View sample biological models of associative learning research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Over the course of the past century extraordinary progress has been made in our understanding of the behavioral properties of basic associative learning and memory and the manner in which the nervous system codes, stores, and retrieves these memories.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Current views recognize a number of different forms or aspects of learning and memory involving different neural systems (Figure 18.1; Squire & Knowlton, 1994). Many workers distinguish between declarative and nondeclarative or procedural memory. Declarative memory generally refers to explicit memories of “what,” that is, one’s own previous experiences, recognition of similar scenes and objects, and so on. That is clearly slanted toward human verbal memory; some workers have even equated it with the information one can be aware of. However, recognition memory clearly occurs in all mammals studied and even in some invertebrate preparations.
Here we focus on nondeclarative, implicit, or procedural memory: memory of “how.” The vast majority of memory processes in infrahuman animals, and many aspects of memory in humans, is of this sort. Consider all your likes and dislikes, all the skilled movements you perform (tennis, golf, swimming, bicycle riding, not to mention walking and talking), and so on. Procedural is really a grab-bag category; it even includes some aspects of recognition memory, as in visual priming memory.
The categories of memory shown in Figure 18.1 are of course somewhat arbitrary and by no means mutually exclusive. When an organism learns something important, several of these memory systems can become engaged. At a more general level, all aspects of learning share a common thrust.As Rescorla (1988) stressed, basic associative learning is the way organisms, including humans, learn about causal relationships in the world. It results from exposure to relations among events in the world. For both modern Pavlovian and cognitive views of learning and memory, the individual learns a representation of the causal structure of the world and adjusts this representation through experience to bring it in tune with the real causal structure of the world, striving to reduce any discrepancies or errors between its internal representation and external reality (see also Dudai, 1989; Rescorla & Wagner, 1972; Wagner & Rescorla, 1972).
Nonassociative learning involves the effect of a single event on response probability. The three examples of nonassociative learning that have received the most attention are habituation, dishabituation, and sensitization. Habituation is defined as a reduction in responding to a repeatedly delivered stimulus where adaptation and fatigue do not contribute to the decremented response (see R. F. Thompson & Spencer, 1966). Dishabituation referstotherestorationorrecoveryofahabituatedresponseby the presentation of another, typically strong stimulus to the animal. Sensitization is an enhancement or augmentation of a response produced by the presentation of a strong stimulus. In vertebrate systems, at least, dishabituation appears to be an instance of sensitization (Groves &Thompson, 1970).
Associative learning is a very broad category that includes much of the learning we do, from learning to be afraid to learning to talk to learning a foreign language to learning to play the piano. In essence, associative learning involves the formation of associations among stimuli and responses. It is generally subdivided into classical versus instrumental conditioning or learning. Classical or Pavlovian conditioning refers to the procedure in which a neutral stimulus, termed a conditioned stimulus (CS), is paired with a stimulus that elicits a response, termed an unconditioned stimulus (US), for example, food that elicits salivation or a shock to the foot that elicits limb withdrawal. Instrumental learning or operant conditioning describes a situation in which the animal or person must perform some response in order to obtain a reward or avoid punishment. That is, the subject can control the occurrence of the US.
Classical or Pavlovian conditioning according to the traditional view is a procedure that refers to the operation of pairing one stimulus, the CS, with a second stimulus, the US, as just noted. The US reliably elicits a response prior to pairing with the CS, termed the unconditioned response (UR). Repeated pairings of the CS and US result in the CS eliciting a response defined as the conditioned response (CR). Critically important variables are order (the CS proceeds the US), timing (the interval between CS and US is critical for most examples of conditioning), and contiguity (the pairing or contiguity of the CS and US is necessary for conditioning). Conditioning procedures in which the CS and US overlap in time are called delay conditioning, whereas trace conditioning consists of a procedure in which a time interval of no stimulation exists between the CS and US. It is often, but not always, the case that CR is similar to the UR (i.e., in Pavlov’s experiment both are salivation).
A more general and contemporary view of Pavlovian conditioning emphasizes the relationship between the CS and US. That is, the information that the CS provides about the occurrence of the US is the critical feature for learning. This perspective on Pavlovian conditioning is consistent with current cognitive views of learning and memory, as noted earlier. Thus, the generation of a new response to the CS that has properties similar to the US is viewed as less important. Indeed, in some situations the CR is quite different from the UR: foot shock causes an increase in activity (UR) in the rat; fear learned to a tone paired with this same foot shock is expressed as freezing (CR). But note that both responses are adaptive. Instead, as noted earlier, conditioning involves learning about the relations between events in the organism’s environment. In this view, the key process is the contingencies among events in the organism’s environment. Consider the following experiment. A group of rats is given a series of paired-tone CS–foot-shock US trials and learns very well to freeze (the CR) when the CS occurs. Another group of rats is given the same number of paired CS-US trials but is also given a number of US-alone presentations as well. Animals in this group do not learn to freeze to the CS at all. Both groups had the same number of contiguous pairings of CS and US, but the contingency—the probability that CS would be followed by US—was very much lower in the group given US-alone trials as well (see Rescorla, 1988).
Our focus in this research paper is on classical conditioning, but we also consider examples of instrumental learning, particularly processes of instrumental avoidance that relate closely to the phenomena of classical conditioning with an aversive US. Analysis of possible mechanisms underlying processes of learning and memory has been greatly facilitated by the use of model systems—simplified preparations in animals in which cellular and molecular mechanisms underlying behavioral plasticity can be analyzed. A number of invertebrate preparations have been used as model systems; spinal reflexes have served as a vertebrate model system. We review this literature briefly here; the major focus of this research paper is brain substrates of learning and memory in behaving mammals.
Invertebrate Preparations
Certain invertebrate nervous systems have several advantages for analysis of mechanisms of plasticity: They may contain from hundreds to thousands of neurons in contrast to the billions of neurons in vertebrate nervous systems. Many of the neurons are large and can be identified as unique. Circuits can be identified that exhibit plasticity and have only a small number of neurons. As J. M. Beggs et al. (1999) noted,
For many years, the general belief was that the small number of neurons found in most invertebrates limits their behavioral capabilities to only the simplest forms of behavioral modifications such as habituation and sensitization. However, it has become clear that even invertebrates exhibit more complex behavioral modifications such as classical conditioning, operant conditioning, and higher-order forms of classical conditioning (p. 1415).
In the chapter on basic mechanisms and systems of learning and memory in the recent text on Fundamental Neuroscience (Zigmond, Bloom, Landis, Roberts, & Squire, 1999), J. M. Beggs et al. (1999) provided a very helpful summary of invertebrate preparations that have proved useful for providing insights into possible mechanisms underlying learning. We present their summary, unchanged by any editorial comments we might make (J. M. Beggs et al., 1999, Box 55.1, pp. 1416–1417). They treat Aplysia and Hermissenda separately, as do we. The focus of their summary is on laboratory studies, where analysis of mechanisms can to some degree be done, as opposed to the often-rich behavioral phenomena exhibited by some invertebrate species in the natural or “ethological” state, as in the dance of the honeybee.
Gastropod Mollusks
Pleurobranchaea
The opisthobranch Pleurobranchaea is a voracious marine carnivore. When exposed to food, the animal exhibits a characteristic bite-strike response. After pairing a food stimulus (CS) withastrongelectricshocktotheoralveil(US),theCS,instead of eliciting a bite strike response, elicits a withdrawal and suppression of feeding responses (conditionedresponse, CR). The task is acquired within a few trials and is retained for up to 4 weeks. Neural correlates of associative learning have been analyzed by examining responses of various identified neurons in the circuit to chemosensory inputs in animals that have been conditioned. One correlate is an enhanced inhibition of command neurons for feeding (London and Gillette, 1986).
Tritonia
The opisthobranch Tritonia diomedea undergoes a stereotypic rhythmic swimming behavior in an attempt to escape a noxious stimulus. This response exhibits both habituation and sensitization and involves changes in multiple components of swim behavior in each case (Frost et al., 1996). The neural circuit consists of sensory neurons, pre-central pattern generating (CPG) neurons, CPG neurons and motor neurons. Habituation appears to involve plasticity at multiple loci, including decrement at the first afferent synapse. Sensitization appears to involve an enhanced excitability and synaptic strength of one of the CPG interneurons.
Pond Snail (Lymnaea stagnalis)
The pulmonate Lymnaea stagnalis exhibits fairly rapid nonaversive conditioning of feeding behavior. A neutral chemical or mechanical stimulus (CS) to the lips is paired with a strong stimulant of feeding such as sucrose (US) (Kemenes and Benjamin, 1994). Greater levels of rasping, a component of the feeding behavior, can be produced by a single trial, and this response can persist for at least 19 days. The circuit consists of a network of three types of CPG neurons, ten types of motor neurons and a variety of modulatory interneurons. An analogue of the learning occurs in the isolated central nervous system. The enhancement of the feeding motor program appears to be due to an increased activation of the CPG cells by mechanosensory inputs from the lips.
Land Snail (Helix)
Food-avoidance conditioning procedures similar to those used with Pleurobranchaea have been adopted for use in the land snail. A food stimulus such as a piece of carrot (CS) is paired with an electric shock to the dorsal surface of the snail (US). After 5–15 pairings, the carrot, instead of eliciting a feeding response, elicits a withdrawal and suppression of feeding responses. The transmitter serotonin appears to have a critical role in learning. Animals injected with a toxin that destroys serotonergic neurons exhibit normal responses to the food and the shocks alone, but are incapable of learning. Helix also exhibit habituation and sensitization of avoidance responses elicited by tactile stimuli (Balaban, 1993).
Limax
The pulmonate Limax is an herbivore that locomotes toward desirable food odors making it well suited for foodavoidance conditioning. The slug’s normal attraction to a preferred food odor (CS) is significantly reduced when the preferred odor is paired with a bitter taste (US). In addition to this example of classical conditioning, food-avoidance in Limax exhibits higher-order features of classical conditioning such as blocking and second-order conditioning. An analogue of taste-aversion learning has been shown to occur in the isolated central nervous system, which will facilitate subsequent cellular analyses of learning in Limax. The procerebral (PC) lobe in the cerebral ganglion processes olfactory information and is a likely site for the plasticity (Gelperin, 1994).
Arthropods
Cockroach (Periplaneta americana) and Locust (Schistocerca gregaria)
Learned modifications of leg positioning in the cockroach and locust may serve as a valuable preparation for the cellular analysis of operant conditioning. In this preparation, the animal is suspended over a dish containing a fluid. Initially, the insect makes many movements, including those that cause the leg to come in contact with the liquid surface. If contact with the fluid is paired with an electric shock, it learns rapidly to hold its foot away from the fluid. Neural correlates of the conditioning have been observed in somata of the leg motor neurons. These correlates include changes in intrinsic firing rate and membrane conductance.
Crayfish (Procambarus clarkii)
The crayfish tailflip response exhibits habituation and sensitization. A key component of the circuit is a pair of large neurons called the Lateral Giants (LGs), which run the length of the animal’s nerve cord. The LGs are the decision and command cells for the tailflip. Learning is related to changes in the strength of synaptic input driving the LGs.
Honeybee (Apis mellifera)
Honeybees, like other insects, are superb at learning. For example, sensitization of the antenna reflex of Apis mellifera is produced as a result of presenting gustatory stimuli to the antennae. Classical conditioning of feeding behavior can be produced by pairing visual or olfactory CSs with sugar solutions (US) to the antennae. The small size of bee neurons is an obstacle in pursuing detailed cellular analyses of these behavioral modifications. Nevertheless, regions of the brain necessary for associative learning have been identified, and some neural correlates have been described. In particular, intracellular recordings have revealed that one identified cell, the ventral unpaired median (VUM) neuron, is sufficient to mediate the reinforcing effects of the US (Hammer and Menzel, 1995).
Drosophila
Since the neural circuitry in the fruit fly is both complex and inaccessible, the fly might seem to be an unpromising subject for studying the neural basis of learning. However, the ease with which genetic studies are performed compensates for the difficulty to perform electrophysiological studies (DeZazzo and Tully, 1995). A frequently used paradigm is a two-stage differential odor-shock avoidance procedure, which is performed on large groups of animals simultaneously rather than on individual animals. Animals learn to avoid odors paired (CS) with shock but not one explicitly unpaired (CS). This learning is typically retained for 4–6 hours, but 24 hours to 1-week retention can be produced by a spaced training procedure. Several mutants deficient in learning have been identified. Many of these mutants affect elements of the cAMP-signaling pathway. Recent experiments using inducible genes demonstrate a role for cAMP-responsive transcription factors in the induction of long-term memory. These transcription factors are also important for long-term memory in Aplysia, and in vertebrates.
Annelids
Leech
Defensive reflexes in the leech (Hirudo medicinalis) exhibit habituation, dishabituation, sensitization and classical conditioning.Forexample, the shortening response is enhanced following pairing of a light touch to the head (CS) with electric shock to the tail (US). The identified S cells appear critical for sensitization, as their ablation disrupts sensitization. Interestingly, ablation of the S cells only partly disrupts dishabituation, indicating that separate processes contribute to dishabituation and sensitization (Sahley, 1995). Separate processes also contribute to dishabituation and sensitization in Aplysia. The transmitter serotonin (5-HT) appears to mediate at least part of the reinforcing effects of sensitizing stimuli and the US. Serotonin appears to play similar roles in Aplysia, Helix, Hermissenda and Tritonia.
Nematoda
Caenorhabditis elegans
Although analyses in C. elegans are just beginning, this animal promises to be a valuable preparation for the cellular and molecular studies of learning. Its principal advantages are threefold. First, its nervous system is extremely simple. It has a total of 302 neurons, all of which have been described in terms of their locations and synaptic connections. Second, the developmental lineage of each neuron is completely specified. Third, it is amenable to genetic and molecular manipulations. Recently, the animal has been shown to exhibit several forms of learning. When a vibratory stimulus is applied to the medium upon which they locomote, adult C. elegans will swim backward. This reaction, known as the tap withdrawal reflex, exhibits habituation, dishabituation, sensitization, long-term (24-hr) retention of habituation training, and context conditioning.Although the neurons are small and difficult to record, aspects of the neural circuit have been described. The particular role of individual neurons is being elucidated using laser ablation to remove specific neurons from the circuit (Wicks and Rankin, 1995).
Aplysia
The marine mollusk Aplysia has a relatively simple nervous system with large, identifiable neurons that are accessible for detailed anatomical, biophysical, and biochemical studies. Neurons and neural circuits that mediate many behaviors in Aplysia have been identified in heroic studies by Eric Kandel andhismanyassociates(Kandel,1976).Inseveralcases,these behaviors have been shown to be modified by experience.
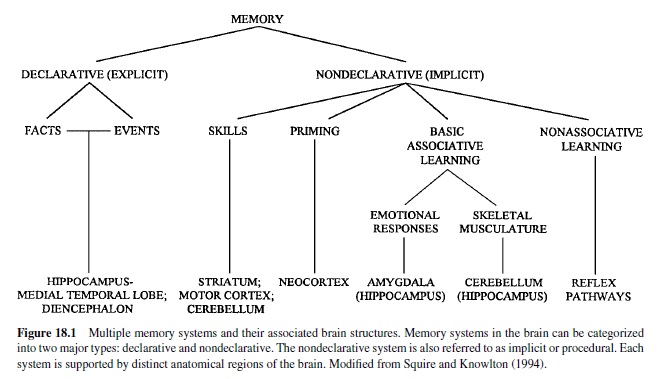
Two preparations have been particularly useful: thesiphongill withdrawal reflex and the tail-siphon withdrawal reflex (Figure 18.2). In the siphon-gill reflex, tactile or electrical stimulation of the siphon causes withdrawal of the siphon and gill, asimple defensive reflex.Stimulation of the tail of the animal elicits a set of defensive responses, including withdrawal of the tail and siphon. Relatively simple neuronal circuits mediate these reflexes. Indeed, the neural circuits subserving these reflexes can be isolated, with siphon-gill and tail connected, or can be completely isolated from body tissues and studied as neural networks.The key feature of these circuits is that the sensory neurons have monosynaptic connections to the motor neurons.
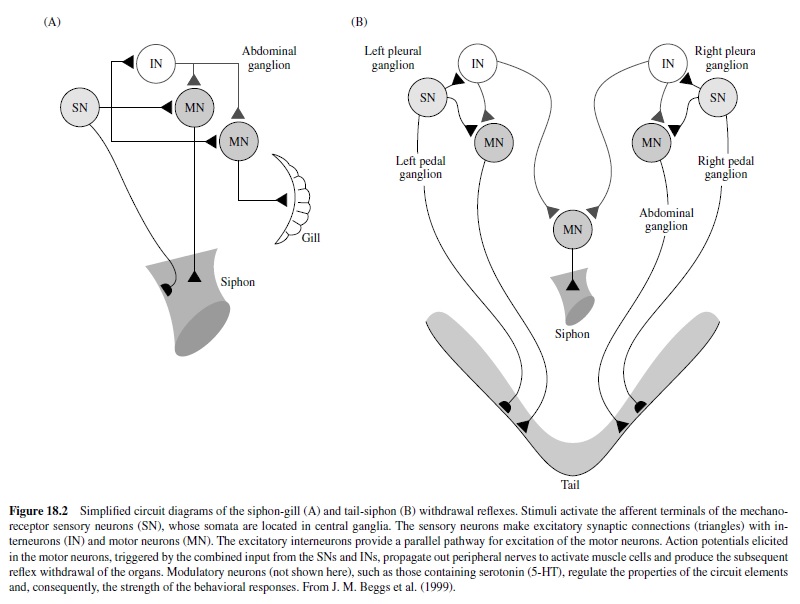
These circuits exhibit habituation, sensitization, and classical conditioning (see Byrne & Kandel, 1996). Most of the analytic work on classical conditioning has actually been done with sensitization. Short-term sensitization is induced by a single brief train of shock to the body wall (or appropriate nerves) that cause release of modulatory neurotransmitters (e.g., serotonin) from interneurons onto the sensory neurons to enhance transmitter release. The mechanism involves activation of adenylyl cyclase, which leads to increased cAMP in the sensory neurons. This results in protein phosphorylation, which alters membrane channels in the neurons, resulting in membrane depolarization, enhanced excitability, and an increase in the duration of the action potential (Figure 18.3). Other synergistic processes also occur. The net result is that stimulation of the sensory neurons results in increased probability of transmitter release at their terminals and a larger postsynaptic response in the motor neurons. Note that the plasticity here is a presynaptic phenomenon.
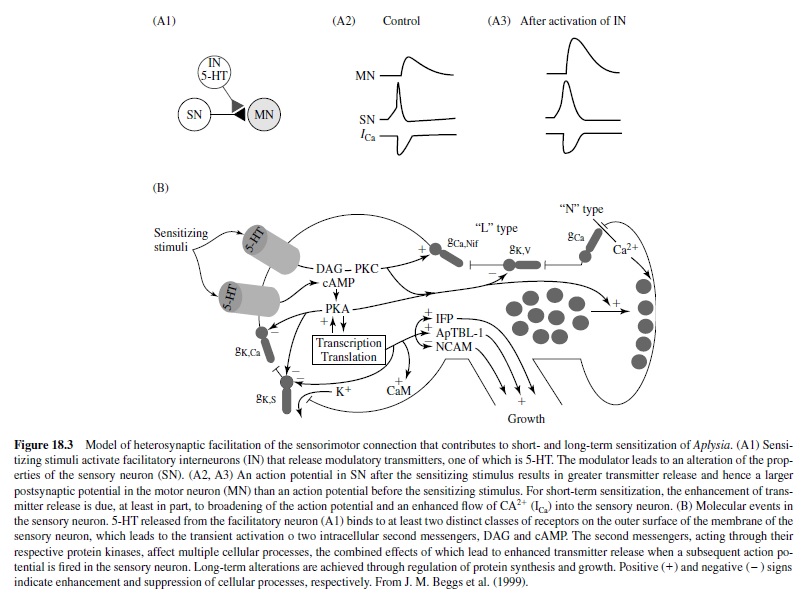
Long-term sensitization, unlike short-term sensitization, requires protein synthesis. The repeated sensitizing stimulus leads to more prolonged phosphorylation and activation of nuclear regulatory proteins by protein kinase A (PKA). The key step involves translocation of the catalytic subunit of PKA into the nucleus of sensory neurons where it appears to activate CREB (cAMP responsive element binding protein) that results in long-term sensitization (Figure 18.3). Following this discovery of the key role of CREB by Dash, Hochner, and Kandel in 1990, much work has been done on the role of CREB in memory processes in mammalian models (see Silva, Kogan, Frankland, & Kida, 1998). One of the newly synthesized proteins initiates internalization and degradation of neuronal cell adhesion molecules (NCAMs), allowing restructuring of the axon terminal arbors. Other synergistic biochemical processes also occur. Eric Kandel received the Nobel prize in physiology and medicine in 2000 in part for his elucidation of these biochemical processes underlying behavioral plasticity in Aplysia.
Hermissenda
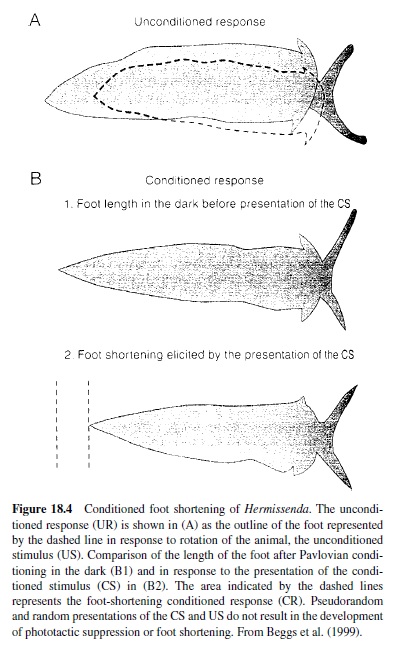
Another invertebrate model that has proved amenable to cellular and molecular analysis of mechanisms of behavioral plasticity is the Pacific nudibranch Hermissenda crassicornis, studied in detail by Daniel Alcon and his many associates. The behavioral CR studied involves pairing a light CS with high-speed rotation (stimulating hair cell gravity receptors) as a US. Conditioning results in a CS-elicited suppression of the normal positive phototaxic response and a foot shortening (the normal response to a light is foot lengthening), lasting for days (Figure 18.4). Because the sensory systems activated by the CS and US are central, the conditioning process can be studied in the isolated nervous system (see Alkon, 1989; Crow, 1988). The eyes of Hermissenda are very simple, no-image-forming photoreceptors labeled Type A (two) and Type B (three).
Cellular correlates of the CR involve a significant increase in CS-elicited spike frequency and enhanced excitability in the Type B photoreceptor and similar changes in the Type Aphotoreceptor. Note that, like sensitization in Aplysia, these are changes in sensory neurons. Several mechanisms have been discovered to cause this increased excitability in the photoreceptor neurons, most particularly two potassium currents that are reduced as a result of conditioning. Because outward potassium currents reduce cell excitability, reduction in the currents would increase cell excitability, as seen in Hermissenda conditioning. Note that these charges are intrinsic to the neurons and not necessarily due to any synaptic processes.
It appears that the phosphoinositide system is responsible for the reduction in K+ currents in Hermissenda conditioning. Activation of protein kinase C (PKC) may be initiated by actions of an agonist released by stimulation of the US pathway (Figure 18.5). Serotonergic neurons may provide polysynaptic input to the visual system, acting synergistically (see Alkon, 1989; Crow, 1988; Matzel, Ledehendler, & Alkon, 1990).
