View sample auditory processing in the primate brain research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
For all mammals, including primates, the auditory system makes it possible to obtain information from the environment that may or may not be detected by other sensory modalities. Sounds can be perceived, localized, and identified, often at great distances, without confirmation by the other senses. Depending on the species, input to the auditory system may be crucial for navigation, evasion of predators, location of food and water, and communication between other members of the same species. Some species of bats, for example, rely on auditory input for both navigating and finding food. Primates, by comparison, are not dependent on audition for these activities but have specializations that enable vocal communication between individuals (Ghazanfar & Hauser, 1999). Auditory-related specializations such as these equip each species with unique mechanisms that ultimately enhance survival and propagation.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
To accomplish these tasks, the auditory system must encode the relevant acoustic cues and distribute this information to the auditory and multisensory areas of the brain that make use of it. This complex process involves a wide variety of neuronal cell types, specialized circuitry, and vast networks of subcortical nuclei and cortical fields. These pathways and their elements are only partially understood, but the available data allow us to describe certain processes competently. For the purposes of this research paper, therefore, we discuss auditory-related processing and behaviors in terms of the two major tasks of the auditory system: object recognition and sound localization. We highlight the subcortical and cortical mechanisms that underlie these aspects of audition, in general, with special emphasis given to the organization of the auditory system in primates.
The Auditory Pathways
In mammals the major components of the auditory system are the outer ear, middle ear, inner ear, and central pathways, including the cerebral cortex. Peripherally, the outer and middle ears are responsible for the conduction of sound energy to the inner ear, where the signal is encoded by specialized sensory receptors (hair cells) and the eighth cranial nerve (CN VIII). The central auditory pathways consist of an elaborate network of interconnected nuclear complexes in the brain stem and thalamus and a number of cortical areas or fields (Figure 7.1). Serial and parallel inputs to each level are processed and passed on to other nuclei or fields, where additional processing occurs. In addition to the ascending network for the processing of sensory input, there is an extensive descending network that modulates activity at all levels, including the cochlea, enabling neurons to modify their input.
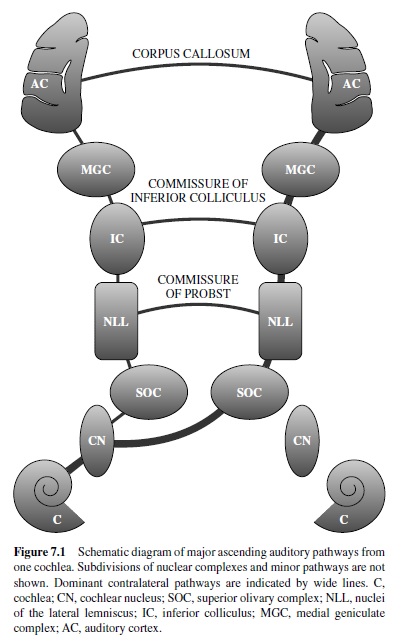
Subcortical Auditory Processing
Compared to the somatosensory and visual systems, the organization of the subcortical auditory pathways is exceptionally complex. The pathways involve five major nuclear groups, each of which can be parceled into discrete subdivisions. In addition, the pathways from each ear cross the midline shortly after entering the brain stem; thus, input from both ears is available to both sides of the brain at nearly every level of processing. This complexity creates an abundance of opportunities for signal processing below the level of cortex; thus our discussion of auditory processing would be incomplete without the inclusion of this information.
Compared with some other mammals, anatomical and physiological studies of subcortical auditory structures in primates are relatively few in number. Most of what is known about subcortical auditory processing in mammals comes from studies of nonprimates, especially cats, bats, and rodents. The findings from primate studies largely complement those of other mammals; thus it has been common to generalize principles of auditory subcortical organization across taxonomic groups, including humans. The extent to which this is valid depends on the presence of species-dependent specializations are expressed in the organization of the subcortical auditory pathways. Because of the lack of relevant primate data, however, such differences cannot be ruled out, so we must rely on data from nonprimates to discuss subcortical auditory processing in primates. In contrast, functional specializations are well known in the organization of auditory cortex, where the number of cortical fields devoted to a given sensory modality varies across taxa, and animals with larger brains tend to have more neocortical areas (Kaas, 1993, 2000). We must remind ourselves, however, that the identification of species differences in cortical organization does not rule out attendant subcortical differentiation. It may be that technical limitations hinder identification of the subcortical substrates that underlie, or contribute to, cortical specializations. Nevertheless, for our purposes we assume that the functional organization of subcortical auditory structures in primates corresponds to that of species for which data are more abundant. We begin with an overview of the sensory transduction process in the cochlea, followed by a brief description of auditory processing associated with each nuclear complex.Although much of the information is based on findings in nonprimates, we make reference to relevant primates studies where appropriate.
Cochlea
The cochlea is a coiled fluid-filled tubular structure carved out of the petrous portion of the temporal bone. The sensory receptors responsible for neural transduction of the acoustic signal, the hair cells, are located in the cochlea within the organ of Corti. The organ of Corti rests on the basilar membrane, which runs the entire length of the cochlear spiral. In primates the length of the basilar membrane is about 20 mm (Fernandez, Butler, Konishi, & Honrubia, 1962; Rose, Hind, Anderson, & Brugge, 1971). Deflection of the basilar membrane by fluid movements within the cochlea depolarizes the hair cells, giving rise to the neural signal. Afferent innervation of the cochlea is mediated by bipolar neurons located in the spiral ganglion of the cochlea within its central bony core. The distal (peripheral) processes of these neurons terminate on the hair cells. The proximal (central) processes comprise the auditory portion of CN VIII and synapse with neurons of the ipsilateral cochlear nucleus in the medulla. A single row of inner hair cells is the principal source of afferent information in the auditory system. Each inner hair cell is innervated by approximately 10 to 20 myelinated afferent fibers (Type I). The three rows of outer hair cells are innervated by a different class of afferent neurons (Type II). Type II neurons are unmyelinated, and collaterals of a single fiber innervate many outer hair cells; thus, the afferent contribution of the outer hair cells is minor compared with that of the inner hair cells. A more important function of the outer hair cells may be related to their efferent innervation. Efferent modulation of outer hair cell properties affects the physical attributes of the organ of Corti and appears to serve as a mechanism for fine tuning the afferent output of the cochlea (discussed later).
One important property of the cochlea concerns the arrangement of hair cells along the basilar membrane. Because of the physical properties of the cochlear structures, hair cells at the base of the cochlea (i.e., nearest the stapes bone and middle ear) are maximally responsive to highfrequency sounds, whereas hair cells at the apex of the cochlea respond to low-frequency sounds. This feature enables the cochlea to separate a complex acoustic signal into its component frequencies. The resulting tonotopic organization of the cochlea is preserved in CN VIII and subsequent stages of central auditory processing and therefore represents an important organizational feature of the auditory system.
Eighth Cranial Nerve
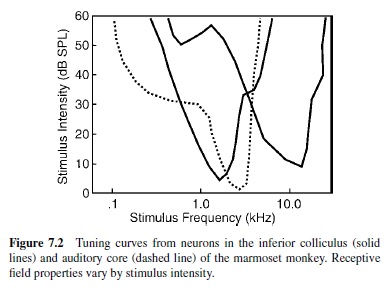
Also known as the vestibulocochlear nerve, CN VIII has two divisions containing fibers from the vestibular and cochlear structures of the inner ear. CN VIII passes through the temporal bone via the internal auditory canal in the skull base, exiting medially at the junction of the medulla, pons, and cerebellum. Alving and Cowan (1971) estimated that the number of fibers in the macaque monkey ranges from about 28,000 to 33,500, considerably less than the 50,000 to 55,000 estimated for cats (Gacek & Rasmussen, 1961). The auditory division of CN VIII is comprised of two classes of bipolar neurons. Type I auditory neurons comprise 90% to 95% of the total fiber population and carry most of the afferent information from the inner hair cells of the cochlea. Type II afferent and efferent neurons make up the rest. Type I neurons can be subclassified on the basis of spontaneous activity, activation threshold, and spectral response profile. The spectral and temporal features of the acoustic signal are preserved in the firing patterns and topography of CN VIII neurons. Consistent with the tonotopic organization of the cochlea, units that innervate hair cells in the basal segment of the cochlea respond best to high-frequency stimuli, whereas those that innervate the apical portion of the cochlea are most sensitive to low-frequency stimuli. Typically, CN VIII neurons respond to a narrow range of frequencies over a broad intensity range. The spectral response profile of each neuron is commonly referred to as its receptive field. The frequency to which the neuron is most sensitive (i.e., lowest threshold) is known as the characteristic frequency (CF). The unit also responds to frequencies above and below the CF, but response thresholds are higher (i.e., greater intensity is required to elicit a response). By plotting response frequency as a function of threshold intensity, the receptive field of the unit can be represented in the form of a tuning curve (Figure 7.2). Tuning curves can be obtained from auditory-responsive neurons at all levels of the central auditory system, and the distributions of CFs are used to construct tonotopic maps of a given nucleus or cortical field.
Cochlear Nucleus
The cochlear nucleus represents only the first stage of subcortical auditory processing, yet the anatomical and physiological diversity of this structure indicates that substantial auditory processing is mediated at this level. The cochlear nuclei contain several subdivisions and a wide variety of cell types (Figure 7.3). The tonotopic organization of CN VIII is maintained in the orderly pattern of projections of Type I and II fibers among the various subdivisions of the cochlear nucleus, which bifurcate on entering the brain stem. The ascending branches innervate the anteroventral division of the cochlear nucleus (AVCN). The posterior branches synapse in the posteroventral (PVCN) and/or dorsal (DCN) divisions. Neurons in the cochlear nuclei project to nearly every auditory nucleus on both sides of the brain stem, including the reticular formation. Each division exhibits a distinct pattern of projections to higher auditory centers and also receives patterned modulatory projections from higher auditory and nonauditory centers. Three major fiber bundles form the principal output connections of the cochlear nuclei. The largest band from the AVCN forms the trapezoid body with bilateral projections to the superior olivary complexes (SOCs), and contralateral projections to the lateral lemniscus and inferior colliculus (IC). Fibers from the PVCN form the intermediate acoustic stria of Held with projections to the contralateral lateral lemniscus and IC. The dorsal acoustic stria of von Monakow is formed by fibers of the DCN that also project primarily to the contralateral lateral lemniscus and IC. The anatomical features of the cochlear nuclei are generally consistent across primate species (Barnes, Magoun, & Ranson, 1943; Moskowitz & Liu, 1972; Strominger & Strominger, 1971; Strominger, Nelson, & Dougherty, 1977) and compare well with nonprimates, although some structural variations have been found (e.g., J. K. Moore, 1980).
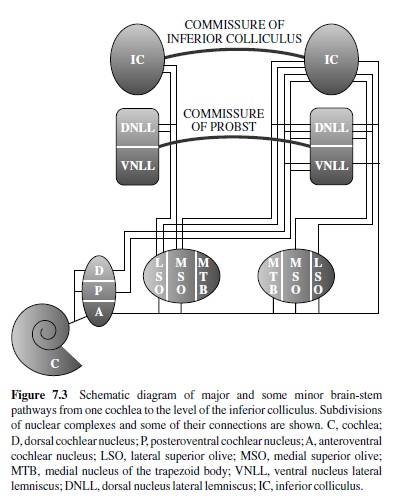
In the cochlear nucleus each of the major cell types is associated with a unique response profile reflecting particular attributes of the original acoustic signal. Different populations of neurons appear to be specialized to extract particular aspects of the encoded auditory stimulus for delivery to other centers for further processing (Romand & Avan, 1997). In the AVCN, for example, only the globular and bushy spherical cells exhibit a primary-like response similar to that of CN VIII fibers (Smith, Joris, Carney, & Yin, 1991). By comparison, certain pyramidal cells in the DCN respond only after a variable delay (Oertel & Wu, 1989; Rhode, Smith, & Oertel, 1983). The distribution and connectivity of each cell type varies among the subdivisions of the cochlear nucleus and contributes to the range of response properties observed; thus each subdivision has a unique anatomical and physiological profile. The diverse cell populations also give rise to a number of segregated pathways that are functionally distinct (Figure 7.3). Division of the auditory pathways into multiple subsystems has been observed at all levels of subcortical processing; however, one pathway may not be functionally independent of the others at any level, nor must a pathway be strictly hierarchical across levels. The complex network of connections between auditory nuclei provides numerous opportunities for interaction between pathways at all levels.
Superior Olivary Complex
The next major level of auditory brain-stem processing involves the SOC. The primary ascending pathway from the contralateral AVCN synapses in the SOC and then projects to the ipsilateral central nucleus of the IC (ICc; see Figure 7.3). Additional ascending pathways also synapse in the SOC, whereas others bypass the SOC with targets in the lateral lemniscus or IC. The SOC consists of several nuclei that vary morphologically among mammals, birds, reptiles, and amphibians. In mammals the three main subnuclei are the lateral (LSO) and medial (MSO) superior olivary nuclei and the medial nucleus of the trapezoid body (MNTB). These nuclei are surrounded by a variable number of periolivary nuclei, depending on the species. Anatomical studies in several primate species recognize the three major divisions, but descriptions of the periolivary nuclei reveal some variations (Barnes et al., 1943; Harrison & Irving, 1966; Irving & Harrison, 1967; J. K. Moore & Moore, 1971; J. K. Moore, 2000). The major divisions of the SOC are tonotopically organized and can be distinguished on the bases of their anatomy and physiology; however, their small size has limited their study to some extent, and only anatomical data are available for primates.
