View sample Infant Visual Development Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Immediately after birth, the visual system has access to structured visual input from the environment that could be used to guide behavior in, and perception of, the external world. Developmental events prior to birth establish rudimentary neural mechanisms that enable some limited perceptual capacities, but further neural developments as well as interactions with the visual environment lead to substantial improvements in these capacities after birth. This research paper covers the emergence of these perceptual capacities during the early postnatal period in human infants (see also recent monographs by Atkinson 2000, Daw 1995, Kellman and Arterberry 1998, Simons 1993). After considering methods that have been used to assess infant vision, the most fundamental aspects of visual development will be reviewed.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
1. Methods For Assessing Infant Vision
Prior to 1960, most anecdotal and published accounts of the visual capacities of human newborns concluded that they were blind or, at best, severely visually impaired. These assessments, however, were based largely on insensitive methods, such as moving a single, small spot of light to elicit from the newborn a change in eye alignment (an ocular following or tracking response). Four methodological breakthroughs (see Gottlieb and Krasnegor 1985) occurred in the early 1960s which revealed that the visual capacities of young infants, although considerably less mature than in adults, were nevertheless quite sophisticated.
1.1 Preferential Looking
In the late 1950s, Robert Fantz revolutionized the study of infant vision by modifying a two-alternative preference technique originally developed for testing visual sensitivity in chickens. Fantz substituted the infant’s looking response for the chicken’s pecking response and presented infants with two side-by-side stimulus locations, only one of which contained a set of black-and-white stripes. Even newborns have a natural tendency to fixate (look at) the side of the display containing the stripes over the side of the display devoid of stripes. An observer viewing the infant’s eyes, and estimating the direction of gaze through a peephole in the stimulus display, judges which of the two stimulus locations is fixated more often or for a longer duration. To guard against bias, the observer is ‘blind’ (i.e., unaware of the location) of the stripes. By systematically varying the width of the stripes, the smallest stripe-width that is just barely resolvable (significantly greater than chance, or 50 percent) can be determined across a series of trials in which the location of the stripes is randomly varied.
Teller (1979) established rigorous standards for using infant looking preferences to assess stimulus detection (a choice between a stimulus and no-stimulus). Teller coined the term forced-choice preferential looking (FPL) to describe this technique. FPL can also be used to assess stimulus discrimination by presenting two stimuli simultaneously. Evidence of a significant preference allows for the conclusion that infants can discriminate the difference between the two stimuli. However, the absence of a significant preference is ambiguous because the two stimuli may be discriminable but fail to elicit a clear preference in looking behavior.
1.2 Visual Evoked Potentials
Surface electrodes attached to the skull can record small changes in synchronized electrical activity from various brain regions. The amplitude and latency of several peaks and troughs in the visual evoked potential (VEP) vary with the size and intensity of a visual stimulus. As with preferential looking, VEPs can be used to assess the smallest stripe-width that is just detectable. VEPs can also be used to assess visual discrimination by measuring the averaged response to a change from one stimulus to a different stimulus and comparing it to the averaged response to no-change control trials.
1.3 Fixations And Eye-Movement Scanpaths
Newborns show poor evidence of steady and consistent fixations of a small visual target, and their ability to track (follow) a small moving target is very poor. They will, however, follow a set of moving stripes. This following response is called optokinetic nystagmus (OKN) because it consists of alternations between a smooth tracking response in the direction of stripe movement and a rapid saccadic eye movement in the direction opposite to stripe movement (as the eyes move from one extreme orbital position to the other). As with FPL and VEP, OKN can be used to assess the smallest stripe-width that is just detectable. OKN can be measured by direct observation or by one of a variety of eye-tracking devices, such as electrooculography (EOG) or video-based images of the pupil and reflections on the front surface (cornea) of the eye. These latter corneal reflection techniques were developed by William Kessen and his colleagues (see Haith 1980) and provided the first detailed assessments of moment-to-moment sequences of visual fixations (scanpaths) by newborns as they viewed a simple visual stimulus.
