View sample emotion research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Fundamental concepts are often the hardest to define. Emotion seems to be one of these concepts. Most people’s first try at a definition is to say, “Emotion is what I feel when I see or think about something, the way I react.” It’s also something hard to control. We get carried away in emotional experience. The sight of a snake frightens us; the smell of a rose pleases us. We all agree that words like love, adore, cherish, hate, and abhor describe our experiences of things we see or imagine. We know what it is to be joyful, happy, anxious, and depressed, and we agree that these mental states can fundamentally change our well-being. When we think further about emotion, we also realize that it is more than mental experience. It is something biological that humans certainly have but machines do not. And emotion is something we feel also going on in other people: We have no trouble inferring that a baby who coos and smiles is happy, even though the baby does not tell us so verbally—although we may be less certain looking at an animal that can never speak to us.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
It seems that everyone knows instinctively what emotion is, yet among scholars the concept of emotion has been the subject of huge debate. Few agree on the proper taxonomy. Are there two fundamental emotions or four or six or many more? Do animals have emotions like people do? Can we have emotion without consciousness? Are emotions innate, or are they all learned? All of these questions have consumed theorists over the years, and a rich literature explores the issues.
It is not the aim of this discourse to resolve these many knotty problems. Rather, it is our purpose to present an organizational framework that will help understanding of the biological foundations of emotion. Considering the data of emotion to be threefold (Lang, 1985, 1994)—affective language, behavior, and physiology—our emphasis here is on the latter two sources. We start with the view that emotions are products of evolution and that their expression in action and physiology is determined in significant part by brain structures and circuits that we share with other species.
Although we cannot know whether animals experience emotion in the same waythat humans do, itis clear that the expression of emotion can be very similar across the mammalian phyla. When a rat is confronted with a predatory cat, the animal’s behavioral and physiological reactions are highly similar to those displayed, for example, by a human confronted with an intruder in the night. Members of both species freeze, and both show a change in heart rate and in breathing. Both release similar chemicals into their blood streams. In each case these events prepare the organism to attend to the potential threat and to ready the body for quick action. Furthermore, if animal and human escape the danger, both organisms will learn to be aroused and wary if cues related to that context should again be presented. These are patterns of behavior that have been carefully preserved in evolution because they are successful in promoting survival. Thus, much can be learned about the subject of this research paper, the biological basis of emotion, by studying the responses that commonly occur (and their determining neural circuitry) when humans and lower animals confront appetitive or aversive events.
A Working Definition of Emotion
For the purpose of scientific study, emotion is best defined not as a single reaction, but as a process: Emotion involves multiple responses, organized according to temporal and spatial parameters.Thus, events that are positive-appetitive or aversive-threatening engage attention. They prompt information gathering, and do so more than other less motivationally relevant stimuli. Motive cues also occasion metabolic arousal, anticipatory responses that are oriented towards the engaging event, and this mobilization of the organism can lead to some action. Human beings report emotional experience, often the same emotion (e.g., fear, joy), while the multiple—and very different—components of the process unfold.
Imagine that you are sitting on a park bench reading the newspaper, and you hear voices in the distance. You immediately stop reading the paper, turn your head to the source of the voices, and try to figure out who is approaching. You soon realize it is a group of school children, talking amiably with each other as they approach. You go back to reading the paper and feel little reaction as they pass by.
Now imagine that you hear voices again. You stop reading the paper, turn your head in the direction of the voices, and listen and watch intently. You see a group of teenaged and even older men. They all have studded leather jackets, shaven heads, and heavily tattooed arms. It is clear that they have been drinking, and they are loud and lewd, and two of them are carrying something you cannot quite identify. As they come closer you see the two are carrying baseball bats, and one of them, who seems to be their leader, is staring directly at you. As they get closer and closer you begin to feel more and more apprehensive—you break out in a cold sweat and your breathing deepens. Suddenly you pick up your paper and quickly cross the street, trying not to look back to make it appear as if you intended to cross the street at that moment any way. One of them shouts something that makes you pick up yourpace even more in order to get around the corner out of sight.
In the third scenario you are back on the park bench, and you hear voices once again. You stop reading the paper, turn your head to the voices, and listen and watch intently. Again you hear the voices of a group of young adults, laughing and talking. As they get closer you notice they are your old high school friends, to whom you once were very close. Suddenly you recognize one of your very best friends in high school, whom you have not seen in many years. As he approaches you put down your paper and run to him and give him a big hug.
Emotional processing can be compressed into fractions of a second or can be considerably extended in time. The longer scenarios described here were chosen to highlight emotion’s separate components. The sound of distant voices captured attention. This resulted in a cessation of what you were doing (reading the paper) and an active change in your behavior to allow you to take in information about the source of sound. You turned your head and listened and looked intently. Once you had stopped doing what you were doing and oriented to the voices, your brain began processing the input, automatically resolving its motivational relevance. Is the situation innocuous, positive, and potentially pleasant, or negative and threatening? As the various groups came closer, more information was available, and different behaviors resulted. Thus, attention was soon disengaged from the school children, and you continued reading. However, as evidence increased for either a happy encounter or a dangerous confrontation, motivation intensified. That is, the brain was more and more active, orchestrating a variety of changes in your body, initially in facilitating sensory acuity and attention, and then, more and more, to mobilize for action. These preparatory changes were physiologically widespread, involving cardiovascular and other autonomic responses, hormonal release into the blood stream, and a decrease and then a progressive increase in muscle tension. It is interesting to note that the two patterns of change—as you began anticipating a happy reunion or feared facing the dangerous-looking men—were in some ways similar (e.g., in initial sensory system response), and in other ways quite different (e.g., in hormonal biochemistry, and clearly in the final action). In this research paper we try to show that these two general reactions are mediated by overlapping brain circuits, connecting some unique and some common neural structures. That is, emotions are associated with two different but interdependent neural networks, activated either by appetitive or by aversive events.
Before leaving these examples it must also be noted that in many instances of emotional arousal the completing actions do not occur. The dangerous-looking men may change their direction, and you need not flee; when the anticipated friend gets closer, you realize that he is actually a stranger, and you return to your reading. More often, action is simply inhibited: If you run, the chase is on. Perhaps it is best to sit here quietly and wait for the danger to go away. Humans find many reasons to report states of affective arousal that are not followed by an action outcome. Indeed, much of emotional life occurs when cues mobilize us for approach or defense, but—for better or for worse—we do not act.
In this research paper we try to explicate what is special about emotional information processing in the brain. We propose that neural networks underlying emotion include direct connections to the brain’s primary motivational systems: appetitive and defensive. These neural circuits were laid down early in our evolutionary history, in primitive cortex, subcortex, and midbrain, to mediate behaviors basic to the survival of individuals and species. Unconditioned and conditioned appetitive and aversive stimuli activate these motivational circuits. They determine the general mobilization of the organism, the deployment of reflexive attentional, approach, and defensive behaviors, and mediate the formation of conditioned associations originally based on primary reinforcement.
Affective Valence, Arousal, and the Psychophysiology of Emotion
The words emotion and motivation are both derived from the Latin word for move: movere. In fact, the behavior of a simple organism such as a flatworm can be characterized almost entirely by two survival movements: direct approach to appetitive stimuli and withdrawal from aversive stimuli (see Schneirla, 1959). This modest motivational repertoire would not, however, implement the many subgoals of more complex beings, nor effectively cope with a richly perceived sensory environment. Chained instrumental acts, behavioral delay, and response inhibition have all evolved, greatly elaborating the simple bidirectional paths of goal-related behavior. Thus danger cues, for example, can prompt an array of coping behaviors—freezing, defensive displays, and fight as well as flight.
Motivated behavior in humans is more adaptive, creative, and less predictable than is that in less evolved species. Its most singularly human feature is the use of complex language—to communicate, to manipulate symbols in problem solving, and to label and catalog experiences of the world. Furthermore, as already noted, language is one of the major mediums through which we can know the emotional feelings of others. Indeed, for many psychologists and laypeople alike, understanding reported feelings is the cornerstone of emotion studies. Thus, despite our focus on the biological foundations of emotion, it will be useful to consider first how, in general, the self-evaluative language of emotion relates to the sensory system adjustments and autonomic and somatic responses that occur when humans confront emotionally arousing cues.
Feelings are often described as if they were actions. We say we were moved by a story we read or a play we enjoyed. It is not difficult to see in words such as love, adore, and cherish a common desire to approach some object or to think about a pleasant event. Words like hate, abhor, and detest all suggest a desire to avoid some object or thought. Indeed, the view that affects—subjective reports of emotion—might be organized by overarching motivational factors has been a common theoretical view at least since Wundt’s (1896) mental chemistry. Contemporary studies of natural language categories (Ortony, Clore, & Collins, 1988; Shaver, Schwartz, Kirson, & O’Connor, 1987) suggest that emotional knowledge is hierarchically organized and that the superordinate division is between positivity (pleasant states: love, joy) and negativity (unpleasant states: anger, sadness, fear). Using the semantic differential, Osgood and his associates (e.g., Osgood, Suci, & Tannenbaum, 1957) showed that emotional descriptors were primarily distributed along a bipolar dimension of affective valence—ranging from attraction and pleasure to aversion and displeasure. A dimension of activation—from calm to aroused—also accounted for substantial variance. Similar conclusions have been drawn by other investigators using factor analysis of verbal reports (e.g., Mehrabian & Russell, 1974; Tellegen, 1985) as well as facial expressions (Schlosberg, 1952).
Emotional language appears highly differentiated and subtle; nevertheless, researchers concur that a single dominant factor accounts for a very considerable proportion of the variance in evaluative reports: Feelings are either pleasant or unpleasant. We either want more or less of an affect-arousing stimulus. Indeed, we may want very much more of a pleasant stimulus—or very much less of one that is unpleasant. Incentives can be scaled as more or less intense, and both pleasant and unpleasant stimulation can lead to different levels of arousal or activation. In the analysis of evaluative language, affective valence is primary, followed by affective arousal. No other factors have ever approached the generality and significance of these two simple variables.
We should not be too surprised, perhaps, to learn that affective valence and arousal find a parallel in motivational theories based on behavioral research with animals. For example, Konorski (1967) founded a motivational typology of unconditioned reflexes, keyed to the reflex’s survival role. Exteroceptive reflexes were either preservative (e.g., ingestion, copulation, nurture of progeny) or protective (e.g., withdrawal from or rejection of noxious agents). He further suggested that affective states were consistent with this biphasic typology: Preservative emotions include such affects as sexual passion, joy, and nurturance; fear and anger are protective affects. Dickinson and Dearing (1979) developed Konorski’s dichotomy into a theory of two opponent motivational systems, aversive and attractive, each activated by a different but equally wide range of unconditioned stimuli that determine perceptual-motor patterns and the course of learning. In this general view, affective valence is determined by the dominant motive system: the appetitive system (preservative-attractive) prompts positive affect; the defense system (protective-aversive) is the source of negative affect. Affective arousal reflects motivational mobilization, appetitive or defensive, modulated by changes in survival need or in the probability of nociception or appetitive consummation.
Attention and Emotion
As our opening scenarios suggested, emotion begins when attention is captured by a provocative stimulus. Following Pavlov (1927), attention begins with reflexive orienting of the activated sense receptors. Sokolov (1963) later developed a cortical model of this orienting reflex, suggesting that the brain held a template of the current sensory environment. He proposed that the reflex was evoked whenever a change in the perceptual field occurred (a pattern modification that did not match the template). Sokolov also described a second sensory reaction that had a protective function—the defense reflex— evoked when stimuli were intense, approaching the threshold of pain. These concepts of orienting and defense have proved useful even though the underlying phenomena are more complex than was at first appreciated.
Orienting is not, of course, a single reflex arc. Rather, this initial reaction to stimulation involves many subreflexes. In reacting to visual input, for example, there is a muscular aiming of the eye and pupillary dilation, coincident with a general inhibition of the gross somatic muscle activity, with accompanying vascular and cardiac changes. As a stimulus is conceptually resolved, the reflex pattern changes. On the one hand, if input has no motivational relevance, the initial attentional response soon habituates. Cues of appetite or aversion, on the other hand, lead to more sustained, singular patterns of attentional processing. For example, an animal (reptile or mammal) orienting to a distant predator or other danger cue shows a profound deceleration in heart rate—fear bradycardia—not found in response to other events (Campbell, Wood, & McBride, 1997). This heart rate change is accompanied by freezing—a statue-like arresting of movement—and a general increase in sensitivity of all the sense receptors. If the predator approaches (shows stalking behavior), there is a increase in systemic activation that culminates in defensive action.
Attentional changes that are similar to the previous—in autonomic and somatic reflexes—occur when humans process affectively engaging stimuli. Furthermore, we respond reflexively even if the stimuli are not actual events, but media representations. Stories, pictures, and films all prompt patterns of bodily change that vary systematically with the reported affective valence (pleasant or unpleasant) and arousal (intensity) of the evoked reactions.
Valence and Arousal
In recent years the psychophysiology of emotional perception has been systematically studied by researchers using a set of standard photographic picture stimuli calibrated for affective response. There are currently over 700 pictures in the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1998), and each picture is rated for experienced pleasure and arousal by a large normative subject sample. A representative group of IAPS pictures is presented in Figure 15.1 (Bradley, 2000) located in a Cartesian space formed by independent dimensions of rated pleasure and arousal. The distribution of these picture stimuli has an overall boomerang shape. Its two arms extend from a common calm, nonaffective base to either the high-arousal pleasant or high-arousal unpleasant quadrant. This distribution is curiously suggestive of an underlying two-system motivational structure: That is, affective pictures appear to be organized around two vectors—one that reflects a parameter of increasing appetitive motivation (higher self-ratings of arousal with increasing pleasantness) and a mirror image of increasing motivation for defense (higher arousal with ratings of increasing unpleasantness). The slopes of these vectors recall Neal Miller’s (1959) description of approach and avoidance gradients, representing response strength over distance, from a remote site to the place of reward or punishment. In Miller’s behavioral research, animals showed a precipitous increase in avoidance motivation with greater proximity to a site of punishment; the gradient of approach to a reward was significantly less steep. Similarly, for the picture distribution, the slope of the pleasant (appetitive input) vector is less steep than that of the unpleasant (aversive input) vector, consistent with the view that defense motivation may increment more rapidly with increases in the incentive value of stimuli. Despite considerable effort to fill gaps in the affective picture space (e.g., in the unpleasant–low arousal quadrant) with a wider range of images, this vector pattern has remained stable over repeated studies. Similar distributions have also been obtained for collections of acoustic stimuli (International Affective Digitized Sounds, or IADS; Bradley, Cuthbert, & Lang, 1998) as well as verbal materials (Affective Norms for English Words, or ANEW; Bradley, Lang, & Cuthbert, 1998).
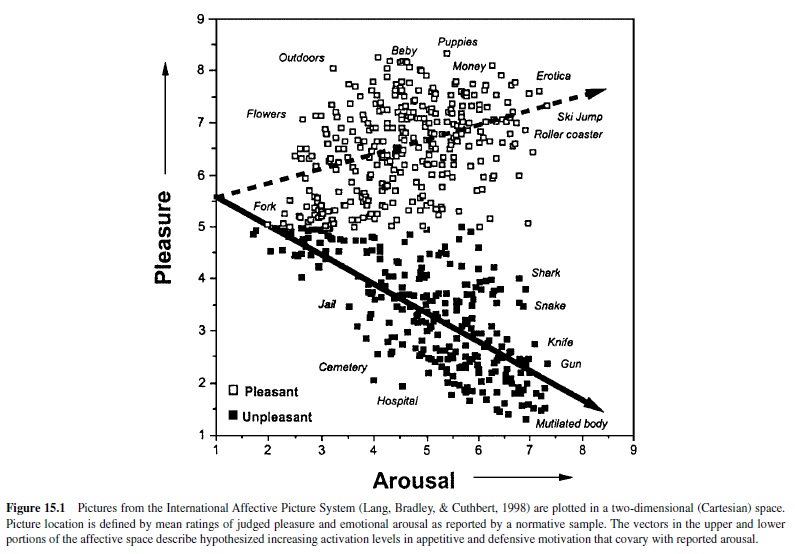
The Psychophysiology of Picture Perception
Studies of IAPS picture stimuli have uncovered highly reliable patterns of physiological and behavioral responses that vary systematically with experienced emotion (see Figure 15.2; Bradley et al., 2001; Greenwald, Bradley, Cuthbert, & Lang, 1998; Greenwald, Cook, & Lang, 1989). Thus, when affective valence ratings are ranked by picture from the most to the least pleasant image, facial muscle activity for each subject during picture viewing shows a strong monotonic relationship with level of affective valence: Corrugator (frown) muscle action increases linearly as pictures are rated more unpleasant; conversely, zygomatic (smile) muscle activity increases with judged pleasantness. Heart rate is also responsive to differences in affective valence: Unpleasant pictures generally prompt marked deceleration during viewing (recalling the fear bradycardia seen in animals), less than is seen when subjects view pleasant pictures.
