View sample Temporal Lobe Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
The temporal lobe subserves disparate functions. The dorsal (or superior) part is involved in the perceptual processing of auditory signals including speech. The primary auditory area is located at the central part of the lower bank of the lateral fissure, with higher auditory areas circumscribing it. Visual information processing about objects spreads from the ventral part of the occipital lobe to the posteroventral part of the temporal lobe. The most anterior and anteroventral parts are essential for the memory and retrieval of the semantic knowledge of objects.
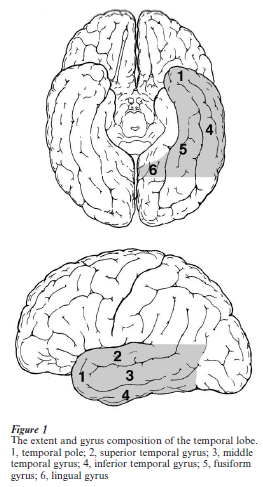
1. Borders And Gyrus Composition
The temporal lobe is separated from the frontal lobe and the anterior part of the parietal lobe by the lateral (or Sylvian) fissure. Its borders with the occipital lobe and the posterior part of the parietal lobe do not have clear delimiting sulci. On the lateral surface, an imaginary line drawn from the temporo-occipital incisure (or the occipital notch) at the inferior margin of the hemisphere to the posterior end of the parietooccipital sulcus defines the anterior border of the occipital lobe. Another imaginary line drawn orthogonally to this first line, from the point at which the lateral fissure turns upward, defines the border between the temporal and parietal lobes on the lateral surface. On the ventromedial surface, an imaginary line drawn from the temporo-occipital incisure to the point at which the parieto-occipital sulcus merges with the calcarine sulcus defines the border between the temporal and occipital lobes. The most medial part of the ventral surface of the hemisphere, medial to the anterior part of the calcarine sulcus and the anterior part of the collateral sulcus, is often treated as a part of the limbic system. The temporal lobe does not border with the parietal lobe on the medial surface.
The anterior end of the temporal lobe, the temporal pole, does not have sulci. The lateral surface of the temporal lobe has three anteroposteriorly elongated gyri separated by two sulci. Going from the more dorsal to the more ventral, these are the superior temporal gyrus, the middle temporal gyrus, and the inferior temporal gyrus. The superior temporal sulcus separates the superior and middle temporal gyri, and the inferior temporal sulcus separates the middle and inferior temporal gyri. The dorsal surface of the temporal lobe, which is concealed in the lateral fissure, is composed, from anterior to posterior regions, of the planum polare, the transverse temporal gyrus (or the transverse gyrus of Heschl), and the planum temporale. From its medial to lateral extent, the ventromedial surface of the temporal lobe is composed of the lingual gyrus, the fusiform gyrus, and the inferior temporal gyrus. The collateral sulcus separates the lingual and fusiform gyri, and the occipitotemporal sulcus separates the fusiform and inferior temporal gyri. The lingual gyrus continues anteriorly to the parahippocampal gyrus, which is the gateway to the hippocampus and related structures implicated in explicit memory. Both the lingual gyrus and the fusiform gyrus continue from the occipital lobe to the temporal lobe with no marks.
2. Auditory Functions
Because microelectrode recordings in human cortex are possible only in very rare clinical cases, and in vivo tracer studies are not possible, the current knowledge of the structure and function of the auditory cortical areas in the temporal lobe is largely due to results obtained in experimental macaque monkeys. Fortunately, the location and basic organization of auditory cortical areas is very similar in humans and macaques, insofar as the available data indicate.