1.4 Habituation
Fantz noted in his early studies of preferential looking that when the same two stimuli were presented side by side, fixation durations declined across repeated trials. This decrement in looking duration is called habituation and indicates that the infant has processed and retained some information about the stimulus across time. Subsequent elaborations on the use of habituation as a measure of visual discrimination (Bornstein 1985) have resulted in an ‘industry standard’ that consists of two phases: habituation and test. During habituation, a single visual stimulus is presented and an observer records how long the infant sustains fixation (interrupted by no more than two seconds of distraction) to that stimulus on a given trial. Identical trials are repeated, tallying fixation duration until there is a preset decrement (typically 50 percent) from the initial level of looking on the first several trials. When this criterion of habituation has been met, the infant enters a test phase in which the same habituation stimulus and a novel stimulus are presented on alternating trials. Continued low levels of looking to the habituation stimulus and significant increases (recovery) of looking to the novel stimulus are taken as evidence of discriminating the habituation (familiar) from the novel stimulus.
2. Resolving Fine Detail
2.1 Acuity
Perhaps the most basic question one could ask about infant vision is how well they can see small objects. This ability to resolve fine detail, called visual acuity, is what is assessed by an ophthalmologist or optometrist using an eye chart. Because infants cannot verbally report the smallest letter-size on an eye chart, one of the foregoing non-verbal techniques must be used to estimate their visual acuity.
FPL, VEP, and OKN have been used to assess visual acuity in young infants. In each case, black-and-white stripes (or checks) are systematically varied in size to determine the smallest size that elicits a reliable response. Although there are some differences in estimates of visual acuity using these three methods, two facts are quite clear. First, visual acuity in newborns is approximately five octaves (an octave is a doubling) worse than in normal adults. Using the Snellen notation, where 20/20 refers to normal visual acuity (average performance at a viewing distance of 20 feet), newborn visual acuity is approximately 20/640. This means that a newborn can see at 20 feet what a normal adult can see at 640 feet. Alternatively, a newborn must be five times closer to a visual stimulus than an adult (7.5 inches vs. 20 feet) to resolve it equally well. Second, there is a rapid improvement in visual acuity between birth and six months of age, with average acuity rising to 20 40 within this postnatal period (Norcia and Tyler 1985).
2.2 Contrast Sensitivity
Most objects are not composed of features at the limit of resolution, but rather contain features sufficiently large to be well above the acuity threshold. For these features, size is less important than contrast. Contrast refers to the difference in luminance between adjacent object features. For example, a pair of black-and- white stripes has very high contrast because the luminance difference is large. As luminance is added to the black stripe and subtracted from the white stripe, the stripes become different shades of gray and have lower contrast. At the limit, when the stripes are equal shades of gray, contrast is zero and the stripes are no longer visible. Thus, for any feature above the acuity threshold, visibility is determined by sensitivity to contrast.
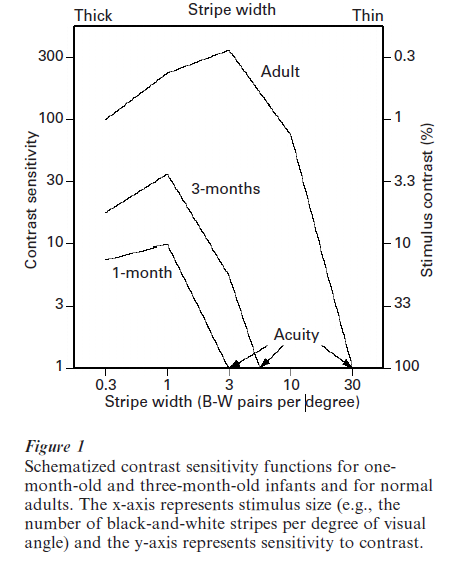
Figure 1 illustrates a typical contrast sensitivity function (CSF) obtained from an adult, as well as CSFs obtained from young infants (Kellman and Banks 1998, Norcia et al. 1990). Notice that the adult CSF peaks at features with medium size and falls off for both smaller and larger sizes. The point on the far right of the CSF, where size is smallest and contrast sensitivity is least (i.e., stimulus contrast is maximal), is the estimate of visual acuity. The lower heights of the infant CSFs indicate that they are much less sensitive to contrast than adults at all feature sizes, and of course at maximal stimulus contrast visual acuity is far poorer. Importantly, even at medium feature sizes where both infants and adults can resolve a stimulus at high contrast, infants require much more contrast for minimal detection of that stimulus. Because many features of objects have low contrast, these features are invisible to the infant’s visual system.
