View sample Synaptic Transmission Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
As explained in detail by J. C. Eccles in 1964, nerve cells communicate via specialized regions of close juxtaposition that were first named ‘synapses’ by C. S. Sherrington, who used this term to denote the anatomical region where the axon terminal of one neuron contacts another neuron. Sherrington always used synapse in its functional sense (for example, to account for the one-way conduction in the reflex arc). However, it must now be considered from an anatomical perspective as well since, despite variations, there are common structural features consistent with some of the physiological properties of synapses. For example, given that there is a high resistance gap of 200 A width separating neuronal membranes at most synapses, as first claimed by Ramon y Cajal, current generated by one cell cannot spread significantly to the next one. Thus, it is advantageous for postsynaptic terminals to utilize a chemical mediator to secure transmission. This concept was only established after decades of controversy and efforts to distinguish between electrical and chemical models, for both excitation and inhibition. Yet, although it is beyond the scope of this overview, electrotonic interactions between nerve cells, in the periphery and in the brain, can indeed occur, due to either (a) areas of continuity between them via gap junctions (Bennett 1977); or (b) the channeling of currents in adjacent cells by an unusually high external resistance. The second case is called ephaptic or, better stated, field effect transmission (Faber and Korn 1989). These two electrical modes, which should not be confused, allow signaling to occur instantaneously, and are well suited for the mediation of rapid behaviors, such as escape. In contrast, the hallmark of chemical synapses is the irreducible delay ( -1 msec) between the incoming stimulus and the postsynaptic response, which reflects activation of the presynaptic machinery involved with transmitter release (Katz 1969, Llinas 1982). Chemical synapses, however, have remarkable adaptive advantages, such as: (a) variable gain; (b) a large repertoire of polarity and kinetics, resulting in excitations and inhibitions of different durations, depending upon their specific chemistry; and (c) the ability to store information on a long-term basis, due to plastic properties which have become a paradigm for studies of learning and memory.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Integrative Properties Of Neurons And Synaptic Correlates
Since any behavior is ultimately produced by combined activity in neuronal assemblies, it is of critical interest to understand how each individual neuron reacts to incoming signals initiated in cells connected to it through synaptic junctions, as in a simple reflex chain (Fig. 1A). In fact, information is distributed widely in the nervous system, such that a single neuron may both have many targets and receive inputs from numerous presynaptic axonal terminals, according to the principles of divergence and convergence, respectively. Recording electrodes inserted in the various parts of a neuron (soma, dendrite, axon), in combination with anatomical studies of the synapses contacting these loci, have revealed electrical signals whose polarity reflects their function. The signals are named excitatory postsynaptic potentials (EPSPs), and inhibitory postsynaptic potentials (ISPSs). Due to nerve cell cable properties, which are determined by the membrane resistivity and capacitance, signals generated at a distance from the axon are attenuated there, but nevertheless contribute to the summation of responses at the so-called trigger zone (Fig. 1B), which decides whether or not some information will be passed on to the next neuronal relay. This essential integrative property of the cell can be quite complex, with there being multiple trigger zones (Llinas et al. 1969), and summation not always being algebraic (non-linear). Yet, whatever the rules are in a given neuronal subtype, the tonic and phasic bombardment of a cell by incoming inputs can only bring it to such a decision when excitation dominates inhibition and reaches a critical value, the threshold for generating an action potential (Fig. 1C) that will propagate along the axon towards the next synaptic relay. It can already be noted that reaching threshold is the result of the collective activity of large populations of inputs, each of which may, however, act randomly, as will be described below. This cooperative nature of the nervous system pertains whether it is viewed from a molecular, synaptic, network, or behavioral perspective.