2.1 Primary Auditory Cortex
The dorsal surface of the monkey temporal lobe does not have a transverse gyrus, but has an anteroposteriorly elongated annectant gyrus. This annectant gyrus in the ventral bank of the lateral fissure shows the histological features of primary sensory cortex such as densely packed granule cells, and dense reactivity to the cytochrome oxidase and calciumbinding protein parvalbumin. Studies with microelectrodes show that cells in this region respond well to pure tones, showing relatively sharp selectivity to a tone’s frequency. These studies have permitted the identification of two separate tonotopically organized areas, named A1 and R. A1 occupies a large part of the annectant gyrus, and R occupies a smaller area anteriorly adjoining A1. Frequencies are mapped from lowest to highest in the anterolateral to posteromedial direction in A1, and the reverse in R. Both areas receive strong fiber projections from the main part (ventral division) of the medial geniculate nucleus (MGv). Although the histological features of primary sensory cortex are more pronounced in A1 than R, R is thought to be parallel to A1 in signal flow because responses of cells in R to pure tones survive ablation of A1.
In the human temporal lobe, a region with the histological features of primary cortex is located in the transverse temporal gyrus within the ventral bank of the lateral fissure. MEG (magnetoencephalography) and functional MRI (magnetic resonance imaging) studies consistently show tonotopy in the transverse temporal gyrus; frequencies are mapped from lowest to highest in the anterolateral to posteromedial direction. This mapping orientation is the same as in monkey A1. Some studies have also shown a second tonotopic mapping in the transverse temporal gyrus, which may correspond to R in the monkey. Bilateral damage to these primary auditory areas results in a profound deficit in sound detection, although the deficit usually recovers within several weeks to several months.
2.2 Higher Auditory Areas
The monkey auditory areas in the dorsal part of the temporal lobe are composed of three or four serial stages (Kaas et al. 1999). The primary auditory areas constitute the ‘core.’ Axonal fibers from the core strongly project to regions in the ‘belt,’ which circumscribe the core in all directions except posteromedially. The belt regions are confined to the lower bank of the lateral sulcus. Fiber projections from MGv to the belt are weak, while there are moderate projections from the dorsal (MGd) and medial (MGm) divisions of the medial geniculate nucleus to the belt. There are at least three subdivisions in the belt as determined by distinct tonotopic mapping and histochemical and connectional properties. Cells in the belt tend to respond more strongly to narrow band noises and more complex sounds than pure tones, and their response tuning to pure tone frequencies tends to be broader than in the core (Rauschecker 1998). There is no consensus on the relative contribution of serial inputs from the core and direct inputs from MGd and MGm to the auditory responses of belt cells, but it is at least known that responses to pure tones in a part of the belt disappear after ablation of A1.
The belt strongly projects to the laterally adjoining regions on the lateral surface of the superior temporal gyrus. These regions have been named the ‘parabelt.’ The projections from the core to the parabelt are weak. Thus, there is a more or less serial projection stream from core to belt to parabelt. Like the belt, the parabelt receives projections from MGd and MGm, but in addition, the parabelt receives projections from other thalamic sites: the suprageniculate, limitans, and medial pulvinar nuclei. The parabelt projects extensively to more rostral and caudal parts of the superior temporal gyrus, adjoining regions in the superior temporal sulcus, prefrontal cortex, parietal cortex, and other brain sites. There are no projections from the core, and only weak projections from the belt, to these fourth-stage regions. Tonotopic mapping has not been found in the parabelt. The parabelt receives projections from the belt in a rostral-to-rostral and caudal-to-caudal manner. Thus, there are at least two serial pathways in parallel. There are some data on the cellular response properties that suggest that the two pathways correspond to the two main pathways in the visual system; the caudal auditory pathway corresponding to the dorsal, or spatial visual pathway, and the rostral auditory pathway to the ventral, or object visual pathway (Rauschecker 1998). Most cells in the rostral parabelt are more selective for sound properties, while cells in the caudal parabelt respond to pure tones of wide frequency ranges. Unlike rostral parabelt cells, most cells in the caudal parabelt, respond only to sounds from contralateral space. Some are more responsive to sounds from moving sound sources, sometimes with sensitivity to the direction of the source motion.
