View sample Groundwater and Terrestrial Water Pollution Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our custom writing service for professional assistance. We offer high-quality assignments for reasonable rates.
1. Introduction
Water is an essential component of life. Plants and animals (including human beings) are dominantly made up of water, and rely on water to regulate temperature, carry dissolved materials inside and between cells, remove waste products, etc. Organisms cannot survive without a supply of water—human beings need to consume approximately 1.5 litres of water per day, and this water needs to be fresh (i.e., lacking in appreciable quantities of dissolved minerals) and clean. Human civilization has traditionally been found in areas where clean water is readily available for consumption and for agriculture, but, with increasing population densities and increasing industrialization, the freshwater resources on which civilization relies are becoming increasingly strained—an estimated 1,200 million people worldwide lack a satisfactory or safe water supply (Pickering and Owen 1997). Along with overconsumption and resulting water shortages, the introduction of substances hazardous to human health into terrestrial water bodies (or pollution) is the most serious threat to the world’s freshwater supplies. This research paper examines the scale and impacts of pollution in terrestrial, freshwater bodies such as rivers, lakes, and groundwater (underground water, specifically that below the water table). Pollution of the marine environment is examined in Water Pollution: Marine.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
2. Water Pollution
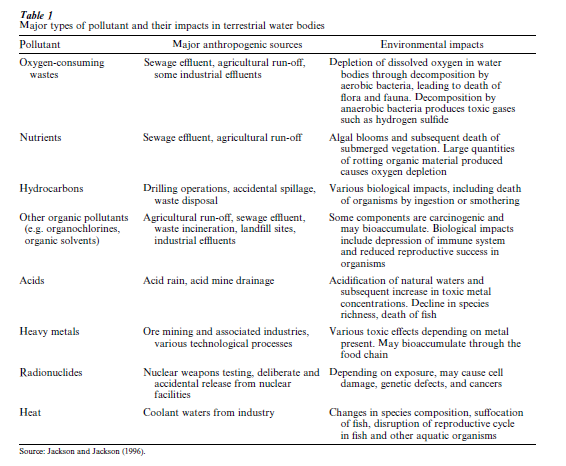
Pollution can be defined as ‘the introduction by man into the environment of substances or energy liable to cause hazards to human health, harm to living resources and ecological systems, damage to structures or amenity, or interference with legitimate uses of the environment’ (Holdgate 1979). The major pollutants in freshwaters vary across the world, but pollution by sewage wastes is a major problem in ‘underdeveloped’ nations, whereas pollution from industrial effluents is, arguably, the dominant problem in overdeveloped nations (although sewage and agricultural pollution may also be serious problems). Industrial effluents may contain a variety of hazardous components, such as radioactive materials, hydrocarbons, heavy metals, and toxic organic compounds. Thermal pollution, due to discharge of cooling waters, may also have a significant effect on the biology of water bodies. A summary of the major types of water pollutants and their impacts is given in Table 1.
Many of the substances that cause pollution of water bodies are naturally present in the environment, and organisms tolerate, or even actively require, certain concentrations of these substances. It is the accumulation of these substances beyond safe levels, or the additive effects of a series of pollutants, that leads to detrimental impacts on the environment. For example, nitrates are essential to plant growth, but excess nitrate may cause eutrophication in lakes and rivers and contamination of groundwater supplies (see Sect. 3.2). In some areas, water bodies may be naturally contaminated to toxic levels. In Bangladesh and West Bengal, large volumes of groundwater used for public water supply are polluted with naturally occurring arsenic, which adversely affects the health of millions of people. Arsenic concentrations of up to 1000 µgl-1 have been measured, well above the limits set for Bangladesh drinking water (50 µg l-1) or the maximum concentrations recommended by the World Health Organisation (10 µg l-1). Consumption of this water has led to widespread death and disease (Nickson et al. 1998). Conversely, many organic pollutants, such as some pesticides and industrial chemicals, are entirely artificial and so have no natural background concentration in the environment.