One of the primary functions associated with the SOC is the encoding of auditory cues pertaining to sound location. The SOC is the lowest level of central auditory processing at which inputs from both ears are represented on both sides of the brain stem. Tonotopic inputs to the MSO and LSO originate bilaterally in the AVCN and MNTB. The interaural differences associated with the location of a sound source can be resolved by the circuitry of the LSO and MSO. The LSO can detect interaural differences in time and intensity. The majority of LSO neurons sensitive to these differences are inhibited (I) by contralateral stimulation and excited (E) by ipsilateral stimulation (Type IE; Caird & Klinke, 1983; Tsuchitani, 1977). By comparison, most of the neurons in the MSO are excited by ipsilateral and contralateral stimulation (Type EE) and sensitive to interaural differences in time or phase (Guinan, Guinan, & Norris, 1972; Guinan, Norris, & Guinan, 1972; Yin & Chan, 1990) but relatively insensitive to differences in intensity. The interaural differences encoded by the LSO and MSO are the primary auditory cues used by later stages of processing to identify the location of a sound source in three-dimensional space. The principal cells in the LSO project tonotopically to the ICc bilaterally via the lateral lemniscus (Glendenning & Masterton, 1983; Henkel & Brunso-Bechtold, 1993). These projections are inhibitory to the ipsilateral ICc (Saint-Marie & Baker, 1990; Saint-Marie, Ostapoff, Morest, & Wenthold, 1989) and excitatory to the contralateral ICc (Glendenning, Baker, Hutson, & Masterton, 1992). Significant projections also target the dorsal nucleus of the lateral lemniscus (DNLL). The majority of MSO neurons project tonotopically to the ipsilateral ICc and DNLL, whereas minor projections target the contralateral ICc (Brunso-Bechtold, Thompson, & Masterton, 1981; Goldberg & Moore, 1967; Henkel & Spangler, 1983). Subsequent projections to the superior colliculus and motor nuclei in the brain stem mediate various reflexive and nonreflexive movements of the eyes, head, and limbs in response to particular types of auditory stimuli.
A second important function of the SOC is related to its centrifugal projections. There is evidence for the modulation of ascending activity by descending inputs at nearly every level of the central and peripheral auditory system. One of the most well-studied pathways involves direct projections from the periolivary region of the SOC to the cochlea. The olivocochlear bundle (OCB) was originally described by Rasmussen (1946, 1953) and has since been the subject of intense study. Activation of the OCB produces a variety of inhibitory effects on the cochlea thought to protect the cochlea from acoustic trauma and possibly improve auditory acuity in the presence of background (masking) noise. Two primary pathways comprise the olivocochlear system (for a review, see Warr, 1992). The lateral olivocochlear system (LOS) is largely uncrossed and involves projections from cells in the vicinity of the LSO to the ipsilateral cochlea. Most of these fibers terminate on the dendrites of Type I auditory neurons innervating the inner hair cells. The medial olivocochlear system (MOS) includes projections from medial periolivary neurons to the contralateral (approximately two thirds) and ipsilateral (approximately one third) cochleas. These fibers terminate primarily at the base of outer hair cells. The LOS and MOS systems of cochlear projections are tonotopic (Guinan, Warr, & Norris, 1983; Guinan, Warr, & Norris, 1984; Robertson, Anderson, & Cole, 1987). The modulation of OHC activity is thought to alter cochlear mechanics in a manner that decreases the sensitivity of inner hair cells (M. C. Brown, Nuttall, & Masta, 1983; Brownell, Bader, Bertrand, & de Ribaupierre, 1985). Activation of the crossed OCB projections by electrical or acoustic stimulation raises response thresholds and reduces the spontaneous activity of Type I afferents (Buno, 1978; Galambos, 1956; Guinan & Gifford, 1988; Liberman, 1989; Wiederhold & Kiang, 1970). In the presence of continuous background noise, OCB activation suppresses responses to the noise but enhances responses to transients by decreasing adaptation in the auditory nerve (Kawase & Liberman, 1993; Kawase, Delgutte, & Liberman, 1993). This antimasking mechanism could actually improve auditory discrimination in noise (Winslow & Sachs, 1987, 1988). OCB-mediated response suppression has also been shown to protect the inner ear from certain types of acoustic trauma (Rajan, 1988a, 1988b, 2000; Rajan & Johnstone, 1988a, 1988b, 1988c, 1989; Reiter & Liberman, 1995; Trahiotis & Elliott, 1970).
Nuclei of the Lateral Lemniscus
The lateral lemniscus is the principal fiber tract between the SOC and IC. In most species at least two primary subnuclei are recognized. The ventral nucleus (VNLL) receives inputs primarily from the contralateral ventral cochlear nuclei (Adams & Warr, 1976; Friauf & Ostwald, 1988; Glendenning, Brunso-Bechtold, Thompson, & Masterton, 1981). Output projections target mainly the ipsilateral ICc (Brunso-Bechtold et al., 1981; Covey & Casseday, 1986; Kudo, 1981). The dorsal nucleus (DNLL) receives bilateral inputs from the AVCN and LSO, an ipsilateral projection from the MSO, and additional inputs from the contralateral DNLL (Adams & Warr, 1976; Glendenning et al., 1981; Henkel & Spangler; 1983: Schneiderman, Oliver, & Henkel, 1988). The major DNLL projections are tonotopically organized and target the ICc bilaterally, with minor outputs to the deep layers of the superior colliculus bilaterally and the ipsilateral medial geniculate complex (Bajo, Merchan, Lopez, & Rouiller, 1993; Brunso-Bechtold et al., 1981; Coleman & Clerici, 1987; Hackett, Neagu, & Kaas, 1999; Hutson, Glendenning, & Masterton, 1991; Kudo, 1981; Merchan, Saldana, & Plaza, 1994; Schneiderman et al., 1988).
The VNLL is one of the few auditory nuclei that does not appear to be tonotopically organized (Aitkin, Anderson, & Brugge, 1970; Glendenning & Hutson, 1998; Whitley & Henkel, 1984). Most neurons in the VNLL are monaural and respond only to contralateral stimulation (Aitkin et al., 1970; Guinan, Norris, et al., 1972). Neurons in the DNLL are tonotopically organized, and most are of the EI type (Aitkin et al., 1970; Markovitz & Pollak, 1993, 1994; Merchan et al., 1994). Brugge, Andersen, and Aitkin (1970) reported that 88% of DNLL neurons sampled were responsive to binaural stimulation. Many units were sensitive to interaural differences in either intensity or phase, reflecting the projections of the LSO and MSO. The DNLL performs a wide range of integrative functions (see Pollak, 1997) and has an important influence on the activity of neurons in the IC (Kelly & Li, 1997; van Adel, Kidd, & Kelly, 1999). Its anatomical and physiological profile is consistent with a role in binaural auditory processing, but the precise functions of the lateral lemnisens remain unclear.
Inferior Colliculus
Multiple ascending and descending auditory pathways converge in the IC (Figures 7.3 and 7.4). Nearly all projections from the cochlear nuclei, SOC, and lateral lemniscus terminate in the IC, as do descending inputs from superior colliculus, thalamus, and cortex (for reviews, see Ehret, 1997; Huffman & Henson, 1990; Spangler & Warr, 1991). Accordingly, the IC is the principal source of ascending input to the medial geniculate complex (MGC) and descending projections to lower levels of the brain stem. As the connection patterns indicate, the IC plans a major role in the integration of monaural and binaural information processed by lower and higher auditory centers, including cortex.
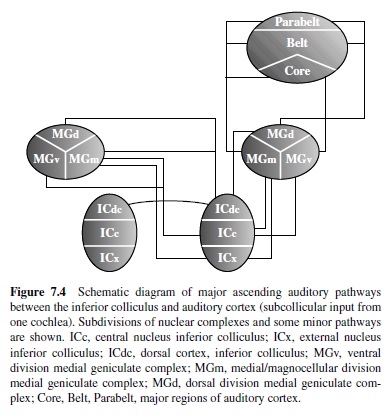
The IC is most commonly divided into three subnuclei: central (ICc), external (ICx), and pericentral (ICp) or dorsal cortex (ICdc). Ipsilateral and contralateral inputs to the ICc originate in the cochlear nuclei, SOC, and lateral lemniscus, as described earlier. Most major projections to the ICc are tonotopically organized. Within the ICc neurons are narrowly tuned and topographically arranged by CF (Aitkin, Webster, Veale, & Crosby, 1975; Fitzpatrick, 1975; Merzenich & Reid, 1974; Rose, Greenwood, Goldberg, & Hind, 1963; Webster, Serviere, Crewther, & Crewther, 1984). Manley and MullerPreuss (1981) studied single-unit responses to tones, clicks, white noise, and species-specific vocalizations in the squirrel monkey IC. Most of the neurons sampled (>90%) were responsive to all classes of stimuli. One review of the literature (Irvine, 1986) indicated that about 75% of the units in the ICc are binaural (types EE and EI) and sensitive to interaural differences in level and phase. By comparison, tuning curves in the ICdc and ICx are broad and variable in shape (Aitkin et al., 1975; Merzenich & Reid, 1974) and tend to be more sensitive to complex sounds than pure tones. Some units in the ICx respond to both auditory and somatic stimulation (Aitkin, Dickhaus, Schulz, & Zimmerman, 1978). Experiments in the barn owl (Knudsen & Konishi, 1978a, 1978b) and guinea pig (Binns, Grant, Withington, & King, 1992) have shown that the auditory midbrain contains a representation of auditory space that is computed from the map of stimulus frequency. In the ICx spatial cues are combined across frequency channels and transformed into a topographic code of auditory space where neurons are tuned for location instead of frequency (for reviews, see Cohen & Knudsen, 1999; Knudsen, du Lac, & Esterly, 1987). As shown in Figure 7.5, the output of these neurons targets the superior colliculus, where there is a coregistration of auditory and visual space (e.g., Hyde & Knudsen, 2000; King & Palmer, 1983). Descending inputs to the IC target the dorsomedial and pericentral regions. The ventrolateral ICc is virtually devoid of descending inputs; thus the ascending and descending tracks in the IC are largely segregated (see Huffman & Henson, 1990; Spangler & Warr, 1991). The major ascending projections of the IC target the MGC bilaterally. Other outputs target the superior colliculi, reticular formation, periaqueductal gray, contralateral IC, and lower auditory nuclei.
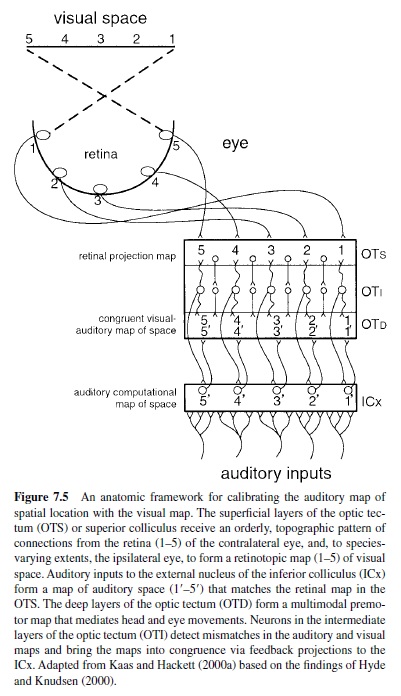
The integration of inputs is a key feature of signal processing in the IC, reflected in the wide variety of response patterns among its neurons. Patterns vary with the frequency, intensity, temporal, and binaural characteristics of the sound and reflect a broad range of acoustic features. In addition to stimulus frequency some of these features, including latency, response threshold, tuning bandwidth, and best azimuth, appear to be arranged topographically in distinct maps in the ICc (Aitkin, Pettigrew, Calford, Phillips, & Wise, 1985; Schreiner & Langner, 1988; Stiebler, 1986). Thus, different acoustic stimuli would be expected to activate unique spatial domains in each feature map, and the resulting pattern of activation would constitute an abstraction of the original stimulus. At higher levels of processing the integration of coactivation patterns across maps could underlie the formation of feature combination maps (see Kohonen & Hari, 1999; Suga, 1988).
Medial Geniculate Complex
The MGC is the final stage of subcortical processing of ascending auditory information. The primary input to the MGC arises bilaterally from the IC (Figure 7.4). Outputs target primary and nonprimary auditory cortical fields. In primates the MGC is commonly divided into three or four divisions: ventral (MGv), dorsal (MGd; anterodorsal, MGad; posterodorsal, MGpd), and magnocellular or medial (MGm; Burton & Jones, 1976; Fitzpatrick & Imig, 1978; Jordan, 1973). These divisions are distinguished on the bases of their unique architecture, patterns of cortical and subcortical connections, and neurophysiological properties. In addition to the MGC, the connection patterns of the suprageniculate nucleus (Sg), posterior nucleus (PO), and medial pulvinar (PM) indicate that these nuclei, among others, also play a role in thalamic auditory processing, although their significance is more uncertain. The functional organization of the MGC incorporates multiple parallel pathways in which distinct aspects of auditory processing appear to be mediated (Andersen, Knight, & Merzenich, 1980; Calford & Aitkin, 1983; Morest, 1965).
The principal target of the primary ascending pathway through the ICc is the MGv. Neurons in the MGv are arranged in distinct laminae corresponding to the tonotopic organization of the MGv and ICc (Calford & Aitkin, 1983; Morest, 1965).The input from the ipsilateral ICc is much stronger than the contralateral projection (monkeys: Hackett, Neagu, et al., 1999; cats: Andersen, Roth, Aitkin, & Merzenich, 1980; Rouiller & de Ribaupierre, 1985). In primates the thalamocortical projections of the MGv target areas in the primary auditory region, known as the core, while the surrounding narrow belt of cortex adjacent to the core has few connections with the MGv (see Hackett, Stepniewska, & Kaas, 1998b, for review). The MGad and MGpd receive inputs from the ICdc (Andersen, Roth, et al., 1980; Calford &Aitkin, 1983; Kudo & Niimi, 1980) and project primarily to the belt and parabelt regions surrounding the core (Molinari et al., 1995; Morel, Garraghty, & Kaas, 1993). The MGm receives projections from the ICc and ICx (Calford & Aitkin, 1983; Kudo & Niimi, 1980). In contrast to the ventral and dorsal divisions of the MGC, the MGm projects diffusely to the core, belt, and parabelt fields of the primate auditory cortex (see Hackett, Stepniewska, et al., 1998b). The heterogeneous cell populations of the MGm project to the supragranular layers of cortex (Oliver, 1984), and there is some evidence that different classes of neurons project to different cortical layers (Hashikawa, Molinari, Rausell, & Jones, 1995; Molinari et al., 1995).
