View sample Psychiatric Aspects of Sleep Disorders Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing services for professional assistance. We offer high-quality assignments for reasonable rates.
Complaints of too little sleep (insomnia) or too much sleep (hypersomnia), or of sleep that is not restorative enough, are termed dyssomnias. This chapter is intended to give an overview on the frequency and risk factors of dyssomnias, and on the psychiatric conditions that may be associated with them, and the physiopathological concepts related to sleep changes in depression. Of the greater than 30 percent of the general population who complain of insomnia during the course of one year, about 17 percent report that it is ‘serious.’ Insomnia is encountered more commonly in women than men and its prevalence increases with age. Insomnia represents one of the major features of depression and appears to be one type of the prodromal symptoms and risk factors for the development of major depression. Since chronic dyssomnia most often occurs as a comorbid disturbance of psychiatric and physical conditions, a thorough evaluation of the patient and his/her sleep complaints is needed to lay the foundation for accurate diagnosis and effective treatment.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
The diagnosis of insomnia is based upon the subjective complaint of sleeping too little. Patients report difficulties in initiating or maintaining sleep or of non-restorative sleep, i.e., not feeling well rested after sleep that is apparently adequate in amount, and tiredness during the day. Insomnia may occur as primary or as secondary disorder due to other psychiatric conditions, due to general medical conditions, and/or due to substance misuse. Compared to secondary insomnia, the prevalence of primary insomnia is relatively small.
Hypersomnia includes complaints of excessive sleepiness characterized either by prolonged sleep episodes and/or excessive sleepiness during the day. These symptoms may interfere with social, occupational, and/or other areas of functioning. Like insomnia, hypersomnia may occur as a primary disorder or as a secondary disorder due to psychiatric and/or medical conditions, and/or due to substance misuse. Especially among patients encountered in psychiatric practices and hospitals, secondary sleep disturbances are more common than primary sleep disturbances. This is particularly important to bear in mind when evaluating a patient with sleep complaints. Dyssomnia is particularly often associated with psychiatric disorders such as depression, schizophrenia, anxiety disorders, or personality disorders, and with misuse of drugs and/or alcohol. Whenever possible, it is clinically useful to assess which is the primary and which is the secondary disturbance. This will facilitate clinical treatment and management and improve preventive measures.
1. Frequency And Risk Factors
Somnipathies are among the most frequent complaints a general practitioner must deal with. According to epidemiological studies, 19–46 percent of the population report sleep problems. Of these, 13 percent suffer from moderate or severe disturbances. In terms of the narrow definition of diagnostic criteria, that is, initial insomnia, interrupted sleep and disturbance of daily well being, 1.3 percent of the population suffers from somnipathies.
Risk factors for somnipathies are psychological stress or psychiatric illness. A high prevalence of somnipathies was reported by volunteers suffering from stress, tension, solitude, or depression. More severe sleep problems were found to be clearly related to psychiatric illness such as depression and anxiety disorders as well as substance misuse.
A recent World Health Organization (WHO) collaborative study in 15 different countries found that insomnia is more common in females than males and increases with age. In fact, it is thought that half of the population over 65 years of age suffers from chronic sleep disturbances. The WHO study also found that 51 percent of people with an insomnia complaint had a well defined International Classification of Diseases-10 (ICD-10) mental disorder (mainly depression, anxiety, alcohol problems). Although many somnipathies develop intermittently, 70–80 percent of the surveyed subjects had been suffering from sleeping problems for more than one year. Disturbances of concentration and memory, difficulties in getting on with daily activities, and depressive mood changes were among the problems most often related to insomnia. The pressure imposed by their ailment leads many patients to seek help in alcohol and drugs.