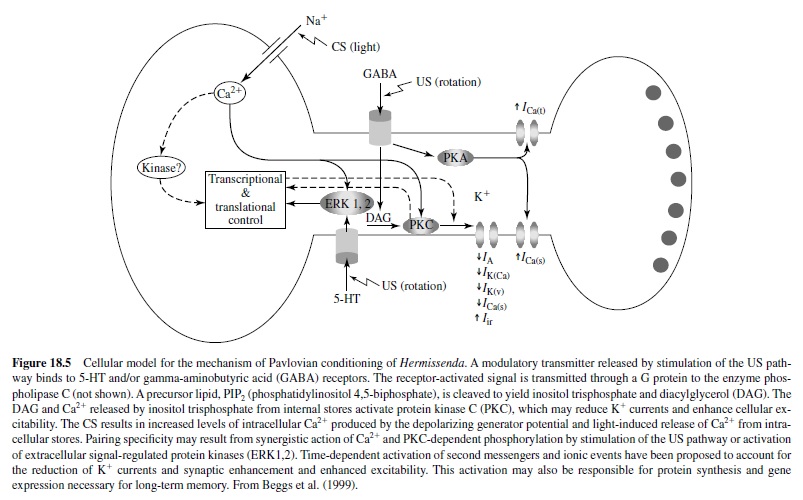
It is important to note that a similar decrease in a calciumdependent slow after-hyperpolarization, mediated by a voltage-gated potassium conductance, results in a learninginduced increase in excitability of pyramidal neurons in the hippocampus of rabbits as a result of eye-blink conditioning (de Jonge, Black, Deyo, & Disterhoft, 1990; Disterhoft, Coulter, & Alkon, 1986; and see later discussion).
Comment
Advances in our understanding of the cellular and molecular mechanisms underlying various forms and aspects of behavioral plasticity and memory in at least some of the invertebrate models, particularly Aplysia and Hermissenda, have been spectacular. There would appear to be clear points of contact with putative mechanisms of memory formation in mammals (e.g., CREB and potassium channel–mediated afterhyperpolarization. Study of these systems in their own right is exciting and eminently worthwhile. However, as egocentric mammals we must ask to what extent these systems and mechanisms apply to mammals. Note that the key processes in both Aplysia and Hermissenda systems are in the sensory neurons; hence the changes are presynaptic. To date, no such changes have been reported for sensory neurons in mammals. A major putative mechanism in mammalian learning, long-term potentiation (LTP), appears to be postsynaptic, at least in CA1. We note that studies by Glanzman and associates (Lin & Glanzman, 1994) have shown that postsynaptic changes in the motor neurons may occur in the monosynaptic Aplysia circuit. Most of the Aplysia results on classical conditioning have actually been obtained for sensitization. In mammalian systems great effort is expanded to rule out sensitization in classical conditioning studies. Indeed, sensitization appears to play no role in mammalian classical conditioning studies.
There are more general issues as well. To what extent do processes of long-term sensitization and classical conditioning play roles in the natural environment in Aplysia or in light rotation in Hermissenda? That is, are the laboratory studies imposing plasticity that may not normally occur? More generally, are the control procedures used in these invertebrate studies, which are taken from the mammalian literature, entirely appropriate? In some instances the learning procedure itself may have problems. For example, in fruit fly learning, individual animals are not trained; the data are single numbers for groups of animals.
The degrees of convergence between mechanisms of memory in invertebrates and mammals will become clearer as we learn more about mechanisms in the mammalian brain. There is much to be done.
Spinal Conditioning
The early history of spinal conditioning—the possibility that classical or instrumental training procedures could induce associative learning-like phenomena in the vertebrate spinal cord—was somewhat controversial. Pavlov’s dictum to the effect that associative learning required the cerebral cortex did not help matters.
Shurrager, working in Culler’s laboratory (where so many pioneering studies of brain substrates of learning and memory were carried out), published the first modern studies of classical conditioning of spinal reflexes (Shurrager & Culler, 1940, 1941). In brief, they used acute spinal dogs, measured the twitch response of a partially dissected flexor muscle, and gave paw shock as a US and weak stimulation of the tail as CS. They obtained robust acquisition in about half of the animals and demonstrated CS-alone extinction and successively more rapid reacquisition in repeated training and extinction sessions. Unfortunately, adequate controls for sensitization and pseudoconditioning were not run in these studies.
Afew years later Kellogg and associates reported negative results in attempts at spinal conditioning (e.g., Deese & Kellogg, 1949; Kellogg, 1947; Kellogg, Deese, & Pronko, 1946). They used chronic spinal dogs and the flexor response of the whole leg. The US was shock to the paw of that leg, and the CS was shock to the opposite hind paw. Kellogg’s choice of CS locus was unfortunate. Paw shock elicits a crossed extension reflex that would work against the development of a conditioned flexion response. Pinto and Bromily (1950) completed an extensive spinal conditioning study with long-term acute spinal animals and found only inconclusive evidence because of passive hindquarter movements caused by anterior limb movements.
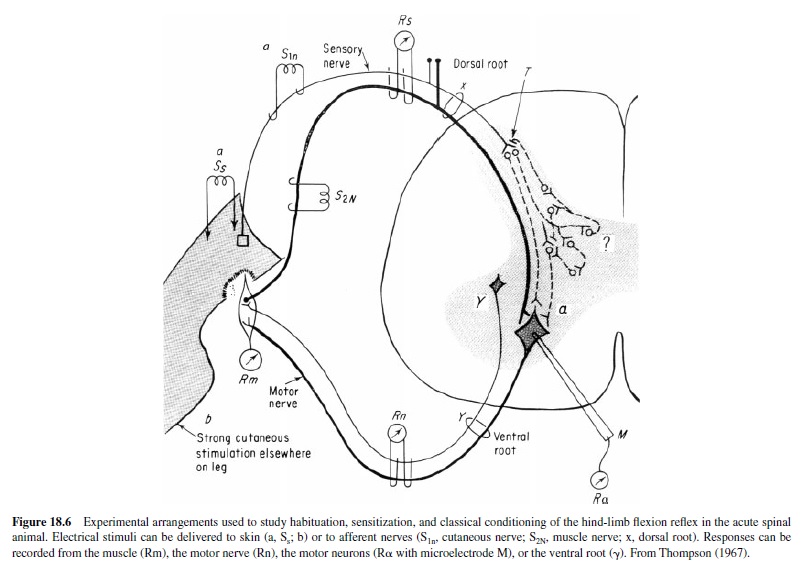
Patterson, Cegavske, and Thompson (1973) completed a detailed and extensive study of spinal conditioning, using a number of control procedures and conditions, that yielded clear positive results. Animals were anesthetized, spinalized at the twelfth thoracic vertebrae (T-12), given local anesthetics, then paralyzed with flaxedil and given artificial respiration (Figure 18.6). The superficial and deep peroneal motor nerves were dissected out and placed on stimulating (CS – S1n in Figure 18.6) and recording (UR, CR – Rn in Figure 18.6) electrodes. The CS was a weak shock to the superficial peroneal nerve of intensity yielding a motor nerve response to the first pulse. The US was a series of pulses to skin of the left ankle (b in Figure 18.6), yielding a UR (response of deep peroneal nerve). The conditioning group received 75 acquisition trials, 250 ms forward interstimulus interval (ISI), and 50 CSalone extinction trials. Control groups received explicitly unpaired CS and US trials (75 each). In one series a CS-alone trials group was also included. Two separate experiments were completed; both showed clear evidence of associative learning.
These experiments clearly ruled out sensitization as a process responsible for the increase in CS response in the paired group. The fact that the animals were paralyzed ruled out movement artifacts. Acquisition was rapid, as was extinction, just as in the original Shurrager and Culler studies. These results were replicated in careful studies by Durkovic (1975). In a recent and most interesting study, Durkovic and Prokowich (1998) infused intrathecally artificial cerebral spinal fluid (CSF; controls) or artificial CSF with the N-methyl-Daspartate (NMDA) blocker DL-2-amino-5-phosphonovaleric acid (APV) during the conditioning period in acute spinal cats, using procedures described earlier. Both groups showed normal acquisition of the spinal CR. However, theAPV group exhibited no retention of the increased response in the 2.5-hr retention period, in contrast to the CSF-alone group. The results suggest that NMDA receptor activation plays a critical role in the establishment of long-term associative plasticity in the spinal cord.
A key issue is the extent to which this form of spinal Pavlovian conditioning resembles Pavlovian conditioning of discrete responses in the intact animal. Patterson and associates completed a heroic series of parametric studies to address this issue, using the same general procedures as Patterson et al. (1973). In brief, spinal conditioning exhibits differential conditioning (A. L. Beggs, Steinmetz, & Patterson, 1985) forward but not backward conditioning (Patterson, 1975), retention of the CR over a period of hours (A. L. Beggs et al., 1983), increasingly effective conditioning with increasing US strength (Polenchar, Romano, Steinmetz, & Patterson, 1984), and best learning with a 250 onset forward ISI. (See Patterson, 1976, for a detailed review of all studies to that time on spinal conditioning.) All these properties resemble the properties of classical conditioning of discrete responses in intact mammals.
In an interesting recent series of studies Grau and associates paired shock to a hind leg as a CS with intense tail shock as a US in the spinal rat and then examined effect of CS presentations on antinociception on the tail-flick test (Joynes & Grau, 1996). This paradigm is complex in that the CR is a variation on the US. Intense tail shock would seem to induce massive sensitization. Grau interpreted the results, incidentally, as protection from habituation.
The spinal conditioning results just reviewed resemble classical conditioning of discrete responses in intact animals in many properties; nonetheless, they appear to differ from intact animal learning in several ways. First, acquisition is very rapid; most increases in response to the CS occur in the first few trials. Second, and perhaps more important, the onset latency of the CR does not appear to move forward in time over the course of learning. Finally, and seemingly most important, spinal conditioning involves an alpha response. In most studies the CS elicits the UR-CR before training. As a result of training there is an associatively produced increase in the amplitude of the response to the CS. The fact that spinal conditioning may be alpha conditioning perhaps accounts for the lack of forward shift of the CR onset with training.
The idea that alpha conditioning differs from normal conditions may be somewhat arbitrary. In one experiment in Patterson et al. (1973), two branches of the deep peroneal nerve were recorded during conditioning. One branch showed responses to the CS (superficial peroneal nerve stimulus) prior to training, but the other branch did not. However, by Trial 10 the nonresponsive branch did show a response to the CS. Is it the case that one branch of the nerve showed alpha conditioning, whereas the other branch did not exhibit alpha conditioning but rather showed only normal conditioning?
In unpublished pilot studies Patterson, Thompson, and associates completed some initial analytic studies in an attempt to localize the sites of synaptic plasticity that underlie spinal conditioning. The preparation itself, involving paralysis, cutaneous nerve stimulation as the CS, strong cutaneous stimulation of the paw as a US, and the recording of motor nerve responses, ruled out changes in sensory receptors or properties of the muscles. Using a monosynaptic test pathway (R in Figure 18.6), they ruled out changes in motor neurons. Similarly, changes in the excitability of the cutaneous afferent fibers were ruled out by stimulation of the terminals (T in Figure 18.6) with antidromic recording (Rs in Figure 18.6). Consequently, the mechanisms of synaptic plasticity must reside within the interneuron circuits (? in Figure 18.6) in the spinal gray (see R. F. Thompson, 2001).
What happens in the spinal cord when the limb flexion response is conditioned in the intact animal? In the otherwise intact animal, lesions in the cerebellar nuclei or rubrospinal tract produce complete and specific abolition of the conditioned limb flexion response (see Thompson & Krupa, 1994; Voneida, 1999; see also the later discussion). In fact, normal animals that undergo leg flexion training prior to spinal transection show no retention or savings of CRs in spinal reflexes following transection (J. Steinmetz, personal communication to R F. Thompson, January, 1984). The isolated spinal cord is thus capable of mediating a kind of associative neuronal plasticity but does not subserve classical conditioning of the limb flexion response in the intact animal. Spinal conditioning is a useful model for studying basic associative plasticity in a simplified neuronal network, but it does not tell us where or how such memories are formed in the intact animal.
Fear Conditioning
Fear as a scientific term describes a brain state in which a set of adaptive (or defensive) responses is activated in the presence of danger (LeDoux, 1996). Although humans and other animals have genetic predispositions to fear certain stimuli, it is also beneficial for animals to have the capacity to learn about new dangers in their environments. For instance, although newborn infants innately exhibit fear to certain stimuli (e.g., loud noises), they do not show inherent fear to flame or heights (two stimuli that most children learn and adults remember to avoid; Fischer & Lazerson, 1984). Accordingly, fear behaviors to many stimuli and events in the environment appear to be acquired.
Classical or Pavlovian fear conditioning has been widely employed for studying the mechanisms by which fear is acquired. Fear conditioning occurs when initially neutral CSs are contingently paired with aversive USs that reflexively activate unconditioned fear responses (URs; Rescorla, 1967; Watson & Rayner, 1920). Through CS-US association formation, the CS comes to elicit various CRs that share similar characteristics to innate fear responses (R. J. Blanchard & Blanchard, 1969, 1971; Bolles, 1970; Fanselow, 1984; Kim, Rison, & Fanselow, 1993; LeDoux, Iwata, Pearl, & Reis, 1986). Perhaps the best-known example of fear conditioning is the LittleAlbert experiment by Watson and Rayner (1920). Little Albert was an 11-month-old infant who initially exhibited curiosity (and no fear) to a white rat by touching and playingwithit.AsAlbert’shandtouchedtherat,theexperimenters banged a steel bar with a hammer behind his head (US), causing him to startle, fall forward, and cry (UR). Afterwards, when the rat (CS) was placed near Little Albert’s hand, he withdrew his hand and began to cry (CR). This exhibition of fear toward the rat was allegedly generalized to other white, furry animals and objects (e.g., rabbits, dogs, fur muffs).
Modern investigations of fear conditioning typically employ small mammals (e.g., rats, mice, and rabbits) as subjects and use a tone (or a light or a distinctive environmental setting) as a CS and a mild electric shock (e.g., a foot shock) as a US. Under these circumstances a small number of CS-US pairingsproducerobustfearlearningasevidencedbyavariety of fear responses exhibited upon subsequent presentations of the CS. In rats typical fear CR measures include freezing (or movement arrest; D. C. Blanchard & Blanchard, 1972; R. J. Blanchard & Blanchard, 1969; Bolles 1970, Fanselow, 1984; LeDoux, Iwata, Pearl, & Reis, 1986), enhancement of musculature reflexes (e.g., startle; Brown, Kalish, & Farber, 1951; Choi, Lindquist, & Brown, 2001; Davis, 1997; Leaton & Borszcz, 1985), analgesia (Fanselow, 1986; Helmstetter, 1992), 22-kHz ultrasonic vocalization (a distress signal; R. J. Blanchard, Blanchard, Agullana, & Weiss, 1991; Lee, Choi, Brown, & Kim, 2001), and alterations in autonomic nervous system activities (e.g., increased heart rate, increased blood pressure, rapid respiration; Iwata, Chida, & LeDoux, 1987; Iwata, LeDoux, & Reis, 1986; Kapp, Frysinger, Gallagher, & Haselton, 1979; Stiedl & Spiess, 1997). Because fear conditioning occurs rapidly and with lasting effect, it has become a popular behavioral tool for investigating the neurobiological mechanisms of learning and memory (see Davis, 1997; Lavond, Kim, & Thompson, 1993; LeDoux, 1996; Maren & Fanselow, 1996).
Fear can also be rapidly acquired through instrumental or operant conditioning in which the presentation of an aversive stimulus is contingent on the behavior of the animal. A widely employed procedure with rodents is the passive (or inhibitory) avoidance task (Grossman, Grossman, & Walsh, 1975; McGaugh, 1989; Nagel & Kemble, 1976), in which the animal’s response (e.g., entering a dark compartment of a box when placed in an adjacent lighted compartment, or stepping down from a platform onto a grid floor) is paired with an aversive experience (e.g., a foot shock). As a function of this response-stimulus pairing, the animal learns to avoid making the response that was followed by the aversive experience.
Amygdala as the Locus of Fear Conditioning
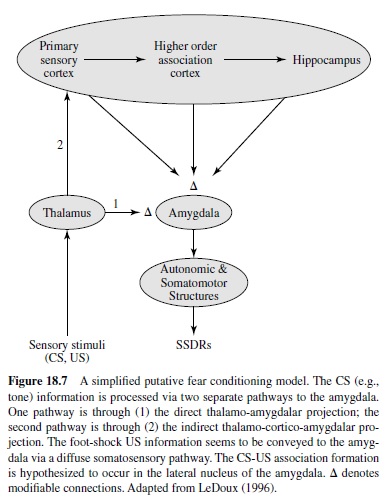
An accumulating body of evidence from lesion, pharmacology, and neural correlates of behavior studies point to the amygdala—an almond-shaped group of nuclei buried deep within the temporal lobes—as the key neural system underlying fear conditioning (see Davis, 1997; Fendt & Fanselow, 1999; Lavond et al., 1993; LeDoux, 1996; Maren & Fanselow, 1996). The amygdala, one of the principal structures of the limbic system (Isaacson, 1974), has long been implicated as a crucial emotive brain center in monkey studies (e.g., Klüver & Bucy, 1937; MacLean & Delgado, 1953; Weiskrantz, 1956). Anatomically, the amygdala is positioned to receive sensory inputs from diverse areas of the brain (e.g., thalamus, hypothalamus, neocortex, olfactory cortex, hippocampus) and to send projections to various autonomic and somatomotor structures that mediate specific fear responses (e.g., bed nucleus of stria terminalis for activating stress hormones, periaqueductal gray matter for defensive behavior, lateral hypothalamus for sympathetic activation; LeDoux, 1996). It is generally accepted that various sensory information enters the amygdala through its basal and lateral nuclei (Aggleton, 2000; LeDoux, 1996), where CS-US association formation is believed to take place (Figure 18.7). These nuclei are reciprocally interconnected with the central nucleus, which appears to be the main amygdaloid output structure that sends projections to various autonomic and somatomotor centers involved in mediating specific fear responses. In this section we examine various types of experimental evidence indicating that the amygdala is the essential site of fear conditioning.
Evidence From Lesion Studies
Permanent and reversible lesions of the amygdala, particularly in the central nucleus (ACe), effectively attenuate or abolish a variety of conditioned fear responses in several mammalian species. In rats, amygdalar lesions impair both acquisition (learning) and expression (performance) of conditioned increaseinbloodpressure(Iwataetal.,1986),potentiatedstartle (Hitchcock & Davis, 1986, 1987) and enhanced eye-blink reflexes (Choi et al., 2001), analgesia (reduction in pain sensitivity; Helmstetter, 1992), 22-kHz ultrasonic vocalization (Antoniadis & McDonald, 2000; Goldstein, Rasmusson, Bunney, & Roth, 1996), and freezing (D. C. Blanchard & Blanchard, 1972; Cousens & Otto, 1998; Iwata et al., 1986; Kim et al., 1993). Similarly, reversible inactivation of neurons (prior to fear conditioning) in the basolateral amygdala complex (BLA), via microinfusing the gamma-aminobutyric acid (GABA) agonist muscimol, blocks the acquisition of conditioned fear; whereas intra-amygdalar muscimol infusions (prior to retention testing) in previously fear-conditioned rats impair the expression of conditioned fear (Helmstetter & Bellgowan, 1994; Muller, Corodimas, Fridel, & LeDoux, 1997). In rabbits, amygdalar lesions have been found to impede conditioned brady cardia (deceleration in heart rate; Kappetal., 1979; Gentile, Jarrell, Teich, McCabe, & Schneiderman, 1986); whereas in cats, reversible cryogenic (cooling) inactivation of the ACe reduces conditioned blood pressure and respiratory responses (Zhang, Harper, & Ni, 1986).
Besides affecting fear CRs, amygdalar lesions also influence the performance of innate unconditioned fear responses (URs). For instance, amygdalectomized rats fail to display normal defensive freezing behavior in the presence of a cat predator (D. C. Blanchard & Blanchard, 1972). Amygdalar lesions have also been reported to reduce reactivity to the foot-shock US and to block shock-induced sensitization of startle (Hitchcock, Sananes, & Davis, 1989). The fact that lesions to the amygdala interfere with both fear CRs and URs indicates that the amygdala receives both CS and US information (Figure 18.7).
Lesions restricted to particular structures afferent to the amygdala can impede fear conditioning to specific CSs. Forexample, lesions to the medial geniculate nucleus (MGN) of the thalamus, which relays auditory information to the amygdala (LeDoux, Farb, & Ruggiero, 1990), block the formation of the tone–foot shock association, but not light–foot shock association (Campeau & Davis, 1995; LeDouxetal., 1986). Similarly, in rabbits, Schneiderman and colleagues made partial lesions in the MGN (limited to the medial border) and found that the lesioned animals did not demonstrate differential bradycardia CRs to CS+ and CS– tones, even though the magnitude of the bradycardia response was not affected (Jarrell, Gentile, McCabe, & Schneiderman, 1986).Amygdalar lesions, by contrast, abolished the retention of differential fear conditioning of bradycardia in rabbits (Gentile et al., 1986). These results suggest that the MGN relays auditory CS to the amygdala, where fear conditioning is likely to take place. It has been shown that the MGN sends auditory information to the amygdala both directly (via the thalamo-amygdala pathway) and indirectly (via the thalamo-cortico-amygdala pathway; Figure 18.7), both of which sufficiently support auditory fear conditioning (Romanski & LeDoux, 1992). On the efferent side, the amygdala sends projections to particular hypothalamic and brainstem areas that mediate specific conditioned fear responses (Francis, Hernandez, & Powell, 1981; Hitchcock et al., 1989; Iwata et al., 1986; Kim et al., 1993). For instance, LeDoux and colleagues showed that lesions to the lateral hypothalamus impair conditioned blood pressure response, whereas lesions to the ventrolateral portion of the periaqueductal gray (PAG) matter abolish conditioned freezing response; the lateral hypothalamus lesions do not affect freezing, and the PAG lesions do not alter blood pressure (LeDoux, Iwata, Cicchetti, & Reis, 1988). Lesions to the ventrolateral PAG also do not affect the expression of the conditioned bradycardia response in rabbits (Wilson & Kapp, 1994).
These examples of double dissociations of CSs and CRs, as a result of damaging afferent and efferent structures to the amygdala, are consistent with the view that the amygdala is a critical mediator of fear conditioning. It is interesting to note that recent lesion studies suggest that different amygdalar nuclei (e.g., the central nucleus vs. the basal nucleus of the amygdala) mediate independent fear-learning systems (e.g., the ACe controls the classical fear responses,whereastheBLAcontrols the instrumental fear responses; Killcross, Robbins, & Everitt, 1997; Nader & LeDoux, 1997; Amorapanth, LeDoux, & Nader, 2000). The possible existence of multiple fear-learning systems is perhaps not unexpected given the evolutionary importance of fear conditioning in survival.
Evidence From Stimulation and Recording Studies
Electrical and chemical stimulation of specific regions in the amygdala can evoke conditioned fear-like responses. In rats, amygdala stimulation produces freezing behavior (Weingarten & White, 1978), cardiovascular changes (Iwata et al., 1987), and acoustically enhanced startle responses (Rosen & Davis, 1988). In rabbits, stimulation of the ACe induces bradycardia, pupillodilation, arrest of ongoing behavior (e.g., movement of the mouth and tongue), and enhanced amplitude of the nictitating membrane reflex (Applegate, Kapp, Underwood, & McNall, 1983; Whalen & Kapp, 1991). Stimulation of the lateral hypothalamus, an efferent target structure of the amygdala, elicits cardiovascular responses in anesthetizedrabbits (M.D. Gellman, Schneiderman, Wallach, & LeBlanc, 1981). In dogs that previously underwent alimentary (or salivary) conditioning, electrical and chemical stimulations of the basolateral area of the amygdala have been found to inhibit conditioned secretory reflexes (Danilova, 1986; Shefer, 1988). These data indicate that the amygdala can directly activate various fear responses and also inhibit competing responses (that are incompatible with fear responses). In some cases, however, stimulation of the amygdala can interfere with aversive learning. For example, immediate posttraining stimulation of the amygdala produces amnesia that impairs the formation of fear memory (Gold, Hankins, Edwards, Chester, & McGaugh, 1975; McDonough & Kesner, 1971).
Unit recording studies reveal that neurons in the ACe respond to both CS and US (Pascoe & Kapp, 1985) and undergo learning-related changes during fear conditioning (Applegate,Frysinger,Kapp,&Gallagher,1982).Usingadifferential conditioning paradigm, Pascoe and Kapp (1985) reportedthatACeneuronsexhibitedselectiveincreasesinsingle unit activity to a tone (CS+ ) that signaled the US, but not to a different tone (CS– ) that did not signal the US. The conditioned bradycardia response paralleled the neuronal response; it was observed preferentially during the reinforced tone presentation. In addition, the magnitude of the amygdalar unit activity correlated with the magnitude of the conditioned bradycardia response. It appears that during fear conditioning some forms of neurophy siological changes strengthen the CS-amygdala pathway such that the CS becomes capable of eliciting conditioned fear responses.
