View sample Thirst And Drinking Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Drinking is one of the triad of motivated ingestive behaviors used by animals to ingest those substances essential for maintaining metabolism, growth, and overall bodily integrity. Feeding controls the intake of nutrients (fat, protein, carbohydrates), vitamins, and cofactors; targeted appetites control the intake of specific ions (principally sodium, but also to a lesser extent other essential ions such as calcium) from a variety of sources; while drinking regulates water intake. These behaviors are controlled by complex neural systems that have components distributed throughout the brain, and transform the need to replenish or anticipate deficits, the desire to find suitable goal objects, and knowledge of their whereabouts into appropriate sets of behavioral and visceral motor output.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Composition And Physiological Regulation Of The Body’s Fluid Compartments
Understanding how body fluids are compartmentalized and which of their components are maintained within regulated limits is central to understanding how drinking behaviors are initiated.
The body consists of two fluid compartments: a cellular compartment comprising the internal fluid of all cells, and an extracellular compartment comprised of all interstitial fluid and plasma in the blood. In terrestrial mammals the water in the cellular compartment makes up about 65–70 percent of the body’s total water. Fluid in the cellular compartment is separated from extracellular fluid (ECF) by the semipermeable membranes that surrounds all cells. This membrane is completely permeable to water, but the transmembrane movement of many other molecules and ions is actively regulated, making the physiology of this membrane critical for water balance.
One of the key features of the cell membrane is its ability of the enzyme Na+/K+ ATPase to promote the active transport of sodium ions out of the cellular compartment. This enzyme is a two-subunit transmembrane protein, is a major utilizer of cellular energy, and exchanges 3 Na+ out of the cell for 2 K+ in. This process maintains the ECF Na+ concentration (150 mM) at about tenfold greater than that inside the cell. This concentration gradient is critical for fluid balance and the control of drinking behavior because it determines the rate of water flux across the membrane.
Two variables of the ECF compartment are regulated tightly by physiological and behavioral process: ECF osmolality and volume. Under conditions of isotonicity the net flow of water across the cell membrane is zero. However, any disturbance of this osmotic balance leads to increased transmembrane water flow. The sodium ion is the most important osmolyte in this regard. In terms of thirst and drinking, increasing the osmolality of the ECF compartment is an important parameter that results in cellular de- hydration, which is a net loss of water from the cell. In turn, the kidney attempts to excrete the excess sodium from the blood, but this cannot be achieved without incurring an accompanying loss of water from the body.
ECF volume is important for long-term maintenance of blood pressure and efficient cardiovascular function. Unlike cellular dehydration, which involves a net loss of water but not sodium, loss of fluid from the extracellular compartment (hypovolemia) involves loss of water and all of its other components, including sodium. Therefore, in addition to triggering an appetite for water (i.e., thirst), hypovolemia also initiates the specific appetite for sodium (Grossman 1990).
2. Types Of Drinking Behavior
Drinking behaviors can be divided usefully into two categories: those that occur in response to a significant disturbance in ECF composition and result in a classic homeostatic response; and those that occur in the absence of such a disturbance, but instead are initiated to prevent the occurrence of such deficits. Each of these types of drinking is associated with distinct neural mechanisms (Grossman 1990, Rolls and Rolls 1982).
2.1 Homeostatic Drinking
The most well-studied aspects of drinking behavior are those initiated in response to significant changes in ECF composition. A large body of work has identified specific types of dipsogenic (i.e., thirst-inducing) sensory signals that are associated with particular disturbances to the composition of the ECF. In humans, the most common form of homeostatic drinking occurs in response to exercise or heat-stress. Pathological causes of homeostatic drinking include severe hemorrhage or conditions associated with severe diarrhea.