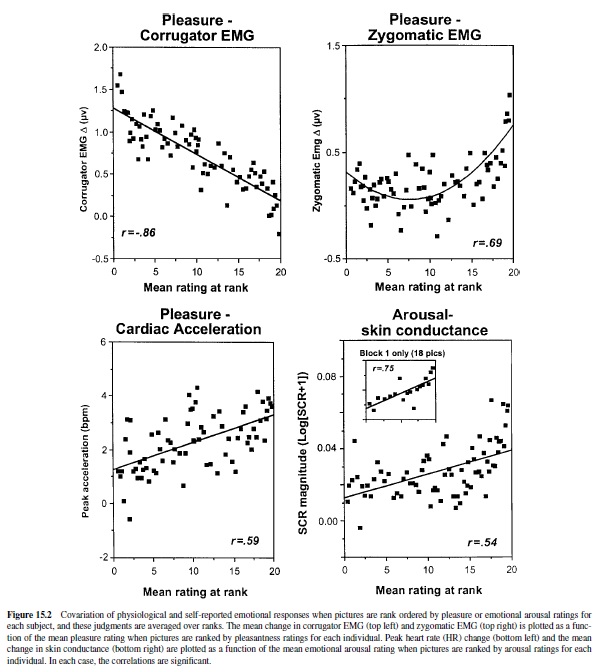
Other physiological responses vary with changes in rated emotional arousal, rather than affective valence. Skin conductance—a good general index of autonomic activation— increments monotonically with increases in rated arousal, regardless of picture valence. Electroencephalographic (EEG) measurement shows a distinct, voltage-positive cortical response, evoked directly by the picture stimuli, that is also positively correlated with stimulus arousal (i.e., it is similarly enhanced for both pleasant and unpleasant arousing pictures; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 1998). These measures appear to index the intensity or activation level of the current motivational state, but they are silent about motivational direction (i.e., appetitive or defensive).
Behaviors elicited in the context of emotional picture perception also covary with motivational parameters. When people are first exposed to a new picture, reaction time responses to probes are significantly slower for emotionally arousing than for affectively calm pictures (Bradley, Greenwald, Petry, & Lang, 1992). These data suggest that new activating images may require more attentional resources at encoding. The amount of time that observers choose to view different pictures also covaries with arousal. When normal subjects are placed in a free-viewing context, arousing unpleasant pictures are viewed as long as arousing pleasant pictures, and both are viewed for a longer duration than are unarousing pictures. As might be inferred from the popularity of slasher movies or from the habitual slowing of traffic at roadside accidents, normal subjects allocate more processing time to arousing, intense images, regardless of affective valence. This relationship does not persist if pictures evoke very high levels of distress: When individuals with phobias view pictures specific to their fears, viewing time is dramatically reduced (see Hamm, Cuthbert, Globisch, & Vaitl, 1997). They also show heart rate acceleration (rather than deceleration), consistent with a precipitous increase in defense motivation and mobilization for active escape.
As the phobia data imply, relationships between specific measures can vary widely for individuals, and to some extent between particular groups. Gender effects are clear. For example, pleasantness ratings covary more closely with facial muscle activity in females than in males; on the other hand, skin conductance changes are more closely correlated with arousal ratings in males than in females (Lang, Greenwald, Bradley, & Hamm, 1993). Overall, however, motivational variables of affective valence and arousal predominate in organizing the picture perception data.
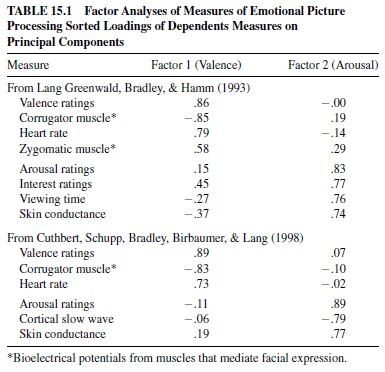
The results of factor analyses of self-report, physiological, and behavioral measures of affect are presented in Table 15.1. The data were obtained from large groups of young, healthy participants. The obtained two-factor solution is clearly very strong: Pleasantness ratings, heart rate, and facial muscles load on a first affective valence factor; arousal and interest ratings, viewing time, skin conductance, and cortical EEG load on a second affective arousal factor. The cross-loadings for all measures are very low. The data are consistent with the view that reported affective experience is determined in significant part by the individual’s motivational state. That is, negative affective valence (unpleasant feelings) is associated with activation of the defense system; positive valence (pleasant feelings) is associated with activation of the appetitive system. Reports of arousal are associated with both states, reflecting an increase in incentive strength and organismic mobilization. The motivational states elicited by these affective cues (and the somatic, cortical, and autonomic substrates of their perception) appear to be fundamentally similar to those occurring when other complex animals stop, look, and listen, sifting through the environmental buzz for cues of danger, social meaning, or incentives to appetite.
Neural Substrates of Affect: Attention, Action, and the Role of the Amygdala
Humans and animals show great similarity in behavioral and physiological response patterns to appetitive and defensive cues. Furthermore, the reports of affect that only humans provide seem to covary systematically with these shared motivational reactions and hence must involve homologous neural pathways. Thus, it is pertinent to ask, What can the neurophysiological study of animals tell us about human emotion? How are the autonomic and somatic reflex reactions in emotion—the signatures of feeling and affect—determined in the brain?
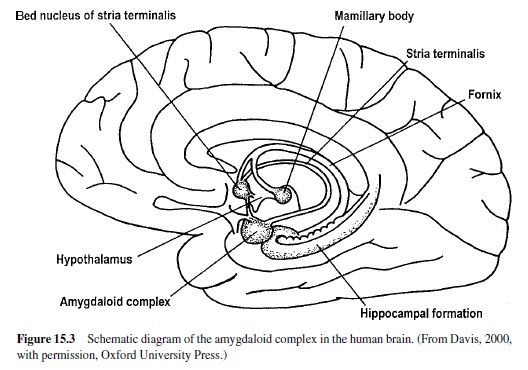
Much recent research has shown that a brain area called the amygdala is a crucial nodal point in a neural network that mediates motivated attention and preparation for action (i.e., what we have called emotional processing). In man, the amygdala lies deep in the brain’s temporal lobe (Figure 15.3). Although this small, almond-shaped structure is made up of several different nuclei, it is popularly imagined as a single unit. Recent research suggests, however, that individual nuclei play very different functional roles. The basolateral amygdala is of particular significance. It projects to several target areas (other amygdala nuclei and nuclei elsewhere in the brain), forming a broad neural network that serves a variety of specialized functions (Figure 15.4). The network is activated by information that comes to the basolateral nucleus (either directly or via the amygdala’s lateral nucleus) from the thalamus, hippocampus, and cerebral cortex (for a highly comprehensive review in rats, monkeys, and cats, see McDonald, 1998). The basolateral nucleus then projects to several brain areas, mediating memory and an array of reflex responses that are the basic stuff of emotional processing.
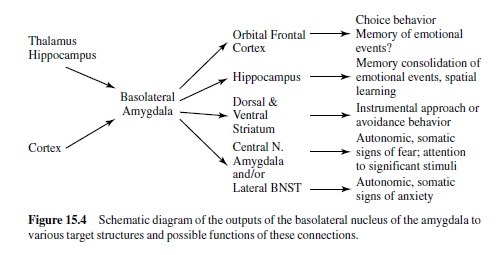
The Anatomy of Emotion: Projections from the Amygdala’s Central Nucleus and the Extended Amygdala
We are now ready to consider more specifically how this emotion network, activated when an animal is under threat or stress, mediates the reflex responses of defense—responses that we often see also in humans when they report unpleasant affect. As noted in Figure 15.4, the projection from the basolateral nucleus to the central nucleus of the amygdala provides a path to target areas that mediate many of the autonomic and somatic changes found in fear and anxiety. A nearby structure, the bed nucleus of the stria terminalis (BNST; sometimes called part of the extended amygdala) has similar projections. Figure 15.5 is a schematic diagram of outputs from the central nucleus (CeA) and the BNST. These nuclei project to specific hypothalamic and brain-stem target areas that mediate most of the visceral and striate muscle events that index emotional processing.
Autonomic and Hormonal Measures of Fear
The amygdala’s central nucleus, as well as the BNST, sends prominent projections to the lateral hypothalamus—a key We are now ready to consider more specifically how this center activating the sympathetic branch of the autonomic emotion network, activated when an animal is under threat or nervous system in emotion (LeDoux, Iwata, Cicchetti, & stress, mediates the reflex responses of defense—responses Reis, 1988). In addition, direct projections from the lateral that we often see also in humans when they report unpleas- extended amygdala go to the dorsal motor nucleus of the ant affect. As noted in Figure 15.4, the projection from the vagus, the nucleus of the solitary tract, and the ventrolateral basolateral nucleus to the central nucleus of the amygdala medulla. These brain-stem nuclei are known to regulate heart provides a path to target areas that mediate many of the auto- rate and blood pressure (Schwaber, Kapp, Higgins, & Rapp, nomic and somatic changes found in fear and anxiety. A 1982) and may thus modulate cardiovascular responses in nearby structure, the bed nucleus of the stria terminalis emotion. Projections to the parabrachial nucleus are likely to (BNST; sometimes called part of the extended amygdala) has be involved in emotion’s respiratory changes (with addisimilar projections. Figure 15.5 is a schematic diagram of tional effects, perhaps indirect, on the cardiovascular sysoutputs from the central nucleus (CeA) and the BNST. These tem), as electrical stimulation and lesions of this nucleus alter nuclei project to specific hypothalamic and brain-stem target breathing patterns. Finally, indirect projections from the areas that mediate most of the visceral and striate muscle amygdala’s central nucleus to the paraventricular nucleus neuroendocrine responses that are particularly prominent when emotional stimuli are sustained.
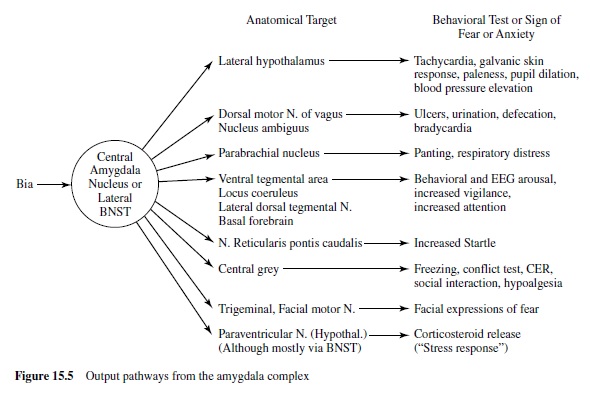
Attention, Vigilance, and Conditioned Fear
During emotional stimulation, projections from the central nucleus or BNST to the ventral tegmental area appear to mediate increases in dopamine metabolites in the prefrontal cortex (Goldstein, Rasmusson, Bunney, & Roth, 1996). Cells in the locus coeruleus are also activated, perhaps mediated by projections to its dendritic field or indirectly via projections to the paragigantocellularis nucleus (Aston-Jones, Rajkowski, Kubiak, Valentino, & Shipley, 1996; Redmond, 1977). Furthermore, there are direct projections to the lateral dorsal tegmental nucleus and parabrachial nuclei. These latter nuclei have cholinergic neurons that project to the thalamus and could mediate increased synaptic transmission of its sensory relay neurons. The sensory thalamus is, of course, a primary processor of environmental input. Thus, this sequence of projections, by augmenting cholinergic activation and facilitating thalamic transmission, may contribute to the increased vigilance and superior signal detection found in the attentional phase of emotional processing.
As already noted, most animals and reptiles react to the appearance of a predator in the distance with immobility and sensory orientation toward the threat. This behavioral pattern is accompanied by a profound decrease in heart rate, referred to as fear bradycardia (Campbell et al., 1997). A similar change in heart rate is associated with increased attention in humans (Graham & Clifton, 1966); furthermore, a greater deceleration is generally found in response to stimuli judged to be unpleasant (Bradley, 2000; Lang et al., 1993). Several lines of research suggest that this cardiac response can be mediated by the central nucleus of the amygdala. During Pavlovian aversive conditioning in rabbits, one sees a rapid development of conditioned bradycardia. Pascoe and Kapp (1985) found a high correlation (.71) between the firing frequency of a population of individual neurons in the amygdala’s central nucleus and the degree to which heart rate decelerated in response to the conditioned stimulus. Furthermore, as emphasized by Kapp and colleagues (Kapp, Whalen, Supple, & Pascoe, 1992), the central nucleus of the amygdala also has the potential for indirect but widespread effects on cortical activity— mediated by projections to cholinergic neurons that in turn project to the cortex. This is a probable path, prompting the low-voltage, fast-EEG activity that readies the cortex for sensory information processing. These changes in the EEG waveform are acquired during Pavlovian aversive conditioning at the same rate as conditioned bradycardia.
Changes in Motor Behavior
Emotional processing involves both attentional engagement and behaviors preparatory to, or part of, motivated action. Emotion’s attentional phase is characterized by immobility. Research by many investigators has implicated projections from the central nucleus of the amygdala to the ventral periaqueductal gray in the freezing response of the rat, whereas projections to the dorsal periaqueductal gray appear to mediate active fight-flight responses. The latter, defensive actions must often be engaged quickly, and both norepinephrine and serotonin facilitate excitation of motor neurons (McCall & Aghajanian, 1979; White & Neuman, 1980). This enhanced motor performance in emotion could be mediated by the lateral extended amygdala’s activation of norepinephrine release in the locus coeruleus, or via its projections to serotonin containing raphe neurons.
Experimental Elicitation of Emotional Responses: Amygdala Stimulation
Electrical and Chemical Stimulation
Electrical stimulation of the amygdala (or abnormal electrical activation via temporal lobe seizures) can produce a complex pattern of behavioral and autonomic changes that, taken together, resemble a state of heightened emotion. This effect is probably attributable to amygdala outputs that simultaneously activate the many target areas seen in Figure 15.4. In humans the most common emotional experience reported after electrical stimulation of the amygdala is one of fear or apprehension, accompanied by autonomic reactions (Chapman et al., 1954; Gloor, Olivier, & Quesney, 1981). In animals, electrical or chemical stimulation of the amygdala produces prominent cardiovascular effects that depend on the species, site of stimulation, and state of the animal. Persistent stimulation can also produce gastric ulceration, increases in blood levels of cortisol and epinephrine, and sustained changes in respiration.
Studies in several species indicate that electrical stimulation of the amygdala’s central nucleus increases processes associated with attention. Thus, stimulation of the same sites in the central nucleus can produce both bradycardia (Kapp, Wilson, Pascoe, Supple, & Whalen, 1990) and low-voltage, fast-EEG activity in rabbits (Kapp, Supple, & Whalen, 1994) and in rats (Dringenberg & Vanderwolf, 1996). Furthermore, depending on the state of sleep, electrical stimulation of the amygdala in some species activates cholinergic cells that are involved in arousal-like effects. Overall, the orienting reflex has been described as the most common response elicited by electrical stimulation of the amygdala (Applegate, Kapp, Underwood, & McNall, 1983; Ursin & Kaada, 1960).
In many species, electrical or chemical stimulation of the amygdala produces a palpable cessation of ongoing behavior. This immobility facilitates sensory orienting and is a critical attentional component of emotion. In rats, the response is measured by freezing, or by the cessation of operant bar pressing. Electrical stimulation of the amygdala also activates facial motoneurons, eliciting jaw movements, and may be the pathway mediating the facial expressions that characterize emotional states. In fact, amygdala stimulation appears to have very broad effects on the motor system, including the modulation brain-stem reflexes, such as the massenteric, baroreceptor nictitating membrane, eye-blink, and startle reflexes.
In summary, the stimulation data show that the amygdala projects to a variety of target areas, each of which is critical for a different aspect of emotional processing. Moreover, it must be assumed that these connections are already formed in an adult organism, as electrical and chemical stimulation produce these effects in the absence of explicit prior learning. This suggests that a significant part of the behavioral pattern evoked by emotional stimuli may have been hardwired during evolution. Thus, it is only necessary that an initially neutral stimulus activate the amygdala—in association, for example, with an aversive event—for this formerly neutral cue then to produce the full constellation of emotional effects. The complex patterns of behavioral changes seen during emotional processing—the modulation of afferent and efferent systems—are produced by virtue of the innate connections between the amygdala and the implementing brain target sites.
Effects of Amygdala Lesions and Drug Infusion on the Emotion Circuit
Attention to Motivational Cues
If the emotion circuit that we have described is truly a unique, hardwired set of connections, then destruction of the amygdala can be expected to disrupt or eliminate emotion’s sensory processing and motor output. Various investigators have provided data in support of this hypothesis, showing, for example, that lesions of the amygdala block attentional responses to stimuli paired with food (cf. Gallagher, Graham, & Holland, 1990). In general, rats with these lesions fail to benefit from procedures that normally facilitate attention to stimuli conditioned to primary reinforcers (Holland & Gallagher, 1993a, 1993b).
As already noted, however, the amygdala is a complex structure in which the different nuclei play different roles. Thus, lesions of the central nucleus and the basolateral nucleus each have unique effects on the phenomenon known as taste-potentiated odor aversion learning. In this test, which requires processing information in two sensory modalities, rats develop aversions to a novel odor paired with illness only when the odor is presented in compound with a distinctive gustatory stimulus. Electrolytic (Bermudez-Rattoni, Grijalva, Kiefer, & Garcia, 1986) or chemical lesions (Hatfield, Graham, & Gallagher, 1992) of the basolateral but not the central nucleus blocked taste-potentiated odor aversion learning even though they had no effect on taste aversion learning itself. Local infusion of N-methyl-D-aspartate (NMDA) antagonists into the basolateral nucleus also blocked the acquisition but not the expression of taste-potentiated odor aversion, but again had no effect on taste aversion learning (Hatfield & Gallagher, 1995). Based on these and other data, Hatfield, Han, Conley, Gallagher, and Holland (1996) suggested that the amygdala’s central nucleus “regulates attentional processing of cues during associative conditioning” (p. 5265), whereas the basolateral nuclei are critically involved in “associative learning processes that give conditioned stimuli access to the motivation value of their associated unconditioned stimuli” (p. 5264). Thus, the different amygdalar nuclei work in concert, orchestrating the components of emotional learning that determine the motivational significance of input and act to maintain the relevant cue as a focus of attention.
