View sample biological bases of personality research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Whether we speak of mice or men, every member of a species is the same as other members in many respects but different in others. One task of personality psychology is to describe the basic behavioral differences and discover their origins. Description of personality is usually in terms of observable traits, and various models have been proposed to classify them. Biology has confronted a similar task in the classification of species (taxonomy). Taxonomy has been based on phenomenal and functional similarities and differences but more recently has been moving in the direction of using evolutionary analyses to define species in terms of their ancestries. Psychology still depends on phenomenal similarities and differences. As the genome reveals its secrets, both fields will eventually turn to DNA for the classification task.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
There are two basic pathways for the second task, the search for the sources of individual differences. These are shown in Figure 4.1. One pathway is the biological beginning in behavioral genetics. Genes make proteins into neurons, and neurons are organized into brain and nervous systems.
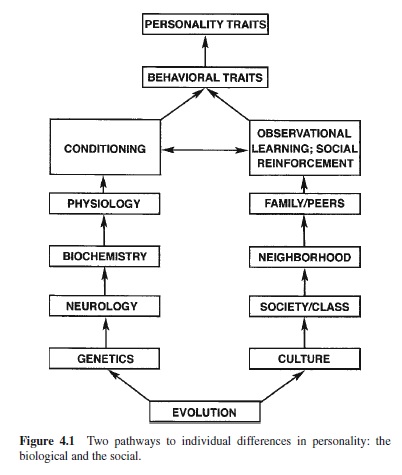
Neurons operate through chemical neurotransmitters and the enzymes that govern their production and catabolism, as well as through hormones produced in other loci. This is the biochemical level. Differences in neurochemical makeup result in differences in neural activity and reactivity or physiology. Physiological differences affect conditionability, both of the classical and operant types. Individuals differ in both their conditionability and their sensitivities to conditioned stimuli associated with reward and punishment.
The second pathway begins with the largest social unit, culture. Cultures are subdivided into specific societies defined by geography or class groupings defined by wealth, occupation, and education. Neighborhood provides the more proximal influences on behavior. The family of origin and peers transmit the influences of society, albeit with individual variations on modal mores, values, and behavior patterns. Observational learning combined with social reinforcement is the mechanism of influence at the next level. At this point there is a convergence of the pathways because the different mechanisms of learning combine to produce behavioral traits. These traits are usually specific to certain types of situations. Depending on their generality and strength they combine to form what we call personality traits.
Both of these pathways have a historical origin in the evolutionary history of the species. Genetic changes account for the origin and changes (over long periods of time) in the species. Cultures represent the collective solutions of the human species to the basic demands of evolution: survival and reproduction. Cultural evolution is more rapid than biological evolution. Significant changes can occur within a generation, as with the sudden impact of computer technology on the current generation.
This research paper describes the biological pathway up to, but not including, conditioning. For each of four dimensions of personality I describe theory and research at each level of analysis along this pathway starting at the top (physiology). At the genetic level I describe primarily the studies of molecular genetics that link specific genes to traits. The molecular studies link genes more directly to the neurological and biochemical levels on the way up to personality traits. An analysis of this type was conducted a decade ago (Zuckerman, 1991). Advances occur rapidly in the neurosciences. Ten years is equivalent to at least several decades in the social sciences. I have made an attempt to survey the changes since my last attempt. In a research paper I can hope only to highlight some of these advances and will reserve a more thorough review for a revision of my 1991 book. My approach draws heavily on comparative studies of other species as any psychobiological model must do (Gosling, 2001; Zuckerman, 1984, 1991), but I cannot do so within the constraints of a single paper. I will limit comparative studies to those in which there are clear biological markers in common between animal and human models.
Temperament and Personality Traits
Researchers of temperament in children and behavioral traits in other species have typically included certain dimensions like emotionality, fearfulness, aggressiveness, approach versus withdrawal (in reactions to novel stimuli), general activity, playfulness, curiosity, sociability versus solitariness, and inhibition versus impulsivity (Strelau, 1998). From the 1950s through the 1970s personality trait classification was dominated by two models: Eysenck’s (1947) three-factor theory (extraversion, neuroticism, and psychoticism) and Cattell’s (1950) 16-factor model. Eysenck’s (1967) model was biologically based with an emphasis on genetics, physiology, and conditioning. Gray’s (1982, 1987) model is a bottom-up model that starts with behavioral traits in animals and extrapolates to human personality. He places his three behavioral dimensions (anxiety, impulsivity, fight-flight) within the axes of Eysenck’s dimensions, but not lying on the axes of those dimensions or being precise equivalents of them.
The first five-factor model originated in lexical studies of trait-descriptive adjectives in language done in the 1960s (Norman, 1963; Tupes & Christal, 1961) with its roots in a much earlier study by Fiske (1949). Interest in this model reawakened in the 1980s (Digman & Inouye, 1986; Goldberg, 1990; Hogan, 1982; McCrae & Costa, 1985). Most of these studies used adjective rating scales. The translation of the model into a questionnaire form (NEO-PI-R; Costa & McCrae, 1992a) increased the use of the scales by personality investigators. The five factors incorporated in this tests are labeled extraversion, neuroticism, agreeableness, conscientiousness, and openness to experience. The five factors have been replicated in studies in many countries although with some differences—particularly on the last factor, openness. The enthusiasts for the Big Five insist it is the definitive and final wordonthestructureofpersonality(Costa&McCrae,1992b), although critics regard this claim as premature (Block, 1995; Eysenck,1992;Zuckerman,1992).Oneofthecriticismsofthe model is its atheoretical basis in contrast to Eysenck’s development of his factors from theory as well as empirical factor analytic studies of questionnaire content. However, recent studies in behavior genetics have used the model, and some of the data from earlier studies has been translated into the form of these five factors (Loehlin, 1992).
Two recent models have been derived from biosocial theories. Based on factor analyses of scales used in psychobiological studies of temperament and personality, Zuckerman and Kuhlman developed a five-factor model dubbed the alternative five (Zuckerman, Kuhlman, & Camac, 1988; Zuckerman, Kuhlman, Thornquist, & Kiers, 1991). This model was translated into a five-factor questionnaire (Zuckerman-Kuhlman Personality Questionnaire, or ZKPQ) on the basis of item and factor analyses (Zuckerman, Kuhlman, Joireman, Teta, & Kraft, 1993). The five factors are sociability, neuroticism-anxiety, impulsive sensation seeking, aggression-hostility, and activity. This model was used as the framework for a volume on the psychobiology of personality (Zuckerman, 1991).
Cloninger (1987) developed a personality model for both clinical description and classification of personality. The theory is biologically based and, like Zuckerman’s, uses the monoamine neurotransmitters as fundamental determinants of personality differences. The factors included in the most recent version of his questionnaire include novelty seeking, harm avoidance, reward dependence, persistence, cooperativeness, persistence, self-directedness, and self-transcendence (Cloninger, Przybeck, Svrakic, & Wetzel, 1994). Much of the recent psychobiological research in personality and psychopathology has used Cloninger’s system and questionnaires.
Builders of personality trait models often give different names to what are essentially the same traits. But even if one goes by the trait labels alone there are obvious similarities in what are considered the basic personality traits. Extraversion and neuroticism appear in nearly every system. Of course, one cannot take their equivalence for granted until empirical studies are done of their correlational relatedness.
Zuckerman et al. (1993) compared Eysenck’s Big Three, Costa and McCrae’s Big Five, and Zuckerman and Kuhlman’s Alternative Five in a factor-analytic study. Afourfactor solution accounted for two thirds of the variance. The first factor was clearly extraversion, and the second was neuroticism with representative scales from all three questionnaires highly loading on their respective factors. The third factor consisted of Eysenck’s psychoticism and Zuckerman and Kuhlman’s impulsive sensation seeking at one pole and the NEO conscientiousness at the other. The fourth factor was defined by NEO agreeableness at one pole and ZKPQ aggression-hostility at the other. The analysis did not yield a fifth factor, possibly because of a lack of representative markers in the three tests. Activity loaded on the extraversion factor, and openness loaded on the agreeableness factor.
Zuckerman and Cloninger (1996) compared the scales of the ZKPQ with those of Cloninger’s Temperament and Character Inventory (TCI). ZKPQ impulsive sensation seeking was highly correlated with TCI novelty seeking (r = .68), ZKPQ neuroticism-anxiety with TCI harm-avoidance (r = .66), ZKPQ aggression-hostility with TCI cooperativeness (r = −.60), and ZKPQ activity with TCI persistence (r = .46). These scales showed convergent and discriminant cross validity, but the other scales in both tests had weaker correlations and correlated equally with several measures on the other scales. In Cloninger’s model there is no specific scale for extraversion or sociability.
The personality systems described thus far have been developed using factor analyses of trait dimensions. Many personologists have developed typologies on a rationaltheoretical basis. Freud (1914/1957), Erikson (1963), and Maslow (1954) described personality types based on their developmental theories, each stressing the adult expressions of types derived from earlier stages of development. No valid methods of assessment were developed to operationalize these theories, although many clinicians continue to use them to describe personality differences among patients or others.
More recently, Millon and Everly (1985) defined eight types based on the interactions of four primary sources of reinforcement and two kinds of instrumental behavior patterns (active and passive). Some of the resultant types resemble different poles of the standard dimensions of personality. Sociable and introversive personality types resemble the two poles of the extraversion dimension; the inhibited type resembles neuroticism; and the cooperative types sounds like agreeableness. The model was developed as a way of integrating personality development of psychopathology, particularly the personality disorders. It has been described as a biosocial theory but has not as yet been widely used in psychobiological research.
The examination of the biosocial bases of personality in this research paper will be organized around four basic personality factors, derived mostly from factor analytic studies, which are the same or quite similar across these studies, have some similarity to traits described in studies of temperament and animal behavior, and have been used in correlational studies of traits and psychobiology in humans. The four traits are extraversion/sociability, neuroticism/anxiety, aggression/ agreeableness, and impulsivity/sensation seeking/psychoticism. Although activity is a widely used trait in studies of children and animals, it has not been widely used in studies of humans except for the pathological extreme of hyperactivity disorder and is recognized as a primary personality trait only in the Zuckerman-Kuhlman model.
Extraversion/Sociability
All models of basic personality, with the exception of Cloninger’s, recognize extraversion (E) as a primary and basic personality factor, but different models have defined it differently. In his earlier model Eysenck regarded E as a combination of two narrower traits: sociability and impulsivity. This amalgam was questioned by Carrigan (1960) and Guilford (1975), who claimed that sociability and impulsivity were independent traits. Sybil Eysenck and Hans Eysenck (1963) initially defended the dual nature of extraversion. However, the introduction of psychoticism (P) into a new version of their questionnaire resulted in a drift of impulsivitytype items to the P dimension, leaving E defined primarily by sociability and activity types of items. Hans and Michael Eysenck (1985) finally defined E in terms of the subtraits: sociable, lively, active, assertive, sensation seeking, carefree, dominant, surgent, and venturesome.
Costa and McCrae (1992a) defined their E superfactor in terms of subscale facets: warmth, gregariousness (sociability), activity, excitement seeking (sensation seeking), and positive emotions. Neither Eysenck nor Costa and McCrae now include impulsivity in the E factor; Eysenck now includes it in the N superfactor, and Costa and McCrae place it in their neuroticism factor. Both Eysenck and Costa and McCrae include activity and sensation seeking as components of their E factors.
Zuckerman et al. (1993) include only sociability and isolation intolerance in their sociability superfactor. In the alternative five, impulsivity and sensation seeking form another primary factor instead of being subsumed under E, and activity comprises another major factor. In spite of these differences in the content of the E factor in the three models, the questionnaire measures of the factors intercorrelate highly and have high loadings on a common factor (Zuckerman et al., 1993).
Cortical Arousal
Eysenck’s (1967) theory of extraversion has shaped much of the psychobiological research on this trait even to the end of the century (Strelau & Eysenck, 1987).The model suggests that introversion-extraversion is based on arousal characteristics of the cerebral cortex as regulated by the reticulocortical activating system. The extravert’s cortex in waking, nonstimulating conditions is underaroused relative to his or her optimal level of arousal. In these conditions the extravert is prone to seek out exciting stimulation in order to increase the level of arousal to a level that makes him or her feel and function better. The introvert is usually closer to an optimal level of arousal in low stimulation conditions and has less need to seek additional stimulation to feel better.The introvert may be overstimulated at a level of stimulation that is positive for the extravert.
The theory was initially tested with measures of brain activity from the electroencephalogram (EEG). Spectrum analyses break the raw EEG into bands characteristic of different degrees of arousal: sleep (delta), drowsiness (theta), relaxed wakefulness (alpha), and alert excitement (beta). Alpha has often been regarded as inversely related to arousal on the assumption that any interruption of this regular wave means an increase in arousal. However, some have used the frequency of alpha within the usual band (8–13 Hz) as a measure of relative arousal or alpha amplitude as an inverse measure of arousal. EEG spectrum characteristics are highly if not completely heritable (Lykken, 1982).
The findings relating extraversion to EEG criteria of arousal in various conditions from nonstimulating to mentally engaged have been summarized by Gale (1983), O’Gorman (1984), and Zuckerman (1991). Gale tried to reconcile the wide variety of results with the hypothesis that differences between introverts and extraverts appear only in moderately active conditions and not in either low stimulation (eyes closed, no stimulation) or activating conditions. Both O’Gorman and Zuckerman concluded that neither Eysenck’s broad hypothesis nor Gale’s narrow hypothesis, limiting the prediction to specific experimental conditions, were consistently supported by studies. Zuckerman noted that among the best studies, those confirming Eysenck’s hypothesis used samples with either all female or equal male and female participants, whereas those with all male or a preponderance of male participants did not support the hypothesis.
A large study utilizing the full spectrum range of EEG, three levels of activating conditions, measures of impulsivity as well as E, and a test of the interaction of personality, arousal level, and performance, found only weak evidence supporting Eysenck’s hypothesis (Matthews & Amelang, 1993). Correlations of .16 (about 3% of the variance) were found between activation in the low arousal bands (delta and theta) and E and one of its components, impulsivity. These correlations controlled for the influence of the other two Eysenck factors, neuroticism and psychoticism. The sociability component of E was not related to any index of cortical arousal. The significant results linking E to low arousal bands were found only in the least stimulating condition (reclining, eyes closed). The fact that the differences were not found in
alpha or beta bands but were found only in the most relaxed condition suggests that the weak correlation may have been due to impulsive extraverts’getting drowsy or actually falling asleep. Regardless of interpretation, the low level of relationship between personality and arousal in this study could explain the inconsistency of previous studies testing the hypothesis: They simply did not have enough power to detect the relationship with any reliability.
Consistent with Eysenck’s model was the finding that while performing six tasks extraverts tended to perform worse than introverts at higher levels of alpha (indicating lower levels of arousal). Only the alpha band, however, supported the hypothesis of better performance of introverts at lower levels of arousal. Brain imaging using positronemission tomography (PET) and cerebral blood flow (CBF) have an advantage over EEG because they assess subcortical as well as cortical activation and analyze activity in particular structures or brain loci. The problem with studies using these new techniques is that because of the expense, low numbers of subjects are used and many brain areas are analyzed, increasing the possibilities of both Type I and Type II errors. Replication across studies is one solution to the problem.
Mathew, Weinman, and Barr (1984) found negative correlations between E and CBF indices of activation in all cortical areas in both hemispheres, supporting Eysenck’s hypothesis of higher cortical arousal in introverts than in extraverts. All of their participants were female. Stenberg, Wendt, and Risberg (1993) also found an overall negative correlation (r = −.37), but this was a function of the high correlation among the female participants; the correlation among the males was close to zero. As with the EEG data, confirmation of the hypothesis was more common in female than in male samples.
Some studies have found hemispheric differences in the relationships between E and activation, but these have not been consistent (Johnson et al., 1999; Stenberg et al., 1993). Studies of subcortical areas of brain have also yielded little in the way of consistent findings except for one: E is associated positively with activation of the anterior cingulate area (Ebmeier et al., 1994; Haier, Sokolski, Katz, & Buchsbaum, 1987; Johnson et al., 1999). The cingulum is the major pathway between the frontal cortex and the limbic system and has been theoretically associated with neuroticism and anxiety rather than E (Zuckerman, 1991).
The results in the two brain imaging studies described, unlike the EEG studies, tend to support Eysenck’s hypothesis of a relationship (albeit a weak one) between E and cortical arousal. There is no clue in his theory, however, why the finding is supported more in females than in males or why subcortical differences in the cingulum, the executive structure of the limbic brain, should be associated with extraversion. In Eysenck’s model limbic arousability is associated with neuroticism, and any association with E would be with introversion rather than extraversion.
General arousal may be too broad a construct to be associated with personality. Arousal is highly dependent on diurnal variation and general stimulation levels. Arousal as a trait would represent the state of the nervous system at a given time under a given set of conditions. In contrast, arousability is the typical immediate reaction of some part of the nervous system to a stimulus with specified characteristics. Eysenck’s (1967) optimal level of stimulation model says that introverts are more arousable at low to moderate intensities of stimulation, but at higher intensities extraverts are more responsive. Introverts have strong reactive inhibition mechanisms that dampen response to high intensities. Strelau (1987), in a model based on neo-Pavlovian theories, states that persons with strong nervous systems are relatively insensitive to stimuli at lower intensities but can process and react to stimuli at higher intensities. For weak nervous system types the opposite is true: They are highly sensitive to low intensities but show inhibition of response at high intensities.
Cortical Arousability
Cortical arousability is usually assessed with the cortical evoked potential (EP). A brief stimulus, such as a tone or flash of light, is presented a number of times, and the EEG is digitized at a fixed rate, that is time locked to stimulus delivery time and averaged across trials for a given participant. This process averages out the “noise” and produces a clear waveform representing the typical reaction of that subject to the specific stimulus over a 500-ms period. Although latencies of response vary somewhat for individuals, for most one can identify particular peaks of positivity and negativity. For instance, a peak of positive potential at about 100 ms after the stimulus (P1) represents the first impact of the intensity characteristics of stimuli on the cortical centers. Earlier peaks represent stimulus processing at subcortical centers. The peak at 300 ms after the stimulus (P3) is influenced by novelty, surprise, or unexpectedness of the stimulus and thus represents a higher level of cortical processing in that the stimulus must be compared with previous stimuli.
