View sample olfaction and taste research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The ability to detect and interpret the chemicals in our environment affects nearly every aspect of our survival. Most important, perhaps, is the central role of chemical sensation in the detection of what is edible and where it is located. It is well known, for example, that the flavor of our food (i.e., the combination of its taste and smell) is a major determinant of ingestion. One need consider only what happens to our appetite and our affect (see Toller, 1999) when these sensibilities are lost or altered to realize just how essential the chemical senses are to our well-being. Conversely, we utilize our chemical senses to protect us from ingesting or inhaling toxins that can harm us.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Historically, the study of taste and olfaction has progressed at a relatively slow pace when compared to the study of the other sensory modalities such as vision or audition. The reason has been in part the difficulty in defining the dimensions of the stimulus domain of the chemical senses. The original hypotheses regarding chemical structure as a predictor of perceptual quality have at times led to some anomalous results. Likewise, psychophysical analysis of tastes and smells has sometimes produced controversy. Without adequate confidence that any given array of stimuli would span the limits of chemical sensibility, investigators have been slow to agree on schemes with which taste and olfactory stimuli are encoded by the nervous system. However, as the present review of the recent scientific literature hopefully reveals, technological advances, particularly in the realm of molecular neurobiology, are providing the tools for unraveling some of the long-standing mysteries of the chemical senses.
Taste
Taste Stimuli
Before one can define a taste stimulus, one must define what constitutes a taste sensation. From a purely literal view a taste sensation is the sum of the neural activity in a tasterelated pathway in the nervous system. Obviously, this definition is circular in that taste-related pathways are defined by the observation that their activation produces a taste sensation. The chemosenses have this tautology in common with all sensory systems. Thus, the definition of a taste stimulus is a stimulus that evokes a taste sensation or, by extension, any stimulus that evokes activity in a taste-related portion of the nervous system. Historically, some sensations that we think of as taste, such as metallic or alkaline, have turned out not to be taste sensations at all, but rather arise from the concurrent stimulation of trigeminal or olfactory pathways. In fact, most of what we think of ordinarily as taste sensations are actually combinations of taste, olfactory, tactile, and thermal sensations; there are very few purely taste sensations. In part, that accounts for the observation that an individual can lose a large part of his or her sense of taste and not be aware of any loss at all (Kveton & Bartoshuk, 1994).
From a practical point of view taste stimuli are chemicals that are capable of dissolving in saliva because saliva is the medium that conveys the stimulus to the taste receptors. Taste stimuli express a variety of chemical structures but, as some would argue, evoke only a handful of different sensations.
These are commonly known as the four basic taste qualities: sweet, sour, salty, and bitter. Prototypical stimuli associated with these basic qualities are sucrose (sugars) for sweet, citric or hydrochloric acid for sour, sodium chloride (NaCl) for salty, and quinine for bitter. Many have argued that another taste, called umami and exemplified by monosodium glutamate (MSG) and L-aspartate, is unique enough to be considered a fifth basic taste quality. Although it was discovered nearly 100 years ago, investigation of umami in this regard has intensified only in the last two decades (Brand, 2000; Kurihara & Kashiwayanagi, 2000; Yamaguchi, 1991). Table 10.1 shows a list of chemicals that have been used in experiments on the gustatory system.
Defining what constitutes a taste stimulus is not a trivial exercise because it affects an entire cascade of research on the taste system. For example, if one argues that a group of chemicals taste alike, say salty, then psychophysicists will study the “salty” sensation of fluids; chemists will look for molecular similarities that define whether a chemical tastes salty; cell biologists will look for salt receptors on the taste receptor cells; physiologists will look for nerve fibers and neurons that respond well to salt; and theorists will devise schemes for how salt is encoded. To provide guidance as to how the taste world is organized, students of gustation have turned to the field of psychophysics.
A Word About Psychophysics
Early psychophysicists set about to examine the inherent organization of the world of sapid (defined as having a taste) stimuli. They quickly discovered that there were groups of stimuli that tasted alike and that each group could be characterized by a single descriptor (e.g., sweet or salty). From these observations came the idea of taste primaries, defined as qualities that in combination could reconstruct any gustatory experience. The most active challenge to this idea has been offered by Erickson (2000) and Schiffman (2000), who argued that the taste world is organized as a continuum, rather than parceled into discrete groups. Their argument, in part, is that the historical categorization into four taste qualities is an artifact of our language, in that the English language has a limited number of words available to describe a taste and that most of us have a long history of describing what we taste using just these few words. It has been shown, for example, that in Asian cultures umami is a commonly recognized taste whereas in the United States the taste of umami is described as salty-bitter (O’Mahony & Ishii, 1986).
In general, the problem of reconciling language with function is thorny and not easily solved, though many have tried. Many studies of the organization of the taste world ask subjects to describe the taste stimulus presented to them without giving any specific suggestions. In that case, one cannot divorce the subject’s history from the likelihood of his or her choices of descriptors. This inherent conflict may be reinforced in studies where subjects are asked to choose from among a list of descriptors that are restricted to those associated with the four or five basic taste qualities. Erickson (2000) discussed this point in detail in a recent review.
One of the more persuasive paradigms that has been used to study the idea that there are four (or five) independent taste qualities is that of adaptation. The phenomenon of adaptation is defined as a response decrement following prolonged exposure to a given stimulus. The overall strategy is to adapt the tongue to one stimulus and test for a response to another; if the adapting and test stimuli share a common receptor mechanism, the response to the test stimulus should be attenuated. If the two stimuli are independent in the sense that they evoke independent (i.e., nonoverlapping) sensations, adaptation to one will not alter the perception of the other. In general, results of this kind of experiment have concluded that, psychophysically, there is little cross-adaptation among the four basic taste qualities and, conversely, much cross-adaptation among stimuli classified as belonging to the same class of taste quality (e.g., McBurney & Bartoshuk, 1973).
In addition to producing an outline of the taste world, modern psychophysicists have identified several areas of research where their contributions can have potentially significant medical and societal impact. One of these areas is in the genetic variability of taste as a marker for people who are at risk for a variety of disorders. For example, it has been known since the 1930s that the ability to perceive phenylthiocarbamide (PTC) and the chemically related 6-n-propylthiouracil (PROP) as bitter is genetically transmitted through a dominant gene (Bartoshuk, Duffy, Reed, & Williams, 1996). More recently, the study of people who taste PROP, called tasters, has suggested that there is a subset of tasters who experience PROPas extremely bitter (i.e., more bitter than most other tasters do). This group of supertasters has not only an enhanced perception of PROP but also a greater number of fungiform taste papillae than do medium tasters or nontasters, as well as an enhanced perception for other bitter compounds, for salty, sweet, fatty, and viscous substances, and for oral burn produced by capsaicin (Bartoshuk, Duffy, Lucchina, Prutkin, & Fast, 1998). In the United States about 25% of the public are nontasters; 50% are medium tasters; and 25% are supertasters. There is some evidence that the ability to perceive PROP may be a genetic marker for the propensity to become alcoholic (DiCarlo & Powers, 1998; Pelchat & Danowski, 1992), although this remains controversial (see Kranzler, Moore, & Hesselbrock, 1996). In any case, these studies suggest that genetic variability in taste perception may prove to be a useful marker for at-risk populations for a variety of behavioral problems.
Another application for the study of taste psychophysics has been in the study of the relationship of taste perception to the molecular biology of signal transduction. In general, there is an interplay between the perceptual phenomena that psychophysicists can define (i.e., commonalities among the things we taste) and the transduction mechanisms that molecular biologists search for and study to account for these commonalities. A good example of this is the study of the perception of saltiness. When the amiloride-sensitive sodium channels were discovered, it was widely hypothesized that the direct entry of Na ions through these channels could account entirely for salt perception (see Halpern, 1998, for a review). However, when it turned out that amiloride mixed with a NaCl solution did not eliminate the perception of saltiness entirely, it became apparent that additional transduction mechanisms for NaCl must be present. Indeed, it is now known that NaCl can affect neurotransmitter release from receptor cells through other pathways (as discussed later).
Transduction of Taste Stimuli
Some of the most exciting discoveries in the field of taste in recent years have been in the study of transduction mechanisms. A thorough treatment of these advances is beyond the scope of the present research paper; however, the reader is referred to some excellent reviews (Brand, 2000; Gilbertson, Damak, & Margolskee, 2000; Herness & Gilbertson, 1999; Kinnamon & Margolskee, 1996; Lindemann, 1996; Miyamoto, Fujiyama, Okada, & Sato, 2000; Spielman, 1998; R. E. Stewart, DeSimone, & Hill, 1997).
Most treatments of the subject of taste transduction mechanisms are divided into sections according to taste quality. That is, there are usually separate sections for encoding of sweet, sour, salty, and bitter substances. This is partly a natural extension of the results from psychophysical work, as mentioned earlier, and it has proven a fruitful strategy, all things considered. However, as this work has unfolded, it has become clear that there may be more than one transduction mechanism for each of the basic tastes. The fact that multiple transduction pathways contribute to a taste experience may underlie the singular taste experiences.
Saltiness
The sensation of saltiness in its purest form is produced by NaCl or lithium chloride (LiCl; Murphy, Cardello, & Brand, 1981). Other salts produce taste qualities (e.g., bitter or sour) in addition to saltiness. The rather narrow spectrum of pure tastants associated with saltiness endows the system with the ability to pick out NaCl specifically when needed, as in the case of Na deprivation (Fitzsimmons, 1979). This ability may be beneficial to survival because NaCl is essential for a variety of biological functions, including nervous function and homeostatic regulation of water in the body.
The transduction of NaCl is thought to involve the entry of Na ions directly into the taste receptor cell (TRC) through two separate pathways. The first is through Na channels that are reversibly blockable by the diuretic amiloride. These amiloride-sensitive channels (ASCs) are located on the apical portion of the TRC. There are large species differences in the effectiveness of amiloride to block these channels, and these differences are reflected in the varying effectiveness of amiloride to block the perception of saltiness (see Halpern, 1998, for a review). Na is thought to enter the TRC through these channels and depolarize the TRC membrane, causing voltage-sensitive Na, K, and Ca channels to open. The entry of Ca into the TRC then causes the release of transmitter from the TRC onto the taste nerve endings. The second transduction pathway for NaCl is by Na entry through Na channels located on the basolateral TRC membrane below the tight junctions.
There is also a role for the anion in the transduction and subsequent perception of saltiness. Small anions such as Clact through a paracellular shunt by diffusing past the tight junctions. This produces a negative region at the basal portion of the TRC that further promotes the influx of positive ions. The tight junctions control this diffusion and current flow; they pass small cations more freely than they do larger ones. This accounts for the observation that salts with larger cations (e.g., sodium gluconate) do not taste as salty as do those with smaller ones (e.g., NaCl).
Sourness
The sensation of sourness is produced by acids. Sourness is thought to be associated with rotting fruit and, by extension, with toxicity. Its detection is therefore important for survival.
The perception of sourness appears to be directly related to the concentration of the hydrogen ion (Ganzevles & Kroeze, 1987).Avariety of transduction mechanisms have been studied for sourness, and it appears that there are large species differences in the mechanisms that are present. For example, in the mud puppy (Kinnamon, Dionne, & Beam, 1988) and tiger salamander (Sugimoto & Teeter, 1991), H ions block a normally open K channel located on the apical region of the TRC. This then depolarizes the TRC, resulting in the eventual entry of Ca and subsequent release of neurotransmitter. In the hamster there is good evidence that H enters the TRC through amiloride-blockable channels that may be identical to those involved in Na transduction (Gilbertson, Avenet, Kinnamon, & Roper, 1992); however, this mechanism is not present in the rat (DeSimone, Calahan, & Heck, 1995). Still other mechanisms that have been proposed include a proton exchange mechanism, a stimulus-gated Ca channel, and the direct entry through a H channel that has yet to be identified (see Lindemann, 1996). Clearly, there is much work yet to be done in this area.
Bitterness
As is the case for sweet substances, a wide variety of chemicals produces a bitter sensation. These include some plant alkaloids such as the prototypical bitter substance quinine, nicotine, strychnine, and caffeine, as well as hydrophilic salts of Ca, Mg, Ba, Cs, K, and NH4 (R. E. Stewart et al., 1997). Other compounds such as urea, sucrose octa-acetate (SOA), denatonium, and many of the D-isomers of amino acids also taste bitter. The variety of chemical structures represented by bitter substances has led investigators to conclude that many different receptors must be involved in their transduction. Furthermore, observations that some bitter tastants utilize more than one transduction pathway (see R. E. Stewart et al., 1997) only complicate the question of how bitterness is encoded at the TRC level.
Fortunately, some dramatic discoveries in the last decade have significantly advanced our knowledge of the transduction of bitterness. These began with the cloning of a G protein called gustducin, which is localized exclusively in TRCs (Spielman, 1998) and was reported by McLaughlin, McKinnon, and Margolskee in 1992. Gustducin shares an 80% homology with transducin, the G protein once thought to be restricted to the retina. In fact, transducin has also been found in TRCs (McLaughlin, McKinnon, Spickofsky, Danho, & Margolskee, 1994). Because of the gustducin’s similarity to transducin, the former is thought to work in much the same way: Binding of a bitter substance to a receptor (discussed later) would cause gustducin to activate phosphodiesterase, which would then decrease the level of a cyclic nucleotide. Lower levels of cyclic nucleotides would affect a cyclic nucleotide-activated membrane channel and thereby depolarize the membrane potential. Conversely, high levels of cyclic nucleotides in frog TRCs produce a decrease in conductance (Kolesnikov & Margolskee, 1995).
In addition to the cyclic nucleotide pathway for bitter transduction, other transduction mechanisms are also under investigation. For example, there is evidence that some bitter substances, such as SOA (Spielman, Huque, Nagai, Whitney, & Brand, 1994) and denatonium (Akabus, Dodd, & Al-Awqati, 1988), stimulate the inositol triphosphate (IP3) system to increase Ca levels derived from intracellular stores. SOA is also known to block K channels directly (Spielman, Huque, Whitney, & Brand, 1992), as does quinine at low concentrations (Ozeki, 1971).
Sweetness
Awide variety of compounds produces a sweet sensation, including mono-, di-, and polysaccharides, polyalcohols, amino acids, peptides, and some proteins. In addition to the variety of chemical structures, there are wide species differences in sensitivity to sweet-tasting substances. For example, it is well known that gerbils are about 40 times more sensitive to sucrose than are humans. However, some chemicals that taste sweet to humans, such as aspartame, probably do not taste sweet to rats. For example, Sclafani and Abrams (1986) demonstrated that rats showed only a weak preference for aspartame over water when aspartame was presented at a variety of concentrations in a two-choice paradigm. It is interesting to note that cats do not have any sweet taste receptors and thus are completely insensitive to sweetness.
Considering the diversity of the molecular structure of this group of chemicals, it is not surprising that a number of transduction mechanisms exist. There is growing evidence that natural sweeteners like sucrose activate the production of cAMP through a receptor–G protein interaction. The production of cyclic adenosine monophosphate (cAMP) would then activate protein kinase A, leading to the closure of K channels. The subsequent depolarization would result in Ca entry through voltage-activated Ca channels and eventual neurotransmitter release. There is also evidence that a decrease in cAMP may be involved in sweet taste transduction. This evidence is derived from the observation by Wong, Gannon, and Margolskee (1996) that knockout mice that are lacking gustducin are also deficient in their sensitivity to sweet-tasting compounds. As with bitter compounds, gustducin may activate a phosphodiesterase and thereby decrease the concentration of cyclic nucleotides. The resolution of evidence implying both an increase and a decrease in cyclic nucleotides in response to sweet substances requires additional investigation.
Yet another transduction mechanism for sweet tastes involves the IP3 cascade. Sweet chemicals such as saccharin and amino acids also close K channels but do not activate cAMP. These substances stimulate Ca release from intracellular stores (Bernhardt, Naim, Zehavi, & Lindemann, 1996). There is evidence (Behe, DeSimone, Avenet, & Lindemann, 1990) that TRCs may contain the molecular machinery for both cAMP and IP3 transduction pathways.
Finally, there is evidence that sugars stimulate an influx of cations through an ASC in dogs but not in rats (Simon, Labarca, & Robb, 1989).
Umami
Although still controversial to some, it has been argued that the taste of umami is just as distinct as that of salty, sweet, bitter, or sour and should therefore be considered a fifth basic taste quality. The prototypical stimulus for umami is MSG, although L-aspartate also evokes umami. Other amino acids evoke umami as well.
The transduction of MSG as a tastant is presumably accomplished through a glutamate receptor. Although much of the work on glutamate receptors related to gustation has been done in the channel catfish (see Caprio et al., 1993, for a review), studies in mammalian systems have also begun to bear fruit. In this context there is evidence for both ionotropic and metabotropic receptors in TRCs. Recently, a G-protein coupled receptor for MSG that is localized to mouse TRCs (Chaudhari, Landin, & Roper, 2000) has been identified, and evidence has been provided implicating it as a taste receptor for MSG.