Conditioned Emotional States
One of the most widely studied examples of emotion in animals is conditioned fear. Fear is here defined by the pattern of behavior evoked by stimuli previously paired with an aversive event (e.g., electric shock). A large literature indicates that lesions of the amygdala block many measures used to assess conditioned and unconditioned fear (cf. Davis, 2000). These include autonomic measures such as changes in heart rate, blood pressure, ulcers, respiration, and secretion of adrenocorticotropic hormone (ACTH), or corticosteroids into the blood or release of dopamine, norepinephrine, or serotonin in certain brain areas.They include behavioral measures such as freezing, fear-potentiated startle and vocalization, and several operant measures (operant conflict test, conditioned emotional response, avoidance of a electrified shock probe). Furthermore, lesions of the amygdala cause a general taming effect in many species (Goddard, 1964), perhaps analogous to the increase in trust found in humans following surgical amygdala lesions (Adolphs, Tranel, & Damasio, 1998).
Effects of Local Infusion of Drugs
Fear levels vary depending on many circumstances, and they can be mild or extremely intense. It is not surprising, therefore, that the intensity of fear is determined by the interplay of a variety of chemicals in the brain, many of which act directly in the amygdala. Local infusion of compounds that inhibit neuronal activity in the amygdala by acting through gammaaminobutyric acid (GABA), a major inhibitory neurotransmitter in the brain, reduce fear. These include GABA itself, GABA agonists, and benzodiazepines, such as valium, which increase GABA transmission. Drugs that decrease excitatory transmission in the amygdala, such as glutamate antagonists, have similar actions. Neurotransmitters that modulate glutamate excitation or GABA inhibition in the amygdala, such as norepinephrine, dopamine and serotonin, and peptides such as corticotropin-releasing hormone (CRH), cholecystokinin (CCK), neuropeptide Y, vasopressin, thyroid-releasing hormone (TRH), and opiates also are important.
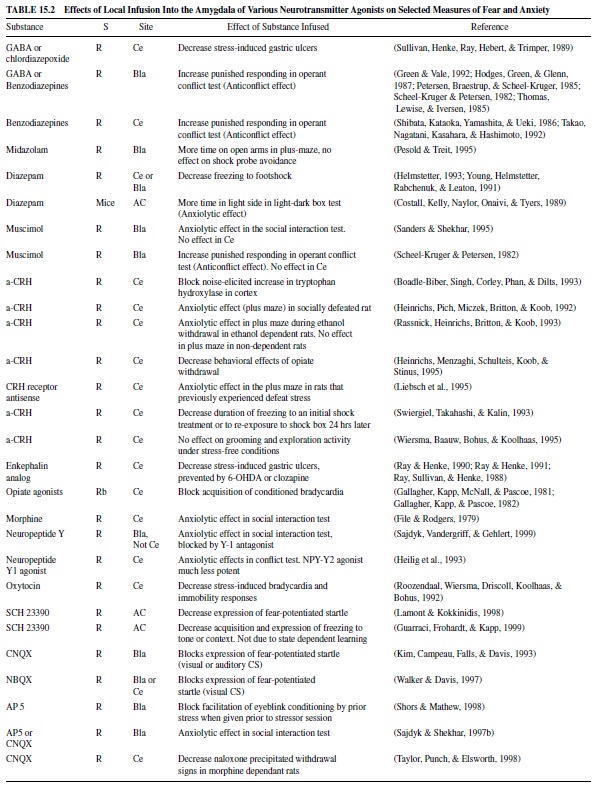
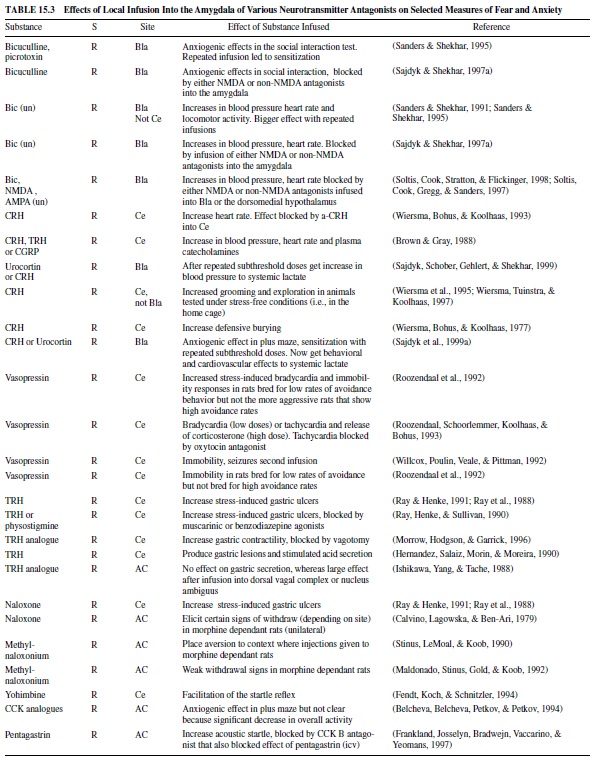
Table 15.2 gives selected examples of some of these studies in which local infusion of various compounds reduces measures of fear. These include GABA or GABA agonists, benzodiazepines, CRH antagonists, opiate agonists, neuropeptide Y, dopamine antagonists, and glutamate antagonists. Table 15.3 gives selected examples of studies in which local infusion of various compounds increase measures of fear. These include GABA antagonists, CRH or CRH analogues, vasopressin, TRH, opiate antagonists, CCK, and CCK analogues. More extensive tables can be found in Davis (2000).
The Role of the Central Nucleus of the Amygdala in Appetitive and Approach Behavior
In a systematic and comprehensive series of experiments, Barry Everitt, Trevor Robbins, and colleagues (cf. Everitt, Cardinal, Hall, Parkinson, & Robbins, 2000) provided a new and very important theory about the role of the amygdala in appetitive conditioning. These studies emphasize the projection from the central nucleus of the amygdala to dopamine neurons in the ventral tegmental area that project to the ventral striatum (nucleus accumbens), a region of the brain that is important for approach behavior when Pavlovian conditioning is measured in appetitive situations.
In one series of experiments using the phenomenon of autoshaping, a light (CS+ ) is presented followed by delivery of food in a different location, regardless of what the rat is doing. Another stimulus (CS– ) is also presented but never followed by food. Under these conditions, rats learn to approach the CS+ light before going to the food hopper to retrieve the food. Bilateral lesions of the central nucleus of the amygdala, but not lesions of the basolateral nucleus of the amygdala (Bla), markedly disrupted this approach behavior (Parkinson, Robbins, & Everitt, 2000). The importance of the central nucleus in this form of approach behavior seems to be mediated by its projection to dopamine-containing neurons in the ventral tegmental area and the consequent release of dopamine in the nucleus accumbens because depletion of dopamine in the nucleus accumbens core eliminates the acquisition of autoshaping (Parkinson et al., 1998). In contrast, the approach behavior itself seems to be mediated by the anterior cingulate cortex (Bussey, Everitt, & Robbins, 1997) via projections to the nucleus accumbens core (Parkinson, Willoughby, Robbins, & Everitt, 2000).
In another series of experiments, rats were first trained to associate an auditory CS with delivery of food. In a second phase these rats were trained to press a lever to obtain food. In the test phase, presentation of the auditory CS led to an increase in lever pressing for food. Lesions of the central nucleus of the amygdala, but not the Bla, reduced this facilitatory effect (Everitt et al., 2000). These authors speculate that this is mediated by projections from the central nucleus to the mesolimbic dopamine system.
On the other hand, just the opposite effects have been reported when a CS previously paired with food increases the actual consumption of food (Gallagher, 2000). In this case, projections from the posterior division of the basolateral amygdala to the hypothalamus are thought to be involved.
The Basolateral Nucleus Projects Beyond the Amygdala and the Extended Amygdala
It is clear that connections between the amygdala’s central nucleus and the BNST are critically involved in many of the autonomic and motor responses seen in emotion. As described earlier, projections from the central nucleus to the mesolimbic dopamine system are involved in modulating certain types of approach behavior, as well as the invigorating effects of a stimulus previously paired with reward on instrumental behavior. However, it is also the case that there are direct connections between the basolateral nucleus and other target areas in the brain. These latter targets are also important mediators of emotional behaviors (see Figure 15.4).
The Ventral Striatum Pathway: Secondary Reinforcement
The Bla projects directly to the nucleus accumbens in the ventral striatum (McDonald, 1991), in close apposition to dopamine terminals of A10 cell bodies in the ventral tegmental area (cf. Everitt & Robbins, 1992). Morgenson and colleagues suggested that the ventral striatum was the site where affective processes in the limbic forebrain gained access to subcortical elements of the motor system that resulted in appetitive actions (cf. Morgenson, 1987).
Projections from the Bla to the nucleus accumbens are critically involved in secondary reinforcement. In this paradigm, a light is paired with food. Animals are then presented with two levers. Pressing one lever turns on the light, whereas pressing the other one does not. Normal rats press the lever that turns on the light much more often than they press the other lever. Hence, the light serves to reinforce new behavior via its prior association with food and is called a secondary reinforcer. Rats with lesions of the Bla fail to learn this discrimination, whereas rats with lesions of the CeA do (Burns, Robbins, & Everitt, 1993; Cador, Robbins, & Everitt, 1989). Connections between the Bla and the ventral striatum also are involved in conditioned place preference (Everitt, Morris, O’Brien, & Robbins, 1991).
However, the central nucleus of the amygdala also has an important modulatory role on instrumental behavior in these secondary reinforcement paradigms. Drugs that release dopamine (e.g., amphetamine) increase the rate of bar pressing for a light previously paired with food. These facilitative effects also occur after local infusion of amphetamine into the nucleus accumbens (Taylor & Robbins, 1984) and are blocked by local depletion of dopamine in this area via 6-hydroxydopamine (6-OHDA; Taylor & Robbins, 1986). However, 6-OHDA did not block the expression of conditioned reinforcement itself, consistent with the idea that the reinforcement signal comes from some other brain area, such as the Bla, that projects to the nucleus accumbens. These results suggest that two relatively independent processes operate during conditioned reinforcement. First, information from the amygdala concerning the CS-US association is sent to the nucleus accumbens to control instrumental behavior as a conditioned reinforcer. Second, dopamine in the nucleus accumbens modulates this instrumental behavior. The central nucleus of the amygdala, via its projections to the mesolimbic dopamine system, seems to be critical for this invigorating or arousing effect of dopamine. Thus, lesions of the central nucleus block the increase in bar pressing normally produced by infusion of amphetamine into the nucleus accumbens (Robledo, Robbins, & Everitt, 1996), probably by preventing dopamine in the nucleus accumbens shell (Everitt et al., 2000).
The Dorsal Striatum Pathway
As emphasized by McGaugh, Packard, and others, the amygdala modulates memory in a variety of tasks such as inhibitory avoidance and motor or spatial learning (Cahill & McGaugh, 1998; McGaugh et al., 1993; McGaugh, IntroiniCollison, Cahill, Kim, & Liang, 1992; Packard, Cahill, & McGaugh, 1994; Packard & Teather, 1998). For example, posttraining intracaudate injections of amphetamine enhanced memory in a visible platform water maze task but had no effect in the spatially guided hidden-platform task (Packard et al., 1994; Packard & Teather, 1998). Conversely, posttraining intrahippocampal infusion of amphetamine enhanced memory in the hidden-platform water-maze task but not in the visible-platform task. However, posttraining intra-amygdala injections of amphetamine enhanced memory in both water-maze tasks (Packard et al., 1994; Packard & Teather, 1998). These findings indicate that the amygdala exerts a modulatory influence on both the hippocampal and caudate-putamen memory systems. Indeed, more recent brain imaging studies in humans show correlations between memory recall of emotional stories and blood flow in the amygdala (Cahill, 2000).
Perhaps similarly, lesions of the central nucleus block freezing but not escape to a tone previously paired with shock, whereas lesions of the basal nucleus of the basolateral complex have just the opposite effect (Amorapanth, LeDoux, & Nader, 2000). However, lesions of the lateral nucleus, which receive sensory information required by both measures, block both freezing and escape. Lesions of the Bla, but not the CeA, also block avoidance of a bar associated with shock (Killcross, Robbins, & Everitt, 1997). Thus basolateral outputs to the dorsal or the ventral striatum may mediate escape or avoidance behavior, given the importance of the striatum in several measures of escape or avoidance learning.
Projections to the Cortex
Research with primates has shown that the basal nucleus of the amygdala projects to several areas in the inferior temporal cortex, continuing into prestriate and striate areas of the occipital lobe (Amaral & Price, 1984; Iwai & Yukie, 1987). Furthermore, the lateral nucleus of the amygdala gets input from an adjacent site in the visual system, which in turn receives hierarchical projections from the several nuclei along the ventral visual stream, extending to the retinal mapping area of the calcarine fissure. These projections could potentially close the loop with the visual system (Amaral, Price, Pitkanen, & Carmichael, 1992), representing an amygdala feedback circuit that may be significant for the sustained perceptual evaluation seen in the early stages of emotional processing.
Following Pavlovian conditioning, presentation of a conditioned stimulus appears to elicit some neural representation of the unconditioned stimulus (US) with which it was paired. In the family cat, for example, the sound of an electric can opener or of a refrigerator door opening may elicit a neural representation of food. This representation prompts predigestive responses and leads the cat to come into the kitchen in expectation of dinner. Based on a procedure called US devaluation, several studies suggest that the basolateral amygdala—perhaps via connections with cortical areas such as the perirhinal cortex (cf. Gewirtz & Davis, 1998)—is critical for retaining these US representations (e.g., Hatfield et al., 1996). Second-order conditioning also depends on a US representation elicited by a conditioned stimulus. Again, lesions of the Bla, but not the central nucleus, block second-order conditioning (Everitt, Cador, & Robbins, 1989; Everitt et al., 1991; Hatfield et al., 1996). This same effect occurs with local infusions of NMDA antagonists into the basolateral nucleus of the amygdala (Gewirtz & Davis, 1997).
Converging evidence also now suggests that the connection between the basolateral nucleus and the prefrontal cortex is critically involved in the way in which a representation of an unconditioned stimulus (e.g., very good, pretty good, very bad, pretty bad) guides approach or avoidance behavior. Analogous to the animal data, patients with late- or earlyonset lesions of the orbital regions of the prefrontal cortex frequently ignore important information that could usefully guide their actions and decision making (S. W. Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Bechara, Damasio, Tranel, & Damasio, 1997; Damasio, 1994). For example, on a gambling task the patients chose high, immediate reward associated with long-term loss rather than low reward associated with positive long-term gains. Clinically, they are reported to have a severe deficit in social skills, to fail to anticipate future consequences, and to make poor life decisions.
Studies using single-unit recording techniques in rats indicate that cells in both the Bla and the orbitofrontal cortex fire differentially to an odor, depending on whether the odor predicts a positive (e.g., sucrose) or negative (e.g., quinine) US. These differential responses emerge before the development of consistent approach or avoidance behavior elicited by that odor (Schoenbaum, Chiba, & Gallagher, 1998). Many cells in the Bla reverse their firing patterns during reversal training (i.e., the cue that used to predict sucrose now predicts quinine and vice versa; Schoenbaum, Chiba, & Gallagher, 1999), although this has not always been observed (e.g., Sanghera, Rolls, & Roper-Hall, 1979). In contrast, many fewer cells in the orbitofrontal cortex showed selectivity before the behavioral criterion was reached, and many fewer reversed their selectivity during reversal training (Schoenbaum et al., 1999). These investigators suggest that cells in the Bla encode the associative significance of cues, whereas cells in the orbitofrontal cortex are active when that information, relayed from the basolateral nucleus, is required to guide motivated choice behavior, presumably via both the motor cortex and the dorsal striatum.
Taken together, these data suggest that the connection between the basolateral complex and the frontal cortex could determine how an expected US is represented in memory, and thus play an important role in guiding motivated behavior and determining the choices that animals make. The effect may depend, however, on direct communication between an amygdala (right or left side) and the adjacent frontal cortex. Thus, when rhesus monkeys had amygdala lesions on one side of the brain and lesions of the frontal cortex on the other side, the degree of unconditioned stimulus devaluation was decreased (Baxter, Parker, Lindner, Izquierdo, & Murray, 2000). That is, lesioned monkeys continued to approach objects signalling a food on which they had recently been satiated (whereas control monkeys consistently chose objects not associated with satiated food).