Stelmack (1990) reviewed the relationship between E and cortical EPs. As might be expected, the results depend on the characteristics of the stimuli used to evoke the EPs as well as the reactor’s age and personality characteristics. For instance, Stelmack said that introverts have greater amplitude EPs in response to low-frequency tones, but there are no differences between introverts and extraverts for high-frequency tones.
If the stimulus attribute had been intensity, these kinds of results might be compatible with Eysenck’s theory of increased sensitivity of introverts to low-intensity stimuli. But the evolutionary type of explanation offered by Stelmack for the greater survival significance of low-frequency sounds is not convincing.
Recent studies have focused on the P300 EP component, many using the “odd-ball” paradigm in which the participant listens with eyes closed to a sequence of tones in which one tone is presented frequently and another one (the oddball) rarely. The rare tone is the signal for some task. These are usually vigilance tasks on which extraverts’ performances and EP reactions are expected to decline more rapidly than those for introverts. However, when the task is made less montonous or response requirements are high, the differences may disappear or even be reversed with larger EP amplitudes in extraverts (Stenberg, 1994).
The intensity of the stimulus is another factor in the I-E difference. Brocke, Tasche, and Beauducel (1997) found that introverts showed larger P3 reactions to a 40-db stimulus, whereas extraverts showed a larger amplitude of P3 in response to a 60-db stimulus. Introverts’ EP amplitudes decreased going from 40 db to 60 db, whereas extraverts increased going from the less intense to the more intense stimulus. These effects were a function of the impulsivity component rather than the sociability component of the E scale used in the study. The results of studies that vary the experimental conditions suggest that attention and inhibition may be the basic mechanisms governing the nature of the relationship between E and cortical EPs. Responses at the brain-stem level are probably less susceptible to these mechanisms, and Eysenck’s theory does involve the brain stem and other points along the reticulocortical arousal system in I and E.
Stelmack and Wilson (1982) found that extraverts had longer latencies for the EP subcortical wave V (inferior colliculus) for stimulus intensity levels up to but not including 90 db. The direction of the finding was confirmed in a second experiment (Stelmack, Campbell, & Bell, 1993) and in a study by Bullock and Gilliland (1993). Different doses of caffeine and levels of task demand were used in the latter study, but the differences between extraverts and introverts held across all levels of caffeine and task demand. The results support Eysenck’s theory more strongly than those using cortical EPs, which seem more susceptible to stimulus, task, and background arousal factors. A study by Pivik, Stelmack, and Bylsma (1988), however, suggested that Eysenck’s arousalinhibition hypothesis may not be broad enough. These researchers measured the excitability of a spinal motoneuronal reflex in the leg and found that extraverts showed reduced motoneuronal excitability as measured by reflex recovery functions. These results show that the inhibitory properties of the nervous system related to E may extend well below the reticulocortical level.
Another line of EP research is based on Gray’s (1982, 1987) model of personality. Gray proposed that impulsivity, a dimension close to extraversion, is related to sensitivity to signals (conditioned stimuli) of reward whereas anxiety, close to neuroticism, is related to sensitivity to signals of punishment. This model suggests that the learned biological significance of stimuli, in addition to the intensity of stimulation, governs the strength of reaction to them.
Bartussek, Diedrich, Naumann, and Collet’s (1993) results supported the theory by showing a stronger EP response (P2, N2) of extraverts than introverts to tones associated with reward (winning money) but no differences in tones associated with punishment (losing money). In a later experiment, however, extraverts showed larger P3 EP amplitudes to stimuli associated with both reward and punishment compared to neutral stimuli (Bartussek, Becker, Diedrich, Naumann, & Maier, 1996).
DePascalis and his colleagues also presented findings supporting Gray’s theory. In one study they used a questionnaire scale developed more directly from Gray’s theory measuring the approach tendency (DePascalis, Fiore, & Sparita, 1996). Although they found no effect for E itself, the participants scoring high on the approach scale had higher EP (P6) amplitudes in response to stimuli (words) associated with winning than to those associated with losing, and the reverse was true for low-approach motive subjects.
Eysenck’s and Gray’s theories have also been tested using peripheral autonomic measures of activity like the electrodermal activity (EDA), or skin conductance (SC), heart rate (HR), and blood pressure (BP). These are only indirect measures of cortical activity and reactivity because they occur in the autonomic nervous system (ANS) and are controlled by limbic system centers, which in Eysenck’s model are associated more closely with neuroticism than with E. The results in relation to E are similar to those obtained with more direct cortical measures. Reviews by Smith (1983) and Stelmack (1990) showed mixed and inconclusive findings relating tonic EDA arousal to E, but some evidence of stronger SC responses of introverts than extraverts in response to low-to moderate-intensity stimuli and stronger responses of extraverts in response to high-intensity stimulation. Tonic (base-level) measures of HR (Myrtek, 1984) and BP (Koehler, Scherbaum, Richter, & Boettcher, 1993) are unrelated to E. Young children rated as shy and inhibited had higher and less variable HRs, and a high HR at 21 months is the same behavior pattern at 48 months (Kagan, Reznick, & Snidman, 1988). Shyness and inhibition, however, are traits that are a mixture of introversion and neuroticism or anxiety; therefore, the correlation with HR could be due to the anxiety component rather than to E.
Eysenck’s model for the trait of extraversion produced a great deal of research in the area of psychophysiology. But psychophysiology has its problems as a branch of neuroscience. Both tonic and phasic psychophysiological measures are highly reactive to environmental conditions. Tonic levels can vary as a function of reactions to the testing situation itself, and phasic reactions depend on the specific qualities of stimulation such as intensity and novelty. It is not surprising that the relationships of physiological measures with personality traits often interact with these stimulus characteristics in complex ways. Eysenck’s theory based on optimal levels of stimulation has received some support. Those based on differences in basal arousal levels are beginning to receive some support from PET studies, although the earlier results with EEG measures remain problematic.
Monoamines
The monoamine neurotransmitter systems in the brain have been the focus of most biosocial theories of personality. The reasons are the evidence of their involvement in human emotional and cognitive disorders and basic emotional and motivational systems in other species. Much of the work with humans has been correlational, comparing basal levels of the neurotransmitters, as estimated from levels of their metabolites in cerebrospinal fluid (CSF), blood, or urine, to personality traits as measured by questionnaires. Of these sources CSF is probably the best because the CSF is in direct contact with the brain. But the indirect relationship of these indicators with brain levels of activity (which can differ in different brain loci) and the fact that some of the metabolites in plasma and urine are produced in the peripheral nervous system make the putative measures of brain amine activity problematic. New imaging methods may eventually overcome these problems by directly viewing the monoamine activities in the brain itself. Added to these problems of validity of measurement is the use of small numbers of subjects in most studies, as well as the use of subjects with certain types of disorders rather than normal subjects. The ethical constraints of giving drugs that affect activity in the brain systems is another barrier, although some of the more recent studies have used such drugs in normals.
The freedom of investigators to experiment directly with the brain in other species has given us a fairly coherent picture of the emotional and motivational functions of the monoamine systems in the brain, and bottom-up theorists have used these findings to extend animal models to human motivations and personality (Gray, 1982, 1987; Mason, 1984; Panksepp, 1982; Soubrié, 1986; Stein, 1978). Top-down theorists have drawn on these findings from the comparative research but have attempted to reconcile them with the relevant research on humans, including clinical and personality studies (Cloninger, Svrakic, & Prszybeck, 1993; Depue & Collins, 1999; Netter, Hennig, & Roed, 1996; Rammsayer, 1998; Zuckerman, 1991, 1995). The problem with building a bridge from two banks is to make it meet in the middle. With these caveats let us first examine the case for extraversion.
The primary monoamines in the brain are norepinephrine, dopamine, and serotonin. The first two are labeled catecholamines because of the similarities in their structures. Serotonin is an indoleamine. These are not independent neurotransmitter systems because activity in one may affect activity in another. Serotonin, for example, may have antagonistic effects on the catecholamines. These kinds of interaction must be kept in mind because most studies relate one neurotransmitter to one personality trait. Some models suggest that this kind of isomorphism of trait and transmitter is the rule. This is a new kind of phrenology based on biochemistry rather than bumps on the head.
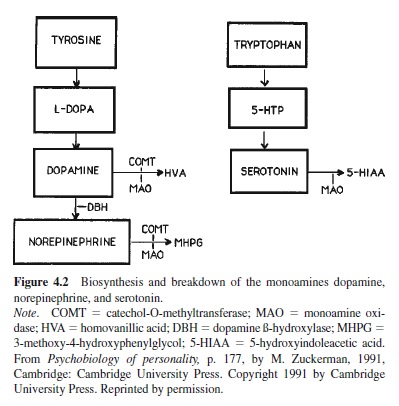
To understand the human research one needs to know the pathways of biosynthesis and catabolism (breakdown) of the monoamines because some experiments block the precursors of the transmitter to see its effect on behavior and most use metabolite products of the catabolism to gauge activity in the systems. Figure 4.2 is a simplified diagram showing the stages of production of the monoamines and some of the enzymes (DBH, COMT, MAO) involved in the conversions from one stage to another. The metabolite for dopamine is homovanillic acid (HVA), for norepinephrine it is 3-methoxy-4hydroxyphenylglycol (MHPG), and for serotonin it is 5-hydroxyindoleacetic acid (5-HIAA).
Theorists are in fair agreement on the role of dopaminergic systems in motivation based on studies of other species: approach and sensitivity to stimuli associated with reward (Crow,1977;Gray,1982,1987;Stein,1978);foragingandexploration and positive emotions like hope, desire, and joy in humans (Panksepp, 1982; Zuckerman, 1991); and novelty or sensation seeking in animals and humans (Bardo, Donohew, & Harrington, 1996; Cloninger et al., 1993; Le Moal, 1995; Zuckerman, 1984, 1991). I have proposed that the activity of the mesolimbic dopamine system is related to a broad approach trait that includes extraversion, sensation seeking, and impulsivity (Zuckerman, 1991). Considering that dopaminergic reactivity is also related to aggression and sexuality in many species, it is also possible that the third dimension of personality, low socialization, or psychoticism, may also be involved. Gray’s (1987) model linked dopamine and reward sensitivity with impulsivity, a dimension related to high E, P, and N, although his more recent remarks (Gray, 1999) suggest that he is linking dopamine more closely with the P dimension because of this transmitter’s involvement in schizophrenia.
Depue and Collins (1999) defined a broad view of extraversion with two main factors: interpersonal engagement, or affiliation and warmth, and agency, which includes social dominance, exhibitionism, and achievement motivation. Positive affect and positive incentive motivation are more strongly associated with the agentic extraversion factor. Impulsivity and sensation seeking are regarded as constituting an emergent factor representing a combination of extraversion and constraint (a dimension related to Eysenck’s P and Costa and McCrae’s conscientiousness). The “lines of causal neurobiological influence” are suggested to lie along the orthogonal dimensions of extraversion and constraint rather than along the dimension of impulsive sensation seeking. Although Depue and Collins say that this structural system does not mean that positive incentive motivation and its dopaminergic basis are related only to extraversion, the expectation is that they will be more strongly related to agentic extraversion than to impulsive sensation seeking or constraint.
Only a few correlational studies of monoamine CSF metabolites and personality traits were done prior to 1991 (Zuckerman, 1991), and they generally showed few significant relationships between the dopamine metabolite HVA and either extraversion or sensation seeking. This is still the case with studies that simply correlate CSF levels of HVA with questionnaire measures of extraversion, even when there is sufficient power to detect weak relationships (Limson et al., 1991). In fact, the Limson et al. study failed to find any correlations between CSF metabolites of serotonin (5-HIAA), norepinephrine (MHPG), norepinephrine itself, and Dopac and any of the personality measures assessed by the Minnesota Multiphasic Personality Inventory (MMPI), Eysenck Personality Questionnaire (EPQ), or Cloninger’s Temperament Character Inventory (TCI). As with psychophysiological measures, levels of neurotransmitter activity in a resting basal state are not sensitive to variations in personality, at least as the latter is measured in self-report questionnaires. However, studies that attempt to potentiate or attenuate activity in neurotransmitters with agonists or antagonists have yielded some significant findings in regard to personality, even though they typically use very small sample sizes.
Depue, Luciana, Arbisi, Collins, and Leon (1994) challenged the dopamine system with bromocriptine, a potent agonist at D2 receptor sites, and measured the effects using inhibition of prolactin secretion and activation of eye-blink rate, two measures of dopamine activation. The correlations between Positive Emotionality (PE) and baselline measures of the dopamine activity indicators were small and insignificant, but they found significant correlations between the putative measures of dopamine response to the agonist and the PE (an extraversion type measure) factor from Tellegen’s MPQ. Rammsayer (1998, 1999) challenged Depue et al.’s interpretation of their findings as indicative of higher dopamine reactivity in high-PE persons (extraverts) than in lows, suggesting that the prolactin response would indicate just the reverse (i.e., higher reactivity in the low-PE persons). The disagreements on the meaning of the data are too complicated to elucidate here.
Rammsayer’s interpretation of the findings is supported by PET measures of higher cerebral blood flow to the dopaminerich basal ganglia areas in introverts than in extraverts (Fischer, Wik, & Fredrikson, 1997); but another PET study found no relationship between E and dopamine binding in the basal ganglia (N. S. Gray, Pickering, & Gray, 1994), and still another found a positive relationship with E (Haier et al., 1987). The first two of these studies used normal controls as subjects whereas the Haier et al. study used patients with Generalized Anxiety Disorder, a possible confounding factor.
Rammsayer, Netter, and Vogel (1993), using an inhibiter of tyrosine hydroxlase, thereby blockading dopamine synthesis, found no difference between introverts and extraverts in either baseline dopamine or reactivity to the blockading agent. Despite the lack of difference in dopaminergic activity or reactivity, they found that reaction time performance was markedly impaired in introverts but not in extraverts by the dopamine blockading agent. In another study, using a chemical that selectively blocks D2 receptors and inhibits dopamine neurons in the limbic and cortical regions of the brain, Rammsayer (1998) again found a detrimental effect on reaction (liftoff) time in introverts but not in extraverts. The agent that was used caused a marked decrease in alertness and cortical arousal, but this effect was equivalent in introverts and extraverts. Both this finding and the performance findings would seem to contradict Eysenck’s arousal explanation for the differences between introverts and extraverts. That theory would predict a more detrimental effect in extraverts because they supposedly start with a lower level of cortical arousal. But the results also raise the question, What is the source of the performance differences between introverts and extraverts if they do not differ in dopamine activity or reactivity?
The answer might lie in the interactions of dopaminergic and other neurotransmitters or hormones or, at another level, in the genetics of the dopaminergic receptors. Considerable interest has developed in a gene associated with the dopamine receptor 4 (DRD4). Allelic variations in this gene have been associated with novelty or sensation seeking, but not with extraversion (Ebstein, Nemarov, Klotz, Gritsenko, & Belmaker, 1997; Ebstein et al., 1996).
Simple correlative studies have found no relationship between serotonin or norepinephrine and E or other personality variables measured by questionnaires given to adult subjects. A study using CSF from newborns in predicting temperamental traits found that infants born with low levels of the serotonin metabolite 5-HIAA showed low sociability at 9 months of age (Constantino & Murphy, 1996). Retest reliability for 5-HIAA in neurologically normal infants was very high (r = .94).
A study of adults with depressive disorder treated with either a noradrenergic or a serotonergic reuptake inhibiter, which increase activity in those systems, showed that there were significant increases in measures of E and gregariousness (sociability) in those treated with these drugs (Bagby, Levitan,Kennedy,Levitt,&Joffe,1999).ThechangeinEwas correlated with the change in depression severity, but the change in sociability was not. Although the result with sociability probably represents a change of state rather than the preillness trait, serotonin and norepinephrine might play some role in the trait as well. Studies of serotonin transporter genes have not shown any relationship to E, although they have to other personality traits (Hamer, Greenberg, Sabol, & Murphy, 1999; Jorm, Henderson, Jacomb, Croft, & Easteal, 1997).
Monoamine Oxidase
Monoamine oxidase (MAO) is an enzyme involved in the catabolic deamination of monoamines. Evidence using selective monoamine inhibitors suggests that MAO-Type B, assayed from blood platelets in humans, is preferentially involved in the catabolic breakdown of dopamine more than the other two brain monoamines, norepinephrine and dopamine (Murphy, Aulakh, Garrick, & Sunderland, 1987). Although no direct correlation of platelet and brain MAO has been found, indirect assessments and the effects of MAO inhibitors on depression, as well as a large body of behavioral data, suggest that there must be a connection, if only one limited to certain brain areas. Platelet MAO is normally distributed in the human population, is highly reliable although it increases in brain and platelets with age, and is lower in men than in woman at all ages, and variations are nearly all genetic in origin. Unlike other biochemical variables it does not vary much with changes in state arousal. Thus, MAO has all of the characteristics of a biological trait.
Low levels of MAO-B taken from umbilical cord blood samples in newborn infants were related to arousal, activity, and good motor development (Sostek, Sostek, Murphy, Martin, & Born, 1981). High levels of the enzyme were related to sleep time and general passivity. The relationship with motor development is particularly suggestive of development of the dopamine-influenced basal ganglionic areas of the brain involved in motor coordination. In a study of monkeys living in a colony in a natural environment, low-platelet MAO was related to high sociability, activity, dominance, and sexual and aggressive activity, a broad array of E-type traits described by Depue and Collins (1999) as agentic extraversion. However, in human correlative studies the results relating MAO-B to questionnaire-measured extraversion have been inconsistent (Zuckerman, 1991). The enzyme has more consistently correlated (inversely) with the trait of sensation seeking. But using reported behavioral indices of sociability in college students, low MAO was related to sociability and high MAO to social insolation (Coursey, Buchsbaum, & Murphy, 1979).
Hormones
The hormone testosterone (T) is produced by both men and women but is 8 to 10 times as high in men as in women. Plasma T is highly heritable (66%) in young adult males and moderately heritable (41%) in females (Harris, Vernon, & Boomsa, 1998). In rats T has reward effects in the nucleus accumbens, the major site of dopaminergic reward. Administration of a dopamine receptor blocker eliminates the rewarding effects of T in rats, suggesting that its rewarding effects are mediated by an interaction with dopamine in the mesolimbic system (Packard, Schroeder, & Gerianne, 1998).