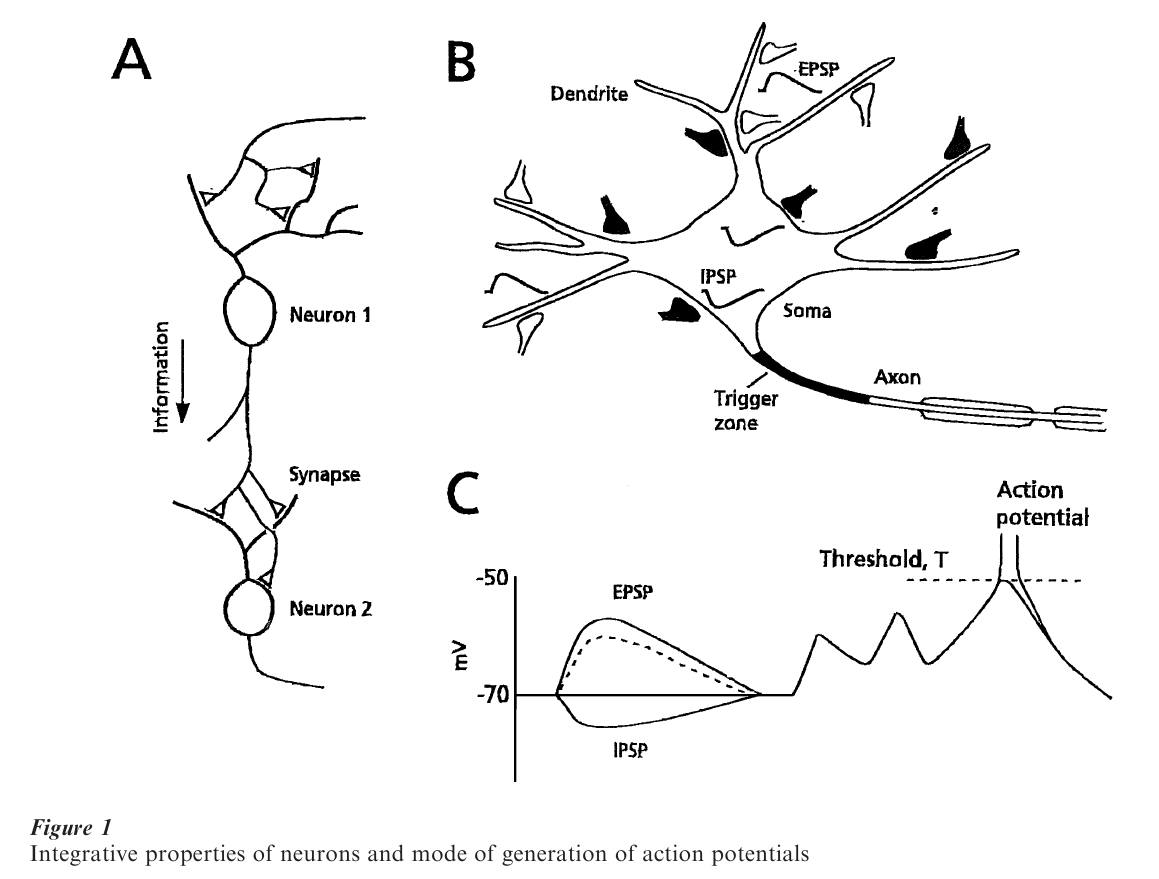
2. What Synapses Have In Common
The meaning of the term ‘synapse’ has evolved over time, in parallel with the development of the morphological and physiological tools available for studies of the brain. Today, its strict definition requires electron microscopic demonstration of ultrastructural features associated with chemical transmission. A comparison of central synapses with the neuromuscular junction (nmj), which was the Holy Grail of pioneering studies, allows one to recognize their basic unity.
At the frog nmj, the terminals of the motor axon occupy the end plate, a specialized depression in the muscle membrane, which forms folds. Opposite the crest of each fold, where transmitter receptors are the most abundant, the axon terminal contains dense bands and spherical organelles that package 5,000– 10,000 molecules of the transmitter acetylcholine (Kuffler and Yoshikami 1975) and are called synaptic vesicles. It was later demonstrated that the opening of synaptic vesicles (exocytosis) takes place at the borders of the bands, which therefore were designated active zones (Couteaux and Pecot-Dechavassine 1970). An equivalent term for physiologists is ‘release site,’ as vesicle openings were observed there (Heuser et al. 1974). The motor end plate can thus be considered as a number of synaptic units, each represented by a dense band with its associated vesicles and a postsynaptic density (for review, see Peters et al. 1991).
Synapses in the central nervous system (CNS) have more restricted apposition zones. They most often consist of synaptic boutons (Fig. 2A, B) that contain the vesicles and are separated from the postsynaptic element by the 10–20 nm wide cleft. The plasma membranes on both sides exhibit marked densities which, together with the assembly of vesicles, are referred to as a synaptic complex (Palay 1958). Here the analog of the active zone is the presynaptic grid, formed by electron-opaque densities called presynaptic dense projections, which are arranged in triangular arrays and, in en face view, take the form of an ‘egg box.’ This structure is postulated to ‘guide’ the vesicles towards the plasma membrane (Pfenninger et al. 1972). As at the nmj, the postsynaptic membrane facing this release apparatus contains the receptors which, however, are restricted to localized clusters (Triller et al. 1985). Thus, as further shown by physiological studies, the basic functional unit of synaptic transmission is a complex containing the presynaptic active zone and its neighboring synaptic vesicles, together with the aggregate of ligand-gated receptors in the opposing postsynaptic membrane. It thus corresponds to the synaptic complex.