Neurons in the MGv are tonotopically arranged in distinct laminae (Gross, Lifschitz, & Anderson, 1974). Tuning curve configurations vary widely among MGv cells (Morel, Rouiller, de Ribaupierre, & de Ribaupierre, 1987) and with anesthetic conditions (Allon, Yeshurun, & Wollberg, 1981; see also Rouiller, 1997), thus a range of response types has been observed. Symmes, Alexander, and Newman (1980) found that units in the squirrel monkey MGv were responsive to tones, clicks, white noise, and species-specific vocalizations. Neurons were most responsive to vocalizations that contained significant spectral energy at the CF of the unit, but neurons were generally not selective for particular vocal stimuli. The majority of cells are binaural (EE or EI) and are sensitive to interaural differences in intensity or time (Heierli, de Ribaupierre, & de Ribaupierre, 1987; Imig & Adrian, 1977; Ivarsson, de Ribaupierre, & de Ribaupierre, 1988), but there is no evidence for a map of auditory space in the MGC. As in the ICc, response features such as stimulus frequency, tuning bandwidth, latency, and binaural class are distributed as gradients within isofrequency laminae in the MGv (Rodrigues-Dagaeff et al., 1989). Thus, the MGv appears capable of simultaneous processing of complex signals. In the MGm neuron response properties vary widely, and patterns of organization are difficult to identify. There is some evidence of tonotopic organization rostrally (Rouiller et al., 1989), but tuning curves are often broad or multipeaked, and response latencies are highly variable (Aitkin, 1973; Gross et al., 1974; Symmes et al., 1980). Some units in the MGm respond also to vestibular and somatic stimulation (Blum, Abraham, & Gilman, 1979; Curry, 1972; Love & Scott, 1969; Wepsic, 1966), reflecting connections with multisensory nuclei such as the ICx; however, it is unclear how these inputs influence the processing of auditory information. The dorsal nuclei of the MG have not been found to be tonotopically organized. Most units are broadly tuned and do not respond well to simple acoustic stimuli like pure tones (Calford & Aitkin, 1983; Toros-Morel, de Ribaupierre, & Rouiller, 1981), and many units respond selectively to complex sounds (e.g., Buchwald, Dickerson, Harrison, & Hinman, 1988).
Serial and Parallel Processing in Subcortical Pathways
Although it may be tempting to ascribe a specific function to individual nuclei, it is important to emphasize that in the SOC, like most other auditory structures, each nuclear subdivision contains a variety of cell types that contribute to major and minor pathways. Most participate in diverse circuits involving feed-forward and feedback connections linking multiple levels of processing. Thus, the full scope of auditory processing mediated by any given nucleus is indefinite because comprehensive descriptions are lacking.
The picture of auditory processing that emerges is that each major stage of hierarchical processing in the brain stem and thalamus initiates and integrates multiple segregated parallel pathways involving functionally distinct populations of neurons. These pathways are responsible for the distribution of specialized acoustic information to higher centers, including cortex, and to lower stages, including the cochlea. Thus, the modulation and integration of auditory input occurs in multiple pathways at all levels, indicating that the system is not strictly hierarchical. Although the unique anatomical and physiological features of these pathways support their segregation into functionally distinct subsystems, interpretations vary. Poljak (1926) proposed that the pathways originating in the ventral and dorsal cochlear nuclei may mediate processing related to auditory localization and discrimination, respectively. Subsequent anatomical and physiological studies were used to support the notion that separate pathways were specialized for the extraction of features important for sound localization and pattern recognition (see Evans, 1974).
Parallel channels were also included in subsequent proposals, but less emphasis was placed on their putative functional significance. Andersen, Knight, et al. (1980) identified two segregated pathways in their study of the thalamic connections with auditory cortex in cats: a cochleotopic system involving AI (auditory area 1), MGv, and ICc; and a diffuse system with uncertain cochleotopic organization involving AII (auditory area 2), medial geniculate divisions outside of the ventral division, and ICp. Calford andAitkin (1983) identified four tectothalamic pathways through the MGv, MGd, MGm, and Sg in cats. Their proposal included a core pathway through the ICc and MGv and a diffuse pathway involving the ICp and subdivisions of the MGC surrounding the MGv. Rouiller, Simm, Villa, de Ribaupierre, and de Ribaupierre (1991) organized the auditory pathways into three parallel channels: a tonotopic system involving the MGv, a nontonotopic/diffuse system involving the MGd, and a polysensory system involving the MGm. The various proposals share obvious anatomical and physiological similarities, but it remains unclear whether spatial and nonspatial auditory functions, for example, are mediated by separate parallel channels, as suggested by Poljak. Some clues may lie in the functional organization of the auditory cortex, described in the next section.
Cortical Auditory Processing
The organization of the auditory cortex in humans and nonhuman primates has received sporadic attention for more than 100 years (for reviews, seeAitkin, 1990; Hackett, 2002; Kaas & Hackett, 1998; Newman, 1988; Woolsey & Walzl, 1982). Early anatomical and lesion studies were useful in identifying the location of the auditory cortex in the brain, and subsequent studies have refined and expanded certain details of its organization. Studies of auditory cortex in other mammals, however, have outpaced work in primates both in number and in scope. Consequently, more is known about the organization of auditory cortex in cats and bats, for example, than in any primate species. Fortunately, interest in primate auditory cortex is rising, and considerable progress has been made in recent years. In the following sections we present what is currently known about the functional organization of the primate auditory cortex and relate these details to more general issues of auditory processing in the brain.
Anatomical Organization
In humans and nonhuman primates the auditory cortex occupies a large portion of the superior temporal region in which a network of interconnected fields processes information in parallel three or more serial stages. Corticocortical connections of these areas include auditory-related fields in frontal, parietal, and temporal cortex, whereas corticofugal projections target numerous subcortical nuclei. Based on anatomical and physiological data from several primate species, including our own recent studies, we have developed a working model of auditory cortical processing in primates to provide a platform for more detailed investigation (Hackett, Stepniewska, & Kaas, 1998a; Hackett, Preuss, & Kaas, 2001; Kaas & Hackett, 1998; Kaas, Hackett, & Tramo, 1999). In the schematic diagram of this model (Figure 7.6) auditory cortex contains three hierarchically arranged regions: core, belt, and parabelt. The core region is comprised of two or three primary-like areas (AI, R, [RT]), each of which has independent parallel inputs from the MGv. The core is surrounded by a belt region of possibly seven or eight areas (CM, CL, ML, AL, RTL, RTM, RM) at a second level of processing with major inputs from the core, MGd, MGm, Sg, and uncertain inputs from the MGv. The lateral portion of the belt region is bordered by a parabelt region of at least two divisions (RP, CP) located on the exposed superior temporal gyrus. The parabelt receives direct projections from the belt, MGd, MGm, Sg, and PM, but not the core or MGv; thus, it represents a third stage of auditory cortical processing. The patterns of thalamocortical and corticocortical connections suggest that areas within a region may process information in parallel before output to a later stage. This parallel arrangement is not known to constrain activity to the simultaneous processing of identical information; rather, significant differences are likely given the topographically distinct patterns of cortical connections noted among areas at all levels.
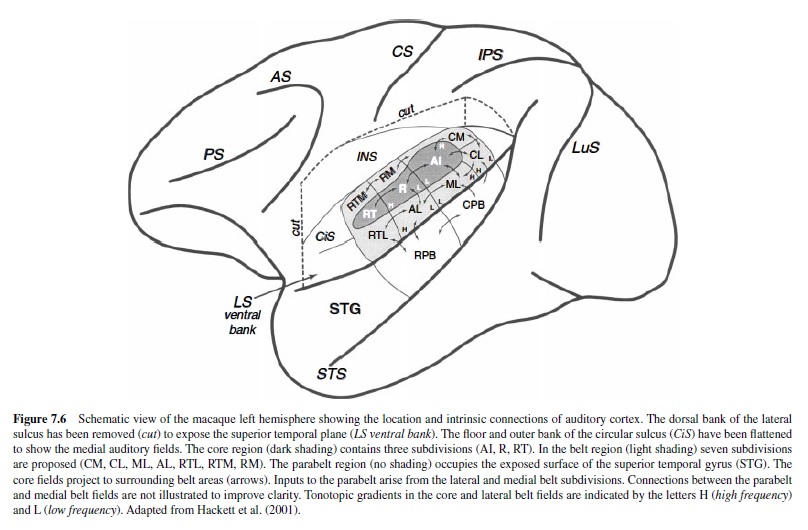
Adjacent areas within and between regions tend to share the densest connections (Figure 7.6). AI, for example, appears to have denser connections with R, CM, CL, and ML than with nonadjacent areas RT, RM,AL, RTM, and RTL. Similarly, the caudalbelt areas (CM,CL,ML) have stronger connections with areas in the caudal belt and parabelt (CPB) than with fields in the rostralbelt and parabelt (RPB;Hackettetal.,1998a). The topographical differences between the rostral and caudal auditory fields is maintained in their projections to other cortical regions, including the prefrontal cortex (Hackett,Stepniewska, & Kaas, 1999; Romanski, Bates, & Goldman-Rakic, 1999; Romanski, Tian, et al., 1999) and ventral intraparietal cortex (Lewis & Van Essen, 2000). The orderly topography of auditory cortical connections among functionally distinct cortical regions has been used to support the hypothesis that there are two streams of processing in primate auditory cortex: one devoted to spatial processing, the other involved with nonspatial auditory processing (Colombo, Rodman, & Gross, 1996; Hackett, Stepniewska, et al., 1999; Kaas & Hackett, 1998, 2000b; Rauschecker, 1998; Rauschecker, Tian, Pons, & Mishkin, 1997; Romanski, Bates, et al., 1999; Romanski, Tian, et al., 1999). This idea is analogous to the ventral “what” and dorsal “where” pathways of the visual system (Mishkin, Ungerleider, & Macko, 1983; Ungerleider & Mishkin, 1982). In the visual system these functionally distinct pathways originate in visual area 1 (VI) and form dual streams through ventral and dorsal visual cortical areas subserving visual object (what) and visual spatial (where) processing, respectively. The evidence for the segregation of auditory function into dual processing streams is discussed later.
Functional Organization
Microelectrode mapping studies of auditory cortex have focused primarily on the tonotopic organization of the core and belt regions (Aitkin, Merzenich, Irvine, Clarey, & Nelson, 1986; Brugge, 1982; Imig, Ruggero, Kitzes, Javel, & Brugge, 1977; Kosaki, Hashikawa, He, & Jones, 1997; Luethke et al., 1989; Merzenich & Brugge, 1973; Morel & Kaas, 1992; Morel et al., 1993; Pfingst & O’Connor, 1981; Rauschecker,Tian, & Hauser, 1995; Rauschecker et al., 1997; Recanzone, Guard, & Phan, 2000; Tian, Reser, Durham, Kustov, & Rauschecker, 2001). Each of the core areas is tonotopically organized, and the tuning curves of single neurons tend to be narrow, especially when compared with units in adjacent belt fields. In AI, neurons with higher CFs are located caudomedially in curved isofrequency lines, and lower frequencies are represented rostrolaterally (Figure 7.6). At the border with R the tonotopic gradient is reversed such that AI and R share a low CF border. The tonotopic organization of RT is not clear, but another reversal in the CF gradient may be present at the border of R and RT(Morel & Kaas, 1992). Mapping in the belt areas surrounding the core has also produced evidence of tonotopic organization. Although less responsive to pure tones, neurons in the belt can also be driven by noise bands (e.g., 13 octave, 12 octave) with a defined center frequency. Experiments using tones and narrow bands of noise have shown that the tonotopic gradient in a given belt area is parallel to that of the adjacent core area (Figure 7.6). Thus, tonotopic organization represents an underlying functional property that is maintained in the auditory cortex through the second major stage of processing in the belt.
Other aspects of functional organization have also been explored in the core and belt regions using stimuli other than pure tones and noise bands. Many of these findings have been interpreted as relevant to the processing of either spatial or nonspatial auditory information, as neurons in some areas are more responsive to certain classes of acoustic stimuli than to others. Accordingly, these findings relate in various ways to the dual-streams hypothesis pertaining to functional segregation in auditory cortex.
Nonspatial Processing
Primates produce a repertoire of species-specific calls that, for at least some species, may refer to objects or events in the environment and may thus convey “what” information about food, predators, social relationships, the caller’s identity, and the caller’s emotional state (Ghazanfar & Hauser, 1999). Calls produced by other animals and a wide range of environmental sounds are also likely to provide useful “what” information. For these sounds to be meaningful, they must ultimately beassociated with a specific entity or event. However, because the acoustic structure of a given auditory object (e.g., a “grunt” call) varies significantly within and between sources, the auditory system must be able to extract and make use of the invariant acoustic cues that convey meaning (see Beecher, Petersen, Zoloth, Moody, & Stebbins, 1979; Green, 1975; May, Moody, & Stebbins, 1989; Wang, 2000; Zoloth et al., 1979). The dynamic nature of the natural acoustic environment suggests that both hardwired and plastic mechanisms contribute to this process in development and throughout life.