Long-term potentiation, which is commonly suggested as a candidate synaptic mnemonic mechanism (Collingridge, Kehl, & McLennan, 1983; R. G. Morris, Davis, & Butcher, 1990; Teyler & DiScenna, 1987), has been demonstrated in the amygdala, for example, the external capsule-lateral nucleus of the amygdala (LA) pathway in vitro (Chapman & Bellavance, 1992; Chapman, Kairiss, Keenan, & Brown, 1990), the internal capsule-LApathway in vitro (Huang & Kandel, 1998), the auditory thalamus-LA pathway in vivo (Clugnet & LeDoux, 1990), and the subiculum-BLA pathway in vivo (Maren & Fanselow, 1995). The auditory inputs from the MGN to the LA—a pathway involved in tone fear conditioning (LeDoux, 2000)—demonstrate an enhancement in auditory-evoked potentials (or LTP-like changes) after tone fear conditioning (Rogan & LeDoux, 1995; Rogan, Staubli, & LeDoux, 1997). Similarly, amygdalar slices prepared from fear-conditioned rats demonstrate enhanced synaptic transmission in the MGN-amygdala pathway (McKernan & Shinnick-Gallagher, 1997). Thus, it has been postulated that LTP or LTP-like changes in the amygdala are involved in fear conditioning (Clugnet & LeDoux, 1990; Davis, 1997; Fanselow & Kim, 1994; Maren & Fanselow, 1996; LeDoux, 2000; Miserendino, Sananes, Melia, & Davis, 1990).
Evidence From Pharmacological Studies
Immediate posttraining drug manipulations in the amygdala can impair or enhance aversive memories. In 1978 Gallagher and Kapp first demonstrated that intra-amygdalar infusions of the opioid receptor antagonist naloxone enhance fear conditioning. In contrast, infusions of the opioid agonist levorphanol reduced fear conditioning (Gallagher, Kapp, McNall, & Pascoe, 1981). Subsequent studies indicated that the memory-enhancing effect of opiate antagonists is induced partly by blocking the endogenously released opioids from inhibiting the release of norepinephrine in the amygdala (McGaugh, 1989). For instance, intra-amygdalar infusions of the noradrenergic receptor antagonist propranol impair the retention of an inhibitory avoidance memory (Gallagher et al., 1981) and block the memory-enhancing effect of naloxone (McGaugh, Introini-Collison, & Nagahara, 1988). In contrast to propranol, posttraining intra-amygdalar infusions of norepinephrine enhance the retention of inhibitory avoidance memory (Liang, Juler, & McGaugh, 1986). Based on these and other pharmacological studies, McGaugh and colleagues proposed that interactions of opioid, GABA, noradrenergic, and cholinergic neurochemical systems in the amygdala modulate aversive learning (McGaugh, 2000; McGaugh, Cahill, & Roozendaal, 1996). Recently, pretraining intra-amygdalar infusions of the dopamine (D2) receptor antagonist eticlopride have been shown markedly to attenuate conditioned freezing, indicating that amygdaloid dopamine transmission also contributes to the formation of fear memories (Guarraci, Frohardt, Falls, & Kapp, 2000). Finally, drugs (e.g., diazepam) that decrease fear or anxiety in humans, when infused directly into the amygdala, have been shown to attenuate conditioned fear in rats (Helmstetter, 1993).
Several studies suggest that the NMDA subtype of the glutamate receptor in the amygdala might be involved in the synaptic plasticity process (e.g., LTP) underlying fear conditioning. Because NMDA receptors have been demonstrated to be critical for the induction (but not expression) of LTP in the hippocampus, a similar type of synaptic plasticity in the amygdala has been proposed as a possible cellular mechanism subserving fear conditioning. Consistent with this notion, intra-amygdalar administrations of APV—a competitive NMDA receptor antagonist—have been found to block the acquisition of conditioned fear effectively, as measured by fearpotentiated startle response (Miserendino et al., 1990) and freezing (Fanselow & Kim, 1994). Other studies, however, also found thatAPV infusions into the amygdala significantly impair the expression of conditioned fear (in previously fearconditioned rats), as measured by a variety of fear responses including freezing, 22-kHz ultrasonic vocalization, analgesia, and potentiated startle (Fendt, 2001; Lee, Choi, Brown, & Kim, 2001; Lee & Kim, 1998; Maren, Aharonov, Stote, & Fanselow, 1996). It is evident that amygdalar NMDA receptors participate in normal synaptic transmission and, therefore, in the overall functioning of the amygdala.
Several recent studies indicate that the acquisition of fear conditioning in rats requires RNAand protein synthesis in the amygdala. For example, pretraining intra-BLA infusions of the RNA synthesis inhibitor actinomycin-D significantly attenuate fear conditioning (to tone and context CSs) and RNA synthesis in the amygdala (Bailey, Kim, Sun, Thompson, & Helmstetter, 1999). Similarly, immediate posttraining infusions of anisomycin (a protein synthesis inhibitor) and Rp-cAMPS (an inhibitor of PKA) into the LA impair fear conditioning (Schafe & LeDoux, 2000). Once fear conditioning has been established (or consolidated), intra-amygdalar infusions of actinomycin-D, anisomycin and Rp-cAMPS do not affect conditioned fear memories (Bailey et al., 1999; Schafe & LeDoux, 2000). It is interesting that previously consolidated fear memories, when reactivated during retrieval (i.e., during a conditioned tone test), appear to return to a labile state that again requires protein synthesis in the amygdala for reconsolidation (Nader, Schafe, & LeDoux, 2000).
Evidence From Human Studies
Recent findings from human neuropsychological and brain imaging studies are also consistent with findings from animal studies. For example, patients with damage to the amygdala display a selective impairment in the recognition of facial expressions of fear (Adolphs, Tranel, Damasio, & Damasio, 1994) and also exhibit deficits in fear conditioning (LaBar, LeDoux, Spencer, & Phelps, 1995) in contrast to normal subjects. Patients with amygdalar damage are also impaired in recalling emotionally influenced memory (Cahill, Babinsky, Markowitsch, & McGaugh, 1995). Correspondingly, imaging studies show that there is a significantly increased blood flow to the amygdala (as measured by functional magnetic resonance imaging, or fMRI) when normal subjects are presented with pictures of fearful faces (J. S. Morris et al., 1996) or are undergoing fear conditioning (Knight, Smith,
Stein, & Helmstetter, 1999; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). Functional activation of the amygdala has also been observed (via positron-emission tomography, or PET) during free recall of emotional information (Cahill et al., 1996). These sources of evidence further support the view that the amygdala is crucially involved in fear conditioning or in processing emotional information.
Other Brain Areas
Most of the evidence presented so far indicates that the amygdala is the essential neuronal substrate underlying fear conditioning. It is not clear, however, whether the amygdala is the permanent storage site for long-term fear memory. The site of learning is not necessarily the site of memory storage. For example,fearretentionisabolishediftheamygdalaislesioned (electrolytically) or reversibly inactivated (via infusions of a local anesthetic agent lidocaine) shortly (1 day) but not long (21 days) after inhibitory avoidance training (Liang et al., 1982), suggesting that long-term fear memory is not stored in the amygdala. In contrast to inhibitory avoidance, however, amygdalar lesions made either shortly (1 day) or long (7 or 28 days) after training effectively abolish conditioned freezing response (Maren,Aharonov, & Fanselow, 1996).
The insular cortex that receives and relays sensory (e.g., visual) information to the amygdala (Turner & Zimmer, 1984) may have some role in the storage of fear memory. Lesions to the most caudal aspect of the insular cortex impair retention of conditioned light-potentiated startle (Rosen et al., 1992). Similarly, reversible inactivation of the insular cortex by a Na-channel blocker tetrodotoxin impairs retention of inhibitory avoidance memory (Bermudez-Rattoni, Introini-Collison, & McGaugh, 1991).
The hippocampus seems to be involved in certain types of conditioned fear memory. In rats, conditioned fear to a diffuse contextual cue, but not to a discrete tone cue, is abolished when the hippocampus is lesioned shortly (1 day) after conditioning (Anagnostaras, Maren, & Fanselow 1999; Kim & Fanselow, 1992; Maren, Aharonov, & Fanselow, 1997). However, animals retain a considerable amount of contextual fear when a long delay (28 days) is imposed between the time of conditioning and the time of hippocampectomy. Thus, it appears that the hippocampus is transiently involved in storing contextual fear memory. Similarly, pretraining hippocampal lesions selectively block the acquisition of context fear memory, but not tone fear memory (Phillips & LeDoux, 1992). It is interesting to note that lesions to the nucleus accumbens (a target of hippocampal efferents) also selectively impair contextual fear conditioning without affecting auditory fear conditioning (Riedel, Harrington, Hall, & Macphail, 1997). Hippocampal lesions also impair trace (but not delay) fear conditioning to an auditory CS in rats (as measured by freezing; McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998) and rabbits (as measured by heart rate; McEchron, Tseng, & Disterhoft, 2000). The notion that the hippocampus is involved in contextual fear memory and trace fear conditioning is also supported by various knockout-transgenic mice studies. In brief, mutant mice with deficient LTP in the hippocampus also exhibit impairments in contextual (but not tone) fear conditioning and trace fear conditioning (e.g., Abeliovich et al., 1993; Bourtchuladze et al., 1994; Huerta, Sun, Wilson, & Tonegawa, 2000).
The perirhinal cortex, which is reciprocally connected to the hippocampus (both directly and indirectly via the entorhinal cortex), also seems to be involved in consolidation and storage of hippocampal-dependent contextual memory. Neurotoxic lesions of the perirhinal cortex made 1 day (but not 28 days) after training produce marked deficits in contextual fear memory (Bucci, Phillips, & Burwell, 2000).
Finally, lesions of the cerebellar vermis in rats have been found to abolish the conditioned autonomic response (heart rate) without affecting the unconditioned autonomic response (Supple & Leaton, 1990). The vermal lesioned rats also exhibit less freezing to a cat predator and fewer signs of fear in an open field (Supple, Leaton, & Fanselow, 1987). In rabbits, during fear conditioning, single unit recordings of Purkinje cells in the vermis demonstrate selective increases in activity to a tone (CS+ ) that signaled the US, but not to a different tone (CS– ) that did not signal the US. The differential unit activities of the Purkinje cells correlated with the behavioral conditioned autonomic response (Supple, Sebastiani, & Kapp, 1993). These results indicate that the cerebellar vermis is an important part of the autonomic fear conditioning circuit that modulates fear-related behaviors.
Some Unresolved and Critical Issues
Although much is known about the neuroanatomy and neural mechanisms underlying fear conditioning, several unresolved and conflicting issues in the field warrant discussion. This section highlights three major critical issues in fear conditioning.
First, whereas the CS pathway (specifically the auditory projection) to the amygdala is relatively well defined, the foot-shock (US) pathway to the amygdala has not been adequately delineated. A recent study reported that combined lesions of the posterior extension of the intralaminar complex (PINT) and caudal insular cortex (INS) block acquisition of fear-potentiated startle and proposed that PINT-INS projections to the amygdala constitute the essential US pathways involved in fear conditioning (Shi & Davis, 1999). However, another study (Brunzell & Kim, 2001) reported that fear conditioning (as assessed by freezing) was unaffected by either pretraining or posttraining PINT-INS lesions. Specifically, Brunzell and Kim found that pretraining lesions in naive animals do not block the acquisition of fear conditioning, and posttraining lesions in previously fear conditioned animals do not lead to extinction of the CR with continued CS-US training (as would be predicted if the US information does not indeed reach the site of learning). Thus, it appears that the foot-shock (US) pathway is comprised of diffuse, multiple somatosensory pathways to the amygdala (Brunzell, & Kim, 2001). Additional research is required to understand the specific role of the US information—as relayed via tactile versus nociception pathways—in fear conditioning.
Second, as previously mentioned, LTP in the amygdala (demonstrated both in vivo and in vitro) is commonly suggested as a putative synaptic mechanism through which acquired fear is encoded in the amygdala. However, the receptor mechanisms responsible for the induction and expression of amygdalar LTP remain ambiguous and may depend on the particular synapses and input pathway (Chapman et al., 1990; LeDoux, 2000; Weisskopf & LeDoux, 1999), as demonstrated in the hippocampus (Grover & Teyler, 1990; Harris & Cotman, 1986; Johnston, Williams, Jaffe, & Gray, 1992; Zalutsky & Nicoll, 1990). One study (Chapman & Bellavance, 1992) found that APV (an NMDA receptor antagonist) blocks LTP induction in the BLA, but only in such high concentrations that the drug markedly impairs normal synaptic transmission (but see Huang & Kandel, 1998). Similarly, single-unit recordings indicate that normal auditory-evoked responses in the amygdala are considerably attenuated by APV, suggesting that NMDA receptors are involved in normal synaptic transmission of the auditory pathway to the LA that mediates auditory fear conditioning (Li, Phillips, & LeDoux, 1995). Davis and colleagues initially reported that APV infusions into the amygdala selectively block acquisition, but not expression, of conditioned fear, as measured by fear-potentiated startle (Campeau, Miserendino, & Davis, 1992; Miserendino et al., 1990). Their finding is remarkably similar to the effects of APV on hippocampal LTP, that is, blocking induction without affecting expression of the Schaffer collateral/commissural-CA1 LTP (Collingridge et al., 1983). However, recent studies found that intraamygdalar infusions of APV dramatically interfere with the expression of multiple measures of conditioned fear, such as freezing (Lee & Kim, 1998; Maren et al., 1996), 22-kHz ultrasonic vocalization, analgesia, defecation (Lee et al., 2001), and fear-potentiated startle (Fendt, 2001). These results indicate that amygdalar NMDA receptors participate in normal synaptic transmission and thus the overall functioning of the amygdala. Clearly, additional studies are necessary to understand the receptor mechanisms of synaptic plasticity underlying fear conditioning in the amygdala.
Finally, if the notion that the amygdala is the locus of fear learning is correct, then amygdalar damage should completely and permanently block fear conditioning. However, evidence from conditioned fear studies and inhibitory (or passive) avoidance studies provides conflicting results. Recall that Pavlovian fear conditioning and inhibitory avoidance are considered to be two procedurally different fear tasks. McGaugh and colleagues found that although amygdalar lesions affect inhibitory avoidance learning, animals can still learn and retain fear when they are overtrained, which indicates that the amygdala is not necessary for fear learning (Parent, Tomaz, & McGaugh, 1992). Rats that received more training prior to lesions also exhibited far greater retention of inhibitory avoidance memory. Similarly, amygdalectomized rats learned inhibitory avoidance task when trained extensively. Furthermore, retention of inhibitory avoidance memory is abolished if amygdalar lesions are made shortly after training, but not several days after training (Liang et al., 1982). In contrast to inhibitory avoidance results, the retention of conditioned fear (as measured by freezing) is completely abolished whether amygdalar lesions are made shortly or long after training (Maren et al., 1996), which indicates that the amygdala is necessary in Pavlovian fear conditioning. Recently, it has been reported that amygdalar lesioned rats, exhibiting impairments in conditioned freezing, are capable of demonstrating inhibitory avoidance behavior when both responses are simultaneously assessed in aY-maze task (Vazdarjanova & McGaugh, 1998). Based on the observation that amygdalar lesions abolish both conditioned and unconditioned freezing but not avoidance behavior, Cahill, Weinberger, Roozendaal, and McGaugh (1999) suggested that the amygdala is critical for the expression (or performance) of reflexive fear reactions rather than the actual learningandstorageoffearmemory.Instead,basedonaseries of inhibitory avoidance and immediate posttraining drug injection studies, McGaugh and colleagues proposed that the amygdala critically modulates the consolidation of memory occurring in extra-amygdalar structures (McGaugh, 2000; McGaugh et al., 1996). It appears then that studies employing classical fear conditioning and inhibitory (passive) avoidance provide different insight into the neuronal substrates underlyingfearlearningandmemory.Ifacommonneuralmechanism mediates both conditioned fear and inhibitory avoidance, then pharmacological manipulations influencing inhibitory avoidance learning should affect fear conditioning in a similar manner. However, several studies employing rats and mice found that conditioned fear is not susceptible to memory modulation by various drugs when conducted in the manner described in inhibitory avoidance tasks (Lee, Berger, Stiedl, Spiess, & Kim, 2001; Wilensky, Schafe, & LeDoux, 1999). Given the discrepancy of these findings from conditioned fear and inhibitory avoidance studies, it is clear that further studies are necessary for understanding the precise role of the amygdala in fear conditioning.
Classical and Instrumental Conditioning of Discrete Responses
Overview
Over the years, the study of the neurobiology of learning and memory has been significantly advanced when standard brain research techniques have been used together with classical or instrumental conditioning of discrete responses such as eye blinks, limb flexions, and jaw movements. For example, classical eye-blink conditioning, used in conjunction with brain recording, lesion, stimulation, and neuropharmacological techniques, has advanced our understanding of the brain systems and processes involved in simple associative learning more than any other behavioral procedure.
There are several reasons for the relatively high degree of success that has been obtained when classical conditioning of discrete responses has been used as a behavioral tool for understanding brain function. First, the stimuli used in classical conditioning are discrete, well defined, and simpler than other more complicated behavioral procedures. Second, the responses measured (e.g., eye blinks, limb flexions, and jaw movements) are relatively simple and discrete. This enables the experimenter to measure easily and accurately the various properties of the response including variables related to response amplitude and timing. Third, in classical conditioning experiments the experimenter controls when stimuli are delivered and thus when responses are expected. This has made lesion, stimulation, and recording experiments relatively easy to interpret. Finally, due to a wide variety of studies conducted by Gormezano and his colleagues as well as other researchers, a huge behavioral database exists concerning the classical conditioning of discrete responses, especially classical eye-blink conditioning (see Gormezano, Kehoe, & Marshall, 1983, for review). This behavioral database has proven useful for designing experiments and interpreting data collected from studies that have been conducted to delineate the neural bases of associative learning. In this section we review the rather large literature that has been generated concerning the neural bases of the classical and instrumental conditioning of discrete responses.
Classical Eye-Blink Conditioning
By far, the most popular paradigm for studying associative learning has been classical conditioning of the eye-blink response. For purposes of this research paper, the eye-blink response refers to a constellation of responses that include movement of the nictitating membrane (in species with this third eyelid) and movement of the external eyelid. In classical eye-blink conditioning, a neutral stimulus called the CS is presented shortly before a second stimulus, called the US. The US reliably elicits a reflexive eye-blink response called the UR. Typically, a tone or a light is used as a CS while a periorbital shock or corneal air puff is used as a US.After 100 or so pairings of the CS and the US, the organism begins blinking to the CS (i.e., the organism has learned that the CS reliably precedes the US and thus can be used as an anticipatory cue). The learned anticipatory eye blink is called the CR. Over the years, a number of parametric features of the conditioning process have been delineated. For example, (a) the rate of acquisition of the CR generally increases as the intensity of the CS or the US increases; (b) the rate of acquisition is affected by the length of the interstimulus interval (ISI) between the onsets of the CS and the US; (c) conditioning of discrete responses occurs only when ISIs between about 80 and 3,000 ms are used; (d) CSalone presentations after acquisition training result in extinction of the CR; and (e) unpaired presentations of the CS and the US do not result in CR acquisition.
For several reasons, the rabbit has been the favorite subject for classical eye-blink conditioning. The rabbit is docile and adapts well to mild restraint, and this has facilitated the collection of behavioral and neural data. Also, it is relatively easy to measure accurately movements of the rabbit nictitating membrane or external eyelids. Eye-blink conditioning studies involving other species have also been successfully undertaken. For example, Patterson, Olah, and Clement (1977) developed a nictitating membrane conditioning procedure for the cat. Also, Hesslow and colleagues have published a series of studies concerning the involvement of the cerebellum and brain stem in classical eye-blink conditioning using ferrets as behavioral subjects (e.g., Hesslow & Ivarsson, 1994, 1996). Recently, several investigators have developed rat eye-blink conditioning preparations (e.g., Green, Rogers, Goodlett, & Steinmetz, 2000; Schmajuk & Christiansen, 1990; Skelton, 1988; Stanton, Freeman, & Skelton, 1992), and there has been a renewed interest in human eye-blink conditioning (e.g., see Woodruff-Pak & Steinmetz, 2000, for review).
Early Studies of the Brain Correlates of Classical Eye-Blink Conditioning
Among the earliest studies concerning the neural substrates of classical eye-blink conditioning were those by Oakley and Russell (1972, 1974, 1976, 1977), who examined the possibility that the cerebral cortex was involved in the storage of eye-blink CRs. They showed that rather extensive lesions of cerebral neocortex did not abolish eye-blink CRs that have been established in rabbits trained before the lesions. The cortical lesions had no effect on the acquisition of new CRs when training was delivered to naive rabbits. More recently, Mauk and R. F. Thompson (1987) used decerebration to separate neocortex from lower brain areas. They showed that the decerebrate rabbits retained eye-blink CRs. Together, the decortication and decerebration studies provide solid evidence that the cerebral cortex was not critically involved in acquisition and storage of classical eye-blink CRs.
There is evidence that under some circumstances classically conditioned-related plasticity does occur in neocortex. In an extensive series of studies, Woody and colleagues studied the involvement of portions of neocortex in eye-blink conditioning in cats. In their behavioral paradigm, an auditory CS was paired with a blink-producing glabellar tap US. After several trials, the tone CS produced an eye-blink CR. Cats given unpaired CS and US presentations did not show eye blinks to the CS. Using extracellular and intracellular recording techniques, Woody and colleagues showed that learning-related patterns of CS-evoked unit activity could be found in cortical motor areas and that persistent differences in neuronal excitability could be found in these regions after conditioning (e.g., Woody & BlackCleworth, 1973; Woody & Engel, 1972). These data suggest that the excitability of neurons in motor neocortical areas may change during this type of eye-blink conditioning. There are several differences between the cat and rabbit preparations, however. For example, the cat conditioned eye-blink response was of very short latency (i.e., less than 20 ms), whereas the rabbit CR is typically longer in latency.Also, many more trials are needed to produce conditioning in the cat preparation. The cerebellumisnotcriticalfortheacquisitionandperformanceof the short-latency CR in cats, whereas (as detailed later) the cerebellum is essential for acquisition and performance of the longerlatencyCRinrabbits(andothermammalianspecies,for that matter). In addition, extensive lesions of motor cortex in the rabbit do not affect acquisition or performance of classical eye-blink CRs (Ivkovich & Thompson, 1997). Nevertheless, the data from Woody and colleagues demonstrate that under some conditions classical conditioning-related plasticity can occur in regions of neocortex.
In other early studies investigators used brain stimulation techniques to study stimulus pathways in the brain that could potentially be involved in eye-blink conditioning. For example, in a pair of studies, Patterson (1970, 1971) implanted stimulating electrodes into the inferior colliculus and substituted microstimulation of the inferior colliculus for the peripheral tone CS. He observed robust conditioning when the collicular stimulation was paired with a US. These early data suggested that the inferior colliculus might be a portion of the auditory pathway that normally conveyed acoustic CSs used in conditioning. Kettner and Thompson (1982) used signal detection methods in a neural recording study to examine further the involvement of the inferior colliculus in eye-blink conditioning. They showed that while the inferior colliculus effectively encoded a tone CS, patterns of activation did not differ on CR versus non-CR trials, thus indicating that the inferior colliculus was not likely a brain region where CRs were critically encoded. This was contrasted with recording from the hippocampus and cerebellum where CR-related responding could be isolated (as we describe later).
Early studies also examined the motor components of the basic eye-blink conditioning circuitry, in essence defining the essential cranial nerve nuclei and relay nuclei involved in generating the unconditioned and conditioned eye-blink responses (e.g., Cegavske, Patterson, & Thompson, 1979; Cegavske, Thompson, Patterson, & Gormezano, 1976; Young, Cegavske, & Thompson, 1976). In brief, these studies showed that for the rabbit, activation of motoneurons in the abducens and accessory abducens nuclei produced nictitating membrane movement through activation of the retractor bulbi muscle, which caused eyeball retraction and passive movement of the nictitating membrane. The oculomotor and trochlear nerves were found also to be involved to some extent in the eye-blink response along with the facial nerve, which controlled external eyelid closure via activation of the orbicularis oculi muscles (Figure 18.8). Although species like the rabbit and cat have functional nictitating membranes, other species like the human and rat do not. Nevertheless, control of the reflexive eye-blink involves similar collections of brain stem nuclei across species. Further, McCormick, Lavond, and Thompson (1982) showed that the occurrences of conditioned nictitating membrane movements and conditioned external eyelid movements were highly correlated, a part of a constellation of responses that are produced by the CS-US pairings.