The hormone T affects personality traits and may account in part for many of the personality trait differences between menandwomen.Menandwomendonotdifferonthepuresociability or affiliative type of extraversion, but they do on the agentic type, which includes dominance, assertiveness, surgency, and self-confidence. To the extent that sensation seeking is associated with extraversion, it is with the agentic type.
Daitzman and Zuckerman (1980) found that T in young males was positively correlated with sociability and extraversion, as well as with dominance and activity and inversely with responsibility and socialization, indicating an association with the agentic type of extraversion. Windle (1994) also found that testosterone was associated with a scale measuring behavioral activation, characterized by boldness, sociability, pleasure seeking, and rebelliousness. Dabbs (2000) also found that Tis associated with a type of extraversion characterized by high energy and activity levels and lower responsibility.
Summary
Eysenck’s theory relating cortical arousal to extraversion has been extensively tested using the EEG and, in more recent times, the brain scanning methods. The EEG studies yielded mixed results in which the sources of differences between studies were not clearly apparent. Two cerebral blood flow studies did confirm that extraverts were cortically underaroused related to introverts in female subjects but not in males. Studies measuring cortical arousability have also not clarified the picture. Apparently, experimental conditions affecting attention or inhibition may confound the relationship with E. Some more consistent results have been obtained from EP studies of responses at subcortical levels in which conscious attention is less of a factor.Although Eysenck’s theory is confined to cortical arousal and reactivity, differences between introverts and extraverts have been found at lower levels of the central nervous system, even in a spinal motoneuronal reflex.
Theories of the biochemical basis of extraversion have focused on the monoamine neurotransmitters, particularly dopamine. Simple correlational studies between the monoamine metabolites and trait measures of E have not yielded significant findings, although there is some evidence that drugs that increase noradrenergic or serotonergic activity in depressed patients also increase their extraversion and sociability. This may be an indirect effect of the reduction in depression rather than a direct effect on E. The enzyme MAO-B is involved in regulation of the monoamines, particularly dopamine. Low levels of MAO have been related to arousal and activity in newborn human infants and to sociable behavior in adult humans and monkeys. These results suggest that a dysregulation of the dopamine system may be a factor in extraversion even in its earliest expression in the behavior of newborns. The hormone testosterone is related to E, but more so to E of the agentic type, which is the type characterized by dominance, assertiveness, surgent affect, high energy levels, activity, and irresponsibility, rather than simple sociability and interest in social relationships. This distinction between the two types of E has been hypothesized to be crucial for the relationship between dopamine and E as well (Depue & Collins, 1999).
Neuroticism/Anxiety/Harm Avoidance
Although the broad trait of neuroticism/anxiety includes other negative emotions, such as depression, guilt, and hostility, and character traits such as low self-esteem, neuroticism and anxiety are virtually indistinguishable as traits. Neuroticism is highly correlated with measures of negative affect, but when the negative affect was broken down into anxiety, depression, and hostility components, anxiety had the highest correlation, and hostility the lowest, with the N factor while depression was intermediate (Zuckerman, Joireman, Kraft, & Kuhlman, 1999). Hostility had a higher relationship to a factor defined by aggression.
Eysenck (1967) assumed a continuity between N as a personality trait and anxiety disorders. Indeed, N is elevated in all of the anxiety and depressive mood disorders, and longitudinal studies show that the trait was evident in most persons before they developed the symptoms of the clinical disorder (Zuckerman, 1999). In the first half of the twentieth century, when little was known about the role of the limbic system in emotions, the biological basis of neuroticism and anxiety trait was related to overarousal or arousability of the sympathetic branch of the autonomic nervous system. Such arousal is apparent in state anxiety elicited by anticipation of some kind of aversive stimulus or conditioned stimuli associated with aversive consequences.
Autonomic overarousal is apparent in the primary symptoms of many anxiety disorders. On the assumption of continuity between the N trait and these disorders, it was expected that autonomic arousal, as assessed by peripheral measures such as heart rate (HR), breathing rate (BR), blood pressure (BP), and electrodermal activity (EDA), would be correlated with N. In Eysenck’s (1967) theory, N was ultimately based
on reactivity of the limbic system, which regulates the ANS, but he did not distinguish particular pathways, structures, or neurotransmitters within that system that were involved in N. Some theories did not even make a distinction between cortical and autonomic arousal in emotions. Eysenck felt that there was some correlation between the two kinds of arousal because of collaterals between the limbic and ascending reticulocortical system. Gray (1982) and others, extrapolating from experimental studies of animals, delineated specific limbic systems involved in anxiety and the neurotransmitters involved in these systems. Neuroimaging studies have attempted to extend these brain models to humans.
Autonomic Arousal
Large-scale studies of the relationship between cardiovascular measures, either in resting levels of activity or reactivity to stressful experimental situations, and Measures of N failed to reveal any significant relationships (Fahrenberg, 1987; Myrtek, 1984). On the assumption that high cardiovascular activity put high-N subjects at risk for cardiovascular disease, Almada et al. (1991) investigated the relation between measures of N and subsequent health history in nearly 2,000 men. N was not associated with systolic BP or serum cholesterol but was associated with cigarette smoking and alcohol consumption. When tobacco and alcohol consumption were held constant there was no relationship between N and cardiovascular disease. Similar studies have failed to find any relationships between electrodermal activity and N or trait anxiety (Fahrenberg, 1987; Hodges, 1976; Naveteur & Baque, 1987).
Given the fact that many anxiety disorders do show elevated heart rate and electrodermal reactivity, how can we explain the lack of correlation with N? The answer may lie in the difference between generalized anxiety disorder (GAD) and panic disorder (PD), agoraphobia (Ag), and obsessivecompulsive disorder (OCD). Whereas the latter (PD, Ag, OCD) show elevated basal HRs and frequent spontaneous SCRs, GAD patients show little evidence of this kind of autonomic arousal (Zuckerman, 1991). Their anxiety is expressed cognitively (worry) and in symptoms of muscle tension such as fatigue. In contrast, PD, Ag, and OCD patients complain of autonomic symptoms, such as accelerated heart rate, even when they are not experiencing an actual panic attack (Zuckerman, 1999). Most persons who are high on N probably represent subclinical GAD disorder rather than the other types of anxiety disorders.
Brain Arousal
Studies of general cortical arousal using the EEG have historically focused on E, but some of these studies found interactions with N. These effects were inconsistent; some found higher and some reported lower arousal for high-N persons. Application of PET methods has not shown any association of general cortical or limbic arousal with N in situations that were not emotionally provoking (Fischer et al., 1997; Haier et al., 1987). Similar results are seen in anxiety patients; but when anxiety is provoked in patients by presenting them with feared stimuli, increased activity is seen in areas like the orbitofrontal cortex, insular cortex, temporal cortex, and anterior cingulate (Breier et al., 1992; Rauch et al., 1995). These studies identify an anxiety pathway in humans (orbitofrontal-frontal to cingulate to temporal lobe and amygdala) already established in animals, but they do not show a preexisting sensitivity of this pathway in normals scoring high in N. Another study of anxiety patients in nonstimulated conditions, which did use normal controls, found that whole brain blood flow did not distinguish anxiety patients from normals but did find a negative correlation between a depression scale and caudate activation. The previously mentioned study by Canli et al. (2001) found that in a small sample of normal women N correlated with increased brain activation to negative pictures (relative to activation by positive pictures) in left-middle frontal and temporal gyri and reduced activation in the right-middle frontal gyrus. Taken together, the clinical studies and this last study of normals suggests that whole brain activation does not vary with NAnx, but given negative emotional provocation there may be a reactive disposition in frontal cortex of high-N persons that activates a pathway through the orbitofrontal cortex around the cingulum to the temporal lobe and amygdala.
Davis (1986) argued that the central nucleus of the amygdala is a major center where the input of fear-provoking stimuli is organized and where output to various intermediate nuclei organizes the entire range of behavioral, autonomic, and neurotransmitter reactions involved in panic or fear. A recent MRI study (van Elst, Woermann, Lemieux, & Trimble, 1999) found an enlargement of left and right amygdala volumes in epileptic patients with dysthymia (a chronic kind of neurotic depression). Amygdala volume within the group did not correlate with trait or state anxiety but did correlate positively with a depression inventory. Because anxiety and depression are usually highly correlated and both correlate highly with N, it is not clear why depression alone was related to amygdala volume.
Monoamines
Much of the recent exploration of the role of the monoamines in N-Anx have been based on Cloninger’s (1987) biosocial model of personality and therefore used his scale of Harm Avoidance (HA) instead of the N or anxiety trait scales used by other investigators. HA, however, is not a pure scale of the N factor but lies between the E and N dimensions, constituting a measure of introverted neuroticism. It is defined in the same way that Gray defines trait anxiety: a sensitivity to cues associated with punishment and nonreward (frustration) and a tendency to avoid them.
Gray’s (1982) model suggests that norepinephrine in the dorsal ascending noradrenergic system (DANA) originating in the locus coeruleus is the major neurotransmitter involved in anxiety, although high levels of serotonin may mediate the behavioral inhibition that is associated with high levels of anxiety. Redmond (1977), from a psychiatric viewpoint, sees the DANA as an alarm system at lower levels and a panic provoker at high levels of activity. In contrast to these two theorists, Cloninger, Svrakic, and Przybeck (1993) proposed that high levels of serotonin activity underlie the trait of HA whereas norepinephrine activity is related to another trait called Reward Dependence.
In patients there has been little evidence of higher levels of the norepinephrine metabolite MHPG in anxiety patients compared to normals, although a more recent study by Spivak et al. (1999) showed higher levels of MHPG in plasma of patients with combat-related posttraumatic stress disorder than in controls.
The alpha-2 receptor functions as a homeostatic regulator of the norepinephrine systems, tuning them down when excessive neurotransmitter levels are detected in the synapse. Yohimbine is a antagonist to this receptor and therefore potentiates the activity of the norepinephrine system, just as a broken thermostat results in an overheated room. Yohimbine increases MHPG levels and provokes panic attacks in patients with panic disorders, although it does not have these effects in normal controls (Charney & Heninger, 1986). Cameron et al. (1996) replicated a previous result finding a decreased number of alpha-2 receptors in panic disorder. One might extrapolate that MHPG should correlate with N or anxiety over the range in normals and other patient groups. However, as noted earlier, high N in normals may resemble GAD more than panic disorder. Heinz, Weingarten, Hommer, Wolkowitz, and Linnoila (1999) reported a high correlation between CSF MHPG and an anxiety scale in a combined group of abstinent alcoholics and normals. A stress resistant group, defined by N and similar measures, had lower plasma MHPG after a mild stressor than did a nonresistant (high-N) group (de Leeuwe, Hentschel, Tavenier, & Edelbroek, 1992). Norepinephrine may be one of the factors underlying N, but it may be the dysregulation of norepinephrine by a lack of the receptors needed for this and a consequent tendency to be unable to cope with stress, rather than the basal level of activity in the norepinephrine system, which is related to N.
Cloninger’s biosocial theory of personality proposes that the trait of harm avoidance is related to behavioral inhibition mediated by serotonergic activity in the brain. Earlier studies showed no correlation between between CSF levels of the serotonin metabolite, 5-HIAA, and N. A more recent study has found a positive correlation between CSF 5-HIAA and N but in a sample of depressed patients (Roy, 1999). Constantino and Murphy’s (1996) study of the prediction of infant temperament from CSF levels of 5-HIAA showed no relationships between this metabolite and emotionality, soothability, or activity in infants.
Studies of normals using serotonin challenges, drugs that stimulate serotonergic activity, and indirect measures of serotonin response in normals have yielded mixed results including both positive (Gerra et al., 2000; Hansenne & Ansseau, 1999), nonsignificant (Ruegg et al., 1997), and a negative relationship (Mannuck et al., 1998) with N. The first three of these studies used the HA scale, whereas the last used the N scale, but with a much larger number of normal subjects than in the other studies. Serotonin seems to be implicated in harm avoidance, but the nature of that relationship is open to question. As with other neurotransmitters, the personalityrelevant aspects of serotonin may have more to do with receptor number and sensitivity than with basal levels of transmitter activity.
Hormones
Daitzman and Zuckerman (1980) found that testosterone (T) in males correlated negatively with various MMPI indexes of anxiety, depression, and neuroticism; that is, subjects with neurotic tendencies were low on T. Dabbs, Hopper, and Jurkovic (1990) reported a significant negative correlation between T and N in one study, but this was not replicated in another larger study of males; and in an even larger study of over 5,000 veterans T was not correlated with any MMPI indexes of trait anxiety or N. In still another study Dabbs et al. report significant negative correlations between T and a measure of pessimism in both males and females. T reflects both trait and state characteristics; that is, it is affected by immediate stressful experiences, particularly those involving success or defeat in competitive activities (Dabbs, 2000). The relationship with pessimism may reflect a history of defeat and consequent expectations for future failures. This depressive attitude may underlie negative relationships with N if any such relationships do exist.
Cortisol is one of the end products of activation of the hypothalamic-pituitary adrenocortical (HYPAC) system, a stress-reactive hormonal system. Like T, cortisol reactivity has both trait and state characteristics. Elevated cortisol is associated with major depressive disorder as a trait but is found in anxiety disorders only when activated by an immediate stressor.
Molecular Genetics
Lesch, Bengal, Hells, and Sabol (1996) found an association between a serotonin transporter gene (5-HTTLPR) and the trait of neuroticism, as assessed by three different scales including the NEO N scale and Cloninger’s TCI harm avoidance scale. Individuals with either one or two copies of the short form had higher N scores than individuals homozy gous for the long variant of the gene. The association was limited to the N factor of the NEO and the harm avoidant factor of the TCI; none of the other factors in these test was associated with the genetic variant. However, in a second study by this group (Hamer, Greenberg,Sabol,&Murphy,1999)theassociationofthegene with harm avoidance was weaker, and associations were found with TCI traits of cooperativeness and self-directiveness.
Several other studies have not been able to replicate the relationship between the gene variants and N or harm avoidance. This is a common outcome in the hunt for specific genes associated with personality traits or types of psychopathology, even when studies have adequate power and use good methodology. Population differences may account for some of these failures. Even in the studies that are significant the particular gene accounts only for a small portion of the genetic variance. In the Lesch et al. study the 5-HTT polymorphism accounted for 3% to 4% of the total variance for the trait and 7% to 9% of the genetic variance, and 10 to 15 more genes were estimated to be involved. If there is any replication of a gene-trait association, that finding should not be immediately dismissed by subsequent failures of replication, particularly if the finding has a theoretical basis. In this case Cloninger’s theory has suggested the involvement of serotonin in harm avoidance.
The short form of the gene, which is associated with high neuroticism, reduces serotonin uptake and therefore increases serotonergic transmission. Reduced uptake has been associated with anxiety in animal and human models, but paradoxically the serotonin uptake inhibitors are therapeutic agents in depressive disorders and several forms of anxiety disorders. These drugs could achieve their results through the inhibitory effects of serotonin on other systems such as the noradrenergic ones.
Summary
A sudden intense surge in anxiety is characterized by arousal of the sympathetic branch of the autonomic nervous system as expressed in elevated heart and breathing rates, blood pressure, sweating, and other signs of activation of this system. This led to the expectation that N or trait anxiety would be related to measures of these indicators either in the basal state or in reaction to stress. Research has generally failed to support this correlational hypothesis. EEG and brain scan studies also fail to reveal a difference in arousal levels as a trait distinguishing high- and low-N individuals. However, PET scan studies, done primarily on patients with anxiety disorders in reaction to fearful stimuli, show heightened reactivity of frontal, insular, and temporal cortex and anterior cingulate to such stimuli. Evidence from studies of animals has implicated the amygdala as a center for organization of the fear response, but brain imaging studies in humans have not yet supplied evidence for this localization.
Much of the research on other species identifies activation of the dorsal ascending noradrenergic system originating in the locus coeruleus as an alarm system activating the entire cortex in states of fear or anxiety. Reactivity of this system is a characteristic of panic disorders during panic attacks compared to the reactions of other types of anxiety disorders and normal controls. Correlational studies of norepinephrine metabolites and N-type trait measures in the basal state have not found a relationship, but at least one study has found a relationship between N and reactivity of a norepinephrine metabolite and response to stress. A hypothesized relationship with the monoamine serotonin has also shown no relationship with N in the basal state and no consistent findings relating N to reactions to drugs that stimulate serotonergic activity. Initial findings of a relationship between a serotonin transporter gene and N-type scales have not been replicated. Hormones like testosterone and cortisol show similar negative findings in the basal state and few findings relating N to reactivity to stress.
The research attempting to find a biological basis for N has had a disappointing outcome, particularly in view of the positive results in experimental research with animals and with humans that suffer from anxiety and mood disorders. Longitudal research has shown that N is a personality precursor of these disorders, so why does N not show relationships with some of the same biological indicators that characterize the disorders? There may be a kind of threshold effect so that the dysregulation of neurotransmitter systems characteristic of the disorders only emerges at some critical level of persistent stress that is not reproducible in controlled laboratory studies.
Psychoticism/Impulsivity/Sensation Seeking/Conscientiousness/Constraint
The third major personality factor goes under a variety of names depending on the various trait classification systems. Our factor analyses of personality scales have shown that Eysenck’s psychoticism scale is one of the best markers for the dimension that consists of scales for impulsivity and sensation seeking at one pole and scales for socialization, responsibility, and restraint at the other pole (Zuckerman et al., 1988, 1991, 1993). In a three-factor solution this factor also includes aggression and capacity to inhibit aggression, but in a four- or five-factor solution aggression and hostility versus agreeableness form a separate factor (Zuckerman et al., 1993). This research paper is organized by the four-factor model.