2.3 Limiting Factors
The two most obvious limitations on acuity and contrast sensitivity in early infancy are optical quality and neural immaturity. Adults with an optical error requiring spectacles or contact lenses show deficits in both acuity and contrast sensitivity when they fail to wear their correction. Although a small proportion of infants have optical errors (myopia: nearsightedness; hyperopia: farsightness; astigmatism: errors that vary by stimulus orientation), the five-fold deficit in newborn acuity cannot be accounted for by optical errors (Banks and Bennett 1988). Another potential contributor to optical quality is accommodation (change in shape) of the lens in the eye, which optimizes the focus of the retinal image for different viewing distances. Although newborns show poor accommodative control, this deficit is the product of poor visual acuity; that is, the immature visual system is unable to detect subtle changes in stimulus blur that trigger an accommodative response (Kellman and Banks 1998).
These results suggest that the fundamental limitation on acuity and contrast sensitivity is neural, not optical. Classic data on the developmental anatomy of the visual cortex in human infants (Conel 1939) documented substantial elaborations of neural structures (e.g., density of synapses) during the first two postnatal years. However, more recent data on the developmental anatomy of the retina suggest that much of the five-fold improvement in acuity can be accounted for by the increasing density of photo- receptors in the center (fovea) of the retinas and the inefficiency of photoreceptors (the outer segments) in capturing light (Banks and Bennett 1988).
Both maturational and experiential factors influence the development of acuity. The time-course of developmental improvement in acuity is more closely linked to the infant’s age post-conception than to its age post-birth, suggesting that normal visual experience after birth plays a relatively minor role in this developmental process. However, the absence of normal visual experience after birth leads to substantial deficits in acuity. Visual deprivation in the form of cataracts (opacities in the eye that prevent a clear retinal image) slows down the time-course and eventual level of acuity unless the cataract is removed very early in infancy (Maurer and Lewis 1993).
3. Color
Chromatic discrimination requires at least two classes of photoreceptors (cones) that are sensitive to slightly different wavelengths of light. FPL, VEP, and OKN assessments confirm that the medium-wavelength (green) and long-wavelength (red) photopigments are functional by two months of age. However, the immaturity of the photoreceptor outer segments (see Sect. 2.3) limits the number of different hues that young infants can discriminate (Banks and Bennett 1988). Clear evidence that infants are trichromats (i.e., have the red, green, and short-wavelength sensitive blue cones required for normal color vision) is not present until four months of age (Teller and Bornstein 1987). Color constancy, the ability of infants to perceive the correct hue despite variations in lighting (e.g., ignoring the reddish tint at sunset), emerges between two and five months of age (Dannemiller 1989).
4. Motion
Moving stimuli are more effective at capturing infants’ attention than stationary stimuli. This natural bias to attend to moving stimuli has been used in preferential looking studies to estimate motion thresholds: the minimum stimulus speed required to discriminate a moving from an otherwise identical stationary stimulus. Motion thresholds improve dramatically in early infancy despite nearly adult-like temporal sensitivity at birth, suggesting that these improvements in motion sensitivity rely critically on improvements in spatial resolution (Roessler and Dannemiller 1997).