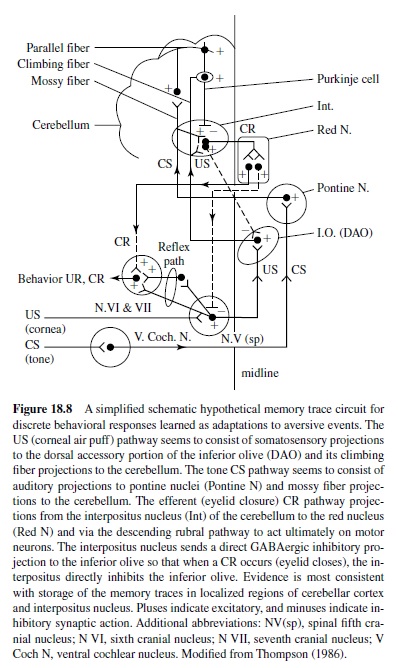
A variety of data demonstrate that the periorbital shock and air puff USs used in classical conditioning activate the reflexive UR rather directly at the level of the brain stem (Figure 18.8). For example, an air puff US activates neurons in the trigeminal complex, which projects to nuclei involved in generating eye blinks both directly and indirectly (Hiroaka & Shimamura, 1977). Neural recordings taken from the motor nuclei (e.g., the abducens nucleus) revealed that the nuclei were activated when either a UR or a CR occurred and the amplitude-time course of the unit activity was very highly correlated with the CR or the UR that was executed (Cegavske et al., 1979; Cegavske et al., 1976). Lesions of the various motor nuclei abolished portions of the CR and UR, but only those features of the eye-blink response activated by the nuclei that were removed by the lesion (Disterhoft, Quinn, Weiss, & Shipley, 1985; Steinmetz, Lavond, Ivkovich, Logan, & Thompson, 1992). For example, lesions of the abducens nucleus abolished nictitating membrane response while preserving external eyelid responses. Lesions of the facial nucleus produced the opposite effect.
Studies of the Hippocampus and Limbic System
In the 1960s and 1970s a rapidly growing body of literature suggested that the hippocampus and related limbic systems structures were involved in a variety of learning and memory processes. During this time, Thompson and his colleagues recognized the power of using the classical eye-blink conditioning paradigm to study hippocampal function during learning and memory. Specifically, Berger, Thompson, and their colleagues recorded multiple- and single-unit activity from the hippocampus and other limbic system structures during conditioning (Berger, Alger, & Thompson, 1976; Berger, Rinaldi, Weisz, & Thompson, 1983; Berger & Thompson, 1978a, 1978b). They showed that even before behavioral CRs emerged, pyramidal neurons in the hippocampus were activated. At first, pyramidal cell activation was seen during the trial period that was coincident with US presentation. Over time, as additional paired CS-US trials were delivered, the hippocampal activity could be seen during the CS-US interval. Eventually, the pattern of hippocampal activity formed an amplitude-time course model of the CR. Other limbic system structures were also found to be involved in the eye-blink conditioning process. For example, recordings from the medial septum, which sends cholinergic projections to the hippocampus, revealed stimulus-evoked responses to the CS and the US that declined with training (Berger & Thompson, 1978a). Patterns of action potentials recorded from the lateral septum were similar to the patterns seen in the hippocampus. Many studies have supported the idea that cholinergic activity in the septohippocampal system may play a very important role in eye-blink conditioning. Solomon, Solomon, Van der Schaaf, and Perry (1983), for example, showed that systemic administration of scopolamine, an anticholinergic drugs that alters hippocampal activity, severely impairs delay eye-blink conditioning (and, in fact, is more disruptive than hippocampal abalation). Salvatierra and Berry (1989) later showed that systemic scopolamine suppressed neuronal responses in the hippocampus and lateral septum while slowing the rate of delay eye-blink conditioning. Kaneko and Thompson (1997) more recently showed that central cholinergic blockade essentially blocked trace eye-blink conditioning while slowing the rate of delay conditioning. These studies suggest that the brain’s cholinergic system is centrally involved in the modulation of eye-blink conditioning, an idea that is compatible with a large body of research that suggests an important role for the cholinergic system in learning and memory.
Interestingly, an earlier study by Schmaltz and Theios (1972) had shown that rabbits could learn and retain the classically conditioned eye-blink response after the hippocampus was removed. Together with the recording data, these lesion results suggest that while the hippocampus was not critically involved in CR acquisition, it likely plays an important modulatory role in classical eye-blink conditioning. Research conducted after the early lesion and recording studies has concentrated mainly on trying to delineate what role the hippocampus plays in simple associative learning. For example, trace conditioning, a variation of the basic classical eye-blink conditioning procedure, has been used to study the possibility that the hippocampus is involved in memory processing associated with learning. In trace conditioning, the CS is turned on and then turned off; a time period is allowed to elapse; and then the US is presented. Unlike delay conditioning, there is no overlap of the CS and the US during individual trials. In essence, the subject must form a memory of the CS that bridges the trace interval before the US is presented. It has been established that the hippocampus is necessary for this variation of training. For example, lesions of the hippocampus have been shown to abolish or significantly impair trace conditioning without affecting basic delay conditioning (Moyer, Deyo, & Disterhoft, 1990; Port, Romano, Steinmetz, Mikhail, & Patterson, 1986; Solomon, Van der Schaaf, Thompson, & Weisz, 1986). Similar to the recording studies of Berger, Thompson, and colleagues, pyramidal cells in the hippocampus become active during trace conditioning in a CR-related fashion (see Disterhoft & McEchron, 2000, for review). Also, at a more cellular level of analysis, Disterhoft and colleagues have shown that calcium-dependent afterhyperpolarization potentials recorded from hippocampal pyramidal cells are significantly reduced after trace conditioning training (Coulter, Lo Turco, Kubota, Disterhoft, Moore, & Alkon, 1989; Disterhoft, Golden, Read, Coulter, & Alkon, 1988).
A large part of the interest in exploring the involvement of the hippocampus in simple associative learning tasks such as classical eye-blink conditioning was generated by the observation that individuals with amnesia and hippocampal damage,suchasthewell-knownH.M.,demonstratedrathersevere anterograde amnesia and time-limited retrograde amnesia. Kim, Clark, and Thompson (1995) demonstrated similar amnesia effects for classical eye-blink conditioning. Rabbits were trained using a trace conditioning procedure then given hippocampal lesions either immediately or 1 month after training. Whereas lesions delivere dimmediately after training effectively abolished CRs, lesions given one month after training had no effect. If the rabbits were trained with a delay procedure and then immediately lesioned, no decrement in responding was seen. However, if these rabbits were then switched to trace conditioning, CR extinction occurred. Together with the Disterhoft data, these data suggested that the hippocampus is involved in memory processing of eye-blink conditioning when stimulus memory demands on the system are relatively high (e.g., during trace conditioning). During simple delay conditioning, however, the CS and the US overlap, and there appears to be no need to hold the CS in memory in anticipation of the US. Although the recording studies show that the hippocampus is engaged during the simpler delay task, apparently the structure is not necessary for learning (and memory) to take place. This implies that critical plasticity for eye-blink conditioning lies in lower brain areas.
Over the last several years, the conceptualization of memory systems in the brain has been dominated by the view that distinct brain systems exist for processing declarative and nondeclarative memories (e.g., Clark & Squire, 2000; Squire, 1992). In the human literature, declarative memories are those memories of one’s own experience, as is exemplified by one’s memories for events and facts. Nondeclarative memories are essentially all other memories, including memories for skills, habits, procedures, and simple conditioning. Because hippocampal lesions appear to cause amnesia for declarative memories, the hippocampus has therefore been regarded as critically important for the storage of declarative but not nondeclarative memories.
Because hippocampal lesions affect classical eye-blink conditioning in a manner that is very similar to the effects of hippocampal lesions on other memory tasks (i.e., severe anterograde effects with mild, short-term retrograde effects), eye-blink conditioning has provided an excellent model system for exploring the distinction in memory systems (as is evidenced by the data cited above). In addition to the nonhuman animal studies, several human eye-blink conditioning studies have recently been conducted to explore further the multiple memory system idea. For example, McGlincheyBerroth, Carrillo, Gabrieli, Brawn, and Disterhoft (1997) demonstrated deficits in long-trace eye-blink conditioning in individuals with hippocampal amnesia. Participants given short-trace or delay eye-blink conditioning have not shown the learning and memory deficits. More recently, Squire and colleagues suggested that whether the hippocampus is critically engaged in the learning and memory process may depend for the most part on whether the subjects are aware of the memories they are forming (see Clark & Squire, 2000, for review). In one study they trained participants with amnesia and participants without amnesia on both a delaydifferential conditioning procedure and a long (1,000 ms) trace-differential conditioning procedure (Clark & Squire, 1998). Subjects in both groups learned the delay procedure normally although those with amnesia could not recall the experience when questioned about it later. The control subjects could easily learn the long-trace procedure, but the subjects with amnesia could not. These results are compatible with previous literature concerning hippocampal involvement in declarative (trace) versus nondeclarative (delay) memory procedures. Using data from the control subjects in this study, Clark and Squire (1999) also demonstrated that awareness was important for the learning. The control subjects showed a great deal of variability in learning the trace procedure. In examining the individual data, Clark and Squire noted that the subjects who learned the procedure could verbalize the stimulus contingencies whereas the subjects who did not learn the procedure could not (i.e., the subjects who learned trace conditioning were aware of the stimulus contingencies).
In another study, Clark and Squire (1999) directly manipulated awareness and studied conditioning in normal, older adults. This study involved four groups of subjects: Two were given a secondary, attention-demanding task designed to reduce awareness of the conditioning contingencies; a third group was given an explicit explanation of the conditioning contingencies; and a fourth group was given the explicit explanation and the attention-demanding task. Clark and Squire showed that those subjects given trace conditioning and the distraction task did not acquire differential CRs, whereas those given the delay procedure with distraction did acquire differential CRs. The group given knowledge of the CS-US contingency and trace conditioning learned the differential CR, but subjects given knowledge together with the distraction task did not. These data indicate that awareness of the contingency affects trace conditioning although the individuals apparently must have access to the knowledge during the conditioning session to produce CRs.
At the very least, these data provide a wonderful demonstration of the power of analysis afforded by the use of eyeblink conditioning for the study of basic learning and memory phenomena. Future studies will undoubtedly continue to use this paradigm to explore the hippocampus and other brain systems involved in declarative memory.
Studies of the Involvement of Other Higher Brain Areas in Eye-Blink Conditioning
Overtheyearsanumberofstudieshaveexaminedtheinvolvement of other higher brain areas, such as the cerebral cortex, thalamus, amygdala, and neostriatum, in classical eye-blink conditioning. Even though there is ample evidence that higher brain areas are not necessary for conditioning, many studies have provided evidence that these brain areas are recruited during conditioning.
Although Oakley and Steele (e.g., 1972) showed that eyeblink conditioning could be achieved without cerebral neocortex, some studies have demonstrated that neocortex is engaged in eye-blink conditioning and may be encoding the learning process. For example, Fox, Eichenbaum, and Butter (1982) showed that lesions of frontal cortex in rabbits decreased the latencies of conditioned eye-blink responses and retarded extinction of the CRs. Through systematic lesions of brain stem nuclei that interact with frontal cortex, they provided evidence that the frontal cortex may normally provide inhibitory effects on conditioned behavior indirectly through brain stem nuclei that make contact with motor neurons responsible for CR formation.
Given the central nature of the thalamus in distributing information to higher brain areas, several studies have explored whether this structure is involved in eye-blink conditioning. Buchanan and Powell (1988) showed that knife cuts that severed afferent and efferent connections between the prefrontal cortex and the mediodorsal nucleus of the thalamus retarded the rate of conditioning established with a tone CS and periorbital shock US. These lesions abolished the late-occurring tachycardiac component of the conditioned heart rate response that was measured concomitantly. Similar results were obtained when ibotenic acid lesions of the mediodorsal thalamic nuclei were used (Buchanan & Thompson, 1990). These data suggested that the mediodorsal thalamic–prefrontal circuitry was involved in the sympathetic control associated with somatomotor learning. Other thalamic regions have also been studied. For example, Sears, Logue, and Steinmetz (1996) recorded neuronal activity from the ventrolateral thalamus, which receives input from the cerebellum, among other areas of the brain. Learning-related neuronal activity was recorded from the ventrolateral thalamus, and this activity was abolished after cerebellar lesions. These data suggest that the ventrolateral thalamus receives an efferent copy of learningrelatedactivitythatisgeneratedinthecerebellum—anefferent copy that is perhaps used to integrate the learned movement into the ongoing motor activity of the organism.
In agreement with other data suggesting a role for the brain’s dopaminergic system in sensorimotor learning and integration, there is evidence that the dopamine system is activated during eye-blink conditioning. Kao and Powell (1988) bilaterally infused 6-hydroxydopamine into the substantia nigraandobservedaretardationofbotheye-blinkconditioning and heart-rate conditioning. The lesions produced significant norepinephrine depletion in the nucleus accumbens, frontal cortex, and hypothalamus and also produced dopamine depletion in the caudate nucleus. Furthermore, the rate of conditioning was highly correlated with the level of dopamine found in the caudate. In another study, White et al. (1994) recorded unit activity from the neostriatum during eye-blink conditioning (see also Richardson &Thompson, 1985). Neostriatal neurons were responsive to the tones and air puffs used as CSs and USs, respectively, and some neurons showed a CR-related pattern of discharge with an onset that preceded the behavioral response. Haloperidol, a dopamine antagonist, caused a disruption of behavioral and neural responding that appeared to be related to CS intensity. This observation was consistent with an earlier study that suggested a role for dopamine in CS processing (Sears & Steinmetz, 1990). These data suggest that the neostriatum may be activated during eye-blink conditioning; consistent with other studies (e.g., Schneider, 1987), the data support the idea that the neostriatum may be modulating the access of sensory inputs to motor output.
Finally, there has been a great deal of recent interest in the role of the amygdala in learning and memory, especially in processing emotional aspects (as discussed earlier). Given that eye-blink conditioning is an aversive conditioning procedure, it was reasonable to assume that the amygdala might be activated during this type of learning. This appears to be the case. Whalen and Kapp (1991) showed that stimulation of the central nucleus of the amygdala increased the amplitude of an eye-blink UR that was subsequently elicited by an air puff US. Further, Weisz, Harden, and Xiang (1992) showed that large electrolytic lesions of the amygdala disrupted the maintenance of reflex facilitation of the eye-blink UR and retarded the acquisition of the eye-blink CR. These data suggest that the amygdala may be involved in US processing, perhaps in processing information concerning the aversiveness of the US (see Richardson & Thompson, 1984). This would be compatible with other studies suggesting a role for the amygdala in emotional processing (e.g., Hitchcock & Davis, 1991; LeDoux, 1995). Consistent with this view, Wagner and associates have developed a theoretical model (AESOP; Wagner & Brandon, 1989) that incorporates both emotive and sensory aspects of classical conditioning and have presented considerable empirical evidence to support the model (Brandon & Wagner, 1991).
In related work, Shors and associates have explored effects of behavioral stress on processes of learning and memory (Shors, 1998). In general, severe stress can markedly impair performance in learning tasks that might be categorized as declarative in rodents (Overmier & Seligman, 1967; see Figure 18.1). This is perhaps consistent with the fact that behavioral stress markedly impairs the subsequent induction of LTP in the hippocampus (rat, CA1 slice; Foy, Foy, Levine, & Thompson, 1990; Foy, Stanton, Levine, & Stanton, 1987; Shors, Levine, & Thompson, 1990; Shors, Seib, Levine, & Thompson, 1989). Indeed, these observations support the view that LTP is a mechanism of declarative memory storage in the hippocampus (Bliss & Collingridge, 1993). Shors, Weiss, and Thompson (1992) discovered that stress actually facilitates classical eye-blink conditioning in rats. It is interesting that a much earlier literature reported a similar effect in humans: High anxious subjects learn eye-blink conditioning better than do low anxious subjects (Taylor, 1951). Shors subsequently showed that this stress facilitation of conditioning is sexually dimorphic, facilitating learning in males but impairing learning in females. The facilitation in males involves the amygdala, whereas the impairment in females is dependent on activational influences of estrogen (Shors, Beylin, Wood, & Gould, 2000; Shors, Lewczyk, Pacynski, Mathew, & Pickett, 1998; Wood & Shors, 1998).
The Critical Involvement of the Cerebellum in Eye-Blink Conditioning
The results of lesion studies involving the cerebral neocortex and limbic system strongly suggested that the learning and memory of classical eye-blink CRs was not critically dependent on higher brain areas but more likely critically involved lower brain stem areas. With this in mind, Thompson and colleagues used a variety of techniques, including systematic lesion and recording methods, in an attempt to find regions of the lower brain that were essential for the acquisition and performance of eye-blink CRs. These experiments suggested a critical role for the cerebellum in eye-blink conditioning and launched 20 years of research that has strongly supported these early results.
In early studies large aspirations of the cerebellum that included cortex and the deep cerebellar nuclei were found to abolish eye-blink CRs in rabbits trained before the lesion and prevent acquisition of eye-blink CRs in rabbits trained after the lesion (Lincoln, McCormick, & Thompson, 1982; McCormick et al., 1981). Subsequent studies showed that small electrolytic lesions (McCormick & Thompson, 1984a; Steinmetz, Lavond, et al., 1992) as well as kainic acid lesions (Lavond, Hembree, & Thompson, 1985) delivered to a dorsolateral region of the anterior interpositus nucleus on the side ipsilateral to the training permanently abolished the learned responses (Figure 18.8).There appears to be no recovery from the cerebellar lesion (Steinmetz, Logue, & Steinmetz, 1992). Lesions delivered to cerebellar cortex have produced mixed results. Some investigators have reported that lesions delivered to cerebellar cortex abolished CRs (Yeo & Hardiman, 1992; Yeo, Hardiman, & Glickstein, 1985); others have reported little or no effect of the lesion (Woodruff-Pak, Lavond, Logan, Steinmetz, & Thompson, 1993); and others have reported effects on the rate of acquisition and CR amplitude (Lavond & Steinmetz, 1989) and CR timing (Perrett, Ruiz, & Mauk, 1993). These lesion studies established that both the interpositus nucleus and cerebellar cortex were critically involved in eye-blink conditioning.
More recent studies using temporary inactivation techniques such as muscimol infusion (Krupa, Thompson, & Thompson, 1993) or cold probe cooling (Clark, Zhang, & Lavond, 1992) have provided compelling evidence that the cerebellum is necessary for eye-blink conditioning (Figure 18.9). In these studies, the region of the interpositus nucleus was temporarily inactivated by either infusing muscimol (which hyperpolarizes affected neurons) or by cooling with a cold probe (which shuts down neural function). Inactivation of the cerebellum after training abolished conditioned responding for the duration of infusion or cooling. It is even more interesting that when the cerebellum was inactivated during training trials delivered to naive rabbits, the animals showed no signs that paired training had been delivered. That is, after several days of training while the cerebellum was inactivated, no savings in acquisition were seen during subsequent training when the inactivation was removed. One would expect to see savings if essential neuronal plasticity processes were active at other brain sites during training. Because no savings were seen, these studies provided very strong evidence that the basic cellular processes important for plasticity that underlie classical eye-blink conditioning resided in the region of the cerebellum that was inactivated by the muscimol infusion or cold-probe cooling. These data provide some of the most compelling evidence to date that plasticity in the cerebellum is essential for classical eye-blink conditioning.
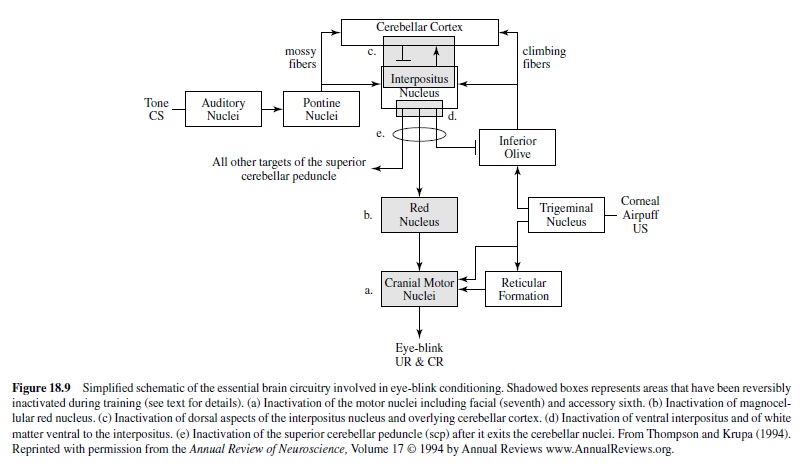
Neural recording studies have also provided evidence for the involvement of the cerebellum in encoding classical eye-blink conditioning. Multiple- and single-unit recordings made in the dorsolateral anterior interpositus nucleus have shown patterns of neuronal spiking that correlated well with the behavioral CR (Berthier & Moore, 1990; Gould & Steinmetz, 1996; McCormick & Thompson, 1984b). Neurons that respond to the CS or the US have been found along with neurons that discharge ina pattern that, when summed, formed amplitude-time course models of the behavioral response. Moreover, the onset of interpositus nucleus spiking typically preceded the behavioral response by 30 ms to 60ms, a time interval that can be accounted for by synaptic and neural processing delays between the cerebellum and the motor nuclei responsible for producing the CR. Recordings of Purkinje cell activity in cerebellar cortex have also revealed learningrelated patterns of unit discharges (Berthier & Moore, 1986; Gould & Steinmetz, 1996; Katz & Steinmetz, 1997). Some cells show CS- or US-related activation patterns, whereas other cells seem to fire in relation to the behavioral response. Purkinje cells have been isolated that increase their firing rate during the CS-US interval while other Purkinje cells that have been isolated show decreases in their firing rate (see King, Krupa, Foy, & Thompson, 2001; Thompson, 1986). These recording studies provide additional supportive evidence for the involvement of both cerebellar cortex and the interpositus nucleus in eye-blink conditioning. Specifically, the patterns of action potentials recorded in both the nucleus and the cortex appear to be encoding the delivery of the CS and US used during training as well as the learned response that is executed.
The essential stimulus pathways used in projecting the CS and US used in eye-blink conditioning from the periphery to the cerebellum have been delineated. On the CS side, it appears that CSs are projected to the basilar pontine nuclei, which send mossy fiber projections to the interpositus nucleus as well as to the cerebellar cortex (Figure 18.8). The basic CS pathway was established by stimulation, lesion, and recording studies. For example, CS-related responses were evoked in discrete regions of the pontine nuclei, and lesions delivered to these regions abolished conditioned responding (Steinmetz et al., 1987). Microstimulation delivered to these same regions could be substituted for the peripherally administered CS, and robust conditioning was produced (e.g., Steinmetz, 1990; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986; Tracy, Thompson, Krupa, & Thompson, 1998). The essential US appears to involve a projection from the region of the eye where the US is delivered to the trigeminal nucleus, which sends projections to a discrete region of the dorsal accessory inferior olive (R. Gellman, Houk, & Gibson, 1983). The inferior olive, in turn, sends climbing fiber projections to the cerebellar cortex and the deep cerebellar nuclei (Figure 18.9). Again, recording, stimulation, and lesion studies were used to establish the connectivity in the US system. Neurons in the dorsal accessory inferior olive were found to be responsivetostimulationoftheface,includingtopresentations of a corneal air puff (Sears & Steinmetz, 1991). Lesions of the inferior olive caused extinction (McCormick, Steinmetz, & Thompson, 1985; Voneida, Christie, Bogdanski, & Chopko, 1990;seealsolater)orabolition(Yeo,Hardiman,&Glickstein, 1986) of conditioned responding, whereas stimulation of the inferior olive, which produced a variety of discrete responses including eye blinks (depending on precise location of the stimulation), could be substituted for peripheral US to produce robust conditioning (Mauk, Steinmetz, & Thompson, 1986).