Cortical Arousal and Arousability
At the time the original studies were done relating conditioning to arousal and the construct “strength of the nervous system” to extraversion, E was measured by scales with two components: E and Impulsivity (Imp). In a theoretical shift, not receiving much attention, Eysenck and Eysenck (1985) reassigned Imp to the P rather than the E dimension. Although nearly all the earlier arousal and conditioning studies focused on E, it was shown that the relationship of E to conditionability (introverts more conditionable than extraverts) depended on the Imp component of E rather than the sociability component (Barratt, 1971; Eysenck & Levey, 1972). A later study showed that classical eyelid conditoning was related most closely to a specific type of Imp, the tendency to act quickly on impulse without thinking or planning. This is the type of Imp, called narrow impulsivity (IMPn), that constitutes a subscale of the older E scale. It is also the type of Imp that has been combined with sensation seeking in the latest ImpSS scale. Conditionability is thought to be a function of arousal; the more aroused a person is, the more conditionable he or she is thought to be. Could this mean that cortical arousal is related to the third dimension (P), including sensation seeking and IMPn, rather than the first (E) dimension of personality? A PET study found negative correlations between Pand glucose use in cortex and in thalamic and cingulate areas of the limbic system (Haier et al., 1987). Low cortical and autonomic arousal is a characteristic of the psychopathic (antisocial) personality, which may represent an extreme manifestation of the P dimension of personality (Zuckerman, 1989).
Evidence for a relationship between cortical arousal (EEG) and P and IMPn was found by some investigators (Goldring & Richards, 1985; O’Gorman & Lloyd, 1987); high P and impulsive subjects were underaroused. Sensation seeking, however, was not related to tonic arousal. Instead, sensation seeking—particularly that of the disinhibitiory type—has been consistently related to a particular measure of cortical arousability called augmenting-reducing (A-R, Buchsbaum, 1971).
A-R asseses the relationship of cortical reactivity, measured as a function of the relationship between the cortical EP and stimulus intensity for any given individual. Astrong positive relationship between the amplitude of the EP and the intensity of stimuli is called augmenting, and a negative or zero relationship is called reducing. A-R differences are most often observed at the highest intensities of stimulation, where the reducers show a marked EP reduction and the augmenters continue to show increased EP amplitude. There is an obvious relevance of this measure to Pavlov’s (1927/1960) construct of “strength of the nervous system,” based on the nervous system’s capacity to respond to high intensities of stimulation without showing transmarginal inhibition.
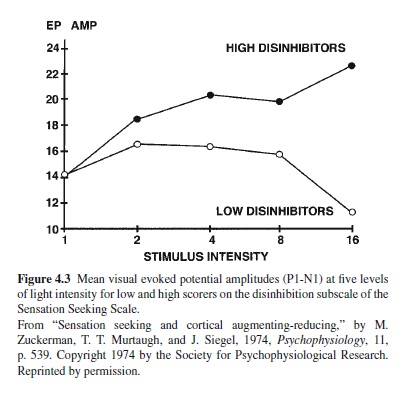
Figure 4.3 shows the first study of the relationship between the Disinhibition (Dis) subscale of the SSS and amplitude of the visual EP. Those scoring high on Dis displayed an augmenting pattern, and those scoring low on this scale showed a strong reducing pattern, particularly at the highest intensity of stimulation. This study was followed by many others, some using visual and others using auditory stimuli. Replications were frequent, particularly for the auditory EP (Zuckerman, 1990, 1991). Replications continue to appear (Brocke, Beauducel, John, Debener, & Heilemann, 2000; Stenberg, Rosen, & Risberg, 1990). A-R has also been found to be related to Imp, particularly cognitive impulsiveness (Barratt, Pritchard, Faulk, & Brandt, 1987).
The A-R model has been extended to other species and used as a biological marker for behavioral traits in animals resembling those in high and low human sensation seekers and impulsive and constrained persons. Cats who showed the augmenting pattern were active, exploratory, and approached rather than withdrew from novel stimuli. Augmenting cats adapted easily to novel situations, were responsive to a simple reward task, but were poor at learning to inhibit responses where they were only reinforced for low rates of response (Hall, Rappaport, Hopkins, Griffin, & Silverman, 1970; Lukas & Siegel, 1977; Saxton, Siegel, & Lukas, 1987).
Siegel extended this paradigm to a study of two genetically selected strains of rats, one actively avoidant or more aggressive and the other passive and frozen in reaction to shock (Siegel, Sisson, & Driscoll, 1993). The first strain consistently showed the augmenting EP pattern, and the second showed the reducing. Other behavioral characteristics of these strains were consistent with the human model of impulsive sensation seeking: The augmenting strain was aggressive, more willing to ingest alcohol, had high tolerance for barbituates, and self-administered higher intensities of electrical stimulation in reward areas of the limbic brain than the reducing strain.
Biochemical reactions suggested the basis for behavioral differences in characteristics of stress-reactive neurotransmitter and hormonal responses. Under stress, the augmenting strain showed more dopaminergic activity in the prefrontal cortex of brain, whereas the reducers had a stronger reaction in the hypothalamic-pituitary-adrenal cortex (HYPAC) stress pathway including increased serotonergic activity and corticotropin releasing factor in the hypothalamus and adrenocorticotropic hormone in the pituitary gland. Dopamine is a neurotransmitter implicated in action tendencies and theorized to be the basis of novelty and sensation seeking. Dopamine release would explain the active avoidance patterns that were the basis for selecting the two strains. Conversely, serotonin activity is associated with behavioral inhibition.
Monoamines
The animal model described earlier suggests that sensation seeking and related traits in humans may be associated positively with dopaminergic and negatively with serotonergic reactivity. Indirect evidence of this association comes from patients with Parkinson’s disease (PD), in which dopamine is depleted 75% in ventral tegmental neurons. A study of personality of PD patients showed that the PD patients were significantly lower on novelty seeking than controls but did not differ from them on harm avoidance or reward dependence (Menza, Golbe, Cody, & Forman, 1993). The PD patients were more depressed than controls, but depression did not correlate with novelty seeking scores.
Simple correlations between sensation seeking and dopamine and serotonin metabolites (HVA and 5-HIAA) assayed from CSF reveal no correlations between these metabolites and sensation seeking or the P scale or impulsivity scales (Ballenger et al., 1983; Limson et al., 1991). However, the correlational study by Ballenger et al. found a significant negative correlation between norepinephrine in the CSF and sensation seeking. A significant correlation was found between P and dopamine D2 binding in left and right basal ganglia in a PET study of a small group of normal subjects (Gray, Pickering, & Gray, 1994).
An experimental study by Netter, Hennig, and Roed (1996) used drugs that stimulate (agonist) or inhibit (antagonist) activity in the serotonergic and dopaminergic systems and measured their effects on hormonal, emotional-state, and behavioral reactions. Their findings suggested a low responsivity of the serotonergic system in high sensation seekers, but no association of dopaminergic response to an agonist and sensation seeking. However, craving for nicotine was increased by a dopamine agonist in high sensation seekers, suggesting that dopamine stimulation may induce more approach behavior in high than in low sensation seekers. Experiments in which nicotine or amphetamine is given to participants high or low in sensation seeking or novelty seeking showed that the high sensation/novelty seekers had more intense “highs” or subjective effects in response to these drugs than did low sensation seekers (Hutchison, Wood, & Swift, 1999; Perkins, Wilson, Gerlach, Broge, & Grobe, 2000). The effect for nicotine was most intense for nonsmokers, and the study on amphetamine did not use persons with a drug history. These special reactions of high sensation/novelty seekers to the novel drugs suggests some sensitivity to these dopamine agonists, perhaps in the receptors.
Another study by the German group found that the disinhibition type of sensation seeking and impulsivity, as well as aggression, were correlated with a response to a serotonin antagonist indicating low serotonergic responsivity in impulsive sensation seekers (Hennig et al., 1998).
Monoamine Oxidase
Fairly consistent negative relationships have been found between sensation seeking and MAO. A survey of results in 1994 showed low but significant negative correlations between platelet MAO and sensation seeking trait in 9 of 13 groups, and in 11 of 13 groups the correlations were negative in sign. The gender and age differences in sensation seeking are consistent with the gender and age differences in MAO described previously. Low MAO levels are characteristic of disorders characterized by impulsive, antisocial behavior including antisocial and borderline personality disorders, alcoholism and heavy drug abuse, pathological gambling disorder, bipolar
disorder, and attention deficit and hyperactivity disorder in children. MAO is low even in children of alcoholics and bipolar disorders who have not yet manifested the disorders, suggesting that it is a genetic risk marker for these disorders.
In a general normal population, low MAO was associated with use of tobacco, alcohol, and illegal drugs, convictions for crimes other than traffic offenses, and sociability in terms of hours spent with friends (Coursey et al., 1979). A study of low-MAO monkeys living in a natural environment showed that they were more aggressive, dominant, sexually active, and sociable than were high-MAO monkeys (Redmond, Murphy, & Baulu, 1979). Monkeys with high MAO levels were social isolates and passive. This study of another species suggests the evolutionary advantage of sensation seeking as mediated by MAO and possibly dopaminergic systems in the brain. Low MAO, however, is also associated with impulsivity in laboratory tests (Klinteberg et al., 1991), as is sensation seeking (Breen & Zuckerman, 1999; Thornquist & Zuckerman,1995), and impulsivity in risky situations could be a disadvantaged trait that may lead to premature death. However, the advantage in securing and dominating mates by intimidation of rivals may have outweighed the evolutionary disadvantages of reckless behavior.
In the public mind testosterone is identified with sexual drive and aggressiveness. However research shows that testosterone (T) is associated with a broader range of traits than these two. Androgens and T assayed from blood are correlated with sensation seeking (Daitzman & Zuckerman, 1980; Daitzman, Zuckerman, Sammelwitz, & Ganjam, 1978). Dabbs (2000) and Bogaert and Fisher (1995), using T from saliva, found only nonsignificant tendencies in that direction. A comparison of hypogonadal (low-T) and normal-T men, all referred for complaints of erectile dysfunction, showed that the low T-men were lower on sensation seeking than were the normal-T men (O’Carroll, 1984).
Hormones
Testosterone and sensation seeking in young males are both correlated with their sexual experience, in terms of the number of sex partners they have had (Bogaert & Fisher, 1995; Dabbs, 2000; Daitzman & Zuckerman 1980). Testosterone levels affect sexuality in women as well as men. Androgen levels of married women were related to sexual responsivity, frequency of intercourse, and sexual gratification (Persky et al., 1982). As with MAO, we can see the evolutionary advantage of the behavioral trait based on its biochemical substrate. However, other correlates of T include sociability, dominance, and activity, as well as inverse relationships to socialization and self-control.
The high-T male tends to be assertive, impulsive, and low in self-control, as well as high in sensation seeking. There is much less work on T in women, but what data there are suggest the same kind of personality correlates as in men. Apart from aggression, high-T men were more likely than others to misbehave in school as children, get into legal difficulties as adults, use drugs and alcohol, and go AWOL (absent without leave) while in the army (Dabbs, 2000). Fraternities with high average T levels were generally disorderly and chaotic, and their members were described by an observer as “crude and rude.” The high-T fraternities had more parties, worse grades, and fewer community service activities. Dabbs (2000) suggested that the total effect among members is an outcome of an interaction between T levels of its members and reinforcement of each other for antisocial behavior. In this case, high T is clearly a predisposing factor for low socialization, which these authors describe as “rambunctiousness.”
Testosterone levels reflect both trait and state moods. Although reliability can be found in T levels taken at the same time of day in the same setting, T levels can also be affected by experiences in competition (Dabbs, 2000). Competitors show increases in T when victorious and decreases when defeated. Even sports spectators show increases in T when their team wins and decrease when their team loses.
High levels of cortisol are associated with prolonged stress and depression. Ballenger et al. (1983) found that low levels of CSF cortisol were associated with a P dimension factor that included the P scale, the disinhibition subscale of the Sensation Seeking Scale (SSS), the MMPI hypomania scale, and lifetime number of sexual partners. Low levels of cortisol have been found in prisoners who have a history of psychopathic and violent behavior (Virkkunen, 1985). Low cortisol was also associated with novelty seeking in veterans with posttraumatic stress disorder (Wang, Mason, Charney, & Yehuda, 1997). Low cortisol may indicate a low reactivity to stress, which can be an advantage in some situations but carries the dangers inherent in lack of control and impulsivity. Traits that may have been adaptive in the warrior societies of the past may now confer a disadvantage in more socialized civilizations.
Genetics
Twin studies have found relatively high heritabilities (58%) for sensation seeking whether based on twins raised together (Fulker, Eysenck, & Zuckerman, 1980) or on twins separated shortly after birth and raised in different families (Hur & Bouchard, 1997). Heritability for Cloninger’s NS scale is somewhat lower (40%) but typical of that found for other personality traits (Heath, Cloninger, & Martin, 1994), but that for impulsivity is lower (15–40%) albeit significant (Eysenck, 1983).
Ebstein et al. (1996) were the first to report an association between the trait of novelty seeking and the gene for the D4 dopamine receptor (D4DR). The longer, usually the 7 repeat form of the 48 base pair sequence, was associated with high scores on Cloninger’s NS scale in an Israeli population. An immediate replication was reported by Benjamin et al. (1996) in an American population using scales from the NEO that approximate the NS factor such as Excitement Seeking and Deliberation (vs. Impulsiveness). Within a year Ebstein and Belmaker (1997) summarized the rapidly growing literature reporting two more replications and three failures to replicate. Since then two more failures to replicate have been reported, one in a Swedish population (Jönsson et al., 1998) and the other in a New Zealand sample (Sullivan et al., 1998). One partial replication was reported in Finland (Ekelund, Lichtermann, Jaervelin, & Peltonen, 1999). The variations in populations among the studies may have something to do with the inconsistent results. The distribution of alleles differs among populations. For instance, in a Japanese population the 7 repeat allele was not found but a comparison of the longer (5 and 6 repeats) with the shorter (2 to 4 repeats) still showed the former to be more characteristic of high novelty seekers (Ono et al., 1997).
As with MAO, the association between sensation or novelty seeking and this genetic marker is given some credence by its association with behavioral traits or disorders characterized by impulsivity and sensation seeking. The longer form of the D4DR has been found in high proportions of opiate abusers (Kotler et al., 1997), persons with pathological gambling disorder (Castro, Ibanez, Torres, Sáiz-Ruiz, & Fernández-Piqueras, 1997), those with attention-deficithyperactivity disorder (Swanson et al., 1998), and infants showing less distress in reaction to novel stimuli (Auerbach et al., 1998).
A comparative study was done on the effects of knocking out the D4R gene in mice on tests of approach-avoidance in reaction to novel objects or situations (Dulawa, Grandy, Low, Paulus, & Geyer, 1999). The D4 knockout mice showed reductions in behavioral response to novelty or a decrease in novelty related exploration in comparison to D4 intact mice.
Despite some failures of direct replication the association between novelty seeking and the D4DR receptor gene is given credence by these extensions to psychopathology and behavior in humans and mice. The D4DR association accounts for only about 10% of the genetic variation in the human trait, so other genes are certainly involved. The search is on for such genes. A crucial question is the functional significance of the difference between the alleles associated with high or low sensation seeking. The D4DR gene is expressed mainly in the limbic brain regions associated with emotional and motivational characteristics of sensation seeking. Dopaminergic activity is certainly involved, as has been postulated. But the significance of the D4DR gene in this activity is far from certain.An interesting finding is that the density of D4 receptors is elevated in brains of schizophrenics and that this receptor is the primary target for the antipsychotic drug clozapine (Seeman, 1995).
Summary
The underarousal hypothesis related to E has been more successfully applied to this third dimension of personality. Both EEG and brain imaging studies have found some preliminary evidence of cortical underarousal related to the P dimension and impulsivity. Sensation seeking and impulsivity have been related to the characteristic cortical response to a range of intensities of stimulation. Disinhibited and impulsive persons show an augmentation of cortical response at high intensities of stimulation relative to low intensities, whereas inhibited and constrained individuals show a reducing pattern, particularly at high intensities. This augmentingreducing paradigm of cortical reactivity has been extended to cats and rats, where it is associated with similar kinds of behavioral reactions and with other kinds of biological reactivity postulated to be the basis of the behavioral traits in humans.
The clinical model for this dimension of personality lies in the psychopathic or antisocial personality disorder. One of the characteristics of this disorder is a lack of emotional reactivity to stimuli associated with punishment and therefore a deficit in learning to avoid reacting to such stimuli. This leads to seeking of high-intensity rewarding stimuli regardless of the risk involved. It is not surprising that psychopaths are all high impulsive sensation seekers and share some of the same biological traits with nonpsychopathic sensation seekers such as low levels of the monoamine oxidase enzyme and high levels of testosterone.
One psychopharmacological theory of the P dimension is that it is based on high dopaminergic reactivity and low serotonergic and noradrenergic reactivity to highly stimulating situations. The low serotonergic reactivity is particularly related to the lack of restraint or behavioral inhibition and the low noradrenergic reactivity to the lack of arousal characteristic of high P, impulsive, and sensation seeking individuals. There is some evidence from studies of humans of a weaker response to serotonin stimulants in high sensation seekers than in low sensation seekers. There is no demonstrated relationship between the Pdimension and dopaminergic reactivity although animal and clinical research would support such a relationship.
High levels of testosterone and low levels of cortisol have been associated with disinhibition and psychopathic traits. But high levels of testosterone have also been associated with sociability and low levels with neuroticism, as discussed in previous sections. There is no necessary one-to-one relationship between biological and personality traits. Neurotransmitters like dopamine and hormones like testosterone may be related to two or more of the basic dimensions of personality or to a higher order dimensions like approach or inhibition.
Personality in the third dimension shows a high degree of heritability compared to other major dimensions. A specific gene, the dopamine receptor D4, has been associated with the trait of novelty seeking, although replication has been spotty. The association is supported by animal and clinical studies. Disorders characterized by impulsivity like opiate abuse, pathological gambling disorder, and attention-deficithyperactivity disorder share the same form of the gene as found in high novelty seekers. Mice with the gene removed show decreases in exploration and responses to novel situations. The third dimension of personality has been a rich lode of biological findings from the psychophysiological down to the most basic genetic level.
Aggression/Hostility/Anger/Agreeableness
Problems of definition confuse the fourth dimension of personality. Aggression refers to behavior, hostility to attitude, and anger to emotion. One can be aggressive without being hostile or angry, as in certain kinds of competition; or one can be chronically hostile and angry without expressing the negative attitude and feelings in overt aggression. One may be disagreeable without being aggressive or being aware of hostile attitudes or anger. Hostility without aggression is more closely associated with the N factor whereas aggression, with or without hostility, is more closely associated with this fourth factor.