The Search for Taste Receptors
Researchers have only recently begun to identify candidate genes and their respective proteins that may serve as taste receptors. The first of these is a small family of two G-protein coupled receptors, T1R-1 and T1R-2, which have been localized specifically to TRCs (Hoon et al., 1999). Each of these has a differential spatial distribution across the receptive fields of the oropharyngeal area:T1R-1 is expressed primarily in the fungiform papillae and palatal taste buds, and T1R-2 is expressed more prominently in foliate and circumvallate taste buds. This regional specificity led Hoon et al. (1999) to suggest that these putative receptors encode sweet and bitter modalities; however, this idea remains speculative at present. It is interesting that these proteins are absent from TRCs expressing gustducin.
Another recent report has identified a family of putative taste receptors, called T2Rs, that are specifically localized to TRCs in the mouse (Adler et al., 2000). Further evidence suggested that TRCs may express a large repertoire of T2Rs, which may account for the ability to perceive a wide variety of substances as bitter (Chandrashekar et al., 2000). This line of research is quite recent and can certainly be expected to progress rapidly in the next few years.
Anatomy of the Taste System
Peripheral Nervous System
Taste buds are located in the oropharyngeal area in structures called papillae. Papillae can be seen as bumps on the surface of the tongue. Filliform papillae do not contain any taste buds and are located on the dorsal middle of the tongue. Fungiform papillae, found on the tip and sides of the tongue, resemble mushrooms in shape. In the rat only one taste bud is located in each fungiform papilla, and nearly all fungiform papillae have taste buds in them (Mistretta & Oakley, 1986). In humans, however, fungiform papillae contain on average one to four taste buds each (Arvidson, 1979; Miller, 1986). In general, only about one third of these papillae contain any taste buds (Cheng & Robinson, 1991). The great majority of fungiform papillae and the taste buds contained in them are located on the tip of the tongue in both rat (Miller & Preslar, 1975) and human (Cheng & Robinson, 1991). Circumvallate papillae are located at the rear of the tongue and look like flattened disks; taste buds are also located in surrounding trenches as with fungiform papillae. In the human they are arranged in an inverted V with the apex oriented toward the back of the tongue. Foliate papillae are located along the sides of the tongue behind the molar teeth. They appear as parallel folds and ridges with taste buds buried in the folds. There are also taste buds on the soft palate and epiglottis.
In general, there is a good deal of variation from person to person in the number of papillae and in the number of taste buds per papillae; this variation may be genetically determined and may be related to taste sensitivity (Bartoshuk, 2000a, 2000b). For an excellent review of the location and distribution of papillae and taste buds in the human, see Miller and Bartoshuk (1991). Stimulation of individual taste papillae and individual taste buds can evoke all four taste qualities (Bealer & Smith, 1975).
One of the most common misconceptions about taste is that different parts of the tongue are differentially sensitive to the various taste qualities. For example, it is usually stated that the tip of the tongue is most sensitive to sweet, the back to bitter, and so on. These assertions are based on work in the nineteenth century showing that there are differences in taste thresholds for the basic taste qualities across different regions of the tongue. In truth, these differences are slight, and all of the basic taste qualities may be perceived everywhere there are taste buds (Collings, 1974).
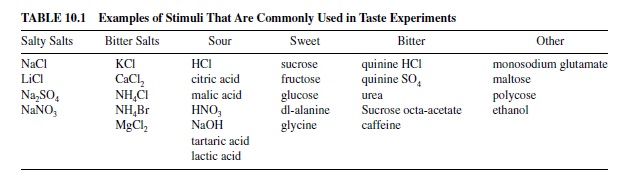
The sensory organ that contains the receptor cells for taste is the taste bud. The arrangement of the spindle-shaped TRCs within the taste bud is usually described as resembling the slices of an orange (see Figure 10.1). Slender cilia protrude from the apical end of TRCs into the oral environment through the taste pore. Tight junctions between TRCs prevent taste stimuli from entering the taste bud. There are generally three types of TRCs described in mammalian taste buds: Type I (dark), Type II (light), and Type III, which synapse on the gustatory nerves. These types are generally considered to represent different developmental stages; TRCs are known to have a life span of about 10 days. In the mouse all types of taste cells receive synapses from gustatory nerve fibers (Royer & Kinnamon, 1994). In the basal portion of the taste bud in the rat, nerve fibers form a dense plexus, sending thin beaded branches between the taste cells to synapse on Type III cells (Kanazawa & Yoshie, 1996; Muller & Jastrow, 1998). It is now known that TRCs can contain the transduction mechanisms for all four taste qualities, can produce action potentials themselves, and can thus respond broadly across the basic taste qualities. For a recent review of this topic see Herness (2000).
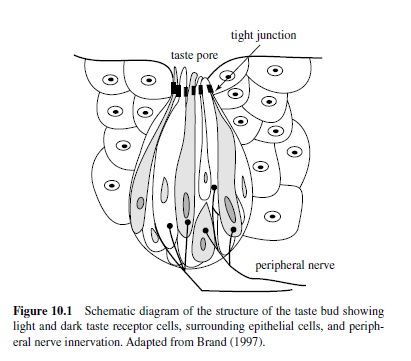
Three cranial nerves innervate taste buds. The first is the facial (VII) with the greater superficial petrosal branch innervating taste buds within the nasoincisor ducts and the chorda tympani (CT) innervating the rostral two thirds of the tongue. The rat CT contains about 1,500 fibers (Ferrell, Tsuetaki, & Chole, 1985), whereas the human CT contains about 5,500 fibers (Ylikoski, Gamoletti, & Galey, 1983). The second is the glossopharyngeal (IX), or GP, with the lingual branch innervating the caudal third of the tongue. The remaining taste buds are innervated by the superior laryngeal branch of the vagus (X) nerve. The VIIth, IXth, and Xth nerve innervations of the oropharyngeal area project to the medulla via the geniculate, petrosal, and nodose ganglia, respectively.
Central Taste Pathways
Figure 10.2 is a schematic diagram of the central neural pathways associated with gustation in the rodent.
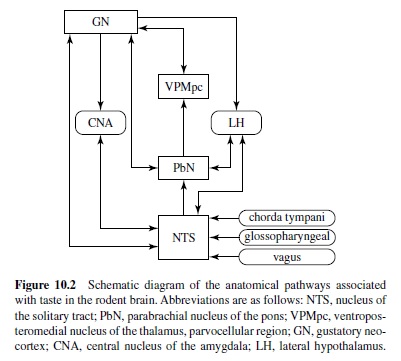
The three cranial nerves associated with taste terminate centrally in a roughly topographical arrangement within the rostral portion of the nucleus of the solitary tract (NTS), although there is considerable overlap (Halsell, Travers, & Travers, 1993; McPheeters et al., 1990; S. P. Travers & Norgren, 1995). These primary afferents form excitatory synapses on the distal dendrites and spines of cells in the rostral central and rostral lateral subdivisions of the NTS (Whitehead, 1986; Whitehead & Frank, 1983). They terminate in glomeruli in which a variety of synaptic relationships is located (Davis, 1998; Whitehead & Frank, 1983). These include both axodendritic (possibly inhibitory) and dendrodendritic connections (Whitehead & Frank, 1983).
Most investigations of the morphological characteristics of neurons in the gustatory NTS have identified three cell types: fusiform (elongate), stellate (multipolar), and ovoid. Fusiform cells have at least two primary dendrites that are preferentially oriented in the mediolateral plane, are perpendicular to the solitary tract, and can extend distances of several hundred micrometers (Whitehead, 1986; Whitehead & Frank, 1983). (More recent evidence using three-dimensional reconstruction of neurobiotin-labeled cells suggests that very few NTS cells are actually bipolar; Renehan, Jin, Zhang, & Schweitzer, 1994.) It has been suggested that this arrangement maximizes the opportunity to synapse with a large number of incoming fibers (Davis & Jang, 1986; Whitehead, 1986; Whitehead & Frank,1983). A more recent analysis of the morphology of NTS cells has defined six cell types, based on a cluster analysis of six morphological features (Renehan et al., 1994). One of these features, cell size, may correlate with immunohistochemical features (i.e., large cells are associated with immunoreactivity to tyrosine hydroxylase; Davis, 1998), and small cells are immunoreactive to gamma-aminobutyric acid (GABA; Davis, 1993; Lasiter, & Kachele, 1988b) and may be inhibitory interneurons (Whitehead, 1986).
A significant proportion of cells in the rostral central and rostral lateral subdivisions of the NTS are responsive to exogenously applied GABA (Davis, 1993; Liu, Behbehani, & Smith, 1993; Smith & Li, 1998, 2000). Evidence that both GABAA and GABAB receptors are present has been reported (Liu et al., 1993; Smith & Li, 1998). These observations have fueled speculation that inhibitory processes may be important in the neural processing of gustatory stimuli in the NTS.
From the NTS the gustatory pathway in the rodent is known to project mainly to the parabrachial nucleus of the pons (PbN; Norgren, 1974; Saper & Loewy, 1980). Some taste-sensitive projections from the NTS, however, may bypass the PbN and terminate in the lateral hypothalamus (LH), central nucleus of the amygdala (CNA), and the gustatory neocortex (GN; Horst, de Boer, Luiten, & van Willigen, 1989; Ricardo & Koh, 1978). (In the primate, the main projection of the taste-related pathway from the NTS is directly to the thalamus without a synapse in the PbN; see Pritchard, 1991.) Approximately one third of the taste-responsive NTS cells send axons to the PbN (Monroe & Di Lorenzo, 1995; Ogawa, Imoto, & Hayama, 1984; Ogawa, Imoto, Hayama, & Kaisaku, 1982; Ogawa & Kaisaku, 1982).Anatomical studies have shown that only fusiform and stellate cells send axons to the gustatory portions of the PbN (Whitehead, 1990). These cells receive input also from primary gustatory afferents and are therefore second-order neurons in the gustatory pathway.
In the PbN taste-responsive cells are found in the medial and lateral subdivisions and scattered among the fibers of the brachium, in the so-called waist area (Davis, 1991; Lasiter & Kachele, 1988a). Two types of cells in the areas that receive afferents from the gustatory portions of the NTS have been described: fusiform and multipolar cells (Davis, 1991; Lasiter & Kachele, 1988a). Compared with cells in the NTS, neurons in gustatory subdivisions of the PbN apparently show more elaborate dendritic arborizations that do not extend large distances (Davis, 1991).
From the PbN gustatory neurons project rostrally in the central tegmental bundle and terminate bilaterally in the parvicellular region of the ventromedial thalamus (VPMpc; Bester, Bourgeais,Villanueva,Besson,&Bernard,1999;Karimnamazi &Travers, 1998; Krout & Loewy, 2000; Norgren, 1974). In addition, ascending fibers from the PbN project also to the GN (Saper, 1982), the LH (Bester, Besson, & Bernard, 1997), the CNA (Bernard, Alden, & Besson, 1993; Karimnamazi & Travers, 1998), the substantia innominata (Karimnamazi & Travers, 1998), and the bed nucleus of the stria terminalis (Alden, Besson, & Bernard, 1994). From the VPMpc there is a reciprocal connection with the GN (Norgren & Grill, 1976; Wolf, 1968). The GN in the rodent is located in the agranular and dysgranular insular cortex, on either side of the middle cerebral artery, just above the rhinal fissure. (See also the later discussion of the location of the GN in primates.)
Centrifugal Influence in Relation to Gustation
Like all sensory systems, the gustatory system is characterized by a rich centrifugal influence. In addition to projections to the VPMpc (Norgren & Grill, 1976; Shi & Cassell, 1998; Wolf, 1968), the GN projects to the CNA (Norgren & Grill, 1976; Shi & Cassell, 1998) and to the PbN (Lasiter, Glanzman, & Mensah, 1982; Norgren & Grill, 1976; Wolf, 1968). The LH (Bereiter, Berthoud, & Jeanrenaud, 1980; Hosoya & Matsushita, 1981) also sends direct input to the PbN. Direct projections from the LH (Bereiter et al., 1980; Hosoya & Matsushita, 1981; Whitehead, Bergula, & Holliday, 2000), the CNA (Whitehead et al., 2000), and the GN (Norgren & Grill, 1976; Whitehead et al., 2000) to the NTS have been described. Additionally, projections from the contralateral NTS have recently been discovered in the hamster (Whitehead et al., 2000).
Although centrifugal input to gustatory neural structures has been described anatomically, little is known of the physiological mechanisms involved or of their functional significance. A few studies, however, have been reported. For example, Yamamoto, Matsuo, and Kawamura (1980) investigated the effects of electrical stimulation of the GN on the VPMpc. They found two types of changes in excitability of thalamic cells: inhibitory (for about 60 ms) and inhibitory (for about 10 ms)-facilitory (for about 60 ms). The researchers suggested that their results could be partially explained by a corticofugal feedback loop. In an earlier study, Ganchrow and Erickson (1972) recorded synaptic activation from the GN of some cells in the VPMpc, which might involve thalamic interneurons.
Corticofugal input to the CNA has also been investigated. Although anatomical evidence suggests a direct input from the GN to the CNA (Norgren & Grill, 1976; Shi & Cassell, 1998), Yamamoto, Azuma, and Kawamura (1984) found evidence only for a mutual polysynaptic relationship between these structures. They reported that 13 out of 18 CNA units showed facilitation or inhibition to electrical shocks in the GN. These effects occurred with a mean latency of 16 ms. Behavioral data suggested that interruption of the corticoamygdaloid projection impairs conditioned taste-aversion retention. A polysynaptic influence of the GN on the LH has also been reported (Kita & Oomura, 1981), which showed two types of responses in the LH following GN stimulation: initial excitation followed by inhibition and inhibition alone.
The GN is also known to affect the processing of taste stimuli in the brain stem. When taste responses were recorded in the NTS before and after infusions of procaine (a local, short-acting anesthetic) into the GN on both sides of the brain, 22 of 30 units (73%) were affected by infusions on at least one side of the brain (Di Lorenzo & Monroe, 1995). Both infusions had the effect of decreasing the number of taste stimuli to which a unit responded. It is interesting that the most profound effects of the elimination of GN input were seen in taste responses in those units that did not evidence projections to the PbN (as determined by the lack of an antidromically driven response to electrical stimulation of the PbN). It is therefore likely that the influence of the GN in the NTS is on interneurons, many of which may be inhibitory.
More recently, Smith and Li (2000) recorded from tasteresponsive cells in the NTS following stimulation of the ipsilateral GN. They found that spontaneous activity was both enhanced and attenuated by cortical input in 34% of 50 cells in the NTS. Infusion of bicuculline, a GABAA antagonist, into the NTS blocked only the inhibition. This inhibitory influence was found to be most often associated with NaCl best cells. These results confirm previous reports (Smith & Li, 1998) showing that GN input is selective in that it affects neither all taste-responsive NTS cells nor the responses to all taste stimuli within a cell (Di Lorenzo, 1990; Di Lorenzo & Monroe, 1995).
In a similar experiment 40 of 42 (95%) of the tasteresponsive units in the PbN were affected by procaine infusions into the ipsilateral GN (Di Lorenzo, 1990). As in the NTS, taste responses could be either enhanced or attenuated, and the effects were stimulus selective within the response profile of a given cell. Only about one third of the tasteresponsive units in the PbN could be directly activated by electrical stimulation of the GN (Di Lorenzo & Monroe, 1992). These results imply that much of the corticofugal influence of the GN on the PbN is relayed through the NTS.
Physiology of Taste
Studies of the physiology of the taste system show that participants are multisensitive at all levels of processing from receptor cell to cortex. That is, they respond to more than one of the representatives of the four basic taste qualities. However, within each structure the breadth of tuning across taste qualities can vary widely among the responsive elements, as well as the preponderance of sensitivity to one taste quality or another. For example, the majority of fibers in the CT of the rat generally respond well to NaCl and to acid (Frank, 1973, 1974; Frank, Bieber, & Smith, 1988; Frank, Contreras, & Hettinger, 1983) and less well to sucrose and quinine. The GP nerve also responds well to salt and acid but is more sensitive to quinine than are the other nerves (Frank, 1991; Hanamori, Miller, & Smith, 1988). In the monkey there is a similar division of sensitivity between the CT and GP nerves (Hellekant, Danilova, & Ninomiya, 1997). The greater superficial petrosal nerve, on the other hand, is relatively more sensitive to sucrose and NaCl (S. P. Travers & Norgren, 1991; S. P. Travers, Pfaffmann, & Norgren, 1986). Collectively, it appears that although the peripheral nerves that convey taste information are multisensitive, some specialization in their sensitivity among taste qualities is apparent. These specializations are reflected in the effects of selective nerve cuts on the perceptual capabilities of animals.