Discrimination of different directions of motion is the definitive test of a motion mechanism because the responses of single neurons in primary visual cortex and many extrastriate cortical areas in the dorsal visual pathway have a preferred direction of stimulus motion. However, both FPL and habituation techniques have failed to demonstrate infants’ discrimination of opposite directions of stimulus motion until two months of age. This is surprising because even newborns show directionally appropriate eye movements to stripe motion during OKN. Asymmetries in the direction of OKN (temporal ward stripe motion is ineffective in eliciting monocular OKN in newborns) are mirrored in the VEP. Thus, the processing of motion direction appears to be mediated by at least two different neural mechanisms. One of these mechanisms is presumably subcortical and is capable of controlling directional eye movements (i.e., OKN) in newborns, whereas the other mechanism is presumably cortical and does not support direction discrimination until several weeks after birth (Banton and Bertenthal 1997).
5. Orientation And Vernier Acuity
Another fundamental property of neurons in the visual cortex is orientation sensitivity, and this property is present in rudimentary form in newborn cats and monkeys. Human newborns are also sensitive to orientation, but only for gross differences (e.g., horizontal vs. vertical stripes). A VEP masking technique suggests that orientation sensitivity continues to improve throughout the first postnatal year (Skoczenski 2001).
Vernier or displacement acuity refers to the ability to discriminate the spatial misalignment of two line segments that are abutted end to end. In adults, vernier acuity is far superior to grating (stripe-width) acuity, whereas in infants prior to four months of age vernier acuity is poorer than grating acuity. This developmental difference in the relation between vernier and grating acuity has been documented using both FPL and VEPs (Skoczenski 2001). One potential cue for solving a vernier acuity task is the presence of a slight orientation change at the point where the two line segments meet. Thus, the protracted development of orientation sensitivity may acount for the developmental crossover in the relation between Vernier and grating acuity.
6. Depth And Binocular Rivalry
The relative distance (depth) of objects can be appreciated using three different sources of information: motion, retinal disparity, and pictorial cues. A rapidly approaching (looming) stimulus elicits a blink response in one-month-olds, and motion parallax (more rapid image speed for near than for far objects) enables three-month-olds to discriminate small differences in object distance. Thus, sensitivity to depth from motion is present in very early infancy and does not require the use of both eyes (Kellman and Arterberry 1998).
Retinal disparity refers to the subtle differences in the images projected to the two retinas from an object at near (less than five meters) viewing distances. FPL and VEP studies have demonstrated that sensitivity to retinal disparity does not emerge until three to four months after birth. Moreover, the smallest retinal disparity that is just discriminable by infants improves very rapidly between three and five months of age, progressing from no sensitivity to nearly adult values (less than one minute of arc) in this age range (Birch et al. 1985).
During this same age range, infants become sensitive to binocular rivalry: the perceptual conflict induced by presenting grossly different images to the two retinas (e.g., horizontal stripes in one eye and vertical stripes in the other). Binocular rivalry occurs when the discrepant retinal images cannot be fused into a single percept. Prior to three months of age, infants appear to have a much greater tolerance for fusing discrepant images than adults, perhaps because of their poor acuity and contrast sensitivity (Birch et al. 1985).
In adults, failure to align both foveas onto a stimulus typically leads to binocular rivalry and prevents stereopsis (the appreciation of depth from retinal disparity). Some individuals, including some infants, have an ocular misalignment (strabismus) that eliminates fusion and stereopsis. If uncorrected in infancy, this misalignment can result in a permanent loss of the capacity for stereopsis, even if the eyes are surgically realigned in childhood (Banks et al. 1975). Thus, there is a sensitive period during which a normally developing neural mechanism for stereopsis, present by four months of age, can be permanently disabled by subsequent abnormal binocular experience (strabismus). These same processes were earlier demonstrated in the visual cortex of cats and monkeys, and subsequently confirmed behaviorally.
Pictorial cues to depth are contained in flat (two-dimensional) representations of actual (three-dimensional) scenes. These cues include shading, occlusion, and linear perspective (e.g., receding railroad tracks that converge in the picture plane). A preferential reaching technique was developed by Albert Yonas to determine, under monocular viewing conditions, whether infants perceive the depth information in pictures. At approximately seven months of age, infants under these testing conditions become sensitive to a variety of pictorial cues to depth, as indicated by their reliable reaching for the apparently nearer picture (Yonas and Granrud 1985).