Another source of differences is in the way aggression is expressed. Aggression in other species is classified by the sourceor context of the aggression: predatory, intermale, fearinduced, maternal, sex-related, instrumental, territorial, or merely irritable (Volavka, 1995). Human aggression is more often characterized by the form of expression. For instance, the widely used Buss-Durkee (1957) Hostility Scale (BDHS) classifies aggression as assault, indirect hostility, verbal hostility, irritability, negativism, resentment, and suspicion.
A new form of the scale has reduced the number of subscales to four, using factor analyses: physical aggression, verbal aggression, anger, and hostility (Buss & Perry, 1992). Although the subscales are moderately intercorrelated, quite different results have been found for the different subscales of the test in biological studies. Another important distinction in the literature is whether aggression is impulsive. The impulsive type of aggression seems more biologically rooted than instrumental types of aggression, but this confounds two different dimensions of personality.
Although aggression and hostility are correlated in tests and life, they are separated in two of the major trait classification systems. Eysenck’s system includes negative feelings like anger (moodiness) in neuroticism, but aggression and hostility are at the core of the psychoticism dimension. Costa and McCrae (1992a) have angry-hostility as a facet of neuroticism but regard aggression as the obverse of agreeableness. My colleagues and I found that hostility and anger load more highly on N and aggression on P in a three-factor model, but all three correlate with a common factor in a fivefactor analysis (Zuckerman et al., 1991).
Aggression has been defined by several methods, including self-report ratings or questionnaires, observer or ratings by others, and life-history variables like membership in groups characterized by violent acts or crimes. Aggression is not a socially desirable trait and this may limit the usefulness of self-report methods in some settings. Laboratory observations may be too specific to the experimental conditions. Persons who committed a violent crime, like murder, may differ depending on how characteristic their violent behavior was before they committed the crime. All methods have methodological problems, but in spite of this there are certain consistencies in results across methods in the literature.
Cortical Arousal and Arousability
Early studies of the EEG in abnormal populations, like violent criminals, used crude qualitative judgments of the EEG records as “abnormal” or “normal” (Volavka, 1995). EEG abnormalities included diffused or focal slowing, spiking or sharp waves in certain areas, and generalized paroxysmal features resembling minor epileptic seizures. The incidence of abnormal records found in samples of prisoners convicted of homicides and habitually violent prisoners was quite high (50–65%) compared to nonviolent prisoners or normal controls (about 5–10%; Volavka, 1995). However, some other studies found no differences between violent and nonviolent offenders.
Studies using quantitative methods showed EEG slowing in offenders, including slowing of alpha activity and an excess of slow wave (theta) activity. Volavka (1995) pointed out that these results could be due to a variety of factors including developmental retardation, brain injuries, decreased arousal level, cortical disinhibition, or genetic factors. Actually, twin research suggests that most of the activity in spectrum parameters of the EEG is genetically determined (Lykken, 1982).
One limitation of most of these earlier studies was that only prisoners referred for neuropsychiatric evaluation were used. A study by Wong, Lumsden, Fenton, and Fenwick (1994) selected subjects from a population of prisoners who had all been rated for violent behavior, and 70% had received EEG assessment. The prisoners were divided into three groups based on their history of violence. Going from the lowest to the highest violent groups, the percentages of abnormal EEG’s were, respectively, 26%, 24%, and 43%. The most frequent EEG characteristics differentiating the most violent from the less violent groups was focalized EEG abnormalities, particularly in the temporal lobes. Twenty percent of the most violent patients showed abnormal temporal lobe readings compared to 2% to 3% in the other two groups. Computerized tomography (CT) scans confirmed the high incidence of temporal lobe abnormalities in the most violent group.
The cortical EP has also been used to study cortical arousability. A study comparing detoxified alcoholic patients with and without histories of aggression found lower amplitudes of the P300 in the aggressive group (Branchey, BuydensBranchey, & Lieber, 1988). Aggressive alcoholics often have other characteristics, such as impulsivity and alcoholism, which might have produced the weaker P300 signal. Another study found that impulsive aggressive subjects screened from a college student population also showed lower P300 amplitudes at frontal electrode sites (Gerstle, Mathias, & Stanford, 1998). Still another study showed that a drug that reduced frequency of aggressive acts among prisoners with a history of impulsive aggression also increased the amplitude of the P300 in this group (Barratt, Stanford, Felthous, & Kent, 1997). This effect of the drug was not found in a group of prisoners who had committed premeditated murders. A reduced P300, particularly in the frontal lobes, may be symptomatic of a weakened inhibition from the frontal lobes and may account for the impulsive aspect of the aggression.
Visual imaging methods have been used in the study of violent behavior. Two structural methods are computed tomography (CT) and magnetic resonance imaging (MRI). MRI yields better images for precise assessment of brain structure. PET is used to assess brain activity in specific areas of brain including regions not accessible by ordinary EEG methods. Mills and Raine (1994) reviewed 15 studies of structural
Aggression/Hostility/Anger/Agreeableness
brain imaging (MRI, CT) and 5 studies using PET and regional CBF. Subject groups were violent prisoners, convicted murderers, pedophiles, incest offenders, property offenders, and, in some studies, normal controls. Property offenders were regarded as controls for violent offenders. Sexual offenders were not necessarily violent. Nine of the 15 studies using CT or MRI showed some type of structural abnormality, about evenly divided between frontal and temporal or frontotemporal deficits. Frontal abnormalities characterized the violent offenders and frontotemporal the sexual offenders, according to the authors of the review. However, most studies used small samples.The two studies of violent offenders using large samples (Ns of 128 and 148) found no particular localization of abnormalities (Elliot, 1982; Merikangus, 1981). The only study using MRI with any kind of N (another had only 2 cases) found evidence of temporal lobe lesions in 5 of 14violent patients (Tonkonogy, 1990).The large study by Wong et al. (1994), not included in the review, found that 55% of the most violent group had abnormal CT findings, and 75% of these were temporal lobe findings. Contrary to the hypothesis of Mills and Raine, temporal lobe lesions alone seem to be characteristic of violent patients. More MRI studies are needed to clarify the issue of localization.
The temporal lobe overlays the amygdala and has connections with it. Animal lesion and stimulation studies have found sites in the amygdala that inhibit and others that trigger aggression. Total amygdalectomies in monkeys produce a drop in the dominance hierarchy and an inability to defend against aggression from other monkeys. The comparative data suggest loci for aggression in the amygdala.
Mills and Raine reviewed five PET studies, but of these only one had a near-adequate number of subjects (3 had less than 10) and another compared child molesters with controls. The one study remaining compared 22 murderers with 22 normals and found selective prefrontal dysfunction in the group of murderers (Raine et al., 1994). Temporal lobe damage and functional hypofrontality are not unique to violent offenders but are also found in patients with schizophrenia.
Cardiovascular Arousal and Arousability
Numerous studies show that persons who score high on hostility scales show greater anger and cardiovascular arousal, especially blood pressure, in response to stress or perceived attack than do low hostile persons. As an example, a recent study found that among participants who were deliberately harassed in an experiment, the high hostile group who was harassed showed enhanced and prolonged blood pressure, heart rate, forearm blood flow and vascular resistance, and increased norepinephrine, testosterone, and cortisol responses than did low hostile subjects who were harassed (Suarez, Kuhn, Schanberg, Williams, & Zimmermann, 1998). This kind of cardiovascular reactivity may occur in frequent situations like stressful marital interactions (Smith & Gallo, 1999), and general day-to-day living (Räikkönen, Matthews, Flory, & Owens, 1999), and thus put a strain on the cardiovascular system that can result in cardiovascular disease, including hypertension (Lawler et al., 1998; Miller, Dolgoy, Friese, & Sita, 1998) and isochemic heart disease (IHD; Gallagher, Yarnell, Sweetnam, Elwood, & Stansfied, 1999). Persons with a family history of hypertension exhibit the same pattern of hostility and anger arousal with elevated blood pressure as do those who have developed the disorder suggesting that there may be a genetically influenced source to the cardiovascular overreactivity associated with anger arousability. However, how the anger is dealt with is a factor in vulnerability to heart disease. In a prospective study of nearly 3,000 men in their 50s and 60s Gallager et al. (1999) found that suppressed anger was most predictive of the incidence of IHD even when other risk factors were statistically controlled.
Monoamines
Åsberg’s (1994) review of the role of the monoamine neurotransmitters in human aggressiveness and violence attributes a primary importance to the role of serotonin. In animals serotonin is associated with inhibition of aggressive behavior and lowered serotonin with disinhibition of such behavior (Soubrié, 1986). In humans low levels of the serotonin metabolite, 5-HIAA, have been consistently found in those who attempt or complete suicide using violent means and in violent criminal offenders, particularly those characterized by impulsive violence or murder (Åsberg, 1994). Personality disorders like antisocial and borderline disorder have a high incidence of aggressive behaviors and suicide attempts. Homicide and suicide are not antithetical; homicide offenders have increased suicide rates. Within a group with personality disorders a negative correlation was found between CSF 5-HIAA and lifetime aggressive behavior (Brown et al., 1982).
Hormonal responses to serotonergic agonists and antagonists have also been used to assess the reactivity of the system in relation to aggression. They generally support the hypothesis of an inverse relationship between serotonin function and aggression/hostility (Cleare & Bond, 1997; Coccaro, Kavoussi, Sheline, Berman, & Csernansky, 1997; Moss, Yao, & Panzak, 1990).
Tryptophan is a precursor of serotonin in the brain (see Figure 4.2). Tryptophan depletion provides an experimental approach to the serotonin-aggression hypothesis, and unlike correlational studies it can provide evidence of a causal link with aggression. Studies have found that tryptophan depletion increases aggressive responses in laboratory behavioral tests (Cleare & Bond, 1995; Dougherty, Bjork, Marsh, & Moeller, 1999) as well as subjective feelings of anger, aggression, and hostile mood (Cleare & Bond, 1995; Finn, Young, Pihl, & Ervin, 1998), but the effect is limited to persons who are high in trait measures of hostility. The inference is that hostile persons, who are already low in serotonergic activity, tend to react aggressively with even more lowering of serotonin stores. There is the further suggestion that serotonin agonists or selective serotonin reuptake inhibitors (SSRIs) may reduce aggression in aggression-prone persons. A study by Knutson et al. (1998) showed that an SSRI reduced focal indices of hostility through a general decrease in negative affect without altering positive affect. In addition, the SSRI increased agreeableness on a behavioral index and cooperativeness in a puzzle-solving task.
SSRI’s are used to treat depression, but can they change other emotions like anger-hostility? A study of SSRI therapy for depressed outpatients showed a significant decrease in anger-hostility as well as neuroticism (Bagby et al., 1999). The decrease in neuroticism, however, was correlated with the decrease in clinical depression severity, whereas the decrease in anger-hostility was independent of the reduction of depression.
NE mediates a primary arousal system in the brain beginning in the locus-coeruleus and extending through limbic structures to innervate all areas of cerebral cortex. As such it has been implicated in the arousal of anxiety as previously discussed. But anger is also associated with an arousal effect as shown by the cardiovascular reactivity in highly hostile and angry persons as previously discussed. A study of aggression in free-ranging monkeys found a negative correlation between aggressivity and CSF 5-HIAA, congruent with the serotonin-aggression hypothesis, but it also found an equally strong positive correlation between aggressivity and CSF MHPG, the NE metabolite. The Ballenger et al. (1983) study of humans (normals) found a very high positive correlation (.64) between plasma MHPG and the Assault scale from the BDHS. Use of a noradrenergic challenge revealed a correlation of noradrenergic reactivity and irritability and assault scales (Coccaro et al., 1991).
On the other hand, low levels of the catecholamines epinephrine and NE, obtained from urine, are inversely related to aggressiveness (Magnusson, 1987). Psychopathic youths have low reactivity in these peripheral catecholamine systems in stressful situations (Lidberg, Levander, Schalling, & Lidberg, 1978). The difference may lie between the central noradrenergic and the peripheral autonomic stress system. Another possibility is that there is a difference between the psychopathic type of aggression, which is often not accompanied by high arousal, and the impulsive-angry type of aggression in which emotional disinhibition is typical. Netter, Hennig, and Rohrmann (1999) believed that they can distinguish the two types of aggressiveness on the basis of selective types of challenges to the monoamine systems. The serotonergic challenge was primarily correlated with Eysenck’s P scale, assessing the psychopathic type of aggression, whereas another type of challenge more closely related to dopamine reactivity was related to the nonpsychopathic type of aggression.
Increasing levels of brain dopamine in rats increases impulsive aggressive responding, but it takes a great deal of dopamine depletion to reduce aggressive behavior (Volavka, 1995). Little research has been done on dopamine specifically although the aggression producing effects of amphetamine may be a function of stimulation of dopaminergic as well as noradrenergic systems. Astudy of the neuroendocrine responses to glucose challenge in a group of substance abusers showed that those participants characterized by antisocial hostility had responses suggestive of increased dopaminergic activity (Fishbein, Dax, Lozovsky, & Jaffe, 1992).
Hormones
The hypothesis of an influence of T on aggressive behavior has a prescientific origin in that the pacifying effects of castration in animals were known and used for that purpose. Sexual competition among males is one form of aggression influenced by T, but other forms are also affected. Castration reduces aggression in males in most species, and T replacement reverses this effect.
Studies of the relationships between T and hostility or aggression in humans have produced mixed results, but a metaanalysis of such studies found a moderate effect size of .40 over all studies (Archer, Birring, & Wu, 1988). An earlier review by Archer (1991) suggested that results were more positive in studies where behavioral assessments (usually in prisoners) were used and less powerful in studies of trait (self-report) hostility or aggression (usually in college student samples). The newer meta-analysis failed to support this hypothesis. Better results were obtained in studies using salivary T as opposed to T derived from blood. A study using salivary T in 306 students found T positively correlated with aggression and negatively with prosocial scales in both men and women (Harris, Rushton, Hampson, & Jackson, 1996), but in other studies using either blood (Archer et al., 1998) or salivary T (Campbell, Muncer, & Odber, 1997) in large samples of male students no relationship was found. In still another study of blood T in students, both T and estradiol were postively correlated with self-reported aggression in men, but the correlations were negative in women (Gladue, 1991).
More consistent results have been obtained with behavioral (non-self-report) assessments. A study of nearly 700 male prison inmates found that salivary T was related to a history of violent crimes, particularly rape, homicide, and child molestation, as well as violations of prison rules, particularly those involving assault (Dabbs, Carr, Frady, & Riad, 1995). A study of female inmates showed a relationship of T with aggressive dominance in prison but not with the history of criminal violence. A group of alcoholics with a history of violence had elevated levels of serum T relative to other alcoholics (Bergman & Brismar, 1994).
Even among nonclinical samples there is correlational evidence of a relationship between T and aggression. T correlated with more aggressive fighting in men during judo contests (Salvador, Suay, Martinez, Simon, & Brain, 1999) and in amount of shock given to an opponent in a contrived laboratory situation (Berman, Gladue, & Taylor, 1993).
Whether self-report or behavioral, correlational studies cannot establish cause and effect. There is ample evidence in both animal and human studies that aggressive experience in competition may raise T levels in victors or lower them in those who are defeated. Experimental studies in which the effects of raised T levels on aggression are observed might be helpful. Clinical studies of steroid users have shown increased aggressiveness in some of them (Pope & Katz, 1994). Archer (1991) reviewed studies in which T or T-stimulating hormones were given and effects on aggression assessed by self-report. Although there is some evidence that T can affect hostility, there are also some negative findings from other studies. In all likelihood there is a continuous interaction between endogenous levels of T and life experiences (affecting current levels) during life. T makes one more likely to aggress, and aggression or its anticipation raises T levels.
Longitudinal studies may also elucidate the complex causal pattern. In one study a group of boys was followed from 6 to13 years of age (Tremblay et al., 1998).Tat age 12 and body mass predicted social dominance in adolescence but only body mass independently predicted physical aggression.The authors suggest that the relation between aggression and T in adolescents may be mediated by the effect of T in the change in physique in the context of dominance. A similar study followed males from pre- or early adolescence (12–13 years) and found little relationship between early or concurrent measure of T and aggression; the few that were found did not persist over time (Halpern, Udry, Campbell, & Suchindran, 1993).
Short time periods of prediction may confound environmental-developmental interactions that could mask the influence of endogenous levels of T. Windle and Windle (1995), in a retrospective longitudinal study, examined the adult levels of plasma T in four groups: (a) those who were aggressive only in childhood; (b) those who became aggressive as adults; (c) those who were aggressive in both childhood and adulthood (continuity); and (d) those who were low in aggression in childhood and adulthood. Adult onset and continuity (in aggressiveness) groups had higher T levels as adults than the other two groups. Other than aggressiveness, the high-T adult groups had higher rates of antisocial personality and a history of various signs of antisocial behavior. Was the high level of T in these groups a product of their history or a sign of an earlier level of T that affected the development of these behaviors? The authors admit that it is impossible to answer this question.
High levels of cortisol are associated with stress and inhibition and low levels with impulsivity and sensation seeking, as noted previously. In baboons dominant and aggressive males usually have low levels of cortisol and subordinate and nonaggressive primates have higher levels of cortisol. As with testosterone, cortisol varies considerably with recent and long-term patterns of experience such as winning or losing in fights. Low levels of cortisol have been found in psychopathic, violent offenders (Virkkunen, 1985), but high levels of cortisol are positively associated with hostility as measured by hostility questionnaires (Keltikangas, Räikkönen, & Adlercreutz, 1997; Pope & Smith, 1991). Chronic feelings of hostility are often associated with anxiety and depression, but the type of impulsive aggression seen in antisocial personality represents a brief state of anger in a generally unemotional personality.
Genetics
Behavior genetic studies of general hostility scales or aggression in children have shown significant heritabilities. However, it is possible that some aspects of hostility or aggression may be more heritable than others. A twin study of adult males using the BDHS revealed heritabilities ranging from 28% for verbal hostility to 47% for assault (Coccaro, Bergeman, Kavoussi, & Seroczynski, 1997). Verbal hostility is the most common form and yet it had the least heritability and the strongest environmental influence. An analysis of the genetic influence on the correlations among the scales that the assault scale had different underlying influences than the other scales which shared a common genetic influence. With the exception of the assault scale the genetic influence underlying the scales is of a nonadditive type suggesting Mendelian dominant or recessive or epistatic mechanisms. If it is the former, there is the likelihood of finding a gene of major effect in the general trait of aggression, apart from physical assault type.