For example, behavioral assessments of taste perception have suggested that damage to the facial nerve (VII), composed of the greater superficial ptrosal (GSP) and CT branches, or to the GP nerve has distinctly different effects. In both hamsters (Barry, Larson, & Frank, 1996) and rats (Spector & Grill, 1992), transection of the CT nerve disrupts the discrimination of NaCl versus KCl. Recovery of this task depends on the regeneration of the CT (St. John, Markison, & Spector, 1995). Damage to the GP nerve had no effect on discrimination between NaCl and KCl (Spector & Grill, 1992). Transection of the CT nerve in hamsters (Barry, Larson, & Frank, 1993) has been shown to disrupt conditioned taste aversions to NaCl but not KCl. The opposite finding has been reported in rats (St. John, Markison, & Spector, 1997). However, sensitivity to low concentrations of NaCl, but not sucrose, was disrupted (O’Keefe, Schumm, & Smith, 1994), and the threshold for detection of NaCl, but not sucrose, was elevated in rats with CT damage (Spector, Schwartz, & Grill, 1990). NaCl appetite was also impaired after CT, but not GP, transection (Markison, St. John, & Spector, 1995). Whereas these studies point to a role of the CT nerve in the perception of salt, other work suggests that a combined CT and GSP transection is required to disrupt the sensitivity to sweet tastes (Spector, Markison, St. John, & Garcea, 1997; Spector, Redman, & Garcea, 1996; Spector, Travers, & Norgren, 1993). Likewise, combined CT and GP transection is most effective in disrupting the perception of quinine (St. John & Spector, 1996; St. John, Garcea, & Spector, 1994).
There is some evidence that GP nerve damage alone has disruptive effects on the processing of quinine. For example, Markison, St. John, and Spector (1999) showed that rats without any preexposure to quinine drank more of highconcentration quinine solutions after GP transection than did sham-operated controls. Furthermore, recent work in rats has shown that GP damage eliminates the expression of fos produced by quinine in the NTS (King, Travers, Rowland, Garcea, & Spector, 1999) and that this returns after GP regeneration (King, Garcea, & Spector, 2000).
Several reports in the literature point to the idea that input from the CT can affect the responses produced by GP stimulation, and vice versa, at their central projection sites in the brain stem. Perhaps the first hint of such an interaction was published by Halpern and Nelson (1965), who reported that anesthetization of the CT in rats enhanced taste responses in the NTS produced by stimulation of the posterior tongue. They interpreted their results as indicating a general inhibitory influence of the CT input on the input from the GP. This interpretation has been buttressed by psychophysical studies using anesthesia of the CT in humans (Kroeze & Bartoshuk 1985; Lehman, Bartoshuk, Catalanotto, Kveton, & Lowlocht, 1995). The appearance of taste phantoms following such a procedure has prompted these authors to suggest also that the input from the CT inhibits the input from the GP. Since the early work of Halpern and Nelson (1965), several other physiological studies in rodents have reported results here and there that have been consistent with the idea that such an interaction occurs (e.g., Sweazy & Smith, 1987).
However, in a study designed to test the idea of CT inhibition of GP input directly, Dinkins and Travers (1998) failed to replicate Halpern and Nelson’s (1965) earlier findings.
Dinkins and Travers recorded the multiunit and single-unit responses to a cocktail of taste stimuli before and after anesthetization of the CT. Receptive fields within the oropharyngeal area innervated by different taste nerves were tested individually. Results showed that CT anesthesia produced pervasive attenuation, rather than enhancement, of taste responses in NTS cells that received input from the CT and the GP. These data suggest that, rather than input from the CT inhibiting input from the GP, these inputs may instead be additive.
Grabauskas and Bradley (1996) showed that electrical stimulation of the solitary tract at levels where either the CT or GP input terminate produced both excitatory postsynaptic potentials (EPSPs) or inhibitory postsynaptic potentials (IPSPs) in NTS cells in vitro. Their evidence suggests that both excitatory and inhibitory input from both the CT and GP are combined in complex ways.
In the brain stem, taste responses reflect convergence of input from the various peripheral taste fields. Neurons in the NTS, for example, are more broadly tuned than are those of peripheral nerve fibers that drive them (see J. B. Travers, Travers, & Norgren, 1987, for a review). In addition, the overall magnitude of response is magnified in the NTS compared with peripheral nerve responses (Ganchrow & Erickson, 1970). In the rat only about one third of the taste-responsive NTS neurons project directly to the PbN (Monroe & Di Lorenzo, 1995; Ogawa et al., 1984; Ogawa et al., 1982). PbN cells with a given response profile (e.g., NaCl best or HCl best) receive direct input from cells in the NTS with a variety of response profiles, both those with the same and those with different best stimuli (Di Lorenzo & Monroe, 1997). This suggests that there is a convergence of cell types at the level of the PbN. Taste responses in the PbN are again amplified with respect to the NTS (Di Lorenzo & Monroe, 1997), but this amplification is already apparent in the enhanced responses of NTS-PbN relay neurons. This implies that taste responses are enhanced before they are relayed to the PbN.
Similarly, taste responses in PbN-thalamic relay neurons (about 60% of all PbN taste-responsive cells) were larger than were nonrelay neurons (Ogawa, Hayama, & Ito, 1987). PbN-thalamic relay neurons respond to taste stimuli at about a threefold amplification compared with NTS-PbN neurons.
At the level of the thalamus, taste-responsive cells retain their broad sensitivity across taste qualities. In the rat, tasteevoked response magnitude is attenuated with respect to the PbN (Nomura & Ogawa, 1985; Scott & Erickson, 1971). This deamplification results in more similar response magnitudes for all taste stimuli as well as broader tuning compared with cells in the PbN (Scott & Erickson, 1971). In the monkey, electrophysiological data suggest that taste stimuli are processed similarly in the thalamus and brain stem (Pritchard, Hamilton, & Norgren, 1989). In both rat (Scott & Erickson, 1971) and monkey (Pritchard et al., 1989) there appears to be a more prominent response to sucrose in the thalamus compared with lower centers; however, the relatively small sensitivity of the brain stem to sucrose may be a by-product of anesthetics. Recordings from the NTS (Nakamura & Norgren, 1991, 1993) and PbN (Nishijo & Norgren, 1997) from awake, unanesthetized rats show a significantly larger proportion of sucrose best cells and an overall larger response magnitude associated with sucrose compared with recordings from anesthetized rats.Approximately 56% of the taste-responsive neurons in the thalamus project to the GN (Ogawa & Nomura, 1988). Thalamocortical relay neurons do not differ from nonrelay neurons in their response properties (Ogawa & Nomura, 1988).
Reponses of GN neurons in rats show a trend toward equalization of effectiveness among the four basic taste qualities. As a result, cells in the GN are more broadly tuned than tasteresponsive cells at lower levels (reviewed in Ogawa, 1994). Two areas within the GN have been identified: the granular insula, where fine gustatory discrimination is thought to occur, and the dysgranular insula, where integration of taste informationoccurs(Ogawa, Hasegawa, & Murayama, 1992).Cells in the dysgranular insula have larger receptive fields in the oral cavity than do those in the granular insula, although no evidence of orotopic mapping was found in either area of cortex (Ogawa, Murayama, & Hasegawa, 1992).Attempts to discover a columnar organization within the GN have revealed that adjacent neurons can show overlapping sensitivities (Kosar & Schwartz, 1990). Cross-correlational analyses of simultaneously recorded pairs of cortical neurons have, however, provided some evidence for the existence of functional columns measuring about 50 m in diameter (T. Nakamura & Ogawa, 1997).
In the macaque monkey two cortical areas have been identified that process information about taste. In the primary GN (rostrodorsal insula and frontal opercular cortex) only a small percentage of cells (6%) respond to gustatory stimuli, and their response profiles are more narrowly tuned than those in lower structures (Scott & Plata-Salaman, 1999; Yaxley, Rolls, & Sienkiewicz, 1990). Cells in the secondary GN (caudolateral orbitofrontal cortex) are the first in the gustatory pathway to reflect motivational variables associated with food (Rolls,Yaxley, & Sienkiewicz, 1990).
The investigation of taste processing in the human cortex is ongoing (seeSmalletal., 1999, forareview). In general, a primary GN has been located in the insula and the parietal and opercular region of the neocortex using magnetic imaging (Faurion et al., 1999; Faurion, Cerf, Le Bihan, & Pillias, 1998; Kobayakawa et al., 1999; Small et al. 1999) and positronemission tomography (PET) scans (Frey & Petrides, 1999; Kinomuraetal.,1994). An area that may correspond to the secondary GN described in monkey cortex has been localized to the caudolateral orbitofrontal cortex in the right hemisphere (Smalletal.,1999). There seems to be some lateralization associated with the cortical representation of gustation in the human (Faurion et al. 1999; Pritchard, Macaluso, & Eslinger, 1999).
Theories of Taste Coding
In the study of the gustatory system two main theories of neural coding have dominated the literature. Both of these theories were originally based on observations of the taste sensitivity of single fibers in the CT nerve of the rodent. Both are based on the observation that nearly all fibers are multisensitive. That is, they respond to tastants representing more than one taste quality when stimuli are presented at midrange concentrations. (The relative response rates evoked by the spectrum of taste stimuli representing the various taste qualities define a unit’s response profile.) Because other studies have shown that neural elements at all levels of the taste pathway— from the TRCs to the cortex—are generally multisensitive, these two theories have also been used to account for taste coding in all parts of the system (see Scott & Giza, 2000, for a recent review).
One theory, called the labeled line (LL) theory, emphasizes the commonalities among response profiles. That is, given a fixed array of taste stimuli, cells that respond most vigorously to a particular stimulus will tend to respond similarly to the other tastants in the array. Cells may then be grouped according to their best stimuli. For example, knowing that a taste cell responds to sucrose as its best stimulus will predict that its second best stimulus will be NaCl. In effect, the implication of these observations is that groups of units that share the same best stimulus represent unit “types” that are functionally homogeneous in that they serve the same role in the coding process. Original conceptualizations identified four groups or neuron types, each labeled by one of the four tastants that are prototypical of the four basic taste qualities (e.g., NaCl for salty, sucrose for sweet, HCl for sour, and quinine for bitter). In its extreme incarnation each neuron type was posited to be exclusively responsible for encoding the taste quality represented by its best stimulus.
More recent investigations have divided fiber types in the CT (see Frank, 2000; M. Sato, Ogawa, & Yamashita, 1994) and cell types in the geniculate ganglion (Lundy & Contreras, 1999) into specialists and generalists. Specialists, as one might imagine, respond nearly exclusively to a single class of taste stimuli representing a single basic taste quality. Generalists respond well to several tastants but are named for their best stimuli. In the rat and hamster CT there are sucrose and NaCl specialists and HCl and quinine generalists (see Frank, 2000, for a review). Contreras and Lundy (2000) have recently identified a class of NaCl generalists in the rat geniculate ganglion.
In aid of the LL theory many studies have aimed at identifying functional characteristics, other than the response properties under normal conditions, that would correlate with a neuron’s best stimulus and thus contribute to the definition of these types as distinct. Some manipulations, such as salt deprivation (Contreras, 1977; Contreras & Frank, 1979; Jacobs, Mark, & Scott, 1988; McCaughey, Giza, & Scott, 1996; Shimura, Komori, & Yamamoto, 1997; Tamura & Norgren, 1997) and conditioned taste aversion (Chang & Scott, 1984; McCaughey, Giza, Nolan, & Scott, 1997), have effects on only one best-stimulus neuron type, whereas others, such as hormonal manipulations (Di Lorenzo & Monroe, 1989, 1990) and adaptation (Di Lorenzo & Lemon, 2000), have more widespread effects across these putative neuron types. At present there seems to be ample evidence that not all units behave in the same way under all experimental conditions. That implies that there are types of units, defined functionally. Although most investigators still use the best-stimulus nomenclature to define these types, other, less theoretically laden labels may be more appropriate.
The second major theory of taste coding, the across-fiber (or -neuron) pattern (AFP) theory emphasizes the differences or varieties among response profiles. Proponents of this theory suggest that the perception of a given tastant is captured by the pattern of firing across the population of fibers or neurons. To compare AFPs generated by two taste stimuli, most investigators have used the Pearson correlation coefficient. Support for the AFP theory has been derived from the observation that correlation coefficients of AFPs generated by similar-tasting stimuli tend to be larger than those generated by dissimilar tastants (e.g., Doetsch & Erickson, 1970; Ganchrow & Erickson, 1970; Woolston & Erickson, 1979). The observation that tastants evoking similar AFPs also evoke similar behavioral reactivity (Scott, 1974; Yamamoto & Yuyama, 1987; Yamamoto, Yuyama, Kato, & Kawamura, 1985) has lent further support to this theory.
In its purest form the AFP theory assumes that all neural elements within a taste-related nerve or neural structure contribute equivalently to the neural code for a taste stimulus. That includes both fibers and neurons that respond and those that do not. In a slight variation of this idea, Erickson and Gill (Erickson, 1986; Gill & Erickson, 1985) proposed that the discrimination between two taste stimuli is encoded by the difference in the amount of neural activity produced by both tastants across neurons. They called this quantity the neural mass difference. It is calculated as the difference between the responses evoked by two taste stimuli for each unit in a sample, averaged across units. In the description of the rationale behind this metric, the authors proposed that the absolute amount, rather than the relative amount, of neural activity produced by a taste stimulus is an important feature of the neural code.
Despite several decades of research, there is still debate over whether the LL or AFP theories are closer to the truth. Recent studies have produced interpretations that incorporate some aspects of both theories. One of these lines of research has involved the use of amiloride, a Na channel blocker. Because it is believed that NaCl is transduced through these amiloride-sensitive Na channels (as discussed earlier), it has been argued that recording the electrophysiological responses to NaCl before and after blocking these channels can provide insight into how NaCl is encoded. Whereas some reports concluded that only NaCl responses in NaCl best neurons in the NTS were affected by amiloride (Giza & Scott, 1991; Scott & Giza, 1990), more recent studies in the hamster concluded that NaCl responses in both NaCl best and sucrose best NTS neurons were modified by amiloride (Boughter & Smith, 1998; Smith, Liu, & Vogt, 1996). The upshot of these studies is that the neural code for NaCl is represented by the neural activity that is distributed across both types of neurons (Smith, St. John, & Boughter, 2000). The idea of neuron types is retained, but it is the pattern of activity across these types that conveys the information.
A third aspect of the neural code for taste is based on the observation that different taste stimuli appear to produce different temporal patterns of response. The first strategy for the investigation of temporal patterns has been to find ways to quantify the time-dependent aspects of the response (Bradley, Stedman, & Mistretta, 1983; Nagai & Ueda, 1981; Ogawa, Sato, & Yamashita, 1973; Ogawa, Yamashita, & Sato, 1974). In most cases this process combines some sort of standardization with various methods of comparison of sequences of increases and decreases in firing rate across the time course of the response. The results of these studies, although suggestive, have not definitively determined that information contained in the temporal pattern of response can be used unambiguously to identify a taste stimulus (Di Lorenzo & Schwartzbaum, 1982; Nagai & Ueda, 1981). As an alternative, some accounts suggest that the temporal pattern may signal the hedonic properties of taste stimuli (Di Lorenzo & Schwartzbaum, 1982). That is, those tastants that are rejected (expelled from the mouth) by an organism may produce a different temporal pattern of response than do those that are accepted (ingested).
Two experiments thus far have produced evidence that the temporal pattern of a taste response may have some function in determining the type of behavioral reactivity to a taste stimulus. The first is the work in which Covey (1980) first recorded the electrophysiological responses to a variety of tastants in the NTS. She then used the temporal patterns of response evoked by each tastant to produce electrical pulse trains that followed these temporal patterns. These unique pulse trains were then delivered to the CT of an awake decerebrate rat. Results showed that these rats displayed orofacial reactions that were appropriate to the taste stimuli whose temporal patterns of response were used to drive the electrical pulse trains.
The second example of a study of the functional significance of temporal patterns of response (Di Lorenzo & Hecht, 1993) involved the presentation of lick-contingent electrical pulse trains to the NTS through chronically implanted microelectrodes in awake, intact rats. In that experiment the electrophysiological response to sucrose recorded from the NTS of an anesthetized animal was used as a template for the temporal arrangement of electrical pulses in a 1-s train of electrical stimulation. Animals learned to avoid lick-contingent electrical stimulation when it was paired with injections of LiCl in a conditioned aversion paradigm. When the temporal pattern of electrical stimulation was switched from one that mimicked a sucrose response to one that mimicked a quinine response, the animals avoided the lick-contingent stimulation without any prior training. Collectively, these studies imply that the temporal pattern of response may provide some information that may be used as a guide for behavioral reactivity. Whether the temporal pattern of response is used also to identify more precisely a taste stimulus remains an open question.
Conclusions
To conclude this survey of the study of the gustatory system, it is perhaps fitting to highlight the frontiers of the field because they will undoubtedly be the subject of future reviews. First is the study of the biology of the TRC, aided in recent times by stunning advances in molecular biology.These advances have openedthedoortostudiesonthetransductionmechanismsassociated with taste and have, for the first time, enabled the study of the genetics of chemoreception. Future research will define the structure and physiology of taste receptor molecules, the interrelationship of the various transduction pathways within the receptor cells and the neurotransmitters that are released. Second, the study of the regeneration of TRCs and their innervation is already a very active and exciting field of study, and it promises to become even more so in the future. Again, molecular biology has finally provided the tools for examining how old receptor cells die and how new ones are born, mature, and are innervated to replace the old ones. Finally, there is the study of the relationship of psychophysics to health and disease. In this regard, psychophysicists have already begun to link the genetic variability in chemical receptivity to a variety of behavioral and physical conditions and abnormalities (see Bartoshuk, 2000b; Reed et al., 1999). The further study of what our individual receptive profiles can tell us about our susceptibility to disease and the normal functioning of the body will certainly yield some exciting developments.