Insomnia has also costly financial consequences. The economical impact of insomnia can be divided into direct and indirect costs. Direct costs of insomnia include outpatient visits, sleep-recordings, and medications directly devoted to insomnia. There is too little knowledge about the exact figures. However, the direct costs of insomnia in the US has been estimated in 1990 to be $10.9 billion (with $1.1 billion devoted to substances used to promote sleep and $9.8 billion associated with nursing home care for elderly subjects with sleep problems). The direct costs related to the evaluation of sleep disorders by practitioners seem to be a small part of the total costs of insomnia. The indirect costs of insomnia include the presumed secondary consequences of insomnia such as health and professional problems, and accidents. The exact quantification of these costs is, however, controversial. It is often not known if sleep disorders are the cause or the consequence of various medical or psychiatric diseases. For instance, it has been observed that insomniacs report more medical problems than good sleepers do and have twice as many doctor visits per year than good sleepers do. Furthermore, subjects with severe insomnia appear to be hospitalized about twice as often as good sleepers. It has also been observed that insomniacs consume more medication for various problems than good sleepers do.
These results confirm previous observations showing that insomnia is statistically linked with a worse health status than individuals with good sleep. Again, it can not be established whether insomnia is the cause or the result of this worse status. For instance, one could reasonably hypothesize that insomnia promotes fatigue that could increase the risk of some diseases, or more simply decrease the threshold of others that could more easily develop.
2. Regulation Of Normal Sleep
The quantity and quality of sleep is measured with polysomnographic recordings (PSG) that include the electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). Human sleep consists of two major states, the Rapid-Eye-Movement (REM) sleep, and the non-REM (non-REM) sleep (Fig. 1). Non-REM sleep is characterized by increasing EEG synchronization and is divided into stages 1–4. Stages 3 and 4 are also termed slow wave sleep (SWS) or delta sleep (‘delta waves’). REM sleep has also been termed paradoxical sleep because of its wake-like desynchronized EEG pattern combined with an increased arousal threshold. Typical nocturnal sleep is characterized by 3-5 cycles of non-REM and REM sleep phases of about 60–100 minutes duration. At the beginning of the night, the cycles contain more non-REM sleep, particularly more SWS. Towards the end of the night, the amounts of REM sleep increases and the amount of SWS decreases. The following standard terms are used to describe quality and quantity of sleep: sleep continuity refers to the balance between sleep and wakefulness (i.e., initiating and maintaining sleep), and sleep architecture refers to the amount, distribution, and sequencing of specific sleep stages. Measures of sleep continuity include sleep latency (usually defined as the duration between lights out and the first occurrence of stage 2 sleep), intermittent wake after sleep onset, and sleep efficiency (ratio of the time spent asleep to total time spent in bed). REM latency refers to the elapsed time between sleep onset and the first occurrence of REM sleep. The amount of each sleep stage is quantified by its percentage of the total sleep time. REM density is a measure of eye movements during REM sleep; typically this is low in early REM sleep periods and increases in intensity with successive REM sleep periods. In normal sleep, the cycle of non-REM and REM sleep lasts approximately 60–100 minutes. Sleep usually begins with the non-REM sleep stage 1 and progresses to stage 4 before the appearance of the first REM period.