The MAO type-A gene has become a likely candidate for this trait. Aggression in male mice is heightened by deletion of the MAO-A gene (Cases et al., 1995), and a mutation in the gene in a large Dutch family has been linked to mild retardation and impulsive aggressive behavior (Bruner, Nelsen, Breakfield, Ropers, & van Oost, 1993). The mutation is rare, but the gene has a wide range of alleles varying in repeat length. Subjects with one form, in contrast to those with another form, had lower scores on an index of aggression/ impulsivity and the Barratt impulsiveness scale (Manuck, Flory, Ferrell, Mann, & Muldoon, 2000). The life history of aggression only approached significance and the BDHS did not show significant differences between allele groups. Apparently, the impulsivity was more salient than the aggressiveness in the combination. Consistent with the association between low serotonin and aggression in the finding that the allele group with the higher impulsive aggression score also showed less response to a serotonergic challenge test.
Just as the findings on the MAO-A gene suggest one source of the link between serotonin and aggression, another gene has been found that suggests a genetic mechanism for the association of norepinephrine with aggression. The adrenergic-2Areceptor gene (ADRA2A) plays a role in modulating norepinephrine release in the locus coeruleus. Alleles of this gene were associated with scales for hostility and impulsivity in a younger student sample and impulsivity alone in an older sample (Comings et al., 2000).
Summary
Extreme violence has been associated with EEG evidence of cortical abnormality usually in the form of an excess of slow wave activity (underarousal) or focalized EEG abnormalities in the temporal lobes. Brain scans have confirmed the temporal lobe abnormalities and also found an equal incidence of frontal lobe abnormalities. A reduced P300 cortical EP response has also been found in prisoners with a history of extremely violent behavior. The reduced activity and reactivity in the frontal lobes may reflect a deficit in inhibitory capacity, which is part of the executive function of these lobes. The abnormal activity of the temporal lobe may be symptomatic of abnormal amygdala function because this lobe is in close proximity to the underlying amygdala. An MRI study has revealed temporal lobe lesions in about one third of violent patients. Hostility or anger proneness is related to a high level of cardiovascular, noradrenergic, and testosterone and cortisol response to stress or perceived attack. Suppressed hostility can lead to cardiovascular disease.
Among the monoamines, serotonin deficit is most highly associated with impulsive aggression. However, low serotonin is associated with depression and suicide as well as aggression and homicide, another example of the multiple trait associations of biological markers. Lack of emotional and behavioral control is the likely consequence of serotonin deficit. Depletion of tryptophan, the precursor of serotonin in the production chain, increases aggressive responses and angry and hostile feelings in laboratory experiments. Augmentation of serotonin, through reuptake inhibitors, can reduce aggression in aggression-prone persons.
Unlike depression, in which both serotonin and norepinephrine depletions are seen, brain norepinephrine (from CSF) tends to be positively correlated with aggressive tendencies in monkeys and humans. However, low levels of peripheral levels of the catecholamines norepinephrine and epinephrine are also related to aggressiveness. We need to distinguish between the type of aggression that occurs in states of high emotional arousal and the cold type of aggression more characteristic of the psychopath. The latter type may be reflected in the low levels of peripheral catecholamine reactivity.
Testosterone is associated with aggression based on behavioral records, but results using self-report measures of hostility or aggression are less conclusive. Prisoners with either histories of extremely violent crimes or characterized by aggression in prison show high levels of testosterone. Testosterone is increased by victory in competitive contests and sexual stimulation and decreased by defeat, raising the old “chicken or egg” problem of causation. The influence of testosterone during development may be mediated by its influence on physique in male adolescents where it is associated with a more muscular mesomorhpic body build. Low cortisol levels are found in aggressive types and are also influenced by the outcomes of fights.
Aggression trait is moderately heritable, but its heritability depends on the form it takes. Assaultive aggression is moderately heritable but verbal aggression is only weakly heritable. The gene for MAO of the A type has been linked to aggression in a human family study. Deletion of the MAO-Agene in mice increases their aggressivity, suggesting that the gene is involved in the inhibition or regulation of aggression.
Conclusions
Wilson (1998) described consilience as a quality of science that links knowledge across disciplines to create a common background of explanation. Personality psychology, extending from social psychology at the higher level to biopsychology at the more fundamental level, provides a daunting challenge to consilience. The introduction to this research paper presented a model of levels along the biological and social pathways leading up to a merger in personality traits.
Such a levels approach suggests a goal of reductionism, a pejorative term for critics of science and many scientists as well. The artist is contemptuous of the critic’s attempts to reduce his or her art to a textual formula, and the social scientist may resent the presumptious intrusion of the biological scientist into his or her own complex type of explanation. Wilson, however, views reductionism as a natural mode of science:
The cutting edge of science is reductionism, the breaking apart of nature into its natural constituents. . . . It is the search strategy employed to find points of entry into otherwise impenetrably complex systems. Complexity is what interests scientists in the end, not simplicity. Reductionism is the way to understand it. The love of complexity without reductionism makes art; the love of complexity with reductionism makes science. (pp. 58–59)
Later, Wilson (1998) admits that reductionism is an oversimplification that may sometimes be impossible. At each level of organization the phenomena may require new laws and principles that cannot be predicted from those at more general levels. My view is that this is always true for levels that involve an interaction between biological traits or genes and experience in the social environment. A learned association cannot be reduced to a specific set of neural events, at least not in the complex brain of a higher organism. It is not inconceivable, however, that the difference in general neural events that make an association more likely in one individual than another is not only explicable but also essential for a complete understanding of the event. Consilience is more possible at the borders of two levels, and this is where the breakthroughs are most likely to take place. As Wilson puts it, “The challenge and the cracking of thin ice are what gives science its metaphysical excitement” (p. 60).
This research paper was organized around a top-down approach, starting with four broad classes of personality traits that are empirically identifiable across several systems of trait description: extraversion/sociability, neuroticism/anxiety, impulsiveness/ conscientousness, and aggression/agreeableness. One way to bypass the complex social determinants of these traits in human societies is to look for appropriate animal models and biological links between behavior in these species and our own.This approach has identified certain biological markers for analogous behavioral traits such as the monoamine neurotransmitters and enzymes like MAO that regulate them; hormones like testosterone and cortisol; psychophysiological characteristics such as augmenting/ reducing of the cortical evoked potential; brain structure and physiology as assessed by brain imaging methods in humans and lesion and stimulation studies in other species; and molecular genetic studies that link genes, biological mechanisms, and behavioral and personality traits.
Simple-minded reductionism would expect one personality or behavioral trait to be associated with one brain structure, one neurotransmitter, one hormone, one physiological pattern of reactivity, and one gene in both humans and other animals. The paper is organized by personality traits, but if one reads across the traits it is clear that this neat kind of phrenological isomorphism is not the rule. Evolution may have shaped the nervous system around behavioral mechanisms necessary for adaptation, but evolution did not select for personality traits. The tendency to explore, forage, and approach novel but nonthreatening objects or creatures is part of that adaptation and is important in survival, as is competitive and defensive aggression, cooperation, and even altruism.
If we reverse direction and work up from the biological mechanisms to the personality trait and behavioral levels the fourfold classification at the top becomes blurred. Monoamine reactivities, MAO, testosterone, cortisol, and reactivity of cortical EPs to stimulus variation are related to sociability and sensation seeking, impulsivity and aggression, asocialization, neuroticism, anxiety, and inhibition, but in no simple one-to-one manner. Low levels of serotonergic activity are related to both depression and impulsive aggression producing both violent and impulsive homicides and suicides, sometimes in the same person. Is it the impulsivity, the aggression, or the neuroticism that is related to a serotonin deficiency? High levels of testosterone are related to sociability and social dominance, disinhibitory sensation seeking, aggressivity, asocialization, and low levels to neuroticism and agreeableness. Low levels of MAO are related to sensation seeking, impulsivity, asocial tendencies, and sociability.
Personality traits may be orthogonal, but biological traits do not respect these boundaries. It is almost as if the functional biology of the organism is organized around two basic traits: approach (including sociability, impulsivity, sensation seeking, and aggression) and inhibition/avoidance (or neuroticism/anxiety at the personality trait level). The comparative psychologist Schneirla (1959) put this idea into a postulate: “For all organisms in early ontogenetic stages, low intensities of stimulation tend to evoke approach reactions, high intensities withdrawal reactions” (p. 3). In evolved or more mature organisms Schneirla used the terms “seeking” and “avoidance” in place of “approach” and withdrawal.” The latter terms convey the idea of reflexive or tropistic mechanisms, whereas the former imply learned behavior. Approach-withdrawal describes a basic dimension of temperament and inhibition/shyness another in infant scales of temperament. These individual differences in infants may represent two biologically based dimensions found in other species, and they may develop into more diffentiated characteristics in adult humans.
Genetic dissection is one method of defining the boundaries of biological influence in traits. If both biological and behavioral traits are included in biometric or molecular genetic studies, the genetic covariance between the genetic and the other two can be determined. Rarely are genetic, biological, and behavioral traits all included in one study.
A biosocial approach cannot ignore the complex interactions between biological traits and environmental experiences. In both animals and humans the levels of the hormones testosterone and cortisol influence behavioral interactions with the environment but are in turn influenced by the outcomes of these interactions. There is no reason to think that similar interactions do not occur for the monoamine neurotransmitters. All of these systems are regulated by internal mechanisms. For instance, if there is overactivity in a system, regulators like MAO may catabolize the excess neurotransmitter. There may be more trait stability in the regulator than in the transmitter itself. After repeated experiences, however, there may be changes in the activity of a biological system that are relatively enduring if not irreversible. Environment may even influence the effect of genes by affecting their release. Given the constant interaction between the biological and environmental pathways (Figure 4.1), reductionism of one to the other is impossible. It would be like describing the biological activity of the lungs in the absence of oxygen, the digestive organs in the absence of food, or, using a more relevant analogy, the brain in the absence of stimulation.
Psychology emerged from the biological sciences more than a century ago, although its origins were forgotten by those who wanted a science that would emulate physics and those who wanted to cut all connections with the biological sciences. Fifty years ago, when I entered the field, the founder of behaviorism, Watson, had declared that the outcome of personality was entirely a matter of life experience (conditioning) and had nothing to do with genetics, and Skinner had declared the irrelevancy of the brain in behavior. Despite Freud’s own view that the mysteries of the psyche would one day be understood in terms of biology, his followers advocated an environmental determinism that put the entire weight of explanation on society, the family, and early experience. These early prophets of our science are now historical footnotes, and the science is more cognitive and biosocial with new cross disciplines like cognitive neuroscience emerging. The changes are in large measure due to the rapid advances in the neurosciences that have opened new, unforeseen vistas in psychology. Further progress will also procede apace with the development of new methodologies and the refinement of current ones.
Behavior genetics has challenged the radical environmentalist position by showing that nearly all personality traits and even some broad attitudinal traits have a significant degree of genetic determination. It is becoming a truism that genes interact with environment throughout life. But the precise nature of this complex interaction remains obscure. Genes do not make personality traits; they make proteins. The development of molecular behavioral genetics will help solve some of these problems. When we know some of the major genes involved with a personality trait and what these genes make and influence in the nervous system, we will be in a better position to define the biological mechanisms that lie between gene and behavior. Knowing the gene-biological trait link is not sufficient until we can understand the way the biological mechanism interacts with the environment, or more specifically the brain-behavior relationship.
Until recent decades the study of the brain was limited to peripheral measures like the EEG. The brain-imaging methods are only in their infancy but are already influencing the course of our science. The ones like PET or the more effective fMRI can tell us exactly what is happening in the brain after the presentation of a stimulus or condition, as well as where it is happening. The expensiveness of these methods has limited their use to medical settings and to clinical populations. Studies of personality in normals are rare and incidental to the objectives of clinical studies. They usually involve small numbers of subjects with a consequent unreliability of findings. Sooner or later the application of these methods to the study of personality dimensions in nonclinical populations will help to understand exactly what a personality predisposition is in the brain. Longitudinal studies starting with genetic and neurochemical markers and tracking the fate of individuals with these markers through life will enable us to predict both normal variant outcomes and psychopathology.
Bibliography:
- Almada, S. J., Zonderman, A. B., Shekelle, R. B., Dyer, A. R., Daviglus, M. L., Costa, P. T., & Stamler, J. (1991). Neuroticism and cynicism and risk of death in middle-aged men: The Western Electric Study. Psychosomatic Medicine, 53, 165–175.
- Archer, J. (1991). The influence of testosterone on human aggression. British Journal of Psychology, 82, 1–28.
- Archer, J., Birring, S. S., & Wu, F. C. W. (1998). The association between testosterone and aggression in young men: Empirical findings and a meta-analysis. Aggressive Behavior, 24, 411–420.
- Åsberg, M. (1994). Monoamine neurotransmitters in human aggressiveness and violence: A selected review. Criminal Behaviour and Mental Health, 4, 303–327.
- Auerbach, J., Faroy, M., Kahanna, M., Ebstein, R., Geller, V., Levine, J., & Belmaker, H. (1998, October). Dopamine D4 receptor and serotonin transporter promoter in the determination of infant temperament. Poster presented at 12th Occasional Temperament Conference, Philadelphia.
- Bagby, R. M., Levitan, R. D., Kennedy, S. H., Levitt, A. J., & Joffe, R. T. (1999). Selective alteration of personality in response to noradrenergic and serotonergic antidepressant medication in a depressed sample: Evidence on non-specificity. Psychiatry Research, 86, 211–216.
- Ballenger, J. C., Post, R. M., Jimerson, D. C., Lake, C. R., Murphy, D. L., Zuckerman, M., & Cronin, C. (1983). Biochemical correlates of personality traits in normals: An exploratory study. Personality and Individual Differences, 4, 615–625.
- Bardo, M. T., Donohew, R. L., & Harrington, N. G. (1996). Psychobiology of novelty seeking and drug seeking behavior. Behavioural Brain Research, 77, 23–43.
- Barratt, E. S. (1971). Psychophysiological correlates of classical differential eyelid conditioning among subjects selected on the basis of impulsivity and anxiety. Biological Psychiatry, 3, 339–346.
- Barratt, E. S., Pritchard, W. S., Faulk, D. M., & Brandt, M. E. (1987). The relationship between impulsiveness subtraits, trait anxiety, and visual N100-augmenting-reducing: A topographic analysis. Personality and Individual Differences, 8, 43–51.
- Barratt, E. S., Stanford, M. S., Felthous, A. R., & Kent, T. A. (1997). The effects of phenytoin on impulsive and premeditated aggression: A controlled study. Journal of Clinical Psychopharmacology, 17, 341–349.
- Bartussek, D., Becker, G., Diedrich, O., Naumann, E., & Maier, S. (1996). Extraversion, neuroticism, and event-related brain potentials in response to emotional stimuli. Personality and Individual Differences, 20, 301–312.
- Bartussek, D., Diedrich, O., Naumann, E., & Collet, W. (1993). Introversion-extraversion and event-related potential (ERP): A test of J. A. Gray’s theory. Personality and Individual Differences, 14, 565–574.
- Benjamin, J., Ebstein, R. P., & Belmaker, R. (1997). Personality genetics. Israel Journal of Psychiatry and Related Sciences, 34, 270–280.
- Benjamin, J., Li, L., Patterson, C., Greenberg, B. D., Murphy, D. L., & Hamer, D. H. (1996). Population and familial association between the D4 dopamine receptor gene and measures of sensation seeking. Nature Genetics, 12, 81–84.
- Bergman, B., & Brismar, B. (1994). Hormone levels and personality traits in abusive and suicidal male alcoholics. Alcoholism: Clinical and Experimental Research, 18, 311–316.
- Berman, M., Gladue, B., & Taylor, S. (1993). The effects of hormones, Type A behavior pattern, and provocation on aggression in men. Motivation and Emotion, 17, 125–138.
- Block, J. (1995). A contrarian view of the five-factor approach to personality description. Psychological Bulletin, 117, 187–215.
- Bogaert, F., & Fisher, W. A. (1995). Predictors of university men’s number of sexual partners.Journal of Sex Research, 32,119–130.
- Branchey, M. H., Buydens-Branchey, L., & Lieber, C. S. (1988). P3 in alcoholics with disordered regulation of aggression. Psychiatry Research, 25, 49–58.
- Breen, R. B., & Zuckerman, M. (1999). “Chasing” in gambling behavior: Personality and cognitive determinants. Personality and Individual Differences, 27, 1097–1111.
- Breier, A., Buchanan, R. W., Elkashef, A., Munson, R. C., Kirkpatrick, B., & Gellad, F. (1992). Brain morphology and schizophrenia: A magnetic resonance imaging study of limbic, prefrontal cortex and caudate structures. Archives of General Psychiatry, 49, 921–924.
- Brocke, B., Beauducel, A., John, R., Debener, S., & Heilemann, H. (2000). Sensation seeking and affective disorders: Characteristics in the intensity dependence of acoustic evoked potentials. Neuropsychobiology, 41, 24–30.
- Brocke, B., Tasche, K. G., & Beauducel, A. (1997). Differential effort reactivity and state control. Personality and Individual Differences, 22, 447–458.
- Brown, G. L., Ebert, M. H., Gover, P. F., Jimerson, D. C., Klein, W. J., Bunney, W. E., & Goodwin, F. K. (1982). Aggression, suicide, and serotonin: Relationships to CSF amine metabolites. American Journal of Psychiatry, 139, 741–746.
- Bruner, H. G., Nelsen, M., Breakefield, X. O., Ropers, H. H., & van Oost, B. A. (1993). Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase-A. Science, 262, 578–580.
- Buchsbaum, M. S. (1971). Neural events and the psychophysical law. Science, 172,
- Bullock, W. A., & Gilliland, K. (1993). Eysenck’s arousal theory of introversion-extraversion: A converging measures investigation. Journal of Personality and Social Psychology, 64, 113–123.
- Buss, A. H., & Durkee, A. (1957). An inventory for assessing different kinds of hostility. Journal of Consulting Psychology, 21, 343–349.