Studies of the Amygdala in Humans
Neurological Disorders
Clinical studies of neurological patients have shown that some aspects of emotional behavior, particularly the perception of fear, may depend on an intact amygdala. Thus, removal of the amygdala (e.g., in the context of surgery for epilepsy) has been associated both with impairment of emotional face recognition, and with misinterpretation of another’s gaze angle (Broks et al., 1998; Calder et al., 1996; A. W. Young et al., 1995). In a very rare case involving a confined, bilateral calcification of the amygdala (Urbach-Wiethe disease), the patient (SM046) could not identify the emotion of fear in pictures of human faces. Moreover, she could not draw a fearful face, even though other emotional faces—happy, sad, angry, and disgusted—were identified and more successfully rendered (Adolphs, Tranel, Damasio, & Damasio, 1994, 1995). Curiously, this patient had no deficit in judging the emotional quality of music (Adolphs & Tranel, 1999). Another patient (SP) with extensive bilateral amygdala damage showed a deficit in her ability to rate levels of fear in human faces. Nevertheless, she had not lost the ability to evaluate vocal expressions of fear correctly (A. K.Anderson & Phelps, 1998), and she appeared perfectly normal in generating a fearful facial expression (A. K.Anderson & Phelps, 2000).
Bilateral amygdalotomy has recently been employed as a treatment for intractable aggression: It is reported that individuals who have undergone this surgery show both a reduction in autonomic arousal levels in response to stressful stimuli and a reduction in the number of aggressive outbursts. The behavior pattern is not, however, wholly suppressed, as these patients continue to have difficulty controlling aggression (G. P. Lee et al., 1998). Patients with unilateral (LeBar, LeDoux, Spencer, & Phelps, 1995) or bilateral (Bechara et al., 1995) lesions of the amygdala are reported to have deficits in classical aversive conditioning of the skin conductance response. In another patient with resection of the right temporal lobe (including the amygdala), M. Morris, Bradley, Bowers, Lange, and Heilman (1991) found both reduced skin conductance responses and low arousal ratings to unpleasant emotional pictures.
Overall, the effects of amygdala lesions in neurological patients suggest the presence of an emotional deficit. However, the pattern of results is less consistent and specific than that found in experimentally lesioned animals.There is agreement that loss of an amygdala may compromise a patient’s ability to interpret facial expression (notably the fear face). However, patients with this difficulty—which could relate to more general visual discrimination problems or difficulty discriminating among facial expressions—do not have problems interpreting emotional cues that come through other sensory systems (e.g., the auditory system). Furthermore, they usually show no deficit in overt expression emotion. The skin conductance data are suggestive. However, given the great variance in skin conductance responding in the normal population (which also includes many nonresponders), they are difficult to evaluate. Finally, although radical amygdalotomy appears to alter some features of aggression, the loss did not have a persistent effect on emotion regulation.
There are of course many reasons why the neurological data may be less robust and less clear in their implications than are the results of animal experimentation. It is important to consider that the primary lesions in patients are random and that the secondary surgical lesions can rarely be wholly precise in terms of the anatomical structure ablated. Furthermore, clinical considerations must always rule over experimental control. Drug intake, the general health status of patients, and the time since the lesion and its evaluation can all be highly variable. Surgery (as in epilepsy) is usually reserved for patients intractable to other treatments. In these cases, the brain disorder has persisted for a considerable time, with unknown effects on brain structure and organization. Thus, defining a comparison group and replicating a result exactly are often not feasible. Finally, it should also be considered that the brain changes with development and often has redundant neural circuits. Although the amygdala may be necessary—in humans as it is in animals—in much basic emotional learning, its functional role in emotional expression could be less critical in the mature human adult.
Brain Imaging
The emergence of neuroimaging technologies has opened a new window into the human brain. Positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI) are two technologies that make possible functional analysis of the amygdala and other brain structures in intact, normal human beings. It is significant that neither method directly assesses neural activation. Rather, fMRI and PET measure regional blood flow in the capillaries of the cerebral parenchyma associated with stimulus presentation. This BOLD (blood oxygen level–dependent) effect has been shown to be systematically correlated with neural firing. Thus, it is possible to assess indirectly the activation of brain structures that mediate the language, the reflexes (autonomic and somatic), and the behavioral acts that are emotion’s output.
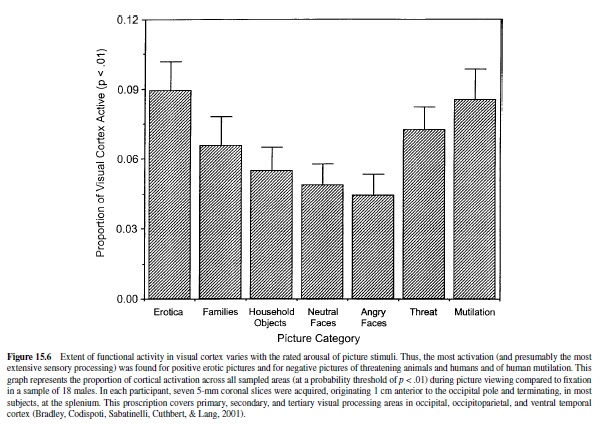
For technical reasons, the functioning of cortical motor and sensory systems are the easiest to image, and considerable progress has been made in, for example, mapping visual functioning in the human brain (Schneider, Noll, & Cohen, 1993). As already noted, appetitive and threatening stimuli capture attention and appear to accentuate processing in primary sensory areas. Primate research indicates, furthermore, that the amygdala projects to occipital and ventral temporal processing areas of the visual system (Amaral et al., 1992). To evaluate emotional processing in the visual system, Lang, Bradley, Fitzsimmons, et al. (1998) presented evocative picture stimuli (from the IAPS) to normal subjects, recording blood flow changes in the caudal cortex. Compared to affectively neutral pictures, participants showed dramatically larger areas of activation for picture stimuli rated pleasant or unpleasant. These fMRI findings were particularly strong in areas 18 and 19 of the occipital cortex, as well as in the fusiform cortex. More recent research by this group (Sabatinelli, Bradley, Cuthbert, & Lang, 1996) examined different categories of picture stimuli, confirming previous results and indicating clearly that activation increases monotonically with emotional arousal (see Figure 15.6). Thus, the greatest activity was found for pictures of attack (animal or human) made toward the viewer, for erotic pictures, and for pictures of mutilated bodies. Pictures rated less affectively arousing—household objects, neutral and angry faces, or mildly pleasant family groups showed— significantly less activation. Consistent with these results, PET studies have shown greater activation in the occipital visual system with individuals with phobias viewing pictures of relevant phobic objects (Fredrikson, Wik, Annas, Ericson, & StoneElander, 1995; Fredrikson et al., 1993), as well as increased activation, relative to neutral stimulation, in normal subjects viewing a range of unpleasant pictures (Lane et al., 1997).
Structures more anterior in the cortex, and ventrally near the midline where the amygdala is found, generally show smaller, less reliable BOLD effects in fMRI. Nevertheless, human neuroimaging studies generally support a role for the amygdala that may be particular to the processing of emotional stimuli. Irwin et al. (1996) reported that fMRI signal intensity is greater when subjects view graphic photographs of negative material (e.g., mutilated human bodies) compared to when they view neutral pictures. PET images suggest that metabolic activity increases during film clips (Reiman et al., 1997) or IAPS picture stimuli (Lane et al., 1997) that are unpleasant in content, and some data suggest that the amount of amygdala activity during affect-arousing film clips predicts later recall (Cahill et al., 1996). Furthermore, following Pavlovian fear conditioning, the formerly neutral, conditioned stimuli prompt increased fMRI signal intensities from the amygdala (cf. Davis & Whalen, 2001).
There are several studies (e.g., Whalen, 1998) suggesting that pictures of emotional faces engage the amygdala. These are interesting data, as face stimuli generally do not arouse strong emotion as defined by peripheral physiology, activation of the cortical visual system, or verbal report. One hypothesis is that the amygdala serves as a first-stage stimulus discriminator that screens stimuli of potential motivational significance. The finding that fearful faces are sometimes more effective (than other expressions) in activating the amygdala may reflect the inherent ambiguity of the fear face, rather than the exact content of the emotion itself as first suggested by Whalen (1998). Thus, angry faces might be less effective, paradoxically, because they provide more complete information. That is, they suggest a threat presence and also define the source of that threat. Fearful faces provide information about threat presence but give less information about the source, and thus require further amygdalar analysis. For similar reasons, amygdala activation might be expected to be greatest early in training, when reinforcement schedules are variable, or when stimulus contingencies change—all examples of ambiguity. In fact, in both nonhuman and human subjects, several amygdala-mediated responses (Applegate et al., 1983; Whalen & Kapp, 1991) reach their peak during early conditioning trials and subside thereafter (Masur, Dienst, & O’Neal, 1974; Schneiderman, 1972; Weisz & McInerney, 1990; see also Kapp et al., 1990). Moreover, when stimulus contingencies change (e.g., when a CS is suddenly not followed by shock at the beginning of extinction), there is a reemergence of singleunit activity in the lateral amygdala nucleus in rats (Quirk, Repa, & LeDoux, 1995). In analogous conditions, humans show a resurgence of amygdalar blood flow (LeBar, Gatenby, Gore, LeDoux, & Phelps, 1998).
The imaging results are exciting. However, a more detailed explication of these issues may well require a more refined analysis than imaging technology can currently deliver. As the animal model informs us, the amygdala is not a single structure, but a collection of many nuclei with different functions. Based on imaging and neurological findings, we might speculate that emotional face recognition requires, in particular, activation of the basolateral nuclei of the amygdala. Furthermore, we might conclude that such activation occurs without a coincident transmission to the central nucleus (i.e., the structure that has consequences for the autonomic and somatic reflexes and the experience of emotion). Unfortunately, the gross spatial resolution provided by PET permits us to say only that activation is in the region of the amygdala, and even fMRI cannot yet discriminate individual nuclei.
Emotional Arousal and Emotional Memory
From the perspective of the animal model presented here, input to the amygdala’s basolateral nucleus begins the sequence of neural events that underlay emotion, namely, orienting and attention, approach, and defensive behaviors such as avoidance. Basolateral outputs to the central nucleus and the extended amygdala appear to be critical in the increased processing of emotionally significant stimuli, whether pleasant or aversive. Outputs from the central nucleus and BNST in turn mediate many of the autonomic and somatic components of overt action. Direct output to the dorsal striatum, or indirect output via the orbital frontal cortex, appears to be involved in the actual avoidance response. Furthermore, output from the central nucleus and the BNST to the ventral striatum, as well as the orbitofrontal cortex, is also a likely contributor to the execution of approach and choice behavior.
The circuitry just described constitutes a motivational system that is finely tuned to the probability that events will require survival action (e.g., that a remote threat will become an imminent danger or that a sexual provocation will likely to lead to pleasant consummation). In animals, increasing imminence prompts a more general mobilization of the organism, mediated by various neurotransmitters such as acetylcholine, dopamine, norepinephrine, and many peptides such as CRH. These substances act either within the amygdala or at various central target areas to facilitate neural transmission, and they are associated with increasing intensity of appetitive or defensive motivation (and are also roughly correlated with reports of increasing arousal in humans).
This of course is not a new idea. There is a large literature on the role of neurotransmitters and neuromodulators in activating the organism, especially as they pertain to the sleepwakefulness continuum. However, it is activation specific to emotional arousal that is under consideration, as well as how the strength of this activation might vary with different provocations. For example, what differentiates our remembrance of a walk in the park from our recall of an erotic encounter? What is different about looking at a picture of a garbage can and looking at a picture of a mutilated body? Most of us would say immediately that for each comparison, the latter experience (the erotic encounter or the mutilated body) is more emotionally arousing. What is the physiology of this experience? How does looking at a picture lead to a release of acetylcholine, dopamine, norepinephrine, or CRH?
From the perspective of animal research, we know that the central nucleus of the amygdala and the BNST have direct connections to the neurons in the brain stem that release acetylcholine, dopamine, and norepinephrine and to neurons in the basal forebrain that releaseACH. Electrical stimulation of the amygdala has been shown to increase cell firing in many of these neuronal groups. In addition, cells in the lateral division of the amygdala’s central nucleus sends CRHcontaining terminals to the BNST (Sakanaka, Shibasaki, & Lederis, 1986), where many of the actions of CRH may actually be mediated (Y. Lee & Davis, 1996).Thus, more arousing images and thoughts could activate more cells in amygdala that automatically lead to a release of these neurochemicals.
However, it seems more difficult to account for why one image is considered emotionally upsetting to almost everyone whereas another is not (e.g., a picture of a dental chair vs. a picture of a rocking chair)—or why representation connotes more excitement than another picture (e.g., a picture of a chair on a roller coaster vs. a picture of a rocking chair). In addition, it seems more difficult to account for why one image is especially frightening in one situation but not in another. A picture of a chainsaw in a hospital operating room might well generate a stronger emotional reaction than a picture of the same chainsaw in a hardware store. In addition, it is not obvious why, neurophysiologically, one picture is especially frightening to one individual but less so to another. That is, a picture of a chainsaw in a hospital operating room produces considerably more emotion to the patient facing an amputation than the same picture does to a surgeon who has carried out this operation many times before. The intensity of an emotion generated by a picture depends not only on the particular item (e.g., chainsaw) but also on the content of the picture (hospital operating room vs. hardware store), as well as on the history of the person viewing the picture (future amputee vs. doctor). How then does this translate into levels of transmitter release, producing the many outputs of emotion, with varying affective intensity, almost instantly?
Cognitive Networks
These considerations raise fundamental questions about how information is processed and memories are stored in the human brain. Cognitive psychologists (e.g., J. R.Anderson & Bower, 1974; Kintsch, 1974) suggest that knowledge about events is represented in networks of representations linked by laws of association. They are instantiated by stimuli that match elements in the network. Lang (1994) suggested that emotional memories may be considered networks of this type. These networks include associated information about emotional episodes, coding stimulus events and context, behavior, and interpretive elaborations. When cues match units in the network (e.g., chainsaw, surgical operating room), network processing is initiated: Activity in one unit is transmitted to adjacent units, and depending on the strength of activation, the entire structure may be engaged. The probability of network processing is likely to be increased with the number of units initially stimulated.Ahypothesized fear memory network for asnake-phobic individual is presented in Figure 15.7. Itcanbe thought of as a net of linked representations (which, in turn, might be individual neural subnetworks). Only a fraction of its representational units may have higher level language representation, and thus—passing through awareness—be the formative stuff of affective reports.
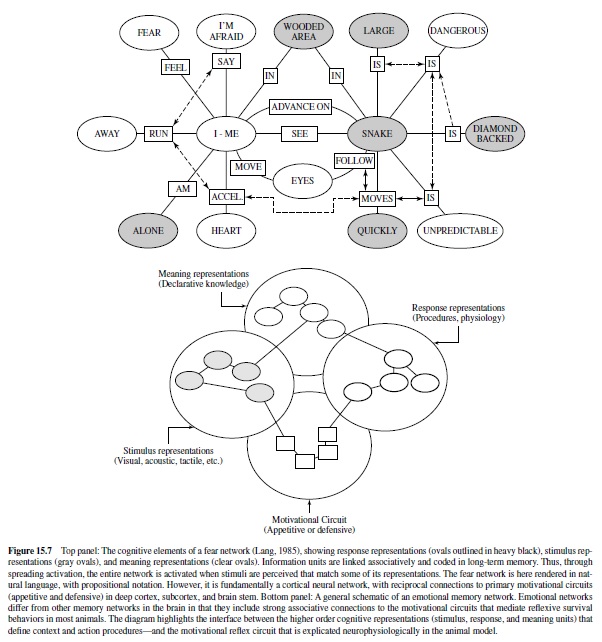
How do emotional networks differ from other knowledge structures in the brain? It is proposed that emotion networks are singular because they include associative connections to the primary motivational systems in the brain that are the focus of this discourse. In brief, reciprocal projections from cortex to the amygdalar circuit engage the somatic and autonomic reflexes that evolved to ensure the survival of individuals and species.
Levels of Activation
It may be that differences in level of arousal occur because networks activate different numbers of cells in the amygdala depending on the associative history of those stimuli. In fact, most stimuli or situations that produce an emotional reaction do so by virtue of prior conditioning. Monkeys reared in the lab, where serpents are not normally encountered, are generally not afraid of snakes compared with monkeys raised in the wild (Mineka, Davidson, Cook, & Keir, 1984). A baby with his or her finger in a light socket does not feel afraid when the light switch is turned on, whereas a child who was once shocked in a similar situation certainly does. After this association is formed, putting a finger in a socket may be presumed to engage many cells in the amygdala, leading to a large release of neurochemicals and strong activation of the defense motivation system.
Similar amygdala activations can be assumed to occur when we think about an unpleasant experience or look at emotional pictures. The site of a chainsaw in an operating room would activate a neural representation of pain or blood and mutilation, which would activate the amygdala by virtue of prior association. The site of a chainsaw in a hardware store would activate a network of cutting wood, which would probably not include strongly associated representations— pain, blood, and mutilation—that may be unconditioned instigators of amygdala activation.
In summary, emotional arousal occurs when a stimulus activates a matching representation in an emotional network. The immediacy and intensity of the affective reaction depends on the general associative strength of the network, and specifically on the associative strength of connections to the amygdala circuit. In humans, this net is broadly cast. Affects can be instantiated vicariously, by language representation and other media or, indeed, by cues not discriminated linguistically (and therefore outside awareness). Thus, many different stimuli, varying across individuals, can prompt an amygdala-dependent release of neurochemicals, with a potentially widespread modulation of sensory and motor systems.
Measuring Emotionalarousal in Rats and Humans: The Startle Reflex and the Motivational Priming Hypothesis
From an evolutionary perspective, human emotions such as fear or anger are usefully considered as dispositions to action. That is, they may have evolved from preparatory states evoked by threat cues, in which survival depended on delay or inhibition of overt behavior. In this sense, they derive from the first stage of defense that is associated with vigilance and immobility, when the organism is primed to respond, but not yet active.