2.2 Anticipatory Drinking
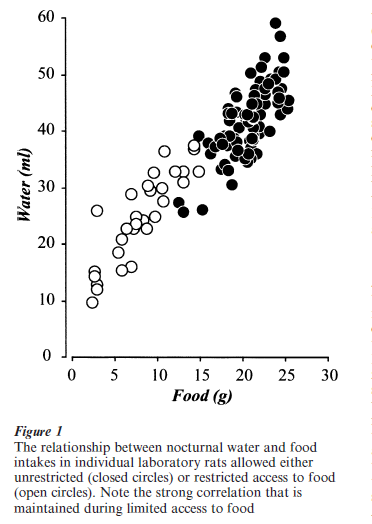
Drinking that occurs without a major disturbance to ECF composition requires regulation by regions of the brain concerned with the anticipatory components of motivated behaviors. Two groups of brain regions are particularly concerned with regulating this type of drinking; those involved with controlling arousal state and circadian timing (particularly the circadian clock in the suprachiasmatic nucleus), and those cortical regions concerned with the complex processing of sensory objects and their representation in processes such as reward/aversion, and learning and memory (Watts 2001). In these circumstances, drinking is often closely associated with feeding behavior, both temporally and in quantity (Fig. 1). Food-associated water intake (either as a component of the food itself or as a separate drinking episode) represents the most common route of water entry into the body for humans living in economically developed countries.
3. Sensory Signals And Their Transduction
3.1 Extracellular Fluid Volume
Physiological mechanisms concerned with maintaining ECF volume are evolutionarily older than those that maintain ECF osmolality. This is because the challenge from water deprivation and increasing ECF osmolality did not pose a physiological threat until the evolution of terrestrial life. Indeed in primitive vertebrates, the physiologically more important actions of two of the hormones most closely associated with the response to dehydration—angiotensin II (ATII) and the vasopressin-related peptides—is vasoconstriction driven by AT1 and V1 receptors, respectively, which leads to a reduction in renal blood flow, and consequently reduced fluid loss from the kidneys. The antidiuretic actions of vasopressin are evolutionarily more recent and are mediated by specific water channel proteins called aquaporins. An elevated insertion rate of these proteins into the basal membrane of convoluted tubule is mediated by vasopressin’s interactions with V2 receptors. Although vasopressin has significant effects on water conservation, it has no dipsogenic actions. Decreases in ECF volume are transduced into appropriate motor actions by two sensory mechanisms.
3.1.1 Angiotensin II. Reduced blood flow through the specialized juxtaglomerular apparatus in the kidney stimulates the release of an renin, an enzyme that catalyzes the conversion of angiotensinogen to angiotensin I. Once in the general circulation angiotensin I is converted to ATII by angiotensin-converting enzyme in the lungs. ATII has four major actions associated with thirst and fluid balance. First, it acts on vascular AT1 receptors to constrict blood vessels and maintain blood pressure; second it is dipsogenic; third, it stimulates sodium appetite; and finally, it acts on zona glomerulosa cells in the adrenal cortex to stimulate aldosterone release, which then targets the convoluted tubules in the kidney to potentiate sodium retention. To stimulate drinking, circulating ATII binds to AT1 receptors in the subfornical organ (SFO). This structure is the most sensitive ATII responsive site in the brain and many studies place this structure centrally in a circuit that directly and specifically stimulates water intake (Fitzsimons 1998). The SFO provides neural efferent projections—most of which also contain ATII (Swanson 1987)—to a relatively limited set of brain structures, including parts of the prefrontal cortex, substantia innominata, medial preoptic area, bed nuclei of the stria terminalis (BST), zona incerta, paraventricular hypothalamic nucleus (PVH), supraoptic nucleus, and lateral hypothalamic area (Swanson 1987).
3.1.2 Baroreceptors. Inputs from baroreceptors in the walls of blood vessels on low-pressure (venous) side of the circulation (primarily the superior vena cava) provide important neural information that contributes to the thirst generated by hypovolemia. This is demonstrated by the fact that the thirst generated by hypovolemia is inhibited by a small inflatable balloon placed to maintain the output of the baroreceptors in the wall of the superior vena cava as the animal becomes hypovolemic (Kaufman 1984). The afferent information from these receptors is initially processed by the nucleus of the solitary tract (NTS) in the hindbrain, before being relayed to those regions of the brain responsible for organizing drinking behavior.