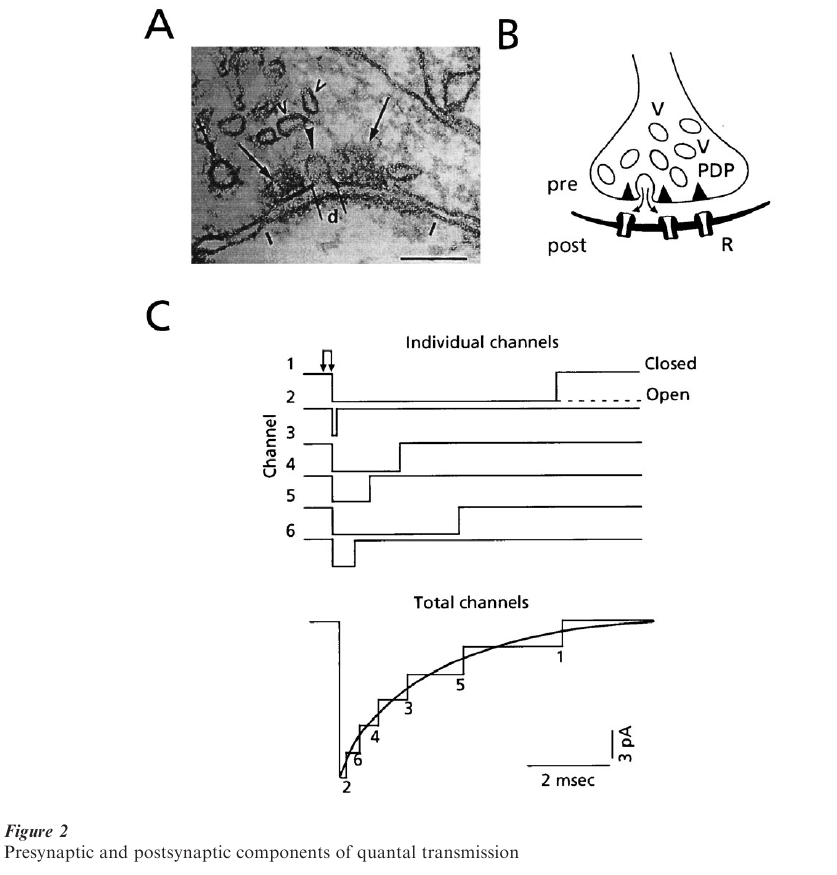
At either type of chemical synapse, the depolarization of the terminals associated with a presynaptic action potential opens voltage sensitive Ca++ channels, with the consequent Ca++ influx initiating a cascade of molecular events that can culminate in the opening of a vesicle fused to the plasma membrane at the release site and the rapid discharge of its contents into the cleft (Fig. 2B). The postsynaptic receptors are thus exposed to a high concentration of transmitter, on the order of mM, albeit briefly (Fig. 2C, double arrow). Consequently, the ligand-gated channels open nearly synchronously, allowing specific ions to flow down their electrochemical gradient. However, as each individual opened channel in the postsynaptic receptor matrix closes randomly, the postsynaptic current, which is the sum of all individual channel activity, decays exponentially. This ‘population’ phenomenon is illustrated in Fig. 2C, where the summed current rises rapidly and relaxes slowly, a time course typical of those at the so-called fast synapses used by the brain for information processing. At the nmj, the contents of one vesicle open about 1000 receptor-channels, while at central synapses the number can be much less.
One should note that in the CNS the current flow through synaptic channels can be either inward (i.e., directed towards the cell interior) and excitatory, or outward and inhibitory, depending upon the selectivity of the channel, i.e., which ion(s) it allows to pass. Furthermore, in most neuronal systems, glycine and γaminobutyric acid (GABA) are inhibitory, and glutamate and acetylcholine are excitatory, although a given transmitter can have either function, depending upon the type of channel associated with that transmitter’s receptor (Hille 1984).
3. Why Synaptic Potentials Fluctuate In Integer Steps
With a recording electrode placed at the nmj, Katz and colleagues made two related discoveries. First, at rest, spontaneous potentials of similar amplitudes occur randomly. Second, when the nerve is stimulated repeatedly, the resulting evoked endplate potentials fluctuate in amplitude in a steplike manner from trial to trial. Furthermore, amplitude histograms show that the apparently discrete size classes are integer (1, 2, 3, … n) multiples of a basic unit which corresponds in time course and average size to the spontaneous event (del Castillo and Katz 1954). These elementary ‘miniature’ endplate potentials were thus called ‘quanta,’ since they are representative of the basic unit of the evoked response. Remarkably, the underlying statistics of transmitter release, which account for the variable number of quanta issued after each impulse (based on experiments conducted i n a low concentration of extracellular calcium ([Ca2+] ), satisfied the Poisson process. Release occurred with statistical independence at any one of a large number, n, of ‘quantal units available for release’ at the junction, with a low release probability ( p) that was identical for each unit. Finally, it was postulated that each quantum corresponded to the action of the transmitter packaged in one synaptic vesicle, and since n is large in the Poisson equation, it was assumed to represent the total number of vesicles ready to be released (Katz 1969).
