View sample Sleep and Neural Systems Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing services for professional assistance. We offer high-quality assignments for reasonable rates.
Since we spend roughly one third of our lives asleep, it is remarkable that so little attention has been paid to the capacity of sleep to organize the social behavior of animals including humans. To grasp this point, try to imagine life without sleep: no bedrooms and no beds; no time out for the weary parent of a newborn infant; and nothing to do all through the long, cold, dark winter nights. Besides demonstrating the poverty of the sociobiology of sleep, this thought experiment serves to cast the biological details which follow in the broader terms of social adaptation. Family life, reproduction, childrearing, and even economic commerce all owe their temporal organization to the power of sleep. The dependence of these aspects of our lives on sleep also underlines its importance to deeper aspects of biological adaptation—like energy regulation—that are only beginning to be understood by modern scientists.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Behavioral Physiology Of Sleep
Sleep is a behavioral state of homeothermic vertebrate mammals defined by: (a) characteristic relaxation of posture; (b) raised sensory thresholds; and (c) distinctive electrographic signs.
Sleep tends to occur at certain times of day and becomes more probable as sleeplessness is prolonged. These two organizing principles are the pillars of Alex Borbely’s two-factor model: factor one captures the temporal or ‘circadian’ aspect while factor two captures the energetic or ‘homeostatic’ aspect. Sleep is usually associated with a marked diminution of motor activity and with the assumption of recumbent postures. Typically the eyes close and the somatic musculature becomes hypotonic. The threshold to external stimulation increases and animals become progressively less responsive to external stimuli as sleep deepens.
The differentiation of sleep from states of torpor in animals that cannot regulate core body temperature has an important phylogenetic correlation with the neural structures mediating the electrographic signs of sleep. These include the cerebral cortex and thalamus whose complex evolution underlies the distinctive electroencephalographic features of sleep in the higher vertebrate mammals. Sleep also constitutes the state of entry to and exit from hibernation in mammalian species that regulate temperature at lower levels during winter.
In humans, who can report upon the subjective concomitants of these outwardly observable signs of sleep, it is now clear that mental activity undergoes a progressive and systematic reorganization throughout sleep. On first falling asleep, individuals may progressively lose awareness of the outside world and experience microhallucinations and illusions of movement of the body in space; after sleep onset, mental activity persists but is described as thoughtlike and perseverative, if it can be recalled at all upon awakening. (For a description of the subsequent changes in mental activity, see the entry on dreaming.)
2. Electro-Physiological Aspects Of Sleep
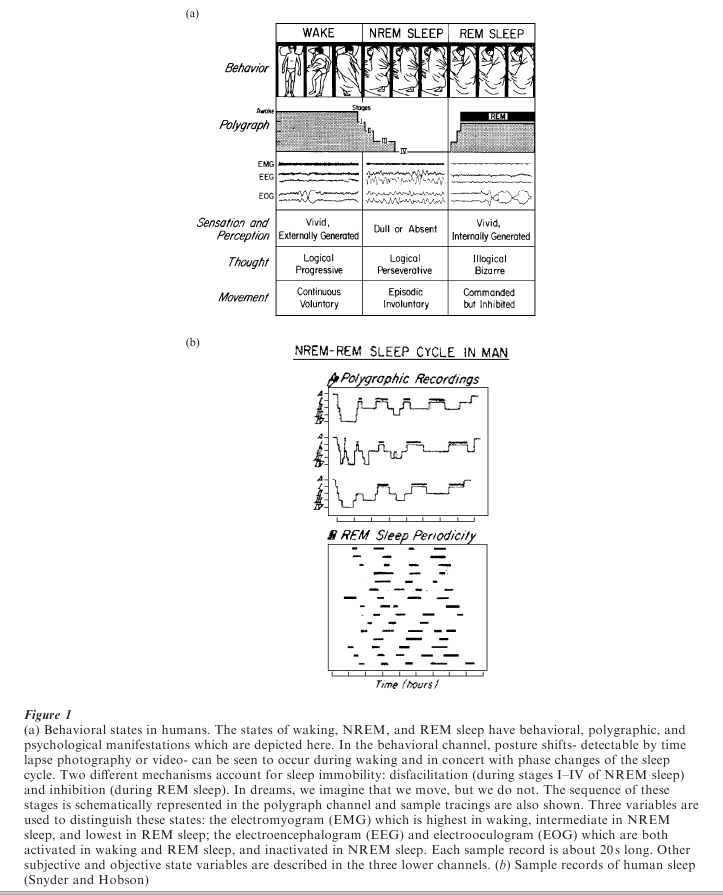
There is a complex organization of behavioral, physiological, and psychological events within each sleep bout. To detect this organization, it is necessary to record the electroencephalogram (EEG) from the surface of the head (or directly from the cortical structures of the brain), to record the movement of the eyes by means of the electrooculogram (EOG), and to record muscle tone by means of the electromyogram (EMG). These three electrographic parameters allow one to distinguish sleep from waking and to distinguish two distinctive and cyclically recurrent phases within sleep: NREM (non-rapid eye movement) and REM (rapid eye movement) sleep. NREM, also known as synchronized or quiet sleep, is characterized by a change in the EEG from a low-amplitude, highfrequency to a high-amplitude, low-frequency pattern (see Fig. 1a).