Following their release, pollutants undergo a variety of physical, chemical, and biological processes which act to change their concentration, and often their chemical form. Of the physical processes, mixing and dilution are particularly important. Waste discharged into a fast-flowing river will rapidly be washed downstream and diluted, whereas wastes which seep into reservoirs or groundwater may have long residence times—the water in lakes may take between 10 and 100 years to be replaced, while for groundwater the timescales may be even longer. Consequently, pollutants may build up over time, providing a long-term contamination problem. Other processes which can dramatically affect the concentration of pollutants in water bodies are adsorption and removal onto sediments, and uptake and breakdown by organisms. Sediments and soil particles, particularly those rich in organic material or clays, effectively adsorb a range of polluting substances, such as heavy metals, many organic pollutants, and some radioactive contaminants. Organisms may break down pollutants, e.g., the microbial oxidation and decomposition of sewage sludges, or may influence the transport of the pollutant, e.g., the methylation of elements such as arsenic and mercury by micro-organisms into more volatile (easily evaporated) forms. The impact of any pollutant discharged into a water body will largely depend on:
(a) the scale of the input (i.e., is the substance(s) discharged in large enough amounts to have a discernible effect on biological function?);
(b) the chemical form of the pollutant (e.g., will it undergo rapid removal onto bottom sediments, will it tend to accumulate in organisms?);
(c) the physical, chemical, and biological characteristics of the receiving water body (e.g., is the water body vigorously mixed, is it acidic or alkaline, oxygenrich or reducing, etc.?).
As discussed above, a diverse range of pollutants may be present in freshwater systems. A detailed synthesis of the environmental behavior and impacts of each of these individual pollutants is beyond the scope of this research paper, and the reader is referred to the Bibliography:. Instead, the potential impacts and environmental behavior of these pollutants in terrestrial water bodies is perhaps best shown by considering three case studies which illustrate the impacts of three major waste categories on terrestrial water bodies: (a) wastes from mineral extraction and processing, (b) agricultural wastes, and (c) urban and industrial hazardous wastes.
3. Case Studies
3.1 Legacy of Current and Historical Mining Activity
None of the 40 main elements that are economically important to modern industrial society can be synthetically made. Instead, they must be mined, extracted, and purified, which inevitably generates waste material and frequently causes water pollution. Mining of coal and metal ores generates vast amounts of waste material that contains significant quantities of metals. These wastes are generally dumped to form spoil heaps around the mining area, or may be washed into rivers. Sulfide minerals, most commonly iron sulfide (FeS ) or pyrite, are commonly associated with coal seams and metal ores, and exposure of these minerals to oxygen and water produces reactions of the following type:
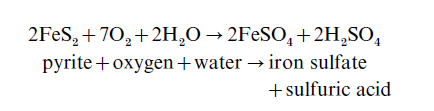
Hence, the pyrite oxidizes to form iron sulfate and sulfuric acid. This generates acid mine drainage, which lowers the pH of water bodies into which it flows, dramatically affecting ecosystem characteristics, and which may solubilize toxic heavy metals associated with the mine waste. Extraction and purification of the mined material may also cause large-scale pollution of water bodies. Use of mercury to extract gold from river sediments in the Amazon region of Brazil has caused poisoning in people living around and downstream from mining areas (e.g., Homewood 1991), while cyanide-contaminated water accidentally released from the Baia Mare goldmine in northwest Romania recently devastated freshwater ecosystems along the River Tisza in Hungary.
In countries which underwent early industrialization, such as the UK, the rapid development and subsequent decline of the mining industry means that pollution from abandoned mines is arguably a bigger threat to water quality than contemporary mining activity. In Cornwall, the former center of the UK tin mining industry, a land area of around 50 km2 is covered by mine wastes derived from historical working of tin and copper deposits and large-scale extraction of kaolinite. Some of these wastes contain elevated concentrations of heavy metals and have a low pH from the oxidation of sulfide minerals, which promotes the leaching of heavy metals. The release of waste particles from the extraction and washing of mined materials has led to extensive siltation of rivers and estuaries, while groundwater percolating through disused mines has produced acid mine drainage. The most obvious example of this is the 1992 Wheal Jane incident, where 50 × 106l of low pH, heavy-metalenriched mine water was released into Restronguet Creek following overflow of groundwater from Wheal Jane (a recently closed mine). The biological impact of this event, however, was relatively minor, and the heavy metal input small in comparison to the existing amounts of heavy metals in the sediments derived from historical mining activity.