Attempts to understand these mechanisms have produced a wide range of findings. Ablation studies of primate auditory cortex indicate that lesions of auditory cortex disrupt auditory pattern discrimination. Animals with unilateral or bilateral ablation of core, belt, or parabelt regions exhibit problems discriminating between sounds ranging in complexity from pure tones to vocalizations (Colombo et al., 1996; Cowey & Dewson, 1972; Cowey & Weiskrantz, 1976; Dewson, Pribram, & Lynch, 1969; Dewson, Cowey, & Weiskrantz, 1970; Heffner & Heffner, 1984, 1986; Hupfer, Jurgens, & Ploog, 1977; Iversen & Mishkin, 1973; Jerison & Neff, 1953; Massopust, Wolin, Meder, & Frost, 1967; Massopust, Wolin, & Frost, 1970; Pratt & Iversen, 1978; Symmes, 1966; Wegener, 1976), although they are still able to detect an auditory stimulus. Microelectrode recordings within the core region have revealed neurons responsive to a similar broad range of acoustic stimuli, including species-specific calls (Funkenstein & Winter, 1973; Glass & Wollberg, 1983; Lu & Wang, 2000; Manley & Muller-Preuss, 1978; Newman, 1978a, 1978b; Newman & Symmes, 1979; Newman & Wollberg, 1973a, 1973b; Pelleg-Toiba & Wollberg, 1991; Wang, Merzenich, Beitel, & Schreiner, 1995; Winter & Funkenstein, 1973; Wollberg & Newman, 1972). Because many neurons in the core were found to be responsive to vocalizations, it was initially proposed that these neurons may also be selective for a particular vocalization (i.e., “call detectors”), but this hypothesis was not supported by subsequent studies. Based on the wide range of response types encountered in AI, for example, Newman (1978b, 1979) classified neurons into seven categories: (a) tuned filters, responsive to stimuli restricted to a particular bandwidth; (b) specialists, responsive only to a single vocalization; (c) class detectors, responsive to all tonal or atonal calls, but not both; (d) complex-feature detectors, responsive to tonal, atonal, and mixed vocalizations; (e) generalists, responsive to all vocalizations and possibly responsive to tones or noise; (f) amplitude modulation (AM) detectors, responsive to most stimuli and with a response pattern that matches the temporal pattern of the stimulus; and (g) variant detectors, discriminately responsive to variants within a class of vocalizations. The specialists and class detectors represented 33% and 20% of the sampled population, respectively. In later studies (Glass & Wollberg, 1983; Pelleg-Toiba & Wollberg, 1991; Wang et al., 1995), however, most AI neurons were found to be equally responsive to vocalizations presented normally and in reverse temporal order. These results suggest that only subpopulations of neurons at the first stage of cortical processing in the core function as call detectors in the representation of complex vocalizations. Their contribution is augmented by the activity of synchronized cell assemblies that are spatially distributed along and across the tonotopic axis, as described in marmoset monkeys by Wang et al. (1995). Their findings suggest that the spectral and temporal discharge pattern of a large population of AI neurons forms an abstract representationoftheacousticpatternofthevocalization.Although abrupt changes in complex waveforms can be followed for some stimuli (Bieser & Muller-Preuss, 1996; Steinschneider, Arezzo, & Vaughan, 1980; Steinschneider, Reser, Fishman, Schroder,&Arezzo,1998;Steinschneider,Reser,Schroeder,& Arezzo, 1995; Steinschneider, Schroeder, Arezzo, & Vaughan, 1995), precise replication of the spectrotemporal acoustic pattern is not preserved because few cortical neurons are able to follow rapid temporal changes faster than 20 ms to 30 ms (Lu & Wang, 2000). The collective findings indicate that a smaller population of neurons inAI is more selective to specific calls or callers and that a larger nonselective population is responsive to a wide range of sounds (Wang, 2000). The selective population could signal the detection of a specific call or caller, whereas the nonselective population processes and distributes detailed information about the sound (Suga, 1994).
Outside of the core, neuron response profiles are notably different, and greater selectivity has been observed. Symmes, Newman, and Alexander (1976) reported that neurons in cortex lateral toAI of squirrel monkeys were generally less responsive to acoustic stimulation, but the incidence of call-selective units was two to three times higher than in AI. This may be related to spectrotemporal integration by neurons in the belt areas, where tuning has been found to be broader (Kosaki et al., 1997; Merzenich & Brugge, 1973; Morel et al., 1993; Rauschecker et al., 1995, 1997; Recanzone, Guard, & Phan, 2000). Recording from fields in the lateral belt region of macaques, Rauschecker et al. (1995) reported that neurons were generally much more responsive to narrow bands of noise with a defined center frequency than to pure tones. Moreover, neurons were found to be cochleotopically arranged by best center frequency (BFc). On the basis of reversals in BFc, Rauschecker et al. identified three lateral belt areas (AL, ML, CL) adjacent to areas R,AI, and CM, respectively. The tonotopic gradient in each lateral belt field matched that of the adjacent core field (see Figure 7.6). The anterior (AL) and caudal (CL) fields of the lateral belt could also be distinguished by preference for frequency modulated (FM) sweep rates (Tian & Rauschecker, 1995). Neurons in AL responded better to lower sweep rates (approximately 10 kHz/s), whereas units in CL preferred higher rates (approximately 100 kHz/s). Most lateral belt neurons also responded better to species-specific vocalizations than to energy-matched tones or noise bands and preferred certain calls to others. Call preferences could often be predicted from the spectral composition of the call given the frequency response area (receptive field) of the neuron. In addition, temporal integration was found to characterize the responses of some neurons. For example, response strength was greater when both syllables of a two-syllable vocalization were presented in their correct temporal sequence than when either syllable was presented alone. Although response specificity for calls might be expected to increase as a result of spectral and temporal integration, most neurons in the lateral belt responded to several calls, and few responded exclusively to a single call or to all calls. Support for the dual streams hypothesis can be found in a recent study of the lateral belt fields (Tian et al., 2001). Neurons in AL were found to be more selective for a particular call than were neurons in CL, which are more selective for the spatial location (azimuth) of the call. However, neurons rarely responded to a single call. Further integration and greater specificity may be found at later stages of processing (e.g., parabelt, superior temporal sulcus, insula) or among networks of neurons in one or more areas.
In the multimodal insular cortex of squirrel monkeys, neurons respond to simple and complex auditory stimuli, including species-specific vocalizations (Bieser, 1998; Bieser & Muller-Preuss, 1996; Pribram, Rosner, & Rosenblith, 1954; Sudakov, MacLean, Reeves, & Marino, 1971). There is some evidence for cochleotopic organization in the granular insula (Bieser & Muller-Preuss, 1996;). Neurons in AI and the insula exhibited phase-locked encoding of periodic FM stimuli (e.g., tones and twitter calls) at repetition rates up to about 16 Hz (Bieser, 1998). Using AM stimuli, the temporal resolution of AI neurons had a mean best modulation frequency of 17.8 Hz, compared to a mean of 9.9 Hz for insular neurons (Bieser & Muller-Preuss, 1996). These results suggest that complex sound integration in squirrel monkeys occurs in a time window of about 50 ms and that the temporal resolution of insular neurons is sufficient to encode the transient features of a complex call. Interestingly, in AI, R, and the insula, the phase-locked response to the natural call, which is comprised of both AM and FM elements, was better than the response to an FM stimulus alone. These results are comparable to human psychophysical data showing that mixed AM-FM modulated stimuli were easier to detect than were AM or FM stimuli alone, suggesting related mechanisms of detection (B. C. J. Moore & Sek, 1992; Sek & Moore, 1994). Overall, then, these findings indicate that insular neurons are involved in the processing of complex auditory stimuli. Further, as found for most mammals, these auditory cortical neurons appear best suited to process slow temporal modulations of simple and complex acoustic stimuli (see Langner, 1992).
Spatial Processing
Like most mammals, primates are able to localize sounds in space with high precision (C. H. Brown, Beecher, Moody, & Stebbins, 1978; C. H. Brown, Beecher, Moody, & Stebbins, 1980; C. H. Brown, Schessler, Moody, & Stebbins, 1982). In addition to the contribution of subcortical mechanisms of spatial encoding, numerous studies suggest that the auditory cortex plays a role in sound localization. Lesion studies have shown that bilateral (Heffner & Heffner, 1990; Heffner & Masterton, 1975; Ravizza & Diamond, 1974) and unilateral (Thompson & Cortez, 1983) ablation of the auditory cortex caused deficits in sound localization. Unilateral lesions cause greater deficits for sounds presented in the hemisphere contralateral to the lesion, whereas bilateral lesions have more global effects. The deficits observed across studies are somewhat task dependent, and lesions involved multiple fields; thus, the functional implications are uncertain, but it seems clear that lesions of auditory cortex tend to reduce spatial acuity and performance in tasks requiring accurate sound localization. Spatial discrimination of sounds near the midline may be less affected by a bilateral cortical lesion than sounds in the same hemifield further from midline (Heffner & Heffner, 1990).
In experiments using microelectrode recordings, Brugge and Merzenich (1973) found that cells in the core and belt regions of macaque monkeys were sensitive to interaural differences in time and intensity, and many units were most sensitive to a particular stimulus intensity or interaural delay. Recording from the caudal superior temporal gyrus in the area known as the temporoparietal area (Tpt), Leinonen, Hyvarinen, and Sovijarvi (1980) found neurons responsive to auditory, somatosensory, and visual stimuli. Unimodal and bimodal units were encountered for each modality. Most of the auditory responsive neurons in this area were selective for a particular azimuth of the sound source, typically in the contralateral hemifield. In a survey of 196 single units, Benson, Hienz, and Goldstein (1981) found that a majority of units sampled in cortex outside of the core were spatially tuned, but there was no correlation between tuning properties and the cortical field in which the neuron was isolated. More recently, however, the connections of auditory fields in the caudal belt and parabelt regions were correlated with prefrontal (see Hackett, Stepniewska, et al., 1999; Romanski, Bates, et al., 1999; Romanski, Tian, et al., 1999) and parietal (Lewis & Van Essen, 2000) areas involved in auditory and multimodal spatial processing. Such findings have generated renewed interest in the possibility that certain areas of primate auditory cortex are specialized for the processing of spatial information (for reviews, see Rauschecker & Tian, 2000; Recanzone, 2000; Tian et al., 2001). One of these areas is the caudomedial area, CM, adjacent to the caudal and medial borders of AI. Single- and multiple-unit recordings in CM have consistently reported that neurons in CM are generally broadly tuned and respond more variably to pure tones than to complex acoustic stimuli (Imig et al., 1977; Morel & Kaas, 1992; Kosaki et al., 1997; Merzenich & Brugge, 1973; Morel, Garraghty, & Kaas, 1993; Rauschecker et al., 1997; Recanzone, Guard, & Phan, 2000). In addition, Rauschecker et al., 1997 demonstrated that CM is dependent on inputs fromAI for responsiveness to tonal stimuli. In these experiments ablation of AI abolished tonal responses in CM, but not the adjacent core field. Rauschecker (1998) reported finding neurons tuned to specific spatial locations in CM, suggesting that CM may represent the beginning of the spatial pathway in auditory cortex. Support for this hypothesis can be found in a recent study correlating single-unit activity of neurons in AI and CM with sound localization in awake behaving macaque monkeys (Recanzone, Guard, Phan, & Su, 2000). The responses of about 80% of the neurons sampled in AI and CM were correlated with a particular azimuth or elevation (spatially sensitive), usually in the contralateral hemifield. On the psychophysical detection task performance was best for broadband stimuli, and compared with neurons inAI, a slightly greater percentage of neurons in CM were sensitive to the spatial location of both tone and noise stimuli. Further, neurons in AI and CM could predict behavioral thresholds for spatial location, but neurons in CM were generally better predictors of performance. The CM area is also unique among auditory belt fields for its bimodal response properties. Several studies have shown that neurons in CM are responsive to auditory and somatosensory stimulation (Fu et al., 2001; Robinson & Burton, 1980a, 1980b; Schroeder et al., 2001). The significance of bimodal convergence in CM with respect to its role in spatial localization is currently unclear as there is not yet evidence for a correspondence in spatial tuning among the auditory and somatosensory responsive neurons. One possibility is that such convergence would be useful in networks computing head and body position from somatosensory, auditory, visual, and vestibular inputs because neurons in this region have been shown to be responsive to vestibular stimulation (for review, see Gulden & Grusser, 1998). Another caudal auditory field has been shown to demonstrate spatial selectivity. Tian et al. (2001) found a clear dissociation of auditory spatial tuning between neurons in the anterolateral (AL) and caudolateral (CL) auditory belt fields. Neurons in CL were much more selective for spatial location of species-specific vocalizations than were neurons in AL, which responded equally to calls at any azimuth but were more selective for the type of call than were neurons in CL.
Auditory Processing in Prefrontal Cortex
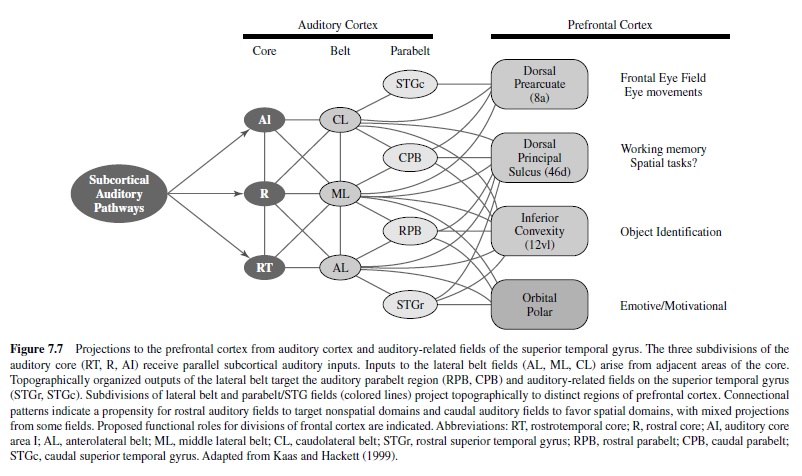
Anatomical and functional connections between nonprimary auditory cortex and the frontal lobe are well established in primates, dating back to some early studies of macaque monkeys (Hurst, 1959; Mettler, 1935; Sugar, French, & Chusid, 1948; Ward, Peden, & Sugar, 1946). Pandya and Kuypers (1969) and Pandya, Hallett, and Mukherjee (1969) subsequently showed that the rostral and caudal auditory regions projected to different domains of frontal cortex. The distinctive patterns of prefrontal projections from rostral and caudal auditory regions have since been elucidated in greater detail (see Hackett, Stepniewska, et al., 1999; Romanski, Bates, et al., 1999; Romanski, Tian, et al., 1999). Rostral belt and parabelt fields are most densely connected with orbitofrontal cortex and Areas 10, 12, 45, and rostral 46 within the inferior convexity and frontopolar cortex (Figure 7.7). By comparison, caudal belt and parabelt fields are primarily connected with the dorsolateral periprincipal region (e.g., caudal 46) and prearcuate cortex (e.g., Area 8). The rostrocaudal topography of auditory corticofrontal connections has been used to support the dual-streams hypothesis (Hackett, Stepniewska, et al., 1999a; Kaas & Hackett, 1998, 2000b; Rauschecker, 1998; Rauschecker et al., 1997; Romanski, Bates, et al., 1999; Romanski, Tian, et al., 1999). Rostral auditory fields target inferior, polar, and orbital prefrontal domains involved in auditory memory, discrimination, and language processing, whereas the caudal auditory fields target dorsolateral and periarcuate prefrontal areas involved in multimodal spatial tasks. The segregation of pathways is not complete, however, as there are connections between rostral and caudal auditory domains and notable overlapping projections from auditory cortex to the dorsal superior temporal sulcus and prefrontal Area 46 (principal sulcus region). Thus, there are numerous opportunities for interaction between streams and for other streams, as well (see Kaas & Hackett, 1999). Nevertheless, there is compelling support for the segregation of spatial and nonspatial processing in some form as revealed by anatomical and physiological studies of the frontal lobe.