The degree to which the EEG is progressively synchronized (that is, of high voltage and low frequency) can be subdivided into four stages in humans: In stage one, the EEG slows to the theta frequency range (4–7 cycles per second or cps) and is of low voltage ( < 50 mV and arrhythmic). Stage two is characterized by the evolution of distinctive sleep spindles composed of augmenting and decrementing waves at a frequency of 12–15 cps and peak amplitudes of 100 mV. Stage three is demarcated by the addition to the spindling pattern of high voltage ( > 100 mV) slow waves (1–4 cps), with no more than 50 percent of the record occupied by the latter. In stage four, the record is dominated by high-voltage (150–250 mV) slow waves (1–3 cps). At the same time that the EEG frequency is decreasing and the voltage increasing, muscle tone progressively declines and may be lost in most of the somatic musculature. Slow rolling eye movements first replace rapid saccadic eye movements of waking and then also subside, with the eyes finally assuming a divergent upward gaze (Fig. 1a).
After varying amounts of time, the progressive set of changes in the EEG reverses itself and the EEG finally resumes the low-voltage, fast character previously seen in waking. Instead of waking, however, behavioral sleep persists; muscle tone, at first passively decreased, is now actively inhibited; and there arise in the electrooculogram stereotyped bursts of saccadic eye movement called rapid eye movements (the REMs, which give this sleep state the name REM sleep). Major body movements are quite likely to occur during the NREM–REM transition. The REM phase of sleep has also been called activated sleep (to signal the EEG desynchronization) and paradoxical sleep (to signal the maintenance of increased threshold to arousal in spite of the activated brain). Human consciousness is associated with the low-voltage, fast EEG activity of waking and REM sleep but its unique character in dreaming depends upon other aspects of REM neurophysiology (see Fig. 1b).
In all mammals (including aquatic, arboreal, and flying species) sleep is organized in this cyclic fashion: sleep is initiated by NREM and punctuated by REM at regular intervals. Most animals compose a sleep bout out of three or more such cycles, and in mature humans the average nocturnal sleep period consists of four to five such cycles, each of 90–100 minutes duration. After a prolonged period of wake activity (as in humans) the first cycles are characterized by a preponderance of high-voltage, slow wave activity (i.e., the NREM phase is enhanced early in the sleep bout) while the last cycles show more low-voltage, fast wave activity (i.e., the REM phase is enhanced late in the sleep bout). The period length is fixed across any and all sleep periods in a given individual but is shorter in immature and smaller animals indicating a correlation of NREM–REM cycle length with brain size.
3. Neural Mechanisms Of Sleep
In all animals, sleep is one of a number of circadian behavioral functions controlled by an oscillator in the suprachiasmatic nucleus of the hypothalamus. It is the interaction of the intrinsic propensity to sleep at about 24 hour (i.e., circadian) intervals with the daily cycles of extrinsic light and temperature that gives sleep its diurnal organization. The exact mechanism by which hypothalamic structures act to potentiate sleep at one or another diurnal period is unknown, but increasing evidence suggests that peptide hormones are involved in regulating this coordination.
The hypothesis that sleep is hormonally regulated is an old one and is linked to the subjective impression of fatigue with its implied humoral basis. The fact that the sleep drive increases with the duration of time spent awake in both normal and sleep-deprived animals clearly indicates the homeostatic regulation function of sleep, and experiments begun by Pieron at the beginning of the twentieth century suggested that a circulating humoral factor might mediate this propensity. Subsequent efforts to test the humoral hypothesis have involved the isolation of an S-muramyl peptide as a slow wave sleep-enhancing factor found both in the cerebrospinal fluid of sleep-deprived animals and in normal human urine. It remains to be established that this so-called factor S is involved in physiological sleep mediation; this is most important since factor S is known to be a product of bacterial cell walls but is not produced by the brain.