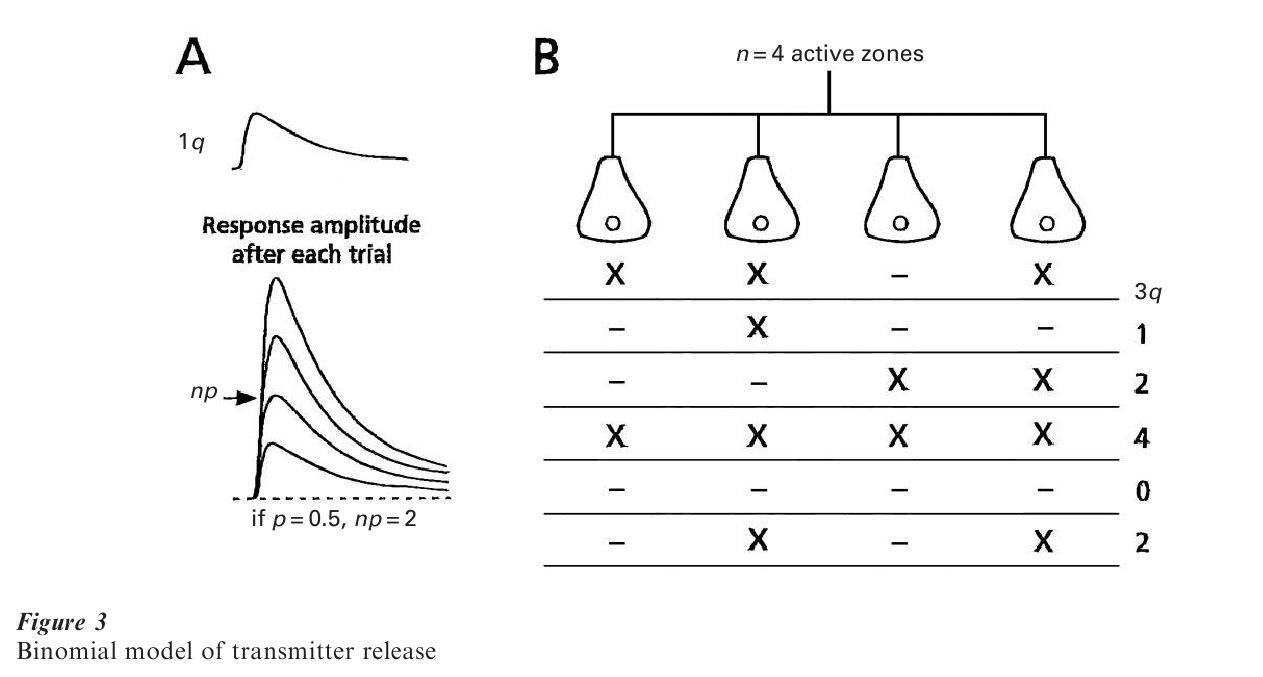
Despite difficulties inherent to the analysis of signals recorded from brain cells, such as the inability to relate any miniature response to one of the numerous junctions impinging on the neuron being investigated, and the low signal to noise ratio, the conclusion illustrated in Fig. 3A, namely that responses evoked by activating a single presynaptic neuron are quantal, was also reached for central synapses (Korn and Faber 1991, Redman 1990, see also Jonas et al. 1993). However, a statistical formulation other than the Poisson most often provides a better description of the amplitude fluctuations. Here the statistics are either a simple binomial model, where n is finite and p is relatively large, or a compound binomial, where p may be different at individual releasing units (see also below). Regardless of the mathematical details (Martin 1977 and see below), n is smaller than originally thought. Also, in experiments combining physiological recordings with anatomical reconstructions of the cells studied, the value derived for this parameter turned out to be equivalent to the number of active zones or release sites in the activated presynaptic terminals. The result lead to the ‘one vesicle’ hypothesis, according to which a presynaptic action potential causes the exocytosis of the contents of at most one vesicle at each active zone (reviewed in Korn et al. 1990). Despite controversies about multivesicular release, for example during development, this concept is still commonly used for interpreting experimental data (see Stevens 1993).
4. Consequences Of Quantal Transmission
At the skeletal nmj, neurotransmission in normal Ca++ has a high safety factor (large n and p), such that a presynaptic impulse always leads to muscle contraction. On the other hand, the full implications and richness of the quantal nature, and hence uncertainty of transmitter release, are better exploited, albeit still puzzling, at central synapses. From a functional point of view, the most novel feature is that communication across central synapses is not guaranteed systematically even when p is relatively high: after each impulse, a synapse flips a coin. This probabilistic aspect is exemplified in Fig. 3A, 3B, which stress that each synaptic unit operates in an ‘all or none’ manner. It also shows that over a large number of trials the average response amplitude equals the n p q, product, where n and p are presynaptic parameters, and q, the size of a quantum, is proportional to the number of channels opened by a single exocytotic event. This product defines the so-called synaptic weight or strength of a connection between two neurons, which is a dynamic term, as it can be modulated pre or postsynaptically. Indeed, persistent increases in synaptic weights are considered to underlie higher-order processes such as learning and memory, from invertebrates to primates. For example, activity-dependent long-term potentiation (LTP; Bliss and Collingridge 1993) in mammalian hippocampus, which is considered a well-suited candidate for a memory mechanism, can result from an increase in any one of the three quantal parameters (Stevens 1993). The reverse form of plasticity is long-term depression (LTD; Bear and Abraham 1996) (first described in the cerebellum), which may be a cellular correlate of erasing memory traces. It presumably represents a second mechanism for allowing neurons to respond preferentially to selected subsets of their inputs.