Olfaction
Overview
The Role of the Olfactory System
Of the senses we possess, the olfactory system is, in evolutionary terms, an ancient sensory system capable of detecting and discriminating among thousands of different odorants. It is well established that olfaction is critical to the survival of many lower animals ranging from insects to mammals. Proper olfactory function is basic to the maintenance of life in a variety of ways, namely, the regulation of reproductive physiology, food intake, and social behaviors (Brown, 1979; Doty, 1986; Hudsen&Distel,1983). It is essential for finding food, and it is the first line of defense from becoming food. Even the basic foundation of animal communication is chemical, relying on odors produced by body glands, feces, and urine (Mech & Peters, 1977; Muller-Schwartz, 1977; Yahr, 1977). For example, the male gypsy moth uses olfactory cues to find his mate many miles away (D. Schneider, 1969), as does the adult salmon to return to its spawning ground (Harden-Jones, 1968). Several species as diverse as cats, dogs, and deer mark their territory with urine or other secretions (Mech & Peters, 1977; Muller-Schwartz, 1977; Yahr, 1977). These chemical signatures provide the animal sampling the scent mark with information regarding whether it came from a conspecific, whether the depositor was male or female, and even the social and reproductive status of the animal.
Studies in a number of different species have also shown a well-documented dependency of reproductive and sexual behavior on olfactory cues. Odors are involved at almost all stages of mammalian reproduction, from initial attraction of the sexes to induction of estrus, maintenance or termination of pregnancy, and maternal-neonate imprinting. For example, introducing the odor of a male mouse or his urine can induce and even accelerate the estrus cycle of a female (Whitten, 1956). In addition, appropriate odor cues from a female are important in attracting the male’s interest during estrus and promoting copulation. Male hamsters will display mating behavior, even with anesthetized males, when presented with vaginal discharge from receptive females (Darby, Devor, & Chorover, 1975).
The importance of this type of chemical communication cannot be overstated. In some animals a reduction or loss of olfaction can result in sexual dysfunction and even altered sexual development (Brown, 1979;Doty, 1986; Hudsen &Distel, 1983). Although not as extensively documented as in lower animals, a relationship between olfaction and sex has been demonstrated in humans. Olfactory acuity in women appears better at ovulation than during menstruation (Henkin, 1974; Schneider & Wolf, 1955), and there is emerging strong evidence that olfactory cues (i.e., human pheromones) among women can synchronize the menstrual cycle (McClintock, 1983; Stern & McClintock, 1998). Similarly, odors in humans may play a role in attracting the opposite sex (Gangestad & Thornhill, 1998).
In humans the sense of smell is generally considered less critical to survival, even though there are times when the detection of odors associated with a potential danger such as smoke, gas, or decaying food can prevent bodily injury. In contrast to the lower animals, the potential importance of this sense should be given consideration because of the tremendous impact it has on our quality of life. In other words, modern society seems to emphasize the hedonic effect of olfaction. As examples of the positive hedonic effect, people add a variety of spices to their foods, often creating dishes with complex aromas and flavors (as discussed later), perfume their bodies, and add pleasing odors to a variety of things such as their cars, homes, and even shopping malls. In contrast, the importance of the negative effect is illustrated by the vast number of commercial products available today that are directed toward eliminating offensive odors.These preferences, of course, depend on a number of variables such as age, sex, socioethnic background, and prior odor experience (Wysocki, Pierce, & Gilbert, 1991).
One instance in which olfaction has been shown to play a major role is in flavor perception (i.e., the integration of taste and smell) and the recognition of tastes (Mozell, Smith, Smith, Sullivan, & Swender, 1969). In fact, people are actually smelling much of what they think they are tasting. That is, individuals often confuse the concept of taste perception (i.e., the identification of salty, sour, bitter, and sweet) with flavor perception. In the Mozell et al. study (1969) subjects were asked to identify 21 common food substances placed on the tongue. The results were rather intriguing in that there was a decrease from an average of 60% correct to 10% correct when, experimentally, no odor vapors given off by the test stimuli were allowed to reach the nasal cavity (i.e., there was no access in either the ortho- or retronasal direction).
Even coffee and chocolate, which were correctly identified by greater than 90% of the test subjects when odorant molecules had access to the nasal cavity, were not identified correctly when the nose was made inaccessible. Thus, at least for humans, olfaction appears to have a tremendous impact on the quality of life, and anything that interferes with proper functioning can be very distressing. Consider what happens to simple food appreciation when people have colds or nasal allergies.
Basic Anatomy
The olfactory organ of vertebrates is a complex structure designed to collect odorant molecules and direct them to the sensory neurons. Although the chemoreceptive endings and neural projections of the olfactory nerve carry the primary information of the sense of smell, other cranial nerves are involved, namely, the trigeminal, GP, and vagus. These cranial nerves, which innervate different regions of the respiratory tract (i.e., nose, pharynx, and larynx) give rise to the pungent or irritating quality often experienced as part of an odor sensation (Cain, 1976, 1990). In addition, they also mediate a variety of reflexes in response to chemical stimulation (James & Daley, 1969).These reflexes serve to minimize the effects of noxious stimuli and protect the animal from continued exposure.
The primary olfactory receptive area is the region of the nasal cavity subserved by the olfactory nerve (cranial nerve I). In humans the olfactory receptors lie deep within the nasal cavity and are confined to a patch of specialized epithelium, the olfactory epithelium, covering roughly 5 cm2 of the dorsal posterior recess of the nasal cavity (Moran, Rowley, & Jafek, 1982). In other lower mammals, such as rats and mice, the olfactory epithelium extends throughout the rostrocaudal extent of the nasal cavity occupying the superior and lateral portions of all nasal turbinates (Pedersen, Jastreboff, Stewart, & Shepherd, 1986; W. B. Stewart & Pedersen, 1987).
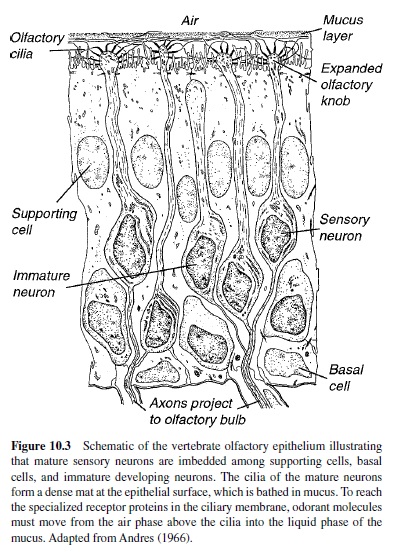
Figure 10.3 illustrates that the olfactory epithelium proper is a pseudostratified structure comprised of three principal cell types: (a) receptor cells, (b) supporting cells, and (c) basal cells. The olfactory receptor is a bipolar neuron that has a short peripheral process and a long central process. The short peripheral process or dendrite extends to the surface of the epithelium (which is in contact with the air space of the nasal cavity), where it ends in an expanded olfactory knob. This knob, in turn, gives rise to several cilia that, along with the cilia from other receptor cells, form a dense mat at the epithelial surface (Moran et al., 1982; Morrison & Costanzo, 1990). Odor transduction is initiated in these cilia as a result of the interaction of odorant molecules with specialized receptor proteins within the ciliary membrane (Buck & Axel, 1991). The longer central process of the olfactory receptor is an unmyelinated axon that projects through the cribriform plate to synapse in the olfactory bulb. In contrast to the receptor cells, supporting cells do not have an axon, nor are they believed to mediate any sensory information. The supporting cells, as their name implies, surround the receptor cells in a columnar fashion. In addition to their role as supporting elements, they also have secretory properties (M. L. Getchell, Zelinski, & Getchell, 1988). Basal cells or stem cells, on the other hand, are found either singly or in clusters next to the basal lamina of the epithelium. Basal cells are mitotically active and give rise to new neurons and supporting cells throughout life and at a markedly increased rate after injury (Huard, Youngentob, Goldstein, Luskin, & Schwob, 1998; Schwob, Youngentob, & Mezza, 1995). This process for self-renewal is quite remarkable because the developing receptor cell must send its newly formed dendrite toward the surface of the epithelium and its axon in the opposite direction to synapse appropriately in the olfactory bulb.
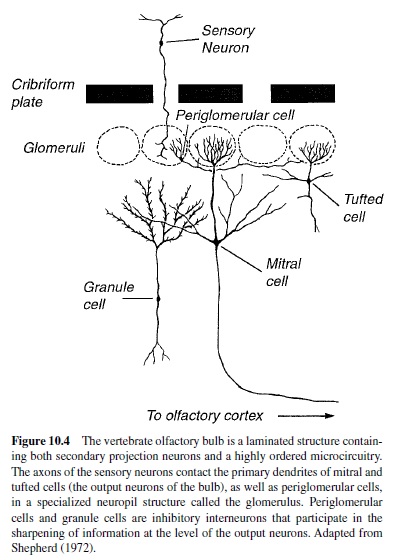
The first relay station in the olfactory pathway is the olfactory bulb, a distinctly laminated structure that receives direct axonal projections from the peripheral sensory neurons (Figure 10.4; Shepherd, 1972). The unmyelinated axons of the olfactory nerve synapse on secondary projection neurons (i.e., mitral and tufted cells) within the bulb. In addition to the massive input from the periphery, the olfactory bulb contains a highly ordered synaptic microcircuitry. Following interaction with local circuits within the olfactory bulb (Shepherd & Greer, 1990), the mitral and tufted cell axons project to higher cortical regions including the piriform cortex, olfactory tubercle, anterior olfactory nucleus, amygdala, and entorhinal cortex (Price, 1987).
Analytical Problem
The olfactory system is a molecular detector of great sensitivity. It has the capacity to discriminate among literally millions of different odorants and can often detect them at concentrations well below the levels of physical instrumentation. It has not been an easy task to understand the mechanisms by which the olfactory system encodes odorant quality information due to the absence of a clear physical energy continuum to describe and control odorant stimuli. In contrast to other sensory systems such as vision and audition, there is no metric analogous to wavelength for color or frequency for pitch. Moreover, unlike mixtures of light or sound, odorant mixtures do not result in predictable perceptions. The situation is further confounded by the knowledge that essentially identical chemical substances can have different quality perceptions. For example, the enantiomers d-carvone and lcarvone have very different odors (Pike, Enns, & Hornung, 1988). D-carvone smells like caraway, whereas l-carvone smells like spearmint. Enantiomers are stereoisomers; that is, their formulas are the same, but the two molecules are mirror images. Precisely why these odorants smell perceptually different still remains unanswered. In contrast, some substances with very different chemical formulas, such as carborane, trisallylrhodium, and cyclopentadienyl-tricarbon monoxide-manganese, all have perceptually the same odor (i.e., camphor; Beets, 1971). How does the olfactory system handle the transduction and encoding of odorant information?
Processing of Odorant Stimuli
Signal Recognition and Transduction
In terrestrial animals, in order for airborne odorant molecules to gain access to the olfactory receptors, these molecules must first traverse the mucus layer covering the olfactory epithelium. The time it takes for odorant molecules to enter and exit the epithelium, as well as the dwell time within the receptor environment, is considered to be an important part of the initial reception process (T. V. Getchell, Margolis, & Getchell, 1984). The discovery of abundant small, water-soluble proteins in the mucus of the vertebrate nasal cavity led to the hypothesis that odorantbinding proteins (OBPs) may accommodate and enhance the access of odorant molecules to the receptors (Pevsner & Snyder, 1990). To date, however, no direct physiological demonstration of function has been reported for vertebrate OBPs. Nonetheless, given the time frame in which olfactory events occur, translocation of odorants from the mucus surface to the receptors must be achieved either by some kind of carrier-bound delivery system or by facilitated diffusion.
The mechanism by which thousands of different odorants that vary widely in structure are readily detected and identified has long been a key problem in understanding the encoding of odorants. However, it is now well established that odorant receptors are G-protein coupled receptors, encoded by a large olfactory-specific multigene family numbering between 500 and 1,000 genes (Buck & Axel, 1991). In addition to being large in number, molecular sequence comparison among members of the receptor gene family has indicated that they are highly divergent. Therefore, it appears that the extremely large size and diversity of this gene family provide the necessary breadth to interact with an immense number of different odorants. Unfortunately, although vigorously characterized, most of these odorant receptors have remained, for the most part, functionally anonymous. That is, there has been a paucity of information regarding the relationship between individual odorant receptors and that portion of the odorant universe to which they respond. One recent study, however, established a relationship between one rat odorant receptor, I7, and the transduction of a restricted odorant set (Zhao et al., 1998). This particular odorant receptor appears to be sensitive to a small subset of aldehydes with chain lengths between 7 and 10 carbons. Aldehydes with both longer and shorter chain lengths failed to elicit responses. Thus, odorant receptors may be highly selective for particular molecular features of an odorant, including chain length.
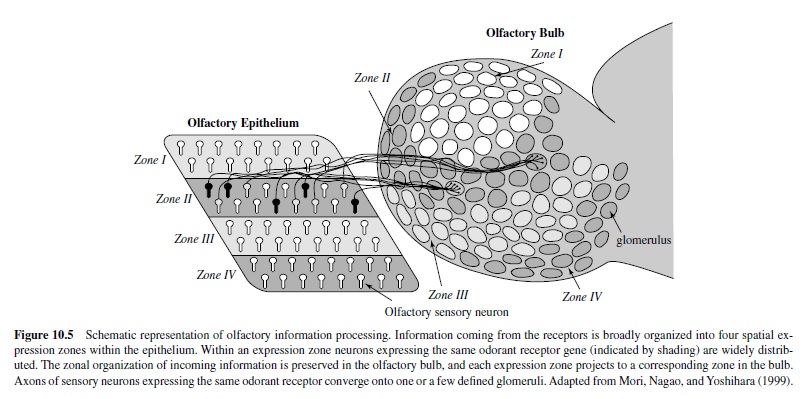
The expression pattern of the large multigene family of receptors also likely plays an important role in the encoding process. In situ hybridization studies examining spatial expression patterns of olfactory receptors in the epithelium indicate that each olfactory receptor gene is expressed by a small subset of sensory neurons (Ressler, Sullivan, & Buck, 1993; Strotmann, Wanner, Helfrich, Beck, & Breer, 1994). On average, each olfactory receptor gene is expressed in approximately 0.1% to 0.2% of the total olfactory sensory neuronal population (or approximately 5,000–10,000 neurons). In addition, neurons expressing a given olfactory receptor are restricted to one of four expression zones within the epithelium (Figure 10.5). Each expression zone occupies a different anatomical domain within the nasal cavity, and each encompasses approximately 25% of the epithelial surface area. In general, within a zone each of the different odorant receptors is homogeneously distributed with each receptor neuron surrounded by other sensory neurons expressing a different receptor type, although exceptions to this rule have been observed (Kubick, Strotmann, Andreini, & Breer, 1997; Strotmann, Wanner, Krieger, Raming, & Breer, 1992). Thus, each receptor zone forms a mosaic of different subtypes of sensory neurons expressing different odorant receptor proteins.
Binding of odorant molecules to the odorant receptor protein sets into motion an intracellular signal cascade leading to the depolarization of olfactory sensory neurons. This depolarization, in turn, is converted into action potentials that are transmitted via olfactory axons to the olfactory bulb.
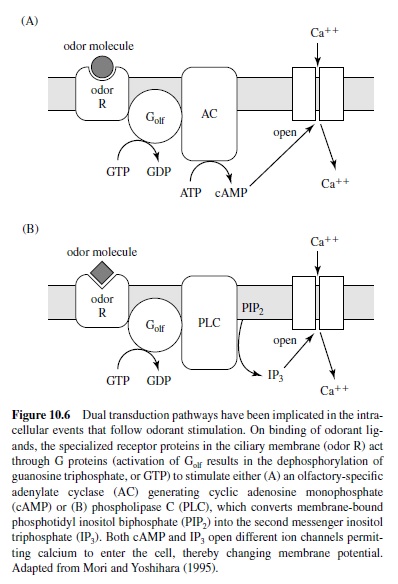
Figure 10.6 illustrates that two different intracellular messengers, cAMP and IP3, have been implicated in the transduction process that follows odorant stimulation, with cAMP generating the response to some odorants (Breer, Boekoff, & Tareilus, 1990; Lowe, Nakamura, & Gold, 1989) and IP3 mediating the response to others (Breer & Boekoff, 1991; Restrepo et al., 1992).At present, the duality of the second messenger system in mammals remains somewhat controversial (Firestein, Darrow, & Shepherd, 1991; Lowe & Gold, 1993; T. Nakamura, Lee, Kobayashi, & Sato, 1996), with recent evidence suggesting that the cyclic nucleotidegated channel subserves excitatory olfactory signal transduction and that cAMP is the sole second messenger mediating the process (Brunet, Gold, & Ngai, 1996). Nonetheless, in either cascade, odorant ligand receptor interactions lead to the activation of G-protein coupled cascades with the former producing cAMP and the latter producing IP3. In turn, these cascades open calcium channels on the plasma membrane, resulting in membrane depolarization and axon potential generation.
