515, 303–308.
- Marson, L., & McKenna, K. E. (1992). A role for 5-hydroxytryptamine in descending inhibition of spinal sexual reflexes. Experimental Brain Research, 88, 313–320.
- Marson, L., & McKenna, K. E. (1994a). Serotonergic neurotoxic lesions facilitate male sexual reflexes. Pharmacology Biochemistry Behavior, 47, 883–888.
- Marson, L., & McKenna, K. E. (1994b). Stimulation of the hypothalamus initiates the urethrogenital reflex in male rats. Brain Research, 638, 103–108.
- Mas, M., Gonzalez-Mora, J. L., Louilot, A., Sole, C., & Guadalupe, T. (1990). Increased dopamine release in the nucleus accumbens of copulating male rats as evidenced by in vivo voltammetry. Neuroscience Letters, 110, 303–308.
- Matteo, S., & Rissman, E. F. (1984). Increased sexual activity during the midcycle portion of the human menstrual cycle. Hormones and Behavior, 18, 249–255.
- Matuszewich, L., Lorrain, D. S., & Hull, E. M. (2000). Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behavioral Neuroscience, 114, 772–782.
- McCarthy, M. M., McDonald, C. H., Brooks, P. J., & Goldman, D. (1996). An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiology and Behavior, 60, 1209–1215.
- McCarthy, M. M, Pfaff, D. W., & Schwartz-Giblin, S. (1991). Midbrain central gray GABA-a receptor activation enhances, and blockade reduces, sexual-behavior in the female rat. Experimental Brain Research, 86, 108–116.
- McCarthy, M. M., Schlenker, E. H., & Pfaff, D. W. (1993). Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology, 133, 433–439.
- McClintock, M. K. (1987). A functional approach to the behavioral endocrinology of rodents. In D. Crews (Ed.), Psychobiology of reproductive behavior: An evolutionary perspective (pp. 176– 203). Englewood Cliffs, NJ: Prentice Hall.
- McDonald, A. J. (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55, 257–332.
- McGinnis, M. Y., & Kahn, D. F. (1997). Inhibition of male sexual behavior by intracranial implants of the protein synthesis inhibitor anisomycin into the medial preoptic area of the rat. Hormones and Behavior, 31, 15–23.
- McGinnis, M. Y., & Mirth, M. C. (1986). Inhibition of cell nuclear androgen receptor binding and copulation in male rats by an antiandrogen, Sch 16423. Neuroendocrinology, 43, 63–68.
- McIntosh, T. K., Vallano, M. L., & Barfield, R. J. (1980). Effects of morphine, beta-endorphin and naloxone on catecholamine levels and sexual behavior in the male rat. Pharmacology, Biochemistry, and Behavior, 13, 435–441.
- McKenna, K. E., Chung, S. K., & McVary, K. T. (1991). Amodel for the study of sexual function in anesthetized male and female rats. American Journal Physiology, 30, R1276–R1285.
- Meisel, R. L., Camp, D. M., & Robinson, T. E. (1993). A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behavioural Brain Research, 55, 151–157.
- Meisel, R. L., Leipheimer, R. E., & Sachs, B. D. (1986). Anisomycin does not disrupt the activation of penile reflexes by testosterone in rats. Physiology and Behavior, 37, 951–956.
- Meisel, R. L., O’Hanlon, J. K., & Sachs, B. D. (1984). Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Hormones and Behavior, 18, 56–64.
- Meisel, R. L., Sachs, B. D., & Lumia, A. R. (1984). Olfactory bulb control of sexual function. In S. Finger & C. R. Almli (Eds.), Early brain damage: Vol. 2. Neurobiology and behavior (pp. 253–268). Orlando: Academic Press.
- Melis, M. R., Argiolas, A., & Gessa, G. L. (1987). Apomorphineinduced penile erection and yawning: Site of action in brain. Brain Research, 415, 98–104.
- Melis,M.R.,Stancampiano,R.,&Argiolas,A.(1992).Hippocampal oxytocin mediates apomorphine-induced penile erection and yawning. Pharmacology, Biochemistry, and Behavior, 42, 61–66.
- Melis, M. R., Succu, S., & Argiolas, A. (1996). Dopamine agonists increase nitric oxide production in the paraventricular nucleus of the hypothalamus: Correlation with penile erection and yawning. European Journal of Neuroscience, 8, 2056–2063.