The most popular models concerning the cerebellar basis of classicaleye-blink conditioning hypothesize that plasticity that is crucial for acquisition and performance of the eye-blink CRs occurs in the cerebellar cortex, the deep cerebellar nuclei, or both regions where a convergence of CS and US input occurs (e.g.,Steinmetz,Lavond,&Thompson,1989;R.F.Thompson, 1986).There is ample electrophysiological and anatomical evidence for convergence of the CS and US in the cortex and deep nuclei of the cerebellum (e.g., Gould, Sears, & Steinmetz, 1993; Steinmetz & Sengelaub, 1992). At this point, it is assumed that paired CS-US presentations somehow produce changes in the firing rate of interpositus neurons, either independently or with critical input from cerebellar cortex, which in turn affects nuclei downstream. In essence, current models assume that before conditioning the CS is not capable of activating cerebellar neurons in a manner that would drive brain stem motor nuclei responsible for the CR. After paired training, however, the firing rates of cerebellar neurons are thought to change such that the CS is now capable of activating brain stem motoneurons involved in conditioned blinking.
The critical CR pathway between the cerebellum and the peripheral eye-blinking musculature has been worked out. Axons from the principle cells of the interpositus nucleus cross the midline and innervate the neurons in the magnocellular region of the red nucleus via the superior cerebellar peduncle (Figure 18.9). Lesions of the peduncle completely abolish the eye-blink (and limb flexion) CRs (McCormick, Guyer, & Thompson, 1982; Voneida, 2000). Similarly, lesions of the red nucleus have been shown to result in eye-blink CR abolition (e.g., Haley, Thompson, & Madden, 1988), and learning-related neuronal activity has been recorded from the red nucleus (Chapman, Steinmetz, Sears, & Thompson, 1990; Desmond & Moore, 1991). Red nucleus output cells then project axons back across midline and make synaptic contact on neurons in the variety of cranial nerve nuclei (Figure 18.9) involved in generating the eye-blink CR (and UR, for that matter).
Wagner and Donegan (1989), incidentally, showed how a theoretical model of classical conditioning, the sometimesopponent-process (SOP) model, maps very closely onto the empirical model of the cerebellar circuitry essential for classical conditioning of discrete responses developed by Thompson and associates shown in Figures 18.8 and 18.9.
Interestingly, there is good evidence that in addition to projecting information to the red nucleus, output from the interpositus nucleus also feeds back on the CS and US pathways. Inhibitory projections from the deep nuclei to the dorsal accessory olive have been found (e.g., Andersson, Garwicz, & Hesslow, 1988), and it is known that when CR-related interpositus activity occurs, the inferior olive is inhibited such that US-related activity is not passed to the cerebellum (Sears & Steinmetz, 1991). On the CS side, there are known projections from the interpositus nucleus to the basilar pontine nuclei (likely excitatory), and that CR-related activation of the interpositus may, for some as of yet undetermined reason, project a copy of the CR to the CS input pathway during conditioning (Bao, Chen, & Thompson, 2000; Clark, Gohl, & Lavond, 1997). It is likely that the projections from the interpositus nucleus to the CS and US input pathways to the cerebellum are important for response timing and response topography and also may be responsible for some higher order variations of eye-blink conditioning, such as blocking (see Kim, Krupa, & Thompson, 1998).
Most studies conducted over the last five years or so have been designed to test various aspects of the general model of cerebellar involvement in eye-blink conditioning that were just presented. These studies have examined relationships between cerebellar cortical and nuclear involvement in the acquisition and performance of eye-blink conditioning and have begun the process of delineating neuronal processes that may be involved in establishing and maintaining plasticity that is associated with learning.
Many researchers have suggested that long-term depression (LTD) may play an important role in the acquisition and performance of classically conditioned eye-blink responses (e.g., R. F. Thompson, 1986). Long-term depression is a relatively long-term suppression of cerebellar Purkinje-cell excitability that is produced when climbing fibers and parallel fibers (which receive input from mossy fibers) are conjointly activated (e.g., Ekerot & Kano, 1985; Ito, 1989). Evidence suggests that LTD occurs through the desensitization of quisqualate receptors on Purkinje cells that receive synaptic contact from parallel fiber inputs (e.g., Hemart, Daniel, Jaillard,&Crepel,1994).Ithasbeenproposedthatthemossyfiber CS and climbing-fiber US used in classical eye-blink conditioning converge on Purkinje cells in cerebellar cortex and that LTD results from their coactivation (R. F. Thompson, 1986). Because Purkinje cells inhibit deep nuclear cells to which they send axons, the net effect of the Purkinje cell suppression would be an increase in excitability in the deep nuclei that could allow for the expression of eye-blink CRs through activation of lower motor nuclei. Indirect evidence for a role for LTD in eye-blink conditioning exists. First, convergence of CS and US from mossy-fiber and climbing-fiber sources, respectively, has been anatomically and electrophysiologically established in regions of cerebellar cortex (Gould et al., 1993; Steinmetz & Sengelaub, 1992). Second, recordings from Purkinje cells have shown that some of the neurons demonstrate a conditioning-related decrease in firing rate, as would be expected if LTD occurred as a result of conditioning (Berthier & Moore, 1986; Gould & Steinmetz, 1996).
Schreurs and colleagues intracellularly recorded from rabbit cerebellar slices to study directly the relationship between LTD and conditioning. In an initial study they showed a conditioning-specific increase in the excitability of Purkinje-cell dendrites in slices taken from cerebellar cortical lobule HVI without significant changes in dendritic membrane potential or input resistance (Schreurs, Sanchez-Andres, & Alkon, 1991). Although this initial result supported the idea that learning-specific plasticity took place in Purkinje cells, a decrease in excitability was not seen, as would be expected if LTD occurred. The increase in excitability did, however, explain earlier studies that showed a large number of learningrelated excitatory responses recorded extracellularly from Purkinje cells of awake rabbits. Other studies showed that the increase in dendritic excitability of Purkinje cells was a result of alternations in a specific potassium channel (Schreurs, Gusev, Tomsic, Alkon, & Shi, 1998; Schreurs, Tomsic, Gusev, & Alkon, 1997).
Using direct stimulation of parallel fibers and climbing fibers, Schreurs and colleagues have produced an LTD effect in the cerebellar slice preparation (Schreurs & Alkon, 1993). In the presence of the GABA antagonist bicuculline, LTD was produced when climbing fibers were stimulated before parallel fibers, but no response depression was seen when the parallel-fiber stimulation preceded the climbing-fiber stimulation (as is hypothesized to occur during eye-blink conditioning). Depression could be obtained in the absence of bicuculline, however, when parallel fibers were stimulated in the presence of a depolarizing current that induced local, calcium-dependent dendritic spikes. Schreurs, Oh, and Alkon (1996) did produce a form of long-term reduction in Purkinje cell excitatory postsynaptic potentials (EPSPs) when parallel fibers were stimulated before climbing fibers were. The authors presented trains of stimulation for durations that mimicked conditioning in the intact rabbit. In fact, the trains were presented in nonoverlapping fashion. Consistent depression of EPSP peak amplitude was seen in the slice recordings when parallel fiber stimulation preceded climbing fiber stimulation but not when unpaired stimulations were delivered. In total, these data demonstrate that depending on the order and parameters of stimulation, both increases and decreases in excitability can be seen in Purkinje cells as a result of conjoint activation of parallel (mossy) fibers and climbing fibers. These results provide some direct evidence that cerebellar neurons are capable of showing associative plasticity that could at least in part account for eye-blink conditioning.
Other studies have demonstrated a role for glutamate receptors in plasticity processes associated with eye-blink conditioning. Hauge, Tracy, Baudry, and Thompson (1998) used quantitative autoradiography to examine changes in the ligand binding properties of alpha-amino-3-hydroxy5-methyl-4-isoxazoleproprionic acid (AMPA) receptors following eye-blink conditioning that was established by pairing a pontine stimulation CS with and an air puff US. Preincubation at 35°C produced significant decreases in AMPA binding, whereas unpaired CS-US presentations produced no significant effect.These data indicated that eye-blink conditioning is associated with a modification of AMPA-receptor properties in the cerebellum, modification in a direction that is compatible with the hypothesis that LTD is involved in the conditioning process. This hypothesis was further supported by Attwell, Rahman, Ivarsson, and Yeo (1999), who used 6-cyano-7nitroquinoxaline-2,3-dione (CNQX) infusions into cerebellar cortical lobule HVI to reversibly block AMPA-kainate receptors. Conditioned responses were reversibly blocked by the infusion, thus suggesting that cortical AMPA receptors were important for the expression of eye-blink CRs.
Other studies have examined the role of NMDA receptors in the interpositus nucleus in eye-blink conditioning. A number of studies had demonstrated that systemic injections of the noncompetitive NMDA antagonist MK-801 or PCP and the competitive NMDA antagonist CGP-39551 impaired the acquisition of classical eye-blink conditioning in rabbits and rats but did not affect the performance of the learned response (e.g., Robinson, 1993; Servatius & Shors, 1996; L. T. Thompson & Disterhoft, 1997). Chen and Steinmetz (2000a) further demonstrated that direct infusions of AP5, an NMDA receptor antagonist, in the region of the interpositus nucleus retarded the rate of conditioning but had little, if any, effect on the number of CRs emitted when infused after learning had taken place (although there was an indication that response timing in some rabbits was affected by the AP5 infusions). These studies suggest that NMDA receptors may play a role in the acquisition of classically conditioned eyeblink responses, a result that is compatible with a variety of literature suggesting a rather ubiquitous role for NMDA receptors in plasticity processes.
There is also evidence that protein synthesis in the cerebellumisinvolvedineye-blinkconditioning.Brachaetal.(1998) showed that microinjections of anisomycin, a general protein synthesis inhibitor, into the intermediate cerebellum near the interpositus nucleus impaired the acquisition of conditioning. The anisomycin had no effect on the expression of CRs when infused after asymptotic response levels had been reached. Gomi et al. (1999) further showed that infusion of the transcription inhibitor actinomycin D into the interpositus nucleus of rabbits reversibly blocked the learning but not the performance of eye-blink CRs. In this study, using differential-display polymerase chain reaction (PCR) analysis of interpositus RNAs from trained and control rabbits, they also demonstrated the existence of a 207-base-pair (bp) band that was induced by the conditioning and revealed that training had increased expression of KKIAMRE, a cdc2-related kinase, in interpositus neurons. This result was in agreement with a recent report by Chen and Steinmetz (2000b) that provided evidence that direct infusions of H7, a general protein kinase inhibitor, impaired the acquisition but not the retention of eye-blink CRs. Together, these data provide strong support for the idea that protein synthesis and, more specifically, protein kinase activity in the interpositus nucleus are involved in the acquisition of CRs.
New behavioral and molecular genetics techniques have proven useful for advancing our understanding of cellular processes that might underlie classical eye-blink conditioning. Chen, Bao, Lockard, Kim, andThompson (1996) showed that partial learning could be seen in Purkinje cell-degeneration (pcd) mutant mice that were given classical eye-blink conditioning trials. Lesions of the interpositus nuclei of the pcd mice, however, produced complete response abolition, thus demonstrating that some residual learning appears to take place in the interpositus nucleus (Chen, Bao, & Thompson, 1999). Qiao et al. (1998) showed a severe deficit in the acquisition of classical eye-blink conditioning in the spontaneous ataxic mutant mouse stargazer that have a selective reduction of brain-derived neurotrophic factor (BDNF) mRNAexpression in the cerebellum. Impaired eye-blink conditioning was also observed in the waggler mutant, which also has a selective deficit in BDNF expression involving cerebellar granule cells (Bao, Chen, Qiao, Knusel, & Thompson, 1998). These data suggest that BDNF may play a role in plasticity processes that underlie eye-blink conditioning.
The data concerning cellular processes involved with eyeblink conditioning seem to suggest that important plasticity processes associated with the learning of this simple, discrete response involve neurons in both cerebellar cortex and the deep cerebellar nuclei. Indeed, it is likely that interactions between cortical and nuclear areas, along with conditioningrelated feedback from the cerebellum onto the essential CS and US pathways that project information to the cerebellum, are involved in generating CRs. Some studies designed to examine these interactions are underway, and it is likely that many more will be undertaken in the next several years.
Using Classical Eye-Blink Conditioning to Study Other Behavioral and Neural Phenomena
Many investigators have recognized the usefulness of classical eye-blink conditioning for studying other behavioral and neurological phenomena such as development, aging, neurological impairment, and behavioral and psychological pathologies. Given the well-delineated behavioral bases of this simple form of learning as well as the emerging details concerning the neural substrates that underlie the behavioral plasticity, there has been a growing interest in using eyeblink conditioning to explore a host of basic science issues related to learning and memory as well as a number of clinical and applied phenomena.
Stanton and his colleagues have used eye-blink conditioning to describe the development of associative learning in rats (see Stanton & Freeman, 2000, for review). Using techniques developed by Skelton (1988) for classically conditioning the eye-blink response of freely moving rats, Stanton and his colleagues have conducted a series of studies that have explored the ontogeny of associative learning in the developing rat. For example, they have shown that the development of conditioning parallels the development of the cerebellum: postnatal day (PND) 17 rats do not show evidence of conditioning; PND 20 rats show moderate amounts of learning; and PND 24 rats demonstrate rather robust learning.
At the other end of the life cycle, eye-blink conditioning has been effectively used to study aging in humans and in animal models. Studies have shown that in both humans and nonhuman animals, age-related changes in conditioning can be seen when either the delay or trace conditioning procedure is used (e.g., Woodruff-Pak, Lavond, Logan, & Thompson, 1987;Woodruff-Pak &Thompson, 1988). Further, age-related deficits in conditioning seem to parallel age-associated changes in brain structures that are critical for eye-blink conditioning, such as the cerebellum and hippocampus (see Woodruff-Pak & Lemieux, 2001, for review). In addition, research by Woodruff-Pak and her colleagues has shown that classical eye-blink conditioning is a very sensitive indicator and predictor ofAlzheimer’s disease, a degenerative brain disorder that is known to affect brain regions that are important for eye-blink conditioning (see Woodruff-Pak, 2000, for review).
Classical eye-blink conditioning has proven useful for the study of neurological and psychological impairments. For example, Daum and colleagues have shown that persons with cerebellar damage show impaired eye-blink conditioning (Daum et al., 1993) but not persons with other neurological impairments, including temporal lobe lesions (Daum, Channon, & Gray, 1992), Parkinson’s disease, and Huntington’s disease (see Schugens, Topka, & Daum, 2000, for review). At least some persons with autism are thought to have cerebellar cortical pathologies; and as predicted, deficits in classical eye-blink conditioning have been reported in some persons with autism (Sears, Finn, & Steinmetz, 1994). Specifically, persons with autism show greatly facilitated rates of conditioning and mistimed CRs, which may be due to the pathology in cerebellar cortex and the hippocampus that has been reported. A similar pattern of responding has been reported for individuals with schizophrenia who have been given eye-blink conditioning trials (Sears, Andreasen, & O’Leary, 2000). Finally, eye-blink conditioning has been used to test the basic idea that persons with obsessive-compulsive disorder (OCD) generally acquire aversively motivated CRs more rapidly than do other persons. Tracy, Ghose, Stetcher, McFall, and Steinmetz (1999) showed that under some contextual situations persons with OCD show an extremely rapid acquisition of eye-blink CRs, which supports the idea that these individuals may be sensitive to aversive CRs.
All of these examples demonstrate the great utility of eyeblink conditioning in testing hypotheses concerning behavioral and neural function, a utility that is due to the impressive database that has been assembled concerning behavioral and neural correlates of this simple form of associative learning.
Classical Conditioning of Other Discrete Responses
Although classical eye-blink conditioning has certainly been the most popular paradigm for studying the conditioning of discrete somatic responses, over the years classical conditioning of other response systems has been attempted.
In an early attempt to evaluate the potential role of the inferior colliculus in classical conditioning, Halas and Beardsley (1970) classically conditioned the hind-limb flexion responses of four cats while recording neural activity from the inferior colliculus. Training consisted of paired presentations of a tone CS with a mild electrical shock to the hind paw as a US. In the same cats they also delivered some instrumental conditioning trials where a leg flexion resulted in avoidance of the shock US. Halas and Beardsley reported largely negative results: For three cats the CS produced an inhibition of collicular activity that was not modified by training. For one cat CR-related activity was observed during instrumental but not classical conditioning trials.
Several researchers have studied forelimb flexion conditioning in the cat.Tsukahara and associated conducted a series of studies aimed at delineating the involvement of corticorubrospinal system in classical conditioning of forelimb flexion in cats (e.g., Tsukahara, Oda, & Notsu, 1981). In their studies the CS was electrical stimulation that was delivered to the cerebral peduncle, and the US was an electric shock delivered to the skin of the forelimb. Tsukahara and his colleagues used this preparation to study possible conditioning-related plasticity in the corticorubral system.
Voneida and colleagues have also classically conditioned the limb-flexion response in cats by pairing a tone CS and a mild electric shock to the forelimb as a US (Voneida et al., 1990). They have explored the involvement of the olivocerebellar system in encoding classical forelimb conditioning by delivering lesions to various regions of the inferior olivary complex. Rostromedial olivary lesions, which included spinoolivary and cortico-olivary forelimb projection zones and the olivocerebellar projection area, produced extinction and severe deficits in conditioned responding. This result parallels nicely the results of olivary lesion studies during eye-blink conditioning in the rabbit (McCormick et al., 1985; Yeo, Hardiman, & Glickstein, 1986).
Finally, Marchetti-Gauthier, Meziane, Devigne, and Soumireu-Mourat (1990) examined the effects of bilateral lesions of the cerebellar interpositus nucleus on forelimb flexion conditioning in mice. They used lights and tones as CSs and an electric shock delivered to the forelimb as a US. The micewererestrainedinPlexiglasrestraintboxes,andforelimb flexion responses were measured using EMG methods. These researchers showed that bilateral lesions of the interpositus nucleus prevented conditioning. However, unlike previous rabbit studies (e.g., Steinmetz, Lavond, et al., 1992), in which bilateral lesions of the interpositus nucleus were delivered after training, no effects of the lesions could be detected in their paradigm. They concluded that in the mouse the interpositus nucleus was necessary for acquisition, but not retention, of classically conditioned forelimb flexion, a most puzzling result and conclusion.
Classical Jaw-Movement Conditioning
In the 1960s Gormezano and colleagues developed a rabbitappetitive classical conditioning procedure that involves recording a relatively discrete jaw-movement response (e.g., Coleman, Patterson, & Gormezano, 1966; Sheafor & Gormezano, 1972; Smith, DiLollo, & Gormezano, 1966). In this procedure a light or tone CS is paired with a rewarding intraoral water (or saccharin) US. The water US causes a rhythmic jaw movement that is related to consumption. After repeated pairings with the appetitive US, the CS eventually becomes capable of eliciting the jaw-movement response in the absence of the US. In behavioral studies this procedure has proven useful for studying motivational factors that are associated with conditioning. For example, water deprivation prior to conditioning can produce a different motivational state for jaw-movement conditioning and hence alter the rate of acquisition (e.g., Mitchell & Gormezano, 1970). More important for our discussion here, classical jaw-movement conditioning has been effectively used to study the neural bases of simple appetitive learning (for reviews, see Berry, Seager, Asaka, & Borgnis, 2000; Berry, Seager, Asaka, & Griffin, 2001).
Critical CS and US pathways for jaw-movement conditioning have not been delineated. Although the motor pathways for jaw-movement conditioning have not been worked out in as much detail as those for eye-blink conditioning, it is assumed that the trigeminal nucleus, which controls the muscles of mastication (Donga, Dubuc, Kolta, & Lund, 1992), is chiefly involved in generating the UR and CR for this type of learning. The trigeminal is thought to be heavily influence by neocortical and other higher input in the generation of the jaw-movement response, perhaps accounting for the relatively complex rhythmic response pattern that is seen (Dellow & Lund, 1971). Thus, important differences between jaw-movement conditioning and eye-blink conditioning are already apparent—the jaw-movement response is relatively more complex and appears not to involve the cerebellum. This was demonstrated in a study published by Gibbs (1992), who showed that lesions of the interpositus nucleus completely abolished eye-blink CRs but had no affect on jawmovement CRs recorded from the same rabbits.
Berry and colleagues have conducted an extensive series of studies on the involvement of the hippocampus in this type of appetitive learning. In multiple- and single-unit recording studies they have shown that pyramidal cells in the CA1 area of the hippocampus increased their firing rates over training (e.g., Oliver, Swain, & Berry, 1993; Seager, Borgnis, & Berry, 1997). Berry and colleagues compared hippocampal firing patterns recorded on eye-blink conditioning and jawmovement conditioning trials by using a discrimination procedure that employed two tones to differentiate air-puff US and water US trials (see Berry et al., 2000). Although excitatory patterns of responding are generally seen, the patterns of spiking during jaw-movement conditioning (i.e., the magnitude, duration, frequency, and rhythmicity of spiking) were somewhat different than that seen during eye-blink conditioning.
Berry and associates have also successfully used the jawmovement conditioning procedure to study aging effects as well as cholinergic brain function. Consistent with data from eye-blink conditioning experiments (Woodruff-Pak et al., 1987), they observed that 40- to 49-month-old rabbits were slower to acquire the conditioned jaw-movement response than were 3- to 7-month-old rabbits (Seager et al., 1997). Deficits in hippocampal unit activity were also seen in the aged rabbits. Early in training, young rabbits showed significantly great hippocampal activity just prior to US onset, and the magnitude of this activity was highly correlated with the rate of learning. The rhythmic CRs recorded in the aging rabbits were found to be of a significantly lower frequency than younger rabbits, but no difference in UR rhythmicity was observed. This suggested that the effect of aging was on a neural system that somehow modulated the central pattern generator during CRs, but not URs.
Given previous data indicating an involvement of disruptions of cholinergic systems in aging effects, Berry and colleagues have also studied the effects of cholinergic impairment on jaw-movement conditioning. They found many parallels between aging effects and cholinergic impairment. For example, subcutaneous injections of cholinergic blockers (e.g., scopolamine hydrobromide) resulted in longer acquisition times and also the suppression of conditioningrelated hippocampal activity (Salvatierra & Berry, 1989). Further, the cholinergic blocker selectively decreased the frequency of CR rhythmicity similar to the level seen in aged rabbits.
The hippocampal recording, aging, and pharmacological studies described here illustrate the potential usefulness of the classical jaw-movement conditioning procedure for studying the neural bases of the associative learning of discrete responses. It is clear that among other things, the major use of this procedure may be in delineating similarities and differences between appetitive and aversive conditioning processes.
Neural Substrates of the Instrumental Conditioning of Discrete Responses
Instrumental conditioning procedures have also been used to advance our understanding of the neural bases of simple associative learning. While formally similar to classical conditioning procedures (e.g., discrete stimuli are typically used and discrete responses are recorded), instrumental conditioning differs from classical conditioning in one important respect—the response made by the organism affects the delivery of the stimuli used in training. Avoidance conditioning best illustrates the difference between classical and instrumental conditioning. In classical eye-blink conditioning, a US is presented after the CS regardless of whether the subject generates a CR. This task can easily turn into an instrumental task by introducing a contingency between the subject’s response and the presentation of the US. For example, in instrumental eye-blink conditioning the US is withheld if the subject executes a CR prior to when the US would normally occur.
Gabriel and colleagues have used an instrumental conditioning procedure to explore extensively the involvement of the forebrain and other structures in simple associative learning (see Gabriel & Talk, 2001, for review). Their procedure, known as discriminative instrumental avoidance learning, is an adaptation of a procedure first described by Brogden and Culler (1936). Rabbits are placed in a large rotating wheel apparatus. Two CSs (typically tones of two different frequencies) are presented. One tone frequency (the CS+ ) is initially followed by a foot-shock US, whereas the second tone frequency (the CS– ) is presented alone. If the rabbit steps forward (thus turning the wheel) after tone onset but before shock onset, the shock is not delivered (i.e., the rabbit has successfully avoided the shock). Over several trials, the rabbit learns to respond to the CS+ and ignore the CS– . More recently, Gabriel and colleagues developed an appetitive procedure that parallels the aversive task. In this task the rabbits can receive a reward by approaching and making oral contact with a drinking spout that is presented for a period of time after CS+ presentation. The reward is not delivered if spout contact is made after CS– presentation. In an elegant series of studies, Gabriel and colleagues have recorded brain activity from a variety of brain regions during these forms of learning and have thus described to a large extent the neural systems involved in this learning.