Microelectrode recordings have uncovered unimodal and multimodal neurons responsive to auditory, visual, and somatic stimulation throughout the frontal lobe of monkeys (e.g., Tanila, Carlson, Linnankoski, & Kahila, 1993). Some but not all of these studies used methods and produced data relevant to the dual-streams hypothesis. Newman and Lindsley (1976) recorded from the periarcuate and periprincipalis regions in squirrel monkeys. They obtained responses in both regions to pure tones, clicks, and species-specific vocalizations, although a greater proportion of cells around the principal sulcus responded to vocalizations. Units responsive to clicks and tones were more evenly distributed between the two regions. Ito (1982) studied unit activity in the prearcuate and caudal principalis regions of macaque monkeys during auditory and visual reaction time tasks. Unimodal and bimodal neurons were found in both regions, but unimodal units tended to exhibit a phasic onset response, whereas a tonic response profile characterized most bimodal units. It was suggested that unimodal phasic units modulated activity of multimodal tonic units to initiate behaviors such as gaze control. Azuma and Suzuki (1984) and Suzuki (1985) recorded from neurons in the caudal principal sulcus and prearcuate regions of prefrontal cortex. The frontal eye field (FEF), associated with visual saccade initiation, is located within the prearcuate cortex. Most of the units they encountered responded maximally in the contralateral hemifield within an azimuthal range of less than 45 deg. Vaadia, Benson, Heinz, and Goldstein (1986) studied the responses of neurons in the periarcuate region of rhesus monkeys in passive and active localization tasks using auditory and visual stimulation. They found that it was more common for units to respond to both auditory and visual modalities in the active tasks. Further, during active localization many units were tuned to one or more spatial locations (i.e., 0, 30, 60, or 90 deg contralateral), whereas the same units exhibited little spatial tuning in the passive detection task. These findings suggest that location-encoding mechanisms in this region are not necessarily based on location-specific units, but on the coordinated activity of multiple neurons that have broad spatial tuning. In a subsequent report expanding on the former, Vaadia (1989) described a large population of bimodal periarcuate units with similar visual and auditory spatial tuning that were not responsive to passive auditory or visual stimulation but exhibited enhanced responses during an active localization task. Russo and Bruce (1994) found that neurons in the FEF that exhibited movement activity prior to saccades to a visual target were also active prior to saccades made to auditory targets, suggesting that targeting signals from both modalities converge at or before this level of processing (see also Hyde & Knudsen, 2000; Kaas & Hackett, 2000b; Schall, 1991; Schall, Morel, King, & Bullier, 1995).
Such results provide strong support for auditory and visual spatial processing in these prefrontal regions; however, unimodal and bimodal units active in passive and nonspatial tasks have been found within fields of the periprincipalis, prearcuate, postarcuate, and inferior convexity regions (Watanabe, 1992; Wollberg & Sela, 1980). Under passive listening conditions in awake squirrel monkeys, Wollberg and Sela (1980) found units responsive to clicks, species-specific vocalizations, and visual stimuli in pre- and postarcuate cortex. Vocalizations and light flashes were the most effective stimuli in generating a response. About 50% of the cells responded to only one or two vocalizations, and the researchers found just one cell responsive to all seven of the calls tested. Thus, there was some selectivity for particular calls. In a study of units in the periprincipal, prearcuate, postarcuate, and inferior prefrontal cortex, Watanabe (1992) studied the encoding of the associative significance (i.e., whether the stimulus was associated with a reward) of auditory and visual stimuli. Bimodal units were found in all four regions, but the highest proportions were found in postarcuate cortex anterior to primary motor cortex. The second highest proportion was found in prearcuate cortex. About one third of the bimodal units were sensitive to associative significance independent of the stimulus properties and were considered to be involved in cross-modal coding of this parameter. Although the paradigms used in both of these studies were not designed to test the spatial properties of auditory stimuli, the influence of spatial cues on neuronal responses cannot be ruled out because directional information was inherent in the acoustic stimuli. Indeed, the location of a species-specific vocalization is a behaviorally important component of the auditory percept that cannot be reliably ignored or discounted under those experimental conditions.
Other studies of rostral, inferior, and orbital prefrontal regions utilizing auditory stimuli are few in number, so little is known about auditory function in areas with connections favoring the rostral auditory fields of the temporal lobe. Benevento, Fallon, Davis, and Rezak (1977) reported that many units in the orbitofrontal cortex respond to auditory or visual stimuli. Over half of these were bimodal and often showed auditory-visual interactions. For example, presentation of an auditory stimulus resulted in inhibition of excitation produced by concurrent visual stimulation and vice versa. Subsequently, Thorpe, Rolls, and Maddison (1983) showed that the selectivity of bimodal neurons in the orbitofrontal cortex could be matched. A unit that responded selectively to the sight of a banana also could exhibit the same selectivity for the taste of the banana (i.e., cross-modal matching).All of these functional data, coupled with the distinctive connectional patterns described earlier, suggest that the inferior and orbital prefrontal cortex may play a role in the processing of nonspatial information involving several sensory modalities, including audition. However, a role in the processing of spatial-related information cannot be ruled out at this time.
Auditory Processing in Parietal Cortex
The posterior parietal cortex contains several functionally distinct areas that contribute to a multimodal representation of space used by motor structures to guide movements. One of these areas, the lateral intraparietal area (LIP), has connections with extrastriate visual cortex and projections to prefrontal and motor areas involved in movements of the eyes, including the frontal eye field (for review, see Andersen, Snyder, Bradley, & Xing, 1997). Connections between the caudal superior temporal gyrus and the LIP region have been known for many years (Divac, Lavail, Rakic, & Winston, 1977; Hyvarinen, 1982; Lewis & Van Essen, 2000; Pandya & Kuypers, 1969). Physiological investigations of LIP were not able to locate auditory responsive neurons (Hyvarinen, 1982; Koch & Fuster, 1989; Mountcastle et al., 1975). In more recent studies, however, auditory responsive neurons have been identified in macaque monkeys performing auditory and auditory-visual spatial tasks (Grunewald, Linden, & Andersen, 1999; Linden, Grunewald, & Andersen, 1999; Mazzoni, Bracewell, Barash, & Andersen, 1996; Stricanne, Andersen, & Mazzoni, 1996). Grunewald et al. (1999) demonstrated that auditory responses appear in LIPonly after training on spatial tasks requiring saccades to a remembered location after a variable delay. In a companion study, Linden et al. (1999) showed that neurons exhibited greater response strength during the memory-saccade task than during a simple fixation task. Thus, auditory responses in LIPappear to be dependent on both training and the behavioral task. These findings led the investigators to conclude that the auditory responses in LIP are supramodal (cognitive or motor) and not modality-specific sensory responses. Thus, it seems clear that the cortical processing of spatial-related auditory information involves circuitry linking the caudal auditory belt and parabelt fields, the lateral intraparietal area, and prefrontal cortex (Rauschecker & Tian, 2000). The extent to which these circuits constitute a “where” pathway for audition is the subject of ongoing investigations.
Bibliography:
- Adams, J. C., & Warr, W. B. (1976). Origins of axons in the cat’s acoustic striae determined by injection of horseradish peroxidase into severed tracts. Journal of Comparative Neurology, 170, 107–121.
- Aitkin, L. M. (1973). Medial geniculate body of cat: Responses to tonal stimuli of neurons in medial division. Journal of Neurophysiology, 36, 275–283.
- Aitkin, L. M. (1990). The auditory cortex: Structural and functional bases of auditory perception. London: Chapman and Hall.
- Aitkin, L. M., Anderson, D. J., & Brugge, J. F. (1970). Tonotopic organization and discharge characteristics of neurons in the nuclei of the lateral lemniscus. Journal of Neurophysiology, 33, 421–440.
- Aitkin, L. M., Dickhaus, H., Schulz, W., & Zimmermann, M. (1978). External nucleus of inferior colliculus: Auditory and spinal somatosensory afferents and their interactions. Journal of Neurophysiology, 41, 837–847.
- Aitkin, L. M., Merzenich, M. M., Irvine, D. R., Clarey, J. C., & Nelson, J. E. (1986). Frequency representation in auditory cortex of the common marmoset (Callithrix jacchus jacchus). Journal of Comparative Neurology, 252, 175–185.
- Aitkin, L. M., Pettigrew, J. D., Calford, M. B., Phillips, S. C., & Wise, L. Z. (1985). Representation of stimulus azimuth by lowfrequency neurons in inferior colliculus of the cat. Journal of Neurophysiology, 53, 43–59.
- Aitkin, L. M., Webster, W. R., Veale, J. L., & Crosby, D. C. (1975). Inferior colliculus: I. Comparison of response properties of neurons in central, pericentral, and external nuclei of adult cat. Journal of Neurophysiology, 38, 1196–1207.
- Allon, N., Yeshurun, Y., & Wollberg, Z. (1981). Responses of single cells in the medial geniculate body of awake squirrel monkeys. Experimental Brain Research, 41, 222–232.
- Alving, B. M., & Cowan, W. M. (1971). Some quantitative observations on the cochlear division of the eighth nerve in the squirrel monkey (Saimiri sciureus). Brain Research, 25, 229–239.
- Andersen, R. A., Knight, P. J., & Merzenich, M. M. (1980). The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: Evidence for two largely segregated systems of connections. Journal of Comparative Neurology, 194, 663–701.
- Andersen, R. A., Roth, G. L., Aitkin, L. M., & Merzenich, M. M. (1980). The efferent projections of the central nucleus and the pericentral nucleus of the inferior colliculus in the cat. Journal of Comparative Neurology, 194(3), 649–662.
- Andersen, R. A., Snyder, L. H., Bradley, D. C., & Xing, J. (1997). Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annual Review of Neuroscience, 20, 303–330.
- Azuma, M., & Suzuki, H. (1984). Properties and distribution of auditory neurons in the dorsolateral prefrontal cortex of the alert monkey. Brain Research, 90, 57–73.
- Bajo, V. M., Merchan, M. A., Lopez, D. E., & Rouiller, E. M. (1993). Neuronal morphology and efferent projections of the dorsal nucleus of the lateral lemniscus in the rat. Journal of Comparative Neurology, 334, 241–262.
- Barnes, W. T., Magoun, H. W., & Ranson, S. W. (1943). The ascending auditory pathway in the brain stem of the monkey. Journal of Comparative Neurology, 79, 129–152.
- Beecher, M. D., Petersen, M. R., Zoloth, S. R., Moody, D. B., & Stebbins, W. C. (1979). Perception of conspecific vocalizations by Japanese macaques. Brain Behavior and Evolution, 16, 443– 460.
- Benevento, L. A., Fallon, J. H., Davis, B. J., & Rezak, M. (1977). Auditory-visual interaction in single cells of the superior temporal sulcus and orbitofrontal cortex of the macaque monkey. Experimental Neurology, 57, 849–872.
- Benson, D. A., Hienz, R. D., & Goldstein, M. H., Jr. (1981). Singleunit activity in the auditory cortex of monkeys actively localizing sound sources: Spatial tuning and behavioral dependency. Brain Research, 219(2), 249–267.
- Bieser, A. (1998). Processing of twitter-call fundamental frequencies in insula and auditory cortex of squirrel monkeys. Experimental Brain Research, 122, 139–148.
- Bieser, A., & Muller-Preuss, P. (1996). Auditory responsive cortex in the squirrel monkey: Neural responses to amplitudemodulated sounds. Experimental Brain Research, 108, 273–284.
- Binns, K. E., Grant, S., Withington, D. J., & King, M. J. (1992). A topographic representation of auditory space in the external nucleus of the inferior colliculus of the guinea-pig. Brain Research, 589, 231–242.
- Blum, P. S., Abraham, L. D., & Gilman, S. (1979). Vestibular, auditory and somatic input to the posterior thalamus of the cat. Experimental Brain Research, 34, 1–9.
- Brown, C. H., Beecher, M. D., Moody, D. B., & Stebbins, W. C. (1978). Localization of primate calls by Old World monkeys. Science, 201(4357), 753–754.
- Brown, C. H., Beecher, M. D., Moody, D. B., & Stebbins, W. C. (1980). Localization of noise bands by Old World monkeys. Journal of the Acoustical Society of America, 68(1), 127–132.
- Brown, C. H., Schessler, T., Moody, D., & Stebbins, W. (1982). Vertical and horizontal sound localization in primates. Journal of the Acoustical Society of America, 72(6), 1804–1811.
- Brown, M. C., Nuttall, A. L., & Masta, R. I. (1983). Intracellular recordings from cochlear inner hair cells: Effects of stimulation of the crossed olivo-cochlear efferents. Science, 222, 69–71.
- Brownell, W. E., Bader, C. R., Bertrand, D., & de Ribaupierre, Y. (1985). Evoked mechanical responses of isolated cochlear outer hair cells. Science, 227, 194–197.
- Brugge, J. F. (1982). Auditory cortical areas in primates. In C. N. Woolsey (Ed.), Cortical sensory organization: Vol. 3. Multiple auditory areas (pp. 97–111). New York: Raven Press.
- Brugge, J. F., Andersen, D. J., & Aitkin, L. M. (1970). Responses of neurons in the dorsal nucleus of the lateral lemniscus of cat to binaural tonal stimulation. Journal of Neurophysiology, 33, 441–458.
- Brugge, J. F., & Merzenich, M. M. (1973). Responses of neurons in auditory cortex of the macaque monkey to monaural and binaural stimulation. Journal of Neurophysiology, 36, 1138–1158.
- Brunso-Bechtold, J. K., Thompson, G. C., & Masterton, R. B. (1981). HRP study of the organization of auditory afferents ascending to the central nucleus of the inferior colliculus in cat. Journal of Comparative Neurology, 197, 705–722.
- Buchwald, J., Dickerson, L., Harrison, J., & Hinman, C. (1988). Medial geniculate body responses to cat cries. In J. Syka & R. B. Masterton (Eds.), Auditory pathway, structure and function (pp. 319–322). New York: Plenum Press.
- Buno, W. (1978). Auditory nerve activity influenced by contralateral ear sound stimulation. Experimental Neurology, 59, 62–74.