Since a neuron may receive information from thousands of junctions, the binary mode of transmission illustrated in Fig. 2A may be better suited for computational purposes than having a graded process at each site, which would lead to excess or redundant information. Along this line, a synapse can be viewed metaphorically as a symbolic communication system with, according to the scheme of Shannon and Weaver, the encoding process localized to the release site. Knowledge of p and n then allows calculation with the classical entropy equation of the amount of information, in bits, at a connection between neurons. For example, with a given value of p, the increased reliability of neuronal connections with larger ns is counterbalanced by a decreased number of bits per quantum (Korn and Faber 1987).
5. Diversity Of Synapses
Central synapses exhibit a great deal of structural and functional heterogeneity, which is built upon the scaffolding described above. For example, the transmitter at adjacent contacts on to a given postsynaptic could be an amino acid such as glycine or acetylcholine, a biogenic amine such as serotonin or dopamine, or a peptide such as somatostatin, substance P, or Vasoactive Intestinal Peptide (VIP), and, furthermore, combinations of these substances may be packaged in one terminal ending (Hokfelt et al. 1984). The consequences of this colocalization and of the cotransmission that is often associated with it are not yet resolved, particularly in puzzling cases where two classical transmitters with the same function can be released together (Jonas et al. 1998). In addition, there are multiple receptor subtypes for most transmitters, and more than one may be colocalized to the same postsynaptic locus (Somogyi 1998). Some are ionotropic, with binding causing channel opening or closing, while others are metabotropic, which means that the ligand-bound receptor activates an intracellular metabolic cascade which can modulate kinases or channel activity. In fact, listing the differences between synapses is almost an insurmountable task. One would first have to consider constitutive variations in pre-or postsynaptic parameters, namely the probability of release, the kinetics and conductances of single channels. The mechanisms for limiting the duration of action of a transmitter such as its diffusion in the synaptic cleft, its uptake by activated terminals or glia, its chemical inactivation, and receptor desensitization once the channels have opened, add another layer of complexity (Johnston and Wu 1995). One then would have to factor in the synergistic or antagonistic effects of the growing inventory of modulators (Kaczmarek and Levitan 1987), which can influence these various functions in normal and pathological conditions, as well as during development. This diversity may confer a higher degree of functional uniqueness to individual synapses and neurons than is commonly recognized. In any case, it influences the richness of animal behaviors and higher functions which result from activity in complex neuronal assemblies that are synchronized via their synaptic connectivity.
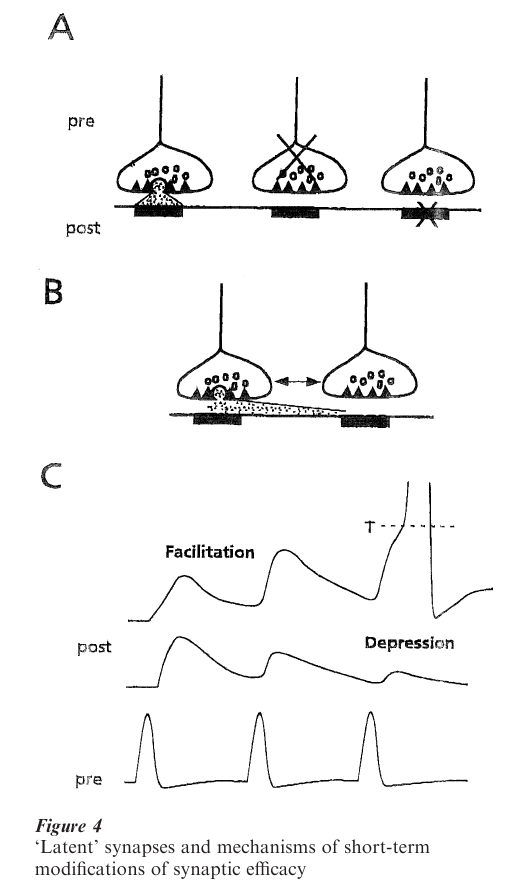
Some elements of synaptic diversity and their functional implications deserve mention. First, although most junctions are fully operational in the normal brain, some of them may remain silent, either because a presynaptic action potential does not evoke transmitter release, or because the postsynaptic receptors are unresponsive (Fig. 4A). These may be viewed as ‘latent’ rather than ‘silent’ synapses, because they may be turned on in specific circumstances, such as during learning (Malenka and Nicoll 1997). Second, by virtue of the close packing of synapses, the transmitter released at one of them can diffuse laterally and interact with the postsynaptic receptors of its neighbors (Fig. 4B). This spillover may violate the principle of independence of individual junctions and result in synergistic interactions (Korn et al. 1990), and it may contribute to phenomena such as LTP. Finally, synaptic strength can vary on a millisecond timescale when a presynaptic neuron discharges repetitively (Fig. 4C), and this plasticity can be expressed as either a facilitation or depression (Johnston and Wu 1995, Markram and Tsodyks 1996). Both processes are presynaptic in origin, due to a corresponding change in the release probability, and typically meet the functional requirements of the related networks. For example, facilitation may contribute to recruit neuronal targets that are otherwise weakly excited.