In vertebrates, olfactory receptor neurons differ in the number and profile of odorants to which they respond (Firestein, Picco, & Menini, 1993; T. V. Getchell & Shepherd, 1978; Revial, Sicard, Duchamp, & Holley, 1982, 1983). The earliest single-cell recordings showed that individual sensory neurons typically responded to a range of odorants that varied from cell to cell. For example, one such study of single neuron responses to 20 different stimuli demonstrated that individual neurons responded to as few as two of the odorants within the panel (Gesteland, Lettvin, Pitts, & Rojas, 1963). Furthermore, even though more than 50 neurons were sampled, each had a distinct odorant response profile. Thus, it would appear that olfactory neuronal responses define the range of odorants that can elicit a response in a given cell (termed its molecular receptive range, or MRR, and analogous to the spatial receptive field in the visual, auditory, or somatosensory systems; Mori, Imamura, & Mataga, 1992; Mori & Shepherd, 1994; Mori & Yoshihara, 1995).
Emerging evidence further suggests that a cell’s MRR reflects interactions with particular ligand determinants. Studies of olfactory sensory neurons using homologous series of odorants have demonstrated regular patterns of neuronal responses to compounds with a similar organization of carbon atoms or functional groups (T. Sato, Hirano, Tonoike, & Takebayashi, 1994). The importance of ligand determinants to the encoding of odorant quality was further extended by the work of Malnic, Hirano, Sato, and Buck (1999) using calcium imaging and single-cell reverse transcriptionpolymerase chain reaction (RT-PCR). In keeping with prior electrophysiology (Firestein et al., 1993; T. V. Getchell & Shepherd, 1978; Revial et al., 1982, 1983), this study demonstrated, at a molecular level, that different odorants are recognized by different combinations of odorant receptors.
In short, the body of evidence suggests that the features of an odorant are dissected and encoded by the types of odorant receptors with which they interact and by the sensory neurons expressing those receptors. Accordingly, if receptor neurons are to be classified as to type on the basis of their response to the odorant universe, then the number of categories might very well approximate the number of different odorant receptors types encoded by the large multigene family (Buck & Axel, 1991).
Processing of Information in the Epithelium and Bulb
In other sensory systems (i.e., audition, vision, and somesthesis), receptor cells encode specificity about the sensory stimulus by virtue of their exact placement in the receptor sheet. In contrast, the receptor sheet in olfaction does not form a spatial map about the environment. Instead, as previously noted, the responsivity of olfactory neurons results from the affinity of their receptors for a particular odorant ligand. So, how is this molecular information mapped into the nervous system? Studies of the ensemble properties of the olfactory epithelium suggest that odorant quality information is encoded in large-scale spatial patterns of neural activity. That is, direct presentation of odorants to the exposed olfactory epithelium revealed intrinsic spatial and temporal differences in the sensitivity to different odorants across this neural tissue (MacKay-Sim & Kesteven, 1994; MacKay-Sim & Kubie, 1981; Moulton, 1976; Mozell et al., 1987; Youngentob, Kent, Sheehe, Schwob, & Tzoumaka, 1995).
In exploring these differential patterns of sensitivity to different odorants, one approach has utilized optical recordings from rat olfactory epithelium stained with a voltage-sensitive dye (Kent, Mozell, Murphy, & Hornung, 1996; Kent, Youngentob, & Sheehe, 1995; Youngentob et al., 1995). The strategy in each of these studies was simultaneously to monitor 100 mucosal sites in an optical matrix and to record the fluorescence changes in response to different odorants. As illustrated in Figure 10.7, different odorants gave their maximal responses at different mucosal regions, even though they gave at least some response in all regions. These activity patterns, which reflect the differential odorant responsiveness of the receptor cell in different regions, were termed inherent activity patterns by Moulton (1976), who initially observed them on the olfactory epithelium of the salamander.
At a molecular level, the underlying mechanism by which these spatial patterns are established appears to be based on the differential responsiveness of different odorant receptor types to different odorants (Malnic et al., 1999) and on the observation that some odorant receptors have topographically distinct clustered patterns of expression, unlike the generally described broad expression zones (Dear, Boehm, Keverne, & Rabbitts, 1991; Kubick et al., 1997; Nef et al., 1992; Strotmann et al., 1992).
Even though the vertebrate olfactory system does not process information about odors by virtue of a single type of receptor neuron’s placement in the receptor sheet, a degree of rhinotopy (analogous to retinotopy in the visual system) does exist in the organization of the central projection.The foundation for this rhinotopy lies in the topographical organization of bulbar glomeruli (neuropil structures comprised of olfactory receptor neuron axon terminals and the distal dendrites of mitral, tufted, and periglomerular cells) in relation to the expression of odorant receptor type in the epithelium. As illustrated in Figure 10.5, an entire subset of olfactory sensory neurons expressing a particular odorant receptor sends its axons to converge on one medially and one laterally positioned glomerulus in each olfactory bulb (Mombaerts et al., 1997; Vassar et al., 1994). With input ratios on the order of, for example, 25,000 receptors to 1 glomerulus in rabbits (Allison, 1952), mammalian glomeruli therefore represent a very large input convergence of information.
More important, this convergence of axons from neurons expressing the same odorant receptor type results in a precise spatial map of olfactory information within the glomerular layer of the bulb, with information gathered by thousands of neurons in the epithelium projecting to precise foci in each bulb. As a result, the odorant information contained in the large-scale differential activation of subsets of olfactory sensory neurons seen in the periphery can be encoded by producing differential spatial patterns of activity across the glomerular layer of the olfactory bulb. Indeed, several lines of evidence using physiologic (Cinelli & Kauer, 1994) and metabolic (Johnson & Leon, 2000; Johnson, Woo, & Leon, 2000) techniques indicate that glomeruli are functional units in odor processing and that each odorant produces a unique pattern of glomerular activation.This activity is further sharpened in the relay neurons of the bulb via complicated local circuits that act both locally and laterally on the signals impinging on the bulb (Mori & Shepherd, 1994; Mori & Takagi, 1978).
In the simplest terms, then, the extraction of odorant features begins with the differential binding affinities of the odorant receptors, which in turn are reflected in the differential activation of the epithelium by different odorants. This information is directly reflected spatially in the olfactory bulb through the existence of anatomically and functionally distinct glomeruli and their associated output neurons, the mitral and tufted cells, dedicated to receiving information from a single receptor type. Studies of the electrophysiological response properties of bulbar output neurons are consistent with this model, indicating that mitral cells are highly selective for particular odorant determinants and respond to similar odorants that have these determinants in common (Imamura, Mataga, & Mori, 1992; Katoh, Koshimoto, Tani, & Mori, 1993; Mori et al., 1992). More important, through the activation of lateral inhibitory mechanisms, the contrast between strongly activated and weakly activated glomeruli is enhanced, thereby sharpening the tuning specificity of individual mitral and tufted cell output neurons to odorant molecules (Buoniviso & Chaput, 1990; Kashiwadani, Sasaki, Uchida, & Mori, 1999; Yokoi, Mori, & Nakanishi, 1995). The second-order mitral and tufted cells may therefore be more sharply tuned to specific molecular features than the sensory neurons themselves. As a result, the ability of the olfactory bulb glomeruli and their output neurons to encode the features of an odorant may allow for an exceedingly large number of odorants to be discriminated.
From Molecules to Perception
In the final analysis, the reception of odorant molecules in the nasal cavity must be translated into a psychological perception. One approach to this problem was directed toward understanding the perceptual coding of olfactory information by comparing the structure of chemical compounds that smelled alike. On the basis of Henning’s (1922) success with primary tastes, the hope was that after ordering a large series of substances into as few groups as possible, based on the perceptual similarity of their odors, one would be able to specify the physical or chemical similarity of the substances in each class. Amoore (1962a, 1962b) examined over 600 different odorants and, on the basis of this work, proposed a stereochemical classification model for describing odor quality and similarity. In this model odorants with similar odors have similar molecular configurations. Amoore first proposed to explain all odor qualities on the basis of a set of seven primary odors or odor dimensions: ethereal, camphoraceous, musky, floral, minty, pungent, and putrid. His concept was that the sense of smell operated on the basis of a limited number of discrete primary odor sensations that can be combined in different proportions to give a large range of distinguishable odors. In this manner, smell would be analogous to taste with its four classic primaries of salty, sour, bitter, and sweet or to color vision with its three primaries of red, yellow, and blue. This would be in contrast to audition, which depends on a continuum of frequencies and has no primary elements.
The evidence for primary odors was provided by a detailed study of smell blindness or specific anosmias (unfortunately misnamed because they actually represented a reduced sensitivity to one or a very limited number of odors in the presence of otherwise normal olfactory functioning; Amoore, 1967, 1977). Amoore believed that these observations were genetic in origin and involved primary odors transduced by specific receptors. Subsequent psychophysical research has, in fact, suggested that a larger number of primaries are needed to account for all of the different perceptual qualities we appreciate and that the number of specific anosmias is far greater than seven (Amoore, 1977). This later observation was consistent with the finding that although many odors fell within the original seven categories, there were, nonetheless, many exceptions. So the original notion of seven primaries appeared to be wrong even though the basic concept might be correct. In this regard, recall that molecular biological techniques have been used to identify a large family of receptor proteins in the olfactory epithelium, which numbers somewhere on the order of 500 to 1,000 (Buck &Axel, 1991). In fact, there may be as many as 500 to 1,000 different odor primaries, each corresponding to a particularreceptor protein.Why such a complex system may have developed is unclear. Once again, it can be suggested that in order to detect a wide range of compounds with the necessary high sensitivity, a large number of primary odor receptor types would be necessary.
Others have attempted to understand the perception of odorant quality by presenting stimuli and monitoring both neurophysiological and behavioral responses. The question of course is whether a relationship exists between a candidate coding mechanism, such as the large-scale distributed patterns of neural activity outlined earlier, and the perception of the animal.
To explore this hypothesis, Kent et al. (1995) trained rats to report differentially (i.e., identify) either a qualitatively unrelated set of five odorants or a homologous series of aldehydes. Once trained, the animals were tested using a confusion matrix design, and the resultant data were used to measure the degree of perceptual dissimilarity between any pair of the five odorants in each set (Youngentob, Markert, Mozell, & Hornung, 1990). The dissimilarity measures were, in turn, subjected to multidimensional scaling analysis in order to establish a perceptual odor space for each animal. At the completion of behavioral testing, optical recording techniques were used to monitor the large-scale activity patterns across a large expanse of epithelium in response to the same odorants. Comparison of these patterns of neural excitation also yielded dissimilarity measures that were subjected to multidimensional scaling analysis in order to establish a neural odorant space for each animal.
Analysis of the behavioral and neurophysiological data indicated a highly significant predictive relationship between the relative position of an odorant’s location in neural space and the relative position of the same odorant in a psychophysically determined perceptual odor space. In other words, the more similar the large-scale activity patterns of two odorants were, the greater was their perceptual similarity. Conversely, the more different the activity patterns of two odorants were, the greater was their degree of perceptual dissimilarity. Thus, on the basis of these data it was suggested that the epithelial representation of an odorant is indeed distributed such that large-scale spatial patterns of activation serve as the basis of odor perceptual space. The results also suggested that the relational information encoded in the odorant-induced mucosal activity patterns were preserved through further neural processing. These conclusions were consistent with observations outlined earlier that there is an organized and stereotyped patterning of information that occurs at the level of the peripheral projection from the olfactory epithelium onto the bulb. That is, a fixed map of bulbar activation would permit the relational information laid down at the level of the epithelium to be transmitted to the brain.
Conclusions
Growing awareness of the importance of olfaction in the lives of many animals, including humans, has led to an increased interest in the study of odor perception and its neural basis. With regard to the olfactory stimulus itself, the olfactory system is faced with a unique problem, namely, the large diversity and potentially unpredictable nature of the odorant universe. As outlined in the foregoing, the basic principles underlying the encoding of odorant quality perception at the level of the olfactory epithelium and bulb are slowly beginning to emerge. The fundamental units of odorant information appear to be contained in the determinants on the odorant molecule. Using a combinatorial code, the molecular features of an odorant are dissected and encoded by the types of odorant receptors with which they interact and the sensory neurons expressing those receptors. The odorant receptors, being members of a large olfactory-specific multigene family, provide the necessary diversity to interact with the breadth of molecular features required to define an immensely large number of odorants. The differential response of the epithelium to different odorants, in turn, can be traced through successive stages of processing in the olfactory bulb. In these processing steps the molecular features of an odorant molecule are first mapped onto bulbar glomeruli and their associated output neurons, constructing a spatial map of activity that is unique for each odorant. Further, the synaptic circuits of the bulb contain mechanisms for fine-tuning the responses of the output neurons and also for comparing the responses of different subsets.
In short, the prevailing data suggest that the differential activation of different subsets of sensory neurons forms the basis for neural coding and further processing by higher centers in the olfactory pathway. Unfortunately, to date, no direct behavioral tests of this emerging model of odorant quality coding have been performed, although a relationship between dissimilarities in epithelial activity patterns and perceptual dissimilarities among odorants has been demonstrated. Nonetheless, it is clear that the integration of data from anatomical, molecular biological, neurophysiological, and behavioral studies are beginning to unravel the basic principles of processing and organization in this phylogenetically ancient sensory system.
Bibliography:
- Adler, E., Hoon, M. A., Mueller, K. L., Chandrashekar, J., Ryba, N. J., & Zuker, C. S. (2000). A novel family of mammalian taste receptors. Cell, 100(6), 693–702.
- Akabus, M. H., Dodd, J., & Al-Awqati, Q. (1988). Abitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science, 242, 1047–1050.
- Alden, M., Besson, J. M., & Bernard, J. F. (1994). Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: A PHA-L study in the rat. Journal of Comparative Neurology, 341(3), 289–314.
- Allison, A. C. (1952). The morphology of the olfactory system in vertebrates. Biological Reviews, 28, 195–244.
- Amoore, J. E. (1962a). The stereochemical theory of olfaction: I. Identification of seven primary odors. Proceedings of the Scientific Section the Toilet Goods Association, 37(Suppl.), 1–12.
- Amoore, J. E. (1962b). The stereochemical theory of olfaction: II. Elucidation of the stereochemical properties of the olfactory receptor sites. Proceedings of the Scientific Section the Toilet Goods Association, 37(Suppl.), 13–23.
- Amoore, J. E. (1967). Specific anosmia: Aclue to the olfactory code. Nature, 214, 1095–1098.
- Amoore, J. E. (1977). Specific anosmia and the concept of primary odors. Chemical Senses and Flavor, 2, 267–281.
- Andres, K. H. (1966). Der Feinbau der Regio Olfactoria von Makrosmatikern (The microstructure of the olfactory area of the xxx). Zeitschrift fur Zellforschung, 69, 140–154.
- Arvidson, K. (1979). Location and variation in number of taste buds in human fungiform papillae. Scandinavian Journal of Dental Research, 87(6), 435–442.
- Barry, M. A., Larson, D. C., & Frank, M. E. (1993). Loss and recovery of sodium-salt taste following bilateral chorda tympani nerve crush. Physiology and Behavior, 53, 75–80.
- Barry, M. A., Larson, D. C., & Frank, M. E. (1996). Effects of chorda tympani transection on long-term salt preference in hamsters. Physiology and Behavior, 60, 347–352.
- Bartoshuk, L. M. (2000a). Psychophysical advances aid the study of genetic variation in taste. Appetite, 34(1), 105.
- Bartoshuk, L. M. (2000b). Comparing sensory experiences across individuals: Recent psychophysical advances illuminate genetic variation in taste perception. Chemical Senses, 25(4), 447– 460.
- Bartoshuk, L. M., Duffy, V. B., Lucchina, L. A., Prutkin, J., & Fast, K. (1998). PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Annals of the New York Academy of Sciences, 855, 793–796.
- Bartoshuk, L. M., Duffy, V. B., Reed, D., & Williams, A. (1996). Supertasting, earaches and head injury: Genetics and pathology alter our taste worlds. Neuroscience and Biobehavioral Reviews, 20(1), 79–87.
- Bealer, S. L., & Smith, D. V. (1975). Multiple sensitivity to chemical stimuli in single human taste papillae. Physiology and Behavior, 14(6), 795–799.
- Beets, M. G. J. (1971). Olfactory response and molecular structure. In L. Beidler (Ed.), Handbook of sensory physiology: Vol. 4. Chemical senses (pp. 257–321). New York: Springer-Verlag.