- Buss, A. H., & Perry, M. (1992). The aggression questionnaire. Journal of Personality and Social Psychology, 63, 452–459.
- Cameron, O. G., Smith, C. B., Neese, R. M., Hill, E. M., Hollingsworth, P. J., Abelson, J. A., Hariharan, M., & Curtis, G. C. (1996). Platelet alpha2-adrenoreceptors, catecholamines, hemodynamic variables, and anxiety in panic patients and their asymptomatic relatives. Psychosomatic Medicine, 58, 289–301.
- Campbell, A., Muncer, S., & Odber, J. (1997). Aggression and testosterone: Testing a bio-social model. Aggressive Behavior, 23 229–238.
- Canli, T., Zhao, Z., Desmond, J. E., Kang, E., Gross, J., & Gabrieli, J. D. E. (2001). An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience, 115, 33–42.
- Carrigan, P. M. (1960). Extraversion-introversion as a dimension of personality: A reappraisal. Psychological Bulletin, 57, 329–360.
- Cases, O., Seif, L., Grimsby, J., Gaspar, P., Chen, K., Pournin, S., Muller, U., Aguet, M., Babinet, C., Shih, J. C., & Demaeyer, E. (1995). Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAO-A. Science, 268, 1763–1766.
- Castro, I. P., Ibanez, A., Torres, P., Sáiz-Ruiz, J., & FernándezPiqueras, J. (1997). Genetic association study between pathological gambling and a fundamental DNA polymorphism at the D4 receptor gene. Pharmacogenetics, 7, 345–348.
- Cattell, R. B. (1950). Personality: A systematic, theoretical, and factual study. New York: McGraw-Hill.
- Charney, D. S., & Heninger, G. R. (1986). Abnormal regulation of noradrenergic function in panic disorders. Archives of General Psychiatry, 43, 1042–1054.
- Cleare, A. J., & Bond, A. J. (1995). The effects of tryplophan depletion and enhancement on subjective and behavioral aggression in normal male subjects. Psychopharmacology, 118, 72–81.
- Cleare, A. J., & Bond, A. J. (1997). Does central serotonergic function correlate inversely with aggression? A study using d-fenfluramine in healthy subjects. Psychiatry Research, 69, 89–95.
- Cloninger, C. R. (1987). A systematic method for clinical classification of personality. Archives of General Psychiatry, 44, 573–588.
- Cloninger, C. R., Svrakic, D. M., & Przybeck, T. R. (1993). A psychobiological model of temperament and character. Archives of General Psychiatry, 50, 975–990.
- Coccaro, E. F., Bergeman, C. S., Kavoussi, R. J., & Seroczynski, A. D. (1997). Heritability of aggression and irritability: A twin study of the Buss-Durkee aggression scales in adult male subjects. Biological Psychiatry, 41, 273–284.
- Coccaro, E. F., Kavoussi, R. J., Sheline, Y. I., Berman, M. E., & Csernansky, J. G. (1997). Impulsive aggression in personality disorder correlates with platelet 5-HT-sub(2A) receptor binding. Neuropsychopharmacology, 16, 211–216.
- Coccaro, E. F., Lawrence, T., Trestman, R., Gabriel, S., Klai, H. M., & Siever, L. (1991). Growth hormone responses to intravenous clonidine challenge correlates with behavioral irritability in psychiatric patients and healthy volunteers. Psychiatry Research, 39, 129–139.
- Comings, D. E., Johnson, J. P., Gonzalez, N. S., Huss, M., Saucier, G., McGue, M., & MacMurray, J. (2000). Psychiatric Genetics, 10, 39–42.
- Constantino, J. N., & Murphy, D. L. (1996). Monoamine metabolites in “leftover” newborn human cerebrospinal fluid: A potential resource for biobehavioral research. Psychiatry Research, 65, 129–142.
- Costa, P. T., Jr., & McCrae, R. R. (1992a). Revised NEO Personality Inventory (NEO-PI-R). Odessa, FL: Psychological Assessment Resources.
- Costa, P. T., Jr., & McCrae, R. R. (1992b). Four ways five factors are basic. Personality and Individual Differences, 13, 653–665.
- Coursey, R. D., Buchsbaum, M. S., & Murphy, D. L. (1979). Platelet MAO activity and evoked potentials in the identification of subjects biologically at risk for psychiatric disorders. British Journal of Psychiatry, 134, 372–381.
- Crow, T. J. (1977). Neurotransmitter related pathways: The structure and function of central monoamine neurons. In A. N. Davison (Ed.), Biochemical correlates of brain structure and function (pp. 137–174). New York: Academic Press.
- Dabbs, J. M., Jr. (2000). Heroes, rogues, and lovers. New York: McGraw-Hill.
- Dabbs, J. M., Jr., Carr, T. S., Frady, R. L., & Riad, J. K. (1995). Testosterone, crime, and misbehavior among 692 male prison inmates. Personality and Individual Differences, 18, 627–633.
- Dabbs, J. M., Hopper, C. H., & Jurkovic, G. J. (1990). Testosterone and personality among college students and military veterans. Personality and Individual Differences, 11, 1263–1269.
- Daitzman, R. J., & Zuckerman, M. (1980). Personality, disinhibitory sensation seeking and gonadal hormones. Personality and Individual Differences, 1, 103–110.
- Daitzman, R. J., Zuckerman, M., Sammelwitz, P. H., & Ganjam, V. (1978). Sensation seeking and gonadal hormones. Journal of Biosocial Science, 10, 401–408.
- Davis, M. (1986). Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behavioral Neuroscience, 100, 814–824.
- De Pascalis, V. F., Fiore, A. D., & Sparita, A. (1996). Personality, event-related potential (ERP) and heart rate (HR): An investigation of Gray’s theory. Personality and Individual Differences, 20, 733–746.
- Depue, R. A., & Collins, P. F. (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–569.
- Depue, R. A., Luciana, M., Arbisi, P., Collins, P., & Leon, A. (1994). Dopamine and the structure of personality: Relationship of agonist induced dopamine activity to positive emotionality. Journal of Personality and Social Psychology, 67, 485–498.
- Digman, J. M., & Inouye, J. (1986). Further specification of the five robust factors of personality. Journal of Personality and Social Psychology, 50, 116–123.
- Dougherty, D. M., Bjork, J. M., Marsh, D. M., & Moeller, F. G. (1999). Influence of trait hostility on tryptophan depletion-induced laboratory Psychiatry Research, 88, 227–232.
- Dulawa, S. C., Grandy, D. K., Low, M. J., Paulus, M. P., & Geyer, M. A. (1999). Dopamine (D4) receptor knock-out mice exhibit reduced exploration of novel stimuli. Journal of Neuroscience, 19, 9550–9556.
- Ebmeier, K. P., Deary, I. J., O’Carroll, R. E., Prentice, N., Moffoot, A. P. R., & Goodwin, G. M. (1994). Personality associations with the uptake of the cerebral blood flow marker-super(99m) Tc-Examtazime estimated with single photon emission tomography. Personality and Individual Differences, 17, 587–595.
- Ebstein, R. P., & Belmaker, R. H. (1997). Saga of an adventure gene: Novelty seeking, substance abuse and the dopamine D4 receptor (D4DR) exon III repeat polymorphism. Molecular Psychiatry, 2, 381–384.
- Ebstein, R. P., Nemarov, L., Klotz, I., Gritsenko, I., & Belmaker, R. H. (1997). Additional evidence for an association between the dopamine D4 receptor (D4DR) exon III repeat polymorphism and the human personality trait of novelty seeking. Molecular Psychiatry, 2, 472–477.
- Ebstein, R. P., Novick, O., Umansky, R., Priel, B., Osher, Y., Blaine, D., Bennett, E. R., Nemanov, L., Katz, M., & Belmaker, R. H. (1996). Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait novelty seeking. Nature Genetics, 12, 78–80.
- Ekelund, J., Lichtermann, D., Jaervelin, M. R., & Peltonen, L. (1999). Association between novelty seeking and type 4 dopamine receptor gene in a large Finnish sample. American Journal of Psychiatry, 156, 1453–1455.
- Elliot, F. A. (1982). Neurological findings in adult minimal brain dysfunction and the dyscontrol syndrome. Journal of Nervous and Mental Disease, 179, 680–687. van Elst, L. T., Woermann, F. G., Lemieux, L., & Trimble, M. R. (1999). Amygdala enlargement in dysthymia: A volumetric study of patients with temporal lobe epilepsy. Biological Psychiatry, 46, 1614–1623.
- Erikson, E. H. (1963). Childhood and society. New York: Norton. Eysenck,H.J.(1947).NewYork:Praeger.
- Eysenck, H. J. (1967). The biological basis of personality. Springfield, IL: Charles C. Thomas.
- Eysenck, H. J. (1983). Abiometrical-genetical analysis of impulsive and sensation seeking behavior. In M. Zuckerman (Ed.), Biological bases of sensation seeking, impulsivity and anxiety (pp. 1–27). Hillside, NJ: Erlbaum.
- Eysenck, H. J. (1992). Four ways five factors are not basic. Personality and Individual Differences, 13, 667–673.
- Eysenck, H. J., & Eysenck, M. W. (1985). Personality and individual differences:Anaturalscienceapproach.NewYork:PlenumPress.
- Eysenck, H. J., & Levey, A. (1972). Conditioning, introversionextraversion and the strength of the nervous system. In V. D. Nebylitsyn & J. A. Gray (Eds.), Biological bases of individual behavior (pp. 206–220). New York: Academic Press.
- Eysenck, S. B. G., & Eysenck, H. J. (1963). On the dual nature of extraversion. British Journal of Social and Clinical Psychology, 2, 46–55.
- Fahrenberg, J. (1987). Concepts of activation and arousal in the theory of emotionality. (Neuroticism): A multivariate conceptualization. In J. Strelau & H. J. Eysenck (Eds.), Personality dimensions and arousal (pp. 99–120). New York: Plenum Press.
- Finn, P. R., Young, S. N., Pihl, R. O., & Ervin, F. R. (1998). The effects of acute plasma tryptophan manipulation on hostile mood: The influence of trait hostility. Aggressive Behavior, 24, 173–185.
- Fischer, H., Wik, G., & Fredrikson, M. (1997). Extraversion, neuroticism and brain function: A PET study of personality. Personality and Individual Differences, 23, 345–352.
- Fishbein, D. H., Dax, E. M., Lozovsky, D. B., & Jaffe, J. H. (1992). Neuroendocrine responses to a glucose challenge in substance abusers with high and low levels of aggression, impulsivity, and antisocial personality. Neuropsychobiology, 25, 106–114.
- Fiske, D. W. (1949). Consistency of factorial structures for personality ratings from different sources. Journal of Abnormal and Social Psychology, 44, 329–344.
- Freud, S. (1957). Various papers. In J. Strachey (Ed.), The standard edition of the complete psychological works of Sigmund Freud. London: Hogarth Press. (Original work published 1914)
- Fulker, D. W., Eysenck, S. B. G., & Zuckerman, M. (1980). The genetics of sensation seeking. Journal of Personality Research, 14, 261–281.
- Gale, A. (1983). Electroencephalographic studies of extraversionintroversion: A case study in the psychophysiology of individual differences. Personality and Individual Differences, 4, 371–380.
- Gallagher, J. E., Yarnell, J. W., Sweetnam, P. M., Elwood, P. C., & Stansfield, S. A. (1999). Anger and incident heart disease in the Caerphilly study. Psychosomatic Medicine, 61, 446–453.
- Gerra, G., Zaimovic, A., Timpano, M., Zambelli, U., Delsignore, R., & Brambilla, F. (2000). Neuroendocrine correlates of temperamental traits in humans. Psychoneuroendocrinology, 20, 479–496.
- Gerstle, J. E., Mathias, C. W., & Stanford, M. S. (1998). Auditory P300 and self-reported impulsive aggression. Progress in Neuropsychopharmacology and Biological Psychiatry, 22, 575–583.
- Gladue, B. A. (1991). Aggressive behavioral characteristics, hormones, and sexual orientation in men and women. Aggressive Behavior, 17, 313–326.
- Goldberg, L. R. (1990). An alternative description of personality: The big-five factor structure. Journal of Personality and Social Psychology, 59, 1216–1229.
- Goldring, J. F., & Richards, M. (1985). EEG spectral analysis, visual evoked potentials and photic-driving correlates of personality and memory. Personality and Individual Differences, 6, 67–76.
- Gosling, S. D. (2001). From mice to men: What can we learn about personality from animal research? Psychological Bulletin, 127, 45–86.
- Gray, J. A. (1982). The neuropsychology of anxiety: An enquiry into the functioning of the septohippocampal system. New York: Oxford University Press.
- Gray, J. A. (1987). The neuropsychology of emotion and personality. In S. M. Stahl, S. D. Iverson, & E. C. Goodman (Eds.), Cognitive neurochemistry (pp. 171–190). Oxford, UK: Oxford University Press.
- Gray, J. A. (1999). But the schizophrenic connection. . . . Behavioral and Brain Sciences, 22, 523–524.
- Gray, N. S., Pickering, A. D., & Gray, J. A. (1994). Psychoticism and dopamine D2 binding in the basal ganglia using single photon emission tomography. Personality and Individual Differences, 17, 431–434.
- Guilford, J. P. (1975). Factors and factors of personality. Psychological Bulletin, 82, 802–814.
- Haier, R. J., Sokolski, K., Katz, M., & Buchsbaum, M. S. (1987). The study of personality with positron emission tomography. In J. Strelau & H. J. Eysenck (Eds.), Personality dimensions and arousal (pp. 251–267). New York: Plenum Press.
- Hall, R. A., Rappaport, M., Hopkins, H. K., Griffin, R. B., Silverman, J. (1970). Evoked response and behavior in cats. Science, 170, 998–1000.
- Halpern, C. T., Udry, J. R., Campbell, B., & Suchindran, C. (1993). Relationships between aggression and pubertal increases in testosterone: A panel analysis of adolescent males. Social Biology, 40, 8–24.
- Hansenne, M., & Ansseau, M. (1999). Harm avoidance and serotonin. Biological Psychology, 51, 71–81.
- Hamer, D. H., Greenberg, B. D., Sabol, S. Z., & Murphy, D. L. (1999). Role of the serotonin transporter gene in temperament and character. Journal of Personality Disorders, 13, 312–328.
- Harris, J. A., Rushton, J. P., Hampson, E., & Jackson, D. N. (1996). Salivary testosterone and self-report aggression and pro-social personality characteristics in men and women. Aggressive Behavior, 22, 321–331.
- Harris, J. A., Vernon, P. A., & Boomsa, D. I. (1998). The heritability of testosterone: A study of Dutch adolescent twins and their parents. Behavior Genetics, 28, 165–171.
- Heath, A., Cloninger, C., & Martin, N. (1994). Testing a model for the genetic structure of personality: A comparison of the personality systems of Cloninger and Eysenck. Journal of Personality and Social Psychology, 66, 702–775.
- Heinz, A., Weingarten, H. G. D., Hommer, D., Wolkowitz, O. M., & Linnoila, M. (1999). Severity of depression in abstinent alcoholics is associated with monoamine metabolites and dehydroepiandrosterone-sulfate concentration. Psychiatry Research, 89, 97–106.
- Hennig, J., Kroeger, A., Meyer, B., Prochaska, H., Krien, P., Huwe, S., & Netter, P. (1998). Personality correlates of Pinodel induced decreases in prolactin. Pharmacopsychiatry, 31, 19–24.
- Hodges, W. F. (1976). The psychophysiology of anxiety. In M. Zuckerman & C. D. Spielberger (Eds.), Emotions and anxiety: New concepts, methods and applications (pp. 175–194). Hillsdale, NJ: Erlbaum.
- Hogan, R. (1982). A socioanalytic theory of personality. In M. M. Page (Ed.), Personality: Current theory and research: Nebraska
- Symposium on motivation (pp. 55–89). Lincoln: University of Nebraska Press.
- Hur, Y., & Bouchard, T. J., Jr. (1997). The genetic correlation between impulsivity and sensation seeking traits. Behavior Genetics, 27, 455–463.
- Hutchison, K. E., Wood, M. D., & Swift, R. (1999). Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Experimental and Clinical Psychopharmacology, 7, 493–501.
- Johnson, D. L., Wiebe, J. S., Gold, S. M., Andreason, N. C., Hichwa, R. D., Watkins, G. L., & Ponton, L. L. B. (1999). Cerebral blood flow and personality: A positron emission tomography study. American Journal of Psychiatry, 156, 252–257.
- Jönsson, E. G., Nöthen, M. M., Gustavsson, J. P., Neidt, H.,
- Forslund, K., Mattila-Evenden, M., Rylander, G., Propping, P., & Åsberg, M. (1998). Lack of association between dopamine D4 receptor gene and personality traits. Psychological Medicine, 28, 985–989.
- Jorm, A. F., Henderson, A. S., Jacomb, P. A., Croft, L., & Easteal, S. (1997). Quantitative trait loci for neuroticism: An allele association study with the serotonin receptor (HTR2) and monoamine oxidase A (MAOA) genes. Personality and Individual Differences, 22, 287–290.
- Kagan, J., Reznick, J. S., & Snidman, N. (1988). Biological bases of childhood shyness. Science, 240, 167–171.
- Keltikangas, J. L., Räikkönen, K., & Adlercreutz, H. (1997). Response of the pituitary adrenal axis in terms of Type Abehaviour, hostility and vital exhaustion in healthy middle-aged men. Psychology and Health, 12, 533–542.
- Klinteberg, B., Oreland, L., Hallman, J., Wirsen, A., Levander, S. E., & Schalling, D. (1991). Exploring the connections between platelet monoamine oxidase (MAO) activity and behavior: Relationships with performance in neuropsychological tasks. Neuropsychobiology, 23, 188–196.
- Knuston, , Wolkowitz, O. M., Cole, S. W., Chan, T., Moore, E.A., Johnson, R. C., Terpstra, J., Turner, R. A., & Reus, V. I. (1998). Selective alteration of personality and social behavior by serotonergic intervention. American Journal of Psychiatry, 155, 373–379.
- Koehler, T., Scherbaum, N., Richter, R., Boettcher, S. (1993). The relationship between neuroticism and blood pressure reexamined: An investigation of a nonclinical sample of military conscripts. Psychotherapy and Psychosomatics, 60, 100–105.