3.2 Extracellular Osmolality
All cells are osmosensitive in that they lose water and shrink in proportion to increases in the osmolality of the ECF. However, these increases are only transduced into neural signals by select groups of osmoreceptive cells located in the brain and periphery. In terms of thirst and drinking behavior, the most important set is located in the vascular organ of the lamina terminalis (OVLT), a hypothalamic structure located at the rostral end of the third ventricle. Supplementary sets of osmoreceptors may also contribute to the motor responses to dehydration, and are located at other sites in the brain (particularly in the SFO and area postrema) as well as in the periphery (the liver and possibly hepatic portal vein).
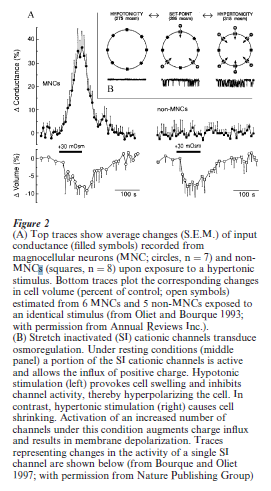
A cellular mechanism that accounts for the osmotransduction exhibited by some neurons has recently been described by Bourque and co-workers (Bourque and Oliet 1997). In this scheme, osmoreceptive magnocellular neurons in the supraoptic nucleus possess stretch-inactivated cationic channels that allow increased entry of positive charge as they shrink in response to increasing ECF osmolality (Fig. 2). In turn, these neurons depolarize and generate action potentials in proportion to the change in ECF osmolality (Fig. 2). Interestingly, unlike many other types of sensory receptors, the osmoreceptors associated with drinking apparently do not adapt when exposed to a slowly changing stimulus intensity (Fitzsimons 1963).
3.3 Sensory Processing
The information derived from these sensory receptors that encodes alterations in ECF composition requires initial processing before being integrated into the motor control networks that select the most appropriate motor action. As with many other motivated behaviors, the exact mechanisms involved with this function are not clear. However, a recent insight was recently provided into those regions important for generating human drinking behaviors in response to cellular dehydration by a positron emission tomographic study (Denton et al. 1999a, 1999b). Volunteers were infused intravenously with hypertonic saline and asked to gauge the subsequent intensity of thirst. In turn, those regions in the brain showing increased blood flow were correlated with the hyperosmolemia and the reported intensity of thirst. These studies identified parts of the cortex as well as the cerebellum as being specifically activated by hyperosmolemia.
4. The Components Of Drinking Behavior
4.1 Motor Actions
As animals become dehydrated the sensory mechanisms described above are activated. However, which of these is specifically engaged depends on the nature of the dehydrating stimulus. In the case of water deprivation, which is likely to be the most commonly encountered stimulus by most animals, approximately 70 percent of the drinking behavior can be attributed to the sensory mechanisms associated with cellular dehydration, while the remainder is mediated by the mechanisms triggered by hypovolemia (Rolls and Rolls 1982). On the other hand, the thirst and drinking that follows loss of body fluids resulting from heat stress, for example, leads to isotonic hypovolemia and consequently ATII and neural baroreceptor sensory mechanisms play critical roles in the subsequent thirst and drinking behavior.
Despite the variety of these sensory mechanisms, there is a strong degree of convergence from the brain regions responsible for organizing drinking behavior onto a well-characterized set of neuroendocrine, autonomic, and behavioral motor mechanisms. These responses should not be considered in isolation and can be usefully categorized as protective, adaptive, or restorative to the body’s fluid compartments (Grossman 1990, Watts 2001).