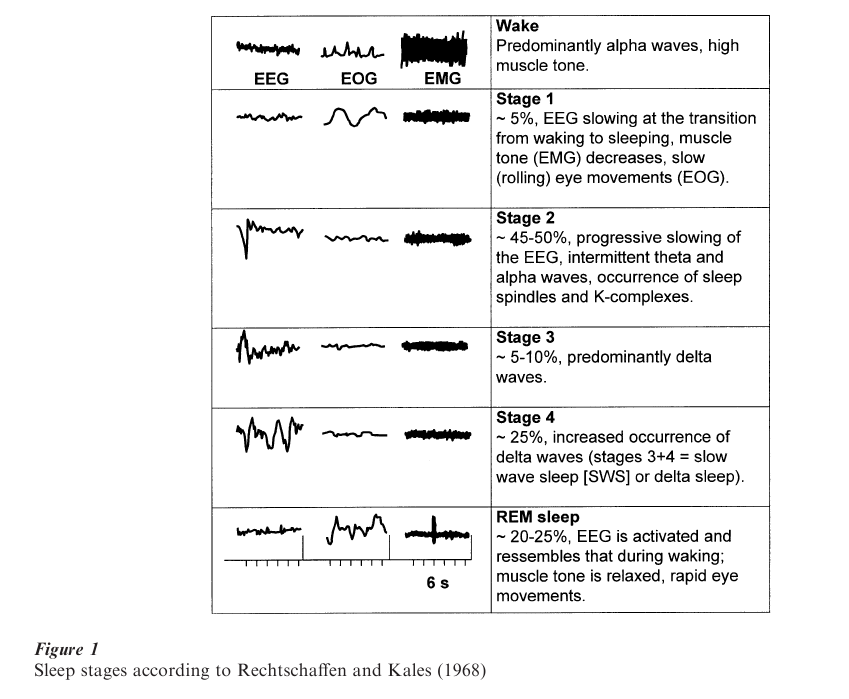
The duration of the REM sleep episodes and REM density usually increases throughout subsequent sleep cycles. As shown in Fig. 2, the pattern of nocturnal growth hormone secretion is associated with the development of SWS during the first non-REM sleep period. Cortisol secretion, on the other hand, appears to be associated with increased amount of REM sleep. Several non-REM and REM sleep measures including the amount of SWS, REM latency and density may be particularly altered in affective and schizophrenic disorders, in aging, and with the administration of certain drugs.
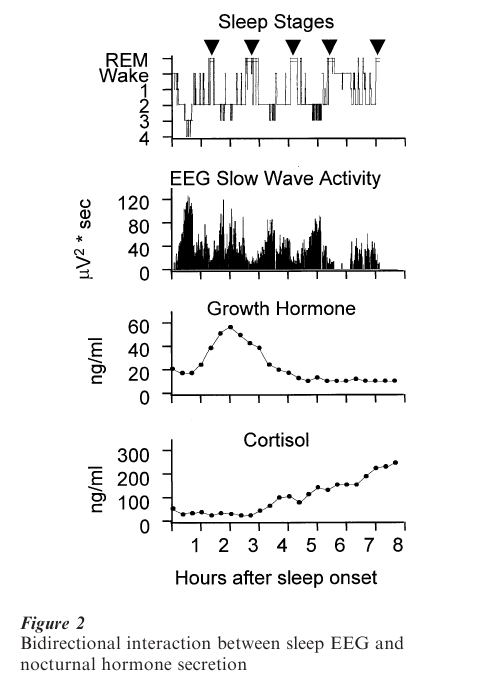
At least three major processes are involved in the regulation of normal sleep. According to the TwoProcess-Model of sleep regulation, sleep and wakefulness are influenced by both a homeostatic and a circadian process that interact in a complex way. The homeostatic Process S (related to SWS) increases with the duration of wakefulness prior to sleep onset and augments sleep propensity. The circadian Process C, driven by the internal clock located in the suprachiasmatic nuclei (SCN), describes the daily cycle of sleepiness and wakefulness and is also related to REM sleep. The third process is the ultradian rhythm of non-REM and REM sleep. The electrophysiological measures are associated with endocrinological and other physiological events.
Wakefulness, non-REM sleep, and REM sleep appear to be controlled by interacting neuronal networks, rather than by unique necessary and sufficient centers. A simplified neuroanatomy of sleep– wakefulness is shown in Fig. 3. The non-REM–REM sleep cycle is regulated within the brainstem. According to current concepts, REM sleep is initiated and maintained by cholinergic neurons originating within the lateral dorsal tegmental and pedunculopontine nuclei in the dorsal tegmentum, and is inhibited by noradrenergic and serotonergic neurons originating in the locus coeruleus and dorsal raphe nuclei. Human pharmacological data are consistent with the neurophysiological concepts of the control of REM and non-REM sleep.