- Melis, M. R., Succu, S., Iannucci, U., & Argiolas, A. (1997). N-methyl-D-aspartic acid-induced penile erection and yawning: Role of hypothalamic paraventricular nitric oxide. European Journal of Pharmacology, 328, 115–123.
- Melis, M. R., Succu, S., Mauri, A., & Argiolas, A. (1998). Nitric oxide production is increased in the paraventricular nucleus of the hypothalamus of male rats during non-contact penile erections and copulation. European Journal of Neuroscience, 10, 1968–1974.
- Melis, M. R., Succu, S., Spano, M. S., & Argiolas, A. (1999a). Morphine injected into the paraventricular nucleus of the hypothalamus prevents noncontact penile erections and impairs copulation involvement of nitric oxide. European Journal of Neuroscience, 11, 1857–1864.
- Melis, M. R., Succu, S., Spano, M. S., & Argiolas, A. (1999b). The oxytocin antagonist d(CH2)5Tyr(ME)2-Om8-vasotocin reduces non-contact penile erections in male rats. Neuroscience Letters, 265, 171–174.
- Meredith, M., Marques, D. M., O’Connell, R. J., & Stem, F. L. (1980). Vomeronasal pump: Significance for male hamster sexual behavior. Science, 207, 1224–1226.
- Mermelstein, P. G., & Becker, J. B. (1995). Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behavioral Neuroscience, 109, 354–365.
- Millan, M. J., Peglion, J. L, Lavielle, G., & Perrin-Monneyron, S. (1997). 5-HT2C receptors mediate penile erections in rats, actions of novel and selective agonists and antagonists. European Journal of Pharmacology, 325, 9–12.
- Mitchell, J. B., & Gratton, A. (1991). Opioid modulation and sensitization of dopamine release elicited by sexually relevant stimuli: A high speed chronoamperometric study in freely behaving rats. Brain Research, 551, 20–27.
- Monaghan, E. P., Arjomand, J., & Breedlove, S. M. (1993). Brain lesions affect penile reflexes. Hormones and Behavior, 27, 122– 131.
- Moore, M. C., Hews, D. K., & Knapp, R. (1998). Hormonal control and evolution of alternative male phenotypes: Generalizations of models for sexual differentiation. American Zoologist, 38, 133– 151.
- Morali, G., Lemus, A. E., Munguia, R., Arteaga, M., Perez-Palacios, G., Sundaram, K., Kumar, N., & Bardin, C. W. (1993). Induction of male sexual behavior in the rat by 7 alpha-methyl-19nortestosterone, an androgen that does not undergo 5 alphareduction. Biology of Reproduction, 49, 577–581.
- Moses, J., & Hull, E. M. (1999). A nitric oxide synthesis inhibitor administered into the medial preoptic area increases seminal emissions in an ex copula reflex test. Pharmacology, Biochemistry, and Behavior, 63, 345–348.
- Moses, J., Loucks, J. A., Watson, H. L., Matuszewich, L., & Hull, E. M. (1995). Dopaminergic drugs in the medial preoptic area and nucleus accumbens: Effects on motor activity, sexual motivation, and sexual performance. Pharmacology, Biochemistry, and Behavior, 51, 681–686.
- Mosig, D. W., & Dewsbury, D. A. (1976). Studies of the copulatory behavior of house mice (Mus musculus). Behavioral Biology, 16, 463–473.
- Murphy, A. Z., & Hoffman, G. E. (2001). Distribution of gonadal steroidreceptor-containingneuronsinthepreoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. Journal of Comparative Neurology, 438, 191–212.
- Murphy, A. Z., Shupnik, M. A., & Hoffman, G. E. (1999). Androgen and estrogen (alpha) receptor distribution in the periaqueductal gray of the male rat. Hormones and Behavior, 36, 98–108.
- Murphy, M. R. (1980). Sexual preferences of male hamsters: Importance of preweaning and adult experience, vaginal secretion, and olfactory or vomeronasal sensation. Behavioral and Neural Biology, 30, 323–340.
- Myers, B. M., & Baum, M. J. (1979). Facilitation by opiate antagonists of sexual performance in the male rat. Pharmacology, Biochemistry, and Behavior, 10, 615–618.