In the Mendip Hills, southwest England, lead concentrations in soils around some former Pb–Zn mine workings are over five percent, over 500 times higher than lead concentrations in uncontaminated soil. Much of the contaminated material is in the form of lead-rich particles, which have been mixed into the local soil and subsequently covered by vegetation. Hence, the danger of human exposure is dramatically reduced, provided the surface remains undisturbed. The neutral to alkaline pH of the soil, and the presence of the lead in a mineral form that is stable in the presence of oxygen, i.e., as a carbonate mineral rather than a sulfide mineral, means that little lead is mobilized or leached from the site. In mine waste dumps generally, toxic heavy metals may be stabilized and water contamination reduced through inhibiting leaching via the insertion of clay or geotextile liners, and maintaining pH conditions suitable for heavy metal containment (e.g., where sulfide minerals are present, constructing a low pH, low oxygen environment such as a wetland).
3.2 Groundwater And River Contamination From Intensive Agriculture
Humans have practiced agriculture for thousands of years. Shifting cultivation, rough grazing of livestock, and crop rotation systems were the dominant agricultural methods until the 1950s, when these were largely superceded by intensive agricultural techniques in Europe and North America. In intensive agriculture, traditional crop rotation is replaced by continuous cultivation of crops, which requires the addition of large amounts of nutrients to replace those lost during harvesting. This is commonly done through the application of phosphate and nitrate-containing inorganic fertilizers. Wash-off of these fertilizers into terrestrial water bodies may cause artificial eutrophication to occur, where enrichment of nutrients (particularly phosphate) causes excessive growth of surface algae. These so-called algal blooms prevent sunlight reaching deeper-dwelling aquatic species, and on death produce large quantities of organic matter which may cause oxygen depletion (see below). It is estimated that in the USA, 85 percent of large lakes near major urban areas are affected by artificial eutrophication (Jackson and Jackson 1996).
The extensive use of nitrogen fertilizers has also increased the nitrate content of surface and ground waters over the last 50 years. Nitrates are not adsorbed in soils, but are instead leached into surface water bodies and groundwater, waters which may subsequently be used for drinking water supplies. Excessive nitrate in drinking water can cause a severe blood disorder, ‘blue baby syndrome’ or methemoglobinemia, in babies under six months of age, and may possibly contribute to the synthesis of carcinogenic nitrosamines in the human body. In the UK, the maximum allowable nitrate concentration in drinking waters is 50 mg NO3 l-1. In intensively farmed areas of the southern UK, such as East Anglia, however, peak winter nitrate values in rivers may exceed 100 mg NO3?l-1 (Alloway and Ayres 1993).
In addition to continuous crop cultivation, the development of intensive agriculture has led to in-creased use of factory farming methods for livestock. The organic wastes produced by large concentrations of livestock can have a serious effect on the oxygen content of water bodies. The bacterially driven break-down of organic wastes released into natural waters consumes oxygen, e.g.
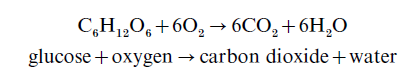
Hence, the introduction of concentrated organic wastes can deplete water bodies of oxygen, with detrimental impacts on flora and fauna. In extreme cases, where the rate of utilization of oxygen is greater than the rate at which oxygen is replenished (e.g., in canals or slow-flowing rivers), all the available oxygen may be consumed. These conditions favor anaerobic bacteria, which produce toxic hydrogen sulfide and ammonia.
3.3 The Love Canal Disaster And Landfill Of Hazardous Wastes
All industrial processes produce waste. Modern lifestyles are dependent upon a vast range of chemicals and their by-products, e.g., a typical office contains plastics, artificial floor coverings, computer components, etc. Synthesizing and producing these components inevitably produces waste, which needs to be recycled or disposed. A fraction of the waste produced may contain substances hazardous to human health, and disposal of these wastes needs to be carefully controlled. A variety of recycling and disposal options exist, ranging from uncontrolled direct dumping in water bodies such as rivers to removal and recycling of the hazardous wastes for use in other industrial processes. Where it is uneconomic or unfeasible to recycle the toxic components, incineration at high temperature is often used to break down various toxic species, although the incineration process is never 100 percent efficient, and may increase the toxicity of some waste products. For example, incomplete combustion of polychlorinated biphenyls (PCBs) in wastes can lead to the formation of toxic dioxin-containing products. An alternative and widely used disposal method is disposal at landfill sites. Landfill sites receive large quantities of domestic and commercial waste, mixed with varying quantities of hazardous wastes. Current landfill design uses a multibarrier disposal approach, where a series of barriers are put in place which are designed to slow the release of toxic components.