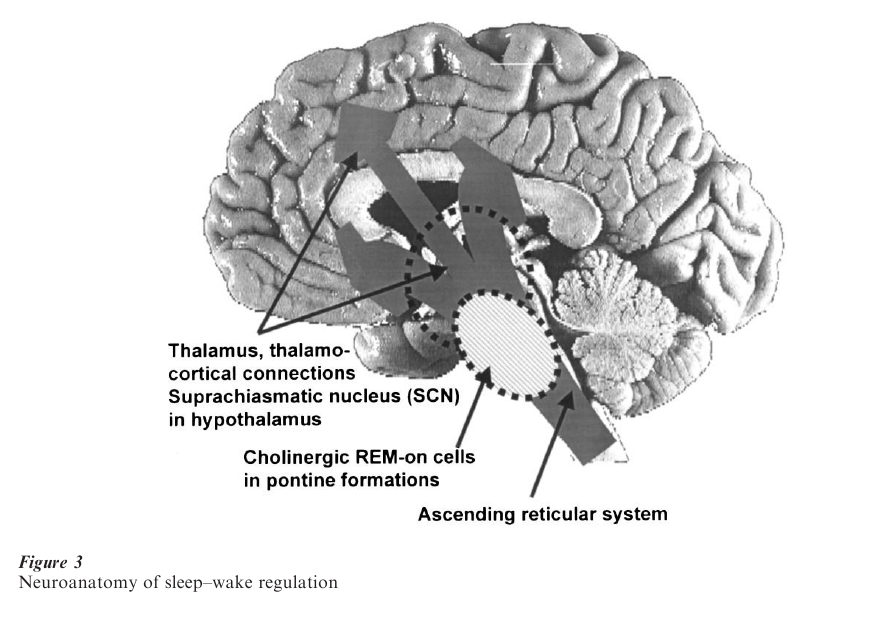
In contrast to the brain-activated state of REM sleep, non-REM sleep is characterized by synchronized, rhythmic inhibitory–excitatory potentials in large numbers of neurons in cortical and thalamic regions. Other areas implicated in the control of non-REM sleep include cholinergic and noncholinergic neurons in the basal forebrain, the hypothalamus, and the area of the solitary tract.
In addition to neuronal mechanisms in the control of sleep, more than 30 different endogenous substances have been reported to be somnogenic. These include delta sleep-inducing peptide, prostaglandin D , vasoactive intestinal peptide, adenosine, growth hormonereleasing hormone, and several cytokines including interleukins, tumor necrosis factor-α, and interferons. The significance of endogenous sleep factors in normal sleep physiology remains to be proven, but the possibility has interesting implications. As the Two Process Model of sleep regulation suggests, increased sleep propensity with progressive duration of wakefulness might be associated with the accumulation of a sleep factor and the homeostatic function of sleep or at least delta sleep might reflect its ‘catabolism.’
3. Sleep And Psychiatric Disorders
Polygraph sleep research helped paving the way to the modern age of scientific, biological psychiatry. Much of the early sleep research beginning in the mid-1960s was descriptive mapping of events taking place during sleep and laid out the currently rich picture of polysomnographic features in the different psychiatric disorders. Although many of the objective sleep abnormalities associated with psychiatric disorders appear not to be diagnostically specific, these studies have also established sleep measures as neurobiological windows into the underlying pathophysiology associated with psychiatric illnesses.
Psychiatric disorders are among the most common primary causes of secondary sleep complaints, particularly of insomnia. Sleep abnormalities may be caused by central nervous system abnormalities associated with psychiatric illnesses as well as by accompanying behavioral disturbances. Patients with depression, anxiety disorders, misuse of alcohol or drugs, schizophrenia and personality disorders may complain of difficulty falling asleep, staying asleep, or of inadequate sleep. Although specific sleep patterns are not necessarily diagnostic of particular psychiatric disorders, there are relationships between certain sleep abnormalities and categories of psychiatric disorders (Table 1).