- Nance, D. M., Shryne, J. E., Gordon, J. H., & Gorski, R. A. (1977). Examination of some factors that control the effects of septal lesions on lordosis behavior. Pharmacology, Biochemistry, and Behavior, 6, 227–234.
- Nelson, R. J. (2000). An introduction to behavioral endocrinology (2nd. ed.). Sunderland, MA: Sinauer.
- Newman, S. W. (1999). The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Annals of the New York Academy of Sciences, 877, 242–257.
- Nock, B., & Feder, H. H. (1979). Noradrenergic transmission and female sexual behavior of guinea pigs. Brain Research, 166, 369–380.
- O’Carroll, R., Shapiro, C., & Bancroft, J. (1985). Androgens, behaviour and nocturnal erection in hypogonadal men: The effects of varying the replacement dose. Clinical Endocrinology, 23, 527–538.
- Ogawa, S., Olazabal, U. E., Parhar, I. S., & Pfaff, D. W. (1994). Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. The Journal of Neuroscience, 14, 1766–1774.
- Ogawa, S., Robbins, A., Kumar, N., Pfaff, D. W., Sundaram, K., & Bardin, C. W. (1996). Effects of testosterone and 7 alpha-methyl19-nortestosterone (MENT) on sexual and aggressive behaviors in two inbred strains of male mice. Hormones and Behavior, 30, 74–84.
- Ogawa, S., Washburn, T. F., Taylor, J., Lubahn, D. B., Korach, K. S., & Pfaff, D. W. (1998). Modification of testosteronedependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology, 139, 5058–5069.
- O’Hanlon, J. K., Meisel, R. L., & Sachs, B. D. (1981). Estradiol maintains castrated male rats’ sexual reflexes in copula, but not ex copula. Behavioral and Neural Biology, 32, 269–273.
- O’Hanlon, J. K., & Sachs, B. D. (1980). Penile reflexes in rats after different numbers of ejaculations. Behavioral and Neural Biology, 29, 338–348.
- Oldenburger, W. P., Everitt, B. J., & de Jonge, F. H. (1992). Conditioned place preference induced by sexual interation in female rats. Hormones and Behavior, 26, 214–228.
- Paredes, R. G., & Ågmo, A. (1995). The GABAB antagonist CGP 35348 inhibits the effects of baclofen on sexual behavior and motor coordination. Brain Research Bulletin, 36, 495–497.
- Paredes, R. G., & Alonso, A. (1997). Sexual behavior regulated (paced) by the female induces conditioned place preference. Behavioral Neuroscience, 111, 123–128.
- Paredes, R. G., & Baum, M. J. (1995). Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. The Journal of Neuroscience, 15, 6619–6630.
- Paredes, R. G., Highland, L., & Karam, P. (1993). Socio-sexual behavior in male rats after lesions of the medial preoptic area: Evidence for reduced sexual motivation. Brain Research, 618, 271–276.
- Paredes, R. G., Karam, P., Highland, L., & Ågmo, A. (1997). GABAergic drugs and socio-sexual behavior. Pharmacology, Biochemistry, and Behavior, 58, 29l–298.
- Paredes, R. G., Tzschentke, T., & Nakach, N. (1998). Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Research, 813, 8l–83.
- Parfitt, D. B., Coolen, L. M., Newman, S. W., & Wood, R. I. (1996). Lesions of the posterior medial nucleus of the amygdala delay sexual satiety. Society for Neuroscience Abstracts, 22,
- Parfitt, D. B., & Newman, S. W. (1998). Fos-immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Hormones and Behavior, 34, 17–29.
- Parrott, R. F. (1986). Minimal effects of 17 beta-hydroxy-17 alphamethyl-estra-4,9,11-triene-3-one (R1881) on sexual behaviour in prepubertally castrated rams. Journal of Endocrinology, 110, 481–487.
- Pehek, E.A., Thompson, J. T., & Hull, E. M. (1989a). The effects of intracranial administration of the dopamine agonist apomorphine on penile reflexes and seminal emission in the rat. Brain Research, 500, 325–332.
- Pehek, E. A., Thompson, J. T., & Hull, E. M. (1989b). The effects of intrathecal administration of the dopamine agonist apomorphine on penile reflexes and copulation in the male rat. Psychopharmacology, 99, 304–308.