Whatever its mediating mechanism, the onset of sleep has long been linked by physiologists to the concept of deafferentation of the brain. In early articulations of the deafferentation hypothesis it was thought that the brain essentially shut down when deprived of external inputs. This idea was put forth by Frederic Bremer to explain the results of midcollicular brain stem transection after which the forebrain persistently manifested the EEG synchronized pattern of slow wave sleep. Bremer thought that the so-called cer eau isole (or isolated forebrain) preparation was asleep because it had been deprived of its afferent input. That interpretation was upset by Bremer’s own subsequent finding that transection of the neuraxis at C-1 produced intense EEG desynchronization and hypervigilance in the so-called encephale isole (or isolated brain) preparation.
The possibility that active neural mediation of arousal by brain stem structures situated somewhere between the midcollicular and high spinal levels was thus suggested. The faint possibility that afferent input (via the trigeminal nerve) might account for the difference between the two preparations was eliminated by Giuseppe Moruzzi’s finding that the mediopontine pretrigeminal transected preparation was also hyperalert. Thus the notion of sleep mediation via passive deafferentation was gradually replaced by the idea of sleep onset control via the active intervention of brain stem neural structures. This new idea does not preclude a contribution from deafferentation. And indeed, they appear to be complementary processes.
The clear articulation of the concept of active brain stem control of the states of sleep and waking came from the 1949 finding by Giuseppe Moruzzi and Horace Magoun that high-frequency stimulation of the mid-brain reticular formation produced immediate desynchronization of the electroencephalogram and behavioral arousal in drowsy or sleeping animals. The most effective part of the reticular activating system was its rostral pole in the diencephalon where the circadian clock is located.
These data indicated that wakefulness was actively maintained by the tonic firing of neurons in an extensive reticular domain. Arousal effects could be obtained following high-frequency stimulation anywhere in the reticular formation from the medullary to the pontine to the midbrain and diencephalic levels. The activating effect was thought to be mediated by neurons in the thalamus, which in turn relayed tonic activation to the entire neocortex. This concept has been recently confirmed at the cellular level by Mircea Steriade, following up on the earlier work of Dominic Purpura which had indicated that the spindles and slow waves of NREM sleep were a function of a thalamocortical neuronal interaction that appeared whenever brain stem activation subsided.
Once Moruzzi and Magoun had substantiated the concept of active neural control of the states of waking and sleep, other evidence supporting this notion promptly followed. For example, it was found by Barry Sterman and Carmine Clemente that highfrequency stimulation of the basal forebrain could produce slow wave sleep in waking animals. When the same region was lesioned by Walle Nauta, arousal was enhanced. The cellular basis of these basal forebrain effects and their possible link to both the circadian oscillator of the hypothalamus and the arousal system of the reticular activating system are currently under intense investigation.
The second dramatic demonstration that sleep states were under active neural control was Michel Jouvet’s lesion and transection work suggesting a role of the pontine brain stem in the timing and triggering of the REM phase of sleep. The pontine cat preparation (with ablation of all forebrain structures above the level of the pontine tegmentum) continued to show periodic abolition of muscle tone and rapid eye movements at a frequency identical to the REM periods of normal sleep. The implications were (a) that the necessary and sufficient neurons for timing and triggering by REM were in the pons and (b) that this system became free-running when the pontine generator was relieved of circadian restraint from the hypothalamus.
Small lesions placed in the pontine tegmentum between the locus coeruleus and the pontine reticular formation resulted in periods of REM sleep without atonia: the cats exhibited all the manifestations of REM sleep except the abolition of muscle tone, indicating that intrapontine connections were essential to mediate and coordinate different aspects of the REM sleep period.