- Behe, P., DeSimone, J. A., Avenet, P., & Lindemann, B. (1990). Membrane currents in taste cells of the rat fungiform papilla: Evidence for two types of Ca currents and inhibition of K currents by saccharin. Journal of General Physiology, 96, 1061–1084.
- Bereiter, D. A., Berthoud, H. R., & Jeanrenaud, B. (1980). Hypothalamic input to brain stem neurons responsive to oropharyngeal stimulation. Experimental Brain Research, 39, 33–39.
- Bernard, J. F., Alden, M., & Besson, J. M. (1993). The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. Journal of Comparative Neurology, 329(2), 201–229.
- Bernhardt, S. J., Naim, M., Zehavi, U., & Lindemann, B. (1996). Changes in IP3 and cytosolic Ca2 in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. Journal of Physiology, 490(Pt. 2), 325–336.
- Bester, H., Besson, J. M., & Bernard, J. F. (1997). Organization of efferent projections from the parabrachial area to the hypothalamus: APhaseolus vulgaris-leucoagglutinin study in the rat. Journal of Comparative Neurology, 383(3), 245–281.
- Bester, H., Bourgeais, L., Villanueva, L., Besson, J. M., & Bernard, J. F. (1999). Differential projections to the intralaminar and gustatory thalamus from the parabrachial area:APHA-Lstudy in the rat. Journal of Comparative Neurology, 405(4), 421–449.
- Boughter, J. D., & Smith, D. V. (1998). Amiloride blocks acid responses in NaCl-best gustatory neurons of the hamster solitary nucleus. Journal of Neurophysiology, 80, 1362–1372.
- Bradley, R. M., Stedman, H. M., & Mistretta, C. M. (1983). Superior laryngeal nerve response patterns to chemical stimulation of sheep epiglottis. Brain Research, 276(1), 81–93.
- Brand, J. G. (1997). Biophysics of taste. In G. K. Beauchamp & L. M. Bartoshuk (Eds.), Tasting and smelling (pp. 1–24). San Diego, CA: Academic Press.
- Brand, J. G. (2000). Receptor and transduction processes for umami taste. Journal of Nutrition, 130(4, Suppl.), 942S–945S.
- Breer, H., & Boekoff, I. (1991). Odorants of the same odor class activate different second messenger pathways. Chemical Senses, 16, 19–29.
- Breer, H., Boekoff, I., & Tareilus, E. (1990). Rapid kinetics of second messenger formation in olfactory transduction. Nature, 345, 65–68.
- Brown, R. E. (1979). Mammalian social odors: A critical review. Advanced Study in Behavior, 10, 104–162.
- Brunet, L. J., Gold, G. H., & Ngai, J. (1996). General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron, 17, 681–693.
- Buck, L., & Axel, R. (1991). A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell, 65, 175–187.
- Buoniviso, N., & Chaput, M. A. (1990). Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: Electrophysiological study using simultaneous single unit recordings. Journal of Neurophysiology, 63, 447–454.
- Cain, W. S. (1976). Olfaction and the common chemical sense: Some psychophysical contrasts. Sensory Processes, 1, 57–67.
- Cain, W. S. (1990). Perceptual characteristics of nasal irritation. In B. G. Green, J. R. Mason, & M. R. Kare (Eds.), Chemical senses: Vol. 2. Irritation (pp. 43–58). New York: Marcel Dekker.
- Caprio, J., Brand, J. G., Teeter, J. H., Valentincic, T., Kalinoski, D. L., Kohbara, J., Kumazawa, T., & Wegert, S. (1993). The taste system of the channel catfish: From biophysics to behavior. Trends in Neuroscience, 16(5), 192–197.
- Chandrashekar, J., Mueller, K. L., Hoon, M. A., Adler, E., Feng, L., Guo, W., Zuker, C. S., & Ryba, N. J. (2000). T2Rs function as bitter taste receptors. Cell, 100(6), 703–711.
- Chang, F.-C. T., & Scott, T. R. (1984). Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. Journal of Neuroscience, 4, 1850–1862.
- Chaudhari, N., Landin,A. M., & Roper, S. D. (2000).Ametabotropic glutamate receptor variant functions as a taste receptor. Nature Neuroscience, 3(2), 113–119.
- Cheng, L. H., & Robinson, P. P. (1991). The distribution of fungiform papillae and taste buds on the human tongue. Archives of Oral Biology, 36(8), 583–589.
- Cinelli, A. R., & Kauer, J. S. (1994). Voltage-sensitive dyes and functional activity in the olfactory pathway. Annual Review of Neuroscience, 15, 321–351.
- Collings, V. B. (1974). Human taste response as a function of the locus of stimulation on the tongue and soft palate. Perception and Psychophysics, 15, 169–174.
- Contreras, R. J. (1977). Changes in gustatory nerve discharges with sodium deficiency: A single unit analysis. Brain Research, 121, 373–378.
- Contreras, R. J., & Frank, M. E. (1979). Sodium deprivation alters neural responses to gustatory stimuli. Journal of General Physiology, 73, 569–594.
- Contreras, R. J., & Lundy, R. F. (2000). Gustatory neuron types in the periphery: A functional perspective. Physiology and Behavior, 69(1-2), 41–52.
- Covey, E. (1980). Temporal coding in gustation. Unpublished doctoral dissertation, Duke University, Durham, North Carolina.
- Darby, E. M., Devor, M., & Chorover, S. L. (1975). A presumptive sex pheromone in the hamster: Some behavioral effects. Journal of Comparative Physiological Psychology, 88, 496–502.
- Davis, B. J. (1991). The ascending gustatory pathway: A Golgi analysis of the medial and lateral parabrachial complex in the adult hamster. Brain Research Bulletin, 27, 63–73.
- Davis, B. J. (1993). GABA-like immunoreactivity in the gustatory zone of the nucleus of the solitary tract in the hamster: Light and electronmicroscopicstudies.BrainResearchBulletin,30,69–77.
- Davis, B. J. (1998). Synaptic relationships between the chorda tympani and tyrosine hydroxylase-immunoreactive dendritic processes in the gustatory zone of the nucleus of the solitary tract inthehamster.JournalofComparativeNeurology,392(1),78–91.
- Davis, B. J., & Jang, T. (1986). The gustatory zone of the solitary tract in the hamster: Light microscopic morphometric studies. Chemical Senses, 11, 213–228.
- Dear, T. N., Boehm, T., Keverne, E. B., & Rabbitts, T. H. (1991). Novel genes for potential ligand-binding proteins in subregions of the olfactory mucosa. The EMBO Journal, 10, 2813–2819.
- DeSimone, J. A., Calahan, E. M., & Heck, G. L. (1995). Chorda tympani taste responses of rat to hydrochloric acid subject to voltage-clamped lingual receptive field. American Journal of Physiology, 268(5, Pt. 1), C1295–C1300.
- DiCarlo, S. T., & Powers, A. S. (1998). Propylthiouracil tasting as a possible genetic association marker for two types of alcoholism. Physiology and Behavior, 64(2), 147–152.
- Di Lorenzo, P. M. (1990). Corticofugal influence on taste responses intheparabrachialponsoftherat.BrainResearch,530(1),73–84.
- Di Lorenzo, P. M., & Hecht, G. S. (1993). Perceptual consequences of electrical stimulation in the gustatory system. Behavioral Neuroscience, 107, 130–138.
- Di Lorenzo, P. M., & Lemon, C. H. (2000). The neural code for taste in the nucleus of the solitary tract of the rat: Effects of adaptation. Brain Research, 852, 383–397.
- Di Lorenzo, P. M., & Monroe, S. (1989). Taste responses in the parabrachial pons of male, female and pregnant rats. Brain Research Bulletin, 23, 219–227.
- Di Lorenzo, P. M., & Monroe, S. (1990). Taste responses in the parabrachial pons of ovariectomized rats. Brain Research Bulletin, 25, 741–748.
- Di Lorenzo, P. M., & Monroe, S. (1992). Corticofugal input to tasteresponsive units in the parabrachial pons. Brain Research Bulletin, 29(6), 925–930.
- Di Lorenzo, P. M., & Monroe, S. (1995). Corticofugal influence on taste responses in the nucleus of the solitary tract in the rat. Journal of Neurophysiology, 74(1), 258–272.
- Di Lorenzo, P. M., & Monroe, S. (1997). Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons. Brain Research, 763, 167–181.
- Di Lorenzo, P. M., & Schwartzbaum, J. S. (1982). Coding of gustatory information in the pontine parabrachial nuclei of the rabbit: Temporal patterns of neural response. Brain Research, 251, 245–257.
- Dinkins, M. E., & Travers, S. P. (1998). Effects of chorda tympani nerve anesthesia on taste responses in the NST. Chemical Senses, 23(6), 661–673.
- Doetsch, G. S., & Erickson, R. P. (1970). Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rat. Journal of Neurophysiology, 33, 490–507.
- Doty, R. L. (1986). Odor-guided behavior in mammals. Experentia, 42, 257–271.
- Erickson, R. P. (1986). A neural metric. Neuroscience and Biobehavioral Reviews, 10(4), 377–386.
- Erickson, R. P. (2000). The evolution of neural coding ideas in the chemical senses. Physiology and Behavior, 69(1-2), 3–16.
- Faurion, A., Cerf, B., Le Bihan, D., & Pillias, A. M. (1998). fMRI study of taste cortical areas in humans. Annals of the New York Academy of Sciences, 855(12), 535–545.
- Faurion, A., Cerf, B., Van De Moortele, P. F., Lobel, E., Mac Leod, P., & Le Bihan, D. (1999). Human taste cortical areas studied with functional magnetic resonance imaging: Evidence of functional lateralization related to handedness. Neuroscience Letters, 277(3), 189–192.
- Ferrell, F., Tsuetaki, T., & Chole, R. A. (1985). Myelination in the chorda tympani of the postnatal rat: A quantitative electron microscope study. Acta Anatomica, Basel, 123(4), 224–229.
- Firestein, S., Darrow, B., & Shepherd, G. M. (1991). Activation of the sensory current in salamander olfactory receptor neurons depends on a G-protein-mediated cAMPsecond messenger system. Neuron, 6, 825–835.
- Firestein, S., Picco, C., & Menini, A. (1993). The relation between stimulus and response in the olfactory receptor cells of tiger salamander. Journal of Physiology, London, 468, 1–10.
- Fitzsimmons, J. T. (1979). The physiology of thirst and sodium appetite (Physiological Society Monograph No. 35). London: Cambridge University Press.
- Frank, M. E. (1973). An analysis of hamster afferent taste nerve response functions. Journal of General Physiology, 61, 588– 618.
- Frank, M. E. (1974). The classification of mammalian afferent taste fibers. Chemical Senses, 1, 53–60.
- Frank, M. E. (1991). Taste-responsive neurons of the glossopharyngeal nerve of the rat. Journal of Neurophysiology, 65(6), 1452–1463.
- Frank, M. E. (2000). Neuron types, receptors, behavior, and taste quality. Physiology and Behavior, 69(1-2), 53–62.
- Frank, M. E., Bieber, S. L., & Smith, D. V. (1988). The organization of taste sensibilities in hamster chorda tympani nerve fibers. Journal of General Physiology, 91, 861–896.
- Frank, M. E., Contreras, R., & Hettinger, T. (1983). Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. Journal of Neurophysiology, 50(4), 941–960.
- Frey, S., & Petrides, M. (1999). Re-examination of the human taste region: A positron emission tomography study. European Journal of Neuroscience, 11(8), 2985–2988.
- Ganchrow, D., & Erickson, R. P. (1970). Neural correlates of gustatory intensity and quality. Journal of Neurophysiology, 33(6), 768–783.
- Ganchrow, D., & Erickson, R. P. (1972). Thalamocortical relations in gustation. Brain Research, 36, 289–305.
- Gangestad, S. W., & Thornhill, R. (1998). Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings of the Royal Society of London, 265B, 927– 933.
- Ganzevles, P. G. J., & Kroeze, J. H. A. (1987). Effects of adaptation and cross-adaptation to common ions on sour intensity. Physiology and Behavior, 40, 641–646.
- Gesteland, R. C., Lettvin, J. Y., Pitts, W. H., & Rojas, A. (1963). Odorant and autonomic regulation of secretion in the olfactory mucosa. In Y. Zotterman (Ed.), Olfaction and taste (pp. 19–44). Oxford, UK: Pergamon Press.
- Getchell, M. L., Zelinski, B., & Getchell, T. V. (1988). Odorant and autonomic regulation of secretion in the olfactory mucosa. In F. L. Margolis & T. V. Getchell (Eds.), Molecular biology of the olfactory system (pp. 71–78). New York: Plenum Press.
- Getchell, T. V., Margolis, F. L., & Getchell, M. L. (1984). Perireceptor and receptor events in vertebrate olfaction. Progress in Neurobiology, 23, 317–345.
- Getchell, T. V., & Shepherd, G. M. (1978). Responses of olfactory receptor cells to step pulses of odour at different concentrations in salamander. Journal of Physiology, 282, 521–540.
- Gilbertson, T. A., Avenet, P., Kinnamon, S. C., & Roper, S. D. (1992). Proton currents through amiloride-sensitive Na channels in hamster taste cells: Role in acid transduction. Journal of General Physiology, 100, 941–960.
- Gilbertson, T. A., Damak, S., & Margolskee, R. F. (2000). The molecular physiology of taste transduction. Current Opinions in Neurobiology, 10(4), 519–527.
- Gill, J. M., II, & Erickson, R. P. (1985). Neural mass differences in gustation. Chemical Senses, 10, 531–548.
- Giza, B. K., & Scott, T. R. (1991). The effect of amiloride on tasteevoked activity in the nucleus tractus solitarius of the rat. Brain Research, 550, 247–256.
- Grabauskas, G., & Bradley, R. M. (1996). Synaptic interactions due to convergent input from gustatory afferent fibers in the rostral nucleus of the solitary tract. Journal of Neurophysiology, 76(5), 2919–2927.
- Halpern, B. P. (1998). Amiloride and vertebrate gustatory responses to NaCl. Neuroscience and Biobehavioral Reviews, 23(1), 5–47.
- Halpern, B. P., & Nelson, L. M. (1965). Bulbar gustatory responses to anterior and to posterior tongue stimulation in the rat. American Journal of Physiology, 209, 105–110.
- Halsell, C. B., Travers, J. B., & Travers, S. P. (1993). Gustatory and tactile stimulation of the posterior tongue activate overlapping but distinctive regions within the nucleus of the solitary tract. Brain Research, 632, 161–173.
- Hanamori, T., Miller, I. J., & Smith, D. V. (1988). Gustatory responsiveness of fibers in the hamster glossopharyngeal nerve. Journal of Neurophysiology, 60(2), 478–498.
- Harden-Jones, F. R. (1968). Fish migration. London: EdwardArnold.
- Hellekant, G., Danilova, V., & Ninomiya, Y. (1997). Primate sense of taste: Behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta. Journal of Neurophysiology, 77(2), 978–993.
- Henkin, R. I. (1974). Sensory changes during the menstrual cycle. In M. Ferin, F. Halberg, R. M. Richart, & R. L. Vande Wiele (Eds.), Biorhythms and human reproduction (pp. 277–285). New York: Wiley.
- Henning, H. (1922). Psychologische studien au geschmacksinn (Psychological studies of taste). Handbuch der Biologische Arbeit-methoden (6A, pp. 627–740). Berlin: Urban & Schwarzenberg.
- Herness, S. (2000). Coding in taste receptor cells: The early years of intracellular recordings. Physiology and Behavior, 69(1-2), 17–28.
- Herness, M. S., & Gilbertson, T. A. (1999). Cellular mechanisms of taste transduction. Annual Review of Physiology, 61, 873– 900.
- Hoon, M. A., Adler, E., Lindemeier, J., Battey, J. F., Ryba, N. J., & Zuker, C. S. (1999). Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell, 96(4), 541–551.
- Horst, G. J. T., de Boer, P., Luiten, P. G. M., & van Willigen, J. D. (1989). Ascending projections from the solitary tract nucleus to the hypothalamus: A phaseolus vulgaris lectin tracing study in the rat. Neuroscience, 31, 785–797.
- Hosoya, Y., & Matsushita, M. (1981). Brainstem projections from the lateral hypothalamic area in the rat, as studied with autoradiography. Neuroscience Letters, 24, 111–116.
- Huard, J. M.T.,Youngentob, S. L., Goldstein, B. J., Luskin, M. B., & Schwob, J. E. (1998). Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neuronal cells. Journal of Comparative Neurology, 400, 469–486.
- Hudsen, R., & Distel, H. (1983). Nipple location by newborn rabbits: Behavioral evidence for pheromonal guidance. Behavior, 85, 260–275.
- Imamura, K., Mataga, N., & Mori, K. (1992). Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb: I. Aliphatic compounds. Journal of Neurophysiology, 68, 1986–2002.
- Jacobs, K. M., Mark, G. P., & Scott, T. R. (1988). Taste responses in the nucleus tractus solitarius of sodium-deprived rats. Journal of Physiology, London, 406, 393–410.