In most mammals, any abrupt sensory event will prompt a startle response, a chained series of rapid extensor-flexor movements that cascade throughout the body (Landis & Hunt, 1939). This reaction is a defensive reflex, facilitating escape in simpler organisms, and perhaps still serving a protective function in more complex animals (i.e., in avoiding organ injury as in the eye blink or in retraction of the head and torso in the full body startle reflex to avoid attack from above; Li & Yeomans, 1999). Abruptness is the key to startle elicitation: To be effective, the rise time of the eliciting stimulus should be perceptually instantaneous. In human subjects, sudden closure of the eyelids is one of the first, fastest (occurring within 25 ms to 40 ms after startle stimulus onset), and most stable elements in the reflex sequence. It is the preferred measure of startle in humans. In rats, whole body startle is measured in specially designed cages.
When under threat (of pain or predation), animals show an exaggerated startle reflex. As first described by Brown, Kalish, and Farber (1951), the amplitude of the acoustically elicited startle reflex in rats is increased when elicited in the presence of a light previously paired with foot shock. This effect is considered an animal model of fear or anxiety because drugs that reduce fear or anxiety in humans, such as diazepam or buspirone, block the increase in startle in the presence of the conditioned light stimulus but do not affect the basic startle reflex itself (see Davis, Falls, Campeau, & Kim, 1993, for a review). In contrast, during an appetitive state, the startle reflex appears to be partially inhibited; that is, startle amplitude is reduced when elicited in the presence of a light previously paired with food (Schmid, Koch, & Schnitzler, 1995) or rewarding electrical brain stimulation (Yeomans, Steidle, & Li, 2000).
These effects are very like what cognitive psychologists call priming in research on human associative behavior. Cognitive priming occurs when a prior stimulus raises the activation level of an associated S-R event. For example, the prime “bread” prompts a faster reaction time response to the word “butter.” States of the organism may also prime particular behavior. Thus, clinically depressed individuals respond to nearly all cues with associations that are affectively negative. The potentiated startle observed in animal conditioning can be understood as an instance of motivational state priming. That is, the induced defensive state of the organism primes (increments) an independently instigated reflex that is connected to the defense system (i.e., the startle response).
According to the motivational priming hypothesis (Lang, Bradley, & Cuthbert, 1990, 1997), the defensive startle reflex will be of significantly greater amplitude (and faster) when a prior stimulus has induced a consonant, defensive motivational state. Alternatively, if the appetitive system has been activated, as in states of pleasure, the defensive startle reflex should undergo a reciprocal attenuation. Assuming our postulate that emotions are driven by underlying motive systems, any instance of emotional perception should prompt startle reflex modulation—increasing with unpleasant percepts and decreasing with pleasant percepts. Thus, the startle reflex can serve as a remarkably simple, objective measure of affective valence. It is also a powerful tool for assessing the role of the amygdala circuit in emotion, as well as the premier method that solidly links animal neuroscience research and human psychophysiology.
Startle Modulation in Humans
Like rats, human subjects also show elevated startle amplitude in the presence of cues previously paired with shock (Grillon & Davis, 1997; Hamm, Greenwald, Bradley,& Lang, 1993;Lipp,Sheridan,&Siddle,1994)orsimplywhentheyare told they might receive a shock (Grillon, Ameli, Woods, Merikangas, & Davis, 1991; Grillon, Ameli, Merikangas, Woods, & Davis, 1993). Lang et al. (1990) further theorized that startle modulation was a more general phenomenon and that this reflex could be used to probe the full range of affective responses. That is, to the extent that any perceptual event evoked a state of unpleasant arousal, the probe startle response would be increased in magnitude; to the extent that a foreground stimulus is pleasantly arousing, the startle probe response would be diminished.
When startle probes are administered while subjects view pictures that vary systematically in emotional arousal, results have consistently conformed to the motivational priming hypothesis.As Figure 15.8 illustrates, there is a startle inhibition during pleasant stimuli and potentiation when pictures were judged to be unpleasant: The largest startle blink responses occurred during unpleasant content, and the smallest during pleasant pictures (e.g, Lang, 1995; Lang et al., 1990; Vrana, Spence, & Lang, 1988; and see Bradley, 2000, for a recent review). These emotion-perceptual effects have also been reported in 5-month-old infants viewing smiling, neutral, and angry faces (Balaban, 1995).
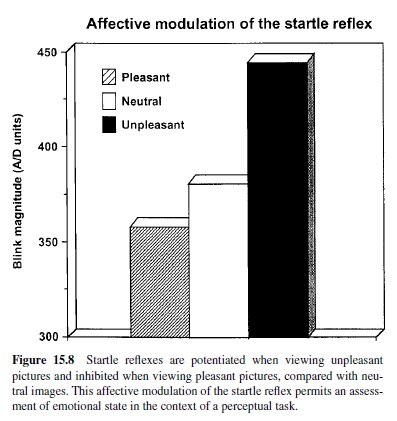
Affective modulation of startle is observed for picture stimuli regardless of whether the startle probe is visual, acoustic, or tactile (e.g, Bradley, Lang, & Cuthbert, 1990; Hawk & Cook, 1997), suggesting that modality-specific processes are not primary in these modulatory effects. Furthermore, affective modulation is not confined to visual percepts: When the foreground stimuli consist of short, 6-s sound clips of various affective events (e.g., sounds of love making, babies crying, or bombs bursting) and the startle probe is a visual light flash, the same affect-reflex effect is obtained (Bradley, Zack, & Lang, 1994). Other researchers have found startle potentiation in subjects smelling unpleasant odors (Ehrlichman, Brown, Zhu, & Warrenburg, 1995; Miltner, Matjak, Braun, & Diekmann, 1994), supporting the view that affective reflex modulation is broadly motivational and thus consistent across affective foregrounds of different stimulus modalities.
Emotional Arousal
Consistent with the motivational priming hypothesis, modulatory effects on the startle reflex appear to increase with greater activation in each motive system. Probe startle potentiation is largest for unpleasant pictures that are rated most arousing; conversely, the most arousing pleasant pictures prompt the greatest probe startle inhibition (Cuthbert, Bradley, & Lang, 1996). Thus, individuals with specific phobias who look at pictures of the phobic object (e.g., snakes or spiders) show startle potentiation well beyond that routinely seen in normal subjects (Hamm et al., 1997). The potentiation is, however, clearly selective: Individuals with phobias show normal reflex inhibition to startle probes when looking at arousing pleasant stimuli (Sabatinelli et al., 1996).
Probe reflex inhibition reflects attentional engagement (Graham, 1992), as well as an appetitive motive state. Consistent with this view, probes presented during less arousing, unpleasant stimuli—pictures of sad events, pollution, ill people—tend to prompt some reflex inhibition. Startle magnitude then increases progressively with reported arousal, and the strongest potentiation occurs during viewing of the most arousing unpleasant stimuli (Cuthbert et al., 1996). In contrast, the most pleasantly arousing percepts show the largest probe startle inhibition.
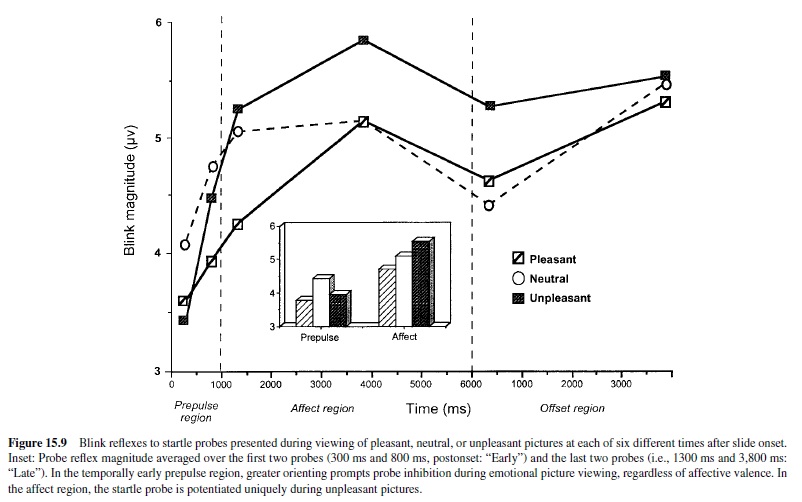
Startle probes have been used to examine the time course of emotional perception (Bradley et al., 2001), beginning with the first apprehension of a cue meaning, milliseconds after an image first appears, and continuing until the percept is fully resolved (Figure 15.9). In this paradigm, startle probes are presented at various times in the stimulus presentation interval on different trials. For early probes, picture onset constitutes a prepulse, clearly drawing attentional resources away from the startle stimulus, resulting in a partial probe startle inhibition for all image contents, whether emotional or not. This diminished reflex in the first few hundred milliseconds of picture exposure is nevertheless significantly more profound for emotionally arousing than for neutral stimuli. That is, compared to neutral pictures, the pleasant and unpleasant stimuli appear already to receive more processing, even though their motivational type, appetitive or defensive, has not yet been determined. This early discrimination of motivationally relevant stimuli has been confirmed by EEG studies. Evoked potential analyses of dipole sources show a distinct difference in bioelectric activity, relative to neutral input, for pictures described as more emotionally arousing—in occipital and parietal cortex—between 150 ms and 250 ms after picture onset (Junghoefer, Bradley, Elbert, & Lang, 2001).
As can be seen in Figure 15.9, when startle is elicited after longer picture exposure and the picture has been resolved as to exact content, the decrease in startle amplitudes continues for pleasurable stimuli; however, unpleasant, arousing stimuli now prompt startle potentiation. In a more than symbolic way, this progression from attentive evaluation of potentially motivationally relevant stimuli to clear emotional content discrimination—with potentiated startle (a motor action) consequent on a resolved unpleasant cue— seems like a microcosm of the defense motivational sequence (attention to action) discussed previously. That is, this rapid change in processing parallels events in a slower time frame, for example, as a friend or possible foe approaches us in the park.
The Role of the Amygdala in Startle Modulation in Rats
Conditioned Fear
Figure 15.10 is a diagrammatic sketch of the pathways that we believe mediate fear-potentiated startle in rats using visual conditioned stimuli. Visual information from the retina projects to both the dorsal lateral geniculate nucleus and lateral posterior nucleus of the thalamus. The dorsal lateral geniculate nucleus projects to the visual cortex, whereas the lateral posterior nucleus projects directly to the basolateral nucleus of the amygdala as well as the perirhinal cortex (Shi & Davis, 1996), which then projects to the lateral or basolateral nuclei of the amygdala. The basolateral nucleus then projects to the central nucleus, which in turn has neural projections to the startle pathway. Electrolytic or chemical lesions of visual thalamus (Shi & Davis, 1996), perirhinal cortex (Campeau & Davis, 1995a; Rosen et al., 1992), or basolateral amygdala (Campeau & Davis, 1995b; Sananes & Davis, 1992) completely block the expression of fear-potentiated startle using a visual CS. None of these lesions affect startle amplitude itself. Lesions (Campeau & Davis, 1995b; Hitchcock & Davis, 1986) and local infusion of glutamate antagonists into the amygdala’s central nucleus (Kim, Campeau, Falls, & Davis, 1993; Walker & Davis, 1997) also block fear potentiated startle.
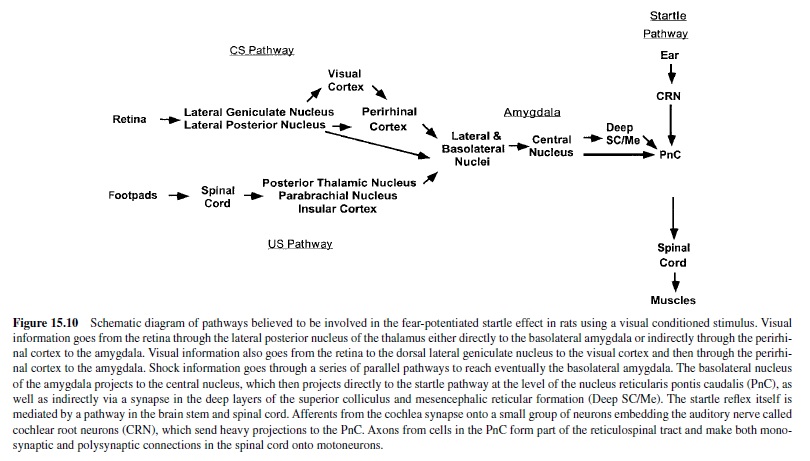
Both conditioned fear and sensitization of startle by foot shocks appear to modulate startle at the level of the nucleus reticularis pontis caudalis (Berg & Davis, 1985; Boulis & Davis, 1989; Krase, Koch, & Schnitzler, 1994). The central nucleus of the amygdala projects directly to the nucleus reticularis pontis caudalis (Rosen, Hitchcock, Sananes, Miserendino, & Davis, 1991), and electrolytic lesions along this pathway block the expression of fear-potentiated startle (Hitchcock & Davis, 1991). However, an obligatory synapse appears to exist in this pathway because fiber-sparing chemical lesions in the deep layers of superior colliculus or periaqueductal gray also block fear-potentiated startle (Fendt, Koch, & Schnitzler, 1996; Frankland & Yeomans, 1995), as does local infusion of muscimol into the same general area (Meloni & Davis, 1999).
Pleasure-Attenuated Startle
As mentioned earlier, startle amplitude is decreased when elicited in the presence of a cue previously paired with food (Schmid et al., 1995). Moreover, the nucleus accumbens is important for this pleasure-attenuated startle effect because pretraining local infusion of the neurotoxin 6-OHDA into the nucleus accumbens blocks pleasure-attenuated startle (Koch, Schmid, & Schnitzler, 1996). It is possible that the connection between the basolateral nuclei and the ventral striatum may be involved in pleasure-attenuated startle. Unilateral lesions of the basolateral nuclei on one side of the brain, and the nucleus accumbens ablation on the other, would test this hypothesis.
Emotion and the Amygdala in Humans
There are still only a few studies with human subjects that have attempted to relate the startle response directly to amygdala function. Bower et al. (1997) studied a group of 18 epilepsy patients who had undergone anterior temporal resections, involving the amygdala, and who were subsequently evaluated for acoustic startle reactivity (binaurally presented white noise bursts). Volumetric measures of the amygdala were obtained before and after surgery. These investigators reported a significant relationship between a reduction in base startle magnitude and the extent of amygdala loss—a relationship that was particularly strong for the right amygdala. Funayama, Grillon, Davis, and Phelps (2001) reported that epilepsy patients, treated by resection of the right temporal lobe, failed to show an increase in startle amplitude when they viewed highly unpleasant, arousing pictures. Nevertheless, they did show potentiated startle when presented with a light cue that they were told signaled possible electric shock. In dramatic contrast, patients with resection of the left temporal lobe showed normal startle potentiation when viewing highly unpleasant, arousing pictures, but not when exposed to the light-shock paradigm. Thus, the results indicated a double dissociation in affective modulation of startle eye blink, mediated separately by the two amygdalae. In humans, the amygdala’s role in startle potentiation appeared to depend on both laterality and type of task. These ablation data are consistent with a recent fMRI study showing preferential activation of the left amygdala when subjects see a cue that they are told may predict shock (Phelps et al., 2001), a procedure that consistently increases the startle reflex (Grillon et al., 1991).
Phelps et al. (2000) used the startle response as an implicit measure of good-bad evaluative judgments, hypothesizing that one race might view other races negatively. While in a MRI scanner, White American participants viewed pictures of Black and White male faces. The pictures were presented again to the same subjects one week later, and during this viewing, startle was elicited by brief bursts of white noise. Focusing on the two amygdalae, the strength of activation to Black versus White faces was correlated with startle potentiation to these same stimuli. The highest correlation with startle potentiation was found for the left-superior amygdala and two small regions in the right insular cortex. An interesting result was that this relationship was not found in a second control experiment in which the Black and White faces were of famous, positively evaluated people.
These data encourage the general hypothesis that startle potentiation in humans is associated with amygdala activation. The laterality effects are provocative but currently hard to interpret. Lateralization of the brain varies widely across species, suggesting that research with mammals other than humans will offer limited guidance. Nevertheless, other investigators have reported differences between the amygdalae in human emotional perception. For example, using PET, J. S. Morris, Frith, Perrett, and Rowland (1996) found more activation (regional cerebral blood flow, or rCBF) in the left amygdala when subjects viewed fearful as opposed to happy facial expressions. In contrast, Cahill et al. (1996) found no increase in either amygdala while their subjects watched unpleasant, arousing films (compared to neutral documentaries). During a subsequent film-recall session, however, these same subjects showed significant relative activation uniquely in the left amygdala when they were cued to retrieve memories of the unpleasant films. J. S. Morris, Ohman, and Dolan (1998) also reported lateralized activation that, curiously, depended on whether previously conditioned pictures of faces (electric shock used as an unconditioned stimulus) were presented masked, and presumably subliminal (right amygdala), or unmasked and supraliminal (left amygdala). Given these provocative but highly various results, laterality will surely continue to be a focus in research, and these efforts should in future yield a better understanding of the phenomenon.