- Burton, H., & Jones, E. G. (1976). The posterior thalamic region and its cortical projection in New World and Old World monkeys. Journal of Comparative Neurology, 168, 249–302.
- Caird, D., & Klinke, R. (1983). Processing of binaural stimuli by cat superior olivary complex neurons. Experimental Brain Research, 52, 385–399.
- Calford, M. B., & Aitkin, L. M. (1983). Ascending projections to the medial geniculate body of the cat: Evidence for multiple, parallel auditory pathways through thalamus. Journal of Neuroscience, 3, 2365–2380.
- Cohen, Y. E., & Knudsen, E. I. (1999). Maps versus clusters: Different representations of auditory space in the midbrain and forebrain. Trends in Neuroscience, 22, 128–135.
- Coleman, J. R., & Clerici, W. J. (1987). Sources of projections to subdivisions of the inferior colliculus in the rat. Journal of Comparative Neurology, 262, 215–226.
- Colombo, M., Rodman, H. R., & Gross, C. G. (1996). The effects of superior temporal cortex lesions on the processing and retention of auditory information in monkeys (Cebus apella). Journal of Neuroscience, 16, 4501–4517.
- Covey,E.,&Casseday,J.H.(1986).Connectionalbasisforfrequency representation in the nuclei of the lateral lemniscus of the bat, Eptesicus fuscus. Journal of Neuroscience, 6, 2926–2940.
- Cowey, A., & Dewson, J. H. (1972). Effects of unilateral ablation of superior temporal cortex on auditory sequence discrimination in Macaca mulatta. Neuropsychologia, 10, 279–289.
- Cowey, A., & Weiskrantz, L. (1976). Auditory sequence discrimination in Macaca mulatta: The role of the superior temporal cortex. Neuropsychologia, 14, 1–10.
- Curry, M. J. (1972). The exteroceptive properties of neurones in the somatic part of the posterior group (PO). Brain Research, 44, 439–462.
- Dewson, J. H., Cowey, A., & Weiskrantz, L. (1970). Disruptions of auditory sequence discrimination by unilateral and bilateral cortical ablations of superior temporal gyrus in the monkey. Experimental Neurology, 28, 529–548.
- Dewson, J. H., Pribram, K. H., & Lynch, J. C. (1969). Effects of ablations of temporal cortex upon speech sound discrimination in the monkey. Experimental Neurology, 24, 579–591.
- Divac, I., Lavail, J. H., Rakic, P., & Winston, K. R. (1997). Heterogeneous afferents to the inferior parietal lobule of the rhesus monkey revealed by the retrograde transport method. Brain Research, 197–207.
- Ehret, G. (1997). The auditory midbrain, a “shunting yard” of acoustical information processing. In G. Ehret & R. Romand (Eds.), The central auditory system (pp. 259–316). New York: Oxford University Press.
- Evans, E. F. (1974). Neural processes for the detection of acoustic patterns and for sound localization. In F. O. Schmitt & F. G Worden (Eds.), The neurosciences (pp. 131–145). Cambridge, MA: MIT Press.
- Fernandez, C., Butler, R., Konishi, T., & Honrubia, V. (1962). Cochlear potentials in the rhesus and squirrel monkey. Journal of the Acoustical Society of America, 34, 1411–1417.
- Fitzpatrick, K. A. (1975). Cellular architecture and topographic organization of the inferior colliculus of the squirrel monkey. Journal of Comparative Neurology, 164, 185–208.
- Fitzpatrick, K. A., & Imig, T. J. (1978). Projections of auditory cortex upon the thalamus and midbrain in the owl monkey. Journal of Comparative Neurology, 177, 537–556.
- Friauf, E., & Ostwald, J. (1988). Divergent projections of physiologically characterized rat ventral cochlear nucleus neurons as shown by intra-axonal injection of horseradish peroxidase. Experimental Brain Research, 73, 263–284.
- Fu, K. G., Johnston, T. A., Shah, A. S., Arnold, L., Smiley, J., Hackett, T. A., Garraghty, P. E., & Schroeder, C. E. (2001). Characterization of somatosensory input to auditory association cortex in macaques. Society for Neuroscience Abstracts, 27.
- Funkenstein, H. H., & Winter, P. (1973). Responses to acoustic stimuli of units in the auditory cortex of awake squirrel monkeys. Experimental Brain Research, 18, 464–488.
- Gacek, R. R., & Rasmussen, G. L. (1961). Fiber analysis of the statoacoustic nerve of guinea pig, cat, and monkey. Anatomical Record, 139, 455–463.
- Galambos, R. (1956). Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. Journal of Neurophysiology, 19, 424–437.
- Ghazanfar, A. A., & Hauser, M. C. (1999). The neuroethology of primate vocal communication: Substrates for the evolution of speech. Trends in Cognitive Sciences, 3, 377–384.
- Glass, I., & Wollberg, Z. (1983). Responses to cells in the auditory cortex of awake squirrel monkeys to normal and reversed species-specific vocalizations. Hearing Research, 9, 27–33.
- Glendenning, K. K., Baker, B. N., Hutson, K. A., & Masterton, R. B. (1992). Acoustic chiasm V: Inhibition and excitation in the ipsilateral and contralateral projections of LSO. Journal of Comparative Neurology, 318, 100–122.
- Glendenning, K. K., Brunso-Bechtold, J. K., Thompson, G. C., & Masterton, R. B. (1981). Ascending auditory afferents to the nuclei of the lateral lemniscus. Journal of Comparative Neurology, 197, 673–703.
- Glendenning, K. K., & Hutson, K. A. (1998). Lack of topography in the ventral nucleus of the lateral lemniscus. Microscopy Research and Technique, 41(4), 298–312.
- Glendenning, K. K., & Masterton, R. B. (1983). Acoustic chiasm: Efferent projections to the lateral superior olive. Journal of Neuroscience, 3, 1521–1537.
- Goldberg, R. B., & Moore, R. Y. (1967). Ascending projections of the lateral lemniscus in the cat and monkey. Journal of Comparative Neurology, 129, 143–156.
- Green, S. (1975). Auditory sensitivity and equal loudness in the squirrel monkey (Saimiri sciureus). Journal of the Experimental Analysis of Behavior, 23, 255–264.
- Gross, N. B., Lifschitz, W. S., & Anderson, D. J. (1974). The tonotopic organization of the auditory thalamus of the squirrel monkey (Saimiri sciureus). Brain Research, 65, 323–332.
- Grunewald, A., Linden, J. F., & Andersen, R. A. (1999). Responses to auditory stimuli in macaque lateral intraparietal area: I. Effects of training. Journal of Neurophysiology, 82, 330–342.
- Guinan, J. J., & Gifford, M. L. (1988). Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers: III. Tuning curves and thresholds at CF. Hearing Research, 37, 29–46.
- Guinan, J. J., Guinan, S. S., & Norris, B. E. (1972). Single auditory units in the superior olivary complex: I. Responses to sound and classifications based on physiological properties. International Journal of Neuroscience, 4, 101–120.
- Guinan, J. J., Norris, B. E., & Guinan, S. S. (1972). Single auditory units in the superior olivary complex: II. Locations of unit categories and tonotopical organization. International Journal of Neuroscience, 4, 147–166.
- Guinan, J. J., Warr, W. B., & Norris, B. E. (1983). Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. Journal of Comparative Neurology, 221, 358–370.
- Guinan, J. J., Warr, W. B., & Norris, B. E. (1984). Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. Journal of Comparative Neurology, 226, 21–27.
- Guldin, W. O., & Grusser, O. J. (1998). Is there a vestibular cortex? Trends in Neuroscience, 21, 254–259.
- Hackett, T. A. (2002). The comparative anatomy of the primate auditory cortex. In A. Ghazanfar (Ed.), Primate audition: Ethology and neurobiology (pp. 199–225). Boca Raton: CRC Press.
- Hackett, T. A., Neagu, T. A., & Kaas, J. H. (1999). Subcortical connections of the inferior colliculus in primates. Association for Research in Otolaryngology Abstracts, 22,
- Hackett, T. A., Preuss, T. M., & Kaas, J. H. (2001). Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. Journal of Comparative Neurology, 441, 197–222.
- Hackett, T. A., Stepniewska, I., & Kaas, J. H. (1998a). Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. Journal of Comparative Neurology, 394, 475–495.
- Hackett, T. A., Stepniewska, I., & Kaas, J. H. (1998b). Thalamocortical connections of parabelt auditory cortex in macaque monkeys. Journal of Comparative Neurology, 400, 271–286.
- Hackett, T. A., Stepniewska, I., & Kaas, J. H. (1999). Prefrontal connections of the auditory parabelt cortex in macaque monkeys. Brain Research, 817, 45–58.
- Harrison, J. M., & Irving, R. (1966). Visual and nonvisual auditory systems in mammals. Science, 154, 738–743.
- Hashikawa, T., Molinari, M., Rausell, E., & Jones, E. G. (1995). Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. Journal of Comparative Neurology, 362, 195–208.
- Heffner, H. E., & Heffner, R. S. (1984). Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science, 226, 75–76.
- Heffner, H. E., & Heffner, R. S. (1986). Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. Journal of Neurophysiology, 56, 683–701.
- Heffner, H. E., & Heffner, R. S. (1990). Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. Journal of Neurophysiology, 64(3), 915–931.
- Heffner, H., & Masterton, B. (1975). Contribution of auditory cortex to sound localization in the monkey (Macaca mulatta). Journal of Neurophysiology, 38, 1340–1358.
- Heierli, P., de Ribaupierre, F., & de Ribaupierre, Y. (1987). Functional properties and interactions of neuron pairs simultaneously recorded in the medial geniculate body of the cat. Hearing Research, 25, 209–225.
- Henkel, C. K., & Brunso-Bechtold, J. K. (1993). Laterality of superior olivary projections to the inferior colliculus in adult and developing ferret. Journal of Comparative Neurology, 331, 458–468.
- Henkel, C. K., & Spangler, K. M. (1983). Organization of the efferent projections of the medial superior olivary nucleus in the cat as revealed by HRP and autoradiographic tracing methods. Journal of Comparative Neurology, 221, 416–428.
- Huffman, R. F., & Henson, O. W. (1990). The descending auditory pathway and acousticomotor systems: Connections with the inferior colliculus. Brain Research Reviews, 15, 245–323.
- Hupfer, K., Jurgens, U., & Ploog, D. (1977). The effect of superior temporal lesions on the recognition of species specific calls in the squirrel monkey. Experimental Brain Research, 30, 75–87.
- Hurst, E. M. (1959). Some cortical association systems related to auditory functions. Journal of Comparative Neurology, 112, 103–119.
- Hutson, K. A., Glendenning, K. K., & Masterton, R. B. (1991). Acoustic chiasm IV: Eight midbrain decussations of the auditory system in the cat. Journal of Comparative Neurology, 312, 105–131.
- Hyde, P. S., & Knudsen, E. I. (2000). Topographic projection from the optic tectum to the auditory space map in the inferior colliculus of the barn owl. Journal of Comparative Neurology, 421, 146–160.
- Hyvarinen, J. (1982). Posterior parietal lobe of the primate brain. Physiological Reviews, 62, 1060–1129.
- Imig, T. J., & Adrian, H. O. (1977). Binaural columns in the primary field (AI) of cat auditory cortex. Brain Research, 138, 241–257.
- Imig, T. J, Ruggero, M. A., Kitzes, L. M., Javel, E., & Brugge, J. F. (1977). Organization of auditory cortex in the owl monkey (Aotus trivirgatus). Journal of Comparative Neurology, 171, 111–128.
- Irvine, D. R. F. (1986). The auditory brainstem. In D. Ottoson (Ed.), Progress in sensory physiology: Vol. 7 (pp. 1–279). Berlin: Springer-Verlag.
- Irving, R., & Harrison, J. M. (1967). The superior olivary complex and audition: A comparative study. Journal of Comparative Neurology, 130, 77–86.
- Ito, S.-I. (1982). Prefrontal unit activity of macaque monkeys during auditory and visual reaction time tasks. Brain Research, 247, 39–47.
- Ivarsson, C., de Ribaupierre,Y., & de Ribaupierre, F. (1988). Influence of auditory localization cues on neuronal activity in the auditory thalamus of the cat. Journal of Neurophysiology, 59, 586–606.
- Iversen, S. D., & Mishkin, M. (1973). Comparison of superior temporal and inferior prefrontal lesions on auditory and nonauditory tasks in rhesus monkeys. Brain Research, 55, 355–367.
- Jerison, H. J., & Neff, W. D. (1953). Effect of cortical ablation in the monkey on discrimination of auditory patterns. Federation Proceedings, 12, 73–74.
- Jordan, H. (1973). The structure of the medial geniculate nucleus (MGN): A cyto- and myeloarchitectonic study in the squirrel monkey. Journal of Comparative Neurology, 148, 469–480.
- Kaas, J. H. (1993). Evolution of multiple areas and modules within neocortex. Perspectives on Developmental Neurobiology, 1, 101–107.
- Kaas, J. H. (2000). Why is brain size so important? Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind, 1, 7–23.
- Kaas, J. H., & Hackett, T. A. (1998). Subdivisions and levels of processing in primate auditory cortex. Audiology and Neurootolology, 3, 73–85.
- Kaas, J. H., & Hackett, T. A. (1999). “What” and “where” processing in auditory cortex. Nature Neuroscience, 2(12), 1045–1047.
- Kaas, J. H., & Hackett, T. A. (2000a). How the visual projection map instructs the auditory computational map. Journal of Comparative Neurology, 421, 143–145.
- Kaas, J. H., & Hackett, T. A. (2000b). Subdivisions of auditory cortex and processing streams in primates. Proceedings of the National Academy of Science, USA, 97(22), 11793–11799.
- Kaas, J. H., Hackett, T. A., & Tramo, M. J. (1999). Auditory processing in primate cerebral cortex. Current Opinion in Neurobiology, 9(2), 164–170.
- Kawase, T., Delgutte, B., & Liberman, M. C. (1993). Antimasking effects of the olivocochlear reflex: II. Enhancement of auditorynerve response to masked tones. Journal of Neurophysiology, 70, 2533–2549.
- Kawase, T., & Liberman, M. C. (1993). Antimasking effects of the olivocochlear reflex: I. Enhancement of compound action potentials to masked tones. Journal of Neurophysiology, 70, 2519–2532.