Gabriel and colleagues have described the neural system involved in discriminative instrumental avoidance (and approach) learning as functional modules (Freeman, Cuppernell, Flannery, & Gabriel, 1996; Gabriel & Talk, 2001). Unlike eye-blink conditioning, in which relatively few critical sites for CS-US convergence appear to exist (and most seem to be in the cerebellum), Gabriel and colleagues have proposed that there are many CS-US convergence sites for discriminative instrumental learning, each of which has a rather unique and necessary function for learning to take place. Other CS-US convergence sites are thought to have important modulatory functions. These various sites are referred to as modules, and each module is assumed to receive CS and US input during learning as well as input from other modules.
In an extensive and comprehensive series of studies conducted over the last 25 years or so, Gabriel and colleagues have used mainly lesion and neural recording methods to define and study the modules involved in this type of instrumental learning. An in-depth review of the impressive data set is beyond the scope of this research paper, but a few generalities concerning their findings can be raised. First, the cingulate cortex and associated thalamic nuclei play a very important role in this learning (e.g., Freeman & Gabriel, 1999; Gabriel, 1990; Gabriel, Sparenborg, & Kubota, 1989). These areas seem to comprise a module that is specialized for processing associative attention and also retrieval of information in response to the presentation of task-relevant cues. The involvement of the hippocampus in this type of instrumental learning has also been evaluated (e.g., Kang & Gabriel, 1998). Lesion and recording studies seem to suggest that the hippocampus is a module that is involved in context-based retrieval (i.e., processing the context in which the simple associative cuebased learning occurs). A third major module that has been defined involves the amygdala, which Gabriel and colleagues have identified as important for initiating learning-relevant plasticity in other areas of the brain (e.g., Poremba & Gabriel, 1999). This idea is compatible with the view championed by McGaugh and his colleagues that amygdala efferents are involved mainly in the establishment of memory in a host of nonamygdalar brain areas (e.g., McGaugh & Cahill, 1997).
There appears to be little, if any, overlap in the neural circuitry that encodes classical eye-blink conditioning and discriminative avoidance learning. In two collaborative studies Gabriel and colleagues and Steinmetz and colleagues evaluated the effects of cerebellar lesions (Steinmetz, Sears, Gabriel, Kubota, & Poremba, 1991) and limbic thalamus lesions (Gabriel et al., 1996) on the two procedures. A complete dissociation of lesion effects was observed: Lesions of the interpositus nucleus completely abolished classical eye-blink CRs but had no effect on the discriminative avoidance learning, whereas lesions of the limbic thalamus severely impaired discriminative avoidance learning but had no effect on classical eye-blink conditioning. Differences in the two learning tasks most likely account for the observed dissociation. The avoidance task involves a relatively complex, goal-directed movement in a discriminative context. The classical conditioning task involves a relatively discrete movement in a nondiscriminative context. Also, the interstimulus intervals for the two tasks are widely different—the CS-US interval for the instrumental learning task is usually greater than 5 s, whereas the CS-US interval for the classical conditioning task is never more than 2 se(and more often in the range of 250–500 ms).
Steinmetz and colleagues have recently developed an instrumental bar-press conditioning procedure in rats that has been successfully used to study behavioral, neural, and pharmacological phenomena (Steinmetz et al., 1993). In the aversive version of this task, a tone CS is presented for a period of time, and rats are required to press a response bar during the tone presentation to avoid a mild foot-shock US. A bar press made during the shock presentation terminates the shock, thus allowing an escape response. In the appetitive version of this task, a tone CS is presented for a period of time, and rats receive a food pellet reinforcement if they press the response bar while the tone is on. This preparation has been used in within-subject design experiments. The same rat is trained in both the aversive and appetitive versions of the task using the same tone CS, the same training context, and the same response requirements (i.e., in essence, the only difference between the appetitive and aversive training is the consequences of the bar press). Variations of the task have also been used such as training using a discriminative stimulus to signal appetitive and aversive trials, training on partial or delay reinforcement schedules, and training in conjunction with autonomic recording.
In an initial study Steinmetz et al. (1993) demonstrated that bilateral lesions of the cerebellar interpositus nuclei prevented learning of the aversively motivated bar-press learning when relatively short tone presentation intervals were used. The same rats could acquire the appetitive task normally, thus demonstrating that the lesion-induced deficit was not sensory or motor in nature. This within-subject instrumental learning procedure has also been used successfully to study basic approach and avoidance learning in rats that were bred specifically for alcohol preference (e.g., Blankenship, Finn, & Steinmetz, 1998, 2000) as well as in studies by Garraghty and colleagues designed to assess cognitive impairments associated with the administration of antiepileptic compounds (Banks, Mohr, Besheer, Steinmetz & Garraghty, 1999).
Conclusion
Study of the brain substrates of learning and memory is at a most exciting stage. We are rapidly gaining an appreciation of the neural circuits and pathways that form the essential substrates for different forms of learning and memory. On the other hand, analyses of the basic mechanisms of synaptic plasticity, LTP, and LTD are proceeding rapidly. At present, these mechanisms are viewed by many as the most likely candidates for memory storage in the brain (Baudry, Davis, & Thompson, 2000; Bliss & Collingridge, 1993; but see Shors & Matzel, 1997). However, these two approaches have yet to meet. At present, LTP and LTD are mechanisms in search of phenomena, and the various forms of learning and memory are phenomena in search of mechanisms. It is our fervent hope that the two will meet. The fundamental problem, posed by Karl Lashley in 1929, remains that in order to analyze mechanisms of memory storage, it is first necessary to localize the sites of storage in the brain. This is now close to being accomplished for simpler forms of learning in mammals: classical conditioning of fear (amygdala) and discrete behavioral responses (cerebellum). Only when this has been done can we build a tight causal chain from, for example, LTP in the amygdala or LTD in the cerebellum to the behavioral expression of memory. The problem is more severe in the hippocampus, a structure that prominently displays LTP and LTD and is clearly necessary for declarative-experiential storage (and retrieval) in humans and seemingly in other mammals as well. Virtually nothing is known about the readout, the neural circuitry, from hippocampus to behavioral expression of memory.
To return to molecular biology, the bases of LTP, LTD, and other aspects of synaptic plasticity are likely to be understood indetailatthebiochemical,structural,andgeneticlevelsinthe next decade or so. Assume for the moment that LTP and LTD are the mechanisms of memory storage in the mammalian brain. Having established this, will we understand memory storage in the brain? The answer is clearly no. All LTP and LTD do is to increase or decrease transmission of information at the synapses where they occur. The nature of the memories so coded is determined entirely by particular complex neural circuits in the brain that form the memories. Moleculargenetic analysis can someday tell us the nature of the mechanisms of memory storage and perhaps even the loci of storage, but it can never tell us what the memories are. Only a detailed characterization of the neural circuitries that code, store, and retrieve memories, the focus of this research paper, can do this.
Bibliography:
- Abeliovich, A., Paylor, R., Chen, C., Kim, J. J., Wehner, J. M., & Tonegawa, S. (1993). PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell, 75, 1263–1271.
- Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372, 669–672.
- Aggleton, J. P. (2000). The amygdala (2nd ed.). Oxford, England: Oxford University Press.
- Alkon, D. L. (1989). Memory storage and neural systems. Scientific America, 261, 42–50.
- Amorapanth, P., LeDoux, J. E., & Nader, K. (2000). Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature Neuroscience, 3, 74–79.
- Anagnostaras, S. G., Maren, S., & Fanselow, M. S. (1999). Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. Journal of Neuroscience, 19, 1106–1114.
- Andersson, G., Garwicz, M., & Hesslow, G. (1988). Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat. Experimental Brain Research, 72, 450–456.
- Antoniadis, E. A., & McDonald, R. J. (2000). Amygdala, hippocampus and discriminative fear conditioning to context. Behavioural Brain Research, 108, 1–19.
- Applegate, C. D., Frysinger, R. C., Kapp, B. S., & Gallagher, M. (1982). Multiple unit activity recorded from the amygdala central nucleus during Pavlovian heart rate conditioning in the rabbit. Brain Research, 238, 457–462.
- Applegate, C. D., Kapp, B. S., Underwood, M. D., & McNall, C. L. (1983). Autonomic and somatomotor effects of amygdala central nucleus stimulation in awake rabbits. Physiology and Behavior, 31, 353–360.
- Attwell, P. J. E., Rahman, S., Ivarsson, M., & Yeo, C. H. (1999). Cerebellar cortical-AMPA-kainate receptor blockage prevents performance of classically conditioned nictitating membrane responses. Journal of Neuroscience, 19, 41–46.
- Bailey, D. J., Kim, J. J., Sun, W., Thompson, R. F., & Helmstetter, F. J. (1999). Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behavioral Neuroscience, 113, 276–282.
- Balaban, P. (1993). Behavioral neurobiology of learning in terrestrial snails. Progress in Neurobiology, 41, 1–19.
- Banks, M. K., Mohr, N. L., Besheer, J., Steinmetz, J. E., & Garraghty, P. E. (1999). The effects of phenytoin on instrumental appetitive-to-aversive transfer in rats. Pharmacology Biochemistry and Behavior, 63, 465–472.
- Bao, S., Chen, L., Qiao, X., Knusel, B., & Thompson, R. F. (1998). Impaired eye-blink conditioning in waggler, a mutant mouse with cerebellar BDNF deficiency. Learning and Memory, 5, 355–364.
- Bao, S., Chen, L., & Thompson, R. F. (2000). Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behavioral Neuroscience, 114, 254–261.
- Baudry, M., Davis, J. L., & Thompson, R. F. (2000). Advances in synaptic plasticity. Cambridge, MA: MIT Press.
- Beggs, A. L., Steinmetz, J. E., & Patterson, M. M. (1985). Classical conditioning of a flexor nerve response in spinal cats: Effects of tibial nerve CS and a differential conditioning paradigm. Behavioral Neuroscience, 99, 496–508.
- Beggs, A. L., Steinmetz, J. E., Romano, A. G., & Patterson, M. M. (1983). Extinction and retention of a classically conditioned flexor nerve response in acute spinal cat. Behavioral Neuroscience, 97, 530–540.
- Beggs, J. M., Brown, T. H., Byrne, J. H., Crow, T., LeDoux, J. E., LeBar, K., & Thompson, R. F. (1999). Learning and memory:
- Basic mechanisms. In M. J. Zigmond, F. E. Bloom, S. C. Landis, L. Roberts, & L. R. Squire (Eds.), Fundamental neuroscience (pp. 1411–1454). New York: Academic Press.
- Berger, T. W., Alger, B., & Thompson, R. F. (1976). Neuronal substrate of classical conditioning in the hippocampus. Science, 192, 483–485.
- Berger, T. W., Rinaldi, P. C., Weisz, D. J., & Thompson, R. F. (1983). Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. Journal of Neurophysiology, 50, 1197–1219.
- Berger, T. W., & Thompson, R. F. (1978a). Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response: I. The hippocampus. Brain Research, 145, 323–346.
- Berger, T. W., & Thompson, R. F. (1978b). Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response: II. Septum and mammillary bodies. Brain Research, 156, 293–314.
- Bermudez-Rattoni, F., Intronini-Collison, I. B., & McGaugh, J. L. (1991). Reversible inactivation of the insular cortex by tetrodotoxin produces retrograde and anterograde amnesia for inhibitory avoidance and spatial learning. Proceedings of the National Academy of Sciences USA, 88, 5379–5382.
- Berry, S. D., Seager, M. A., Asaka, Y., & Borgnis, R. L. (2000). Motivational issues in aversive and appetitive conditioning paradigms. In D. S. Woodruff-Pak & J. E. Steinmetz (Eds.), Eyeblink classical conditioning: Vol. 2. Animal models (pp. 287–312). Boston: Kluwer.
- Berry, S. D., Seager, M. A., Asaka, Y., & Griffin, A. L. (2001). The septo-hippocampal system and classical conditioning. In J. E. Steinmetz, M. A. Gluck, & P. R. Solomon (Eds.), Model systems and the neurobiology of associative learning (pp. 79–110). Mahwah, NJ: Erlbaum.
- Berthier, N. E., & Moore, J. W. (1986). Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Experimental Brain Research, 63, 341–350.
- Berthier,N.E.,&Moore,J.W.(1990).Activityofdeepcerebellarnuclear cells during classical conditioning of nictitating membrane extension in rabbits. Experimental Brain Research, 83, 44–54.
- Blanchard, D. C., & Blanchard, R. J. (1972). Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology, 81, 281–290.
- Blanchard, R. J., & Blanchard, D. C. (1969). Crouching as an index of fear. Journal of Comparative and Physiological Psychology, 67, 370–375.
- Blanchard, R. J., & Blanchard, D. C. (1971). Defensive reactions in the albino rat. Learning and Motivation, 2, 351–362.
- Blanchard, R. J., Blanchard, D. C., Agullana, R., & Weiss, S. M. (1991). Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiology and Behavior, 50, 967–972.
- Blankenship, M. R., Finn, P. R., & Steinmetz, J. E. (1998). A characterization of approach and avoidance learning in alcohol preferring (P) and non-preferring (NP) rats. Alcoholism: Clinical and Experimental Research, 22, 1227–1233.
- Blankenship, M. R., Finn, P. R., & Steinmetz, J. E. (2000). A characterization of approach and avoidance learning in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcoholism: Clinical and Experimental Research, 24, 1778–1784.
- Bliss, T. V. P., & Collingridge, G. L. (1993). A synaptic model of memory: Long term potentiation in the hippocampus. Nature, 361, 31–39.
- Bolles, R. C. (1970). Species-specific defensive reactions and avoidance learning. Psychological Review, 71, 32–48.
- Bourtchuladze, R., Frenguelli, B., Blendy, J., Cioffi, D., Schutz, G., & Silva, A. J. (1994). Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell, 79, 59–68.
- Bracha, V., Irwin, K. B., Webster, M. L., Wunderlich, D. A., Stachowiak, M. K., & Bloedel, J. R. (1998). Microinjections of anisomycin into the intermediate cerebellum during learning affect the acquisition of classically conditioned responses in the rabbit. Brain Research, 788, 169–178.
- Brandon, S. E., & Wagner, A. R. (1991). Modulation of a Pavlovian conditioned reflex by a putative emotive conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 17, 299–311.
- Brogden, W. J., & Culler, E. (1936). Device for motor conditioning of small animals. Science, 83, 269–270.
- Brown, J. S., Kalish, H. I., & Farber, I. E. (1951). Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. Journal of Experimental Psychology, 41, 317–328.
- Brunzell, D. H., & Kim, J. J. (2001). Fear conditioning to tone but not context is attenuated by lesions of insular cortex and posterior extension of intralaminar complex. Behavioral Neuroscience, 115, 365–375.
- Bucci, D. J., Phillips, R. G., & Burwell, R. B. (2000). Contributions of postrhinal and perirhinal cortices to contextual information processing. Behavioral Neuroscience, 114, 882–894.
- Buchanan, S. L., & Powell, D. A. (1988). Parasagittal thalamic knife cuts retard Pavlovian eyeblink conditioning and abolish the tachycardiac component of the heart rate conditioned response. Brain Research Bulletin, 21, 723–729.
- Buchanan, S. L., & Thompson, R. H. (1990). Mediodorsal thalamic lesions and Pavlovian conditioning of heart rate and eyeblink responses in the rabbit. Behavioral Neuroscience, 104, 912– 918.
- Byrne, J. H., & Kandel, E. R. (1996). Presynaptic facilitation revisited: State- and time-dependence. Journal of Neuroscience, 16, 425–435.
- Cahill, L., Babinsky, R., Markowitsch, H., & McGaugh, J. L. (1995). The amygdala and emotional memory. Nature, 377, 295–296.
- Cahill, L., Haier, R., Fallon, J., Alkire, M., Tang, C., Keator, D., Wu, , & McGaugh, J. L. (1996). Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences USA, 93, 8016–8021.
- Cahill, L., Weinberger, N. M., Roozendaal, B., & McGaugh, J. L. (1999). Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron, 23, 227–228.
- Campeau, S., & Davis, M. (1995). Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience, 15, 2312–2327.
- Campeau, S., Miserendino, M. J. D., & Davis, M. (1992). Intraamydaloid infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fearpotentiated startle to an auditory conditioned stimulus. Behavioral Neuroscience, 106, 569–574.
- Cegavske, C. F., Patterson, M. M., & Thompson, R. F. (1979). Neuronal unit activity in the abducens nucleus during classical conditioning of the nictitating membrane response in the rabbit (oryctolagus cuniculus). Journal of Comparative Physiological Psychology, 93, 595–609.
- Cegavske, C. F., Thompson, R. F., Patterson, M. M., & Gormezano, I. (1976). Mechanisms of efferent neuronal control of the reflex nictitating membrane response in rabbit (oryctolagus cuniculus). Journal of Comparative and Physiological Psychology, 90, 411–423.
- Chapman, P. F., & Bellavance, L. L. (1992). Induction of long-term potentiation in the basolateral amygdala does not depend on NMDA receptor activation. Synapse, 11, 310–318.
- Chapman, P. F., Kairiss, E. W., Keenan, C. L., & Brown, T. H. (1990). Long-term synaptic potentiation in the amygdla. Synapse, 6, 271–278.
- Chapman, P. F., Steinmetz, J. E., Sears, L. L., & Thompson, R. F. (1990). Effects of lidocaine injection in the interpositus nucleus and red nucleus on conditioned behavioral and neuronal responses. Brain Research, 537, 149–156.
- Chen, G., & Steinmetz, J. E. (2000a). Intra-cerebellar infusion of NMDA receptor antagonist AP5 disrupts classical eyeblink conditioning in rabbits. Brain Research, 887, 144–156.
- Chen, G., & Steinmetz, J. E. (2000b). Microinfusion of protein kinase inhibitor H7 in the cerebellum impairs the acquisition but not retention of classical eyeblink conditioning in rabbits. Brain Research, 856, 193–201.
- Chen, L., Bao, S., Lockard, J. M., Kim, J. J., & Thompson, R. F. (1996). Impaired classical eyeblink conditioning in cerebellarlesioned and Purkinje cell degeneration (pcd) mutant mice. Journal of Neuroscience, 16, 2829–2838.
- Chen, L., Bao, S., & Thompson, R. F. (1999). Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell-degenerated mutant mice. Behavioral Neuroscience, 113, 204–210.
- Choi, J.-S., Lindquist, D. H., & Brown, T. H. (2001). Amygdala lesions block conditioned enhancement of the early component of the rat eyeblink reflex. Behavioral Neuroscience, 115, 764–775.
- Clark, R. E., Gohl, E. B., & Lavond, D. G. (1997). The learningrelated activity that develops in the pontine nuclei during classical eyeblink conditioning is dependent on the interpositus nucleus. Learning and Memory, 3, 532–544.
- Clark, R. E., & Squire, L. R. (1998). Classical conditioning and brain systems: The role of awareness. Science, 280, 77–81.
- Clark, R. E., & Squire, L. R. (1999). Human eyeblink classical eyeblink conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychological Science, 10, 14–18.
- Clark, R. E., & Squire, L. R. (2000). Awareness and the conditioned eyeblink response. In D. S. Woodruff-Pak & J. E. Steinmetz (Eds.), Eyeblink classical conditioning: Vol. 1. Applications in humans (pp. 229–251). Boston: Kluwer.
- Clark, R. E., Zhang, A. A., & Lavond, D. G. (1992). Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior. Behavioral Neuroscience, 106, 879–888.
- Clugnet, M.-C., & LeDoux, J. E. (1990). Synaptic plasticity in fear conditioning circuits: Induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. Journal of Neuroscience, 10, 2818–2824.
- Coleman, S. R., Patterson, M. M., & Gormezano, I. (1966). Conditioned jaw movement in the rabbit: Deprivation procedure and saccharin concentration. Psychonomic Science, 6, 39–40.
- Collingridge, G. L., Kehl, S. J., & McLennan, H. (1983). Excitatory amino acids in synaptic transmission in the Schaffer collateralcommissural pathway of the rat hippocampus. Journal of Physiology, 334, 33–46.
- Coulter, D. A., Lo Turco, J. J., Kubota, M., Disterhoft, J. F., Moore, J. W., & Alkon, D. L. (1989). Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. Journal of Neurophysiology, 61, 971–981.
- Cousens, G., & Otto, T. (1998). Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behavioral Neuroscience, 112, 1092–1103.
- Crow, T. (1988). Cellular and molecular anyalysis of associative learning and memory in Trends in Neuroscience, 11, 136–142.
- Danilova, L. K. (1986). Inhibitory effect of the amygdala on alimentary-conditioned reflexes in the dog. Zhunal Vysshei Nervnoi Deiatelnosi Imeni I. P. Pavlova, 36, 319–325.
- Dash, P. K., Hochner, B., & Kandel, E. R. (1990). Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature, 345, 718–721.
- Daum, I., Channon, S., & Gray, J. A. (1992). Classical conditioning after temporal lobe lesions in man: Sparing of simple discrimination and extinction. Behavioral Brain Research, 52, 159–165.
- Daum, I., Schugens, M. M., Ackermann, H., Lutzenberger, W., Dichgans, J., & Birbaumer, N. (1993). Classical conditioning after cerebellar lesions in humans. Behavioral Neuroscience, 107, 748–756.
- Davis, M. (1997). Neurobiology of fear responses: The role of the amygdala. Journal of Neuropsychiatry and Clinical Neurosciences, 9, 382–402.
- Deese, J. E., & Kellogg, W. N. (1949). Some new data on “spinal conditioning.” Journal of Comparative Physiological Psychology, 42, 157–160. de Jonge, M. C., Black, J., Deyo, R. A., & Disterhoft, J. F. (1990). Learning-induced afterhyperpolarization reductions in hippocampus are specific for cell type and potassium conductance. Experimental Brain Research, 80, 456–462.
- Dellow, P. G., & Lund, J. P. (1971). Evidence for central timing of rhythmical mastication. Journal of Physiology, 215, 1–13.
- Desmond, J. E., & Moore, J. W. (1991). Single-unit activity in red nucleus during the classically conditioned rabbit nictitating membrane response. Neuroscience Research, 10, 260–279.
- DeZazzo, J., & Tully, T. (1995). Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends in Neuroscience, 18, 212–218.
- Disterhoft,J.F.,Coulter,D.A.,&Alkon,D.L.(1986).Conditioningspecific membrane changes of rabbit hippocampal neurons measured in vitro. Proceedings of the National Academy of Sciences USA, 83, 2733–2737.
- Disterhoft, J. F., Golden, D. T., Read, H. L., Coulter, D. A., & Alkon, D. L. (1988). AHP reduction in rabbit hippocampal neurons during conditioning correlate with acquisition of the learned response. Brain Research, 462, 118–125.
- Disterhoft, J. F., & McEchron, M. D. (2000). Cellular alterations in hippocampus during acquisition and consolidation of hippocampus-dependent trace eyeblink conditioning. In D. S. Woodruff-Pak & J. E. Steinmetz (Eds.), Eyeblink classical conditioning: Vol. 2. Animal models (pp. 313–334). Boston: Kluwer.
- Disterhoft, J. F., Quinn, K. J., Weiss, C., & Shipley, M. T. (1985). Accessory abducens nucleus and conditioned eye retraction nictitating membrane extension in rabbit. Journal of Neuroscience, 5, 941–950.
- Donga, R., Dubuc, R., Kolta, A., & Lund, J. P. (1992). Evidence that the masticatory muscles receive a direct innervation from cell group k in the rabbit. Neuroscience, 49, 951–961.
- Dudai, Y. (1989). The neurobiology of memory. New York: Oxford University Press.
- Durkovic, R. G. (1975). Classical conditioning, sensitization, and habituation of the flexion reflex of the spinal cat. Physiological Behavior, 14, 297–304.
- Durkovic, R. G., & Prokowich, L. J. (1998). D-2-Amino-5phosphonovalerate, an NMDA receptor antagonist, blocks induction of associative long-term potentiation of the flexion reflex in spinal cat. Neuroscience Letters, 257, 162–164.
- Ekerot, C. F., & Kano, M. (1985). Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Research, 342, 357–360.
- Fanselow, M. S. (1984). What is conditioned fear? Trends in Neurosciences, 7, 460–462.