The principal protective responses involve the neuroendocrine and autonomic motor systems. They include the very rapidly initiated release of vasopressin and oxytocin from the posterior pituitary that are activated by neuroendocrine reflexes following dehydration. These hormones help maintain blood pressure, and increase renal water retention and natriuresis. Other protective responses occur after hemorrhage or hypovolemia, and include modifications to autonomic control of cardiovascular function, and the rapid activation of all levels of the hypothalamo-pituitary-adrenal axis.
Both types of dehydration generate a set of adaptive autonomic and behavioral motor responses that target gastrointestinal function to conserve water, although cellular dehydration is more closely associated with actions on the gut. A dry mouth is the sensation that is often identified with thirst. Indeed, one of the earliest theories for the generation of drinking behavior proposed that a dry mouth was the most important sensory trigger (Rolls and Rolls 1982). Although many experiments have now disproved the idea that a dry mouth is the primary instigator of deficit-induced drinking, there is no doubt that it provides important sensory feedback signals that may be important for associating drinking behaviors with particular stimuli that could lead to dehydration. Reduced salivation and a dry mouth may occur as a consequence of reduced blood flow through the salivary glands, as well as reduced parasympathetic activation of salivation in response to increasing plasma osmolality. But a dry mouth also develops as a consequence of, for example, extended talking or eating dry food. Finally, if dehydration is maintained for a significant period decreased gastric motility, and eventually anorexia develop as protective responses to maintain body water by reducing the water investment required by digestion and reducing the inflow of osmolytes into the body (Watts 2001).
Finally, restorative mechanisms modify behavior to return water and sodium (in the case of ECV challenges) back into the body. The selection, timing, and magnitude of all these responses ultimately depends on the intensity and kinetics of the stimulus. For example, mild hyperosmolemia will stimulate vasopressin secretion but not anorexia.
4.2 Feedback And Inhibition
The ability to inhibit drinking when sufficient water has been drunk to cover for the deficit is as important a function as behavioral initiation; overhydration can be as much of a problem as dehydration. Two types of mechanism are available for this purpose, although each varies in importance depending on the species: alterations in plasma osmolality, and oropharyngeal and gastric sensory feedback. For alterations in plasma osmolality to be a major factor in inhibiting drinking behavior, they have to occur rapidly. In small animals this does not occur, and drinking is terminated before plasma osmolality has begun to respond to the absorption of water. In this instance oropharyngeal mechanisms activated by the act of drinking are important. In larger species however, the volumes of water imbibed during a drinking episode may be sufficient to lower plasma osmolality rapidly and inhibit drinking (Rolls and Rolls 1982).
Bibliography:
- Bourque C W, Oliet S H 1997 Osmoreceptors in the central nervous system. Annual Review of Physiology 59: 601–19
- Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P 1999a Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proceedings of the National Academy of Science USA 96: 2532–7
- Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Lancaster J, Fox P 1999b Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proceedings of the National Academy of Science USA 96: 5304–9
- Fitzsimons J T 1963 The effects of slow infusions of hypertonic solutions on drinking and drinking thresholds in rats. Journal of Physiology (London) 167: 344–54
- Fitzsimons J T 1998 Angistensin, thirst, and sodium appetite. Physiological Reviews 78: 583–686
- Grossman S P 1990 Thirst and Sodium Appetite. Academic Press, San Diego, CA
- Kaufman S 1984 Role of right atrial receptors in the control of drinking in the rat. Journal of Physiology (London) 349: 389–96
- Oliet S H, Bourque C W 1993 Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature 364: 341–3
- Rolls B J, Rolls E T 1982 Thirst. Cambridge University Press, Cambridge, UK
- Swanson L W 1987 The hypothalamus. In: Bjorklund A, Hokfelt T, Swanson L W (eds.) Handbook of Chemical Neuroanatomy. Elsevier, Amsterdam, pp. 1–124
- Watts A G 2001 Neuropeptides and the integration of motor responses to dehydration. Annual Review of Neuroscience 24: 357–84