- Kelly, J. B., & Li, L. (1997). Two sources of inhibition affecting binaural evoked responses in the rat’s inferior colliculus: The dorsal nucleus of the lateral lemniscus and the superior olivary complex. Hearing Research, 104, 112–126.
- King, A. J., & Palmer, A. R. (1983). Cells responsive to free-field auditory stimuli in guinea-pig superior colliculus: Distribution and response properties. Journal of Physiology, 342, 361–381.
- Knudsen, E. I., du Lac, S., & Esterly, S. D. (1987). Computational maps in the brain. Annual Review of Neuroscience, 10, 41–65.
- Knudsen, E. I., & Konishi, M. (1978a). Center-surround organization of auditory receptive fields in the owl. Science, 202, 778–780.
- Knudsen, E. I., & Konishi, M. (1978b). Space and frequency are represented separately in auditory midbrain. Journal of Neurophysiology, 41, 870–884.
- Koch, K. W., & Fuster, J. M. (1989). Unit activity in monkey parietal cortex related to haptic perception and temporary memory. Experimental Brain Research, 76, 292–306.
- Kohonen, T., & Hari, R. (1999). Where the abstract feature maps of the brain might come from. Trends in Neuroscience, 22, 135–139.
- Kosaki, H., Hashikawa, T., He, J., & Jones, E. G. (1997). Tonotopic organization of auditory cortical fields delineated by parvalbumin immunoreactivity in macaque monkeys. Journal of Comparative Neurology, 386, 304–316.
- Kudo, M. (1981). Projections of the nuclei of the lateral lemniscus in the cat: An autoradiographic study. Brain Research, 221, 57–71.
- Kudo, M., & Niimi, K. (1980). Ascending projections of the inferior colliculus in the cat: An autoradiographic study. Journal of Comparative Neurology, 191, 545–556.
- Langner, G. (1992). Periodicity coding in the auditory system. Hearing Research, 60, 115–142.
- Leinonen, L., Hyvarinen, J., & Sovijarvi, A. R. (1980). Functional properties of neurons in the temporo-parietal association cortex of awake monkey. Experimental Brain Research, 39, 203–215.
- Lewis, J. W., & Van Essen, D. C. (2000). Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. Journal of Comparative Neurology, 428, 112–137.
- Liberman, M. C. (1989). Rapid assessment of sound-evoked olivocochlear feedback: Suppression of compound action potentials by contralateral sound. Hearing Research, 38, 47–57.
- Linden, J. F., Grunewald, A., & Andersen, R. A. (1999). Responses to auditory stimuli in macaque lateral intraparietal area: II. Behavioral modulation. Journal of Neurophysiology, 82, 343–358.
- Love, J. A., & Scott, J. W. (1969). Some response characteristics of cells of the magnocellular division of the medial geniculate body of the cat. Canadian Journal of Physiology and Pharmacology, 47, 881–888.
- Lu, T., & Wang, X. (2000). Temporal discharge patterns evoked by rapid sequences of wide- and narrowband clicks in the primary auditory cortex of cat. Journal of Neurophysiology, 84, 236–246.
- Luethke, L. E., Krubitzer, L. A., & Kaas, J. H. (1989). Connections of primary auditory cortex in the New World monkey, Journal of Comparative Neurology, 285, 487–513.
- Manley, J. A., & Muller-Preuss, P. (1978). Response variability of auditory cortex cells in the squirrel monkey to constant acoustic stimuli. Experimental Brain Research, 32, 171–180.
- Manley, J. A., & Muller-Preuss, P. A. (1981). A comparison of the responses evoked by artificial stimuli and vocalizations in the inferior colliculus of squirrel monkeys. In J. Syka & L. Aitkin (Eds.), Neuronal mechanisms of hearing (pp. 307–310). New York: Plenum Press.
- Markovitz, N. S., & Pollak, G. D. (1993). The dorsal nucleus of the lateral lemniscus in the mustache bat: monaural properties. Hearing Research, 71, 51–63.
- Markovitz, N. S., & Pollak, G. D. (1994). Binaural processing in the dorsal nucleus of the lateral lemniscus. Hearing Research, 73, 121–140.
- Massopust, L. C., Jr., Wolin, L. R., & Frost, V. (1970). Increases in auditory middle frequency discrimination thresholds after cortical ablations. Experimental Neurology, 28, 299–307.
- Massopust, L. C., Jr., Wolin, L. R., Meder, R., & Frost, V. (1967). Changes in auditory frequency discrimination thresholds after temporal cortex ablations. Experimental Neurology, 19, 245– 255.
- May, B., Moody, D. B., & Stebbins, W. C. (1989). Categorical perception of conspecific communication sounds by Japanese macaques, Macaca fuscata. Journal of the Acoustical Society of America, 85(2), 837–847.
- Mazzoni, P., Bracewell, R. M., Barash, S., & Andersen, R. A. (1996). Spatially tuned auditory responses in area LIP of macaques performing delayed memory saccades to acoustic targets. Journal of Neurophysiology, 75, 1233–1241.
- Merchan, M. A., Saldana, E., & Plaza, I. (1994). Dorsal nucleus of the lateral lemniscus in the rat: Concentric organization and tonotopic projection to the inferior colliculus. Journal of Comparative Neurology, 342, 259–278.
- Merzenich, M. M., & Brugge, J. F. (1973). Representation of the cochlear partition on the superior temporal plane of the macaque monkey. Brain Research, 50, 275–296.
- Merzenich, M. M., & Reid, M. D. (1974). Representation of the cochlea within the inferior colliculus of the cat. Brain Research, 77, 397–415.
- Mettler, F. A. (1935). Corticofugal fiber connections of the cortex of Macaca mulatta: The temporal region. Journal of Comparative Neurology, 61, 25–47.
- Mishkin, M., Ungerleider, L. G., & Macko, K. A. (1983). Object vision and spatial vision: Two cortical pathways. Trends in Neuroscience, 6, 414–417.
- Molinari, M., Dell’Anna, M. E., Rausell, E., Leggio, M. G., Hashikawa, T., & Jones, E. G. (1995). Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. Journal of Comparative Neurology, 362, 171–194.
- Moore, B. C. J., & Sek, A. (1992). Detection of combined frequency and amplitude modulation. Journal of the Acoustical Society of America, 92, 3119–3131.
- Moore, J. K. (1980). The primate cochlear nuclei: Loss of lamination as a phylogenetic process. Journal of Comparative Neurology, 193, 609–629.
- Moore, J. K. (2000). Organization of the human superior olivary complex. Microscopy Research and Technique, 51, 403–412.
- Moore, J. K., & Moore, R. Y. (1971). Acomparative study of the superior olivary complex in the primate brain. Folia Primatologia, 16, 35–51.
- Morel, A., Garraghty, P. E., & Kaas, J. H. (1993). Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. Journal of Comparative Neurology, 335, 437–459.
- Morel, A., & Kaas, J. H. (1992). Subdivisions and connections of auditory cortex in owl monkeys. Journal of Comparative Neurology, 318, 27–63.
- Morel, A., Rouiller, E., de Ribaupierre, Y., & de Ribaupierre, F. (1987). Tonotopic organization in the medial geniculate body (MGB) of lightly anesthetized cats. Experimental Brain Research, 69, 24–42.
- Morest, D. K. (1965). The lateral tegmental system of the midbrain and the medial geniculate body: A study with Golgi and Nauta methods in cat. Journal of Anatomy, 99, 611–634.
- Moskowitz, N., & Liu, J.-C. (1972). Central projections of the spiral ganglion of the squirrel monkey. Journal of Comparative Neurology, 144, 335–344.
- Newman, J. D. (1988). Primate hearing mechanisms. In H. D. Steklis & J. Erwin (Eds.), Neurosciences: 4. Comparative primate biology (pp. 469–499). New York: Liss.
- Newman,J.D.(1978a).Detectionofbiologicallysignificantsoundsby single neurons. In D. Chivers & J. Herbert (Eds.), Recent advances in primatology (Vol. 1, pp. 755–762). London: Academic Press.
- Newman, J. D. (1978b). Perception of sounds used in speciesspecific communication: The auditory cortex and beyond. Journal of Medical Primatology, 7, 98–105.
- Newman, J. D. (1979). Central nervous system processing of sounds in primates. In H. Steklis & M. Raleigh (Eds.), Neurobiology of social communication in primates (pp. 69–109). New York: Academic Press.
- Newman, J. D., & Lindsley, D. F. (1976). Single unit analysis of auditory processing in squirrel monkey auditory cortex. Experimental Brain Research, 25, 169–181.
- Newman, J. D., & Symmes, D. (1979). Feature detection by single units in squirrel monkey auditory cortex. Experimental Brain Research Supplement, 2, 140–145.
- Newman, J. D., & Wollberg, Z. (1973a). Multiple coding of speciesspecific vocalizations in auditory cortex of squirrel monkeys. Brain Research, 54, 287–304.
- Newman, J. D., & Wollberg, Z. (1973b). Responses of single neurons in the auditory cortex of squirrel monkeys to variants of a single call type. Experimental Neurology, 40, 821–824.
- Oertel, D., & Wu, S. H. (1989). Morphology and physiology of cells in slice preparations of the dorsal cochlear nucleus of mice. Journal of Comparative Neurology, 283, 228–247.
- Oliver, D. L. (1984). Neuron types in the central nucleus of the inferior colliculus that project to the medial geniculate body. Neuroscience, 11, 409–424.
- Pandya, D. N., Hallett, M., & Mukherjee, S. K. (1969). Intra- and interhemispheric connections of the neocortical auditory system in the rhesus monkey. Brain Research, 14, 49–65.
- Pandya, D. N., & Kuypers, H. G. J. M. (1969). Cortico-cortical connections in the rhesus monkey. Brain Research, 13, 13–36.
- Pelleg-Toiba, R., & Wollberg, Z. (1991). Discrimination of communication calls in the squirrel monkey: “Call detectors” or “cell ensembles”? Journal of Basic Clinical Physiology and Pharmacology, 2, 257–272.
- Pfingst, B. E., & O’Connor, T. A. (1981). Characteristics of neurons in auditory cortex of monkeys performing a simple auditory task. Journal of Neurophysiology, 45, 16–34.
- Poljak, S. (1926). The connections of the acoustic nerve. Journal of Anatomy, 60, 465–469.
- Pollak, G. D. (1997). Roles of GABAergic inhibition for the binaural processing of multiple sound sources in the inferior colliculus. AnnalsofOtology,Rhinology,andLaryngology,168(Suppl.),44–54.
- Pratt, S. R., & Iversen, S. D. (1978). Selective cortical lesions and auditory behaviour in the monkey. In D. Chievers & J. Herbert (Eds.), Recent advances in primatology (pp. 807–809). London: Academic Press.
- Pribram, K. H., Rosner, B. S., & Rosenblith, W. A. (1954). Electrical responses to acoustic clicks in monkey: Extent of neocortex activated. Journal of Neurophysiology, 17, 336–344.
- Rajan, R. (1988a). Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity: I. Dependence on electrical stimulation parameters. Journal of Neurophysiology, 60, 549–568.
- Rajan, R. (1988b). Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity: II. Dependence on the level of temporary threshold shifts. Journal of Neurophysiology, 60, 569–579.
- Rajan, R. (2000). Centrifugal pathways protect hearing sensitivity at the cochlea in noisy environments that exacerbate the damage induced by loud sound. Journal of Neuroscience, 20, 6684–6693.
- Rajan, R., & Johnstone, B. M. (1988a). Binaural acoustic stimulation exercises protective effects at the cochlea that mimic the effects of electrical stimulation of an auditory efferent pathway. Brain Research, 459, 241–255.
- Rajan, R., & Johnstone, B. M. (1988b). Electrical stimulation of cochlear efferents at the round window reduces auditory desensitization in guinea pigs: I. Dependence on electrical stimulation parameters. Hearing Research, 36, 53–73.
- Rajan, R., & Johnstone, B. M. (1988c). Electrical stimulation of cochlear efferents at the round window reduces auditory desensitization in guinea pigs: II. Dependence on level of temporary threshold shifts. Hearing Research, 36, 75–88.
- Rajan, R., & Johnstone, B. M. (1989). Contralateral cochlear destruction mediates protection from monaural loud sound exposures through the crossed olivocochlear bundle. Hearing Research, 39, 263–277.
- Rasmussen, G. L. (1946). The olivary peduncle and other fiber connections of the superior olivary complex. Journal of Comparative Neurology, 84, 141–219.
- Rasmussen, G. L. (1953). Further observations on the efferent cochlear bundle. Journal of Comparative Neurology, 99, 61–74.
- Rauschecker, J. P. (1998). Parallel processing in the auditory cortex of primates. Audiology and Neurootology, 3, 86–103.
- Rauschecker, J. P., & Tian, B. (2000). Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proceedings of the National Academy of Science, USA, 97, 11800–11806.
- Rauschecker, J. P., Tian, B., & Hauser, M. (1995). Processing of complex sounds in the macaque nonprimary auditory cortex. Science, 268, 111–114.
- Rauschecker, J. P., Tian, B., Pons, T., & Mishkin, M. (1997). Serial and parallel processing in rhesus monkey auditory cortex. Journal of Comparative Neurology, 382, 89–103.
- Ravizza, R., & Diamond, I. T. (1974). Role of auditory cortex in sound localization: A comparative ablation study of hedgehog and bush-baby. Federation Proceedings, 33, 1915–1919.
- Recanzone, G. H. (2000). Spatial processing in the auditory cortex of the macaque monkey. Proceedings of the National Academy of Science, USA, 97, 11829–11835.
- Recanzone, G. H., Guard, D. C., & Phan, M. L. (2000). Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. Journal of Neurophysiology, 83, 2315–2331.
- Recanzone, G. H., Guard, D. C., Phan, M. L., & Su, T. I. K. (2000). Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. Journal of Neurophysiology, 83, 2723–2739.
- Reiter, E. R., & Liberman, M. C. (1995). Efferent-mediated protection from acoustic overexposure: Relation to slow effects of olivocochlear stimulation. Journal of Neurophysiology, 73, 506–514.
- Rhode, W. S., Smith, P. H., & Oertel, D. (1983). Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. Journal of Comparative Neurology, 213, 426–447.
- Robertson, D., Anderson, C. J., & Cole, K. S. (1987). Segregation of efferent projections to different turns of the guinea pig cochlea. Hearing Research, 25, 69–76.