Available data in humans also suggests serial auditory stream (Kaas et al. 1999). Electroencephalogram (EEG) and MEG recordings of auditory evoked potentials show progressively longer response latencies at more distant locations from the primary auditory area. Functional MRI and PET studies have found a few tonotopic maps on the surface of the superior temporal gyrus. As for stimulus selectivity, functional MRI studies have found that complex sounds including human voices more strongly activate these surrounding regions than scrambled voices with matched power spectra (Belin et al. 2000, Scott et al. 2000). A bilateral lesion of the surrounding regions with the primary auditory areas spared is known to cause ‘auditory agnosia,’ a deficit in perceiving meaningful sounds including phonemes, elements of spoken words, and nonvocal familiar environmental sounds. Left-lateralized damage causes a specific deficit in perceiving phonemes (pure word deafness), while right-lateralized damage causes a less specific deficit in perceiving nonvocal, familiar environmental sounds, for which the term auditory agnosia is sometimes specifically used. In any case, a lesion of the surrounding regions leaves the detection of sounds intact. All these findings suggest that auditory information is processed serially in humans as it is in macaques— from the primary auditory areas located within the transverse temporal gyrus, to the surrounding regions at the planum temporale and the lateral surface of the superior temporal gyrus.
Apart from pure word deafness, there are patients who show a deficit in both the perception and vocalization of words. In the latter case, named ‘Wernicke’s aphasia,’ spontaneous speech may be fluent but includes nonsense words or words with syllabic errors. These patients cannot repeat words or sentences spoken by other people. It is assumed that the phonetic memory of words or its access by phonemic inputs is damaged. The posterior third of the left superior temporal gyrus was originally proposed to be involved in this symptom, thus this region is often referred to as ‘Wernicke’s area.’ However, the lesions in patients with permanent Wernicke’s aphasia are larger and include the posterior half of the superior and middle temporal gyri or the white matter under Wernicke’s area.
3. Visual Functions
In macaques, the middle and inferior temporal gyri are mostly dedicated to the purely visual associations involved in object vision. These regions are the latter stages of the ventral visual pathway. Their bilateral ablation causes a specific deficit in performing and learning tasks requiring visual object discrimination and recognition (Gross 1994). Single cell recordings have shown that cells respond to various complex features of visual object images (Tanaka 1996). Some of the cells specifically respond to views of faces.
In humans, however, purely visual functions appear to be restricted to the posterior part of the ventral temporal lobe. Both ‘visual agnosia,’ in which the patient cannot retrieve semantic knowledge including the name of visually presented objects, and ‘prosopagnosia,’ in which the patient cannot retrieve the identity of visually presented faces, are caused by damage to the regions around the border between the occipital and temporal lobes on the ventral surface. These patients retain semantic knowledge of objects and individual persons, which they can retrieve via inputs of other modalities. More anterior parts of the ventral temporal lobe are thought to be essential for the semantic knowledge of objects and persons, with their names localized mostly in the left hemisphere.
Reading can be also specifically degraded (pure alexia) by damage around the border between the occipital and temporal lobes. Classical cases had combined damage to the left occipital lobe and the corpus callosum splenium (the posterior part of the corpus callosum), in which the deficit was explained by the disconnection of the language centers in the left hemisphere from the spared visual cortical regions in the right occipital lobe. However, a similar deficit is caused by a more localized damage to regions around the border between the occipital and temporal lobes in the left hemisphere. The patients have no deficit in writing words. Many patients can read words by picking up letters one by one. Therefore, the deficit is thought to be due to degraded perception of shapes composing words, especially global features covering the whole word. Among Japanese people, who use both visually complex ideograms (Kanji) and relatively simple syllabograms (Kana), there are rare cases in which reading Kanji is specifically degraded. The damage in these patients involve the most posterior part of the inferior temporal lobe in the left hemisphere.