- Pfaff, D. W. (1999). Drive: Neurobiological and molecular mechanisms of sexual motivation. Cambridge, MA: MIT Press.
- Pfaff, D. W., & Pfaffmann, C. (1969). Olfactory and hormonal influences on the basal forebrain of the male rat. Brain Research, 15, 137–156.
- Pfaff, D. W., & Sakuma, Y. (1979). Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. Journal of Physiology, 288, 189–202.
- Pfaff, D. W., & Schwartz-Giblin, S. (1988). Cellular mechanisms of female reproductive behaviors. In E. Knobil, J. Neill, & D. W. Pfaff (Eds.), The physiology of reproduction (pp. 1487–1576). New York: Raven.
- Pfaff, D. W., Schwartz-Giblin, S., McCarthy, M. M., & Kow, L. M. (1994). Cellular and molecular mechanisms of female reproductive behaviors. In E. Knobil & J. Neill (Eds.), The physiology of reproduction (2nd ed.). New York: Raven.
- Pfaus, J. G., Damsma, G., Nomikos, G. G., Wenkstern, D. G., Blaha, C. D., Philips, A. G., & Fibiger, H. C. (1990). Sexual behavior enhances central dopamine transmission in the rat. Brain Research, 530, 345–348.
- Pfaus, J. G., Damsma, G., Wenkstern, D., & Fibiger, H. C. (1995). Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Research, 693, 21–30.
- Pfaus, J. G., & Gorzalka, B. B. (1987). Selective activation of opioid receptors differentially affects lordosis behavior in female rats. Peptides, 8, 309–317.
- Pfaus, J. G., & Heeb, M. M. (1997). Implications of immediateearly gene induction in the brain following sexual stimulation of female and male rodents. Brain Research Bulletin, 44, 397– 407.
- Pfaus, J. G., Mendelson, S. D., & Phillips, A. G. (1990). A correlational and factor analysis of anticipatory and consummatory measures of sexual behavior in the male rat. Psychoneuroendocrinology, 15, 329–340.
- Pfaus, J. G., Pendleton, N., & Gorzalka, B. B. (1986). Dual effect of morphiceptin on lordosis behavior: Possible mediation by different opioid receptor subtypes. Pharmacology, Biochemistry, and Behavior, 24, 1461–1464.
- Phelps, S. M., Lydon, J. P., O’Malley, B. W., & Crews, D. (1998). Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Hormones and Behavior, 34, 294–302.
- Phoenix, C. H. (1974). Effects of dihydrotestosterone on sexual behavior of castrated male rhesus monkeys. Physiology and Behavior, 12, 1045–1055.
- Pirke, K. M., Kockott, G., Aldenhoff, J., Besinger, U., & Feil, W. (1979). Pituitary gonadal system function in patients with erectile impotence and premature ejaculation. Archives of Sexual Behavior, 8, 41–48.
- Pomerantz, S. M., Hepner, B. C., & Wertz, J. M. (1993). 5-HT1A and 5-HT1C /1D receptor agonists produce reciprocal effects on male sexual behavior of rhesus monkeys. European Journal of Pharmacology, 243, 227–234.
- Putnam, S. K., Du, J., Sato, S., & Hull, E. M. (2001). Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Hormones and Behavior, 39, 216–224.
- Putnam, S. K., Panos, J. J., & Hull, E. M. (1998). Hormonal maintenance of copulation in male rat castrates: Association with MPOA dopamine response. Society for Neuroscience Abstracts, 24,
- Qureshi, G. A., Bednar, I., Forsberg, B. G., & Södersten, P. (1988). GABA inhibits sexual behaviour in female rats. Neuroscience, 27, 169–174.
- Raboch, J., Mellan, J., & Starka, L. (1975). Plasma testosterone in male patients with sexual dysfunction. Archives of Sexual Behavior, 4, 541–545.
- Retana-Marquez, S., & Velazquez-Moctezuma, J. (1997). Cholinergicandrogenic interaction in the regulation of male sexual behavior in rats. Pharmacology, Biochemistry, and Behavior, 56, 373–378.
- Richmond, G., & Clemens, L. G. (1986). Evidence for involvement of midbrain central gray in cholinergic mediation of female sexual receptivity in rats. Behavioral Neuroscience, 100, 376–380.