6. Quantal Analysis And Its Limitations
A statistical dissection of postsynaptic electrical recordings, based on the quantal hypothesis, remains an unsurpassed tool for understanding how different classes of nerve cells communicate with each other. The models used, and their corresponding mathematical formulations, are the same as used in game theory and many branches of the social, behavioral and political sciences; that is, the laws of chance. Stated otherwise, the release of a packet of transmitter after an impulse is equivalent to rolling dice. Despite their apparent complexity, these laws are quite straightforward. For all models, the average number of units released over a large series of trials, which is called mean quantal content or m,
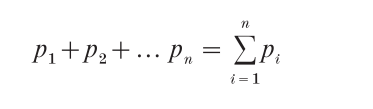
where p is the release probability at each site. This is the essence of the compound binomial release model, which encompasses all others that assume independent probabilities of release.
If there were no ambiguities about quantal size, q, i.e., the magnitude of the postsynaptic response to a quantum, amplitude distribution histograms of evoked responses would have discrete peaks corresponding to transmission failures, single quantum responses, doublets and so on, and these distributions could be described with one of the quantal models.
For a compound binomial:
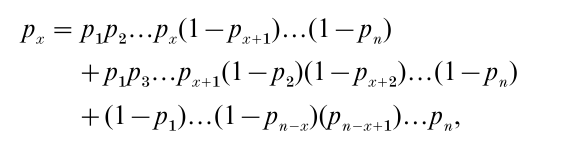
where each line represents one way to get x successes and (n – x) failures. For example, in the first line, units 1 to x release quanta, whereas the other units (x + 1 to n) do not. The probability of failure for one quantal unit is (1 – p ).
For a simple bionomial:
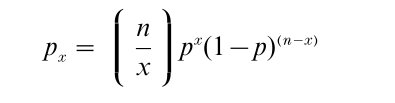
where
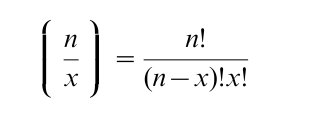
gives the number of combinations by which x quanta can be released out of an available pool of size n.
For a Poisson:
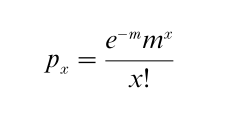
With m = np.
As developed elsewhere (Martin 1977), most investigators have relied on the two latter models and on indirect methods derived from them rather than trying to fit the distributions. These methods involve either counting the number of failures, n0, in N trials or measuring the coefficient of variation, CV, of the amplitude distribution, since according to a Poisson:

In rare cases, m can be determined directly, by counting quanta or by averaging miniature events arising from the same synapses. Corresponding expressions allow the last two methods to be applied to a binomial process if p is finite (see Martin 1977). Also, since the binomial CV = ((1 – p)/np)1/2 and is independent of q, it has been used as a tool for identifying the pre- vs. postsynaptic locus of a change in synaptic strength, but this approach is liable to misinterpretations (Faber and Korn 1991).
Correct application of these tools requires the accurate identification of the quantal response, which is often difficult at central connections due to quantal variance, distortion by cable properties, and instrumental noise. As a consequence, discrete peaks in amplitude histograms are blurred and failures, single quantal responses, doublets, etc., cannot be distinguished. Various fitting algorithms have been developed, with some success (reviewed in Korn and Faber 1987; see also Stricker et al. 1994), their limitations, however, leaving some questions unresolved. One is the size of the quantum, which is related to the number of channels opened by an exocytotic event, and to their unitary conductance, and can be quite small at central contacts (Finkel and Redman 1983, Edwards et al. 1990). Another is that at some junctions there may be a mismatch between the large number of transmitter molecules released and the relatively few receptors available postsynaptically, which led to the suggestion of a saturated response (Jack et al. 1981). If so, the principles of quantal analysis would no longer hold, as quantitization would be imposed post- rather than presynaptically.