Functional MRI studies have shown that the posterior part of the ventral temporal lobe is activated by the passive viewing of object images (Tanaka 1998). Parts of the fusiform gyrus are more strongly activated by viewing faces than other objects. The surrounding regions in the rest of the fusiform gyrus and the inferior occipital gyrus are more activated by non-face objects than faces. There are reports that views of towns, including houses, and views of rooms specifically activate the posterior part of the parahippocampal gyrus (Epstein and Kanwisher 1998).
4. Semantic Knowledge
There are aphasic patients with symptoms that appear to be due to damage or loss of the semantic knowledge of objects. These patients show impaired understanding of spoken words and sentences. Their spontaneous speech is fluent, but their vocabulary is poor. They also have difficulty in naming visually presented objects. Oral repetition of spoken words and sentences is intact, but without understanding. These patients have left-lateralized or bilateral damage of the middle and inferior temporal gyri and temporal pole. This symptom is a subtype of ‘transcortical sensory aphasia,’ but it is also called ‘semantic dementia.’ Semantic dementia is more often observed in ‘Pick’s disease,’ a progressive genetic disease manifesting atrophy of cortical neurons, rather than vascular problems. The atrophy usually starts in both temporal and frontal lobes, but in the limited cases in which the atrophy starts in the temporal lobe, the patients show pure semantic dementia. The knowledge of objects at finer classification levels (individual and subordinate levels rather than categorical levels) tends to be lost first. As the infection progresses to the frontal lobe and the medial aspects of the temporal lobe, the symptoms develop into a more general dementia.
Semantic dementia may show category specificity (Caramazza and Shelton 1998). Three categories may be selectively impaired: animals, tools, and individual persons. Damage to the temporal pole impairs knowledge of familiar persons. In particular, the left temporal pole is important for the association of names with biographical knowledge, while the right temporal pole is important for the retrieval, based on various inputs, of biographical knowledge other than names. Damage to the anteroventral part of the left temporal lobe causes difficulty in naming animals based on various inputs. The conceptual and nominal knowledge of tools is degraded by damage to the posterior region of the lateral surface of the occipital, temporal and parietal lobe junction. Functional imaging studies (PET and functional MRI) show activation patterns in normal subjects consistent with the previously mentioned results obtained from brain-damaged patients (Damasio et al. 1996). In these studies, the subjects named objects from their pictures or made semantic judgments on pictures or visually presented words.
Bibliography:
- Belin P, Zatorre R J, Lafaille P, Ahad P, Pike B 2000 Voiceselective areas in human auditory cortex. Nature 403: 309–12
- Binder J R, Frost M S, Hammeke T A, Cox R W, Rao S M, Prieto T 1997 Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience 17: 353–62
- Caramazza A, Shelton J R 1998 Domain-specific knowledge systems in the brain: The animate-inanimate distinction. Journal of Cognitive Neuroscience 10: 1–34
- Damasio H, Grabowski T J, Tranel D, Hichwa R D, Damasio A R 1996 A neural basis for lexical retrieval. Nature 380: 499–505
- Epstein R, Kanwisher N 1998 A cortical representation of the local visual environment. Nature 392: 598–601
- Gross C G 1994 How inferior temporal cortex became a visual area. Cerebral Cortex 5: 455–69
- Kaas J K, Hackett T A, Tramo M J 1999 Auditory processing in primate cerebral cortex. Current Opinion in Neurobiology 9: 164–70
- Rauschecker J P 1998 Cortical processing of complex sounds. Current Opinion in Neurobiology 8: 516–21
- Scott S K, Blank C C, Rosen S, Wise R J S 2000 Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123: 2400–6
- Tanaka K 1996 Inferotemporal cortex and object vision. Annual Review of Neuroscience 19: 109–39
- Tanaka K 1998 Mechanisms of visual object recognition: Monkey and human studies. Current Opinion in Neurobiology 7: 523–9