- Robinson, C. J., & Burton, H. (1980a). Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory, and granular insula of fascicularis. Journal of Comparative Neurology, 192, 69–92.
- Robinson, C. J., & Burton, H. (1980b). Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of fascicularis. Journal of Comparative Neurology, 192, 93–108.
- Rodrigues-Dagaeff, C., Simm, G., de Ribaupierre, Y., Villa, A., & de Ribaupierre, F., Rouiller, E. M. (1989). Functional organization of the ventral division of the medial geniculate body of the cat: Evidence for a rostro-caudal gradient of response properties and cortical projections. Hearing Research, 39, 103–125.
- Romand, R., & Avan, P. (1997). Anatomical and functional aspects of the cochlear nucleus. In G. Ehret & R. Romand (Eds.), The central auditory system (pp. 97–191). New York: Oxford University Press.
- Romanski, L. M., Bates, J. F., & Goldman-Rakic, P. S. (1999). Auditorybeltandparabeltprojectionstotheprefrontalcortexinthe rhesus monkey. Journal of Comparative Neurology, 403, 141–157.
- Romanski, L. M., Tian, B., Fritz, J., Mishkin, M., Goldman-Rakic, P., & Rauschecker, J. P. (1999). Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nature Neuroscience, 2(12), 1131–1136.
- Rose, J. E., Greenwood, D. D., Goldberg, J. M., & Hind, J. E. (1963). Some discharge characteristics of single neurons in the inferior colliculus of the cat: I. Tonotopical organization, relation of spike-counts to tone intensity, and firing patterns of single elements. Journal of Neurophysiology, 26, 294–320.
- Rose, J. E., Hind, J. E., Anderson, D. J., & Brugge, J. F. (1971). Some effects of stimulus intensity on response of auditory nerve fibers in the squirrel monkey. Journal of Neurophysiology, 34, 685–699.
- Rouiller, E. M. (1997). Functional organization of the auditory pathways. In G. Ehret & R. Romand (Eds.), The central auditory system (pp. 3–96). New York: Oxford University Press.
- Rouiller, E. M., & de Ribaupierre, F. (1985). Origin of afferents to physiologically defined regions of the medial geniculate body of the cat: Ventral and dorsal divisions. Hearing Research, 19, 97–114.
- Rouiller, E. M., Rodrigues-Dagaeff, C., Simm, G., de Ribaupierre, Y., Villa, A., & de Ribaupierre, F. (1989). Functional organization of the medial division of the medial geniculate body of the cat: Tonotopic organization, spatial distribution of response properties and cortical connections. Hearing Research, 39, 127–142.
- Rouiller, E. M., Simm, G. M., Villa, A. E. P., de Ribaupierre, Y., & de Ribaupierre, F. (1991). Auditory corticortical interconnections in the cat: Evidence for parallel and hierarchical arrangement of the auditory cortical areas. Experimental Brain Research, 86, 483–505.
- Russo, G. S., & Bruce, C. J. (1994). Frontal eye field activity preceding aurally guided saccades. Journal of Neurophysiology, 71, 1250–1253.
- Saint-Marie, R. L., & Baker, R. A. (1990). Neurotransmitter-specific uptake and retrograde transport of [3H] glycine from the inferior colliculus by ipsilateral projections of the superior olivary complex and nuclei of the lateral lemniscus. Brain Research, 524, 244–253.
- Saint-Marie, R. L., Ostapoff, E. M., Morest, D. K., & Wenthold, J. R. (1989). Glycine immunoreactive projection of the cat lateral superior olive: Possible role in midbrain ear dominance. Journal of Comparative Neurology, 279, 382–396.
- Schall, J. D. (1991). Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: Comparison with supplementary eye fields. Journal of Neurophysiology, 66, 559–579.
- Schall, J. D., Morel, A., King, D. J., & Bullier, J. (1995). Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. Journal of Neuroscience, 15, 4464–4487.
- Schneiderman, A., Oliver, D. L., & Henkel, D. K. (1988). Connections of the dorsal nucleus of the lateral lemniscus: An inhibitory parallel pathway in the ascending auditory system? Journal of Comparative Neurology, 276, 188–208.
- Schreiner, C. E., & Langner, G. (1988). Periodicity coding in the inferior colliculus of the cat: II. Topographical organization. Journal of Neurophysiology, 60, 1823–1840.
- Schroeder, C. E., Lindsley, R. W., Specht, C., Marcovici, A., Smiley, J. F., & Javitt, D. C. (2001). Somatosensory input to auditory association cortex in the macaque monkey. Journal of Neurophysiology, 85(3), 1322–1327.
- Sek, A., & Moore, B. C. J. (1994). Detection of mixed modulation using correlated and uncorrelated noise modulators. Journal of the Acoustical Society of America, 95, 3511–3517.
- Smith, P. H., Joris, P. X., Carney, L. H., & Yin, T. C. T. (1991). Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. Journal of Comparative Neurology, 304, 387–407.
- Spangler, K. M., & Warr, W. B. (1991). The descending auditory system. In R. A. Altschuler, R. P. Bobbin, B. M. Clopton, & D. W. Hoffman (Eds.), Neurobiology of hearing: The central auditory system (pp. 27–45). New York: Raven Press.
- Stiebler, I. (1986). Tone threshold mapping in the inferior colliculus of the house mouse. Neuroscience Letters, 65, 336–340.
- Steinschneider, M., Arezzo, J., & Vaughan, H. G., Jr. (1980). Phase-locked cortical responses to a human speech sound and low-frequency tones in the monkey. Brain Research, 198, 75–84.
- Steinschneider, M., Reser, D. H., Fishman, Y. I., Schroeder, C. E., & Arezzo, J. C. (1998). Click train encoding in primary auditory cortex of the awake monkey: Evidence for two mechanisms subserving pitch perception. Journal of the Acoustical Society of America, 104, 2935–2955.
- Steinschneider, M., Reser, D., Schroeder, C. E., & Arezzo, J. C. (1995). Tonotopic organization of responses reflecting stop consonant place of articulation in primary auditory cortex (A1) of the monkey. Brain Research, 674, 147–152.
- Steinschneider, M., Schroeder, C. E., Arezzo, J. C., & Vaughan, H. G., Jr. (1995). Physiologic correlates of the voice onset time boundary in primary in auditory cortex (A1) of the awake monkey: Temporal response patterns. Brain and Language, 48, 326– 340.
- Stricanne, B., Andersen, R. A., & Mazzoni, P. (1996). Eye-centered, head-centered, and intermediate coding of remembered sound locations in area LIP. Journal of Neurophysiology, 76, 2071– 2076.
- Strominger, N. L., Nelson, L. R., & Dougherty, W. J. (1977). Second order auditory pathways in the chimpanzee. Journal of Comparative Neurology, 172, 349–366.
- Strominger, N. L., & Strominger, A. I. (1971). Ascending brainstem projections of the anteroventral cochlear nucleus in the rhesus monkey. Brain Research, 252, 353–365.
- Sudakov, K., MacLean, P. D., Reeves, A., & Marino, R. (1971). Unit study of exteroceptive inputs to claustrocortex in awake, sitting, squirrel monkey. Brain Research, 28, 19–34.
- Suga, N. (1988). Auditory neuroethology and speech processing: Complex sound processing by combination-sensitive neurons. In G. M. Edelman, W. E. Gall, & W. M. Cowan (Eds.), Auditory function: Neurobiological bases of hearing (pp. 679–720). New York: Wiley.
- Suga, N. (1994). Multi-function theory for cortical processing of auditory information: Implications of single-unit and lesion data for future research. Journal of Comparative Physiology, 175, 135–144.
- Sugar, O., French, J. D., & Chusid, J. G. (1948). Corticocortical connections of the superior surface of the temporal operculum in the monkey (Macaca mulatta). Journal of Neurophysiology, 11, 175–184.
- Suzuki, H. (1985). Distribution and organization of visual and auditory neurons in the monkey prefrontal cortex. Vision Research, 25, 465–469.
- Symmes, D. (1966). Discrimination of intermittent noise by macaques following lesions of the temporal lobe. Experimental Neurology, 16, 201–214.
- Symmes, D., Alexander, G. E., & Newman, J. D. (1980). Neural processing of vocalizations and artificial stimuli in the medial geniculate body of squirrel monkey. Brain Research, 3, 133–146.
- Symmes, D., Newman, J. D., & Alexander, G. E. (1976). Comparison of temporal auditory areas with respect to reception of species specific sounds. Society for Neuroscience Abstracts, 2,
- Tanila, H., Carlson, S., Linnankoski, I., & Kahila, H. (1993). Regional distribution of functions in dorsolateral prefrontal cortex of the monkey. Behavioral Brain Research, 53, 63–71.
- Thompson, G. C., & Cortez, A. M. (1983). The inability of squirrel monkeys to localize sound after unilateral ablation of auditory cortex. Behavioral Brain Research, 8, 211–216.
- Thorpe, S. J., Rolls, E. T., & Maddison, S. (1983). The orbitofrontal cortex: Neuronal activity in the behaving monkey. Experimental Brain Research, 49, 93–115.
- Tian, B., & Rauschecker, J. P. (1995). FM-selectivity of neurons in the lateral areas of rhesus monkey auditory cortex. Society for Neuroscience Abstracts, 21,
- Tian, B., Reser, D., Durham, A., Kustov, A., & Rauschecker, J. P. (2001). Functional specialization in rhesus monkey auditory cortex. Science, 292, 290–293.
- Toros-Morel, A., de Ribaupierre, F., & Rouiller, E. (1981). Coding properties of the different nuclei of the cat’s medial geniculate body. In J. Syka & L. Aitkin (Eds.), Neuronal mechanisms of hearing (pp. 239–243). New York: Plenum Press.
- Trahiotis, C., & Elliott, D. N. (1970). Behavioral investigation of some possible effects of sectioning the crossed olivocochlear bundle. Journal of the Acoustical Society of America, 47, 592– 596.
- Tsuchitani, C. (1977). Functional organization of lateral cell groups of the cat superior olivary complex. Journal of Neurophysiology, 40, 296–318.
- Ungerleider, L. G., & Mishkin, M. (1982). Two cortical visual systems. In D. J. Ingle, M. A. Goodale, & R. J. W. Mansfield (Eds.), Analysis of visual behaviour (pp. 549–586). Cambridge, MA: MIT Press.
- Vaadia, E. (1989). Single-unit activity related to active localization of acoustic and visual stimuli in the frontal cortex of the rhesus monkey. Brain Behavior and Evoluation, 33, 127– 131.
- Vaadia, E., Benson, D. A., Heinz, R. D., & Goldstein, M. H., Jr. (1986). Unit study of monkey frontal cortex: Active localization of auditory and of visual stimuli. Journal of Neurophysiology, 56, 934–952.
- van Adel, B. A., Kidd, S. A., & Kelly, J. B. (1999). Contribution of the commissure of Probst to binaural evoked responses in the rat’s inferior colliculus: interaural time differences. Hearing Research, 130, 115–130.
- Wang, X. (2000). On cortical coding of vocal communication sounds in primates. Proceedings of the National Academy of Science, USA, 97, 11843–11849.
- Wang, X., Merzenich, M. M., Beitel, R., & Schreiner, C. E. (1995). Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: Temporal and spectral characteristics. Journal of Neurophysiology, 74, 2685–2706.
- Ward, A. A., Jr., Peden, J. K., & Sugar, O. (1946). Cortico-cortical connections in the monkey with special reference to area 6. Journal of Neurophysiology, 9, 453–461.
- Warr, W. B. (1992). Organization of olivocochlear efferent systems in mammals. In D. B. Webster, A. N. Popper, & R. R. Fay (Eds.), The mammalian auditory pathway: Neuroanatomy (pp. 410–448). New York: Springer-Verlag.
- Watanabe, M. (1992). Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Experimental Brain Research, 89, 233–247.
- Webster, W. R., Serviere, J., Crewther, D., & Crewther, S. (1984). Iso-frequency 2-DG contours in the inferior colliculus of the awake monkey. Experimental Brain Research, 56, 425–437.
- Wegener, J. G. (1976). Auditory and visual discrimination following lesions of the anterior supratemporal plane in monkeys. Neuropsychologia, 14, 161–173.
- Wepsic, J. G. (1966). Multimodal sensory activation of cells in the magnocellular medial geniculate nucleus. Experimental Neurology, 15, 299–318.
- Whitley, J. M., & Henkel, C. K. (1984). Topographical organization of the inferior collicular projection and other connections of the ventral nucleus of the lateral lemniscus in the cat. Journal of Comparative Neurology, 229, 257–270.
- Wiederhold, M. L., & Kiang, N. Y. S. (1970). Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. Journal of the Acoustical Society of America, 48, 950–965.
- Winslow, R. L., & Sachs, M. B. (1987). Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. Journal of Neurophysiology, 57, 1002–1021.
- Winslow, R. L., & Sachs, M. B. (1988). Single-tone intensity discrimination based on auditory nerve responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hearing Research, 44, 161–178.
- Winter, P., & Funkenstein, H. H. (1973). The effect of speciesspecific vocalization on the discharge of auditory cortical cells in the awake squirrel monkey (Saimiri sciureus). Experimental Brain Research, 18, 489–504.
- Wollberg, Z., & Newman, J. D. (1972). Auditory cortex of squirrel monkey: Response patterns of single cells to species-specific vocalizations. Science, 175, 212–214.
- Wollberg, Z., & Sela, J. (1980). Frontal cortex of the awake squirrel monkey: Responses of single cells to visual and auditory stimuli. Brain Research, 198, 216–220.
- Woolsey, C. N., & Walzl, E. M. (1982). Cortical auditory area of Macaca mulatta and its relation to the second somatic sensory area (Sm II). In C. N. Woolsey (Ed.), Cortical sensory organization: Vol. 3. Multiple auditory areas (pp. 231–256). Clifton, New Jersey: Humana.
- Yin, T. C. T., & Chan, J. C. K. (1990). Interaural time sensitivity in medial superior olive of cat. Journal of Neurophysiology, 64, 465–488.
- Zoloth, S. R., Petersen, M. R., Beecher, M. D., Green, S., Marler, P., Moody, D. B., & Stebbins, W. (1979). Species-specific perceptual processing of vocal sounds by monkeys. Science, 204, 870–873.