- Fanselow, M. S. (1986). Conditioned fear-induced opiate analgesia: A competing motivational state theory of stress-analgesia. Annals of the New York Academy of Sciences, 467, 40–54.
- Fanselow, M. S., & Kim, J. J. (1994). Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behavioral Neuroscience, 108, 210–212.
- Fendt, M. (2001). Injections of the NMDA receptor antagonist aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. Journal of Neuroscience, 21, 4111–4115.
- Fendt, M., & Fanselow, M. S. (1999). The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and Biobehavioral Reviews, 23, 743–760.
- Fischer, K. W., & Lazerson, A. (1984). Human development. New York: W. H. Freeman.
- Fox, P. C., Eichenbaum, H., & Butter, C. M. (1982). The role of frontal cortex-reticular interactions in performance and extinction of the classically conditioned nictitating membrane response in the rabbit. Behavioral Brain Research, 5, 143–156.
- Foy, M. R., Foy, J. G., Levine, S., & Thompson, R. F. (1990). Manipulationofpituitary-adrenalactivityaffectsneuralplasticity in rodent hippocampus. Psychological Science, 1, 201–204.
- Foy, M. R., Stanton, M. E., Levine, S., & Thompson, R. F. (1987). Behavioral stress impairs long-term potentiation in rodent hippocampus. Behavioral and Neural Biology, 48, 138–149.
- Francis, J., Hernandez, L. L., & Powell, D. A. (1981). Lateral hypothalamic lesions: Effects on Pavlovian cardiac and eyeblink conditioning in the rabbit. Brain Research Bulletin, 6, 155–163.
- Freeman, J. H., Jr., Cuppernell, C., Flannery, K., & Gabriel, M. (1996). Context-specific multi-site cingulate cortical, limbic thalamic, and hippocampal neuronal activity during concurrent discriminative approach and avoidance training in rabbits. Journal of Neuroscience, 16, 1538–1549.
- Freeman, J. H., Jr., & Gabriel, M. (1999). Changes of cingulothalamic topographic excitation patterns and avoidance response incubation over time following initial discriminative conditioning in rabbits. Neurobiology of Learning and Memory, 72, 259– 272.
- Frost, W. N., Brown, G. D., & Getting, P. A. (1996). Parametric features of habituation of swim cycle number in the marine mollusk Tritonia diomedea. Neurobiology of Learning and Memory, 65, 125–134.
- Gabriel, M. (1990). Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. Progress in Brain Research, 85, 467–483.
- Gabriel, M., Kang, E., Poremba, A., Kubota, Y., Allen, M. T., Miller, D. P., & Steinmetz, J. E. (1996). Neural substrates of discriminative avoidance learning and classical eyeblink conditioning in rabbits: A double dissociation. Behavioural Brain Research, 82, 23–30.
- Gabriel, M., Sparenborg, S., & Kubota, Y. (1989). Anterior and medial thalamic lesions, discriminative avoidance learning, and cingulate cortical neuronal activity in rabbits. Experimental Brain Research, 76, 441–457.
- Gabriel, M., & Talk, A. C. (2001). A tale of two paradigms: Lessons learned from parallel studies of discriminative instrumental learning and classical eyeblink conditioning. In J. E. Steinmetz, M. A. Gluck, & P. R. Solomon (Eds.), Model systems and the neurobiology of associative learning (pp. 149–186). Mahwah, NJ: Erlbaum.
- Gallagher, M., & Kapp, B. S. (1978). Manipulation of opiate activity in the amygdala alters memory processes. Life Sciences, 23, 1973–1978.
- Gallagher, M., Kapp, B. S., McNall, C. L., & Pascoe, J. P. (1981). Opiate effects in the amygdala central nucleus alters rabbit heart rate conditioning. Pharmacology, Biochemistry, and Behavior, 14, 497–505.
- Gellman, M. D., Schneiderman, N., Wallach, J. H., & LeBlanc, W. (1981). Cardiovascular responses elicited by hypothalamic stimulation in rabbits reveal a mediolateral organization. Journal of the Autonomic Nervous System, 4, 301–317.
- Gellman, R., Houk, J. C., & Gibson, A. R. (1983). Somatosensory properties of the inferior olive of the cat. Journal of Comparative Neurology, 215, 228–243.
- Gelperin, A. (1994). Nitric oxide, odor processing and plasticity. Netherlands Journal of Zoology, 44, 159–169.
- Gentile, C. G., Jarrell, T. W., Teich, A., McCabe, P. M., & Schneiderman, N. (1986). The role of amygdaloid central nucleus in the retention of differential Pavlovian conditioning of bradycardia in rabbits. Behavioural Brain Research, 20, 263– 273.
- Gibbs, C. M. (1992). Divergent effects on deep cerebellar lesions on two different conditioned somatomotor responses in rabbits. Brain Research, 585, 395–399.
- Gold, P. E., Hankins, L., Edwards, R. M., Chester, J., & McGaugh, J. L. (1975). Memory interference and facilitation with posttrial amygdala stimulation: Effect on memory varies with footshock level. Brain Research, 86, 509–513.
- Goldstein, L. E., Rasmusson, A. M., Bunney, B. S., & Roth, R. H. (1996). Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. Journal of Neuroscience, 16, 4787–4798.
- Gomi, H., Sun, W., Finch, C. E., Itohara, S., Yoshimi, K., & Thompson, R. F. (1999). Learning induces a CDC2-related protein kinase, KKIAMRE. Journal of Neuroscience, 19, 9530– 9537.
- Gormezano, I., Kehoe, E. J., & Marshall, B. S. (1983). Twenty years of classical conditioning with the rabbit. Progress in Psychobiology and Physiological Psychology, 10, 197–275.
- Gould, T. J., Sears, L. L., & Steinmetz, J. E. (1993). Possible CS and US pathways for rabbit classical eyelid conditioning: Electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral and Neural Biology, 60, 172–185.
- Gould, T. J., & Steinmetz, J. E. (1996). Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction and backward classical conditioning. Neurobiology of Learning and Memory, 65, 17–34.
- Green, J. T., Rogers, R. F., Goodlett, C. R., & Steinmetz, J. E. (2000). Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcoholism: Clinical and Experimental Research, 24, 438–447.
- Grossman, S. P., Grossman, L., & Walsh, L. (1975). Functional organization of the rat amygdala with respect to avoidance behavior. Journal of Comparative and Physiological Psychology, 88, 829–850.
- Grover, L. M., & Teyler, T. J. (1990). Two components of long-term potentiation induced by different patterns of afferent activation. Nature, 347, 477–479.
- Groves, P. M., & Thompson, R. F. (1970). Habituation: A dualprocess theory. Psychological Reviews, 77, 419–450.
- Guarraci, F. A., Frohardt, R. J., Falls, W. A., & Kapp, B. S. (2000). The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behavioral Neuroscience, 114, 647–651.
- Halas, E. S., & Beardsley, J. V. (1970). Acomparison of conditioned and unconditioned neuronal responses in the inferior colliculus of cats. Psychonomic Science, 18, 29–30.
- Haley, D. A., Thompson, R. F., & Madden, J. (1988). Pharmacological analysis of the magnocellular red nucleus during classical conditioning of the rabbit nictitating membrane response. Brain Research, 454, 131–139.
- Hammer, M., & Menzel, R. (1995). Learning and memory in the honeybee. Journal of Neuroscience, 15, 1617–1630.
- Harris, E. W., & Cotman, C. W. (1986). Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl-Daspartate antagonists. Neuroscience Letters, 70, 132–137.
- Hauge, S. A., Tracy, J. A., Baudry, M., & Thompson, R. F. (1998). Selective changes in AMPA receptors in rabbit cerebellum following classical conditioning of the eyelid-nictitating membrane response. Brain Research, 803, 9–18.
- Helmstetter, F. J. (1992). The amygdala is essential for the expression of conditional hypoalgesia. Behavioral Neuroscience, 106, 518–528.
- Helmstetter, F. J. (1993). Stress-induced hypoalgesia and defensive freezing are attenuated by application of diazepam to the amygdala. Pharmacology, Biochemistry, and Behavior, 44, 433–438.
- Helmstetter, F. J., & Bellgowan, P. S. (1994). Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behavioral Neuroscience, 108, 1005–1009.
- Hemart, N., Daniel, H., Jaillard, D., & Crepel, F. (1994). Properties of glutamate receptors are modified during long-term depression in rat cerebellar Purkinje cells. Neuroscience Research, 19, 213–221.
- Hesslow, G., & Ivarsson, M. (1994). Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. NeuroReport, 5, 649–652.
- Hesslow, G., & Ivarsson, M. (1996). Inhibition of the inferior olive during conditioned responses in the decerebrate ferret. Experimental Brain Research, 110, 36–46.
- Hiroaka, M., & Shimamura, M. (1977). Neural mechanisms of the corneal blinking reflex in cats. Brain Research, 125, 265–275.
- Hitchcock, J. M., & Davis, M. (1986). Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience, 100, 11–22.
- Hitchcock, J. M., & Davis, M. (1987). Fear-potentiated startle using an auditory conditioned stimulus: Effect of lesions of the amygdala. Physiology and Behavior, 39, 403–408.
- Hitchcock, J. M., & Davis, M. (1991). Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behavioral Neuroscience, 105, 826–842.
- Hitchcock, J. M., Sananes, C. B., & Davis, M. (1989). Sensitization of the startle reflex by footshock: Blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behavioral Neuroscience, 103, 509–518.
- Huang, Y. Y., & Kandel, E. R. (1998). Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron, 21, 169–178.
- Huerta, P. T., Sun, L. D., Wilson, M. A., & Tonegawa, S. (2000). Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron, 25, 473–480.
- Isaacson, R. L. (1974). The limbic system (3rd ed.). New York: Plenum.
- Ito, M. (1989). Long-term depression. Annual Review of Neuroscience, 12, 85–102.
- Ivkovich, D., & Thompson, R. F. (1997). Motor cortex lesions do not affect learning or performance of the eyelid response in rabbits. Behavioral Neuroscience, 111, 727–738.
- Iwata, J., Chida, K., & LeDoux, J. E. (1987). Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses.Brain Research, 418,183–188.
- Iwata, J., LeDoux, J. E., & Reis, D. J. (1986). Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Research, 368, 161–166.
- Jarrell, T. W., Gentile, C. G., McCabe, P. M., & Schneiderman, N. (1986). The role of the medial geniculate region in differential Pavlovian conditioning of bradycardia in rabbits. Brain Research, 374, 126–136.
- Johnston, D., Williams, S., Jaffe, D., & Gray, R. (1992). NMDAreceptor independent long-term potentiation. Annual Review of Physiology, 54, 489–505.
- Joynes, R. L., & Grau, J. W. (1996). Mechanisms of Pavlovian conditioning: Role of protection from habituation in spinal conditioning. Behavioral Neuroscience, 110, 1375–1387.
- Kandel, E. R. (1976). Cellular basis of behavior: An introduction to behavioral neurobiology. San Francisco: W. H. Freeman.
- Kaneko, T., & Thompson, R. F. (1997). Disruption of trace conditioning of the nictitating membrane response in rabbits by central cholinergic blockade. Psychopharmacology, 131, 161–166.
- Kang, E., & Gabriel, M. (1998). Hippocampal modulation of cingulo-thalamic neuronal activity and discriminative avoidance learning in rabbits. Hippocampus, 8, 491–510.
- Kao, K.-T., & Powell, D. A. (1988). Lesions of the substantia nigra retard Pavlovian eye-blink but not heart rate conditioning in the rabbit. Behavioral Neuroscience, 102, 515–525.
- Kapp, B. S., Frysinger, R., Gallagher, M., & Haselton, J. (1979). Amygdala central nucleus lesions: Effects on heart rate conditioning in the rabbit. Physiology and Behavior, 23, 1109–1117.
- Katz, D. B., & Steinmetz, J. E. (1997). Single-unit evidence for eyeblink conditioning in cerebellar cortex is altered, but not eliminated, by interpositus nucleus lesions. Learning and Memory, 4, 88–104.
- Kellogg, W. N. (1947). Is “spinal conditioning” conditioning? Journal of Experimental Psychology, 37, 263–265.
- Kellogg, W. N., Deese, J., & Pronko, N. H. (1946). On the behavior of the lumbospinal dog. Journal of Experimental Psychology, 36, 503–511.
- Kemenes, G., & Benjamin, P. R. (1994). Training in a novel environment improves the appetitive learning performance of the snail, Lymnaea stagnalis. Behavioral and Neural Biology, 61, 139–149.
- Kettner, R. E., & Thompson, R. F. (1982). Auditory signal detection and decision processes in the nervous system. Journal of Comparative and Physiological Psychology, 96, 328–331.
- Killcross, S., Robbins, T. W., & Everitt, B. J. (1997). Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature, 388, 377–380.
- Kim, J. J., Clark, R. E., & Thompson, R. F. (1995). Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience, 109, 195–203.
- Kim, J. J., & Fanselow, M. S. (1992). Modality-specific retrograde amnesia of fear. Science, 256, 675–677.
- Kim, J. J., Krupa, D. J., & Thompson, R. F. (1998). Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science, 279, 570–573.
- Kim, J. J., Rison, R. A., & Fanselow, M. S. (1993). Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral Neuroscience, 107, 1093–1098.
- King, D. A. T., Krupa, D. J., Foy, M. R., & Thompson, R. F. (2001). Mechanisms of neuronal conditioning. International Review of Neurobiology, 45, 313–337.
- Klüver, H., & Bucy, P. C. (1937). “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. American Journal of Physiology, 119, 352–353.
- Knight, D. C., Smith, C. N., Stein, E. A., & Helmstetter, F. J. (1999). Functional MRI of human Pavlovian fear conditioning: Patterns of activation as a function of learning. NeuroReport, 10, 3665– 3670.
- Krupa, D. J., Thompson, J. K., & Thompson, R. F. (1993). Localization of a memory trace in the mammalian brain. Science, 260, 989–991.
- LaBar, K. S., Gatenby, C., Gore, J. C., LeDoux, J. E., & Phelps, E. A. (1998). Amygdalo-cortical activation during conditioned fear acquisition and extinction: A mixed trial fMRI study. Neuron, 20, 937–945.
- LaBar, K. S., LeDoux, J. E., Spencer, D. D., & Phelps, E. A. (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience, 15, 6846–6855.
- Lavond, D. G., Hembree, T. L., & Thompson, R. F. (1985). Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Research, 326, 179–182.
- Lavond, D. G., Kim, J. J., & Thompson, R. F. (1993). Mammalian brain substrates of aversive classical conditioning. Annual Review of Psychology, 44, 317–342.
- Lavond, D. G., & Steinmetz, J. E. (1989). Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research, 33, 113–164.
- Leaton, R. N., & Borszcz, G. S. (1985). Potentiated startle: Its relation to freezing and shock intensity in rats. Journal of Experimental Psychology: Animal Behavioral Processes, 2, 248–259.
- LeDoux, J. E. (1995). Emotion: Clues from the brain. Annual Review of Psychology, 46, 209–235.
- LeDoux, J. E. (1996). The emotional brain. New York: Simon & Schuster.
- LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184.
- LeDoux, J. E., Farb, C., & Ruggiero, D. A. (1990). Topographic organization of neurons in the acoustic thalamus that project to the amygdala. Journal of Neuroscience, 10, 1043–1054.
- LeDoux, J. E., Iwata, J., Cicchetti, P., & Reis, D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience, 8, 2517–2529.
- LeDoux, J. E., Iwata, J., Pearl, D., & Reis, D. J. (1986). Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate body. Brain Research, 371, 395–399.
- Lee, H. J., Berger, S. Y., Stiedl, O., Spiess, J., & Kim, J. J. (2001). Post-training injections of catecholaminergic drugs do not modulate fear conditioning in rats and mice. Neuroscience Letters, 303, 123–126.
- Lee, H. J., Choi, J.-S., Brown, T. H., & Kim, J. J. (2001). Amygdalar N-methyl-D-aspartate (NMDA) receptors are critical for the expression of multiple conditioned fear responses. Journal of Neuroscience, 21, 4116–4124.
- Lee, H. J., & Kim, J. J. (1998). Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. Journal of Neuroscience, 18, 8444–8454.
- Li, X., Phillips, R. G., & LeDoux, J. E. (1995). NMDA and nonNMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Experimental Brain Research, 105, 87–100.
- Liang, K. C., Juler, R., & McGaugh, J. L. (1986). Modulating effects of postraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Research, 68, 125–133.
- Liang, K. C., McGaugh, J. L., Martinez, J. L., Jensen, R. A., Vasquez, B. J., & Messing, R. B. (1982). Post-training amygdaloid lesions impair retention of an inhibitory avoidance response. Behavioral Brain Research, 4, 237–249.
- Lin, X. Y., & Glanzman, D. L. (1994). Hebbian induction of longterm potentiation of Aplysia sensorimotor synapses: Partial requirement for activation of an NMDA-related receptor. Proceedings of the Royal Society of London, B 255, 215–221.
- Lincoln, J. S., McCormick, D. A., & Thompson, R. F. (1982). Ipsilateral cerebellar lesions prevent learning of the classically conditioned nictitating membrane/eyelid response. Brain Research, 242, 190–193.
- London, J. A., & Gillette, R. (1986). Mechanism for food avoidance learning in the central pattern generator of feeding behavior of Pleurobranchaea californica. Proceedings of the National Academy of Sciences USA, 83, 4058–4062.
- MacLean, P. D., & Delgado, J. M. R. (1953). Electrical and chemical stimulation of frontotemporal portion of limbic system in the waking animal. EEG Clinical Neurophysiology, 5, 91–100.
- Marchetti-Gauthier, E., Meziane, H., Devigne, D., & SoumireuMourat, B. (1990). Effects of bilateral lesions of the cerebellar interpositus nuclei on the conditioned forelimb flexion reflex in mice. Neuroscience Letters, 120, 34–37.
- Maren, S., Aharonov, G., & Fanselow, M. S. (1996). Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: Absence of a temporal gradient. Behavioral Neuroscience, 110, 708–717.
- Maren, S., Aharonov, G., & Fanselow, M. S. (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioral Brain Research, 88, 261–274.
- Maren, S., Aharonov, G., Stote, D. L., & Fanselow, M. S. (1996). N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditioned fear in rats. Behavioral Neuroscience, 110, 1365–1374.
- Maren, S., & Fanselow, M. S. (1995). Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. Journal of Neuroscience, 15, 7548–7564.
- Maren, S., & Fanselow, M. S. (1996). The amygdala and fear conditioning: Has the nut been cracked? Neuron, 16, 237–240.
- Matzel, L. D., Ledehendler, I. I., & Alkon, D. L. (1990). Relation of short-term associative memory by calcium-dependent protein kinase. Journal of Neuroscience, 7, 1198–1206.
- Mauk, M. D., Steinmetz, J. E., & Thompson, R. F. (1986). Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences USA, 83, 5349–5353.
- Mauk, M. D., & Thompson, R. F. (1987). Retention of classically conditioned eyelid responses following acute decerebration. Brain Research, 403, 89–95.
- McCormick, D. A., Guyer, P. E., & Thompson, R. F. (1982). Superior cerebellar peduncle lesions selectively abolish the ipsilateral classically conditioned nictitating membrane/eyelid response of the rabbit. Brain Research, 244, 347–350.
- McCormick, D. A., Lavond, D. G., Clark, G. A., Kettner, R. R., Rising, C. E., & Thompson, R. F. (1981). The engram found? Role of the cerebellum in classical conditioning of nictitating membrane and eyelid responses. Bulletin of the Psychonomic Society, 18, 103–105.
- McCormick, D. A., Lavond, D. G., & Thompson, R. F. (1982). Concomitant classical conditioning of the rabbit nictitating membrane and eyelid responses: Correlations and implications. Physiology and Behavior, 28, 769–775.
- McCormick, D. A., Steinmetz, J. E., & Thompson, R. F. (1985). Lesions of the inferior olivary complex cause extinction of the classically conditioned eyelid response. Brain Research, 359, 120–130.
- McCormick, D. A., & Thompson, R. F. (1984a). Cerebellum essential involvement in the classically conditioned eyelid response. Science, 223, 296–299.
- McCormick, D. A., & Thompson, R. F. (1984b). Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid responses. The Journal of Neuroscience, 4, 2811–2822.
- McDonough, J. H., & Kesner, R. P. (1971). Amnesia produced by brief electrical stimulation of amygdala or dorsal hippocampus in cats. Journal of Comparative and Physiological Psychology, 77, 171–178.
- McEchron, M. D., Bouwmeester, H., Tseng, W., Weiss, C., & Disterhoft, J. F. (1998). Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus, 8, 638–646.
- McEchron,M.D.,Tseng,W.,&Disterhoft,J.F.(2000).Neurotoxiclesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus, 10, 739–751.
- McGaugh, J. L. (1989). Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annual Review of Neurosciences, 12, 255–287.
- McGaugh, J. L. (2000). Memory: A century of consolidation. Science, 287, 248–251.
- McGaugh, J. L., & Cahill, L. (1997). Interaction of neuromodulatory systems in modulating memory storage. Behavioural Brain Research, 83, 31–38.
- McGaugh J. L., Cahill, L., Roozendaal, B. (1996). Involvement of the amygdala in memory storage: Interaction with other brain systems. Proceedings of National Academy of Sciences, 93, 13508–13514.
- McGaugh, J. L., Introini-Collison, I. B., & Nagahara, A. H. (1988). Memory-enhancing effects of posttraining naloxone: Involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Research, 446, 37–49.
- McGlinchey-Berroth, R., Carrillo, M. C., Gabrieli, J. D. E., Brawn, C. M., & Disterhoft, J. F. (1997). Impaired trace eyeblink conditioning in bilateral medial temporal lobe amnesia. Behavioral Neuroscience, 111, 873–882.
- McKernan, M. G., & Shinnick-Gallagher, P. (1997). Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature, 390, 607–611.
- Miserendino, M. J. D., Sananes, C. B., Melia, K. R., & Davis, M. (1990). Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature, 345, 716–718.
- Mitchell, D. S., & Gormezano, I. (1970). Effects of water deprivation on classical appetitive conditioning of the rabbit’s jaw movement response. Learning and Motivation, 1, 199–206.
- Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J., & Dolan, R. J. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383, 812–815.
- Morris, R. G., Davis, S., & Butcher, S. P. (1990). Hippocampal synaptic plasticity and NMDA receptors: A role in information storage? Philosophical Transactions of the Royal Society of London, 329B, 187–204.
- Moyer, J. R., Deyo, R. A., & Disterhoft, J. F. (1990). Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience, 104, 243–252.
- Muller, J., Corodimas, K. P., Fridel, Z., & LeDoux, J. E. (1997). Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behavioral Neuroscience, 111, 683–691.
- Nader, K., & LeDoux, J. E. (1997). Is it time to invoke multiple fear learning systems in the amygdala? Trends in Cognitive Sciences, 7, 241–246.
- Nader, K., Schafe, G. E., & LeDoux, J. E. (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature, 406, 722–726.
- Nagel, J. A., & Kemble, E. D. (1976). Effects of amygdaloid lesions on the performance of rats in four passive avoidance tasks. Physiology and Behavior, 17(2), 245–250.
- Oakley, D. A., & Russell, I. S. (1972). Neocortical lesions and Pavlovian conditioning. Physiology and Behavior, 8, 915–926.
- Oakley, D. A., & Russell, I. S. (1974). Differential and reversal conditioning in partially neodecorticate rabbits. Physiology and Behavior, 13, 221–230.
- Oakley, A., & Russell, I. S. (1976). Subcortical nature of Pavlovian differentiation in the rabbit. Physiology and Behavior, 17, 947– 954.
- Oakley, D. A., & Russell, I. S. (1977). Subcortical storage of Pavlovian conditioning in the rabbit. Physiology and Behavior, 18, 931–937.
- Oliver, C. G., Swain, R. A., & Berry, S. D. (1993). Hippocampal plasticity during jaw movement conditioning in the rabbit. Brain Research, 608, 150–154.
- Overmier, J. B., & Seligman, M. E. P. (1967). Effects of inescapable shock on subsequent escape and avoidance learning. Journal of Comparative and Physiological Psychology, 63, 23–33.
- Parent, M. B., Tomaz, C., & McGaugh, J. L. (1992). Increased training in an aversively motivated task attenuates the memory impairing effects of posttraining NMDA-induced amygdala lesions. Behavioral Neuroscience, 106, 791–799.