Emotional Processing: From Attention to Action (With Stops Along the Way)
When a wild rat sees a human at some distance away, the rat freezes in a highly alert, attentive posture. As the human gradually approaches, the rat suddenly darts away if escape is possible. If escape is not possible and the human gets very close, the rat will attack (Blanchard, Flannelly, & Blanchard, 1986). These observations lead Caroline and Robert Blanchard to note that defensive behaviors increase systematically with a reduction in distance from predators and other dangerous or potentially painful stimuli (Blanchard & Blanchard, 1988). Let us recall the scenarios that we discussed at the outset of this research paper—the feelings one might have as the dangerous-looking men approached. Given an available escape route, proximity is associated with an increased probability of active flight. In the absence of an escape option, the best defense may be attack. When the threat stimulus is distant, not clearly discriminable, an animal such as the rat “freezes while oriented toward the predator” (p. S5). The Blanchards noted further that increases in “the amplitude of the startle response to sudden stimuli accompany decreasing defensive distance” (p. S5).
Using the concepts introduced by Timberlake (1993; Timberlake & Lucas, 1989), Fanselow (1991, 1994) has made a parallel analysis of fear behavior, describing three behavioral stages, increasingly proximal to a predator: preencounter defense, preemptive behavior that occurs in a foraging area where predators were previously encountered; postencounter defense, responses prompted by the detection of a distant predator; circa-strike defense, behaviors such as defensive attack that occur in the region of physical contact or its close imminence. Behaviorally, there is a shift from nonspecific threat vigilance at preencounter, to postencounter freezing and orienting to a specific predator or predator cue, to the circa-strike stage when the organism is beyond vigilance and engaged in vigorous defensive action.
During mild electrical stimulation of the amygdala the first reaction is an arrest of ongoing behavior (freezing), bradycardia, and EEG activation. As stimulation increases, the animal suddenly becomes very active and at high levels of stimulation vigorously attempts to escape from the source of stimulation. One may thus conjecture that the site of a predator at some distance leads to a mild activation of the amygdala that produces an increase in attention. As the predator comes closer, activation of the amygdala increases to the point where it now leads toactivedefensivebehavior,includingescape.Assuggestedby Fanselow (1991, 1994) this switch from an attentional mode to an active defense mode may involve a switch from activation of the ventral to the dorsal periaqueductal gray. Work by Bandler and many others (cf. Bandler & Shipley, 1994) has shown that the ventral periaqueductal gray projects to cardiovascular centers that mediate bradycardia as well as motor systems that mediate freezing. In contrast, the dorsal periaqueductal gray projects to cardiovascular systems that mediate tachycardia and active escape behavior. If one assumes that the ventral periaqueductal gray has a lower threshold for amygdala activation, this would explain why we initially go into an attentional mode as the amygdala is activated by a novel stimulus and sends signals to the periaqueductal gray. As the level of amygdala activation increases as a potential predator approaches, suddenly the higher threshold of activation of the dorsal periaqueductal gray is exceeded, leading to an abrupt switch from a high level of attention to action (e.g., quickly crossing the street to avoid the dangerous-looking men).
Lang et al. (1997) recently proposed an adaptation of the predator stage model for explicating human psychophysiological reactions to unpleasant and threatening stimuli. They suggest that the human laboratory participant, responding to stimuli presented by the experimenter, is functioning at a response stage analogous to postencounter responses; that is, like the freezing rat, he is immobile and vigilant, with easy escape blocked (in this case by instructions and social compliance). For the animal subject, increasing proximity to an aversive stimulus (greater nociceptive imminence) prompts an increase in general activation or arousal. It is proposed that this same effect is generated in the human psychophysiological laboratory because social compliance constrains participants to be passive and stimuli are presented that are less or more threatening or aversive. As illustrated in Figure 15.11, the increased vigilance in the postencounter period is characterized by a progressive augmentation of physiological indexes of attention—greater skin conductance, increased heart rate deceleration, and inhibition of the probe startle reflex when arousal is still relatively low.
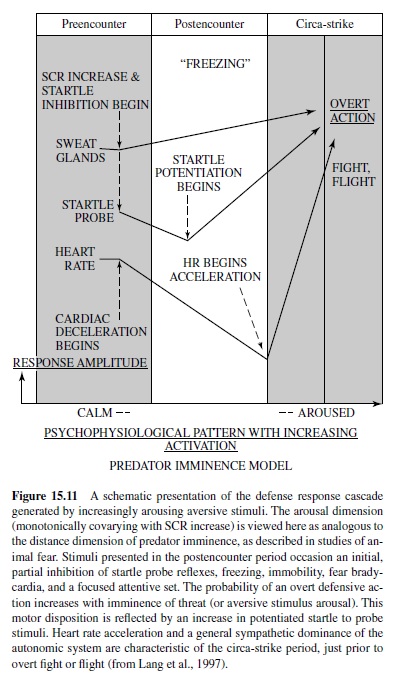
The arousal abscissa of Figure 15.11 also constitutes a dimension of greater probability of motor action that modulates the startle response. Thus, as a close encounter becomes more imminent, the direction of the probe response reverses. In place of motor inhibition, the startle reflex now shows a potentiation that progressively increases in magnitude as probes occur more proximal to actual, overt defensive action. Startle potentiation begins in the context of freezing and vigilance and could be viewed as a premature triggering of defensive action. With a further increment in threat the heart rate response also reverses the direction of change from orienting to defense (Graham, 1979; Sokolov, 1963)—from a vigilance-related fear bradycardia to action mobilization and cardiac acceleration.
The biological model of emotion presented here suggests that, depending on level of stimulus aversion (threat, apprehension), patterns of physiological change (and in humans, reports of experienced emotional arousal) will systematically vary with the level of defense system activation. Furthermore, the overt behaviors that may result with increasing imminence (circa-strike region) could look fearful, angry, or—given overwhelming stress and no available coping behavior—autonomic and somatic collapse, hopelessness, and depression.
The model’s assumed predator is, of course, engaged in parallel dance—first observing quietly the distant prey, stalking forward slowly, increasingly mobilized for what becomes, at circa-strike, a final charge. Overall, it is a parallel progression from attention to action, with a staged increment in appetitive arousal. Although there is currently little data on the predator’s anticipatory pleasures—joy of the hunt, satisfaction in its consumation—the neurophysiology of the process is likely to be, in part, similar to what is observed in defense. In general, we have focused in this research paper on the defensive emotions at the expense of positive, pleasant states. As the allusion to predatory pleasures implies, this is mainly attributable to the larger, more programmatic literature on negative emotion. It is fondly to be wished that neuroscientists, cognitive scientists, and other researchers will in future have more time for consideration of the appetitive-positive affects.
Emotion and the Brain: Concluding Thoughts
We have proposed here that basic emotions are founded on brain circuitry in which the amygdala is a central component. We conceive emotion not as a single reaction, but as a process: In addition to reports of affective experience, emotions engage sequenced somatic and autonomic responses, organized by brain circuits that developed over evolutionary history to ensure the survival of individuals and species. Thus, events that are positive-appetitive or aversivethreatening have an initial advantage in capturing attention. As their affective valence is resolved, these events prompt further information gathering—more according to their degree of motivational significance. Motive cues also occasion the release of neurochemicals, metabolic arousal, anticipatory responses that are oriented toward the engaging event, and a mobilization of the organism that can lead to some action. Sometimes these motivational sequences play out in humans, in the same stimulus-driven way that they occur in less complex organisms. More often, given an elaborate brain that is equipped with language, an interactive memory storage, and a vast behavioral repertoire, motivational reflexes are only being engaged in part, and action is inhibited, delayed, or complexly adapted to the context. Nevertheless, it is this reflex bedrock that generally prompts humans to say that they are emotionally aroused. We may feel joyful, angry, fearful, anxious, or sad and hopeless. Regardless of how the state is labeled, true to emotion’s reflex automaticity, we often feel less in control, as if something is happening to us, rather than that emotion is something we are doing—carried along in pleasure and in pain by the very human experience of emotion.
Bibliography:
- Adolphs, R., & Tranel, D. (1999). Intact recognition of emotional prosody following amygdala damage. Neuropsychologia, 37, 1285–1292.
- Adolphs, R., Tranel, D., & Damasio, A. R. (1998). The human amygdala in social judgment. Nature, 393, 470–474.
- Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. R. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372, 669–672.
- Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. R. (1995). Fear and the human amygdala. Journal of Neuroscience, 15(9), 5879–5891.
- Amaral, D. G., & Price, J. L. (1984). Amygdalo-cortical projections in the monkey (macaca fascicularis). Journal of Comparative Neurology, 230, 465–496.
- Amaral, D. G., Price, J. L., Pitkanen, A., & Carmichael, S. T. (1992). Anatomical organization of the primate amygdaloid complex. In J. Aggleton (Ed.), The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 1–66). New York: Wiley.
- Amorapanth, P., LeDoux, J. E., & Nader, K. (2000). Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature Neuroscience, 3, 74–79.
- Anderson, A. K., & Phelps, E. A. (1998). Intact recognition of vocal expressions of fear following bilateral lesions of the human amygdala. Neuroreport, 9(16), 3607–3613.
- Anderson, A. K., & Phelps, E. A. (2000). Expression without recognition: Contributions of the human amygdala to emotional communication. Psychological Science, 11, 106–111.
- Anderson, J. R., & Bower, G. H. (1974). A propositional theory of recognition memory. Memory and Cognition, 2, 406–412.
- Anderson, S. W., Bechara, A., Damasio, H., Tranel, D., & Damasio, A. R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience, 2, 1032–1037.
- Applegate, C. D., Kapp, B. S., Underwood, M. D., & McNall, C. L. (1983). Autonomic and somatomotor effects of amygdala central n. stimulation in awake rabbits. Physiology and Behavior, 31, 353–360.
- Aston-Jones, G., Rajkowski, J., Kubiak, P., Valentino, R. J., & Shipley, M. T. (1996). Role of the locus coeruleus in emotional activation. Progress in Brain Research, 107, 379–402.
- Balaban, M. (1995). Affective influences on startle in five-monthold infants: Reactions to facial expressions of emotion. Child Development, 66, 23–36.
- Bandler, R., & Shipley, M. T. (1994). Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends in Neuroscience, 17, 379–389.
- Baxter, M. G., Parker, A., Lindner, C. C. C., Izquierdo, A. D., & Murray, E. A. (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience, 20, 4311–4319.
- Bechara, A., Damasio, H., Tranel, D., & Damasio, A. R. (1997). Deciding advantageously before knowing the advantageous strategy. Science, 275, 1293–1294.
- Bechara, A., Tranel, D., Damasio, H., Adolphs, R., Rockland, C., & Damasio, A. R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269, 1115–1118.
- Belcheva, I., Belcheva, S., Petkov, V. V., & Petkov, V. D. (1994). Asymmetry in behavioral responses to cholecystokinin microinjected into rat nucleus accumbens and amygdala. Neuropharmacology, 33(8), 995–1002.
- Berg, W. K., & Davis, M. (1985). Associative learning modifies startle reflexes at the lateral lemniscus. Behavioral Neuroscience, 99, 191–199.
- Bermudez-Rattoni, F., Grijalva, C. V., Kiefer, S. W., & Garcia, J. (1986). Flavor-illness aversion: The role of the amygdala in acquisition of taste-potentiated odor aversions. Physiology and Behavior, 38, 503–508.
- Blanchard, D. C., & Blanchard, R. J. (1988). Ethoexperimental approaches to the biology of emotion. Annual Review of Psychology, 39, 43–68.
- Blanchard, R. J., Flannelly, K. J., & Blanchard, D. C. (1986). Defensive behavior of laboratory and wild Rattus norvegicus. Journal of Comparative Psychology, 100, 101–107.
- Boadle-Biber, M. C., Singh, V. B., Corley, K. C., Phan, T. H., & Dilts, R. P. (1993). Evidence that corticotropin-releasing factor within the extended amygdala mediates the activation of tryptophan hydroxylase produced by sound stress in the rat. Brain Research, 628, 105–114.
- Boulis, N., & Davis, M. (1989). Footshock-induced sensitization of electrically elicited startle reflexes. Behavioral Neuroscience, 103, 504–508.
- Bower, D., Eckert, M., Gilmore, R., Leonard, C. M., Bauer, R., Roper, S., Bradley, M., Lang, P., & Barta, P. (1997). Startle eyeblink magnitude in humans depends on extent of right amygdala removal. Society for Neuroscience Abstracts, 23,
- Bradley, M. M. (2000). Emotion and motivation. In J. T. Cacioppo, L. G. Tassinary, & G. Bernston (Eds.), Handbook of psychophysiology (pp. 602–642). New York: Cambridge University Press.
- Bradley, M. M., Codispoti, M., Sabatinelli, D., Cuthbert, B. N., & Lang, P. J. (2001). Emotion and picture perception: Affective arousal, semantic content, and sex differences. Manuscript submitted for publication.
- Bradley, M. M., Cuthbert, B. N., & Lang, P. J. (1998). International affective digitized sounds (IADS). Gainesville, FL: The Center for Research in Psychophysiology, University of Florida.
- Bradley, M. M., Greenwald, M. K., Petry, M., & Lang, P. J. (1992). Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 18, 379–390.
- Bradley, M. M., Lang, P. J., & Cuthbert, B. N. (1990). Startle reflex modification: Emotion or attention. Psychophysiology, 27, 513–523.
- Bradley, M. M., Lang, P. J., & Cuthbert, B. N. (1998). Affective norms for English words (ANEW). Technical manual and affective ratings. Gainesville, FL: The Center for the Study of Emotion and Attention, University of Florida.
- Bradley, M. M., Lang, P. J., Sabatinelli, D., King, W., Fitzsimmons, J. R., & Desai, P. (2001). Activation of the visual cortex in emotional perception. Manuscript submitted for publication.
- Bradley, M. M., Zack, J., & Lang, P. J. (1994). Cries, screams, and shouts of joy: Affective responses to environmental sounds. Psychophysiology, 31(Suppl. 29).
- Broks, P., Young, A. W., Maratos, E. J., Coffey, P. L., Calder, A. J., Isaac, C., Mayes, A. R., Hodges, J. R., Montaldi, D., Cezayirli, E., Roberts, N., & Hadley, D. (1998). Face processing impairments after encephalitis: Amygdala damage and recognition of fear. Neuropsychologia, 36, 59–70.
- Brown, J. S., Kalish, H. I., & Farber, I. E. (1951). Conditional fear as revealed by magnitude of startle response to an auditory stimulus. Journal of Experimental Psychology, 41, 317–328.
- Brown, M. R., & Gray, T. S. (1988). Peptide injections into the amygdala of conscious rats: Effects on blood pressure, heart rate and plasma catecholamines. Regulatory Peptides, 21, 95–106.
- Burns, L. H., Robbins, T. W., & Everitt, B. J. (1993). Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behavioural Brain Research, 55, 167–183.
- Bussey, T. J., Everitt, B. J., & Robbins, T. W. (1997). Dissociable effects of cingulate and medial frontal cortex lesions on stimulusreward learning using a novel Pavlovian autoshaping procedure in rats: Implications for the neurobiology of emotion. Behavioral Neuroscience, 111, 908–919.
- Cador, M., Robbins, T. W., & Everitt, B. J. (1989). Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience, 30(1), 77–86.
- Cahill, L. (2000). Modulation of long-term memory storage in humans by emotional arousal: Adrenergic activation and the amygdala. In J. P. Aggleton (Ed.), The amygdala (Vol. 2, pp. 425– 445). Oxford, UK: Oxford University Press.
- Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J., & McGaugh, J. L. (1996). Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences, USA, 93, 8016–8021.
- Cahill, L., & McGaugh, J. L. (1998). Mechanisms of emotional arousal and lasting declarative memory. Trends in Neuroscience, 21, 294–299.
- Calder, A. J., Young, A. W., Rowland, D., Perrett, D. I., Hodges, J. R., & Etcoff, N. L. (1996). Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cognitive Neuropsychology, 13, 699–745.
- Calvino, B., Lagowska, J., & Ben-Ari, Y. (1979). Morphine withdrawal syndrome: Differential participation of structures located within the amygdaloid complex and striatum of the rat. Brain Research, 177, 19–34.
- Campbell, B. A., Wood, G., & McBride, T. (1997). Origins of orienting and defense responses: An evolutionary perspective. In P. J. Lang, R. F. Simmons, & M. T. Balaban (Eds.), Attention and orienting: Sensory and motivational processes (pp. 41–67). Hillsdale, NJ: Erlbaum.
- Campeau, S., & Davis, M. (1995a). Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience, 15, 2312–2327.
- Campeau, S., & Davis, M. (1995b). Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience, 15, 2301–2311.
- Chapman, W. P., Schroeder, H. R., Guyer, G., Brazier, M. A. B., Fager, C., Poppen, J. L., Solomon, H. C., & Yakolev, P. I. (1954). Physiological evidence concerning the importance of the amygdaloid nuclear region in the integration of circulating function and emotion in man. Science, 129, 949–950.
- Costall, B., Kelly, M. E., Naylor, R. J., Onaivi, E. S., & Tyers, M. B. (1989). Neuroanatomical sites of action of 5-HT3 receptor agonist and antagonists for alteration of aversive behaviour in the mouse. British Journal of Pharmacology, 96, 325–332.
- Cuthbert, B. N., Bradley, M. M., & Lang, P. J. (1996). Probing picture perception: Activation and emotion. Psychophysiology, 33, 103–111.
- Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (1998). We like to watch: Cortical potentials in emotional perception. Manuscript submitted for publication.
- Damasio, A. R. (1994). Descartes’ error. New York: Grosset/ Putnam.