- Kotler, M., Cohen, H., Segman, R., Gritsenko, I., Nemanov, L., Lerer, B., Kramer, I., Zer-Zion, M., Kletz, I., & Ebstein, R. P. (1997). Excess dopamine D4 receptor (D4DR) exon III seven repeat allele in opioid-dependent subjects. Molecular Psychiatry, 2, 251–254.
- Lawler, K. A., Kline, K., Seabrook, E., Krishnamoorthy, J., Anderson, S. F., Wilcox, Z. C., Craig, F., Adlin, R., & Thomas, S. (1998). Family history of hypertension: A psychophysiological analysis: International Journal of Psychophysiology, 28, 207–222. de Leeuwe, J. N., Hentschel, U., Tavenier, R., & Edelbroek, P. (1992). Prediction of endocrine stress reactions by means of personality variables. Psychological Reports, 70, 791–802.
- Le Moal, M. (1995). Mesocorticolimbic dopaminergic neurons. Functional and regulatory roles. In F. E. Bloom & D. J. Kupfer (Eds.), Psychopharmacology: The fourth generation of progress (pp. 283–294). New York: Raven Press.
- Lesch, K. P., Bengel, D., Heils, A., & Sabol, S. Z. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science, 274, 1527–1531.
- Lidberg, L., Levander, S. E., Schalling, D., & Lidberg, Y. (1978). Urinary catecholamines, stress, and psychopathy: A study of arrested men awaiting trial. Psychosomatic Medicine, 40, 116–125.
- Limson, R., Goldman, D., Roy, A., Lamparski, D., Ravitz, B., Adinoff, B., & Linnoila, M. (1991). Personality and cerebrospinal monoamine metabolites in alcoholics and normals. Archives of General Psychiatry, 48, 437–441.
- Loehlin, J. C. (1992). Genes and environment in personality development. Newbury Park, CA: Sage.
- Lukas, J. H., & Siegel, J. (1977). Cortical mechanisms that augment or reduce evoked potentials in cats. Science, 196, 73–75.
- Lykken, D. T. (1982). Research with twins: The concept of emergenesis. Psychophysiology, 19, 361–373.
- Magnusson, D. (1987). Individual development in interactional perspective. In D. Magnusson (Ed.), Paths through life. Hillsdale, NJ: Erlbaum.
- Manuck, S. B., Flory, J. D., Ferrell, R. E., Mann, J. J., & Muldoon, M. F. (2000). A regulatory polymorphism of the monoamine oxidase-KA gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Research, 95, 9–23.
- Mannuck, S. B., Flory, J. D., McCaffery, J. M., Matthews, K. A., Mann, J. J., & Muldoon, M. F. (1998). Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology, 19, 287–299.
- Maslow, A. H. (1954). Motivation and personality. New York: Harper and Row.
- Mason, S. T. (1984). Catechoamines and behavior. Cambridge, UK: Cambridge University Press.
- Mathew, R. J., Weinman, M. L., & Barr, D. L. (1984). Personality and regional cerebral blood flow. British Journal of Psychiatry, 144, 529–532.
- Matthews, G., & Amelang, M. (1993). Extraversion, arousal theory and performance: Astudy of individual differences in EEG. Personality and Individual Differences, 14, 347–363.
- McCrae, R. R., & Costa, P. T., Jr. (1985). Updating Norman’s “Adequate taxonomy”: Intelligence and personality dimensions in natural languages and in questionnaires. Journal of Personality and Social Psychology, 49, 710–721.
- Menza, M. A., Golbe, L. I., Cody, R. A., & Forman, N. E. (1993). Dopamine-related personality traits in Parkinson’s disease. Neurology, 43(3, Pt. 1), 505–508.
- Merikangus, J. R. (1991). The neurology of violence. In J. R. Merikangus (Ed.), Brain behavior relationships (pp. 155–186). Lexington, MA: Lexington Books.
- Miller, S. B., Dolgoy, L., Friese, M., & Sita, A. (1998). Parental history of hypertension and hostility moderate cardiovascular responses to interpersonal conflict. International Journal of Psychophysiology, 28, 193–206.
- Millon, T., & Everly, G. S., Jr. (1985). Personality and its disorders. New York: Wiley.
- Mills, S., & Raine, A. (1994). Neuroimaging and aggression. Journal of Offender Rehabilitation, 21, 145–158.
- Moss, H. B., Yao, J. K., & Panzak, G. L. (1990). Serotonergic responsivity and behavioral dimensions in antisocial personality disorder with substance abuse. Biological Psychiatry, 28, 325–338.
- Murphy, D. L., Aulakh, C. S., Garrick, N. A., & Sunderland, T. (1987). Monoamine oxidase inhibitors as antidepressants: Implications for the mechanism of action of antidepressants and the psychobiology of the affective disorders and some related disorders. In H. Y. Meltzer (Ed.), Psychopharmacology: The third generation of progress (pp. 545–552). New York: Raven Press.
- Myrtek, M. (1984). Constitutional psychophysiology. London: Academic Press.
- Naveteur, J., & Baque, E. F. (1987). Individual differences in electrodermal activity as a function of subject’s anxiety. Personality and Individual Differences, 8, 615–626.
- Netter, P., Hennig, J., & Roed, I. S. (1996). Serotonin and dopamine as mediators of sensation seeking behavior. Neuropsychobiology, 34, 155–165.
- Netter, P., Hennig, J., & Rohrmann, S. (1999). Psychobiological differences between the aggression and psychoticism dimension. Pharmacopsychiatry, 32, 5–12.
- Norman, W. T. (1963). Toward an adequate taxonomy of personality attributes: Replicated factor structure. Journal of Abnormal and Social Psychology, 66, 574–583.
- O’Carroll, R. E. (1984). Androgen administration to hypogonadal and eugonadal men: Effects on measures of sensation seeking, personality and spatial ability. Personality and Individual Differences, 5, 595–598.
- O’Gorman, J. G. (1984). Extraversion and the EEG I: An evaluation of Gale’s hypothesis. Biological Psychology, 19, 95–112.
- O’Gorman, J. G., & Lloyd, J. E. M. (1987). Extraversion, impulsiveness, and EEG alpha activity. Personality and Individual Differences, 8, 169–174.
- Ono, Y., Manki, H., Yoshimura, K., Muramatsu, T., Muzushina, H., Higuchi, S., Yagi, G., Kanba, S., & Masahiro, A. (1997). Association between dopamine D4 receptor (D4DR) exon III polymorphism and novelty seeking in Japanese subjects. American Journal of Medical Genetics (Neuropsychiatric Genetics), 74, 501–503.
- Packard, M. G., Schroeder, J. P., & Gerianne, M. A. (1998). Expression of testosterone conditioned place preference is blocked by peripheral or intra-accumbens injection of ∂-Flupenthixol. Hormones and Behavior, 34, 39–47.
- Panksepp, J. (1982). Toward a general psychobiological theory of emotions. The Behavioral and Brain Sciences, 5, 407–422.
- Pavlov, I. P. (1960). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex (G. V. Anrep, Trans.). New York: Dover. (Original work published 1927)
- Perkins, K. A., Wilson, A., Gerlach, D., Broge, M., & Grobe, E. (2000). Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation-seeking. Experimental and Clinical Psychopharmacology, 8, 462–471.
- Persky, H., Dresisbach, L., Miller, W. R., O’Brien, C. P., Khan, M. A., Lief, H., Charney, N., & Strauss, D. (1982). The relation of plasma androgen levels to sexual behaviors and attitudes of women. Psychosomatic Medicine, 44, 305–319.
- Pivik, R. T., Stelmack, R. M., & Bylsma, F. W. (1988). Personality and individual differences in spinal motoneuronal excitability. Psychophysiology, 25, 16–24.
- Pope, H. G., & Katz, D. L. (1994). Psychiatric and medical effects of anabolic-androgenic steroid use: A controlled study of 160 athletes. Archives of General Psychiatry, 51, 375–382.
- Pope, K., & Smith, T. W. (1991). Cortisol secretion in high and low cynically hostile men. Psychosomatic Medicine, 53, 386–392.
- Räikkönen, K., Matthews, K. A., Flory, J. D., & Owens, J. F. (1999). Effects of hostility on ambulatory blood pressure and mood during daily living in healthy adults. Health Psychology, 18, 44–53.
- Raine, A., Buchsbaum, M. S., Stanley, J., Lottenberg, S., Abel, L., & Stoddard, J. (1994). Selective reductions in prefrontal glucose in murderers. Biological Psychiatry, 36, 365–373.
- Rammsayer, T. H. (1998). Extraversion and dopamine: Individual differences in response to changes in dopamine activity as a possible biological basis of extraversion. European Psychologist, 3, 37–50.
- Rammsayer, T. H. (1999). Dopamine and extraversion: Differential responsivity may be the key. Behavioral and Brain Sciences, 22, 535–536.
- Rammsayer, T. H., Netter, P., & Vogel, W. H. (1993). A neurochemical model underlying differences in reaction times between introverts and extraverts. Personality and Individual Differences, 14, 701–712.
- Rauch, S. L., Savage, C. R., Alpert, N. M., Miguel, E. C., Baer, L., Breiter, H. C., Fischman, A. J., Manzo, P. A., Moretti, C., & Jenike, M. A. (1995). A positron emission tomographic study of simple phobia. Archives of General Psychiatry, 52, 20–28.
- Redmond, D. E., Jr. (1977). Alterations in the function of the nucleus locus coeruleus. Apossible model for studies of anxiety. In I. Hanan & E. Usdin (Eds.), Animal models in psychiatry and neurology (pp. 293–305). New York: Pergamon Press.
- Redmond, D. E., Jr., Murphy, D. L., & Baulu, J. (1979). Platelet monoamine oxidase activity correlates with social affiliative and agnonistic behaviors in normal rhesus monkeys. Psychosomatic Medicine, 41, 87–100.
- Roy, A. (1999). CSF 5-HIAA correlates with neuroticism in depressed patients. Journal of Affective Disorders, 52, 247–249.
- Ruegg, R. G., Gilmore, J., Ekstrom, R. D., Corrigan, M., Knight, B., Tancer, M., Leatherman, M. E., Carson, S. W., & Golden, R. N. (1997). Clomipramine challenge responses covary with the tridimensional personality questionnaire scores in healthy subjects. Biological Psychiatry, 42, 1123–1129.
- Salvador, A., Suay, F., Martinez, S. S., Simon, V. M., & Brain, P. F. (1999). Correlating testosterone and fighting in male participants in judo contests. Physiology and Behavior, 68, 205–209.
- Saxton, P. M., Siegel, J., & Lukas, J. H. (1987). Visual evoked potential augmenting-reducing slope in cats—2: Correlations with behavior. Personality and Individual Differences, 8, 511–519.
- Schneirla, T. C. (1959). An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In M. J. Jones (Ed.), Nebraska Symposium on motivation (Vol. 7, pp. 1–42). Lincoln: University of Nebraska Press.
- Seeman, P. (1995). Dopamine receptors: Clinical correlates. In F. E. Bloom & D. J. Kupfer (Eds.), Psychopharmacology: The fourth generation of progress (pp. 295–302). New York: Raven Press.
- Siegel, J., Sisson, D. F., & Driscoll, P. (1993). Augmenting and reducing of visual evoked potentials in Roman high- and lowavoidance rats. Physiology and Behavior, 54, 707–711.
- Smith, B. D. (1983). Extraversion and electrodermal activity: Arousability and the inverted U. Personality and Individual Differences, 4, 411–419.
- Smith, T. W., & Gallo, L. C. (1999). Hostility and cardiovascular reactivity during marital interaction. Psychosomatic Medicine, 61, 436–445.
- Sostek, A. J., Sostak, A. M., Murphy, D. L., Martin, E. B., & Born, W. S. (1981). Cord blood amine oxidase activities relate to arousal and motor functioning in human newborns. Life Sciences, 28, 2561–2568.
- Soubrié, P. (1986). Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences, 9, 319–364.
- Spivak, B., Vered, Y., Graff, E., Blum, I., Mester, R., & Weizman, A. (1999). Low platelet-poor plasma concentrations of serotonin in patients with combat-related posttraumatic stress disorder. Biological Psychiatry, 45, 840–845.
- Stein, L. (1978). Reward transmitters: Catecholamines and opioid peptides. In M. A. Lipton, A. DiMascio, & K. F. Killam (Eds.), Psychopharmacology: A generation of progress (pp. 569–581). New York: Raven Press.
- Stelmack, R. M. (1990). Biological bases of extraversion: Psychophysiological evidence. Journal of Personality, 58, 293–311.
- Stelmack, R. M., Campbell, K. B., & Bell, I. (1993). Extraversion and brainstem auditory evoked potentials during sleep and wakefulness. Personality and Individual Differences, 14, 447– 453.
- Stelmack, R. M., & Wilson, K. G. (1982). Extraversion and the effects of frequency and intensity on the auditory brainstem evoked response. Personality and Individual Differences, 3, 373–380.
- Stenberg, G. (1994). Extraversion and the P300 in a visual classification task. Personality and Individual Differences, 16, 543– 560.
- Stenberg, G., Rosen, I., & Risberg, J. (1990). Attention and personality in augmenting/reducing of visual evoked potentials. Personality and Individual Differences, 11, 1243–1254.
- Stenberg, G., Wendt, P. E., & Risberg, J. (1993). Regional blood flow and extraversion. Personality and Individual Differences, 15, 547–554.
- Strelau, J. (1987). Personality dimensions based on arousal theories: Search for integration. In J. Strelau & H. J. Eysenck (Eds.), Personality dimensions and arousal (pp. 269–286). New York: Plenum Press.
- Strelau, J. (1998). Temperament: A psychological perspective. New York: Plenum.
- Strelau, J., & Eysenck, H. J. (Eds.). (1987). Personality dimensions and arousal. New York: Plenum Press.
- Suarez, E. C., Kuhn, C. M., Schanberg, S. M., Williams, R. B., Jr., & Zimmermann, E. A. (1998). Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosomatic Medicine, 60, 78–88.
- Swanson,J.M.,Sunohara,G.A.,Kennedy,J.L.,Regino,R.,Fineberg, E.,Wigal,T.,Lerner,M.,Williams,L.,LaHoste,G.J.,&Wigal,S. (1998).Association of the dopamine receptor (DRD4) gene with a refined phenotype of attention deficit disorder (ADHD):Afamilybased approach. Molecular Psychiatry, 3, 38–41.
- Thornquist, M. H., & Zuckerman, M. (1995). Psychopathy, passiveavoidance learning and basic dimensions of personality. Personality and Individual Differences, 19, 525–534.
- Tonkonogy, J. M. (1990). Violence and the temporal lobe lesion: Head CT and MRI data. Journal of Neuropsychiatry, 3, 189–196.
- Tremblay,R.E.,Schaal,B.,Boulerice,B.,Arseneault,L.,Soussignan, R. G., Paquette, D., & Laurent, D. (1998). Testosterone, physical aggression,dominance,andphysicaldevelopmentinearlyadolescence. International Journal of Behavioral Development, 22, 753–777.
- Tupes, E. C., & Christal, R. E. (1961). Recurrent personality factors based on trait ratings. (USAF ASD Tech. Rep. No. 61–97). Lockland Air Force Base, TX: U.S. Air Force.
- Virkkunen, M. (1985). Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatrica Scandinavica, 72, 40–44.
- Volavka, J. (1995). Neurobiology of violence. Washington, DC: American Psychiatric Press.
- Wang, S., Mason, J., Charney, D., & Yehuda, R. (1997). Biological Psychiatry, 41, 145–151.
- Wilson, E. O. (1998). Consilience: The unity of knowledge. New York: Vintage Books.
- Windle, M. (1994). Temperamental inhibition and activation: Hormonal and psychosocial correlates and associated psychiatric disorders. Personality and Individual Differences, 17, 61–70.
- Windle, R. C., & Windle, M. (1995). Longitudinal patterns of physical aggression: Associations with adult social, psychiatric, and personality functioning and testosterone levels. Development and Psychopathology, 7, 563–585.
- Wong, M. T. H., Lumsden, J., Fenton, G. W., & Fenwick, P. B. C. (1994). Electroencephalography, computed tomography and violence ratings of male patients in a maximum security hospital. Acta Psychiatrica Scandinavica, 90, 97–101.
- Zuckerman, M. (1984). Sensation seeking: A comparative approach to a human trait. Behavioral and Brain Sciences, 7, 413–471.
- Zuckerman, M. (1989). Personality in the third dimension: A psychobiological approach. Personality and Individual Differences, 10, 391–418.
- Zuckerman, M. (1990). The psychophysiology of sensation seeking. Journal of Personality, 58, 313–345.
- Zuckerman, M. (1991). Psychobiology of Personality. Cambridge, UK: Cambridge University Press.
- Zuckerman, M. (1992). What is a basic factor and which factors are basic? Turtles all the way down. Personality and Individual Differences, 13, 675–681.
- Zuckerman, M. (1995). Good and bad humors: Biochemical bases of personality and its disorders. Psychological Science, 6, 325–332.
- Zuckerman,M.(1999).Vulnerabilitytopsychopathology:Abiosocial model. Washington, DC: American Psychological Association.
- Zuckerman, M., & Cloninger, C. R. (1996). Relationships between Cloninger’s, Zuckerman’s, and Eysenck’s dimensions of personality. Personality and Individual Differences, 21, 283–285.
- Zuckerman, M., Joireman, J., Kraft, M., & Kuhlman, D. M. (1999). Where do motivational and emotional traits fit within three factor models of personality? Personality and Individual Differences, 26, 487–504.
- Zuckerman, M., Kuhlman, D. M., & Camac, C. (1988). What lies beyond E and N? Factor analyses of scales believed to measure basic dimensions of personality. Journal of Personality and Social Psychology, 54, 96–107.
- Zuckerman, M., Kuhlman, D. M., Joireman, J., Teta, P., & Kraft, M. (1993). A comparison of three structural models for personality: The big three, the big five, and the alternative five. Journal of Personality and Social Psychology, 65, 757–768.
- Zuckerman, M., Kuhlman, D., Thornquist, M., & Kiers, H. (1991). Five (or three) robust questionnaire scale factors of personality without culture. Personality and Individual Differences, 12, 929–941.