- Pascoe, J. P., & Kapp, B. S. (1985). Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behavioral Brain Research, 16, 117–133.
- Patterson, M. M. (1970). Classical conditioning of the rabbit’s (oryctolagus cuniculus) nictitating membrane response with fluctuating ISI and intracranial CS1. Journal of Comparative Physiological Psychology, 72, 193–202.
- Patterson, M. M. (1971). Inferior colliculus CS intensity effect on rabbit nictitating membrane conditioning. Physiology and Behavior, 6, 273–278.
- Patterson, M. M. (1975). Effects of forward and backward classical conditioning procedures on a spinal cat hind-limb flexor nerve response. Physiological Psychology, 3, 86–91.
- Patterson, M. M. (1976). Mechanisms of classical conditioning and fixation in spinal mammals. In A. H. Riesen & R. F. Thompson (Eds.), Advances in psychobiology (Vol. 3, pp. 381–434). New York: Wiley.
- Patterson, M. M., Cegavske, C. F., & Thompson, R. F. (1973). Effects of classical conditioning paradigm on hindlimb flexor nerve response in immobilized spinal cat. Journal of Comparative and Physiological Psychology, 84, 88–97.
- Patterson, M. M., Olah, J., & Clement, J. (1977). Classical nictitating membrane conditioning in the awake, normal, restrained cat. Science, 196, 1124–1126.
- Perrett, S. P., Ruiz, B. P., & Mauk, M. D. (1993). Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience, 13, 1708–1718.
- Phillips, R. G., & LeDoux, J. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106, 274–285.
- Pinto, R., & Bromily, R. B. (1950). A search for “spinal conditioning” and for evidence that it can become a reflex. Journal of Experimental Psychology, 40, 121–130.
- Polenchar,B.E.,Romano,A.G.,Steinmetz,J.E.,&Patterson,M.M. (1984). Effects of US parameters on classical conditioning of cat hindlimb flexion. Animal Learning and Behavior, 12, 69–72.
- Poremba, A., & Gabriel, M. (1999). Amygdala neurons mediate acquisition but not maintenance of instrumental avoidance behavior in rabbits. Journal of Neuroscience, 19, 9635–9641.
- Port, R. L., Romano, A. G., Steinmetz, J. E., Mikhail, A. A., & Patterson, M. M. (1986). Retention and acquisition of classical trace conditioned responses by rabbits with hippocampal lesions. Behavioral Neuroscience, 100, 745–752.
- Qiao, X., Chen, L., Gao, H., Bao, S., Hefti, F., Thompson, R. F., & Knusel, B. (1998). Cerebellar brain-derived neurotrophic factorTrkB defect associated with impairment of eyeblink conditioning in stargazer mutant mice. Journal of Neuroscience, 19, 6990–6999.
- Rescorla, R. A. (1967). Inhibition of delay in Pavlovian fear conditioning. Journal of Comparative and Physiological Psychology, 64, 114–120.
- Rescorla, R. A. (1988). Behavioral studies of Pavlovian conditioning. Annual Reviews of Neuroscience, 11, 329–352.
- Rescorla, R. A., & Wagner, A. R. (1972). Atheory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning: Vol. 2. Current theory and memory research (pp. 64–99). New York: Appleton-Century-Crofts.
- Richardson, R. T., & Thompson, R. F. (1984). Amygdaloid unit activity during classical conditioning of the nictitating membrane response in the rabbit. Physiology and Behavior, 32, 527–539.
- Richardson, R. T., & Thompson, R. F. (1985). Unit activity recorded from the globus pallidus during classical conditioning of the rabbit nictitating response. Brain Research, 332, 219–229.
- Riedel, G., Harrington, N. R., Hall, G., & Macphail, E. M. (1997). Nucleus accumbens lesions impair context, but not cue, conditioning in rats. NeuroReport, 8, 2477–2481.
- Robinson, G. B. (1993). MK801 retards acquisition of a classically conditioned response without affecting conditioning-related alterations in perforant path-granule cell synaptic transmission. Psychobiology, 21, 253–264.
- Rogan, M. T., & LeDoux, J. E. (1995). LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron, 15, 127–136.
- Rogan, M. T., Staubli, U. V., & LeDoux, J. E. (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature, 390, 604–607.
- Romanski, L. M., & LeDoux, J. E. (1992). Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. Journal of Neuroscience, 12, 4501– 4509.
- Rosen, J. B., & Davis, M. (1988). Enhancement of acoustic startle by electrical stimulation of the amygdala. Physiology and Behavior, 48, 343–349.
- Rosen, J. B., Hitchcock, J. M., Miserendino, M. J., Falls, W. A., Campeau, S., & Davis, M. (1992). Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. Journal of Neuroscience, 12, 4624–4633.
- Sahley, C. L. (1995). What we have learned from the study of learning in the leech. Journal of Neurobiology, 27, 434–445.
- Salvatierra, A. T., & Berry, S. D. (1989). Scopolamine disruption of septo-hippocampal activity and classical conditioning. Behavioral Neuroscience, 103, 715–721.
- Schafe, G. E., & LeDoux, J. E. (2000). Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. Journal of Neuroscience, 20,
- Schmajuk, N. A., & Christiansen, B. A. (1990). Eyeblink conditioning in rats. Physiology and Behavior, 48, 755–758.
- Schmaltz, L. W., & Theios, J. (1972). Acquisition and extinction of a classically conditioned response in hippocampectomized rabbit (oryctolagus cuniculus). Journal of Comparative Physiological Psychology, 79, 328–333.
- Schneider, J. S. (1987). Basal ganglia-motor influences: Role of sensory gating. In J. S. Schneider & T. I. Lidsky (Eds.), Basal ganglia and behavior: Sensory aspects of motor functioning (pp. 103–121). Toronto, Canada: Huber.
- Schreurs, B. G., & Alkon, D. L. (1993). Rabbit cerebellar slice analysis of long-term depression and its role in classical conditioning. Brain Research, 631, 235–240.
- Schreurs, B. G., Gusev, P. A., Tomsic, D., Alkon, D. L., & Shi, T. (1998). Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. Journal of Neuroscience, 18, 5498–5507.
- Schreurs, B. G., Oh, M. M., & Alkon, D. L. (1996). Pairing-specific long-term depression of Purkinje cell excitatory postsynaptic potentials results from a classical conditioning procedure in the rabbit cerebellar slice. Journal of Neurophysiology, 75, 1051– 1060.
- Schreurs, B. G., Sanchez-Andres, J. V., & Alkon, D. L. (1991). Learning-specific differences in Purkinje-cell dendrites of lobule HVI (lobulus simples): Intracellular recording in a rabbit cerebellar slice. Brain Research, 548, 18–22.
- Schreurs, B. G., Tomsic, D., Gusev, P. A., & Alkon, D. L. (1997). Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit’s nictitating membrane response. Journal of Neurophysiology, 77, 86–92.
- Schugens, M. M., Topka, H. R., & Daum, I. (2000). Eyeblink conditioning in neurological patients with motor impairments. In D. S. Woodruff-Pak & J. E. Steinmetz (Eds.) Eyeblink classical conditioning: Vol. 1. Applications in humans (pp. 191–204). Boston: Kluwer.
- Seager, M. A., Borgnis, R. L., & Berry, S. D. (1997). Delayed acquisition of behavioral and hippocampal responses during jaw movement conditioning in aging rabbits. Neurobiology of Aging, 18, 631–639.
- Sears, L. L., Andreasen, N. C., & O’Leary, D. S. (2000). Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biological Psychiatry, 48, 204– 209.
- Sears, L. L., Finn, P. R., & Steinmetz, J. E. (1994). Abnormal classical eyeblink conditioning in autism. Journal of Autism and Developmental Disorders, 24, 737–751.
- Sears, L. L., Logue, S. F., & Steinmetz, J. E. (1996). Involvement of the ventrolateral thalamus in rabbit classical eyeblink conditioning. Behavioural Brain Research, 74, 105–117.
- Sears,L.L.,&Steinmetz,J.E.(1990).Haloperidolimpairsclassically conditioned nictitating membrane responses and conditioningrelated cerebellar interpositus nucleus activity in rabbits. Pharmacology Biochemistry and Behavior, 36, 821–830.
- Sears, L. L., & Steinmetz, J. E. (1991). Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Research, 545, 114–122.
- Servatius, R. J., & Shors, T. J. (1996). Early acquisition, but not retention, of the classical conditioned eyeblink response is N-Methyl-D-Aspartate (NMDA) receptor dependent. Behavioral Neuroscience, 110, 1040–1048.
- Sheafor, P. J., & Gormezano, I. (1972). Conditioning of the rabbit’s (Oryctolagus cuninulus) jaw movement response: US magnitude effects on URs, CRs and pseudo-CRs. Journal of Comparative and Physiological Psychology, 81, 449–456.
- Shefer, S. I. (1988). Food conditioned reflexes in dogs during activation and blockade of the cholinoreactive system of the amygdala. Zhurnal Vysshi Nervnoi Deiatelnosti Imeni I. P. Pavlova, 38, 1010–1016.
- Shi, C., & Davis, M. (1999). Pain pathways involved in fear conditioning measured with fear-potentiated startle: Lesion studies. Journal of Neuroscience, 19, 420–430.
- Shors, T. J. (1998). Stress and sex effects on associative learning: For better or for worse. Neuroscientist, 4, 353–364.
- Shors, T. J., Beylin, A. V., Wood, G. E., & Gould, E. (2000). The modulation of Pavlovian memory. Behavioural Brain Research, 110, 39–52.
- Shors, T. J., Levine, S., & Thompson, R. F. (1990). Effect of adrenalectomyanddemedullatonthestress-inducedimpairmentoflongterm potentiation (LTP). Neuroendocrinology, 51, 70–75.
- Shors, T. J., Lewczyk, C., Pacynski, M., Mathew, P. R., & Pickett, J. (1998). Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. NeuroReport, 9, 419– 423.
- Shors, T. J., & Matzel, L. D. (1997). Long-term potentiation (LTP): What’s learning got to do with it? Behavioral Brain Science, 20, 597–613.
- Shors, T. J., Seib, T. B., Levine, S., & Thompson, R. F. (1989). Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science, 244, 224–226.
- Shors, T. J., Weiss, C., & Thompson, R. F. (1992). Stress-induced facilitation of classical conditioning. Science, 257, 537–539.
- Shurrager, P. S., & Culler, E. (1940). Conditioning in the spinal dog. Journal of Experimental Psychology, 26, 133–159.
- Shurrager, P. S., & Culler, E. (1941). Conditioned extinction of a reflex in a spinal dog. Journal of Experimental Psychology, 28, 287–303.
- Silva, A. J., Kogan, J. H., Frankland, P. W., & Kida, S. (1998). Creb and memory. Annual Reviews of Neuroscience, 21, 127–148.
- Skelton, R. W. (1988). Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behavioral Neuroscience, 102, 586–590.
- Smith, M. C., DiLollo, V., & Gormezano, I. (1966). Conditioned jaw movement in the rabbit. Journal of Comparative and Physiological Psychology, 62, 479–483.
- Solomon, P. R., Solomon, S. D., Van der Schaaf, E. V., & Perry, H. E. (1983). Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science, 220, 329–331.
- Solomon, P. R., Van der Schaaf, E. V., Thompson, R. F., & Weisz, D. J. (1986). Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience, 100, 729–744.
- Squire, L. R. (1992). Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience, 4, 232–243.
- Squire, L. R., & Knowlton, B. J. (1994). Memory, hippocampus and brain systems. In M. S. Gazzaniga (Ed.), The cognitive neurosciences (pp. 825–873). Cambridge, MA: MIT Press.
- Stanton, M. E., & Freeman, J. H., Jr. (2000). Developmental studies of eyeblink conditioning in the rat. In D. S. Woodruff-Pak & J. E. Steinmetz (Eds.), Eyeblink classical conditioning: Vol. 2. Animal models (pp. 105–134). Boston: Kluwer.
- Stanton, M. E., Freeman, J. H., Jr., & Skelton, R. W. (1992). Eyeblink conditioning in the developing rat. Behavioral Neuroscience, 106, 657–665.
- Steinmetz, J. E. (1990). Neuronal activity in the cerebellar interpositus nucleus during classical NM conditioning with a pontine stimulation CS. Psychological Science, 1, 378–382.
- Steinmetz, J. E., Lavond, D. G., Ivkovich, D., Logan, C. G., & Thompson, R. F. (1992). Disruption of classical eyelid conditioning after cerebellar lesions: Damage to a memory trace system or a simple performance deficit? Journal of Neuroscience, 12, 4403–4426.
- Steinmetz, J. E., Lavond, D. G., & Thompson, R. F. (1989). Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse, 3, 225–233.
- Steinmetz, J. E., Logan, C. G., Rosen, D. J., Thompson, J. K., Lavond, D. G., & Thompson, R. F. (1987). Initial localization of the acoustic conditioned stimulus projection system to the cerebellum during classical eyelid conditioning. Proceedings of the National Academy of Sciences USA, 84, 3531–3535.
- Steinmetz, J. E., Logue, S. F., & Miller, D. P. (1993). Using signalled bar-pressing tasks to study the neural substrates of appetitive and aversive learning in rats: Behavioral manipulations and cerebellar lesions. Behavioral Neuroscience, 107, 941–954.
- Steinmetz, J. E., Logue, S. F., & Steinmetz, S. S. (1992). Rabbit classically conditioned eyelid responses do not reappear after interpositus nucleus lesion and extensive post-lesion training. Behavioural Brain Research, 51, 103–114.
- Steinmetz, J. E., Rosen, D. J., Chapman, P. F., Lavond, D. G., & Thompson, R. F. (1986). Classical conditioning of the rabbit eyelid response with a mossy fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience, 100, 871–880.
- Steinmetz, J. E., Sears, L. L., Gabriel, M., Kubota, Y., & Poremba, A. (1991). Cerebellar interpositus nucleus lesions disrupt classical nictitating membrane conditioning but not discriminative avoidance learning in rabbits. Behavioural Brain Research, 45, 71–80.
- Steinmetz, J. E., & Sengelaub, D. R. (1992). Possible CS pathway for classical eyelid conditioning in rabbits: I. Anatomical evidence for direct projections from the pontine nuclei to the cerebellar interpositus nucleus. Behavioral and Neural Biology, 57, 103–115.
- Stiedl, O., & Spiess, J. (1997). Effect of tone-dependent fear conditioning on heart rate and behavior of C57BL/6N mice. Behavioral Neuroscience, 111, 703–711.
- Supple, W. F., & Kapp, B. S. (1993). The anterior cerebellar vermis: Essential involvement in classically conditioned bradycardia in the rabbit. Journal of Neuroscience, 13, 3705–3711.
- Supple, W. F., & Leaton, R. N. (1990). Cerebellar vermis: Essential for classically conditioned bradycardia in the rat. Brain Research, 509, 17–23.
- Supple, W. F., Leaton, R. N., & Fanselow, M. S. (1987). Effects of cerebellar vermal lesions on species-specific responses, neophobia, and taste-aversion learning in rats. Physiology and Behavior, 39, 579–586.
- Supple, W. F., Sebastiani, L., & Kapp, B. S. (1993). Purkinje cell responses in the anterior cerebellar vermis during Pavlovian fear conditioning in the rabbit. NeuroReport, 4, 975–978.
- Taylor, J. A. (1951). The relationship of anxiety to the conditioned eyelid response. Journal of Experimental Psychology, 44, 61–64.
- Teyler, T. J., & DiScenna, P. (1987). Long-term potentiation. Annual Review of Neurosciences, 10, 131–161.
- Teyler, T. J., Roemer, R. A., Thompson, R. F., Thompson, J. K., & Voss, J. F. (1976). The role of sensory feedback in EMG response. In D. I. Mostofsky (Ed.), Behavior control and modification of physiological activity (pp. 253–267). New York: Prentice-Hall.
- Thompson, L. T., & Disterhoft, J. F. (1997). N-methyl-D-aspartate receptors in associative eyeblink conditioning: Both MK-801 and phencyclidine produce task- and dose-dependent impairments. Journal of Pharmacology and Experimental Therapeutics, 281, 928–940.
- Thompson, R. F. (1967). Foundations of physiological psychology. New York: Harper & Row.
- Thompson, R. F. (1986). The neurobiology of learning and memory. Science, 233, 941–947.
- Thompson, R. F. (2001). Spinal plasticity. In M. Patterson & J. Grau (Eds.), The handbook of spinal cord plasticity (pp. 1–19). Norwell, MA: Kluwer Press.
- Thompson, R. F., & Krupa, D. J. (1994). Organization of memory traces in the mammalian brain. Annual Review of Neuroscience, 17, 519–549.
- Thompson, R. F., & Spencer, W. A. (1966). Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review, 173, 16–43.
- Tracy, J. A., Ghose, S. S., Stetcher, T., McFall, R. M., & Steinmetz, J. E. (1999). Classical conditioning in a nonclinical obsessivecompulsive population. Psychological Science, 10, 9–13.
- Tracy, J. A., Thompson, J. K., Krupa, D. J., & Thompson, R. F. (1998). Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience, 112, 267–285.
- Tsukahara, N., Oda, Y., & Notsu, T. (1981). Classical conditioning mediated by the red nucleus in the cat. Journal of Neuroscience, 1, 72–79.
- Turner, B. H., & Zimmer, J. (1984). The architecture and some of the interconnections of the rat’s amygdala and lateral periallocortex. Journal of Comparative Neurology, 227, 540–557.
- Vazdarjanova, A., & McGaugh, J. L. (1998). Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proceedings of the National Academy of Sciences USA, 95, 15003–15007.
- Voneida, T. J. (1999). The effect of rubrospinal tractotomy on a conditioned limb response in the cat. Behavioural Brain Research, 105, 151–162.
- Voneida, T. J. (2000). The effect of brachium conjunctivum transection on a conditioned limb response in the cat. Behavioural Brain Research, 109, 167–175.
- Voneida, T. J., Christie, D., Bogdanski, R., & Chopko, B. (1990). Changes in instrumentally and classically conditioned libflexion responses following inferior olivary lesions and olivocerebellar tractotomy in the cat. Journal of Neuroscience, 10, 3583–3593.
- Wagner, A. R., & Brandon, S. E. (1989). Evolution of a structured connectionist model of Pavlovian conditioning (ÆSOP). In S. B. Klein & R. R. Mowrer (Eds.), Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theories (pp. 149–189). Hillsdale, NJ: Erlbaum.
- Wagner, A. R., & Donegan, N. (1989). Some relationships between a computational model (SOP) and an essential neural circuit for Pavlovian (rabbit eyeblink) conditioning. In R. D. Hawkins & G. H. Bower (Eds.), Computational models of learning in simple neural systems: Vol. 23. The psychology of learning and motivation (pp. 157–203). New York: Academic
- Wagner, A. R., & Rescorla, R. A. (1972). Inhibition in Pavlovian conditioning: Application of a theory. In R. A. Boakes & M. S. Halliday (Eds.), Inhibition and learning (pp. 301–336). London: Academic Press.
- Watson, J. B., & Rayner, R. (1920). Conditioned emotional reactions. Journal of Experimental Psychology, 3, 1–14.
- Weingarten, H., & White, N. (1978). Exploration evoked by electrical stimulation of the amygdala in rats. Physiological Psychology, 6, 229–235.
- Weiskrantz, L. (1956). Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology, 49, 381–391.
- Weisskopf, M. G., & LeDoux, J. E. (1999). Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. Journal of Neurophysiology, 81, 930–934.
- Weisz, D. J., Harden, D. G., & Xiang, Z. (1992). Effects of amygdala lesions on reflex facilitation and conditioned response acquisition during nictitating membrane response conditioning in rabbit. Behavioral Neuroscience, 106, 262–273.
- Whalen, P. J., & Kapp, B. S. (1991). Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behavioral Neuroscience, 105, 141– 153.
- White, I. M., Miller, D. P., White, W., Dike, G. L., Rebec, G. V., & Steinmetz, J. E. (1994). Neuronal activity in rabbit neostriatum during classical eyelid conditioning. Experimental Brain Research, 99, 179–190.
- Wicks,S.R.,&Rankin,C.H.(1995).Integrationofmechanosensory stimuli in Caenorhabditis elegans. Journal of Neuroscience, 15, 2434–2444.
- Wilensky, A. E., Schafe, G. E., & LeDoux, J. E. (1999). Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. Journal of Neuroscience, 19(24),
- Wilensky, A. E., Schafe, G. E., & LeDoux, J. E. (2000). The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. Journal of Neuroscience, 20, 7059–7066.
- Wilson, A., & Kapp, B. S. (1994). Effect of lesions of the ventrolateral periaqueductal gray on the Pavlovian conditioned heart rate response in the rabbit. Behavioral and Neural Biology, 62, 73–76.
- Wood, G. E., & Shors, T. J. (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Science USA, 95, 4066–4071.
- Woodruff-Pak, D. S. (2000). Human eyeblink conditioning in normal aging and Alzheimer’s disease. In D. S. Woodruff-Pak & J. E. Steinmetz (Eds.), Eyeblink classical conditioning: Vol. 1. Applications in humans (pp. 163–189). Boston: Kluwer.
- Woodruff-Pak, D. S., Lavond, D. G., Logan, C. G., Steinmetz, J. E., & Thompson, R. F. (1993). Cerebellar cortical lesions and reacquisition in classical conditioning of the nictitating membrane response in rabbits. Brain Research, 608, 67–77.
- Woodruff-Pak, D. S., Lavond, D. G., Logan, C. G., & Thompson, R. F. (1987). Classical conditioning in 3-, 30- and 45-month old rabbits: Behavioral learning and hippocampal unit acitivity. Neurobiology of Aging, 8, 101–108.
- Woodruff-Pak, D. S., & Lemieux, S. K. (2001). The cerebellum and associative learning: Parallels and contrasts in rabbits and humans. In J. E. Steinmetz, M. A. Gluck, & P. R. Solomon (Eds.), Model systems and the neurobiology of associative learning (pp. 271–294). Mahwah, NJ: Erlbaum.
- Woodruff-Pak, D. S., & Steinmetz, J. E. (Eds.). (2000). Eyeblink classical conditioning: Vol. 1. Human applications. Boston: Kluwer.
- Woodruff-Pak, D. S., & Thompson, R. F. (1988). Classical conditioning of the eyelid response in the delay paradigm in adults aged 18–83 years. Psychology and Aging, 3, 219–229.
- Woody, C. D., & Black-Cleworth, P. (1973). Differences in excitability of cortical neurons as a function of motor projection in conditioned cats. Journal of Neurophysiology, 36, 1004–1116.
- Woody, C. D., & Engel, J., Jr. (1972). Changes in unit activity and thresholds to electrical microstimulation at coronal-precurciate cortex of cat with classical conditioning of different facial movements. Journal of Neurophysiology, 31, 851–864.
- Yeo, C. H., & Hardiman, M. J. (1992). Cerebellar cortex and eyeblink conditioning: A reexamination. Experimental Brain Research, 88, 623–638.
- Yeo, C. H., Hardiman, M. J., & Glickstein, M. (1985). Classical conditioning of the nictitating membrane response of the rabbit: II. Lesions of the cerebellar cortex. Experimental Brain Research, 60, 99–113.
- Yeo, C. H., Hardiman, M. J., & Glickstein, M. (1986). Classical conditioning of the nictitating membrane response of the rabbit: IV. Lesions of the inferior olive. Experimental Brain Research, 63, 81–92.
- Young, R. A., Cegavske, C. F., & Thompson, R. F. (1976). Toneinduced changes in excitability of abducens motoneurons and of
- the reflex path of nictitating membrane response in rabbit (oryctolagus cuniculus). Journal of Comparative and Physiological Psychology, 90, 424–434.
- Zalutsky, R. A., & Nicoll, R. A. (1990). Comparison of two forms of long-term potentiation in single hippocampal neurons. Science, 248, 1619–1624.
- Zhang, J. X., Harper, R. M., & Ni, H. (1986). Cryogenic blockade of the central nucleus of the amygdala attenuates aversively conditioned blood pressure and respiratory responses. Brain Research, 386, 136–145.
- Zigmond, M. J., Bloom, F. E., Landis, S. C., Roberts, J. L., & Squire, L. R. (Eds.). (1999). Fundamental neuroscience. San Diego, CA: American Press.