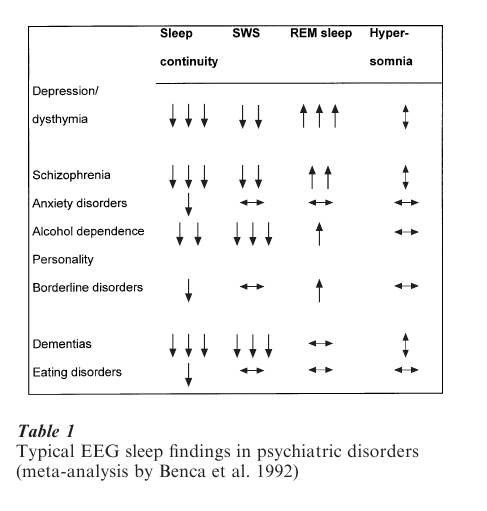
A review of the literature on sleep in psychiatric disorders showed that EEG sleep in patients with affective disorders differed most frequently from those of normal control subjects.
4. Sleep Abnormalities In Depression
Sleep abnormalities in patients with major depressive disorders, as assessed by laboratory studies, can be classified as difficulties of sleep continuity, abnormal sleep architecture, and disruptions in the timing of REM sleep. Sleep initiation and maintenance difficulties include prolonged sleep latency (sleep onset insomnia), intermittent wakefulness and sleep fragmentation during the night, early morning awakenings with an inability to return to sleep, reduced sleep efficiency, and decreased total sleep time. With regard to sleep architecture, abnormalities have been reported in the amounts and distribution of nonREM sleep stages across the night and include increased shallow stage 1 sleep and reductions in the amount of deep, slow-wave (stages 3 + 4) sleep. REM sleep disturbances in depression include a short REM latency ( < 65 minutes), a prolonged first REM sleep period, and an increased total REM sleep time, particularly in the first half of the night.
Sleep disturbances are generally more prevalent in depressed inpatients, whereas only 40–60 percent of outpatients show sleep abnormalities. Moreover, a recent meta-analysis indicated that no single polysomnographic variable might reliably distinguish depressed patients from healthy control subjects or from patients with other psychiatric disorders. Table 1 gives an overview of the typical EEG sleep changes in major psychiatric conditions. This prompted some researchers to conclude that clusters or combinations of sleep variables better describe the nature of sleep disturbances in depression.
Although there is some disagreement as to which specific sleep EEG variables best characterize depressed patients, the importance of sleep to depression is clear. Persistent sleep disturbance is associated with significant risk of both relapse and recurrence, and increased risk of suicide. Sleep variables such as REM latency has also been shown to predict treatment response and clinical course of illness in at least some studies. It has also recently been suggested that the nature of the sleep disturbance at initial clinical presentation may be relevant to the choice of anti-depressant medications and the likelihood of experiencing treatment-emergent side effects.
One of the most sensitive parameter for discrimination of patients with major depression from patients with other psychiatric disorders and healthy subjects is REM density which is substantially elevated only in depressed patients. The persistence of a depression- like sleep pattern in fully remitted depressed patients suggests that the pattern is a trait characteristic of the sleep measurements. However, in the past, subjects have undergone investigations only after the onset of the disorder, and therefore the altered sleep pattern may merely represent a biological scar. The answer to the question ‘trait or scar’ lies in the investigation of potential patients before the onset of the disorder. The EEG sleep patterns of subjects without a personal history but with a strong family history of an affective disorder differed from those of the controls without any personal history of family history of psychiatric disorders showing a depression-like sleep pattern with diminished SWS and increased REM density. Follow- up studies will determine whether the sleep pattern indeed represents a trait marker indicating vulnerability.
The importance of sleep in depression is also shown in other ways. Many well-documented studies show that total and partial sleep deprivation or selective REM sleep deprivation has antidepressant effects. Additionally, following total or partial sleep deprivation, patients with depression appear to be uniquely susceptible to clinical mood changes when they return to sleep. Patients who have shown a clinically significant antidepressant response to sleep deprivation are at risk of awakening depressed again, even after very short naps.
5. Neurobiology Of Sleep: Relevance To Psychiatric Disorders
Inspired by the growing knowledge of the underlying neurobiology of sleep, investigators proposed theories into the pathophysiology of psychiatric disorders. Because depression has been studied more than any other psychiatric syndrome in recent decades, the models have attempted to explain features of sleep in depression, such as short REM latency, decreased delta sleep, and the antidepressant effects of sleep deprivation in depressed patients.