- Rissman, E. F. (1991). Frank A. Beach Award: Behavioral endocrinology of the female musk shrew. Hormones and Behavior, 25, 125–127.
- Rissman, E. F., Early, A. H., Taylor, J. A., Korach, K. S., & Lubahn, D. B. (1997). Estrogen receptors are essential for female sexual receptivity. Endocrinology, 138, 507–510.
- Rizvi, T. A., Murphy, A. Z., Ennis, M., Behbehani, M. M., & Shipley, M. T. (1996). Medial preoptic area afferents to periaqueductal gray medullo-output neurons: A combined Fos and tract tracing study. Journal of Neuroscience, 16, 333– 344.
- Robertson, G. S., Pfaus, J. G., Atkinson, L. J., Matsumura, H., Phillips, A. G., & Fibiger, H. C. (1991). Sexual behavior increases c-fos expression in the forebrain of the male rat. Brain Research, 564, 352–357.
- Robinson, T. J. (1954). The necessity for progesterone with estrogen for the induction of recurrent estrus in the ovariectomized ewe. Endocrinology, 55, 403–408.
- Rodríguez-Manzo, G. (1999). Yohimbine interacts with the dopaminergic system to reverse sexual satiation: Further evidence for a role of sexual motivation in sexual exhaustion. European Journal of Pharmacology, 372, 1–8.
- Rodríguez-Manzo, G., Pellicer, F., Larsson, K. & Fernandez-Guasti, A. (2000). Stimulation of the medial preoptic area facilitates sexual behavior but does not reverse sexual satiation. Behavioral Neuroscience, 114, 553–560.
- Romano, G. J., Harlan, R. E., Shivers, B. D., Howells, R. D., & Pfaff, D. W. (1988). Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Molecular Endocrinology, 2, 1320–1328.
- Rosen, R. C., Lane, R. M., & Menza, M. (1999). Effects of SSRIs on sexual function: A critical review. Journal of Clinical Psychopharmacology, 19, 67–85.
- Rubin, B., & Barfield, R. (1983). Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology, 37, 218–224.
- Sachs, B. D. (1978). Conceptual and neural mechanisms of masculine copulatory behavior. In T. E. McGill, D. A. Dewsbury, & B. D. Sachs (Eds.), Sex and behavior (pp. 267–295). New York: Plenum Press.
- Sachs, B. D. (1980). Sexual reflexes of spinal male house mice. Physiology and Behavior, 24, 489–492.
- Sachs, B. D. (1983). Potency and fertility: Hormonal and mechanical causes and effects of penile actions in rats. In J. Balthazart, E. Pröve, & R. Gilles (Eds.), Hormones and behaviour in higher vertebrates (pp. 86–110). Berlin: Springer-Verlag.
- Sachs, B. D. (2000). Contextual approaches to the physiology and classification of erectile function, erectile dysfunction, and sexual arousal. Neuroscience and Biobehavioral Reviews, 24, 541–560.
- Sachs, B. D., Akasofu, K., Citron, J. H., Daniels, S. B., & Natoli, J. H. (1994). Noncontact stimulation from estrous females evokes penile erection in rats. Physiology and Behavior, 55, 1073–1079.
- Sachs, B. D., & Bitran, D. (1990). Spinal block reveals roles for brain and spinal cord in the mediation of reflexive erection in rats. Brain Research, 528, 99–108.
- Sachs, B. D., & Garinello, L. D. (1979). Spinal pacemaker controlling sexual reflexes in male rats. Brain Research, 171, 152–156.
- Sachs, B. D., & Leipheimer, R. E. (1988). Rapid effect of testosterone on striated muscle activity in rats. Neuroendocrinology, 48, 453–458.
- Sakuma, Y., & Pfaff, D. W. (1979). Mesencephalic mechanisms for integration of female reproductive behavior in the rat. American Journal of Physiology, 237, R285–R290.
- Sakuma, Y., & Pfaff, D. W. (1980). LH-RH in the mesenphalic central grey can potentiate lordosis reflex of female rats. Nature, 283, 566–567.
- Sato, Y., Christ, G. J., Horita, H., Adachi, H., Suzuki, N., & Tsukamoto, T. (1999). The effects of alterations in nitric oxide levels in the paraventricular nucleus on copulatory behavior and reflexive erections in male rats. Journal of Urology, 162, 2182–2185.