- Davis, M. (2000). The role of the amygdala in conditioned and unconditioned fear and anxiety. In J. P. Aggleton (Ed.), The amygdala(Vol.2,pp.213–287).Oxford,UK:OxfordUniversityPress.
- Davis, M., Falls, W. A., Campeau, S., & Kim, M. (1993). Fearpotentiated startle: A neural and pharmacological analysis. Behavioral Brain Research, 58, 175–198.
- Davis, M., & Whalen, P. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13–34.
- Dickinson,A., & Dearing, M. F. (1979).Appetitive-aversive interactionsandinhibitoryprocesses. InA.B.Dickinson&R.A.Boakes (Eds.), Mechanisms of learning and motivation (pp. 203–231). Hillsdale, NJ: Erlbaum.
- Dringenberg, H. C., & Vanderwolf, C. H. (1996). Cholinergic activation of the electrocorticogram: A8n amygdaloid activating system. Experimental Brain Research, 108, 285–296.
- Ehrlichman, H., Brown, S., Zhu, J., & Warrenburg, S. (1995). Startle reflex modulation during exposure to pleasant and unpleasant odors. Psychophysiology, 32, 150–154.
- Everitt, B. J., Cador, M., & Robbins, T. W. (1989). Interactions between the amygdala and ventral striatum in stimulus-reward associations: Studies using a second-order schedule of sexual reinforcement. Neuroscience, 30, 63–75.
- Everitt, B. J., Cardinal, R. N., Hall, J., Parkinson, J. A., & Robbins, T. W. (2000). Differential involvement of amygdala subsytems in apetitive conditioning and drug addiction. In J. P. Aggleton (Ed.), The amygdala (Vol. 2, pp. 353–390). Oxford, UK: Oxford University Press.
- Everitt, B. J., Morris, K. A., O’Brien, A., & Robbins, T. W. (1991). The basolateral amygdala-ventral striatal system and conditioned place preference: Further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience, 42(1), 1–18.
- Everitt, B. J., & Robbins, T. V. (1992). Amygdala-ventral striatal interactions and reward related processes. In J. P. Aggleton (Ed.), The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 401–429). New York: Wiley-Liss.
- Fanselow, M. S. (1991). The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In A. Depaulis & R. Bandler (Eds.), The midbrain periaqueductal gray matter: Functional, anatomical and neurochemical organization (pp. 151–173). New York: Plenum.
- Fanselow, M. S. (1994). Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin and Review, 1, 429–438.
- Fendt, M., Koch, M., & Schnitzler, H.-U. (1996). Lesions of the central gray block conditioned fear as measured with the potentiated startle paradigm. Behavioural Brain Research, 74, 127–134.
- File, S. E., & Rodgers, R. J. (1979). Partial anxiolytic actions of morphine sulphate following microinjection into the central nucleus of the amygdala in rats. Pharmacology, Biochemistry, and Behavior, 11, 313–318.
- Frankland, P. W., Josselyn, S. A., Bradwejn, J., Vaccarino, F. J., & Yeomans, J. S. (1997). Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. Journal of Neuroscience, 17, 1838–1847.
- Frankland, P. W., & Yeomans, J. S. (1995). Fear-potentiated startle and electrically evoked startle mediated by synapses in rostrolateral midbrain. Behavioral Neuroscience, 109, 669–680.
- Fredrikson, M., Wik, G., Annas, P., Ericson, K. A. J., & StoneElander, S. (1995). Functional neuroanatomy of visually elicited simple phobic fear: Additional data and theoretical analysis. Psychophysiology, 32, 43–48.
- Fredrikson, M., Wik, G., Greitz, T., Stone-Elander, S., Ericson, K.A. J., & Sedvall, G. (1993). Regional cerebral blood flow during experimental phobic fear. Psychophysiology, 30, 126–130.
- Funayama, E. S., Grillon, C. G., Davis, M., & Phelps, E. A. (2001). A double dissociation in the affective modulation of startle in humans: Effects of unilateral temporal lobectomy. Journal of Cognitive Neuroscience, 13, 721–729.
- Gallagher, M. (2000). The amygdala and associative learning. In J. P. Aggleton (Ed.), The amygdala (Vol. 2). Oxford, UK: Oxford University Press.
- Gallagher, M., Graham, P. W., & Holland, P. C. (1990). The amygdala central nucleus and appetitive pavlovian conditioning: Lesions impair one class of conditioned behavior. Journal of Neuroscience, 10, 1906–1911.
- Gallagher, M., Kapp, B. S., McNall, C. L., & Pascoe, J. P. (1981). Opiate effects in the amygdala central nucleus on heart rate conditioning in rabbits. Pharmacology, Biochemistry, and Behavior, 14, 497–505.
- Gallagher, M., Kapp, B. S., & Pascoe, J. P. (1982). Enkephalin analogue effects in the amygdala central nucleus on conditioned heart rate. Pharmacology, Biochemistry, and Behavior, 17, 217– 222.
- Gewirtz, J., & Davis, M. (1997). Second order fear conditioning prevented by blocking NMDA receptors in the amygdala. Nature, 388, 471–474.
- Gewirtz, J. C., & Davis, M. (1998). Application of Pavlovian higher-order conditioning to the analysis of the neural substrates of learning and memory. Neuropharmacology, 37, 453–460.
- Gloor, P., Olivier, A., & Quesney, L. F. (1981). The role of the amygdala in the expression of psychic phenomena in temporal lobe seizures. In Y. Ben-Ari (Ed.), The amygdaloid complex (pp. 489– 507). New York: Elsevier/North Holland.
- Goddard, G. V. (1964). Functions of the amygdala. Psychological Bulletin, 62, 89–109.
- Goldstein, L. E., Rasmusson, A. M., Bunney, B. S., & Roth, R. H. (1996). Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. Journal of Neuroscience, 16(15), 4787–4798.
- Graham, F. K. (1979). Distinguishing among orienting, defense, and startle reflexes. In H. D. Kimmel, H. van Olst, & F. Orelebeke (Eds.), The orienting reflex in humans:An international conference sponsored by the Scientific Affairs Division of the North Atlantic Treaty Organization (pp. 137–167). Hillsdale, NJ: Erlbaum.
- Graham, F. K. (1992). Attention: The heartbeat, the blink, and the brain. In B. A. Campbell, H. Hayne, & R. Richardson (Eds.), Attention and information processing in infants and adults (pp. 3–29). Hillsdale, NJ: Erlbaum.
- Graham, F. K., & Clifton, R. K. (1966). Heart rate change as a component of the orienting response. Psychological Bulletin, 65, 305–320.
- Green, S., & Vale, A. L. (1992). Role of amygdaloid nuclei in the anxiolytic effects of benzodiazepines in rats. Behavioural Pharmacology, 3, 261–264.
- Greenwald, M. K., Bradley, M. M., Cuthbert, B. N., & Lang, P. J. (1998). Sensitization of the startle reflex in humans following aversive electric shock exposure. Behavioral Neuroscience, 112, 1069–1079.
- Greenwald, M. K., Cook, E. W. I., & Lang, P. J. (1989). Affective judgement and psychophysiological response: Dimensional covariation in the evaluation of pictorial stimuli. Journal of Psychophysiology, 3, 51–64.
- Grillon, C., Ameli, R., Merikangas, K., Woods, S. W., & Davis, M. (1993). Measuring the time course of anticipatory anxiety using thefear-potentiatedstartlereflex.Psychophysiology,30,340–346.
- Grillon, C., Ameli, R., Woods, S. W., Merikangas, K., & Davis, M. (1991). Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology, 28, 588–595.
- Grillon, C., & Davis, M. (1997). Fear-potentiated startle conditioning in humans: Effects of explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology, 34, 451–458.
- Guarraci, F. A., Frohardt, R. J., & Kapp, B. S. (1999). Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Research, 827, 28–40.
- Hamm, A. O., Cuthbert, B. N., Globisch, J., & Vaitl, D. (1997). Fear and startle reflex: Blink modulation and autonomic response patterns in animal mutilation fearful subjects. Psychophysiology, 34, 97–107.
- Hamm, A. O., Greenwald, M. K., Bradley, M. M., & Lang, P. J. (1993). Emotional learning, hedonic changes, and the startle prove. Journal of Abnormal Psychology, 102, 453–465.
- Hatfield, T., & Gallagher, M. (1995). Taste-potentiated odor conditioning: Impairment produced by infusion of an N-methyl-Daspartate antagonist into basolateral amygdala. Behavioral Neuroscience, 109(4), 663–668.
- Hatfield, , Graham, P. W., & Gallagher, M. (1992). Taste-potentiated odor aversion: Role of the amygdaloid basolateral complex and central nucleus. Behavioral Neuroscience, 106, 286–293.
- Hatfield, T., Han, J.-S., Conley, M., Gallagher, M., & Holland, P. (1996). Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. Journal of Neuroscience, 16, 5256–5265.
- Hawk, L. W., & Cook, E. W. (1997). Affective modulation of tactile startle. Psychophysiology, 34, 23–31.
- Hebb, D. O. (1949). The organization of behavior. New York: Wiley.
- Heilig, M., McLeod, S., Brot, M., Henrichs, S. C., Menzaghi, F., Koob, G. F., & Britton, K. T. (1993). Anxiolytic-like action of neuropeptideY: Mediation byY1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology, 8, 357–363.
- Heinrichs, S. C., Menzaghi, F., Schulteis, G., Koob, G. F., & Stinus, L. (1995). Suppression of corticotropoin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behavioural Pharmacology, 6, 74–80.
- Heinrichs, S. C., Pich, E. M., Miczek, K. A., Britton, K. T., & Koob, F. (1992). Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Research, 581, 190–197.
- Helmstetter, F. J. (1993). Stress-induced hypoalgesia and defensive freezing are attenuated by application of diazepam to the amygdala. Pharmacology, Biochemistry, and Behavior, 44, 433–438.
- Hernandez, D. E., Salaiz, A. B., Morin, P., & Moreira, M. A. (1990). Administration of thyrotropin-releasing hormone into the central nucleus of the amygdala induces gastric lesions in rats. Brain Research Bulletin, 24, 697–699.
- Hitchcock, J. M., & Davis, M. (1986). Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience, 100, 11–22.
- Hitchcock, J. M., & Davis, M. (1991). The efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behavioral Neuroscience, 105, 826–842.
- Hodges, H., Green, S., & Glenn, B. (1987). Evidence that the amygdala is involved in benzodiazepine and serotonergic effects on punished responding but not on discrimination. Psychopharmacology, 92, 491–504.
- Holland, P. C., & Gallagher, M. (1993a). The effects of amygdala central nucleus lesions on blocking and unblocking. Behavioral Neuroscience, 107, 235–245.
- Holland, P. C., & Gallagher, M. (1993b). Amygdala central nucleus lesions disrupt increments, but not decrement, in conditioned stimulus processing. Behavioral Neuroscience, 107, 246–253.
- Irwin, W., Davidson, R. J., Lowe, M. J., Mock, B. J., Sorenson, J.A., & Turski, P. A. (1996). Human amygdala activation detected with echo-planar functional magnetic resonance imaging. NeuroReport, 7, 1765–1769.
- Ishikawa, T., Yang, H., & Tache, Y. (1988). Medullary sites of action of the TRH analogue, RX 77368, for stimulation of gastric acid secretion in the rat. Gastroenterology, 95, 1470–1476.
- Iwai, E., & Yukie, M. (1987). Amygdalofugal and amygdalepetal connetions with modality-specific visual cortical areas in Macaques (Macaca fuscata, M. mulatta, and fascicularis). Journal of Comparative Neurology, 261, 362–387.
- Junghoefer, M., Bradley, M. M., Elbert, T. R., & Lang, P. J. (2001). Pictures in a rapid stream: Early detection of emotion. Psychophysiology, 38, 175–178.
- Kapp, B. S., Supple, W. F., & Whalen, P. J. (1994). Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behavioral Neuroscience, 108, 81–93.
- Kapp, B. S., Whalen, P. J., Supple, W. F., & Pascoe, J. P. (1992). Amygdaloid contributions to conditioned arousal and sensory information processing. In J. P. Aggleton (Ed.), The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 229–254). New York: Wiley-Liss.
- Kapp, B. S., Wilson, A., Pascoe, J. P., Supple, W. F., & Whalen, P. J. (1990). A neuroanatomical systems analysis of conditioned bradycardia in the rabbit. In M. Gabriel & J. Moore (Eds.), Neurocomputation and learning: Foundations of adaptive networks (pp. 55–90). New York: Bradford Books.
- Killcross, S., Robbins, T. W., & Everitt, B. J. (1997). Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature, 388, 377–380.
- Kim, M., Campeau, S., Falls, W. A., & Davis, M. (1993). Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behavioral and Neural Biology, 59, 5–8.
- Kintsch, W. (1974). The representation of meaning in memory. Hillsdale, NJ: Erlbaum.
- Koch, M., Schmid, A., & Schnitzler, H.-U. (1996). Pleasureattenuation of startle is disrupted by lesions of the nucleus accumbens. NeuroReport, 7, 1442–1446.
- Konorski, J. (1967). Integrative activity of the brain: An interdisciplinary approach. Chicago: University of Chicago Press.
- Krase, W., Koch, M., & Schnitzler, H. U. (1994). Substance P is involved in the sensitization of the acoustic startle response by footshock in rats. Behavioral Brain Research, 63, 81–88.
- Lamont, E. W., & Kokkinidis, L. (1998). Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Research, 795, 128–136.
- Landis, C., & Hunt, W. (1939). The startle paradigm. New York: Farrar and Rinehart.
- Lane, R. D., Reiman, E. M., Bradley, M. M., Lang, P. J., Ahern, G. L., Davidson, R. J., & Schwartz, G. E. (1997). Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia, 35, 1437–1444.
- Lang, P. J. (1985). The cognitive psychophysiology of emotion: Fear and anxiety. InA. H. Tuma & J. D. Maser (Eds.), Anxiety and the anxiety disorders (Vol. 3, pp. 131–170). Hillsdale, NJ: Erlbaum.
- Lang, P. J. (1994).The motivational organization of emotion:Affectreflex connections. In S. VanGoozen, N. E. Van De Poll, & J. A. Sargeant (Eds.), Emotions: Essays on emotion theory (pp. 61–93). Hillsdale, NJ: Erlbaum.
- Lang, P. J. (1995). The emotion probe. American Psychologist, 50, 372–385.
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1990). Emotion, attention, and the startle reflex. Psychology Reviews, 97, 377– 395.
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). Motivated attention: Affect, activation and action. In P. J. Lang, R. F. Simons, & M. F. Balaban (Eds.), Attention and orienting: Sensory and motivational processes (pp. 97–135). Hillsdale, NJ: Erlbaum.
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1998). International affective picture system (IAPS). Gainesville, FL: The Center for Research in Psychophysiology, University of Florida.
- Lang, P. J., Bradley, M. M., Fitzsimmons, J. R., Cuthbert, B. N., Scott, J. D., Moulder, B., & Nangia, V. (1998). Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology, 35, 1–13.
- Lang, P. J., Greenwald, M. K., Bradley, M. M., & Hamm, A. O. (1993). Looking at Pictures: Affective, facial, visceral and behavioral reactions. Psychophysiology, 30, 261–273.
- LeBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E., & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20, 937–945.
- LeBar, K. S., LeDoux, J. E., Spencer, D. D., & Phelps, E. A. (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience, 15, 6846–6855.
- LeDoux, J. E., Iwata, J., Cicchetti, P., & Reis, D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience, 8, 2517–2529.
- Lee, G. P., Bechara,A.,Adolphs, R.,Arena, J., Meador, K. J., Loring, D.W., & Smith, J. R. (1998). Clinical and physiological effects of stereotaxic bilateral amygdalotomy for intractable aggression. Journal of Neuropsychiatry and Clinical Neurosciences, 10, 413–420.
- Lee, Y., & Davis, M. (1996). The role of bed nucleus of the stria terminalis in CRH-enhanced startle: An animal model of anxiety. Society for Neuroscience Abstracts, 22,
- Li, L., & Yeomans, J. S. (1999). Summation between acoustic and trigeminal stimuli evoking startle. Neuroscience, 90, 139–152.
- Liebsch, G., Landgraf, R., Gerstberger, R., Probst, J. C., Wotjak, C. T., Engelmann, M., Holsboer, F., & Montkowski, A. (1995). Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regulatory Peptides, 59, 229–239.
- Lipp, O.V., Sheridan, J., & Siddle, D.A. (1994). Human blink startle duringaversiveandnonaversivePavlovianconditioning.Journal of Experimental Psychology: Animal Behavioral Processes, 20, 380–389.
- Maldonado, R., Stinus, L., Gold, L. H., & Koob, G. F. (1992). Role of different brain structures in the expression of the physical morphine withdrawal syndrome. Journal of Pharmacology and Experimental Therapeutics, 261(2), 669–677.
- Masur, J. D., Dienst, F. T., & O’Neal, E. C. (1974). The acquisition of a Pavlovian conditioned response in septally damaged rabbits: Role of a competing response. Physiological Psychology, 2, 133–136.
- McCall, R. B., & Aghajanian, G. K. (1979). Serotonergic facilitation of facial motoneuron excitation. Brain Research, 169, 11–27.
- McDonald, A. J. (1991). Topographic organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striateal-like areas of the rat brain. Neuroscience, 44(1), 15–33.
- McDonald, A. J. (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55, 257–332.