Among the most prominent models of sleep changes in depression are the cholinergic–aminergic imbalance hypothesis for depression, the Two-Process-Model of sleep regulation, the Phase Advance Hypothesis, the Overarousal hypothesis, and the REM sleep hypothesis.
The ‘cholinergic–aminergic imbalance hypothesis’ for depression postulates that depression arises from an increased ratio of cholinergic to aminergic neurotransmission in critical central synapses. Because various features of the sleep of depressed patients (decreased REM latency and delta sleep) have been simulated in normal volunteers by pharmacological probes, the reciprocal interaction hypothesis from basic sleep research and the cholinergic–aminergic imbalance model from clinical psychiatry have been correlated. The reciprocal interaction model assumes that the cycling alternating pattern of non-REM and REM sleep is under the control of noradrenergic serotonergic and cholinergic neuronal networks. Linking these concepts it is suggested if depression results from diminished noradrenergic and serotonergic neurotransmission, cholinergic activity would be expected to increase and lead therefore to the sleep disturbances of depression.
In recent years the role of serotonin in the regulation of sleep has afforded increased attention. Among other neurotransmitters, serotonin plays a role in the pathophysiology of depression and its treatment; chiefly all antidepressants ultimately lead to an enhancement of serotonergic neurotransmission that is believed to be associated with clinical improvement. Depression is associated with a disinhibition of REM sleep (shortened REM latency, increased REM density) and serotonin leads to a suppression of REM sleep. Furthermore, there is evidence that the antidepressant effect of sleep deprivation is related to a modification of serotonergic neurotransmission, thus sleep regulation and depression share common physiopathological mechanisms at the serotonergic level.
According to the ‘Two-Process-Model of sleep regulation,’ depression is thought to result from, or is associated with, a deficiency of Process vs. This model was supported by evidence that EEG power density in the delta frequencies was decreased during sleep in depressed patients compared with controls and by the fact that sleep deprivation increases both Process S and mood. In the ‘Extended Two-Process-Model of sleep regulation’ the interaction of hormones with EEG features has been integrated. Preclinical investigations and studies in young and elderly normal controls and in patients with depression demonstrate that neuropeptides play a key role in sleep regulation. As an example, growth hormone releasing hormone (GHRH) is a common stimulus of SWS and growth hormone release, whereas corticotropin-releasing hormone (CRH) exerts opposite effects. It is suggested that an imbalance of these peptides in favor of CRH contribute to changes in sleep EEG and endocrine activity during depression. Based on the findings that the hypothalamic–pituitary–adrenocortical (HPA) axis is disregulated in depression, and that CRH produces EEG sleep changes reminding to depression on one hand (SWS reduction, REM sleep disinhibition), and that the somatotrophic system reciprocally interacts with the HPA system (decreased GHRH and SWS), it has been postulated that deficient Process S is associated with deficient GHRH and an overdrive of CRH. Although abnormally high values of cortisol secretory activity normalize after recovery from depression, growth hormone release and several characteristic disturbances of the sleep EEG may remain unchanged. These putative trait-dependent alterations suggest that strategies aiming at restoration of these sleep changes are worthy of exploration for their potential antidepressant effect.
The depressed state appears not only to be associated with a disturbance of sleep-wake homeostasis but also of circadian function or of their interaction. The ‘Phase Advance Hypothesis’ suggests that the phase position of the underlying circadian oscillator is ‘phase advanced’ relative to the external clock time. This is supported by studies showing that short REM latency could be simulated in normal controls by appropriate phase shifts of hours in bed.