- Sato, Y., Horita, H., Kurohata, T., Adachi, H., & Tsukamoto, T. (1998). Effect of the nitric oxide level in the medial preoptic area on male copulatory behavior in rats. American Journal of Physiology, 274, R243–R247.
- Scaletta, L. L., & Hull, E. M. (1990). Systemic or intracranial apomorphine increases copulation in long-term castrated male rats. Pharmacology, Biochemistry, and Behavior, 37, 471– 475.
- Scouten, C. W., Burrell, L., Palmer, T., & Cegavske, C. F. (1980). Lateral projections of the medial preoptic area are necessary for androgenic influence on urine marking and copulation in rats. Physiology and Behavior, 25, 237–243.
- Silva, N. L., & Boulant, J. A. (1986). Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. American Journal of Physiology, 250, R625–R632.
- Simerly, R. B., & Swanson, L. W. (1986). The organization of neural inputs to the medial preoptic nucleus of the rat. Journal of Comparative Neurology, 246, 312–342.
- Simerly, R. B., & Swanson, L. W. (1988). Projections of the medial preoptic nucleus: A Phaseolis vulgaris leucoagglutinin anterograde tract-tracing study in the rat. Journal of Comparative Neurology, 270, 209–242.
- Sirinathsinghji, D. J., Whittington, P. E., & Audsley, A. R. (1986). Regulation of mating behaviour in the female rat by gonadotropin-releasing hormone in the ventral tegmental area: Effects of selective destruction of the A10 dopamine neurones. Brain Research, 374, 167–173.
- Slimp, J. C., Hart, B. L., & Goy, R. W. (1978). Heterosexual, autosexual and social behavior of adult male rhesus monkeys with medial preoptic-anterior hypothalamic lesions. Brain Research, 142, 105–122.
- Slob, A. K., Bax, C. M., Hop, W. C., Rowland, D. L., & van der Werff ten Bosch, J. J. (1996). Sexual arousability and the menstrual cycle. Psychoneuroendocrinology, 21, 545–558.
- Steers, W. D. (2000). Neural pathways and central sites involved in penile erection: Neuroanatomy and clinical implications. Neuroscience and Biobehavioral Reviews, 24, 507–516.
- Stefanick, M. L., Smith, E. R., & Davidson, J. M. (1983). Penile reflexes in intact rats following anesthetization of the penis and ejaculation. Physiology and Behavior, 31, 63–65.
- Takeo, T., Chiba, Y., & Sakuma, Y. (1993). Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiology and Behavior, 53, 831–838.
- Takeo, T., & Sakuma, Y. (1995). Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neuroscience Research, 22, 73–80.
- Tallentire, D., McRae, G., Spedding, R., Clark, R., & Vickery, B. (1996). Modulation of sexual behaviour in the rat by a potent and selective alpha 2-adrenoceptor antagonist, delequamine (RS-15385-197). British Journal of Pharmacology, 118, 63–72.
- Uphouse, L. (2000). Female gonadal hormones, serotonin, and sexual receptivity. Brain Research: Brain Research Reviews, 33, 242–257.
- Vagell, M. E., & McGinnis, M. Y. (1997). The role of aromatization in the restoration of male rat reproductive behavior. Journal of Neuroendocrinology, 9, 415–421.
- Vagell, M. E., & McGinnis, M. Y. (1998). The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Hormones and Behavior, 33, 163– 179.
- van de Poll, N. E., & van Dis, H. (1979). The effect of medial preoptic-anterior hypothalamic lesions on bisexual behavior of the male rat. Brain Research Bulletin, 4, 505–511. van Furth, W. R., & van Ree, J. M. (1996). Sexual motivation: Involvement of endogenous opioids in the ventral tegmental area. Brain Research, 729, 20–28.
- Vathy, I., & Etgen, A. M. (1988). Ovarian steroids and hypothalamic norepinephrine release: Studies using in vivo brain microdialysis. Life Sciences, 43, 1493–1499.
- Vathy, I., & Marson, L. (1998). Effects of prenatal morphine and cocaine exposure on spinal sexual reflexes in male and female rats. Physiology and Behavior, 63, 445–450.