- McGaugh, J. L., Introini-Collison, I. B., Cahill, L., Castellano, C., Dalmaz, C., Parent, M. B., & Williams, C. L. (1993). Neuromodulatory systems and memory storage: Role of the amygdala. Behavioural Brain Research, 58, 81–90.
- McGaugh, J. L., Introini-Collison, I. B., Cahill, L., Kim, M., & Liang, K. C. (1992). Involvement of the amygdala in neuromodulatory influences on memory storage. In J. P. Aggleton (Ed.), The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction (pp. 431–451). New York: Wiley-Liss.
- Mehrabian, A., & Russell, J. A. (1974). An approach to environmental psychology. Cambridge, MA: MIT Press.
- Meloni, E. G., & Davis, M. (1999). Muscimol in the deep layers of the superior colliculus/mesencephalic reticular formation blocks expression but not acquisition of fear-potentiated startle in rats. Behavioral Neuroscience, 113, 1152–1160.
- Miller, N. E. (1959). Liberalization of basic S-R concepts: Extensions to conflict behavior, motivation and social learning. In S. Koch (Ed.), Psychology: A study of a science (Vol. 2, pp. 196–292). New York: McGraw-Hill.
- Miltner, W., Matjak, M., Braun, C., & Diekmann, H. (1994). Emotional qualities of odors and their influence on the startle reflex in humans. Psychophysiology, 31, 107–110.
- Mineka, S., Davidson, M., Cook, M., & Keir, R. (1984). Observational conditioning of snake fear in rhesus monkeys. Journal of Abnormal Psychology, 93, 355–372.
- Morgenson, G. M. (1987). Limbic-motor intergration. In A. Epstein & A. R. Morrison (Eds.), Progress in psychobiology and physiological psychology (pp. 117–170). New York: Academic Press.
- Morris, J. S., Frith, C. D., Perrett, D. I., & Rowland, D. (1996). A differential neural response in the human amygdala to fearful and happy facial expression. Nature, 383, 812–815.
- Morris, J. S., Ohman, A., & Dolan, R. J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467–470.
- Morris, M., Bradley, M., Bowers, D., Lang, P., & Heilman, K. (1991). Valence-specific hypoarousal following right temporal lobectomy. Journal of Clinical and Experimental Neuropsychology, 13,
- Morrow, N. S., Hodgson, D. M., & Garrick, T. (1996). Microinjection of thyrotropin-releasing hormone analogue into the central nucleus of the amygdala stimulates gastric contractility in rats. Brain Research, 735, 141–148.
- Ortony, A., Clore, G. L., & Collins, A. (1988). The cognitive structure of emotions. Cambridge, UK: Cambridge University Press.
- Osgood, C., Suci, G., & Tannenbaum, P. (1957). The measurement of meaning. Urbana: University of Illinois.
- Packard, M. G., Cahill, L., & McGaugh, J. L. (1994). Amygdala modulation of hippocampal-dependent and caudate nucleusdependent memory processes. Proceedings of the National Academy of Sciences, USA, 91, 8477–8481.
- Packard, M. G., & Teather, L. A. (1998). Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiology of Learning and Memory, 69, 163–203.
- Parkinson, J. A., Dally, J. W., Bamford, A., Fehrent, B., Robbins, T. W., & Everitt, B. J. (1998). Effects of 6-OHDA lesions of the rat nucleus accumbens on appetitive Pavlovian conditioning. Journal of Psychopharmacology, 12,
- Parkinson, J. A., Robbins, T. W., & Everitt, B. J. (2000). Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. European Journal of Neuroscience, 12, 405–413.
- Parkinson, J. A., Willoughby, P. J., Robbins, T. W., & Everitt, B. J. (2000). Disconnection of the anterior cingulater cortex and nucleus accumbens core impairs Pavlovian approach behavior: Further evidence for limbic cortico-ventral striatopallidal systems. Behavioral Neuroscience, 114, 42–63.
- Pascoe, J. P., & Kapp, B. S. (1985). Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behavioral Brain Research, 16, 117–133.
- Pavlov, I. P. (1927). Conditioned reflexes. Oxford, UK: Oxford University Press.
- Pesold, C., & Treit, D. (1995). The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Research, 671, 213–221.
- Petersen, E. N., Braestrup, C., & Scheel-Kruger, J. (1985). Evidence that the anticonflict effect of midazolam in amygdala is mediated by the specific benzodiazepine receptor. Neuroscience Letters, 53, 285–288.
- Phelps, E. A., O’Connor, K. J., Cunningham, W. A., Funayama, E. S., Gatenby, J. C., Gore, J. C., & Banaji, M. R. (2000). Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience, 12, 729–738.
- Phelps, E. A., O’Connor, K. J., Gatenby, J. C., Gore, J. C., Grillon, C., & Davis, M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4, 437– 441.
- Quirk, G. J., Repa, J. C., & LeDoux, J. E. (1995). Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron, 15, 1029–1039.
- Rassnick, S., Heinrichs, S. C., Britton, K. T., & Koob, G. F. (1993). Microinjection of a corticotroin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Research, 605, 25–32.
- Ray, A., & Henke, P. G. (1990). Enkephalin-dopamine interactions in the central amygdalar nucleus during gastric stress ulcer formation in rats. Behavioural Brain Research, 36, 179–183.
- Ray, A., & Henke, P. G. (1991). TRH-enkephalin interactions in the amygdaloid complex during gastric stress ulcer formation in rats. Regulatory Peptides, 35, 11–17.
- Ray, A., Henke, P. G., & Sullivan, R. M. (1990). Effects of intraamygdalar thyrotropin releasing hormone (TRH) and its antagonism by atropine and benzodiazepines during stress ulcer formationinrats.Pharmacology,Biochemistry,andBehavior,36, 597–601.
- Ray, A., Sullivan, R. M., & Henke, P. G. (1988). Interactions of thyrotropin-releasing hormone (TRH) with neurotensin and dopamine in the central nucleus of the amygdala during stress ulcer formation in rats. Neuroscience Letters, 91, 95–100.
- Redmond, D. E., Jr. (1977). Alteration in the function of the nucleus locus: A possible model for studies on anxiety. In I. E. Hanin & E. Usdin (Eds.), Animal models in psychiatry and neurology (pp. 292–304). Oxford, UK: Pergamon Press.
- Reiman, E. M., Lane, R. D., Ahern, G. L., Schwartz, G. E., Davidson, R. J., Friston, K. J., Yun, L., & Chen, K. (1997). Neuroanatomical correlates of externally and internally generated human emotion. American Journal of Psychiatry, 54, 918– 925.
- Robledo, P., Robbins, T. W., & Everitt, B. J. (1996). Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behavioral Neuroscience, 110, 981–990.
- Roozendaal, B., Schoorlemmer, G. H., Koolhaas, J. M., & Bohus, B. (1993). Cardiac, neuroendocrine, and behavioral effects of central amygdaloid vasopressinergic and oxytocinergic mechanisms under stress-free conditions in rats. Brain Research Bulletin, 32, 573–579.
- Roozendaal, B., Wiersma, A., Driscoll, P., Koolhaas, J. M., & Bohus, B. (1992). Vasopressinergic modulation of stress responses in the central amygdala of the Roman high-avoidance and low-avoidance rat. Brain Research, 596, 35–40.
- Rosen, J. B., Hitchcock, J. M., Miserendino, M. J. D., Falls, W. A., Campeau, S., & Davis, M. (1992). Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. Journal of Neuroscience, 12, 4624–4633.
- Rosen, J. B., Hitchcock, J. M., Sananes, C. B., Miserendino, M. J. D., & Davis, M. (1991). Adirect projection from the central nucleus of the amygdala to the acoustic startle pathway: Anterograde and retrograde tracing studies. Behavioral Neuroscience, 105, 817–825.
- Sabatinelli, D., Bradley, M. M., Cuthbert, B. N., & Lang, P. J. (1996). Wait and see: Aversion and activation in anticipation and perception. Psychophysiology, 33,
- Sajdyk, T. J., Schober, D. A., Gehlert, D. R., & Shekhar, A. (1999). Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behavioural Brain Research, 100, 207–215.
- Sajdyk, T. J., & Shekhar, A. (1997a). Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by y-aminobutyric acid—a receptor blockade in the basolateral amygdala of rats. Journal of Pharmacology and Experimental Therapeutics, 283, 969–977.
- Sajdyk, T. J., & Shekhar, A. (1997b). Excitatory amino acid receptors in the bsolateral amygdala regulate anxiety responses in the social interaction test. Brain Research, 764, 262–264.
- Sajdyk, T. J., Vandergriff, M. G., & Gehlert, D. R. (1999). Amygdalar neuropeptide Y Y-1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. European Journal of Pharmacology, 368, 143–147.
- Sakanaka, M., Shibasaki, T., & Lederis, K. (1986). Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Research, 382, 213–238.
- Sananes, C. B., & Davis, M. (1992). N-Methyl-D-Aspartate lesions of the lateral and basolateral nuclei of the amygdala block fearpotentiated startle and shock sensitization of startle. Behavioral Neuroscience, 106, 72–80.
- Sanders, S. K., & Shekhar, A. (1991). Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Research, 576, 101–110.
- Sanders, S. K., & Shekhar, A. (1995). Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacology, Biochemistry, and Behavior, 52(4), 701–706.
- Sanghera, M. K., Rolls, E. T., & Roper-Hall, A. (1979). Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Experimental Neurology, 63, 610–626.
- Scheel-Kruger, J., & Petersen, E. N. (1982). Anticonflict effect of the benzodiazepines mediated by a GABAergic mechanism in the amygdala. European Journal of Pharmacology, 82, 115–116.
- Schlosberg, J. (1952). The description of facial expression in terms of two dimensions. Journal of Experimental Psychology, 44, 229–237.
- Schmid, A., Koch, M., & Schnitzler, H.-U. (1995). Conditioned pleasure attenuates the startle response in rats. Neurobiology of Learning and Memory, 64, 1–3.
- Schneider, W., Noll, D., & Cohen, J. (1993). Functional topographic mapping of the cortical ribbon in human vision with conventional MRI scanners. Nature, 365, 150–153.
- Schneiderman, N. (1972). Response system divergencies in aversive classical conditioning. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning: Vol. 3. Current research and theory (pp. 341–376). New York: Appleton-Century-Crofts.
- Schneirla, T. (1959). An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In M. Jones (Ed.), Nebraska symposium on motivation (pp. 1–42). Lincoln: University of Nebraska Press.
- Schoenbaum,G.,Chiba,A.A.,&Gallagher,M.(1998).Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience, 1, 155–159.
- Schoenbaum, G., Chiba, A. A., & Gallagher, M. (1999). Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience, 19, 1876–1884.
- Schwaber, J. S., Kapp, B. S., Higgins, G. A., & Rapp, P. R. (1982). Amygdaloid basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. Journal of Neuroscience, 2, 1424–1438.
- Shaver, P., Schwartz, J., Kirson, D., & O’Connor, C. (1987). Emotion knowledge: Further exploration of a prototype approach. Journal of Personality and Social Psychology, 52, 1061–1086.
- Shi, C., & Davis, M. (1996). Anatomical tracing and lesion studies of visual pathways involved in fear conditioning measured with fear potentiated startle. Society for Neuroscience Abstracts, 22,
- Shibata, K., Kataoka, Y., Yamashita, K., & Ueki, S. (1986). An important role of the central amygdaloid nucleus and mammillary body in the mediation of conflict behavior in rats. Brain Research, 372, 159–162.
- Shors, T. J., & Mathew, P. R. (1998). NMDAreceptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learning and Memory, 5, 220–230.
- Sokolov, E. N. (1963). Perception and the conditioned reflex. Oxford, UK: Pergamon Press.
- Soltis, R. P., Cook, J. C., Gregg, A. E., Stratton, J. M., & Flickinger, K. A. (1998). EAA receptors in the dorsomedial hypothalamic area mediate the cardiovascular response to activation of the amygdala. American Journal of Physiology, 275(2, Pt. 2), R624– R631.
- Soltis, R. P., Cook, J. C., Gregg, A. E., & Sanders, B. J. (1997). Interaction of GABA and excitatory amino acids in the basolateral amygdala: Role in cardiovascular regulation. Journal of Neuroscience, 17, 9367–9374.
- Stinus, L., LeMoal, M., & Koob, G. F. (1990). Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience, 37(3), 767–773.
- Sullivan, R. M., Henke, P. G., Ray, A., Hebert, M. A., & Trimper, J. M. (1989). The GABA/benzodiazepine receptor complex in the central amygdalar nucleus and stress ulcers in rats. Behavioral and Neural Biology, 51, 262–269.
- Swiergiel, A. H., Takahashi, L. K., & Kalin, N. H. (1993). Attenuation of stress-induced behavior by antagonism of corticotropinreleasing factor in the central amygdala of the rat. Brain Research, 623, 229–234.
- Takao, K., Nagatani, T., Kasahara, K.-I., & Hashimoto, S. (1992). Role of the central serotonergic system in the anticonflict effect of d-AP159. Pharmacology, Biochemistry, and Behavior, 43, 503–508.
- Taylor, J. R., Punch, L. J., & Elsworth, J. D. (1998). A comparison of the effects of clonidine and CNQX infusion into the locus coeruleus and the amygdala on naloxone-precipitated opiate withdrawal in the rat. Psychopharmacology, 138, 133–142.
- Taylor, J. R., & Robbins, T. W. (1984). Enhanced behavioral control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology, 84, 405–412.
- Taylor, J. R., & Robbins, T. W. (1986). 6-hydroxydopamine lesions of the nucleus accumbens, but not the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology, 90, 390–397.
- Tellegen, A. (1985). Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In A. H. Tuma & J. D. Maser (Eds.), Anxiety and the anxiety disorders (pp. 681–706). Hillsdale, NJ: Erlbaum.
- Thomas, S. R., Lewis, M. E., & Iversen, S. D. (1985). Correlation of [3H]diazepam binding density with anxiolytic locus in the amygdaloid complex of the rat. Brain Research, 342, 85–90.
- Timberlake, W. (1993). Behavior systems and reinforcement: An intergrative approach. Journal of Experimental Analysis of Behavior, 60, 105–128.
- Timberlake, W., & Lucas, G. A. (1989). Behavior systems and learning: From misbehavior to general principles. In S. B. Klein & R. R. Mowrer (Eds.), Instrumental conditioning theory and the impact of biological constraints on learning (pp. 237–275). Hillsdale, NJ: Erlbaum.
- Ursin, H., & Kaada, B. R. (1960). Functional localization within the amygdaloid complex in the cat. Electroencephalography and Clinical Neurophysiology, 12, 109–122.
- Vrana, S. R., Spence, E. L., & Lang, P. J. (1988). The startle probe response: A new measure of emotion? Journal of Abnormal Psychology, 97, 487–491.
- Walker, D. L., & Davis, M. (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in light-enhanced versus fearpotentiated startle. Journal of Neuroscience, 17, 9375–9383.
- Weisz, D. J., & McInerney, J. (1990). An associative process maintains reflex facilitation of the unconditioned nictitating membrane response during the early stages of training. Behavioral Neuroscience, 104, 21–27.
- Whalen, P. J. (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–188.
- Whalen, P. J., & Kapp, B. S. (1991). Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behavioral Neuroscience, 105, 141–153.
- White, S. R., & Neuman, R. S. (1980). Facilitation of spinal motoneuron excitability by 5-hydroxytryptamine and noradrenaline. Brain Research, 185, 1–9.
- Wiersma, A., Baauw, A. D., Bohus, B., & Koolhaas, J. M. (1995). Behavioural activation produced by CRH but not -helical CRH (CRH-receptor antagonist) when microinfused into the central nucleus of the amygdala under stress-free conditions. Psychoneuroendocrinology, 20(4), 423–432.
- Wiersma, A., Bohus, B., & Koolhaas, J. M. (1993). Corticotropinreleasing hormone microinfusion in the central amygdala diminishes a cardiac parasympathetic outflow under stress-free conditions. Brain Research, 625, 219–227.
- Wiersma, A., Bohus, B., & Koolhaas, J. M. (1997). Corticotropinreleasing hormone microinfusion in the central amygdala enhances active behaviour responses in the conditioned burying paradigm. Stress, 1, 113–122.
- Wiersma, A., Tuinstra, T., & Koolhaas, J. M. (1997). Corticotropinreleasing hormone microinfusion into the basolateral nucleus of the amygdala does not induce any changes in cardiovascular, neuroendocrine or behavioural output in a stress-free condition. Unpublished doctoral dissertation, University of Groningen, The Netherlands.
- Willcox, B. J., Poulin, P., Veale, W. L., & Pittman, Q. J. (1992). Vasopressin-induced motor effects: Localization of a sensitive site in the amygdala. Brain Research, 596, 58–64.
- Wundt, W. (1896). Grundriss der Psychologie (Outlines of psychology). Leipzig, Germany: Entgelman.
- Yeomans, J. S., Steidle, S., & Li, L. (2000). Conditioned brainstimulation reward attenuates the acoustic startle reflex in rats. Society for Neuroscience Abstracts, 30,
- Young, A. W., Aggleton, J. P., Hellawell, D. J., Johnson, M., Broks, P., & Hanley, J. R. (1995). Face processing impairments after amygdalotomy. Brain, 118, 15–24.
- Young, B. J., Helmstetter, F. J., Rabchenuk, S. A., & Leaton, R. N. (1991). Effects of systemic and intra-amygdaloid diazepam on long-term habituation of acoustic startle in rats. Pharmacology, Biochemistry, and Behavior, 39, 903–909.