As mentioned above, sleep deprivation has potent antidepressant effects in more than half of all depressed patients. This observation has prompted the hypothesis that depressed patients are ‘overaroused.’ Later, the ‘Overarousal Hypothesis’ has been forwarded and was supported by psychological self-rating studies suggesting that clinical improvement to sleep deprivation in depression may be associated simultaneously with subjective feelings of more energy (arousal), less tension, and more calmness (dearousal). Other data consistent with this hypothesis include the short, shallow, and fragmented sleep patterns, lowered arousal thresholds, and elevated nocturnal core body temperature often seen in depressed patients. This hypothesis has been tested by means of studies of localized cerebral glucose metabolism with 18F-deoxyglucose positron emission tomography in separate studies in depression, sleep deprivation, and the first non-REM period of the night. In these studies it was found that elevated brain metabolism prior to sleep deprivation predicted clinical benefits in depressed patients and that normalization of these measures is associated with clinical improvement. It was also found that local cerebral glucose metabolism in cingulate and amygdala at baseline was significantly higher in clinical responders than in nonresponders or normal controls. Furthermore, it was shown that glucose metabolic rate was increased during the first non-REM period in depressed patients compared with normal controls. Moreover, these studies demonstrated significant ‘hypofrontality,’ that is; reduced ratio of frontal to occipital activity compared with normal controls.
The ‘REM sleep hypothesis’ of depression has been based on the findings that REM sleep is enhanced in depression and that virtually all antidepressant drugs suppress REM sleep. Early, however not replicated, studies have shown that a sustained remission from depression may be achieved by selective REM sleep deprivation carried out repeatedly every night during about two weeks. This treatment modality was not followed because the long-term REM sleep deprivation was too exhausting for the patients. Recently, researchers have proposed a treatment modality that combined some of the above hypotheses. The so-called ‘Sleep-Phase-Advance’ protocol, that is scheduling sleeping times in a way that minimizes the occurrence of REM sleep, has been shown to produce a sustained antidepressant effect. However, also this treatment modality is demanding for both the patients and the institutional staff.
6. Future Perspectives
We have outlined just some aspects of sleep research that are relevant to depression. Linking basic and clinical approaches has been one of the ‘royal roads’ to the neurobiological underpinnings of psychiatric diseases and their treatment in the past, and to narrow the gap between bench and bedside. One of the most impressing links between sleep and depression is the fact that sleep deprivation alleviates depressive symptoms within hours and that sleep may restore the initial symptoms within hours or even minutes. Given the fact that the core symptoms of only few psychiatric and medical conditions may be ‘switched’ on and off, one promising lead of future research in depression is the quest to understanding the mechanisms underlying the neurobiological processes associated with sleep and wakefulness. In the future it is hoped that the rapidly evolving progress in basic neuroscience including recent molecular biology with systematic screening of gene expression and functional brain imaging techniques will help us in progressively uncovering the mystery of sleep, and ultimately improving treatment strategies of depression.
Bibliography:
- Benca R M, Obermayer W H, Thisted R A, Gillin J C 1992 Sleep and psychiatric disorders: A meta-analysis. Arch. Gen. Psychiatry 49: 651–68
- Borbely A A, Achermann P 1999 Sleep homeostasis and models of sleep regulation. Journal of Biological Rhythms 14(6): 557–68
- Gillin J C, Seifritz E, Zoltoski R, Salin-Pascual R J 2000 Basic science of sleep. In: Sadock B J, Sadock V A (eds.) Kaplan & Sadock’s Comprehensi e Textbook of Psychiatry—VII. Lippincott Williams and Wilkins, Philadelphia, pp. 199–208
- Holsboer-Trachsler E, Kocher R 1996 Somnipathies: new recommendations for their diagnosis and treatment. Drugs of Today 32(6): 477–82
- Kupfer D J 1999 Pathophysiology and management of insomnia during depression. Annals of Clinical Psychiatry 11(4): 267–76
- Rechtschaffen A, Kales A 1968 A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Department of Health, Education and Welfare, Neurological Information Network, Bethesda
- Silva J, Chase M, Sartorius N, Roth T 1996 Special report from a symposium held by the World Health Organization and the World Federation of Sleep Research Societies: an overview of insomnia’s and related disorders—recognition, epidemiology, and rational management. Sleep 19(5): 412–6
- Steiger A, Holsboer F 1997 Neuropeptides and human sleep. Sleep 20: 1038–52
- Tononi G, Cirelli C 1999 The frontiers of sleep. Trends in Neuroscience 22(10): 417–8