A related issue is the difficulty in determining the pre or postsynaptic locus of the increased synaptic weight that occurs during LTP, using quantal analysis alone. Raging controversies have not yet been entirely resolved, and mechanisms on both sides of the synapse have been invoked (see Stevens 1993, Liao et al. 1995, Isaac et al. 1996). One of the most intriguing proposals is that although the induction process is postsynaptic, its maintenance can be expressed as an increased release probability, which requires signaling in the reverse direction across the synapse, by a retrograde messenger (reviewed in Bliss and Collingridge 1993). This debate could be obscuring the essential notion of the unity of the synapse where pre-and postsynaptic regulatory factors could be implicated separately or in unison, depending upon functional requirements.
7. Other Avenues For Synaptic Studies
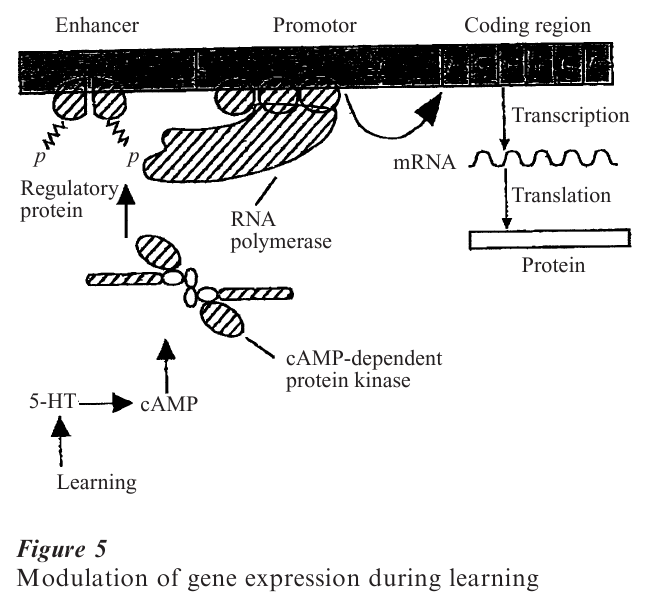
Like all scientific fields, synaptic studies cannot remain confined to their historical roots, such as statistics, and need to be enriched by other approaches. The most direct is imaging of dynamic changes at the synapse, associated with the ability to tag pre- and postsynaptic structural components of activated junctions with fluorophores (Malgaroli et al. 1995). In addition, computer-aided modeling of single synapses, or of populations, is necessary to understand biophysical properties of vesicular exocytosis, the fate of the released molecules in the cleft, and their interactions with the postsynaptic receptors, all of which govern the size and kinetics of the postsynaptic response (Bartol et al. 1991, Kruk et al. 1997). Together with anatomical correlates, these techniques should not only solve issues such as those related to single vs. multivesicular release and receptor saturation, but also deepen our understanding of brain development, and learning and memory, and how synapses are affected in pathological conditions. Along this line, it can already be envisioned how synapses contribute to genetic or acquired illnesses of behavior and cognition, including psychiatric disorders (Kandel 1998). Figure 5 illustrates the manner in which expression of a gene, leading to the production of a specific protein, could be altered following synaptic activation associated with learning. Conversely, genetic alterations can lead to significant psychiatric disorders, and it can be postulated that they do not leave synapses untouched. This example shows once more that synaptic studies represent a bridge between the neural sciences, cognitive psychology, and behavioral sciences.
Bibliography:
- Bartol T M Jr., Land B R, Salpeter E E, Salpeter M M 1991 Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophysicical Journal 59: 1290–307
- Bear M F, Abraham W C 1996 Long-term depression in hippocampus. Annual Review of Neuroscience 19: 437–62
- Bennett M V L 1977 Electrical transmission: A functional analysis and comparison to chemical transmission. In: Brookhart J M, Mountcastle V B, Kandel E R, Geiger S (eds.) Handbook of Physiology: The Ner ous System. Williams and Wilkins, Baltimore, MD, pp. 357–416
- Bliss T V, Collingridge G L 1993 A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–9
- Couteaux R, Pecot-Dechavassine M 1970 Vesicules synaptiques et poches au niveau des zones actives de la jonction neuro-musculaire. Comfetes Rertus le l’Academic des Sciences 271: 2346–9
- Del Castillo J, Katz B 1954 Quantal components of the end-plate potential. Journal of Physiology 124: 560–73
- Eccles J C 1964 The Physiology of Synapses. Springer-Verlag, Berlin
- Edwards F A, Konnerth A, Sakmann B 1990 Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: A patch-clamp study. Journal of Physiology (London) 430: 213–49
- Faber D S, Korn H 1989 Electrical field effects: Their relevance in central neural networks. Physiological Review 69: 821–63
- Finkel A S, Redman S J 1983 The synaptic current evoked in cat spinal motoneurones by impulses in single group la axons. Journal of Physiology 342: 615–32
- Heuser J E, Reese T S, Landis D M D 1974 Functional changes in frog neuromuscular junctions studied with freeze-fracture. Journal of Neurocytology 3: 109–31
- Hille B (ed.) 1984 Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA
- Hokfelt T, Johansson O, Goldstein M 1984 Chemical anatomy of the brain. Science 225: 1326–34
- Isaac J T, Hjelmstad G O, Nicoll R A, Malenka R C 1996 Long-term potentiation at single fiber inputs to hippocampal CA1 pyramidal cells. Proceedings of the National Academy of Science USA 93: 8710–15
- Jack J J, Redman S J, Wong K 1981 The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia aff Journal of Physiology (London) 321: 65–96
- Johnston D, Miao-Sin Wu S (eds.) 1995 Foundations of Cellular Neurophysiology. MIT Press, Cambridge, MA
- Jonas P, Bischofberger J, Sandkuhler J 1998 Corelease of two fast neurotransmitters at a central synapse. Science 281: 419–24
- Jonas P, Major G, Sakmann B 1993 Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. Journal of Physiology (London) 472: 615–63
- Kaczmarek L K, Levitan I B (eds.) 1987 Neuromodulation: The Biochemical Control of Neuronal Excitability. Oxford University Press, Oxford, UK
- Kandel E R 1998 A new intellectual framework for psychiatry. American Journal of Psychiatry 155: 457–69
- Katz B (ed.) 1969 The Release of Neural Transmitter Substances. Charles C. Thomas, Springfield, IL
- Korn H, Faber D S 1987 Regulation and significance of probabilistic release mechanisms at central synapses. In: Edelman G M, Gall W E, Cowan W M (eds.) Synaptic Function. A Neuroscience Institute Publication. Wiley, NY, pp. 57–108
- Korn H, Faber D S 1991 Quantal analysis and synaptic efficacy in the CNS. Trends in Neuroscience 14: 439–45
- Korn H, Faber D S, Triller A 1990 Convergence of morphological, physiological, and immunocytochemical techniques for the study of single Mauthner cells. In: Bjorklund A, Hokfelt T, Wouterlood F G, van den Pol A N (eds.) Handbook of Chemical Neuroanatomy, Vol. 8: Analysis of Neuronal Microcircuits and Synaptic Interactions. Elsevier Science Publishers, Amsterdam, pp. 403–80
- Kruk P J, Korn H, Faber D S 1997 The effects of geometrical parameters on synaptic transmission: A Monte Carlo simulation study. Biophysics Journal 73: 2874–90
- Kuffler S W, Yoshikami D 1975 The number of transmitter molecules in a quantum: An estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. Journal of Physiology (London) 251: 465–82
- Liao D, Hessler N A, Malinow R 1995 Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375: 400–4
- Llinas R R 1982 Calcium in synaptic transmission. Scientific American 4: 56–65
- Llinas R, Nicholson C, Precht W 1969 Preferred centripetal conduction of dendritic spikes in alligator Purkinje cells. Science 163: 184–7
- Malenka R C, Nicoll R A 1997 Silent synapses speak up. Neuron 19: 473–6
- Malgaroli A, Ting A E, Wendland B, Bergamaschi A, Villa A, Tsien R W, Scheller R H 1995 Presynaptic component of long-term potentiation visualized at individual hippocampal synapses. Science 268: 1624–8
- Markram H, Tsodyks M 1996 Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382: 807–10
- Martin A R 1977 Junctional transmission. II: Presynaptic mechanisms. In: Brookhart J M, Mountcastle V B, Kandel E R, Geiger S (eds.) Handbook of Physiology: The Ner ous System. Williams and Wilkins, Bethesda, MD, pp. 357–416
- Palay S L 1958 The morphology of synapses in the central nervous system. Experimental Cell Research 5: 275–93
- Peters A, Palay S L, Webster H DeF (eds.) 1991 The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. Oxford University Press, Oxford, UK
- Pfenninger K H, Akert K, Moor H, Sandri C 1972 The fine structure of freeze-fractured presynaptic membranes. Journal of Neurocytology 1: 129–49
- Redman S J 1990 Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiological Review s 70: 165–98
- Somogyi P 1998 Precision and variability in the placement of pre and postsynaptic receptors in relation to transmitter release sites. In: Faber D S, Korn H, Redman S J, Thompson S M, Altman J S (eds.) Central Synapses: Quantal Mechanisms and Plasticity. pp. 82–95
- Stevens C F 1993 Quantal release of neurotransmitter and long-term potentiation. Neuron 10: 55–63
- Stricker C, Redman S, Daley D 1994 Statistical analysis of synaptic transmission: Model discrimination and confidence limits. Biophysics Journal 67: 532–47
- Triller A, Cluzeaud F, Pfeiffer F, Betz H, Korn H 1985 Distribution of glycine receptors at central synapses: An electron microscopy study. Journal of Cell Biology 101: 683–8