4. A Cellular And Molecular Model Of The Sleep– Wake Cycle
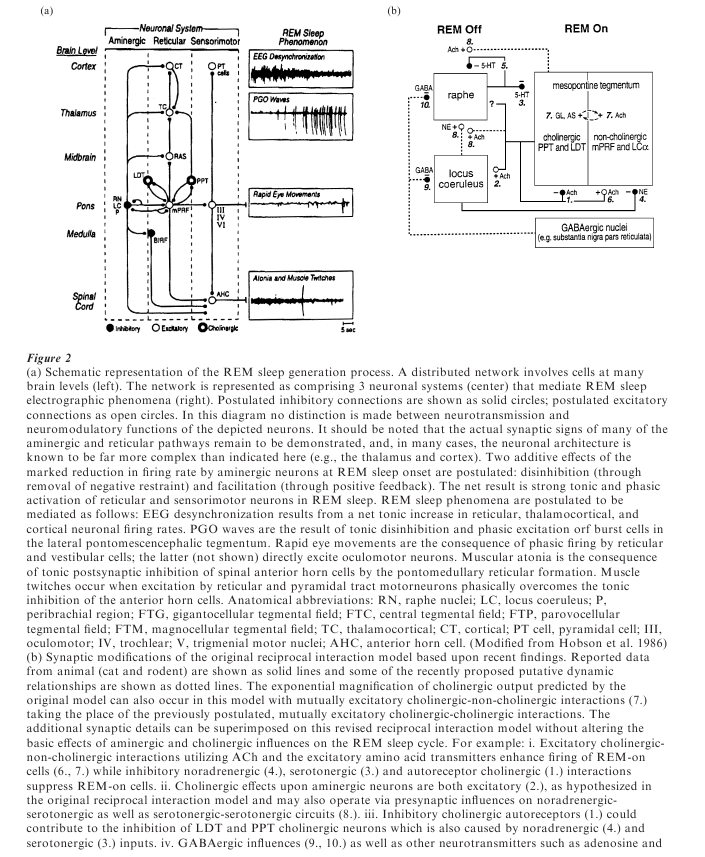
Microelectrode studies designed to determine the cellular mechanism of these effects have indicated that REM sleep is actively triggered by an extensive set of executive neurons ultimately involving at least the oculomotor, the vestibular, and the midbrain, pontine, and medullary reticular nuclei (see Fig. 2). The intrinsic excitability of this neuronal system appears to be lowest in waking, to increase progressively throughout NREM sleep, and to reach its peak during REM sleep. Cells in this system have thus been designated REM-on cells. In contrast, neurons in the aminergic brain stem nuclei (the serotoninergic dorsal raphe and the catacholaminergic locus coeruleus and peribrachial region) have reciprocally opposite activity curves. These aminergic REM-off cells fire maximally in waking, decrease activity in NREM sleep, and reach a low point at REM onset.
The reciprocal interaction model of state control ascribes the progressive excitability of the executive (REM-on) cell population to disinhibition by the modulation (REM-off) cell population. How the aminergic systems are turned off is unknown, but Cliff Saper has recently suggested that GABA-ergic inhibition may arise from neurones in the hypothalamic circadian control system.
5. Cholinergic Rem Sleep Mediation
Confirming the early studies of Jouvet and Raul Hernandez-Peon, numerous investigations have found prompt and sustained increases in REM sleep signs when cholinergic agonist drugs are microinjected into the pontine brain stem of cats. The behavioral syndrome produced by this treatment is indistinguishable from the physiological state of REM sleep, except that it is precipitated directly from waking with no intervening NREM sleep. The cats can be aroused but revert to sleep immediately when stimulation abates. This drug-induced state has all the physiological signs of REM sleep (such as the low voltage fast EEG, EMG atonia, REM, and ponto-geniculo-occipital (PGO) waves), suggesting that it is a valid experimental model for physiological REM. Humans who are administered cholinergic agonist by intravenous injection during the first NREM cycle also show potentiation of REM sleep; this cholinergically enhanced REM sleep is associated, as usual, with dreaming.
Mixed nicotinic and muscarinic agonists (e.g., carbachol), pure muscarinic agonists (e.g., bethanechol) and dioxylane are equally effective potentiators of REM sleep and recent results suggest that activation of the M2 acetylcholine receptor suffices to trigger REM sleep behavior. The inference that acetylcholine induces REM sleep is suggested by Helen Baghdoyan’s finding that neostigmine, (an acetylcholinesterase inhibitor which prevents the breakdown of endogenously released acetylcholine) also potentiates sleep after some delay. All of these effects are dosedependent and are competitively inhibited by the acetylcholine antagonist atropine.
REM sleep induction is obtained only by injection of cholinergic agonists into the pons: Midbrain and medullary injections produce intense arousal with incessant turning and barrel-rolling behavior, respectively. Within the pons, the response pattern differs according to the site of injection, with maximal effects obtained from a region anterodorsal to the pontine reticular formation and bounded by the dorsal raphe and the locus coeruleus nuclei.
In the peribrachial pons, where both aminergic and cholinergic neurons are intermingled, carbachol injection produces continuous PGO waves by activating the cholinergic PGO burst cells of the region. But agonist induction of these drug-induced waves is stateindependent and REM sleep is not potentiated in the first 24 hours following drug injection. It is only later that REM sleep increases reaching its three to fourfold peak at 48–72 hours and thereafter remaining elevated for 6–10 days. By injection of carbachol into the sub and peri-locus coeruleus regions of the pontine reticular formation, where tonic, neuronal activity can be recorded during REM sleep, atonia may be generated, while waking persists, suggesting a possible animal model of human cataplexy.