7. Object Perception
As visual stimuli, objects are bounded three-dimensional collections of features. Even newborns fixate contours (high-contrast edges), suggesting that they can segregate a rudimentary object from its background. However, many objects are comprised of embedded sets of features, such as faces which have external (e.g., hairline) and internal (e.g., eyes) contours. Newborns fail to fixate small, stationary internal contours of embedded-feature objects such as faces, presumably because of limitations in acuity and contrast sensitivity. After two months of age, infants both fixate and discriminate (as indicated by habituation studies) internal and external features, and often prefer to fixate internal features (Maurer and Salapatek 1976).
Objects can be extracted from a camouflaged background using differences in texture or motion. While adults can easily extract a single discrepant target element from an array of background elements, infants require highly salient element differences for such texture segregation, including target motion or multiple discrepant target elements (Sireteanu 2000). Orientation differences are particularly difficult for infants to use in texture segregation, consistent with the evidence (see Sect. 5) that sensitivity to orientation has a lengthy developmental timecourse.
By three months of age, the two-dimensional shape of an object can be extracted from coherent motion of a group of elements in a background of identical, but incoherently moving, elements. The three-dimensional shape of a rigid object can be extracted by four months of age from rotational motion that reveals the hidden perspectives of stationary views of the object. And the three-dimensional shape of non-rigid objects can be extracted by five months of age from displays consisting solely of disconnected points undergoing relative motion that simulates a human walker (Bertenthal 1993).
The actual size and shape of an object must be derived from properties of the retinal image and from object distance. For example, the retinal image of a familiar object (e.g., a dollar bill) varies in size when viewed at arms length or across a room, and in shape when viewed directly or from an oblique angle. Yet in all these cases we perceive the dollar bill to retain a constant physical size and shape. Size and shape constancy have been demonstrated in infants as young as newborns, suggesting that mechanisms for combining retinal image properties and object distance (presumably from motion cues) are functional with little or no postnatal experience (Kellman and Arterberry 1998).
Objects are often partially occluded by other objects, yet adults perceive objects as ‘complete’ despite the absence of various parts behind the occluder. Newborns do not appear to have an intact mechanism for perceptual completion, whereas four-month-olds do (Johnson 2000). Two-month-olds show perceptual completion, but only when given additional information for the continuity of the occluded object (e.g., a smaller occluder or one with gaps in it). And four-month-olds fail to show perceptual completion when the occluded object is stationary, suggesting that coherent motion of the visible object-parts binds them together into a single object.
8. Developmental Mechanisms
The development of mature visual perception during early infancy is influenced by both maturational and experiential mechanisms. Maturational factors include neural developments, such as the migration of photoreceptors (increasing the packing density of cones in the fovea), the elongation of cone outer segments, and cell death (apoptosis). One result of such maturational factors is a reduction in the intrinsic neural noise that limits stimulus detection and discrimination (Skoczenski 2001). Experiential factors include periods of susceptibility to altered visual input. Although the range of visual inputs sufficient to enable ‘normal’ visual development is quite broad, visual deprivation (e.g., cataracts or strabismus) during a sensitive period can lead to permanent deficits in visual development.