- Veening, J. G., & Coolen, L. M. (1998). Neural activation following sexual behavior in the male and female rat brain. Behavioural Brain Research, 92, 181–193.
- Vega Matuszcyk, J., Larsson, K., & Eriksson, E. (1998). The selective serotonin reuptake inhibitor fluoxetine reduces sexual motivation in male rats. Pharmacology, Biochemistry, and Behavior, 60, 527–532.
- Wallen, K. (1990). Desire and ability: Hormones and the regulation of female sexual behavior. Neuroscience and Biobehavioral Reviews, 14, 233–241.
- Wang, Z., Hulihan, T. J., & Insel, T. R. (1997). Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Research, 767, 32l–332.
- Warner, R. K., Thompson, J. T., Markowski, V. P., Loucks, J. A., Bazzett, T. J., Eaton, R. C., & Hull, E. M. (1991). Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile reflexes and sexual motivation in male rats. Brain Research, 540, 177–182.
- Wedekind, C., & Penn, D. (2000). MHC genes, body odours, and odour preferences. Nephrology, Dialysis and Transplantation, 15, 1269–1271.
- Wenkstern, D., Pfaus, J. G., & Fibiger, H. C. (1993). Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive females rats. Brain Research, 618, 4 l–46.
- Wersinger, S. R., Sannen, K., Villalba, C., Lubahn, D. B., Rissman, F., & De Vries, G. J. (1997). Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Hormones and Behavior, 32, 176–183.
- White, N. R., Gonzales, R. N., & Barfield, R. J. (1993). Do vocalizations of the male rat elicit calling from the female? Behavioral and Neural Biology, 59, 76–78.
- Wiesner, J. B., & Moss, R. L. (1986). Suppression of receptive and proceptive behavior in ovariectomized, estrogen-progesteroneprimed rats by intraventricular beta-endorphin: Studies of behavioral specificity. Neuroendocrinology, 43, 57–62.
- Winans, S. S., & Powers, J. B. (1974). Neonatal and two-stage olfactory bulbectomy: Effects on male hamster sexual behavior. Behavioral Biology, 10, 461–471.
- Witt, D. M., & Insel, T. R. (1991). A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology, 128, 3269–3276.
- Wolf, A., Caldarola-Pastuszka, M., DeLashaw, M., & Uphouse, L. (1999). 5-HT2C receptor involvement in female rat lordosis behavior. Brain Research, 825, 146–151.
- Wynne-Edwards, K. E., Terranova, P. F., & Lisk, R. D. (1987). Cyclic Djungarian hamsters, Phodopus campbelli, lack the progesterone surge normally associated with ovulation and behavioral receptivity. Endocrinology, 120, 1308–1316.
- Xiao, L., & Becker, J. B. (1998). Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse, 29, 379–391.
- Yahr, P., & Stephens, D. R. (1987). Hormonal control of sexual and scent marking behaviors of male gerbils in relation to the sexually dimorphic area of the hypothalamus. Hormones and Behavior, 21, 331–346.
- Yamada, K., Emson, P., & Hokfelt, T. (1996). Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. Journal of Chemical Neuroanatomy, 10, 295–316.
- Yamada, Y. (1979). The effects of testosterone on unit activity in rat hypothalamus and septum. Brain Research, 172, 165–169.
- Yells, D. P., Hendricks, S. E., & Prendergast, M. A. (1992). Lesions of the nucleus paragigantocellularis: Effects on mating behavior in male rats. Brain Research, 596, 73–79.
- Zarrindast, M. R., Mamanpush, S. M., & Rashidy-Pour, A. (1994). Morphine inhibits dopaminergic and cholinergic induced ejaculation in rats. General Pharmacology, 25, 803–808.
- Zasorin, N. L., Malsbury, C., & Pfaff, D. W. (1975). Suppression of lordosis in the hormone-primed female hamster by electrical stimulation of the septal area. Physiology and Behavior, 14, 595–599.
- Zumpe, D., Clancy, A. N., Bonsall, R. W., & Michael, R. P. (1996). Behavioral responses to Depo-Provera, Fadrozole, and estradiol in castrated, testosterone-treated cynomolgus monkeys (Macaca fascicularis): The involvement of progestin receptors. Physiology and Behavior, 60, 53l–540.