- James, J. E. A., & Daly, M. de B. (1969). Nasal reflexes. Proceedings of the Royal Society of Medicine, 62, 1287–1293.
- Johnson, B. A., & Leon, M. (2000). Modular representation of odorants in the glomerular layer of the rat olfactory bulb and effects of stimulus concentration. Journal of Comparative Neurology, 422(4), 496–509.
- Johnson, B. A., Woo, C. C., & Leon, M. (2000). Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. Journal of Comparative Neurology, 393, 457–471.
- Kanazawa, H., & Yoshie, S. (1996). The taste bud and its innervation in the rat as studied by immunohistochemistry for PGP 9.5. Archives of Histology and Cytology, 59(4), 357–367.
- Karimnamazi, H., & Travers, J. B. (1998). Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Research, 813(2), 283–302.
- Kashiwadani, H., Sasaki, Y. F., Uchida, N., & Mori, K. (1999). Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. Journal of Neurophysiology, 82, 1786–1792.
- Katoh, K., Koshimoto, H., Tani, A., & Mori, K. (1993). Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb: II. Aromatic compounds. Journal of Neurophysiology, 70, 2161– 2165.
- Kent, P. F., Mozell, M. M., Murphy, S. J., & Hornung, D. E. (1996). The interaction of imposed and inherent olfactory mucosal activity patterns and their composite representation in a mammalian species using voltage-sensitive dyes. Journal of Neuroscience, 16, 345–353.
- Kent, P. F., Youngentob, S. L., & Sheehe, P. R. (1995). Odorantspecific spatial patterns in mucosal activity predict perceptual differences among odorants. Journal of Neurophysiology, 74, 1777–1781.
- King, C. T., Garcea, M., & Spector, A. C. (2000). Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. Journal of Neuroscience, 20(22), 8426–8434.
- King, C. T., Travers, S. P., Rowland, N. E., Garcea, M., & Spector, A. C. (1999). Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: Implications for a functional topography of gustatory nerve input in rats. Journal of Neuroscience, 19(8), 3107–3121.
- Kinnamon, S. C., Dionne, V. E., & Beam, K. G. (1988). Apical localization of K channels in taste cells provides the basis for sour taste transduction. Proceedings of the National Academy of Sciences, USA, 85(18), 7023–7027.
- Kinnamon, S. C., & Margolskee, R. F. (1996). Mechanisms of taste transduction. Current Opinion in Neurobiology, 6(4), 506–513.
- Kinomura, S., Kawashima, R., Yamada, K., Ono, S., Itoh, M., Yoshioka, S., Yamaguchi, T., Matsui, H., Miyazawa, H., Itoh, H., Goto, R., Fujiwara, T., Satoh, K., & Fukuda, H. (1994). Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Research,659(1-2), 263–266.
- Kita, H., & Oomura,Y. (1981). Functional synaptic interconnections between the lateral hypothalamus and frontal and gustatory cortices in the rat. In Y. Katsuki, R. Norgren, & M. Sato (Eds.), Brain mechanisms of sensation (pp. 307–322). NewYork: Wiley.
- Kobayakawa, T., Ogawa, H., Kaneda, H., Ayabe-Kanamura, S., Endo, H., & Saito, S. (1999). Spatio-temporal analysis of cortical activity evoked by gustatory stimulation in humans. Chemical Senses, 24(2), 201–209.
- Kolesnikov, S. S., & Margolskee, R. F. (1995). A cyclic-nucleotidesuppressible conductance activated by transducin in taste cells. Nature, London, 376, 85–88.
- Kosar, E., & Schwartz, G. J. (1990). Cortical unit responses to chemical stimulation of the oral cavity in the rat. Brain Research, 513(2), 212–224.
- Kranzler, H. R., Moore, P. J., & Hesselbrock, V. M. (1996). No association of PROP taster status and paternal history of alcohol dependence. Alcoholism: Clinical and Experimental Research, 20(8), 1496–1500.
- Kroeze, J. H., & Bartoshuk, L. M. (1985). Bitterness suppression as revealed by split-tongue taste stimulation in humans. Physiology and Behavior, 35(5), 779–783.
- Krout, K. E., & Loewy, A. D. (2000). Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. Journal of Comparative Neurology, 428(3), 475–494.
- Kubick, S., Strotmann, J., Andreini, I., & Breer, H. (1997). Subfamily of olfactory receptors characterized by unique structural features and expression patterns. Journal of Neurochemistry, 69, 465–475.
- Kurihara, K., & Kashiwayanagi, M. (2000). Physiological studies on umami taste. Journal of Nutrition, 130(4, Suppl.), 931S– 934S.
- Kveton, J. F., & Bartoshuk, L. M. (1994). The effect of unilateral chorda tympani damage on taste. Laryngoscope, 104(1, Pt. 1), 25–29.
- Lasiter, P. S., Glanzman, D. L., & Mensah, P. A. (1982). Direct connectivity between pontine taste areas and gustatory neocortex in rat. Brain Research, 234, 111–121.
- Lasiter, P. S., & Kachele, D. L. (1988a). Postnatal development of the parabrachial gustatory zone in rat: Dendritic morphology and mitochondrial enzyme activity. Brain Research Bulletin, 21, 79–94.
- Lasiter, P. S., & Kachele, D. L. (1988b). Organization of GABAand GABA-transaminase containing neurons in the gustatory zone of the nucleus of the solitary tract. Brain Research Bulletin, 21, 623–636.
- Lehman, C. D., Bartoshuk, L. M., Catalanotto, F. C., Kveton, J. F., & Lowlocht, R. A. (1995). Effect of anesthesia of the chorda tympani nerve on taste perception in humans. Physiology and Behavior, 57(5), 943–951.
- Lindemann, B. (1996). Taste reception. Physiological Review, 76(3), 718–766.
- Liu, H., Behbehani, M. M., & Smith, D. V. (1993). The influence of GABA on cells in the gustatory region of hamster solitary nucleus. Chemical Senses, 18(3), 285–305.
- Lowe, G., & Gold, G. H. (1993). Contribution of ciliary cyclic nucleotide-gated conductance to olfactory transduction in the salamander. Journal of Physiology, 462, 175–196.
- Lowe, G., Nakamura, T., & Gold, G. H. (1989). Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proceedings of the National Academy of Sciences, USA, 86, 5641–5645.
- Lundy, R. F., & Contreras, R. J. (1999). Gustatory neuron types in rat geniculate ganglion. Journal of Neurophysiology, 82(6), 2970–2988.
- MacKay-Sim, A., & Kesteven, S. (1994). Topographic patterns of responsiveness to odorants in the rat olfactory epithelium. Journal of Neurophysiology, 71, 150–160.
- MacKay-Sim, A., & Kubie, J. L. (1981). The salamander nose: A model system for the study of spatial coding of olfactory quality. Chemical Senses, 6, 249–257.
- Malnic, B., Hirano, J., Sato, T., & Buck, L. B. (1999). Combinatorial receptor codes for odors. Cell, 96, 713–723.
- Markison, S., St. John, S. J., & Spector, A. C. (1995). Glossopharyngeal nerve transection does not compromise the specificity of taste-guided sodium appetite in rats. American Journal of Physiology, 269(1, Pt. 2), R215–R221.
- Markison, S., St. John, S. J., & Spector, A. C. (1999). Glossopharyngeal nerve transection reduces quinine avoidance in rats not given presurgical stimulus exposure. Physiology and Behavior, 65(4-5), 773–778.
- McBurney, D. H., & Bartoshuk, L. M. (1973). Interactions between stimuli with different taste qualities. Physiology and Behavior, 10(6), 1101–1106.
- McCaughey, S. A., Giza, B. K., Nolan, L. J., & Scott, T. R. (1997). Extinction of a conditioned taste aversion in rats: II. Neural effects in the nucleus of the solitary tract. Physiology and Behavior, 61(3), 373–379.
- McCaughey, S. A., Giza, B. K., & Scott, T. R. (1996). Activity in rat nucleus tractus solitarius after recovery from sodium deprivation. Physiology and Behavior, 60(2), 501–506.
- McClintock, M. (1983). Pheromonal regulation of the ovarian cycle: Enhancement, suppression and synchrony. In J. G. Vandenbergh (Ed.), Pheromones and reproduction in mammals (pp. 95–112). New York: Academic Press.
- McLaughlin, S. K., McKinnon, P. J., & Margolskee, R. F. (1992). Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature, 357(6379), 563–569.
- McLaughlin, S. K., McKinnon, P. J., Spickofsky, N., Danho, W., & Margolskee, R. F. (1994). Molecular cloning of G proteins and phosphodiesterases from rat taste cells. Physiology and Behavior, 56, 1157–1164.
- McPheeters, M., Hettinger, T., Nuding, S. C., Savoy, L. D., Whitehead, M. C., & Frank, M. E. (1990). Taste-responsive neurons and their locations in the solitary nucleus of the hamster. Neuroscience, 34(3), 745–758.
- Mech, L. D., & Peters, R. P. (1977). The study of chemical communication in free-ranging mammals. In D. Muller-Schwartz & M. M. Mozell (Eds.), Chemical signals in vertebrates (pp. 321– 333). New York: Plenum Press.
- Miller, I. J. (1986). Variation in human fungiform taste bud densities among regions and subjects. Anatomical Record, 216(4), 474–482.
- Miller, I. J., & Bartoshuk, L. M. (1991). Taste perception and taste bud distribution. In T. V. Getchell, L. M. Bartoshuk, R. L. Doty, & J. B. Snow (Eds.), Smell and taste in health and disease (pp. 205–234). New York: Raven Press.
- Miller, I. J., & Preslar, A. J. (1975). Spatial distribution of rat fungiform papillae. Anatomical Record, 181(3), 679–684.
- Mistretta, C. M., & Oakley, I. A. (1986). Quantitative anatomical study of taste buds in fungiform papillae of young and old Fischer rats. Journal of Gerontology, 41(3), 315–318.
- Miyamoto, T., Fujiyama, R., Okada, Y., & Sato, T. (2000). Acid and salt responses in mouse taste cells. Progress in Neurobiology, 62(2), 135–157.
- Mombaerts, P., Wang, F., Dulac, C., Chao, S. K., Nemes, A., Mendelsohn, M., Edmondson, J., & Axel, R. (1997). Visualizing an olfactory sensory map. Cell, 87, 675–686.
- Monroe, S., & Di Lorenzo, P. M. (1995). Taste responses in neurons in the nucleus of the solitary tract that do and do not project to the parabrachial pons. Journal of Neurophysiology, 74(1), 249–257.
- Moran, D. T., Rowley, J. C., & Jafek, B. W. (1982). Electron microscopy of human olfactory epithelium reveals a new cell type: The microvillar cell. Brain Research, 253, 39–46.
- Mori, K., Imamura, K., & Mataga, N. (1992). Differential specificities in mitral cells in rabbit olfactory bulb for a homologous series of fatty acid molecules. Journal of Neurophysiology, 67, 786–789.
- Mori, K., Nagao, H., & Yoshihara, Y. (1999). The olfactory bulb: Coding and processing of odor molecule information. Science, 286, 711–715.
- Mori, K., & Shepherd, G. M. (1994). Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Seminars in Cell Biology, 5(1), 65–74.
- Mori, K., & Takagi, S. F. (1978). An intracellular study of dendrodendritic inhibitory synapses on mitral cells in the rabbit olfactory bulb. Journal of Physiology, London, 279, 569–588.
- Mori, K., & Yoshihara, Y. (1995). Molecular recognition and olfactory processing in the mammalian olfactory system. Progress in Neurobiology, 45, 585–619.
- Morrison, E. E., & Costanzo, R. M. (1990). Morphology of the human olfactory epithelium. Journal of Comparative Neurology, 297, 1–13.
- Moulton, D. G. (1976). Spatial patterning response to odors in the peripheral olfactory system. Physiological Reviews, 56, 578– 593.
- Mozell, M. M., Sheehe, P. R., Hornung, D. E., Kent, P. F., Youngentob, S. L., & Murphy, S. J. (1987). “Imposed” and “inherent” mucosal activity patterns: Their composite representation of olfactory stimuli. Journal of General Physiology, 90, 625–650.
- Mozell, M. M., Smith, B. P., Smith, P. E., Sullivan, R. L., & Swender, P. (1969). Nasal chemoreception in flavor identification. Archives of Otolaryngology, 90, 131–137.
- Muller, T., & Jastrow, H. (1998). The innervation of taste buds in the soft palate and circumvallate papilla of the rat as revealed by the zinc iodide-osmium tetroxide technique. Archives of Histology and Cytology, 61(4), 327–336.
- Muller-Schwartz, D. (1977). Complex mammalian behavior and pheromone bioassay in the field. In D. Muller-Schwartz & M. M. Mozell (Eds.), Chemical signals in vertebrates (pp. 413–435). New York: Plenum Press.
- Murphy, C., Cardello, A. V., & Brand, J. B. (1981). Tastes of fifteen halide salts following water and NaCl: Anion and cation effects. Physiology and Behavior, 26, 1083–1095.
- Nagai, T., & Ueda, K. (1981). Stochastic properties of gustatory impulse discharges in rat chorda tympani fibers. Journal of Neurophysiology, 45(3), 574–592.
- Nakamura, K., & Norgren, R. (1991). Gustatory responses of neurons in the nucleus of the solitary tract of behaving rats. Journal of Neurophysiology, 66(4), 1232–1248.
- Nakamura, K., & Norgren, R. (1993). Taste responses of neurons in the nucleus of the solitary tract of awake rats: An extended stimulus array. Journal of Neurophysiology, 70(3), 879– 891.
- Nakamura, T., Lee, H. H., Kobayashi, H., & Sato, T. O. (1996). Gated conductances in native and reconstituted membranes from frog olfactory cilia. Biophysical Journal, 70, 813–817.
- Nakamura, T., & Ogawa, H. (1997). Neural interaction between cortical taste neurons in rats: Across-correlation analysis. Chemical Senses, 22(5), 517–528.
- Nef, P., Hermans-Borgmeyer, I., Artieres, H., Beasely, L., Dionne, V. E., & Heinemann, S. F. (1992). Spatial pattern of receptor gene expression in the olfactory epithelium. Proceedings of the National Academy of Sciences, USA, 89, 8948– 8952.
- Nishijo, H., & Norgren, R. (1997). Parabrachial neural coding of taste stimuli in awake rats. Journal of Neurophysiology, 78(5), 2254–2268.
- Nomura, T., & Ogawa, H. (1985). The taste and mechanical response properties of neurons in the parvicellular part of the thalamic posteromedial ventral nucleus of the rat. Neuroscience Research, 3(2), 91–105.
- Norgren, R. (1974). Gustatory afferents to ventral forebrain. Brain Research, 81, 285–295.
- Norgren, R., & Grill, H. J. (1976). Efferent distribution from the cortical gustatory area in rats. Neuroscience Abstracts, 2,
- Ogawa, H. (1994). Gustatory cortex of primates: Anatomy and physiology. Neuroscience Research, 20(1), 1–13.
- Ogawa, H., Hasegawa, K., & Murayama, N. (1992). Difference in taste quality coding between two cortical taste areas, granular and dysgranular insular areas, in rats. Experimental Brain Research, 91(3), 415–424.
- Ogawa, H., Hayama, T., & Ito, S. (1987). Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Experimental Brain Research, 68(3), 449–457.
- Ogawa, H., Imoto, T., & Hayama, T. (1984). Responsiveness of solitario-parabrachial relay neurons to taste and mechanical stimulation applied to the oral cavity in rats. Experimental Brain Research, 54, 349–358.
- Ogawa, H., Imoto, T., Hayama, T., & Kaisaku, J. (1982). Afferent connections to the pontine taste area: Physiologic and anatomic studies. In Y. Katsuki, R. Norgren, & M. Sato (Eds.), Brain mechanisms of sensation (pp. 161–176). New York: Wiley.
- Ogawa, H., & Kaisaku, J. (1982). Physiological characteristics of the solitario-parabrachial relay neurons with tongue afferent inputs in rats. Experimental Brain Research, 48(3), 362–368.
- Ogawa, H., Murayama, N., & Hasegawa, K. (1992). Difference in receptive field features of taste neurons in rat granular and dysgranular insular cortices. Experimental Brain Research, 91(3), 408–414.
- Ogawa, H., & Nomura, T. (1988). Receptive field properties of thalamo-cortical taste relay neurons in the parvicellular part of the posteromedial ventral nucleus in rats. Experimental Brain Research, 73(2), 364–370.
- Ogawa, H., Sato, M., & Yamashita, S. (1973). Variability in impulse discharges in rat chorda tympani fibers in response to repeated gustatory stimulations. Physiology and Behavior, 11(4), 469–479.
- Ogawa, H., Yamashita, S., & Sato, M. (1974). Variation in gustatory nerve fiber discharge pattern with change in stimulus concentration and quality. Journal of Neurophysiology, 37(3), 443–457.