Bibliography:
- Atkinson J 2000 The Developing Visual Brain. Oxford University Press, New York
- Banks M S, Bennett P J 1988 Optical and photoreceptor immaturities limit the spatial and chromatic vision of human neonates. Journal of the Optical Society of America A 5: 2059–79
- Banks M S, Aslin R N, Letson R D 1975 Sensitive period for the development of human binocular vision. Science 190: 675–7
- Banton T, Bertenthal B I 1997 Multiple developmental pathways for motion processing. Optometry and Vision Science 74: 751–60
- Bertenthal B I 1993 Infants’ perception of biomechanical motions: Intrinsic image and knowledge-based constraints. In: Granrud C (ed.) Visual Perception and Cognition In Infancy: Carnegie Mellon Symposium On Cognition. Erlbaum, Hillsdale, NJ, pp. 175–214
- Birch E E, Shimojo S, Held R 1985 Preferential-looking assessment of fusion and stereopsis in infants aged 1 to 6 months. Investigative Ophthalmology and Visual Science 26: 366–70
- Bornstein M H 1985 Habituation of attention as a measure of visual information processing in human infants: Summary, systematization, and synthesis. In: Gottlieb G, Krasnegor N A (eds.) Measurement of Audition and Vision in the First Year of Postnatal Life: A Methodological Overview. Ablex, Norwood, NJ, pp. 253–300
- Conel J L 1939 The Postnatal Development of The Human Cerebral Cortex. Harvard University Press, Cambridge, MA
- Dannemiller J L 1989 Computational approaches to color constancy: Adaptive and ontogenetic considerations. Psycho-logical Review 96: 255–66
- Daw N W 1995 Visual Development. Plenum Press, New York
- Gottlieb G, Krasnegor N A 1985 Measurement of Audition and Vision in the First Year of Postnatal Life: A Methodological Overview. Ablex, Norwood, NJ
- Haith M M 1980 Rules That Babies Look By. Erlbaum, Hillsdale, NJ
- Johnson S P 2000 The development of visual surface perception: Insights into the ontogeny of knowledge. In: Rovee-Collier C, Lipsitt L P, Hayne H (eds.) Progress in Infancy Research. Ablex, Mahwah, NJ, Vol. 1
- Kellman P J, Arterberry M E 1998 The Cradle of Knowledge: Development of Perception in Infancy. MIT Press, Cambridge, MA
- Kellman P J, Banks M S 1998 Infant visual perception. In: Damon W, Kuhn D, Siegler R S (eds.) Handbook of Child Psychology: Cognition, Perception, and Language. Wiley, New York, Vol 2
- Maurer D, Lewis T L 1993 Visual outcomes in infant cataract. In: Simons K (ed.) Early Visual Development: Normal and Abnormal. Oxford University Press, New York, pp. 454–84
- Maurer D, Salapatek P 1976 Developmental changes in the scanning of faces. Child Development 47: 523–7
- Norcia A M, Tyler C W 1985 Spatial frequency sweep VEP: Visual acuity during the first year of life. Vision Research 25: 1399–405
- Norcia A M, Tyler C W, Hamer R D 1990 Development of contrast sensitivity in the human infant. Vision Research 30: 1475–86
- Roessler J S, Dannemiller J L 1997 Changes in human infants’ sensitivity to slow displacements over the first 6 months. Vision Research 37: 417–23
- Simons K 1993 Early Visual Development: Normal and Abnormal. Oxford University Press, New York
- Sireteanu R 2000 Texture segmentation, ‘pop-out,’ and feature binding in infants and children. In: Rovee-Collier C, Lipsitt L P, Hayne H (eds.) Progress in Infancy Research. Ablex, Mahwah, NJ, Vol 1
- Skoczenski A M 2001 Limitations on visual sensitivity during infancy: Contrast sensitivity, vernier acuity and orientation processing. In: Rovee-Collier C, Lipsitt L P, Hayne H (eds.) Progress in Infancy Research. Ablex, Mahwah, NJ, Vol 2
- Teller D Y 1979 The forced-choice preferential looking procedure: A psychophysical technique for use with human infants. Infant Behavior and Development 2: 135–53
- Teller D Y, Bornstein M H 1987 Infant color vision and color perception. In: Salapatek P, Cohen L (eds.) Handbook of Infant Perception: From Sensation to Perception. Academic Press, New York, Vol. 1, pp. 185–236
- Yonas A, Granrud C E 1985 Reaching as a measure of infants’ spatial perception. In: Gottlieb G, Krasnegor N A (eds.) Measurement of Audition and Vision in the First Year of Postnatal Life: A Methodological Overview. Ablex, Norwood, NJ, pp. 301–22