Dilution and dispersion, chemical reactions and biological processes (e.g., bacteria may break down some toxic organic components into less toxic forms, acid wastes may be neutralized by contact with surrounding alkaline rocks), reduce the toxic chemical species to acceptable concentrations before they come into contact with the public via surface and groundwater. The safe and long-term disposal of hazardous wastes, however, requires a thorough understanding of the physical, chemical, and biological processes occurring in and around the landfill site. In particular, groundwater movement and groundwater quality need to be monitored to assess whether toxic chemicals are being leached into water supplies. An illustration of the importance of groundwater movement in the landfill disposal of toxic wastes is provided by the disaster at New York State’s Love Canal.
Love Canal was initially dug in the 1890s by William Love to connect the upper and lower Niagara River and provide hydroelectric power. The canal was subsequently abandoned until 1942, when it was purchased by the then Hooker Electrochemical Company. Over the next 10 years, the company dumped 22,000 tons of toxic chemical waste at the site, including carcinogenic lindane, chlorobenzenes, and dioxin-contaminated trichlorophenol. Population pressure from the City of Niagara Falls led to Hooker selling the site to the city school board. Prior to the sale the wastes were covered with a protective clay layer, and a school, houses, and roads were subsequently built over the landfill.
The clay cap overlying the canal, coupled with the fact that the canal was cut into the underlying clay geology, largely isolated the waste by limiting water migration (clay tends to retard water flow, and many pollutants will adsorb on to clays, see Sect. 2). The construction of a major road along the southern end of the canal in the 1960s, however, significantly altered the site hydrology. The road blocked the natural through-flow of groundwater at the site, which, until then, had been southward towards the Niagara River. Heavy rain and snow melt in the winter of 1977 caused a rise in groundwater levels, and unable to flow southwards, this groundwater filled the canal, which eventually overflowed. Contaminants migrated through surface soil layers and along sewer pipes, eventually entering the basements of houses around the site. Some buried drums of waste also broke through the overlying soil. Following national press coverage in 1978, a federal state of emergency was declared, and the worst-affected houses were evacuated, purchased by the state, and destroyed. A full review of the disaster and its aftermath is given in Hoffman (1995).
Although the Love Canal disaster is an extreme case, it does illustrate the potential long-term threat to terrestrial water quality of inadequately managed hazardous wastes. Disposing of any waste underground needs a careful consideration of water infiltration and groundwater movement—for example one of the major limitations regarding deep geological disposal of nuclear waste is the extent to which contaminants buried underground may interact with, and contaminate, groundwater.
4. Water Pollution In The Twenty-first Century
The twentieth century was a period of rapid technological development and population growth. The second half of the century saw the development of intensive agricultural systems, widespread nuclear power generation, and the synthesis and use of a wide variety of new organic chemicals. The wastes arising from these, and other, processes contain a wide variety of polluting substances, some of which are extremely mobile and persistent in the environment. There is now, however, a much greater knowledge of the impacts of such pollutants, and the need for controlled disposal and subsequent environmental monitoring is widely recognized. Technological developments have taken place which produce cleaner effluents and allow remediation of contaminated areas, and a much stronger legal framework exists in many countries to control polluting processes which may pose a threat to water quality. Pressure on terrestrial water resources will inevitably increase over the twenty-first century as the world’s population grows, however, and it remains to be seen whether the quality of this water can be maintained.
Bibliography:
- Alloway B J, Ayres D C 1993 Chemical Principles of Environmental Pollution. Blackie Academic and Professional, London
- Hoffman A J 1995 An uneasy rebirth at Love Canal. Environment 37(2): 4–9, 25–31
- Holdgate M W 1979 A Perspective of Environmental Pollution. Cambridge University Press, Cambridge, UK
- Homewood B 1991 Mercury poisoning confirmed among Amazon villagers. New Scientist 132: 18
- Jackson A R W, Jackson J M 1996 Environmental Science: the Natural Environment and Human Impact. Longman, Harlow, UK
- Nickson R, McArthur J, Burgess W, Ahmed K M, Ravenscroft P, Rahman M 1998 Arsenic poisoning of Bangladesh groundwater. Nature 395: 338
- Pickering K T, Owen L A 1997 An Introduction to Global Environmental Issues, 2nd ed. Routledge, London