Cholinergic cells of the PGO wave generator region of the peribrachial pons are known to project to both the lateral geniculate nucleus and the perigeniculate sectors of the thalamic reticular nucleus, where they are probably responsible for the phasic excitation of neurons in REM sleep. This supposition is supported by the finding that the PGO waves of the LGN are blocked by nicotinic antagonists.
Wolf Singer’s recent work suggests the functional significance of these internally generated signals. Singer proposes that the resulting resonance contributes to the use-dependent plasticity of visual cortical networks. Besides providing evidence that REM sleep signs are cholinergically mediated, these recent pharmacological results provide neurobiologists with a powerful experimental tool, since a REM-sleeplike state can be produced at will. Thus Francisco Morales and Michael Chase have shown that the atonia produced by carbachol injection produces the same electrophysiological changes in lumbar motoneurons as does REM sleep: a decrease in input resistance and membrane time constant, and a reduction of excitability associated with discrete IPSPs. These findings facilitate the exploration of other neurotransmitters involved in the motorneuronal inhibition during REM sleep which is mediated, at least in part, by glycine.
Another important advance is the intracellular recording of pontine reticular neurons in a slice preparation by Robert McCarley’s group. Neuronal networks can be activated cholinergically, producing the same electrophysiological changes as those found in REM sleep of intact animals. A low threshold calcium spike has been identified as the mediator of firing pattern alterations in brain-stem slice preparations.
The activation process within the pons itself has also invited study, using carbachol as an inducer of REM sleep. Using the moveable microwire technique, Peter Shiromani and Dennis McGinty showed that many neurons normally activated in REM sleep either were not activated or were inactivated by the drug. KenIchi Yamamoto confirmed this surprising finding and found that the proportion of neurons showing REM-sleeplike behavior was greatest at the most sensitive injection sites.
To achieve greater anatomical precision in localizing the carbachol injection site, James Quattrochi conjugated the cholinergic agonist carbachol to fluorescent microspheres, thereby reducing the rate of diffusion tenfold and allowing all neurons projecting to the injection site to be identified by retrograde labeling. Surprisingly, the behavioral effects are no less potent despite the reduced diffusion rate of the agonist. They are maximized by injection into the same anterodorsal site described by Helen Baghdoyan. That site receives input from all the major nuclei implicated in control of the sleep cycle; the pontine gigantocellular tegmental field (glutamatergic), the dorsal raphe nuclei (serotonergic), the locus coeruleus (noradrenergic), and the dorsolateral tegmental and pedunculo-pontine nuclei (cholinergic). Thus the sensitive zone appears to be a point of convergence of the neurons postulated to interact reciprocally in order to generate the NREM– REM sleep cycle. Whether this is also a focal point of reciprocal projection back to those input sites can now be investigated.
6. Conclusions
Because sleep occurs in a social context, and because sleep actively determines many aspects of that context, its study constitutes fertile ground for integration across domains of inquiry. At the basic science level, the cellular and molecular mechanisms emphasized here reach inexorably down, to the level of the genome. The upward extension of sleep psychophysiology to the psychology of individual consciousness, to dreaming, and hence to the mythology that guides cultures is also advancing rapidly. Altogether absent is a sound bridge to the social and behavioral realms where sleep has been ignored almost as if it were a non-behavior and hence devoid of social significance. Yet when, where, and especially with whom we sleep roots us profoundly in our domestic and interpersonal worlds.
Bibliography:
- Hobson J A 1999 Consciousness. Scientific American Library
- Datta S, Calvo J, Quattrochi J, Hobson J A 1991 Long-term enhancement of REM sleep following cholinergic stimulation. NeuroReport 2: 619–22
- Jones B E 1991 Paradoxical sleep and its chemical structural substrates in the brain. Neuroscience 40: 637–56
- Gerber U, Stevens D R, McCarley R W, Greene R W 1991 Muscarinic agonists activate an inwardly rectifying potassium conductance in medial pontine reticular formation neurons of the rat in vitro. Journal of Neuroscience 11: 3861–7
- Steriade M, Dossi R C, Nunez A 1991 Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: Cortically induced synchronization and brainstem cholinergic suppression. Journal of Neuroscience 11: 3200–17
- Steriade M, McCarley R W 1990 Brainstem Control of Wakefulness and Sleep. Plenum Press, New York