- O’Keefe, G. B., Schumm, J., & Smith, J. C. (1994). Loss of sensitivity to low concentrations of NaCl following bilateral chorda tympani nerve sections in rats. Chemical Senses, 19(2), 169– 184.
- O’Mahony, M., & Ishii, R. (1986). A comparison of English and Japanese taste languages: Taste descriptive methodology, codability and the umami taste. British Journal of Psychology, 77(Pt. 2), 161–174.
- Ozeki, M. (1971). Conductance change associated with receptor potentials of gustatory cells in rat. Journal of General Physiology, 58, 688–699.
- Pedersen, P. E., Jastreboff, P. J., Stewart, W. B., & Shepherd, G. M. (1986). Mapping of an olfactory receptor population that projects to a specific region in the rat olfactory bulb. Journal of Comparative Neurology, 250, 93–108.
- Pelchat, M. L., & Danowski, S. (1992). A possible genetic association between PROP-tasting and alcoholism. Physiology and Behavior, 51(6), 1261–1266.
- Pevsner, J., & Snyder, S. H. (1990). Odorant binding protein: Odorant transport function in the vertebrate nasal epithelium. Chemical Senses, 15, 217–222.
- Pike, L. M., Enns, M. P., & Hornung, D. E. (1988). Quality and intensity differences of carvone enantiomers when tested separately and in mixtures. Chemical Senses, 13, 307–309.
- Price, J. L. (1987). The central and accessory olfactory systems. In T. E. Finger & W. L. Silver (Eds.), Neurobiology of taste and smell (pp. 179–204). New York: Wiley.
- Pritchard, T. C. (1991). The primate gustatory system. In T. V. Getchell, L. M. Bartoshuk, R. L. Doty, & J. B. Snow (Eds.), Smell and taste in health and disease (pp. 109–126). New York: Raven Press.
- Pritchard, T. C., Hamilton, R. B., & Norgren, R. (1989). Neural coding of gustatory information in the thalamus of Macaca mulatta. Journal of Neurophysiology, 61(1), 1–14.
- Pritchard, T. C., Macaluso, D. A., & Eslinger, P. J. (1999). Taste perception in patients with insular cortex lesions. Behavioral Neuroscience, 113(4), 663–671.
- Reed, D. R., Nanthakumar, E., North, M., Bell, C., Bartoshuk, L. M., & Price, R. A. (1999). Localization of a gene for bittertaste perception to human chromosome 5p15. American Journal of Human Genetics, 64(5), 1478–1480.
- Renehan, W. E., Jin, Z., Zhang, X., & Schweitzer, L. (1994). The structure and function of gustatory neurons in the nucleus of the solitary tract: I. A classification of neurons based on morphological features. Journal of Comparative Neurology, 347, 531–544.
- Ressler, K. J., Sullivan, S. L., & Buck, L. B. (1993). A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell, 73, 597–609.
- Restrepo, D., Teeter, J. H., Honda, E., Boyle, A. G., Marecek, J. F., Prestwich, G. D., & Kalinoski, D. L. (1992). Evidence for an InsP3-gated channel protein in isolated rat olfactory cilia. American Journal of Physiology, 263, C667–C673.
- Revial, M. F., Sicard, G., Duchamp, A., & Holley, A. (1982). New studies on odour discrimination in the frog’s olfactory receptor cells: I. Experimental results. Chemical Senses, 7, 175–190.
- Revial, M. F., Sicard, G., Duchamp, A., & Holley, A. (1983). New studies on odour discrimination in the frog’s olfactory receptor cells: II. Mathematical analysis of electrophysiological responses. Chemical Senses, 8, 179–194.
- Ricardo, J. A., & Koh, E. T. (1978). Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus amygdala and other forebrain structures in the cat. Brain Research, 153, 1–28.
- Rolls, E. T., Yaxley, S., & Sienkiewicz, Z. J. (1990). Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Journal of Neurophysiology, 64(4), 1055–1066.
- Royer, S. M., & Kinnamon, J. C. (1994). Application of serial sectioning and three-dimensional reconstruction to the study of taste bud ultrastructure and organization. Microscopy Research and Technique, 29(5), 381–407.
- Saper, C. B. (1982). Reciprocal parabrachial-cortical connections in the rat. Brain Research, 242, 33–40.
- Saper, C. B., & Loewy, A. D. (1980). Efferent connections of the parabrachial nucleus of the pons. Brain Research, 197, 291–317.
- Sato, M., Ogawa, H., & Yamashita, S. (1994). Gustatory responsiveness of chorda tympani fibers in the cynomolgus monkey. Chemical Senses, 19(5), 381–400.
- Sato, T., Hirano, J., Tonoike, M., & Takebayashi, M. (1994). Tuning specificity to aliphatic odorants in mouse olfactory receptor neurons and their local distribution. Journal of Neurophysiology, 72, 2980–2989.
- Schiffman, S. S. (2000). Taste quality and neural coding: Implications from psychophysics and neurophysiology. Physiology and Behavior, 69(1-2), 147–160.
- Schneider, D. (1969). Insect olfaction: Deciphering system for chemical messages. Science, 163, 1031–1036.
- Schneider, R. A., & Wolf, S. (1955). Olfactory perception thresholds for citral utilizing a new type olfactorium. Journal of Applied Physiology, 8, 337–342.
- Schwob, J. E., Youngentob, S. L., & Mezza, R. (1995). The reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. Journal of Comparative Neurology, 359, 15–37.
- Sclafani, A., & Abrams, M. (1986). Rats show only a weak preference for the artificial sweetener aspartame. Physiology and Behavior, 37(2), 253–256.
- Scott, T. R. (1974). Behavioral support for a neural taste theory. Physiology and Behavior, 12(3), 413–417.
- Scott, T. R., & Erickson, R. P. (1971). Synaptic processing of tastequality information in thalamus of the rat. Journal of Neurophysiology, 34(5), 868–883.
- Scott, T. R., & Giza, B. (1990). Coding channels in the rat taste system. Science, 249, 1585–1587.
- Scott, T. R., & Giza, B. (2000). Issues of gustatory neural coding: Wheretheystandtoday.PhysiologyandBehavior,69(1-2),65–76.
- Scott, T. R., & Plata-Salaman, C. R. (1999). Taste in the monkey cortex. Physiology & Behavior, 67(4), 489–511.
- Shepherd, G. M. (1972). Synaptic organization of the mammalian olfactory bulb. Physiological Reviews, 52, 864–917.
- Shepherd, G. M., & Greer, C. A. (1990). Olfactory bulb. In G. M. Shepherd (Ed.), The synaptic organization of the brain (pp. 139–169). New York: Oxford University Press.
- Shi, C. J., & Cassell, M. D. (1998). Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. Journal of Comparative Neurology, 399(4), 440–468.
- Shimura, T., Komori, M., & Yamamoto, T. (1997). Acute sodium deficiency reduces gustatory responsiveness to NaCl in the parabrachial nucleus of rats. Neuroscience Letters, 236(1), 33–36.
- Simon, S. A., Labarca, P., & Robb, R. (1989). Activation by saccharides of a cation-selective pathway in canine lingual epithelium. American Journal of Physiology, 256(Regulatory Integrative Comparative Physiology 25), R394–R402.
- Small, D. M., Zald, D. H., Jones-Gotman, M., Zatorre, R. J., Pardo, J. V., Frey, S., & Petrides, M. (1999). Human cortical gustatory areas: A review of functional neuroimaging data. NeuroReport, 10(1), 7–14.
- Smith, D. V., & Li, C.-S. (1998). Tonic GABAergic inhibition of taste-responsive neurons in the nucleus of the solitary tract. Chemical Senses, 23, 159–169.
- Smith, D. V., & Li, C.-S. (2000). GABA-mediated corticofugal inhibition of taste-responsive neurons in the nucleus of the solitary tract. Brain Research, 858(2), 408–415.
- Smith, D. V., Liu, H., & Vogt, M. B. (1996). Responses of gustatory cellsinthenucleusofthesolitarytractofthehamsterafterNaClor amiloride adaptation. Journal of Neurophysiology, 76(1), 47–58.
- Smith, D. V., St. John, S. J., & Boughter, J. D. (2000). Neuronal cell types and taste quality coding. Physiology and Behavior, 69(1-2), 77–85.
- Spector, A. C., & Grill, H. J. (1992). Salt taste discrimination after bilateralsectionofthechordatympaniorglossopharyngealnerves. American Journal of Physiology, 263(1, Pt. 2), R169–R176.
- Spector, A. C., Markison, S., St. John, S. J., & Garcea, M. (1997). Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. American Journal of Physiology, 272(4, Pt. 2), R1210–R1218.
- Spector, A. C., Redman, R., & Garcea, M. (1996). The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behavioral Neuroscience, 110(5), 1096–1109.
- Spector, A. C., Schwartz, G. J., & Grill, H. J. (1990). Chemospecific deficits in taste detection after selective gustatory deafferentation in rats. American Journal of Physiology, 258(3, Pt. 2), R820– R826.
- Spector, A. C., Travers, S. P., & Norgren, R. (1993). Taste receptors on the anterior tongue and nasoincisor ducts of rats contribute synergistically to behavioral responses to sucrose. Behavioral Neuroscience, 107(4), 694–702.
- Spielman, A. I. (1998). Gustducin and its role in taste. Journal of Dental Research, 77(4), 539–544.
- Spielman, A. I., Huque, T., Nagai, H., Whitney, G., & Brand, J. G. (1994). Generation of inositol phosphates in bitter taste transduction. Physiology and Behavior, 56, 1149–1155.
- Spielman, A. I., Huque, T., Whitney, G., & Brand, J. G. (1992). The diversity of bitter taste signal transduction mechanisms. Society of General Physiologists Series, 47, 307–324.
- John, S. J., Garcea, M., & Spector, A. C. (1994). Combined, but not single, gustatory nerve transection substantially alters tasteguided licking behavior to quinine in rats. Behavioral Neuroscience, 108(1), 131–140.
- John, S. J., Markison, S., & Spector, A. C. (1995). Salt discriminability is related to number of regenerated taste buds after chorda tympani nerve section in rats. American Journal of Physiology, 269(1, Pt. 2), R141–R153.
- John, S. J., Markison, S., & Spector, A. C. (1997). Chorda tympani nerve transection disrupts taste aversion learning to potassium chloride, but not sodium chloride. Behavioral Neuroscience, 111(1), 188–194.
- John, S. J., & Spector, A. C. (1996). Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus). Behavioral Neuroscience, 110(6), 1456–1468.
- Stern, K., & McClintock, M. K. (1998). Regulation of ovulation by human pheromones. Nature, 392, 177–179.
- Stewart, R. E., DeSimone, J. A., & Hill, D. L. (1997). New perspectives in a gustatory physiology: Transduction, development, and plasticity.AmericanJournalofPhysiology,272(1,Pt.1),C1–C26.
- Stewart, W. B., & Pedersen, P. E. (1987). The spatial organization of the olfactory nerve projections. Brain Research, 411, 248–258.
- Strotmann, J., Wanner, I., Helfrich, T., Beck, A., & Breer, H. (1994). Rostrocaudal patterning of receptor expressing olfactory neurones in the rat nasal cavity. Cell and Tissue Research, 278, 11–20.
- Strotmann, J., Wanner, I., Krieger, J., Raming, K., & Breer, H. (1992). Expression of odorant receptors in spatially restricted subsets of chemosensory neurones. NeuroReport, 3, 1053–1056.
- Sugimoto, K., & Teeter, J. H. (1991). Stimulus-induced currents in isolated taste receptor cells of the larval tiger salamander. Chemical Senses, 16, 1109–1122.
- Sweazy, R. D., & Smith, D. V. (1987). Convergence onto hamster medullary taste neurons. Brain Research, 408, 173–184.
- Tamura, R., & Norgren, R. (1997). Repeated sodium depletion affects gustatory neural responses in the nucleus of the solitary tract of rats. American Journal of Physiology, 273(4, Pt. 2), R1381– R1391.
- Toller, S. V. (1999). Assessing the impact of anosmia: Review of a questionnaire’s findings. Chemical Senses, 24(6), 705–712.
- Travers, J. B., Travers, S. P., & Norgren, R. (1987). Gustatory neural processing in the hindbrain. Annual Review of Neuroscience, 10, 595–632.
- Travers, S. P., & Norgren, R. (1991). Coding the sweet taste in the nucleus of the solitary tract: Differential roles for anterior tongue and nasoincisor duct gustatory receptors in the rat. Journal of Neurophysiology, 65(6), 1372–1380.
- Travers, S. P., & Norgren, R. (1995). Organization of orosensory responses in the nucleus of the solitary tract of rat. Journal of Neurophysiology, 73(6), 2144–2162.
- Travers, S. P., Pfaffmann, C., & Norgren, R. (1986). Convergence of lingual and palatal gustatory neural activity in the nucleus of the solitary tract. Brain Research, 365, 305–320.
- Vassar, R., Chao, S. K., Sitcheran, R., Nunez, J. M., Vosshall, L. B., & Axel, R. (1994). Topographic organization of sensory projections to the olfactory bulb. Cell, 79, 981–991.
- Whitehead, M. C. (1986). Anatomy of the gustatory system in the hamster: Synaptology of facial afferent terminals in the solitary nucleus. Journal of Comparative Neurology, 224, 1–24.
- Whitehead, M. C. (1990). Subdivisions and neuron types of the nucleus of the solitary tract that project to the parabrachial nucleus in the hamster. Journal of Comparative Neurology, 301(4), 554–574.
- Whitehead, M. C., Bergula, A., & Holliday, K. (2000). Forebrain projections to the rostral nucleus of the solitary tract in the hamster. Journal of Comparative Neurology, 422(3), 429–447.
- Whitehead, M. C., & Frank, M. E. (1983). Anatomy of the gustatory system in the hamster: Central projections of the chorda tympani and the lingual nerve. Journal of Comparative Neurology, 220, 378–395.
- Whitten, W. K. (1956). Modification of oestrus cycle of mouse by external stimuli associated with the male. Journal of Endocrinology, 13, 399–404.
- Wolf, G. (1968). Projections of thalamic and cortical gustatory areas in the rat. Journal of Comparative Neurology, 132, 519–530.
- Wong, G. T., Gannon, K. S., & Margolskee, R. F. (1996). Transduction of bitter and sweet taste by gustducin. Nature, 381(6585), 796–800.
- Woolston, D. C., & Erickson, R. P. (1979). Concept of neuron types in gustation in the rat. Journal of Neurophysiology, 42, 1390–1409.
- Wysocki, C. J., Pierce, J. D., & Gilbert, A. N. (1991). Geographic, cross-cultural and individual variation in human olfaction. In T. V. Getchell, R. L. Doty, L. M. Bartoshuk, & J. B. Snow (Eds.), Smell and taste in health and disease (pp. 287–314). New York: Raven Press.
- Yahr, P. (1977). Central control of scent marking. In D. MullerSchwartz & M. M. Mozell (Eds.), Chemical signals in vertebrates (pp. 549–563). New York: Plenum Press.
- Yamaguchi, S. (1991). Basic properties of umami and effects on humans. Physiology and Behavior, 49, 843–841.
- Yamamoto, T., Azuma, S., & Kawamura, Y. (1984). Functional relations between the cortical gustatory area and the amygdala: Electrophysiological and behavioral studies in rats. Experimental Brain Research, 56, 23–31.
- Yamamoto, T., Matsuo, R., & Kawamura, Y. (1980). Corticofugal effects on the activity of thalamic taste cells. Brain Research, 193, 258–262.
- Yamamoto, T., & Yuyama, N. (1987). On a neural mechanism for cortical processing of taste quality in the rat. Brain Research, 400(2), 312–320.
- Yamamoto, T., Yuyama, N., Kato, T., & Kawamura, Y. (1985). Gustatory responses of cortical neurons in rats: III. Neural and behavioral measures compared. Journal of Neurophysiology, 53(6), 1370–1386.
- Yaxley, S., Rolls, E. T., & Sienkiewicz, Z. J. (1990). Gustatory responses of single neurons in the insula of the macaque monkey. Journal of Neurophysiology, 63(4), 689–700.
- Ylikoski, J., Gamoletti, R., & Galey, F. (1983). Chorda tympani nerve fibers in man. Acta Otolaryngologica, 95(3-4), 291–296.
- Yokoi, M., Mori, K., & Nakanishi, S. (1995). Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proceedings of the National Academy of Sciences, USA, 92, 3371–3375.
- Youngentob, S. L., Kent, P. F., Sheehe, P. R., Schwob, J. E., & Tzoumaka, E. (1995). Mucosal inherent activity patterns in the rat: Evidence from voltage sensitive dyes. Journal of Neurophysiology, 73, 387–398.
- Youngentob, S. L., Markert, L. M., Mozell, M. M., & Hornung, D. E. (1990). A method for establishing a five odorant identification confusion matrix in rats. Physiology and Behavior, 47, 1053–1059.
- Zhao, H., Ivic, L., Otaki, J. M., Hashimoto, M., Mikoshiba, K., & Firestein, S. (1998). Functional expression of a mammalian odorant receptor. Science, 279, 237–242.




